Abstract
Cardiac hypertrophy is widely diagnosed in clinical cardiac disorders. The pathophysiology of hypertrophy is complex and multifactorial, a series of molecular and cellular changes are participated, such as activation of different signaling pathways, a switch of fetal gene program in the myocardium, and apoptosis. Some biomarkers have been applied to assess cardiac hypertrophy including atrial natriuretic peptides (ANP), brain/B-type natriuretic peptides (BNP), and α- or β- Myosin Heavy Chain (MHC) in addition to others. Recently, ubiquitin-protein ligase E3A (UBE3A) has been observed to increase in cardiac hypertrophy. Therefore, UBE3A as a new biomarker seems valuable in the clinic. The cardiac hypertrophy is induced in rat-derived heart cell line H9c2 cells by potassium bromate (KBrO3), high glucose (HG), or isoproterenol (Iso), respectively. As an oxidizing agent, KBrO3 increased cell size at concentrations less than 250 μM. Similarly, HG and Iso also induced cardiac hypertrophy in H9c2 cells. Interestingly, each kind of the cell models promoted the gene expression of the well-known biomarkers of cardiac hypertrophy including atrial natri-uretic peptides (ANP) and brain/B-type natriuretic peptides (BNP). Additionally, UBE3A is also raised with the signals involved in cardiac hypertrophy such as calcineurin and nuclear factor of activated T-cells (NFAT) determined using Western blots. KBrO3 increased the protein levels of these signals and the specific inhibitor, such as cyclosporine A and tacrolimus, attenuated the signaling in H9c2 cells at concentrations sufficient to inhibit calcineurin in addition to the reduction of mRNA levels of UBE3A, similar to ANP or BNP. Moreover, HG or Iso also significantly increased protein levels of UBE3A in H9c2 cells. Taken together, we provided a new view that UBE3A is markedly raised in cardiac hypertrophy using various cell models, mainly through the activation of the calcineurin/
Keywords: Hypertrophic signals, Hyperglycemia, H9c2 cells, Isoproterenol, Potassium bromate
1. Introduction
Cardiac hypertrophy is known to induce heart failure because it is an important compensatory mechanism in response to physiological or pathological stimuli that involve regulation of cellular signaling mediators and transcript factors [1]. Hypertrophic signals result in increased protein synthesis and regulated cell cycles [2]. Cardiac hypertrophy is characterized by cell enlargement, which involves physiological and pathological hypertrophy [3]. Pathological cardiac hypertrophy is often coupled with interstitial and perivascular fibrosis, as well as apoptosis and the release of atrial natriuretic peptides (ANP) and brain/B-type natriuretic peptides (BNP). Upon initiation of the cardiac hypertrophy, concentric hypertrophy is the primary phenotype that resists high after-load and is known as the adaptive phase. Once the cardiac damage progresses, cell length increases, which leads to increased hypertrophy [4]. In cardiac hypertrophy, nuclear factor of activated T-cells (NFAT) is considered to be an important mediator of a number of signal–transduction pathways involved in the coordination of pathological stimulation [5]. In clinics, many biomarkers have been developed in the application. In addition to ANP or BNP, myosin filaments (the expression of α- and β-myosin heavy chain; MHC) and some potential biomarkers such as osteopontin, ST-2 receptor, osteoprotegerin, neopterin, urocortins, growth differentiation factor 15 and urotensin II have been introduced [6]. Most of them belonged to the fetal gene program (FGP) and an activation of FGP in the adult heart occurs after cardiac insults and is ubiquitously used as a biomarker of cardiac hypertrophy [7]. However, the used biomarkers for cardiac hypertrophy seem not provided enough answers to all clinical questions [8]. Therefore, a new, specific and sensitive biomarker is expected in an emergency.
The ubiquitin ligase UBE3A (an E3 ubiquitin ligase encoded by the Ube3a gene) is one of the important members of the ubiquitin proteasome system (UPS) [9]. It has been identified to express in the heart, liver, brain and other tissues. UPS is an ATP-dependent proteolytic system that requires the poly-ubiquitination of a protein intended for degradation [10]. An abnormal pathway in UPS may lead to the disorders of protein metabolism and cause the cardiac hypertrophy [11]. UBE3A mutations are associated with neurological defects in humans with Angelman syndrome [12]. Ube3a mutant mice appeared to have deficits in Ca2+/calmodulin-dependent kinase II (CaMKII) [13]. Moreover, CaMKII and calcineurin pathways played a critical role in cardiac hypertrophy [14,15]. Cardiac-specific activation of calcineurin or its downstream effector nuclear factor of activated T cells (NFAT) is sufficient to induce a robust hypertrophic response in transgenic mice [16]. Recently, gene expression of Ube3a is markedly elevated in cardiac hypertrophy induced by H2O2 [17]. Therefore, we are interested to develop UBE3A as a new biomarker for cardiac hypertrophy.
Role of reactive oxygen species (ROS) in the induction of cardiac hypertrophy has been well-established [18]. In the present study, we used potassium bromate (KBrO3), HG, or isoproterenol (Iso) to induce three models of cardiac hypertrophy in H9c2 cells [19] and investigated the changes of UBE3A in each model. Expression of Ube3a was extremely increased in three kinds of hypertrophic cells. Therefore, we suggest UBE3A as a new biomarker for diagnosis of cardiac hypertrophy.
2. Materials and methods
2.1. Materials
Isoproterenol (Iso), Potassium bromate (KBrO3), Cyclosporine A (CsA), Tacrolimus, and NFAT-inhibitor were purchased from Sigma–Aldrich (St. Louis, MO, USA). All other reagents were obtained from the supplier as indicated and were at least analytical grade. Antibodies used and their sources were also indicated below.
2.2. Cell culture
The H9c2 cells (BCRC No. 60,096) were maintained in Dulbecco's Modified Eagle's Medium (DMEM, pH 7.2; GIBCO-BRL Life Technologies, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum. The H9c2 cells were plated at a density of 6000 cells/cm2 and allowed to proliferate in the growth medium. After plating, the medium was replaced on the second day. On the next day, the cells were incubated with the testing agent(s) as subsequently described.
2.3. Induction of cardiac hypertrophy
H9c2 cells were applied to induce cardiac hypertrophy similar to three models; first one induced by oxidant potassium bromate (KBrO3) similar to H2O2 as described previously [20], second one incubated with HG following the established method [21], and the third was induced by treatment with isoproterenol (Iso) according to previous report [22]. The success of hypertrophic model was then confirmed below.
2.4. Measurement of cardiac hypertrophy
H9c2 cells were arranged on a 24-well plate (Greiner Bio-One, Monroe, North Carolina, USA). Cells were starved for 4 h in a serum-free medium before treatment with KBrO3 for 72 h. Briefly, after washing twice with cold phosphate-buffer solution (PBS), the cells were fixed in 4% paraformaldehyde at room temperature for 15 min and washed with PBS containing 2% bovine serum albumin and 0.1% Triton X-100. Cells were stained with rhodamine phalloidin (Invitrogen, Carlsbad, CA, USA) to identify the actin filaments and with 4–6-diamidine-2-phenylindole dihydrochloride (DAPI) (Abcam, Cambridge, MA, USA) to show the nucleus. An entire field of vision was characterized using a microscope (IX71 Olympus, Tokyo, Japan) connected to an imaging system (DP2-BSW, Olympus, Tokyo, Japan). The cell sizes were magnified 200 times and analyzed by the imaging system. Cell surface area size was determined and quantified by imaging to the complete boundary of individual cells. The results were subsequently expressed as a percentage change in the surface area level in cells based on the analysis using the NIH ImageJ software (Available online: http://imagej.nih.gov/ij/).
2.5. Real-time reverse-transcription-polymerase chain reaction
The mRNA expression levels of each signal were determined. In brief, total RNA was extracted from the cell lysates with TRIzol reagent (Carlsbad, CA, USA). Total RNA (200 ng) was reverse-transcribed into cDNA with random hexamer primers (Roche Diagnostics, Mannheim, Germany). All PCR experiments were performed using a LightCycler (Roche Diagnostics GmbH, Mannheim, Germany). The concentration of each PCR product was calculated relative to a corresponding standard curve. The relative gene expression was subsequently indicated as the ratio of the target gene level to that of β-actin. The primers for ANP, BNP, Ube3a, and β-actin are listed as follows:
ANP F: 5′-CACAGATCTGATGGATTTCAAGA-3’;
ANP R: 5′–CCTCATCTTCTACCGGCATC-3′;
BNP F: 5′-GTCAGTCGCTTGGGCTGT-3’;
BNP R: 5′–CCAGAGCTGGGGAAAGAAG-3′;
UBE3A F: 5′-GAATCACTGTTCTTTACAGCCTAGTTC-3’;
UBE3A R: 5′-GGATTTTCCATAGCGATCATCT-3’;
β-actin F: 5′-CTAAGGCCAACCGTGAAAAG-3′; β-actin R: 5′-GCCTGGATGGCTACGTACA-3′.
2.6. Western blotting analysis
We used ice-cold radio-immuno-precipitation assay (RIPA) buffer to extract the proteins from rat heart homogenates or cell lysates. Western blot analysis was subsequently performed according to our previous method [23]. The target antigens from the protein extracts were detected using primary antibodies specific for UBE3A (Abcam, Rockville, MD, USA), calcineurin (Sigma–Aldrich, St. Louis, Missouri, USA), NFAT3 (Thermo-Fisher Sci., Rockford, IL, USA), or β-actin (Sigma–Aldrich, St. Louis, Missouri, USA) and Histone H3 (Santa Cruz, Dallas, TX, USA). The expression level of histone H3 protein was determined as an internal control [24]. The bound primary antibodies were subsequently hybridized to horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgGs (Calbiochem, San Diego, CA, USA), and the immunoreactive bands were developed with a chemiluminescence kit (Perkin Elmer, Waltham, MA, USA). The optical densities of the bands for UBE3A (100 kDa), calcineurin (18 kDa), NFAT3 (105 kDa), Histone H3 (15 kDa), and β-actin (43 kDa) were quantified as described in our previous report [25].
2.7. Nuclear extraction
We performed the extraction of nuclear fraction using a CNMCS Compartmental Protein Extraction Kit (BioChain Institute, Inc., Hayward, CA, USA). Briefly, H9c2 cells were collected to add with ice-cold lysis buffer (2 ml per 20 million cells). The cell mixture was passed through the needle base 50–90 times to disrupt the cell membranes and to release the nuclei from the cells. The degree of cell membrane disruption and the release of nuclei were monitored with a microscope. The mixture was then centrifuged at 15,000 × g at 4 °C for 20 min. The supernatant, which contained cytoplasmic proteins, was removed and saved in a separate tube. The pellet was re-suspended in ice-cold wash buffer (4 ml per 20 million cells), and the suspension was rotated at 4 °C for 5 min, followed by centrifugation at 15,000 × g at 4 °C for 20 min. The supernatant was then removed and ice-cold nuclear extraction buffer (1 ml per 20 million cells) was added to the pellet. After rotating at 4 °C for 20 min, the suspension was centrifuged at 15,000 × g at 4 °C for 20 min. The supernatant, which contained nuclear proteins, was removed and saved for studies.
2.8. Statistical analysis
The results are presented as the mean ± SEM from the indicated sample size (n) in each group. Statistical analysis was performed using one-way analysis of variance (ANOVA), followed by Tukey's post-hoc analysis to compare the difference. P < 0.05 was considered significant.
3. Results
3.1. Effect of KBrO3 on the cell size of H9c2 cell
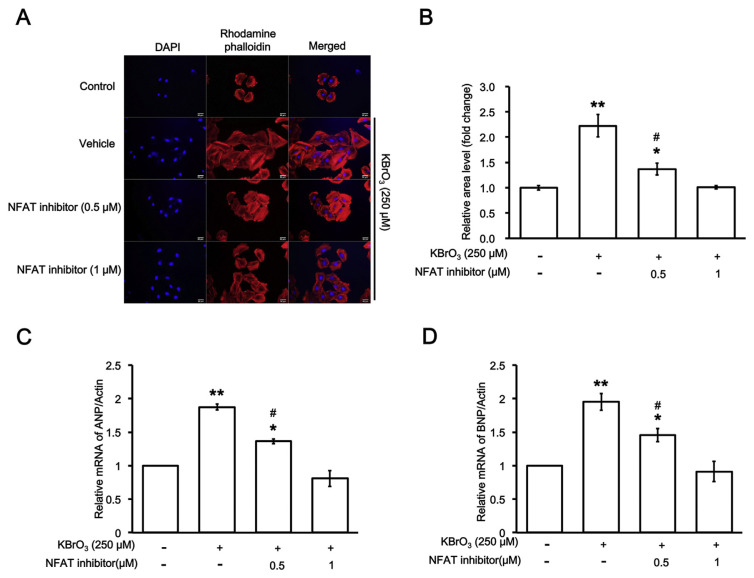
Similar to our previous method [20], KBrO3 was incubated with H9c2 cells for 72 h KBrO3 increased the cell size of H9c2 cells in a dose-dependent manner from 100 to 250 μM. As shown in Fig. 1A, KBrO3 increases cell size of H9c2 cells and it is dose-dependently reduced by NFAT inhibitor (Fig. 1B). Additionally, the biomarkers of cardiac hypertrophy, such as mRNA levels of ANP (Fig. 1C) and BNP (Fig. 1D), were also progressed by KBrO3 in the same manner. Therefore, myocardial hypertrophy did occur in H9c2 cells under the incubation with KBrO3.
Fig. 1.
Effects of NFAT inhibitor on cardiac hypertrophy-induced by potassium bromate (KBrO3). (A) The hypertrophic response of H9c2 cells enhanced by KBrO3 is dose-dependently reversed by NFAT inhibitor at indicated concentration. (B) Change in fluorescent intensity was depicted as fold difference over. The mRNA levels of the biomarkers for cardiac hypertrophy, either (C) atrial natriuretic peptides (ANP) or (D) brain/B-type natriuretic peptides (BNP), were also compared. n = 6, *P < 0.05 and **P < 0.01 vs. normal control (first column). #P < 0.05 vs. samples treated with NFAT inhibitor at high dose (last column).
3.2. Effects of cyclosporine a on the cell signaling in KBrO3-induced H9c2 cells with cardiac hypertrophy
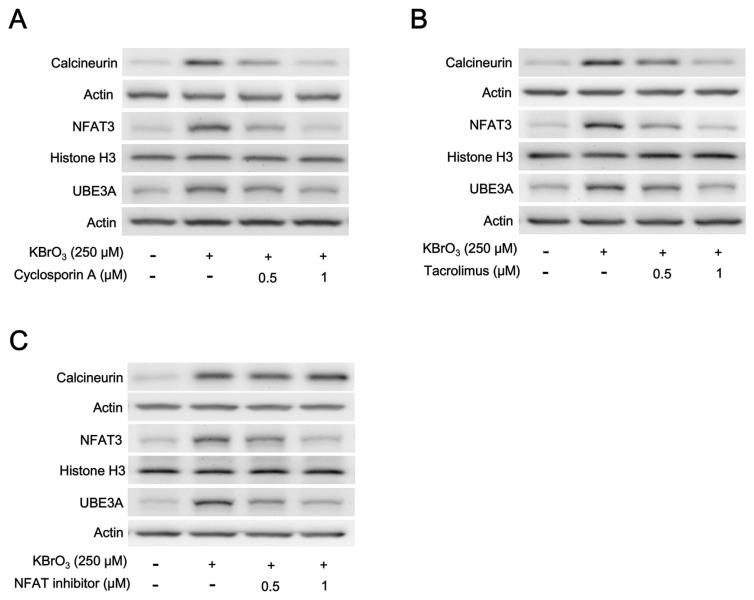
Basically, cardiac hypertrophy is known to be regulated simultaneously by stimulatory (prohypertrophic) and counter-regulatory (antihypertrophic) mechanisms via the calcineurin–NFAT signaling pathway [26]. Therefore, we investigated the changes in signaling pathway using Western blots. KBrO3 elevated both calcineurin and NFAT3 expression levels at the concentration enough to induce cardiac hypertrophy in H9c2 cells. Interestingly, the protein level of UBE3A was also raised markedly by KBrO3 (Fig. 2A). However, these effects of KBrO3 were dose-dependently reduced by a 1-h pretreatment with cyclosporine A (CsA), the well-known inhibitor of calcineurin [27], as shown in Fig. 2A. Moreover, the mRNA levels of UBE3A, as ANP and BNP, were also reduced in the same manner. Quantification of the changes in each signal both the transcriptional and translational levels have been indicated in Table 1.
Fig. 2.
Representative pictures showing Western blots. The cytosolic calcineurin promoted by KBrO3 was dose-dependently reduced by pre-treatment with calcineurin inhibitor, either (A) cyclosporin A or (B) Tacrolimus, and (C) the NFAT inhibitor. Cytosolic UBE3A in H9c2 cells modified after the treatment with each inhibitor was indicated (n = 6). The quantified data were shown in Table 1 for A, Table 2 for B, and Table 3 for C, respectively.
Table 1.
Effects of cyclosporin A (CsA) on the gene expression associated with cardiac hypertrophy-induced by potassium bromate (KBrO3) in H9c2 cells.
| Contents | Control | Vehicle + KBrO3 (250 μM) | CsA (0.5 μM) + KBrO3 (250 μM) | CsA (1 μM) + KBrO3 (250 μM) |
|---|---|---|---|---|
| Relative mRNA of ANP/β-Actin | 1.00 ± 0.00 | 1.87 ± 0.09** | 1.49 ± 0.09*# | 0.99 ± 0.02# |
| Relative mRNA of BNP/β-Actin | 1.00 ± 0.00 | 1.90 ± 0.13** | 1.45 ± 0.14*# | 0.85 ± 0.08# |
| Relative mRNA of UBE3A/β-Actin | 1.00 ± 0.00 | 1.80 ± 0.18** | 1.15 ± 0.14*# | 0.68 ± 0.13# |
| Ratio of calcineurin/β-Actin protein | 0.33 ± 0.06 | 1.04 ± 0.05** | 0.67 ± 0.05*# | 0.37 ± 0.05# |
| Ratio of NFAT3/Histone H3 protein | 0.30 ± 0.03 | 0.69 ± 0.06** | 0.48 ± 0.03*# | 0.32 ± 0.04# |
| Ratio of UBE3A/β-Actin protein | 0.41 ± 0.04 | 0.86 ± 0.04** | 0.52 ± 0.02*# | 0.46 ± 0.03# |
Values (mean ± SEM) were obtained from six samples per group. Effects of CsA at indicated concentration were compared with that treated with vehicle.
P < 0.05 or
P < 0.01 significantly different from the control.
P < 0.05 varied with the vehicle-treated group.
3.3. Tacrolimus, another blocker of calcineurin, inhibits KBrO3-induced cardiac hypertrophy in H9c2 cells
Tacrolimus (FK506) is also a powerful immunosuppressant and it is effective to inhibit calcineurin [28]. Therefore, we followed a previous report [29] to incubate tacrolimus at the effective concentrations with H9c2 cells for 1 h before the treatment with KBrO3. Then, the signals in H9c2 cells and mRNA levels of UBE3A, as ANP and BNP, were measured. As shown in Fig. 2B, tacrolimus inhibits protein level of signals in H9c2 cells with cardiac hypertrophy induced by KBrO3. Changes in each signal both the transcriptional and translational levels were quantified to indicate in Table 2. Therefore, mediation of the calcineurin–NFAT signaling pathway in KBrO3-induced cardiac hypertrophy is further supported.
Table 2.
Effects of Tacrolimus on the gene expression associated with cardiac hypertrophy-induced by potassium bromate (KBrO3) in H9c2 cells.
| Contents | Control | Vehicle + KBrO3 (250 μM) | Tacrolimus (0.5 μM) + KBrO3 (250 μM) | Tacrolimus (1 μM) + KBrO3 (250 μM) |
|---|---|---|---|---|
| Relative mRNA of ANP/β-Actin | 1.00 ± 0.00 | 1.82 ± 0.08** | 1.33 ± 0.06*# | 1.00 ± 0.05# |
| Relative mRNA of BNP/β-Actin | 1.00 ± 0.00 | 1.84 ± 0.11** | 1.09 ± 0.16*# | 0.78 ± 0.13# |
| Relative mRNA of UBE3A/β-Actin | 1.00 ± 0.00 | 1.87 ± 0.1** | 1.33 ± 0.1*# | 0.72 ± 0.13# |
| Ratio of calcineurin/β-Actin protein | 0.23 ± 0.02 | 0.76 ± 0.06** | 0.57 ± 0.03*# | 0.30 ± 0.03# |
| Ratio of NFAT3/Histone H3 protein | 0.28 ± 0.02 | 0.84 ± 0.06** | 0.52 ± 0.06*# | 0.36 ± 0.03# |
| Ratio of UBE3A/β-Actin protein | 0.41 ± 0.05 | 0.81 ± 0.06** | 0.65 ± 0.03*# | 0.45 ± 0.04# |
Values (mean ± SEM) were obtained from six samples per group. Effects of Tacrolimus at indicated concentration were compared with that treated with vehicle.
P < 0.05 or
P < 0.01 significantly different from the control.
P < 0.05 varied with the vehicle-treated group.
3.4. NFAT inhibitor alleviates KBrO3-induced cardiac hypertrophy in H9c2 cells
According to a previous report [30], the NFAT inhibitor is a high-affinity calcineurin-binding peptide that inhibits Nuclear Factor of Activated T cells (NFAT) activation, with the amino acid sequence, Met-Ala-Gly-Pro-His-Pro-Val-Ile-Val-Ile-Thr-Gly-Pro-His-Glu-Glu, to show a molecular weight of 1683 Da. Therefore, we incubate NFAT inhibitor at the effective concentrations of H9c2 cells for 1 h before the treatment with KBrO3. As shown in Fig. 2C, NFAT inhibitor attenuated the protein levels of NFAT and UBE3A in H9c2 cells but not the calcineurin level increased by KBrO3. Changes in each signal both the transcriptional and translational levels were quantified to indicate in Table 3. Therefore, mediation of the calcineurin–NFAT signaling pathway in KBrO3-induced cardiac hypertrophy is also identified.
Table 3.
Effects of NFAT inhibitor on the gene expression associated with cardiac hypertrophy-induced by potassium bromate (KBrO3) in H9c2 cells.
| Contents | Control | Vehicle + KBrO3 (250 μM) | NFAT inhibitor (0.5 μM) + KBrO3 (250 μM) | NFAT inhibitor (1 μM) + KBrO3 (250 μM) |
|---|---|---|---|---|
| Ratio of calcineurin/β-Actin protein | 0.20 ± 0.03 | 0.65 ± 0.06** | 0.62 ± 0.05** | 0.69 ± 0.02** |
| Ratio of NFAT3/Histone H3 protein | 0.28 ± 0.01 | 0.80 ± 0.03** | 0.61 ± 0.06*# | 0.35 ± 0.01# |
| Ratio of UBE3A/β-Actin protein | 0.22 ± 0.01 | 0.69 ± 0.04** | 0.42 ± 0.02*# | 0.31 ± 0.01# |
| Relative mRNA of UBE3A/β-Actin | 1.00 ± 0.00 | 1.95 ± 0.09** | 1.43 ± 0.05*# | 0.99 ± 0.07# |
Values (mean ± SEM) were obtained from six samples per group. Effects of NFAT inhibitor at the indicated concentration were compared with that treated with vehicle.
P < 0.05 or
P < 0.01 significantly different from the control.
P < 0.05 varied with the vehicle-treated group.
3.5. The increase of UBE3A in hypertrophic models induced by HG or isoproterenol in H9c2 cells
In addition to KBrO3-induced cardiac hypertrophy, elevation of UBE3A shall be identified in another model of cardiac hypertrophy. Therefore, we applied HG [21] and isoproterenol (Iso) [22] to reproduce the hypertrophic model as described previously.
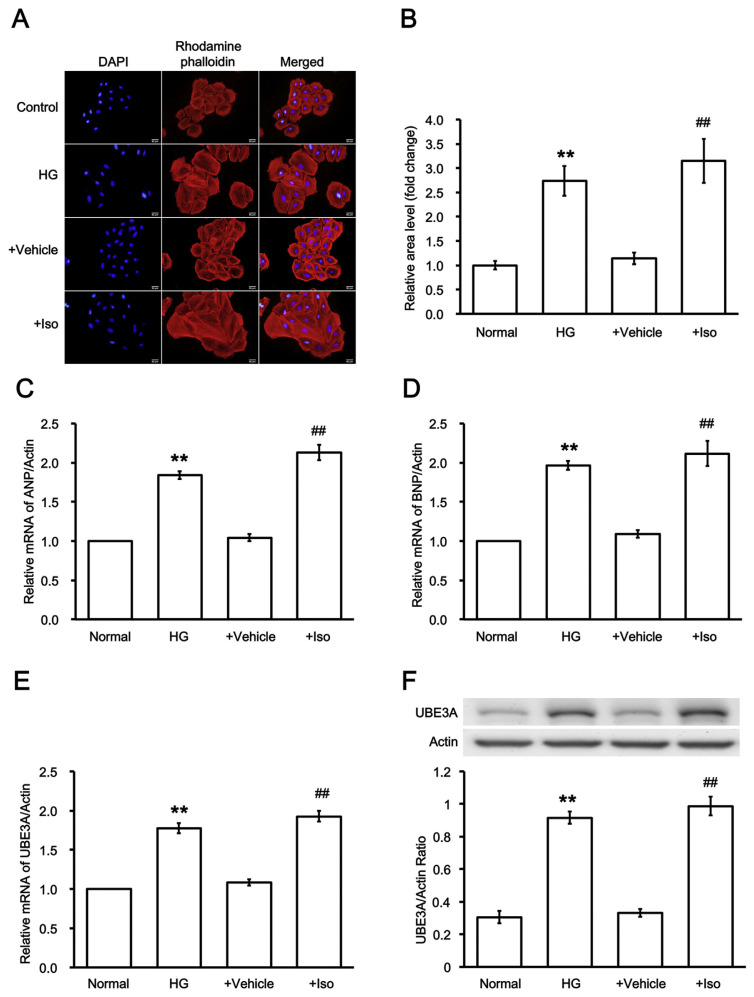
The H9c2 cells were exposed to media containing 30 mM glucose for 48 h. Diabetic cardiac hypertrophy was then characterized as shown in Fig. 3A to compare with control that was exposed to media containing 5.5 mM glucose in the same manner. Additionally, another hypertrophic model was reproduced by incubation with 10 μM Iso for 48 h as described previously [22]. The success of the model was also confirmed in Fig. 3A to compare with the vehicle-treated sample (Vehicle). Quantification of the change has been shown in Fig. 3B.
Fig. 3.
Changes in UBE3A expression in high glucose or isoproterenol treated cardiac cells. (A) Hypertrophic responses in H9c2 cells induced by high glucose (HG) or isoproterenol (Iso) were compared with control or vehicle-treated samples. (B) Quantification of the fluorescence intensity were also compared. Additionally, the mRNA levels of (C) ANP or (D) BNP in two models were both promoted. Similarly, (E) the mRNA levels and (F) the protein levels of UBE3A were both markedly promoted in diabetic and Iso-stimulated models. n = 6, **P < 0.01 vs. normal control (Normal) and ##P < 0.01 vs. vehicle-treated group (Vehicle).
Then, biomarkers of cardiac hypertrophy such as the mRNA levels of ANP and BNP were significantly promoted in two hypertrophic models as shown in Fig. 3C and D, respectively. Additionally, the transcriptional and translational levels of UBE3A were both elevated markedly in two hypertrophic models (Fig. 3E and F).
4. Discussion
In the present study, we found that gene expression of UBE3A is markedly increased in cardiac hypertrophy using 3 kinds of cell model. Additionally, we demonstrated that calcineurin/ NFAT signaling pathway is mediated in the hypertrophic effect of KBrO3. It is fully consistent with two other models, either the HG to mimic diabetes or the widely used compound isoproterenol (Iso). The oxidant KBrO3 induces damage via oxidative stress to resulting cardiac injury in rats [1]. Therefore, it is similar to the effect of hydrogen peroxide (H2O2) for the induction of cardiac hypertrophy in H9c2 cells [31]. Moreover, hypertrophic responses are known as the same between H9c2 cell line and primary neonatal cardiomyocytes [32].
We followed the established method to measure the cell size in H9c2 cells [23]. The direct effect of KBrO3 on cardiac cells may result in an increase in the size of H9c2 cells that have been demonstrated using visual identification and the parallel increased biomarkers of cardiac hypertrophy. Increase in plasma level of ANP or BNP is also used as the biomarker of cardiac hypertrophy in the clinic [33]. In the present study, the mRNA level of ANP or BNP in H9c2 cells is both elevated by KBrO3. Moreover, KBrO3 induces an increase in the size of H9c2 cells (hypertrophy) rather than by enhancing the numbers (hyperplasia). Therefore, the induction of cardiac hypertrophy in H9c2 cells by KBrO3 can be identified and we used it as one of the cell models in the present study.
Then, we investigated the role of the signaling pathway in KBrO3-induced cardiac hypertrophy. It has been established that calcineurin may dephosphorylate NFAT3, the transcription factors, leading to their nuclear translocation [26]. Therefore, the nuclear NFAT3 participates in the promotion of hypertrophic gene expression including ANP and BNP to induce cardiac hypertrophy [4,5]. We applied two specific inhibitors of calcineurin, such as cyclosporine A (CsA) and Tacrolimus (FK506), to interrupt the signaling pathway. Additionally, the peptide functioned as NFAT inhibitor [30] was also employed. Protein levels of calcineurin or nuclear NFAT3 determined by Western blots indicated the effectiveness of CsA as described previously [27] and consisted the influence of Tacrolimus [29]. Also, NFAT inhibitor [30] provided the reliable data showing the mediation of the calcineurin-NFAT signaling pathway in KBrO3-induced cardiac hypertrophy. It has been documented that the calcineurin-NFAT pathway belonged to pathologic but not physiologic cardiac hypertrophy [34]. Otherwise, cardiac hypertrophy by HG, the 2nd model in the present study, is also induced by the same pathway [35]. Additionally, the 3rd model induced by isoproterenol (Iso) is known to produce in the same manner [36]. Therefore, the cell models used in the present study belong to pathologic cardiac hypertrophy.
In the present study, we found that expression of Ube3a is markedly increased in 3 kinds of hypertrophic cells, similar to ANP and BNP. The ubiquitin ligase UBE3A (an E3 ubiquitin ligase encoded by the Ube3a gene) is also elevated in these cardiac cells showing hypertrophy. Ubiquitin ligase is known for protein quality control in cardiomyocytes [37] including E1, E2, E3 (ubiquitin ligase), and deubiquitinating enzymes. Ubiquitin ligases enact the final step in the ubiquitination cascade and give specificity to the UPS by interacting with specific substrates for tagging them with ubiquitin. Therefore, impaired UPS has been mentioned as a pathogenic factor of hypertrophic cardiomyopathy [38]. The UPS is also known to resolve the initial cause of ER stress [39]. Acute activation of the UPS is cytoprotective, but prolonged activation of the UPS initiates a proapoptotic pathway [39]. Additionally, UPS and autophagy are two proteolytic pathways in cardiomyocytes to combat proteotoxicity-related cardiac diseases [40]. In the heart, three subtypes of E3 ubiquitin ligases (E3s) are known to link with cardiac hypertrophy [41]. Therefore, UBE3A is possible to change with the progress of cardiac hypertrophy.
UBE3A expression is really promoted in various kinds of pathologic cardiac hypertrophy as shown in the current study. Therefore, UBE3A could be developed as a biomarker of cardiac hypertrophy in the clinic. The protein UBE3A exists in both the nucleus and cytoplasm. It has been detected in human tissues including the heart. The cellular functions of UBE3A may be influenced by Angelman syndrome, UBE3A-associated autism spectrum disorders, and human papillomavirus-associated cancers [42]. In the heart, E3s regulates autophagy at two critical points through multiple mechanisms [43]. Similar to the changes in cardiac hypertrophy [17], UBE3A was significantly increased in H9c2 cells at the transcriptional and translational levels in response to H2O2 [44]. Moreover, UBE3A is known for initiating the degradation of p53 in rat neonatal cardiomyocytes [45]. UPS impairment has also been mentioned to involve in the pathophysiology of diabetic cardiomyopathy, as described in a review article [46]. However, ablation of UBE3A in the heart has not been performed.
Clinically, some biomarkers are wildly used as the screening tools for cardiac hypertrophy [47]. The plasma levels of high-sensitivity cardiac troponin T (hs TnT) indicating irreversible damage to the heart muscle [48]. The ANP and its precursor N-terminal pro-ANP (NT-proANP) are increased with pressure independent cardiac hypertrophy [49]. Also, BNP and NT-proBNP are the well-known markers of left ventricular wall stress and hypertrophy [50]. Additionally, biomarker kits for the discrimination between myocardial degeneration/necrosis and cardiac hypertrophy have been compared [51]. However, the application of UBE3A as the biomarker of cardiac hypertrophy is still not mentioned before. In the network of key regulators of cardiac mechano-signaling, UBE3A is also not included [52]. Based on the results of the current study, UBE3A is elevated in pathologic cardiac hypertrophy. The changes of UBE3A are similar to that of ANP and BNP in various hypertrophic models of H9c2 cells. Hypertrophic gene is promoted after the binding of NAFT3 with GATA4 [53]. Therefore, UBE3A is possible to develop as a new diagnostic biomarker along with ANP or BNP. Moreover, UBE3A is a product of the UPS in cardiac cells after the hypertrophic stimulation. Therefore, it is not the same as ANP or BNP and it could be developed as an alternative biomarker. Recently, Ube3a gene has been investigated in patients for the development of Tourette syndrome [54] which is valuable for assay of UBE3A in clinic. Limitations of the present study are related to the data from cell line only. Assay of UBE3A in animals and/or in patients with cardiac hypertrophy shall be developed in the near. Commercial kit for assay of blood UBE3A level is particularly required. However, the obtained results are enough to support the application of UBE3A as the biomarker of cardiac hypertrophy in cell models and it could be applied in the screening of drug activity for the alleviation of cardiac hypertrophy.
5. Conclusion
Taken together, we demonstrated a new view that UBE3A is markedly raised in cardiac hypertrophy using 3 kinds of cell model through the activation of the calcineurin-NFAT signaling pathway in H9c2 cells. Therefore, UBE3A is suitable to develop as a new biomarker of cardiac hypertrophy in the future.
Acknowledgments
We thank Yang-Lien Yen and Yi-Zhi Chen for their assistance in the experiments. The present study was partly supported by a grant (CMFHT10503) from Chi-Mei Medical Center, Yong Kang, Tainan City, Taiwan.
Funding Statement
The present study was partly supported by a grant (CMFHT10503) from Chi-Mei Medical Center, Yong Kang, Tainan City, Taiwan.
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
References
- 1. Priscilla DH, Prince PS. Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem Biol Interact. 2009;179:118–24. doi: 10.1016/j.cbi.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 2. Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 3. D'Ascenzi F, Pelliccia A, Corrado D, Cameli M, Curci V, Alvino F, et al. Right ventricular remodelling induced by exercise training in competitive athletes. Eur Heart J Cardiovas Imag. 2016;17:301–7. doi: 10.1093/ehjci/jev155. [DOI] [PubMed] [Google Scholar]
- 4. Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Therapeut. 2010;128:191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 5. Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 6. Malinowski B, Fulgheri G, Wicinski M, Grzesk E, Odrowaz-Sypniewska G, Grzesk G, et al. Potential markers in cardiac hypertrophy? EJIFCC. 2012;23:41–6. [PMC free article] [PubMed] [Google Scholar]
- 7. Taegtmeyer H, Sen S, Vela D. Return to the fetal gene program: a suggested metabolic link to gene expression in the heart. Ann N Y Acad Sci. 2010;1188:191–8. doi: 10.1111/j.1749-6632.2009.05100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cox EJ, Marsh SA. A systematic review of fetal genes as biomarkers of cardiac hypertrophy in rodent models of diabetes. PLoS One. 2014;9:e92903. doi: 10.1371/journal.pone.0092903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou XF, Jafari N, Deng SS, Huang CC. The function of ubiquitin protein ligase E3A and its roles in human diseases. JBMBR. 2015;1:14–8. [Google Scholar]
- 10. Ciechanover A. The ubiquitin proteolytic system: from a vague idea, through basic mechanisms, and onto human diseases and drug targeting. Neurology. 2006;66:S7–19. doi: 10.1212/01.wnl.0000192261.02023.b8. [DOI] [PubMed] [Google Scholar]
- 11. Li HH, Willis MS, Lockyer P, Miller N, McDonough H, Glass DJ, et al. Atrogin-1 inhibits Akt-dependent cardiac hypertrophy in mice via ubiquitin-dependent coactivation of Forkhead proteins. J Clin Invest. 2007;117:3211–23. doi: 10.1172/JCI31757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang HS, Allen JA, Mabb AM, King IF, Miriyala J, Taylor-Blake B, et al. Topoisomerase inhibitors unsilence the dormant allele of Ube3a in neurons. Nature. 2011;481:185–9. doi: 10.1038/nature10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weeber EJ, Jiang YH, Elgersma Y, Varga AW, Carrasquillo Y, Brown SE, et al. Derangements of hippocampal calcium/ calmodulin-dependent protein kinase II in a mouse model for Angelman mental retardation syndrome. J Neurosci. 2003;23:2634–44. doi: 10.1523/JNEUROSCI.23-07-02634.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Obasanjo-Blackshire K, Mesquita R, Jabr RI, Molkentin JD, Hart SL, Marber MS, et al. Calcineurin regulates NFAT-dependent iNOS expression and protection of cardiomyocytes: co-operation with Src tyrosine kinase. Cardiovasc Res. 2006;71:672–83. doi: 10.1016/j.cardiores.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 15. Kreusser MM, Backs J. Integrated mechanisms of CaMKII-dependent ventricular remodeling. Front Pharmacol. 2014;5:36. doi: 10.3389/fphar.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–28. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Song R, Zhang J, Zhang L, Wang G, Wo D, Feng J, et al. H2O 2 induces myocardial hypertrophy in H9c2 cells: a potential role of Ube3a. Cardiovasc Toxicol. 2015;15:23–8. doi: 10.1007/s12012-014-9264-0. [DOI] [PubMed] [Google Scholar]
- 18. Sawyer DB, Siwik DA, Xiao L, Pimentel DR, Singh K, Colucci WS. Role of oxidative stress in myocardial hypertrophy and failure. J Mol Cell Cardiol. 2002;34:379–88. doi: 10.1006/jmcc.2002.1526. [DOI] [PubMed] [Google Scholar]
- 19. Khatua TN, Borkar RM, Mohammed SA, Dinda AK, Srinivas R, Banerjee SK. Novel sulfur metabolites of garlic attenuate cardiac hypertrophy and remodeling through induction of Na+/K+-ATPase expression. Front Pharmacol. 2017;8:18. doi: 10.3389/fphar.2017.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kou SC, Li Y, Cheng YZ, Lee WJ, Cheng JT, Cheng KC. Molecular mechanisms regarding potassium bromate (KBrO3)-induced cardiac hypertrophy without apoptosis in H9c2 cells. Mol Med Rep. 2018;9:206761. doi: 10.3892/mmr.2018.9470. [In Press] [DOI] [PubMed] [Google Scholar]
- 21. Asadi F, Razmi A, Dehpour AR, Shafiei M. Tropisetron inhibits high glucose-induced calcineurin/NFAT hypertrophic pathway in H9c2 myocardial cells. J Pharm Pharmacol. 2016;68:485–93. doi: 10.1111/jphp.12522. [DOI] [PubMed] [Google Scholar]
- 22. Somvanshi RK, Qiu X, Kumar U. Isoproterenol induced hypertrophy and associated signaling pathways are modulated by somatostatin in H9c2 cells. Int J Cardiol. 2013;167:1012–22. doi: 10.1016/j.ijcard.2012.03.077. [DOI] [PubMed] [Google Scholar]
- 23. Lo SH, Hsu CT, Niu HS, Niu CS, Cheng JT, Chen ZC. Ginsenoside Rh2 improves cardiac fibrosis via PPARdelta-STAT3 signaling in type 1-like diabetic rats. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jash A, Sahoo A, Kim GC, Chae CS, Hwang JS, Kim JE, et al. Nuclear factor of activated T cells 1 (NFAT1)-induced permissive chromatin modification facilitates nuclear factor-kappaB (NF-kappaB)-mediated interleukin-9 (IL-9) transactivation. J Biol Chem. 2012;287:15445–57. doi: 10.1074/jbc.M112.340356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yeh MC, Chen LJ, Niu HS, Yang TT, Lin KC, Cheng JT. Signals for increase of mu-opioid receptor expression in muscle by hyperglycemia. Neurosci Lett. 2014;582:109–14. doi: 10.1016/j.neulet.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 26. Fiedler B, Wollert KC. Interference of antihypertrophic molecules and signaling pathways with the Ca2+-calcineurin-NFAT cascade in cardiac myocytes. Cardiovasc Res. 2004;63:450–7. doi: 10.1016/j.cardiores.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 27. Zhu W, Zou Y, Shiojima I, Kudoh S, Aikawa R, Hayashi D, et al. Ca2+/calmodulin-dependent kinase II and calcineurin play critical roles in endothelin-1-induced cardiomyocyte hypertrophy. J Biol Chem. 2000;275:15239–45. doi: 10.1074/jbc.275.20.15239. [DOI] [PubMed] [Google Scholar]
- 28. Williams CR, Gooch JL. Calcineurin inhibitors and immunosuppression - a tale of two isoforms. Expet Rev Mol Med. 2012;14:e14. doi: 10.1017/erm.2012.8. [DOI] [PubMed] [Google Scholar]
- 29. Liu Y, Ji Z. FK506 alleviates proteinuria in rats with adriamycin-induced nephropathy by down-regulating TRPC6 and CaN expression. J Nephrol. 2012;25:918–25. doi: 10.5301/jn.5000192. [DOI] [PubMed] [Google Scholar]
- 30. Xia S, Liu Y, Li X, Thilo F, Tepel M. Insulin increases expression of TRPC6 channels in podocytes by a calcineurin-dependent pathway. Cell Physiol Biochem : Int J Exp Cell Physiol Biochem Pharmacol. 2016;38:659–69. doi: 10.1159/000438658. [DOI] [PubMed] [Google Scholar]
- 31. Schroder E, Eaton P. Hydrogen peroxide as an endogenous mediator and exogenous tool in cardiovascular research: issues and considerations. Curr Opin Pharmacol. 2008;8:153–9. doi: 10.1016/j.coph.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 32. Watkins SJ, Borthwick GM, Arthur HM. The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In vitro cellular & developmental biology. Animal. 2011;47:125–31. doi: 10.1007/s11626-010-9368-1. [DOI] [PubMed] [Google Scholar]
- 33. Sagnella GA. Measurement and significance of circulating natriuretic peptides in cardiovascular disease. Clin Sci. 1998;95:519–29. doi: 10.1042/cs0950519. [DOI] [PubMed] [Google Scholar]
- 34. Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, et al. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–8. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 35. Daskoulidou N, Zeng B, Berglund LM, Jiang H, Chen GL, Kotova O, et al. High glucose enhances store-operated calcium entry by upregulating ORAI/STIM via calcineurin-NFAT signalling. J Mol Med. 2015;93:511–21. doi: 10.1007/s00109-014-1234-2. [DOI] [PubMed] [Google Scholar]
- 36. Somvanshi RK, Zou S, Qiu X, Kumar U. Somatostatin receptor-2 negatively regulates beta-adrenergic receptor mediated Ca(2+) dependent signaling pathways in H9c2 cells. Biochim Biophys Acta. 2014;1843:735–45. doi: 10.1016/j.bbamcr.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 37. Wang X, Su H, Ranek MJ. Protein quality control and degradation in cardiomyocytes. J Mol Cell Cardiol. 2008;45:11–27. doi: 10.1016/j.yjmcc.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carrier L, Schlossarek S, Willis MS, Eschenhagen T. The ubiquitin-proteasome system and nonsense-mediated mRNA decay in hypertrophic cardiomyopathy. Cardiovasc Res. 2010;85:330–8. doi: 10.1093/cvr/cvp247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–90. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang C, Wang X. The interplay between autophagy and the ubiquitin-proteasome system in cardiac proteotoxicity. Biochim Biophys Acta. 2015;1852:188–94. doi: 10.1016/j.bbadis.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bai T, Wang F, Mellen N, Zheng Y, Cai L. Diabetic cardiomyopathy: role of the E3 ubiquitin ligase. Am J Physiol Endocrinol Metab. 2016;310:E473–83. doi: 10.1152/ajpendo.00467.2015. [DOI] [PubMed] [Google Scholar]
- 42. Martinez-Noel G, Luck K, Kuhnle S, Desbuleux A, Szajner P, Galligan JT, et al. Network analysis of UBE3A/E6AP-associated proteins provides connections to several distinct cellular processes. J Mol Biol. 2018;430:1024–50. doi: 10.1016/j.jmb.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Parry TL, Willis MS. Cardiac ubiquitin ligases: their role in cardiac metabolism, autophagy, cardioprotection and therapeutic potential. Biochim Biophys Acta. 2016;1862:2259–69. doi: 10.1016/j.bbadis.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang J, Song R, Li Y, Feng J, Peng L, Li J. Integration of microarray profiles associated with cardiomyopathy and the potential role of Ube3a in apoptosis. Mol Med Rep. 2014;9:621–5. doi: 10.3892/mmr.2013.1848. [DOI] [PubMed] [Google Scholar]
- 45. Long X, Boluyt MO, Hipolito ML, Lundberg MS, Zheng JS, O'Neill L, et al. p53 and the hypoxia-induced apoptosis of cultured neonatal rat cardiac myocytes. J Clin Invest. 1997;99:2635–43. doi: 10.1172/JCI119452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li J, Ma W, Yue G, Tang Y, Kim IM, Weintraub NL, et al. Cardiac proteasome functional insufficiency plays a pathogenic role in diabetic cardiomyopathy. J Mol Cell Cardiol. 2017;102:53–60. doi: 10.1016/j.yjmcc.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Koycheva RY, Cholakov V, Andreev J, Penev M, Iliev R, Nancheva K, et al. Cardiac biomarkers and left ventricular hypertrophy in asymptomatic hemodialysis patients. Open access Macedonian J Med Sci. 2016;4:59–64. doi: 10.3889/oamjms.2016.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim BS, Jeon DS, Shin MJ, Kim YO, Song HC, Lee SH, et al. Persistent elevation of C-reactive protein may predict cardiac hypertrophy and dysfunction in patients maintained on hemodialysis. Am J Nephrol. 2005;25:189–95. doi: 10.1159/000085585. [DOI] [PubMed] [Google Scholar]
- 49. Vinken P, Reagan WJ, Rodriguez LA, Buck WR, Lai-Zhang J, Goeminne N, et al. Cross-laboratory analytical validation of the cardiac biomarker NT-proANP in rat. J Pharmacol Toxicol Meth. 2016;77:58–65. doi: 10.1016/j.vascn.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 50. Codognotto M, Piccoli A, Zaninotto M, Mion MM, Ruzza L, Barchita A, et al. Effect of a dialysis session on the prognostic values of NT-proBNP, troponins, endothelial damage and inflammation biomarkers. J Nephrol. 2010;23:465–71. [PubMed] [Google Scholar]
- 51. Kim K, Chini N, Fairchild DG, Engle SK, Reagan WJ, Summers SD, et al. Cardiac hypertrophy working group of the predictive safety testing C. Evaluation of cardiac toxicity biomarkers in rats from different laboratories. Toxicol Pathol. 2016;44:1072–83. doi: 10.1177/0192623316668276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tan PM, Buchholz KS, Omens JH, McCulloch AD, Saucerman JJ. Predictive model identifies key network regulators of cardiomyocyte mechano-signaling. PLoS Comput Biol. 2017;13:e1005854. doi: 10.1371/journal.pcbi.1005854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Oka T, Maillet M, Watt AJ, Schwartz RJ, Aronow BJ, Duncan SA, et al. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ Res. 2006;98:837–45. doi: 10.1161/01.RES.0000215985.18538.c4. [DOI] [PubMed] [Google Scholar]
- 54. Lei J, Xu H, Liang H, Su L, Zhang J, Huang X, et al. Gene expression changes in peripheral blood from Chinese Han patients with Tourette syndrome. Am J Med Genet B, Neuropsychiatr Genet : Offic Publ Int Soc Psychiatr Genet. 2012;159B:977–80. doi: 10.1002/ajmg.b.32103. [DOI] [PubMed] [Google Scholar]