Abstract
Trigonella foenum graecum L. is a dietary herb used in traditional medicine system. In this study, we investigated the cytotoxicity, antitumor, antimetastatic and antiangiogenic effect of the steroidal compound, ethyl iso-allocholate isolated from T. foenum graecum L. seeds against A549 lung cancer cells in vitro and in vivo. Among all the isolated compounds, the ethyl iso-allocholate rendered the highest cytotoxicity potential. It showed least percentage cell viability in trypan blue assay and lowest nuclei count in hoechst staining. The caspase glo assay and western blot analysis showed the significant caspase 3 cleavage, indicating caspase dependent apoptosis. Consistent with the in vitro data, ethyl iso-allocholate showed highest percentage tumor growth inhibition i.e. 80 ± 5% in zebrafish, equivalent to doxorubicin. It significantly reduced angiogenesis to 5 ± 0.8% (**P < 0.01), compared to negative control group which was 60 ± 2%. The ethyl iso-allocholate showed 55 ± 3% inhibition in liver metastasis. To investigate the safety of the compounds on normal tissues, the percentage mortality was examined. The ethyl iso-allocholate showed zero percent mortality of zebrafish. These results indicate that the steroidal derivative isolated from T. foenum-graecum seeds induces caspase dependent apoptosis in cancer cells and reduces tumor growth, metastasis and angiogenesis in vivo, as well as it is safe on the normal tissues. The in vitro and in vivo anticancer studies suggest that the cytotoxic compound ethyl iso-allocholate has potential application in pharmaceutical industry.
Keywords: Angiogenesis, Apoptosis, Caspase, Metastasis, Safety
1. Introduction
Cancer is a threat for developing as well as developed countries. The genetic variability of the cancer patients is the major issue in the treatment of cancer, which can be overcome by giving personalized treatment to the patient. This approach provides higher treatment efficacy, coupled with lesser side effects [1], however it also raised the demand of newer cytotoxic agents. The plethora of chemical compounds from plants provide higher opportunity to discover new cytotoxic compounds which assist in achieving the goal of multi-targeted therapy, reduce the number of drugs, drug–drug interaction and side effects [2].
Trigonella foenum graecum L. is also known as fenugreek belongs to family Fabaceae. It is an annual herb indigenous to Mediterranean countries and is cultivated throughout Asia. The plant is approximately 60 cm tall with pale yellow triangle shaped flowers and trifolial leaves. It contain pods 7.5 cm in length, each pod contain up to 29 seeds [3]. Traditionally, it is used as an antidiabetic, anti-hyperlipidemic, anti-ulcer, anti-inflammatory, galactogogue, and in wound-healing [4]. It is also used to treat dropsy, paralysis, gout, weakness and piles [5]. The methanol extract of T. foenum graecum L. seeds showed cytotoxicity against HepG2, hepatocellular cancer cells which was mediated by upregulation of PCNA, Bax, and caspase 3 activation [6]. The seed extract also induced apoptosis in breast and colon cancer cells via intrinsic and extrinsic pathway [7,8]. The fenugreek seed extract have protective action on pulmonary fibrosis, it alter T-AOC, MDA, NRF2, NO-level, SOD, GSH, and OH-1 mRNA expression, thereby increase lung antioxidant status and peripheral blood oxygen content [4]. Several lines of evidence show that fenugreek seed also act as antioxidant [9], hepatoprotective [10], neuroprotective [11], antihypertensive [12], and have estrogenic effect [13]. The anti-inflammatory action of fenugreek seed is due to inhibition of cyclooxgenase enzyme (COX-1 and COX-2) and lipid-peroxidation induced by flavonoids-C-glycosides, triglycerides and fatty-acids present in the seeds [14]. The fenugreek seeds increase the secretion of sex hormone in women and is used for breast enhancement [15]. Till now, diosgenin, yamogenin [16], trigonelline [17], polyhydroxystilbenes [18], furostanol saponins [19] and various other constituents [20] have been isolated from fenugreek. In this study, we evaluated the cytotoxicity, antitumor, antimetastatic and antiangiogenic potential of the cytotoxic compounds isolated from T. foenum graecum L. seeds on zebrafish (Danio rerio).
2. Methods
2.1. General experimental procedures
The ultra-violet spectra were recorded on a UV-1800 Shimadzu, UV-spectrophotometer, India. The compounds were visualized by ultra-violet irradiation at 254 and 366 nm. The IR affinity-1 fourier transform infrared spectrophotometer, Shimadzu, India was used for the measurement of the IR spectra. The 1H and 13C NMR spectra were recorded in Bruker’s advance-III 400 MHz, 500 MHz and 700 MHz spectrophotometer. The gas chromatography-mass spectroscopy was performed on Agilent’s (Agilent Technologies, Palo Alto, CA) 7890 GC system with 5975C inert XL EI/CI MSD with triple detector equipped with a firmware version A.01.13 and software driver version 4.01 (054) gas chromatography with FID. All the chemicals and reagents used were purchased from the Sigma Aldrich. The analytical grade organic solvents were utilized for the extraction and column chromatography. The adsorbent and silica gel 60–120 mesh were purchased from the Himedia, India for the column chromatography.
2.2. Extraction and isolation
The fenugreek seeds (1.5 kg) were coarsely powdered, macerated with 50% v/v ethanol, concentrated under reduced pressure in a rotary evaporator (Heidolph; Germany) below 40 °C. The brown color crude extract obtained, was suspended with 95% v/v ethanol (500 ml) and sequentially partitioned with petroleum ether (3 × 500 ml), chloroform (3 × 500 ml), ethyl acetate (3 × 500 ml) and 80% v/v ethanol (3 × 500 ml). The 80% v/v ethanol fraction (6 g) was subjected to silica gel column (60–120 mesh) and eluted with chloroform: methanol: water gradient to provide 93 sub-fractions (20 ml each). These sub-fractions were analyzed by TLC and UV spectroscopy, to give six pure compounds (C1, C2, C3, C4, C5, and C6). After structure elucidation of the isolated compounds, they were tested for the cytotoxicity potential using sulphorhodamine B (SRB) assay against A549 lung cancer cells and compound six (C6) was found to be the most cytotoxic compound.
2.3. Screening of the isolated compounds for cytotoxicity
An experiment using sulphorhodamine B protein binding (SRB) assay for 24 h drug exposure was performed to estimate the percentage control. Consistent with the results of SRB assay, the lower and the higher concentrations i. e 20 μg/ml and 80 μg/ml of all the isolated compounds were selected for the further detailed study. Doxorubicin (also known as adriamycin), treated group was positive control group. After incubation of treated cells for 24 h at 37 °C, cells were trypsinized (2 wells/group) using Trypsin–EDTA (Gibco) (0.05%) then treated with 10 μl of the trypan blue dye (ThermoFisher Scientific) and incubated for 5 min at room temperature. The cells were counted at four corner square of hemocytometer. The total number was averaged and cell viability was determined using the formula: No. of cells/ml = average cell count × 104 × dilution factor. The cells in the remaining wells were treated with 20 mM hoechst 33342 solution (ThermoFisher) and incubated for 10min and imaged for counting nuclei using blue excitation filter in a fluorescent microscope (Olympus, Japan). Further, the MTT assay was performed, to confirm the effectiveness of most potent cytotoxic compounds. The MTT assay is a conventional method used to evaluate the mitochondrial integrity [21]. Cells were treated with 100 μl MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (5 mg/ml) Roche, USA and incubated for 4 h at 37 °C. Media was removed and 150 μl DMSO was added to each well and shaken carefully to dissolve formazan crystals and further incubated for 1–2 h at room temperature. Untreated media was used as a negative control while for positive control Doxorubicin was used. Absorbance was recorded at 570 nm using a plate reader with a reference range of 690 nm. The course of the experiment was repeated three times.
2.4. Caspase assay
The caspase 3/7 assay kit (Promega, USA) was used to measure the caspase activity. The cells in each well including blank were treated with 100 μl of the caspase glo reagent and incubated for 2–3 h at room temperature. The plates were transferred to a luminometer machine (Berthold Technologies, India) for measuring luminescence and results were analyzed.
2.5. Western blot assay
The A549 cancer cells were harvested and total protein was extracted using triple lysis buffer method. To analyze the total protein SDS-PAGE was performed and the gel was transferred to a polyvinylidene difluoride (PVDF) membrane and processed further. After blocking for 1 h, the membrane was incubated overnight at 4 °C with the antibody caspase 3 (1:200) (Santacruz Biotechnology, USA) and β-actin as a loading control for the experiment. The membrane was rinsed three times with the mixture of tris-buffered saline and polysorbate 20 (TBST) and incubated with horseradish peroxidase labeled secondary antibody (1:5000) for 1 h at room temperature. Post incubation, the membrane was rinsed again three times with TBST before scanning the band.
2.6. Antitumor activity of compounds on zebrafish
The wild-type zebrafish, weighing 0.45–0.50 g, length 4–5 cm were housed under 14:10 h light/dark regimen, 27 ± 1 °C, oxygen saturation exceeding 70% and pH 7.4–8 were maintained in the 10 L tanks. The test compounds were dissolved in 3 μl of compound dissolving medium(1ml phosphate buffered saline containing 20% DMSO and 30% fish feed grade coconut oil) and added to a pellet. The pellets were stored at 4 °C. The zebrafish were induced with tumor through xenotransplantation of log phase cells. The fishes were anesthetized using cold water at 10 °C and 3 μl of 1 × 106 log phase A549 cells were injected into the gills section at multiple sites to induce a tumor. The gradual cooling method is used for anesthesia due to its safety and effectiveness for the short term procedure [22]. The fishes were transferred to the housing tanks and maintained for 21 days until tumor reached maximum growth phase. The group sizes of six adult fish were employed. Groups were treated with compounds through oral dosing and treated groups were compared to vehicle treated group, to quantify activity against tumor. The two concentrations i.e. 3 μg and 30 μg of all the samples were given orally per day for 14 days.
After treatment, fishes were anesthetized with 10 °C water and sacrificed by a cut between the brain and the spinal cord. The local tissues near the gills, the gills and liver were isolated and smeared on a glass slide and stained with hematoxylin & eosin respectively for 2 min each followed by phosphate-buffered saline (PBS) washes. The slides were viewed using Labomed 400. The total tumor size was measured using image J software analysis. For quantifying tumor, 100 cells were counted under 40X microscope per field. This was done for 10 field totaling to a 1000 cells. The following formula was used for obtaining the percentage. The total number of tumor cells/total number of cells counted X 100. For quantifying metastasis, the number of secondary tumor sites were quantified. The following formula was used for obtaining the percentage. The total number of secondary tumor sites/4 × 100. A T4 stage tumor zebrafish has four secondary tumor sites and hence, the denominator is four. For quantifying neo-vascularization (angiogenesis), Image J, the software provided by National Institute of Health, USA, was used. The neo-vascularized blood vessels were selected using the area selection tool. The total area quantified in terms of millimeter square by the software was used for scoring. The formula used for percentage neovascularization was, total area of neo-vascularized tissue in the test specimen/80 × 100. A T4 stage tumor zebrafish has a neo-vascularization level of 80 mm square. The mortality was counted on an everyday basis to understand mortality curve over therapeutic intervention.
2.7. Ethical for animal experimentation
The Good Animal Practice as per Institutional Animal Ethical Committee in accordance with Committee for the Purpose of Control and Supervision of Experiments (CPCSEA), India, was followed in adherence to established protocols.
2.8. Statistical analysis
The statistical comparisons were made using Graph Pad prism (Graph Pad Software Inc., CA, USA). The student-t test with a 95% confidence interval was performed using a two-way ANOVA at an alpha = 0.05 (95% confidence interval). The significance is denoted with asterisks, ns not significant, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
2.9. Structural determination of the active compounds
The compound six was obtained as a white amorphous powder (methanol: water; 90:10 v/v). It has a base peak at m/z 43 (ESI-MS), corresponding to the molecular formula of C26 H44 O5. The functional groups of carbonyl (1718.58 cm−1), C–O stretching (1100 cm−1), C–H sp3 stretching (2881.65 cm−1), and aromation of C–H bending (808.17 cm−1) were confirmed by the IR spectrum. The 1H NMR spectra of compound six showed three hydrogen peaks at high field region at δH 5.647 (q, J7.01 Hz), δH 5.608 and δH 5.122 indicating hydrogen attach to carbonyl (C–O) group. The peaks at δH 3.998 (q, J2.79) and δH 3.732 indicate hydrogen positioned near hydroxyl group. The two peaks at δH 2.608 and δH 2.378 (q, J7.27) indicate hydrogen located near carboxyl group. In the low field region, peaks showed rest of the hydrogen attached to sp3 hybridized carbon. The 13C NMR spectrum showed one carboxyl carbon C==O at (δC 177.27), carbon attached with oxygen C–Oat (δC 61.76) and three carbon attached to hydroxyl group i.e. (δC 79.01), (δC 77.27) and (δC 69.01), while other carbons showed peak at low field region ranging from δC 20.01 to δC 31.93. The above spectral data coupled with literature indicate that the compound is homologue of Ethyl iso-allocholate.
3. Results
3.1. Fractionation and isolation of compounds
The fractionation of 50% v/v ethanol extract (32.6 g) of fenugreek seeds with various solvents yielded pale yellow colored petroleum ether fraction 1.027 g (3.15%w/w), brown colored chloroform fraction 1.64 g (5.22%w/w), yellowish brown colored ethyl acetate fraction 4.2 g (14.169%w/w) and brown colored 80% v/v ethanol fraction 7.29 g (28.384%w/w). The 6 g of 80% v/v ethanol fraction was subjected to column chromatography to afford six pure compounds. The structure elucidation of the compounds were performed using GC–MS, IR, 1H NMR and 13C NMR spectroscopy (Supplementary material). The compounds isolated were identified as Dibutyl phthalate (0.78%w/w); Acetamide, N-[4-(4-hydroxybenzylidenamino) phenyl]-(0.1866%w/w); Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy, methyl ester (1.1888%w/w); 2H-Indeno [1,2-b] furan-2-one, 3, 3a,4,5,6,7,8,8b-octahydro-8,8-dimethyl (1.255%w/w); Phenol, 2,4-bis(1,1-dimethylethyl) (1.45%w/w); and Ethyl iso-allocholate (3.46%w/w).
3.2. Screening of isolated compounds for cytotoxicity potential
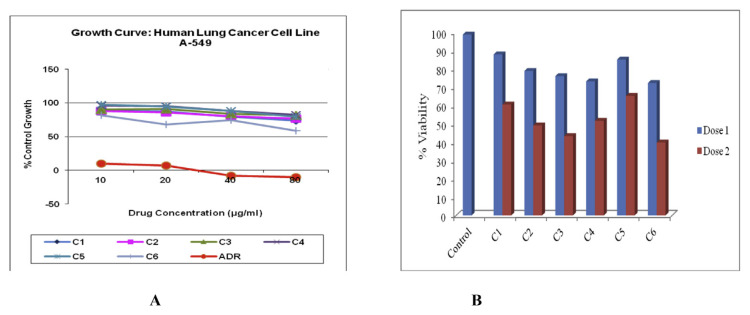
All the isolated compounds were subjected for cytotoxicity screening against A549 cancer cells. The compound six namely ethyl iso-allocholate, at the dose of 80 μg/ml showed 58.7% control growth in SRB assay (Fig. 1A), indicating that compound six has highest cytotoxicity potential as compare to other isolated compounds. The optimum dose of different compounds was determined by trypan blue viability assay. Following treatment, viability was affected in all experimental groups at both the doses i.e 20 μg/ml and 80 μg/ml (Fig. 1B). At the dose 20 μg/ml, compound three, four and six seemed to be effective in suppressing the cell division of the cancer cells, whereas with dose 80 μg/ml, compound two, three and six critically affected viability of the cells after 24 h post treatment. The compound six showed least percentage viability i.e. 39.825%, as compare to control group which was 90.03%. The hoechst staining showed less number of nuclei in the treatment groups with lowest number in compound three, four and six with dose 20 μg/ml and compound two, three, and six with dose 80 μg/ml, consistent with our trypan blue staining results. The compound six showed reduced average nuclei count i.e. 2342.5 nuclei at the dose of 80 μg/ml, as compare to control group, which was 8317.5 (Fig. 2).
Fig. 1.
Cytotoxicity study of isolated compounds of Trigonella foenum graecum L. against A549 cancer cells. (A) All the isolated compounds were tested for cytotoxic potential using SRB assay against A549 cells. (B) Optimum dose for different isolated pure compounds were determined by trypan blue viability assessment. Dose 1 is 20 μg/ml and dose 2 is 80 μg/ml. The x-axis denotes test groups and y-axis denotes percentage viability. Compound 6 (C6) at the dose of 80 μg/ml showed less viable cells i. e 39.825 as compare to control group which was 98.715.
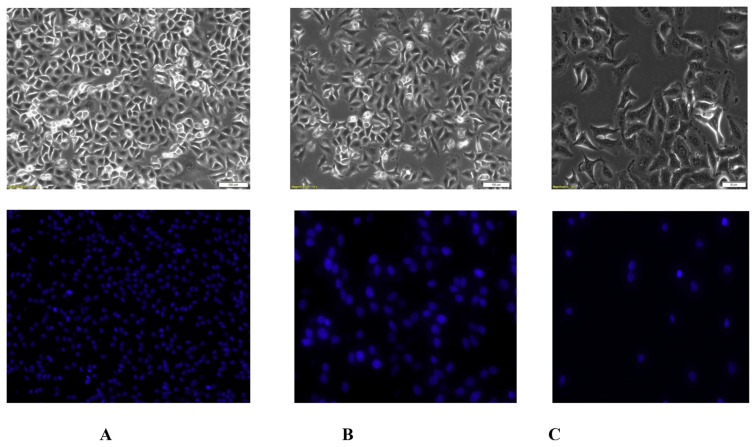
Fig. 2.
Morphological characters and Nuclei count of A549 cancer cells. Effect of isolated compounds on A549 cells was observed after 24 h exposure to the compounds. The bright field images showed confluent growth in control group. The hoechst stained A549 cells were observed under fluorescence microscopy. After treatment, reduced number of cells and lowest nuclei count was observed in compound 6 at both the doses. (A) The negative control group showed highest cell density. (B) Cells treated with compound six at the dose of 20 μg/ml. (C) Cells treated with compound six at the dose of 80 μg/ml showed significantly reduced number of cells.
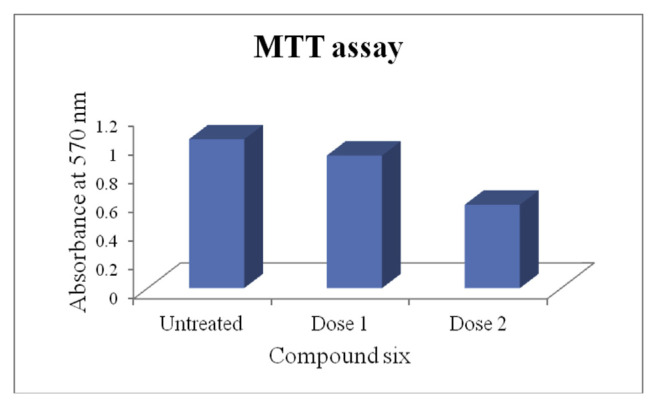
The MTT assay revealed the effectiveness of compound six at two different doses, higher cytotoxicity with dose 80 μg/ml. The untreated cells showed higher absorbance value i.e. 1.035 indicating metabolically active cells, whereas in both the treated groups lower absorbance value, lowest in dose 80 μg/ml apparently i.e. 0.58, indicating susceptible cells as a result of treatment with compound six (Fig. 3).
Fig. 3.
MTT assay. MTT assay revealed effectiveness of compound six at two different doses with higher cytotoxicity at dose 2 than dose 1. Dose 1 is 20 μg/ml and dose 2 is 80 μg/ml.
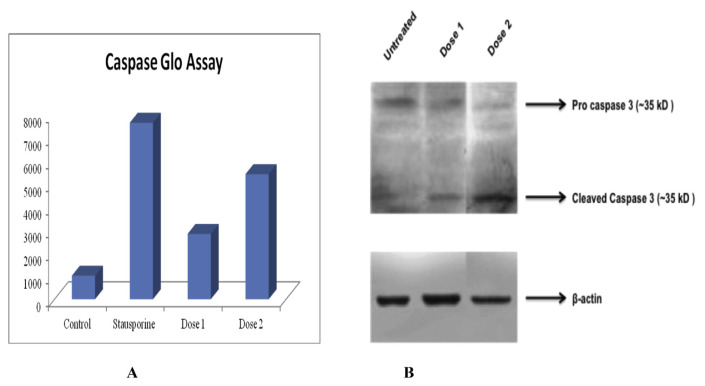
In caspase glo assay, luminescence measured is proportional to the amount of the caspase activity present and thus higher luminescence indicates greater apoptotic cells in an experimental group. In the assay, compound six at the dose of 80 μg/ml showed higher luminescence i.e. 5438.25, indicating extensive apoptotic nature of the cells in that group as compare to negative control group, where lower luminescence i.e. 1021.75 was recorded (Fig. 4A). The stausporine and untreated samples was positive and negative control respectively.
Fig. 4.
Caspase glo assay and western blotting. (A) Caspase glo assay revealed higher luminescence i.e. 5438.25 in compound six treated group at the dose of 80 μg/ml, as compare to control group which was 1021.25. The high luminescence indicates higher caspase activity and more apoptotic cells than control group. Dose 1 is 20 μg/ml and dose 2 is 80 μg/ml. (B) Western blot analysis showed expression of cleaved caspase at both the dose of ethyl iso-allocholate. The β-actin was used as loading control that expressed well in all groups nullifying the loading errors.
Western blot analysis showed expression of cleaved caspase in both the test groups, i.e. 20 μg/ml and 80 μg/ml indicating activation of apoptotic pathways in the cells. At higher dose i.e. 80 μg/ml, the presence of a thick band of cleaved caspase suggest increased activation of pro-apoptotic signal in that particular group as compare to the 20 μg/ml. We used beta actin as loading control that expresses well in all our groups nullifying loading errors (Fig. 4B). The present study implies the use of the compound six at higher dose to be cytotoxic at the given time interval.
3.3. In vivo anticancer activity
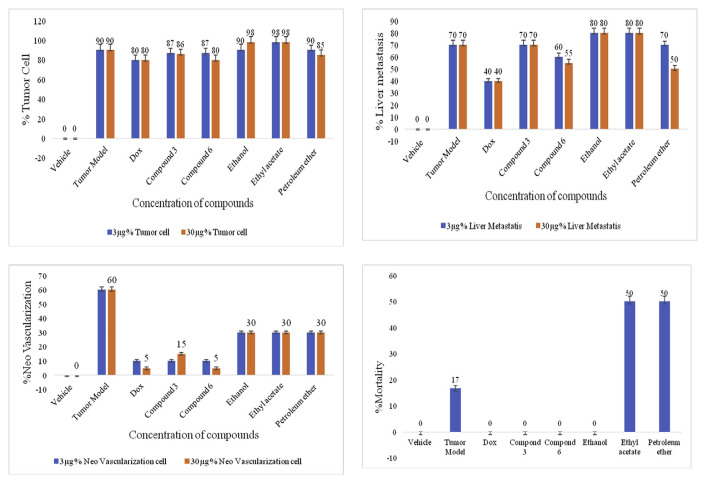
The maximum inhibition in tumor cell proliferation was shown by compound six i.e. 80 ± 5%, equivalent to doxorubicin with the dose of 30 μg (Fig. 5A, Fig. 6A). The negative control group showed maximum tumor migration into the liver called liver metastasis i.e. 70 ± 4%, which was reduced in petroleum ether fraction and compound six treated groups. The petroleum ether fraction treated group reduced the liver metastasis up to 50 ± 3%, followed by compound six i.e. 55 ± 3%, but the results were not equivalent to positive control group (doxorubicin) i.e. 40 ± 2% (Figs. 5B and 6B). The negative control group showed the maximum formation of new blood vessels called neovascularization i.e. 60 ± 2%, which was reduced in all the sample treated groups, with significant reduction in compound six treated group i.e. 5 ± 0.8% (**P < 0.01), equivalent to positive control group (Fig. 5C). During the experiment, negative control group showed 17 ± 1% mortality rate, but no mortality was observed in compound six, compound three and ethanol fraction treated groups (Fig. 5D). Thus, it is considered that the isolated compounds and the mother fraction are safe on normal cells. The vehicle was significantly different from tumor model with ***P < 0.001 indicating validity of the model and compound six was significantly different from tumor model **P < 0.01 indicating curative properties of compound six.
Fig. 5.
In vivo cytotoxicity evaluation of bioactive compounds. (A) Percentage tumor cell after treatment with samples. The ethyl iso-allocholate at the dose of 30 μg showed maximum growth inhibitory effect, equivalent to doxorubicin (positive control) i.e. 80 ± 5. (B) Percentage liver metastasis. The ethyl iso-allocholate and petroleum ether fraction showed least percentage liver metastasis i.e. 55 ± 3 and 50 ± 3, respectively. (C) Percentage neo-vascularization. The ethyl iso-allocholate treated group showed significantly less formation of new blood vessels (**P < 0.01) i.e. 5 ± 0.8 as compared to the negative control group which was 60 ± 2. (D) All the compounds showed zero percent mortality except ethyl acetate and petroleum ether fraction. dox refers to doxorubicin, compound 3 is benzenepropanoic acid, 3,5-bis (1,1-dimethylethyl)-4-hydroxy, methyl ester and compound 6 is ethyl iso-allocholate.
Fig. 6.
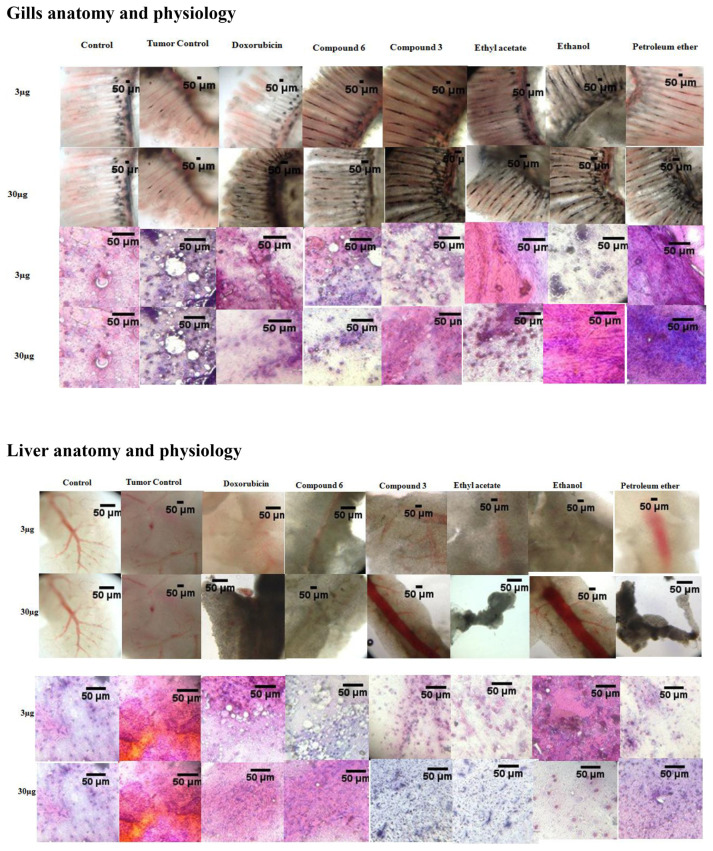
Changes in anatomy and physiology of gills and liver of zebrafish. The compound 3 refers to benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy, methyl ester and compound 6 refers to ethyl iso-allocholate. (A) Gills anatomy and pathology after treatment, (B) Liver anatomy and pathology after treatment.
4. Discussion
In this study, we isolated six pure compounds from the 80% v/v ethanol fraction of 50% v/v ethanol extract of fenugreek seeds using column chromatography. The extraction of fenugreek seeds was carried out using maceration method. The extract was further divided into various fractions by liquid–liquid partitioning method. Only cold processes were selected during the experiment to avoid degradation of natural compounds. All the fractions were tested for cytotoxicity potential against A549 lung cancer cells. The A549 cell line was selected on the basis of our previous study (Table 1 & Fig. 1 Supplementary material). The 80% v/v ethanol fraction showed 90% growth inhibition against A549 cancer cells which was high as compared to the other fractions. The column chromatography of the 80% v/v ethanol fraction was carried out in order to obtain the bioactive compounds. We obtained six pure compounds from the chromatography and structure elucidation of the compounds was carried out using various analytical techniques. The results of IR spectroscopy showed the types of functional group present in the compounds. The GC–MS technique compared the GC graph with the mass spectra taken at different time interval and provided the probable compounds in the sample along with their molecular weight and molecular formula. All the data were compared with NIST library data. The 1H NMR and 13C NMR spectra were obtained which showed the number of proton and 13C carbon, present in different environment, respectively. All the spectral data for the six samples were compared to identify the isolated compounds. The isolated samples were tested against A549 cancer cell, for the cytotoxicity potential. The compound six namely ethyl iso-allocholate, a steroidal derivative showed highest percentage control growth, followed by compound three. To study the mechanism of action of ethyl iso-allocholate, the cell were stained with trypan blue and hoechst 33258 stains.
Apoptosis is a programmed cell death characterized by the morphological changes including cell shrinkage, membrane blebbing, reversal of phosphatydylserine from inside to outside the cell membrane, condensed chromatin, DNA fragmentation and apoptotic bodies formation [23].
The trypan blue dye enters into the dead cells through cell membranes pores, which indicate the altered membrane integrity of the cells [24,25]. The DNA of live and necrotic cells are intact, while apoptotic cell show condensed DNA. The hoechst stain is permeable to the cell membrane, and enters into the cell and migrate inside the nucleus, and bind with the minor groove of condensed double stranded DNA, preferably to adenine-thymine rich region [26,27]. The above staining process indicate that compound six cause cell membrane damage and DNA fragmentation resulting into cell death. Apoptosis is mediated by various signaling pathways including caspases. There are two types of apoptotic pathways that occur inside the cell for the DNA fragmentation namely extrinsic and intrinsic pathways. The activation of caspase 3 is common event in both the pathways [2]. The caspase 3 is a cysteine dependent aspartate directed protease. It recognizes ‘DEVD’ sequence of the protein (d-aspartate, E-glutamate, V-valine). It is present in cytoplasm in an inactive form pro-caspase 3 and cleaves into the active form caspase 3 after proteolytic activation by upstream caspases. It plays a key role in apoptosis. The signaling molecule, caspase 3 is able to cleave a variety of substrates resulting into cell death. The biochemical and morphological characteristics observed in apoptotic cells depends on the types of substrate cleaved by caspase 3. Due to vast range of substrates, caspase 3 is considered as a common mediator for apoptosis [28,29]. The compound six significantly increase the cleavage of pro-caspase 3 into caspase 3 in A549 cells which was detected by caspase glo assay and further confirmed by western blot assay. These results confirmed that apoptosis of A549 cells takes place in a caspase dependent manner.
The in vivo study was performed to evaluate the anticancer potential of compound six and to compare its effect with mother fraction i.e. 80% v/v ethanol fraction on zebrafish. The compound six showed maximum tumor growth inhibition i.e. 80 ± 5%, with the dose of 30 μg, equivalent to the doxorubicin (positive control) which was consistent with in vitro results. The compound six reduced the migration of tumor cells into the liver called liver metastasis. The negative control group showed liver metastasis, confirming uncontrolled tumor, whereas doxorubicin and compound six treated groups showed controlled tumor growth with both the concentration, 3 μg and 30 μg. During metastasis, the tumor cells detach from its signal site and migrate into the extracellular collagen, where they release the matrix metalloproteinase that digests the basement membrane and eventually enters into the blood streams. These tumor cells migrate into the blood circulation and attach to the new tissues where they start cell division, causing the severe condition of cancer [30]. The compound six showed 55 ± 3% liver metastasis, which was much less then negative control group. The formation of new blood vessels from the existing vessels is called angiogenesis or neo-vascularization and is a critical event in cancer. It takes place to fulfill the demand of oxygen and nutrients into the budding tumor cells. The percentage neo-vascularization (new vessels) formed in compound six treated group was significantly less i.e. 5 ± 0.8 (**P < 0.01) as compared to the negative control group which was 60 ± 2%. During the experiment, the percentage mortality of zebrafish was observed, to investigate the safety of compounds in zebrafish. The doxorubicin, compound six, compound three, and ethanol fraction treated group showed zero percent mortality.
5. Conclusion
In conclusion, ethyl iso-allocholate isolated from fenugreek seeds is a steroidal derivative. Moreover, ethyl iso-allocholate induces A546 lung cancer cell apoptosis, by activation of caspase signaling pathway. Notably, it also showed reduction in tumor growth, liver metastasis, and angiogenesis in zebrafish. It was safe on normal tissues as it showed zero percent mortality of zebrafish. Thus, our study provides important information regarding the presence of new potent anticancer agent in fenugreek seeds.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jfda.2018.05.001.
Footnotes
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1. Hutchinson L. Breast cancer: challenges, controversies, breakthroughs. Nat Rev Clin Oncol. 2010;10:669. doi: 10.1038/nrclinonc.2010.192. [DOI] [PubMed] [Google Scholar]
- 2. Kitai Y, Zhang X, Hayashida Y, Kakehi Y, Tamura H. Induction of G2/M arrest and apoptosis through mitochondria pathway by a dimer sesquiterpene lactone from Smallanthus sonchifolius in HeLa cells. J Food Drug Anal. 2017;25:619–27. doi: 10.1016/j.jfda.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patil S, Jain G. Holistic approach of Trigonella foenum-graecum in phytochemistry and pharmacology-A review. Curr Trends Technol Sci. 2014;3:34–48. [Google Scholar]
- 4. Kandhare AD, Bodhankar SL, Mohan V, Thakurdesai PA. Effect of glycosides based standardized fenugreek seed extract in bleomycin-induced pulmonary fibrosis in rats: decisive role of Bax, Nrf2, NF-jB, Muc5ac, TNF-a and IL-1b. Chem Biol Interact. 2015;237:151–65. doi: 10.1016/j.cbi.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 5. Bin-Hafeez B, Haque R, Parvez S, Pandey S, Sayeed I, Raisuddin S. Immunomodulatory effects of fenugreek (Trigonella foenum graecum L.) extract in mice. Int Immunopharmacol. 2003;3:257–65. doi: 10.1016/S1567-5769(02)00292-8. [DOI] [PubMed] [Google Scholar]
- 6. Khalil MIM, Ibrahim MM, El-Gaaly GA, Sultan AS. Trigonella foenum (Fenugreek) induced apoptosis in hepatocellular carcinoma cell line, HepG2, mediated by upregulation of p53 and proliferating cell nuclear antigen. BioMed Res Int. 2015;11 doi: 10.1155/2015/914645. Article ID 914645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prakash E, Gupta DK. Trigonella foenum-graecum (fenugreek) extract exhibit anti-cancer activity when checked against Human Cancer Cell Line MDAMD453. Journal of Environmental Science. Comput Sci Eng Technol. 2014;3(3):1196–202. [Google Scholar]
- 8. Raju J, Patlolla JM, Swamy MV, Rao CV. Diosgenin, a steroid saponin of Trigonella foenum-graecum, inhibits azoxymethane induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol Biomarkers Prev. 2004;13:1392–8. [PubMed] [Google Scholar]
- 9. Kaviarasan S, Naik GH, Gangabhagirathi R, Anuradha CV, Priyadarsini KI. In vitro studies on antiradical and antioxidant activities of fenugreek (Trigonella foenum graecum) seeds. Food Chem. 2007;103:31–7. [Google Scholar]
- 10. Zargar S. Protective effect of Trigonella foenum-graecum on thioacetamide induced hepatotoxicity in rats. Saudi J Biol Sci. 2014;21:139–45. doi: 10.1016/j.sjbs.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaur V, Bodhankar SL, Mohan V, Thakurdesai PA. Neurobehavioral assessment of hydroalcoholic extract of Trigonella foenum-graecum seeds in rodent models of Parkinson’s disease. Pharm Biol. 2013;51:550–7. doi: 10.3109/13880209.2012.747547. [DOI] [PubMed] [Google Scholar]
- 12. Balaraman R, Dangwal S, Mohan M. Antihypertensive Effect of Trigonella foenum-greacum. seeds in experimentally induced hypertension in rats. Pharm Biol. 2006;44:568–75. [Google Scholar]
- 13. Steels E, Steele ML, Harold M, Coulson S. Efficacy of a proprietary Trigonella foenum-graecum L.de-husked seed extract in reducing menopausal symptoms in otherwise healthy women: a double-blind, randomized, placebo-controlled study. Phytother Res. 2017;31:1316–22. doi: 10.1002/ptr.5856. [DOI] [PubMed] [Google Scholar]
- 14. Liu Y, Kakani R, Nair MG. Compounds in functional food fenugreek spice exhibit anti-inflammatory and antioxidant activities. Food Chem. 2012;131:1187–92. [Google Scholar]
- 15. Basch E, Ulbricht C, Kuo G, Szapary P, Smith M. Therapeutic applications of fenugreek. Alternative Med Rev. 2003;8:20–7. [PubMed] [Google Scholar]
- 16. Ouzir M, El Bairi K, Amzazi S. Toxicological properties of fenugreek (Trigonella foenum graecum) Food Chem Toxicol. 2016;96:145–54. doi: 10.1016/j.fct.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 17. Zhou J, Chan L, Zhou S. Trigonelline: a plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr Med Chem. 2012;19:3523–31. doi: 10.2174/092986712801323171. [DOI] [PubMed] [Google Scholar]
- 18. He Y, Lv H, Wang X, Suo Y, Wang H. Isolation and purification of six bioactive compounds from the seeds of Trigonella foenum-graecum L. using high-speed countercurrent chromatography. Separ Sci Technol. 2014;49:580–7. [Google Scholar]
- 19. Pang X, Yu H, Kang L, Feng B, Zhao Y, Xiong C, et al. Two new furostanol saponins from the seeds of Trigonella foenumgraecum. J Asian Nat Prod Res. 2011;13:611–7. [Google Scholar]
- 20. Lambert JP, Cormier A. Potential interaction between warfarin and boldo-fenugreek. Pharmacotherapy. 2001;21:509–12. doi: 10.1592/phco.21.5.509.34492. [DOI] [PubMed] [Google Scholar]
- 21. Yen G, Chen C, Chang W, Wu M, Cheng F, Shiau D, et al. Antioxidant activity and anticancer effect of ethanolic and aqueous extracts of the roots of Ficus beecheyana and their phenolic components. J Food Drug Anal. 2018;26:182–92. doi: 10.1016/j.jfda.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Collymore C, Tolwani A, Lieggi C, Rasmussen S. Efficacy and safety of 5 anesthetics in adult zebrafish (Danio rerio) J Am Assoc Lab Anim Sci. 2014;53:198–203. [PMC free article] [PubMed] [Google Scholar]
- 23. Bai K, Sheu F. A novel protein from edible fungi Cordyceps militaris that induces apoptosis. J Food Drug Anal. 2018;26:21–30. doi: 10.1016/j.jfda.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tran S, Puhar A, Ngo-Camus M, Ramarao N. Trypan blue dye enters viable cells incubated with the pore-forming toxin HlyII of Bacillus cereus. PLoS One. 2011;6:e22876. doi: 10.1371/journal.pone.0022876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garzon I, Carriel V, Marı’n-Fernández AB, Oliveira AC, Garrido-Gómez J, Campos A, et al. A combined approach for the assessment of cell viability and cell functionality of human fibrochondrocytes for use in tissue engineering. PLoS One. 2012;7:e51961. doi: 10.1371/journal.pone.0051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crowley LC, Marfell BJ, Waterhouse NJ. Analyzing cell death by nuclear staining with hoechst 33342. Cold Spring Harb Protoc. 2016;9 doi: 10.1101/pdb.prot087205.pdb.prot087205. [DOI] [PubMed] [Google Scholar]
- 27.Singh US, Kumar J, Sachan A, Singh P. Use of DNA-binding flourochromes for the nuclear staining in fungi. In: Singh RP, Singh US, editors. Molecular methods of plant physiology. United state of America: CRC Press Inc; 1995. pp. 53–9. [Google Scholar]
- 28. Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase 3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–84. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 29. Datta R, Kojima H, Yoshida K, Kufe D. Caspase-3 mediated cleavage of protein kinase C theta in induction of apoptosis. J Biol Chem. 1997;272:20317–20. doi: 10.1074/jbc.272.33.20317. [DOI] [PubMed] [Google Scholar]
- 30. Brábek J, Mierke CT, Rösel D, Veselý P, Fabry B. The role of the tissue microenvironment in the regulation of cancer cell motility and invasion. Cell Commun Signal. 2010;8:22. doi: 10.1186/1478-811X-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]