Abstract
There is an increasing amount of dietary supplements that are adulterated with diuretics and anti-diabetic drugs; this has become a global problem due to the wide distribution of dietary supplements and the serious negative health effects of the adulterants. In this study, a rapid screening method was developed for detection and confirmation of 35 sulfonamides in supplements by ultra-high performance liquid chromatography quadrupole/ time of flight mass spectrometry. For effective extraction of sulfonamides from dietary supplements, four extraction protocols including HLB and WAX solid-phase extraction, Quick Easy Cheap Effective Rugged and Safe method, and pH-controlled liquid–liquid extraction were evaluated, and pH-controlled liquid–liquid extraction method was shown to be the most effective with high recovery efficiency and low matrix effect. Rapid separation of 35 sulfonamides was achieved with the UHPLC C18 column (150 × 2.1 mm, 1.7 um) within 7 min using ammonium acetate aqueous solution (pH 8) and acetonitrile as the mobile phase. From the MS/MS spectra of sulfonamides, common ions (m/z 77.9650 [SO2N]− and m/z 79.9812 [SO2NH2]−) and neutral molecule loss fragments (HCl and SO2) were observed according to their structural characteristics. Extracted common ion chromatograms and neutral loss scan of these characteristic fragments could effectively apply for rapid screening of sulfonamides in various types of supplements. A reduced mass tolerance window of ±5 ppm was useful for detecting targeted and untargeted sulfonamides and could avoid false positive and false negative results. Overall calibration curves within dynamic range for all targets were shown to be linear with a correlation coefficient R2 > 0.995 and limits of detection ranged from 0.04 to 11.18 ng/g for all sulfonamides. The established method was successfully applied for screening and confirmation of sulfonamides in various supplements. The developed method will be helpful for the identification of sulfonamide diuretics and anti-diabetics in dietary supplements, promoting public health and consumer safety.
Keywords: Sulfonamides, Dietary supplements, UHPLC-Q/TOF-MS, Extracted common ion chromatogram, Neutral loss scan
1. Introduction
Sulfonamides (SAs) are an diuretics and anti-diabetics that are extensively used in human medicine due to their high efficiency, low cost, and antibiotic activity [1,2]. Nowadays, supplements illegally adulterated with SAs are being widely distributed via internet markets and local markets because these adulterants are not as strictly regulated as many synthetic drugs. Specifically, dietary supplements for weight loss are sometimes adulterated with diuretic SAs to improve efficacy, but the SAs are not on the label to avoid drug screening. When these illegal adulterated supplements are consumed long term, they can cause serious negative health effects such as irregular heartbeat, confusion, gastrointestinal reactions, and hypoglycemia [3–5]. Thus, it is necessary to establish a rapid and reliable analytical method for the screening and confirmation of sulfonamide adulterants in various types of dietary supplements.
Liquid chromatography (LC) coupled with tandem mass spectrometry (MS/MS) methods have been popularly used for the analysis of SAs in dietary supplements due to their high sensitivity and versatility [6–8]. Especially, when the multiple reaction monitoring (MRM) mode is applied, targeted analytes in complicated sample matrix can be detected with a high selectivity and specificity. However, in some cases, MRM mode can provide ambiguous identification of untargeted or new emerging undeclared chemicals in supplementary foods. Recently, LC-high resolution (HR) tandem mass spectrometry could provide HR-full scan spectra, exact mass data, MS/MS spectra, extracted ion chromatogram (EIC), and neutral loss scans. Moreover, the approach using hybrid tandem quadrupole/ time of flight mass spectrometry (Q/TOF) high-resolution (HR) MS was introduced as a promising alternative that allows screening and confirmation of targeted and untargeted illegal substances in various matrices [9–12].
Despite the advances in separation and detection techniques, appropriate sample preparation is an indispensable process due to high matrix interferences of supplement diets. These sample interferences could hinder the detection of illegal drugs and cause serious contamination of analytical instrument, producing unreliable analytical results. Several methods such as filtration [6,7], solid-phase extraction (SPE) [13,14], and Quick Easy Cheap Effective Rugged and Safe (QuEChERS)method [15] have been applied for the cleanup of targeted illegal drugs in sample extract. In this study, a pH-controlled liquid–liquid extraction (LLE) method based on the chemical properties of SAs was applied for efficient extraction of SAs from supplements [16]. As another advantage, this SA analysis can be effectively applied to pill and capsule samples with complex matrices without the need for co-extraction of interferences.
In this study, to develop a rapid screening method of SAs in various dietary supplements, UHPLC-Q/TOF-MS was applied to screen for targeted related compounds using extracted common ion chromatograms (ECICs) and neutral loss scan (NLS) based on common fragments and specific fragmentation of SAs. The reconstruction of HR-ECICs and HR-NLS provide more specific and selective detection of the illegal drugs with a similar structural moiety compared to low-resolution MS based MRM or EIC mode. Also, with the aid to HR-TOF MS, a reduced mass tolerance window could enable to selective detection of SAs and avoid false positives and negatives within complex sample matrices. This technique can rapidly screen for most SAs using just a few common or specific fragments and serve as an alternative prospective screening method to the conventional MRM transition ion chromatograms or EICs.
In the present study, a generic method for rapid screening and confirmation of 35 SAs adulterants by UHPLC-Q/TOF-MS combined with pH-controlled LLE was developed. The use of four HR-ECIC and HR-NLS chromatograms could successfully be used to screen for targeted and untargeted SAs in various types of supplements. A homemade library including retention times, elemental composition, accurate mass value, and HR MS/MS spectra was constructed for the confirmation of SAs. The developed method was successfully validated in terms of linearity, limits of detection and quantification, precision, and accuracy. Here, it is demonstrated that UHPLC–Q/TOF-MS combined with pH-controlled LLE method was highly specific, selective, and sensitive for comprehensive screening and confirmation of illegal SA adulterants in various types of dietary supplements.
2. Experiments
2.1. Chemicals and materials
Reference standards of the sulfonamide analogs were obtained from Sigma Aldrich (St. Louis, MO, USA) and Toronto Research Chemicals Inc. (Toronto, ON, Canada). Furosemide-d5 and tolbutamide-d9 were purchased from CDN isotopes (Quebec, Canada) and bendroflumethiazide-d5 was obtained from Toronto Research Chemicals Inc. as an isotope-labeled internal standards. The chemical structures of the sulfonamides and deuterium labeled internal standards are depicted in Figs. S1. The dietary supplements free from sulfonamide adulterants were used as blank samples. All reagents and organic solvents were of analytical grade. HPLC grade methanol (MeOH) and acetonitrile (ACN) were purchased from J.T. Baker (Phillipsburg, NJ, USA) and formic acid, hydrochloric acid (HCl) and ammonium acetate (NH4Ac) were purchased from Sigma Aldrich (St. Louis, MO, USA). Acetonitrile was filtered through a 0.45 μm membrane filter and degassed for 10 min. In addition, deionized water was purified using a Milli-Q system (Millipore, Co., Bedford, MA, USA), filtered through a 0.2 μm membrane filter and degassed for 10 min before use.
2.2. Preparation of reference standards
Individual standards were dissolved in methanol at 1000 μg/ mL. Each stock solution was prepared in an amber vial and was vortex mixed for 30 s. Deuterated internal standard stock solutions (furosemide-d5, tolbutamide-d9, and bendroflumethiazide-d5) were prepared in methanol at 1000 μg/mL. The working solutions of all the compounds were prepared by successively diluting the stock solutions. The standard stock and working solutions were stored at −20 °C.
2.3. Sample preparation
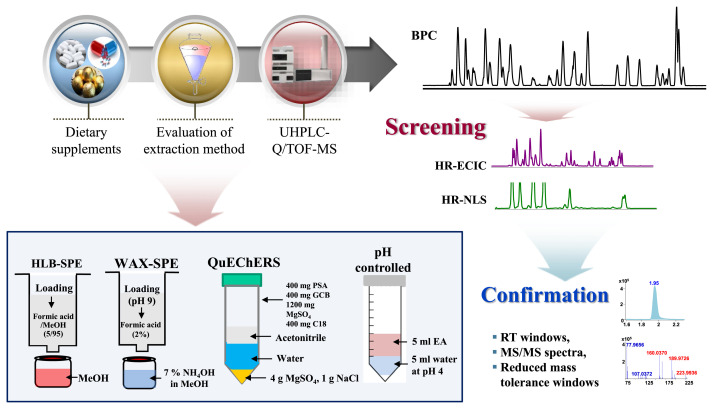
Herbal medicines and dietary supplements are typically in the form of tablets, pills, and capsules. Tablets and pills were pulverized into a homogeneous powder, whereas for capsules, the shells were removed and the powder was mixed. Sample was extracted by four protocols such as HLB-solid phase extraction (SPE), WAX-SPE, QuEChERS, and liquid–liquid extraction (LLE) methods for evaluation of sample cleanup. Approximately 0.5 g of sample was taken and then the extraction of SAs from supplement was performed as shown in Fig. 1. Detail extraction procedures of individual methods are described in Suppl. Information. The overall analytical procedure for the screening and confirmation of sulfonamides in supplement diets is depicted in Fig. 1.
Fig. 1.
Analytical flow for determination of sulfonamides in dietary supplements and demonstration of four extraction methods.
2.4. UHPLC-Q/TOF-MS conditions
Ultra-high-performance liquid chromatography (UHPLC) analysis was performed using an Agilent 1290 UHPLC system (Agilent Technologies, Palo Alto, CA, USA). The chromatographic separation was carried out a Phenomenex Kinetex® UHPLC EVO C18 column (150 × 2.1 mm, i.d., 1.7 μm). The mobile phases were 10 mM ammonium acetate with 1.5 mM ammonium hydroxide (pH 8) (A) and acetonitrile (B). The gradient elution mode was programmed as follows: 20% B for 0.0–1.0 min, 20%–45% B for 1.0–6.0 min, 45%–99% B for 6.0–7.0 min, and 99% B for 7.0–10.0 min. The flow rate, injection volume, and column temperature were set at 0.4 mL/ min, 2 μL, and 30 °C, respectively.
All LC-MS and LC-MS/MS experiments were performed using a 6530 accurate mass quadrupole/time-of-flight mass spectrometer instrument (Agilent Technologies, Palo Alto, CA, USA). This instrument was operated in the extended dynamic range of 2 GHz (m/z 1700 Th) in high resolution mode. Negative ions of analytes were generated using an ESI source with Agilent Jet Stream Technology. Parameters for the ESI source were as follows: the super-heated nitrogen sheath gas temperature, 350 °C; and flow, 11 L/min. Mass spectrometer conditions were set at the following: capillary voltage (VCap), 3000 V; nebulizer pressure, 45 psi; drying gas, 8 L/min; and gas temperature, 300 °C. The fragment, skimmer, and Oct 1 RF Vpp voltages were set at 120, 65, and 750 V, respectively. The mass scan range was m/z 70–1000, and reference masses of m/z 112.985587 (TFA) and 980.016375 (HP-0921) were used to calibrate the mass axis during analysis. For obtaining HR and MS/ MS spectra, the same MS parameters were used for the TOF instrument except the fragmentor voltage was set at 50 V. To construct the MS/MS spectral data for a homemade library, different collision energy conditions (0, 10, 30 and 50 V) were applied for the 35 SAs. All data were recorded with Mass Hunter Qualitative Analysis software (Agilent Technologies, Palo Alto, CA, USA, version B.06.00). An automated targeted screening and confirmation was performed by applying software based on the homemade library.
2.5. Method validation
The analytical method for the analysis of 35 SAs in supplements was validated according to ICH guidelines [17]. Calibration curves were plotted for seven different concentrations in the range of 1–2500 ng/g. The linearity was fitted with the regression equation y = ax + b, with a weighing factor of 1/x. The limit of detection (LOD) and limit of quantification (LOQ) were defined as the concentration with signal-to-noise ratios of 3 and 10, respectively. The inter- and intra-day precision were evaluated using three different concentration (low, medium, and high) in triplicate on one day and three separate days, respectively.
To evaluate the matrix effect (ME), blank samples of three different types (capsule, tablet, and pill) were selected as free SAs. After the extraction procedure, two different concentrations (0.5, and 1.0 μg/g) of standard solutions were spiked in triplicate. Standard solutions were prepared at the same concentrations. The matrix effect was calculated as follows: ME (%) = (B/A) × 100, where A is the peak area ratio of [standard analyte/internal standard] and B is the peak area ratio of [spiked analytes/internal standard] after extraction; the corresponding internal standards to targeted analytes indicate in Table 2.
Table 2.
Method validation data of 35 sulfonamides obtained by UHPLC-Q/TOF-MS.
| No. | Analyte | Linearity range (ng/g) | Correlation coefficient (r2) | Internal standard | LOD (ng/g) | Accuracy and Precision (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Intra day | Inter day | ||||||||||
|
|
|
||||||||||
| 25 | 200 | 400 | 25 | 200 | 400 | ||||||
| 1 | Chlorothiazide | 1–2500 | 0.999 | 1 | 0.04 | 108.4 ± 0.7 | 94.7 ± 9.8 | 96.7 ± 1.7 | 109.1 ± 1.1 | 88.8 ± 7.4 | 98.7 ± 2.9 |
| 2 | Acetazolamide | 1–2500 | 0.999 | 2 | 0.37 | 104.8 ± 4.6 | 116.6 ± 5.8 | 106.8 ± 3.9 | 104.1 ± 5.7 | 103.7 ± 3.7 | 110.5 ± 2.9 |
| 3 | Methazolamide | 5–2500 | 0.998 | 2 | 0.17 | 118.3 ± 4.3 | 83.3 ± 3.7 | 98.8 ± 2.3 | 112.6 ± 1.4 | 87.3 ± 1.4 | 95.1 ± 2.4 |
| 4 | ACB | 5–2500 | 0.996 | 2 | 0.79 | 87.3 ± 5.4 | 81.6 ± 1.5 | 90.9 ± 2.5 | 89.3 ± 3.8 | 88.2 ± 1.0 | 84.5 ± 4.6 |
| 5 | Clofenamide | 1–2500 | 0.997 | 2 | 0.48 | 118.7 ± 7.2 | 85.6 ± 3.2 | 93.0 ± 1.8 | 110.8 ± 3.0 | 85.7 ± 3.5 | 88.5 ± 4.1 |
| 6 | Hydrochlorothiazide | 1–2500 | 1.000 | 1 | 0.13 | 110.9 ± 6.6 | 90.4 ± 7.2 | 106.4 ± 2.4 | 110.7 ± 7.4 | 104.4 ± 7.3 | 109.8 ± 6.0 |
| 7 | Quinethazone | 5–2500 | 0.998 | 1 | 1.35 | 86.6 ± 4.7 | 80.0 ± 2.9 | 84.3 ± 2.2 | 88.4 ± 3.8 | 85.8 ± 5.9 | 90.7 ± 7.0 |
| 8 | Chlorpropamide | 1–2500 | 0.995 | 3 | 0.16 | 97.3 ± 7.7 | 94.2 ± 7.4 | 103.4 ± 3.5 | 110.4 ± 5.1 | 103.3 ± 5.9 | 109.0 ± 3.7 |
| 9 | ATFB | 1–2500 | 0.998 | 2 | 0.07 | 105.0 ± 8.0 | 90.2 ± 2.7 | 84.1 ± 2.5 | 108.4 ± 5.5 | 89.2 ± 3.6 | 85.6 ± 1.7 |
| 10 | Hydroflumethiazide | 1–2500 | 0.999 | 1 | 0.15 | 104.3 ± 2.5 | 96.9 ± 6.8 | 113.1 ± 2.4 | 103.9 ± 4.1 | 113.2 ± 7.9 | 113.9 ± 7.5 |
| 11 | Furosemide | 5–2500 | 0.998 | 2 | 0.93 | 96.8 ± 9.3 | 105.2 ± 6.3 | 109.7 ± 2.1 | 102.9 ± 4.1 | 115.3 ± 2.0 | 112.6 ± 0.9 |
| 12 | Trichlorothiazide | 1–2500 | 0.998 | 1 | 0.27 | 101.6 ± 1.5 | 99.7 ± 7.0 | 98.7 ± 2.2 | 114.1 ± 8.6 | 97.0 ± 4.1 | 105.4 ± 3.2 |
| 13 | Xipamide | 1–2500 | 0.996 | 2 | 0.08 | 114.5 ± 5.7 | 103.0 ± 6.0 | 100.0 ± 1.9 | 117.3 ± 2.6 | 92.6 ± 4.2 | 99.9 ± 1.8 |
| 14 | Tolbutamide | 5–2500 | 0.999 | 3 | 0.75 | 110.2 ± 7.7 | 102.7 ± 8.8 | 106.0 ± 3.8 | 113.6 ± 5.6 | 115.3 ± 1.1 | 113.2 ± 0.7 |
| 15 | Diclofenamide | 1–2500 | 0.998 | 2 | 0.38 | 84.8 ± 2.3 | 92.0 ± 3.1 | 88.1 ± 1.7 | 86.4 ± 4.4 | 93.3 ± 1.0 | 85.5 ± 2.9 |
| 16 | Chlorothalidone | 5–2500 | 0.996 | 2 | 2.14 | 87.1 ± 6.2 | 85.1 ± 6.6 | 89.6 ± 1.7 | 88.4 ± 6.9 | 84.0 ± 2.7 | 93.0 ± 1.3 |
| 17 | Tolazamidea | 50–2500 | 0.999 | 3 | 10.77 | 82.7 ± 7.4 | 95.1 ± 1.1 | 99.7 ± 1.5 | 96.6 ± 8.7 | 96.1 ± 0.8 | 99.6 ± 1.4 |
| 18 | Piretanide | 5–2500 | 0.997 | 2 | 1.40 | 96.4 ± 8.5 | 89.3 ± 6.1 | 97.8 ± 2.6 | 107.4 ± 2.8 | 93.8 ± 3.5 | 96.2 ± 2.2 |
| 19 | Gliclazidea | 50–2500 | 0.996 | 3 | 11.81 | 81.0 ± 2.5 | 103.8 ± 7.2 | 102.5 ± 3.4 | 83.4 ± 3.4 | 104.0 ± 1.6 | 106.1 ± 1.5 |
| 20 | Glipizide | 5–2500 | 1.000 | 3 | 1.05 | 106.3 ± 8.9 | 100.4 ± 7.8 | 101.6 ± 3.8 | 109.2 ± 2.7 | 115.4 ± 7.3 | 112.5 ± 5.3 |
| 21 | Torsemide | 1–2500 | 0.995 | 3 | 0.17 | 116.4 ± 4.0 | 89.3 ± 7.4 | 99.3 ± 3.0 | 117.8 ± 2.3 | 86.9 ± 4.6 | 98.7 ± 4.6 |
| 22 | Bumetanide | 5–2500 | 1.000 | 2 | 1.12 | 80.9 ± 7.3 | 92.7 ± 5.7 | 99.9 ± 1.5 | 84.9 ± 5.4 | 98.2 ± 2.7 | 106.0 ± 1.8 |
| 23 | Clopamide | 1–2500 | 0.998 | 2 | 0.53 | 80.6 ± 2.7 | 92.7 ± 7.7 | 99.9 ± 3.6 | 90.4 ± 6.0 | 87.6 ± 6.9 | 95.0 ± 4.3 |
| 24 | Azosemide | 1–2500 | 0.999 | 2 | 0.10 | 117.5 ± 4.3 | 111.7 ± 6.1 | 110.9 ± 4.7 | 118.4 ± 0.7 | 105.2 ± 6.2 | 107.5 ± 4.8 |
| 25 | Topiramate | 1–2500 | 0.998 | 2 | 0.61 | 92.8 ± 4.1 | 118.1 ± 6.4 | 106.4 ± 3.8 | 97.9 ± 4.6 | 114.5 ± 6.0 | 105.1 ± 3.2 |
| 26 | Methyclothiazide | 1–2500 | 0.999 | 1 | 0.17 | 85.6 ± 3.7 | 88.9 ± 8.8 | 106.4 ± 2.5 | 85.1 ± 4.4 | 100.7 ± 4.2 | 102.4 ± 5.6 |
| 27 | Indapamide | 1–2500 | 0.996 | 2 | 0.46 | 82.7 ± 6.1 | 80.3 ± 2.0 | 83.5 ± 1.3 | 82.3 ± 1.4 | 81.9 ± 1.4 | 85.2 ± 1.6 |
| 28 | Epitizide | 1–2500 | 0.998 | 1 | 0.21 | 100.6 ± 1.6 | 87.6 ± 7.5 | 101.8 ± 3.0 | 100.5 ± 3.0 | 98.8 ± 2.8 | 103.9 ± 5.1 |
| 29 | Metolazone | 1–2500 | 0.997 | 1 | 0.46 | 80.2 ± 1.5 | 92.5 ± 7.6 | 106.7 ± 1.8 | 80.1 ± 1.5 | 108.5 ± 5.2 | 105.9 ± 2.7 |
| 30 | Glibenclamide | 1–2500 | 0.998 | 3 | 0.39 | 106.3 ± 8.0 | 81.1 ± 1.0 | 87.9 ± 3.4 | 102.1 ± 5.7 | 111.0 ± 1.9 | 117.2 ± 6.2 |
| 31 | Cyclothiazide | 5–2500 | 0.998 | 1 | 1.18 | 95.6 ± 1.3 | 90.1 ± 6.0 | 102.6 ± 2.9 | 103.4 ± 6.1 | 107.3 ± 8.7 | 111.3 ± 6.8 |
| 32 | Glimepiride | 5–2500 | 0.999 | 3 | 0.82 | 93.5 ± 7.8 | 106.1 ± 7.5 | 115.3 ± 1.2 | 102.1 ± 2.4 | 111.0 ± 4.3 | 117.2 ± 6.2 |
| 33 | Polythiazide | 1–2500 | 0.999 | 1 | 0.11 | 114.2 ± 2.4 | 94.5 ± 9.1 | 106.2 ± 2.7 | 118.0 ± 4.5 | 92.6 ± 0.4 | 98.9 ± 7.1 |
| 34 | Bendroflumethiazide | 1–2500 | 0.999 | 1 | 0.18 | 106.5 ± 1.6 | 93.7 ± 5.0 | 115.4 ± 2.6 | 108.6 ± 8.4 | 99.3 ± 1.2 | 114.4 ± 4.8 |
| 35 | Cyclopenthiazide | 1–2500 | 1.000 | 1 | 0.50 | 108.7 ± 1.7 | 80.7 ± 6.2 | 95.3 ± 3.3 | 106.9 ± 4.4 | 93.4 ± 6.3 | 101.5 ± 5.4 |
1: Bendroflumethiazide-d5, 2: Furosemide-d5, 3: Tolbutamide-d9.
Spiking at 200, 600, and 800 ng/g.
Recoveries (RE) were calculated by spiking with standard mixture solution (1.0 μg/g) before and after sample extraction to a blank matrix. The internal standards were added when reconstituting the extract. The extraction recovery was calculated as follows: RE (%) = C/B × 100, where C is the peak area ratio of [spiked analytes/internal standard] before extraction.
2.6. Application
Ten dietary supplements (4 pills, 3 capsules, and 3 tablets different brands) were collected from the internet market and analyzed using the developed method. The suitability of the screening method was evaluated by analyzing spiked supplement samples. Confirmation of analytes was accomplished on the basis of matching the retention times, accurate mass values, and MS/MS spectra with their corresponding reference library database. For confirmation of analytes, mass tolerance and retention time deviation were required to be within ±5 ppm and ±0.15 min, respectively.
3. Results and discussion
3.1. Extraction of sulfonamides from supplement diets
For the analysis of SAs in complex supplements matrixes, several extraction methods have been reported [15,18–20], enabling sensitive detection of SAs by LC-MS. For most methods, a limited number of SAs could be simultaneously determined by LC-MS/MS. For efficient extraction, the unique chemical properties of SAs were considered; 1) soluble in polar organic solvents but insoluble in non-polar solvents and water, and 2) different chemical forms depending on the pH of the aqueous solution; most SAs are positively charged below pH 2, neutral between pH 3 and 8, and negatively charged above pH 9 [19]. In this study, four extraction methods were evaluated with regard to their recovery yields and matrix effects for a wide range of SAs.
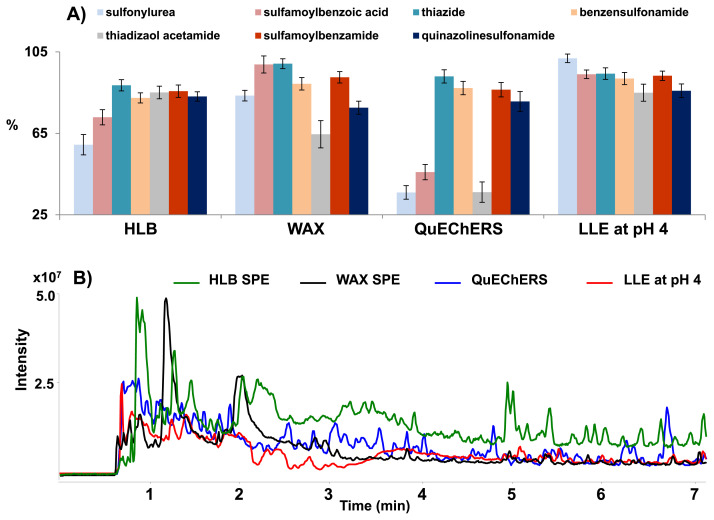
For the SPE procedure, the two Oasis HLB and WAX cartridges were utilized for the extraction of SAs with variation in the eluting solvent and the washing solution [21–23]. The recovery yields of both SPE methods are shown in Fig. 2-A. The HLB-SPE cartridge at neutral pH conditions allowed a reasonable recovery yield for most SAs. However, some of sulfonylureas and sulfamoylbenzoic acids with low pKa < 6.2 exhibited lower recoveries. For this reason, the Oasis WAX cartridges as an alternative SPE method was applied due to their strong interaction with a weak ionic adsorbent. As a result, the extraction recoveries of these SAs were remarkably high for acidic SAs, while those for the thiadiazolacetamides and quinazolinesulfonamides were slightly decreased (shown in Fig. 2-A) Both SPE methods were shown to be unsuitable extraction methods for the simultaneous extraction of a wide range of SAs with different pKa values.
Fig. 2.
A) Comparison of extraction recoveries of sulfonamides according to the different sample preparation procedure, and B) overlay of TICs from pill samples after different sample preparation (SPE, QuEChERS, and pH controlled LLE).
Thirdly, the QuEChERS method was introduced for the extraction of pharmaceutical drugs from various types of matrices due to its easy manipulation [15,24]. The recovery test was performed using acetonitrile as an extracting solvent and the Agilent Bond Elut AOAC extraction kit to purify the matrix. The QuEChERS method achieved unsatisfactory recoveries for sulfonylureas (24.2–64.0%), sulfamoylbenzoic acids (36.3–60.8%), and thiadizaol acetamides (22.7–44.5%). Thus, QuEChERS was not suitable for the extraction of broad range of SAs.
In this study, LLE with pH control was attempted for the extraction of SAs from the sample extract. The pH conditions in LLE can significantly affect the extraction efficiency of SAs due to their unique chemical properties. In order to achieve high extraction recovery, pH conditions of the aqueous phase were evaluated in the range of 3–7. Ethyl acetate was used as an extraction solvent, which was the best extraction solvent for SA in the literature [25,26]. The recovery yields of classified SAs according to the variation of pH are summarized in Fig. S2. At pH 6 and 7, the recovery yield of most SAs was relatively low probably due to the deprotonation of amino groups. As indicated in Fig. S2, the overall recovery yield was similar in the range of pH 3–5. Among them, the LLE at pH 4 provided the highest extraction recovery (ranging from 85.9% to 101.84%) for all types of SA and the lowest RSD values (<5%) of overall SAs. Regardless of a wide range of pKa values of SAs, all targets were successfully extracted from the sample matrix by LLE at pH 4. Compared to SPE and QuEChERS methods, LLE at pH 4 was shown more efficient in view of recovery yield and reproducibility for the extraction of SAs from various types of supplements.
Furthermore, LLE at pH 4 could enable the extraction of 35 SAs and reduce matrix interferences compared to other pretreatment methods. As shown in Fig. 2-B, the two SPE cartridges and QuEChERS methods showed more interference than LLE at pH 4 for the pill samples with complex matrices. Therefore, the established LLE method is simple and effective in terms of simultaneous extraction efficiency and interference effects for a wide range of SAs from supplements.
3.2. Separation of 35 sulfonamides by UHPLC
The optimization of LC separation conditions is crucial for reliable screening and confirmation of SAs in various types of supplements. In this study, several chromatographic parameters including column brand, dimension, mobile phase composition, column temperature, and flow rate were optimized for high peak resolution and MS detection sensitivity of SAs. Three C18 columns with identical dimensions (length, particle size, and inner diameter) were tested by monitoring the separation efficiency, retention behavior, and baseline stability. Although these three columns showed similar elution behavior and MS detection sensitivity, the Kinetex EVO C18 column exhibited the best chromatographic performance in terms of peak shape, separation capability, retention time, and baseline stability [Fig. S3].
The pH of the mobile phase can greatly influence chromatographic elution and the MS detection sensitivity of SAs. In previous studies [10,11,21,26], acidic mobile phase was, in most cases, used for the separation of SAs under dual polarity (positive and negative) mode. In this case, dual analyses for acidic SAs in negative ion mode and basic SAs in positive ion mode were used to achieve high separation capacity and MS detection sensitivity. Thus, in this study, the pH of the mobile phase was set at 8, enabling efficient separation and MS detection sensitivity of SAs in a single run. With a mobile phase of pH 8, a wide range of 35 SAs were successfully separated within 7 min and provided sufficient MS detection sensitivity at the sub-ppb levels in the negative ion mode. The column temperature (20, 30, and 40 °C) and flow rates (0.3, 0.35, and 0.4 mL/min) were also tested. There was no significant difference in sensitivity and peak capacity of SAs. However, a flow rate at above 0.45 mL/min led to a significant decrement of peak separation capacity for specific SAs. Thus, taking consideration of separation efficiency, elution time, and peak sensitivity of 35 SAs, we selected 10 mM NH4Ac in acetonitrile (pH 8) as mobile phase, a flow rate of 0.4 mL/min, and a column temperature at 30 °C.
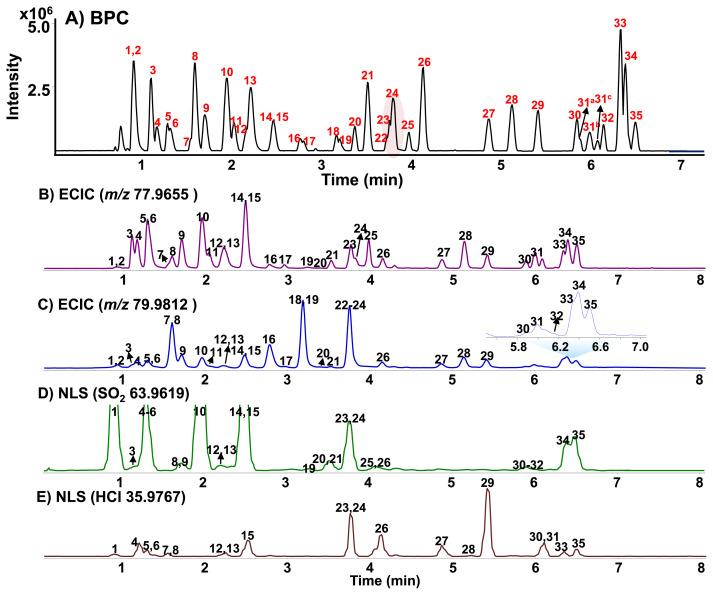
Under optimized UHPLC conditions, the 35 SAs in BPC were well separated within 7 min, as shown in Fig. 3-A. Although some peaks co-eluted and showed poor sensitivity, they could be successfully discriminated with MS because they had different [M−H]− mass values, fragments, and MS/MS spectral patterns and sufficiently detected at sub-ppb level, owing to the high sensitivity of Q/TOF MS. Table 1 reports the retention times, molecular formula, accurate measured precursor ion masses, and the corresponding calculated exact m/z values. In our experiments, a maximum difference of ±2.98 ppm between the experimental m/z values and theoretical values was obtained together with isotopic abundance matching, providing confident identification.
Fig. 3.
A) BPC of 35 sulfonamides by UPLC-Q/TOF-MS in the negative mode and ECICs of B) m/z 77.9655 C) m/z 79.9812 and NLS of D) SO2 63.9619 and E) HCl 35.9767. The peak numbers are the same as in Table 1.
Table 1.
Retention time, molecular formula, and accurate mass of 35 illegal adulterants obtained by UPLC-Q/TOF MS.
| No. | Analyte | RT (min) | Molecular formula | Theoretical m/z ion (Da) | Error (ppm) |
|---|---|---|---|---|---|
| 1 | Chlorothiazide | 0.91 | C7H6ClN3O4S2 | 293.9415 | 1.02 |
| 2 | Acetazolamide | 0.92 | C4H6N4O3S2 | 220.9809 | 0.45 |
| 3 | Methazolamide | 1.10 | C5H8N4O3S2 | 234.9965 | 2.98 |
| 4 | ACB | 1.17 | C6H8ClN3O4S2 | 283.9572 | 1.06 |
| 5 | Clofenamide | 1.28 | C6H7ClN2O4S2 | 268.9463 | 1.12 |
| 6 | Hydrochlorothiazide | 1.31 | C7H8ClN3O4S2 | 295.9572 | 0.34 |
| 7 | Quinethazone | 1.55 | C10H12ClN3O3S | 288.0215 | −0.69 |
| 8 | Chlorpropamide | 1.59 | C10H13ClN2O3S | 275.0262 | 2.18 |
| 9 | ATFB | 1.70 | C7H8F3N3O4S2 | 317.9835 | 1.57 |
| 10 | Hydroflumethiazide | 1.94 | C8H8F3N3O4S2 | 329.9836 | 1.21 |
| 11 | Furosemide | 2.02 | C12H11ClN2O5S | 329.0004 | 1.22 |
| 12 | Trichloromethiazide | 2.16 | C8H8Cl3N3O4S2 | 377.8949 | −0.79 |
| 13 | Xipamide | 2.21 | C15H15ClN2O4S | 353.0368 | 1.42 |
| 14 | Tolbutamide | 2.45 | C12H18N2O3S | 269.0965 | −2.23 |
| 15 | Diclofenamide | 2.46 | C6H6Cl2N2O4S2 | 302.9073 | 0.99 |
| 16 | Chlorothalidone | 2.76 | C14H11ClN2O4S | 337.0055 | −1.19 |
| 17 | Tolazamide | 2.79 | C14H21N3O3S | 310.1231 | −0.64 |
| 18 | Piretanide | 3.17 | C17H18N2O5S | 361.0864 | 0.28 |
| 19 | Gliclazide | 3.19 | C15H21N3O3S | 322.1231 | 0.00 |
| 20 | Glipizide | 3.37 | C21H27N5O4S | 444.1711 | −0.23 |
| 21 | Torsemide | 3.51 | C16H20N4O3S | 347.1183 | 1.44 |
| 22 | Bumetanide | 3.73 | C17H20N2O5S | 363.1020 | −1.10 |
| 23 | Clopamide | 3.76 | C14H20ClN3O3S | 344.0841 | 0.29 |
| 24 | Azosemide | 3.79 | C12H11ClN6O2S2 | 368.9994 | −0.27 |
| 25 | Topiramate | 3.97 | C12H21NO8S | 338.0915 | 0.00 |
| 26 | Methyclothiazide | 4.13 | C9H11Cl2N3O4S2 | 357.9495 | 2.23 |
| 27 | Indapamide | 4.86 | C16H16ClN3O3S | 364.0528 | 0.82 |
| 28 | Epitizide | 5.12 | C10H11ClF3N3O4S3 | 423.9479 | 2.12 |
| 29 | Metolazone | 5.41 | C16H16ClN3O3S | 364.0528 | 1.10 |
| 30 | Glibenclamide | 5.84 | C23H28ClN3O5S | 492.1365 | 0.20 |
| 31 | Cyclothiazide | 5.98 | C14H16ClN3O4S2 | 388.0195 | −0.26 |
| 32 | Glimepiride | 6.14 | C24H34N4O5S | 489.2177 | 0.00 |
| 33 | Polythiaizde | 6.33 | C11H13ClF3N3O4S3 | 437.9636 | 1.14 |
| 34 | Bendroflumethiazide | 6.38 | C15H14F3N3O4S2 | 420.0305 | 0.00 |
| 35 | Cyclopenthiazide | 6.49 | C13H18ClN3O4S2 | 378.0354 | 0.79 |
3.3. Rapid screening of sulfonamide adulterants based on ECIC and NLS
Collision-induced dissociation (CID)-MS/MS spectra for SAs have been intensively investigated in ESI-positive and negative ion modes [3,9,11,12]. In this study, a negative ion mode was applied to obtain high intensity [M−H]− ions due to the acidic property of SAs. Also, MS/MS spectra of [M−H]− ions for SAs were studied to find specific fragments, enabling rapid screening. The MS/MS spectra of SAs with various structures exhibited several characteristic ions through various routes such as neutral molecule losses and weak bonding cleavages. The MS/MS fragmentation pathways of SAs are suggested in Fig. S4–5. Especially, two characteristic ions via weak S–C or S–N bonding cleavages were commonly observed at m/z 77.9655, [NSO2]− and/or m/z 79.9812, [NH2SO2]− ions, reflecting the presence of the SAs in supplements [Fig. S6].
As shown in Fig. 3-B and C, the reconstructed ECICs of m/z 77.9655 and 79.9812 could successfully cover the screening of all sulfonamide analogs. Most SAs were detected with reasonable intensity in ECICs of m/z 77.9655 or 79.9812, except for chlorothiazide (peak #1). Particularly, the MS/MS spectra of sulfamoylbenzoic acids (bumetanide and piretanide) exhibited an abundant fragment at m/z 80 but a weak intensity ion at m/z 78. The MS/MS spectra of the sulfonylurea produced both common ions m/z 78 and 80, but the formation of m/z 80 was more favorable in ESI negative ion mode. As shown in Fig. 3-B, three peaks (#18, 22, and 32) were not detected in ECIC at m/z 77.9655. On the other hand, 34 of 35 SAs were successfully screened with ECIC at m/z 79.9812, except for topiramate (peak #25) [Fig. 3-B]. However, topiramate could be sensitively detected in ECIC at m/z 77.9655. Thus, both HR-ECICs could be used as complementary screening as well as confirmative analysis of SAs. Also, both HR-ECICs could be prospective screening tools in place of conventional extracted ion chromatograms (EICs) or MRM chromatograms for rapid screening and confirmation of SAs in supplements.
Beside both common fragment ions, some characteristic ions formed by the neutral molecule losses of SO2, HCl, HF, CO2 or HCN/SO2 from the [M−H]− ion or the fragment ion were also observed in the MS/MS spectra of SAs [27–29]. Among them, neutral loss fragments of SO2 (63.9619 Da) or HCl (35.9767 Da) molecules were frequently observed for investigated SAs. In this study, NLS chromatograms of SO2 and HCl were applied for screening of SAs [Fig. 3-D and E]. As can be seen in Fig. 3, the advantages of two NLS chromatograms are that peak #1 with very weak intensity in two ECICs could be sensitively detected in the SO2 molecule loss chromatogram and chlorinated sulfonamides could be selectively screened in the NLS chromatogram of HCl molecule. Consequently, the use of these HR-ECICs and HR-NLS could be helpful for the rapid screening of target as well as untargeted SA adulterants in various supplements.
3.4. Method validation
The calibration curves were generated using a linear least squares regression analysis of the peak area ratio of each analyte to the corresponding deuterium-labeled internal standard. The results showed good linearity in the working range (1–2500 ng/g), and the correlation coefficients (R2) of the calibration curves ranged from 0.995 to 1.000. As summarized in Table 2, the LODs and LOQs were within the range of 0.04–11.81 ng/g and 0.13–35.27 ng/g, respectively. The intra-and inter-day precision and accuracy data did not exceed 15% for all concentrations, indicating reasonable quantification method. These results indicate that the proposed method is suitable for the analysis of SAs in dietary supplements.
To evaluate the matrix effect (ME), two different concentration levels (500 and 1000 ng/g) were spiked into post extracts from each different supplement as described in Experimental Section 2.5. The MEs observed for the capsules were negligible (between 81.71% and 115.59%), with the exception of piretanide (59.07%), glibenclamide (76.12%), and glimepiride (69.33%) (Table S1). However, medium matrix effects (out of ranged between 40% and 80% or 120% and 150%) for some SAs were observed for tablet and pill samples. Medium ME was simply eliminated using the dilution of sample extract [7,22]. After diluting tablet and pill extracts 10-fold, ME could be inferred from good relative recovery value, giving acceptable accuracy of all SAs. Despite the decrease of intensity, all SAs could be detected at lower sub-ppm levels. Therefore, it can be concluded that no significant matrix effect was observed in the established method.
3.5. Application to real samples
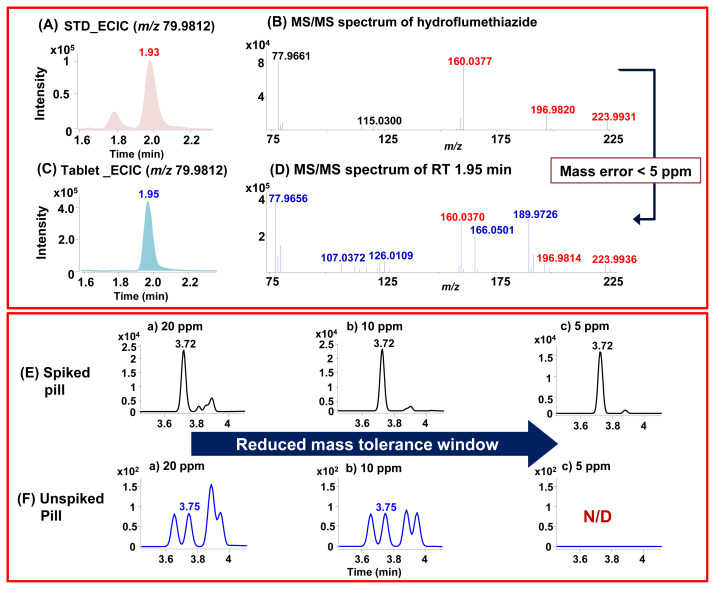
In recent literature [7], some of SAs were detected in the range of 2.62–30.90 mg/g level for capsule and tablet types of supplements. Approximate 0.5 g sample was sufficient for screening and detection of SA targets in supplements due to high sensitivity and selectivity of UHPLC-Q/TOS MS. To evaluate the proposed method, SAs were extracted from 10 dietary supplements by LLE. The screening of SAs in the sample extract was performed by HR-ECICs and NLSs. As typical example, a tablet extract was screened with ECIC of m/z 79.9812, a peak at RT 1.97 min was suspected to be hydroflumethiazide [Fig. 4-A and B]. Also, a peak at the same RT was sensitively detected in ECIC of m/z 77.9655 and NLS of SO2, providing complementary screening. The suspected compound was confirmed by comparing the RT, accurate [M−H]− mass value, and MS/MS spectrum with those of the authentic standard. The difference in RT was 0.02 min and the difference in accurate [M−H]− mass value within 5 ppm [Fig. 4-C and D]. In addition, this compound was well matched with the constructed homemade library with 99%. Besides tablet samples, the SAs were successfully screened for herbal-based supplements with complex matrices.
Fig. 4.
Confirmation of hydroflumethiazide through ECICs of m/z 79.9812 (A) standard; (C) tablet sample and MS/MS mass error values (B) standard; (D) tablet sample. ECICs of m/z 79.9812 for the bumetanide (E) spiked pill sample at 100 ng/g and (F) the unspiked pill sample obtained with different mass tolerances of 20, 10, and 5 ppm.
In some cases, the pill type supplements could lead to false positives due to significant interference, even using ECIC or NSL. To avoid a false positive, reduced mass tolerance window was applied in this study. As examples Fig. 4-E and F present ECICs (m/z 79.9812) with 20, 10, and 5 ppm extraction windows of a pill spiked with bumetanide (100 ng/g) and an unspiked pill sample. As shown in Fig. 4-E, the pill spiked with bumetanide was clearly and sensitively detected with individual ECIC even with a reduced mass tolerance window of 5 ppm. However, the bumetanide suspected peak in an unspiked sample was observed in ECIC at 20 and 10 ppm mass tolerance windows with similar RTs and isobaric mass [Fig. 4-F]. Reducing the mass tolerance window to 5 ppm precluded the detection of bumetanide in the unspiked pill sample. Most of the isobaric interference substances were filtered and background noise was removed virtually to zero by using HR TOF-MS with a mass tolerance window of 5 ppm. Thus, supplements with complex matrices can be screened confidently with only a small chance of false positive results.
4. Conclusions
A reliable screening and confirmation method by UHPLC-Q/ TOF-MS was suggested for the analysis of 35 SAs in various types of dietary supplements. The present method enabled rapid separation of 35 SAs within 7 min and sensitive detection at sub-ppb levels in ESI negative ion mode.
The simple LLE at pH 4 could be used to simultaneously extract a wide range of SAs from complex sample matrix. Both HR-ECICs (m/z 77.9655 and 79.9812) and HR-NSLs (SO2 and HCl) are promising alternative methods to conventional individual EICs or MRM chromatograms for rapid screening of SAs. Also, these ECIC and NSL complement each other and could be used for further evidence of SA adulteration in supplements. Especially, two ECICs could detect possible new emerging SA adulterants in dietary supplements. Thus, HR-ECICs could be of great help for the screening of targeted and untargeted SAs with specific a sulfonamide moiety. Third, for confirmation of SA adulterants in supplements, besides exact mass measurement, RT, and MS/MS spectral data, reduced mass tolerance windows at 5 ppm could help avoid false positive or negative results in the analysis of SAs in complicated sample matrices. UHPLC-HRMS combined with ECICs and NSLs will be a powerful tool for the rapid routine screening and confirmation of a wide range of SAs in various types of dietary supplement samples, ensuring food safety and public health.
Acknowledgments
This study was financially supported by the Ministry of Food and Drug Safety of Korea (2018, 18182MFDS425) and the National Research Foundation of Korea (NRF-2017R1A2B4005616).
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jfda.2018.08.006.
Funding Statement
This study was financially supported by the Ministry of Food and Drug Safety of Korea (2018, 18182MFDS425) and the National Research Foundation of Korea (NRF-2017R1A2B4005616).
Footnotes
Conflicts of interest
All contributing authors have no conflicts of interest to declare.
REFERENCES
- 1. Krentz A, Bailey C. Oral antidiabetic agents; current role in type 2 diabetes mellitus. Drugs. 2005;65:385–411. doi: 10.2165/00003495-200565030-00005. [DOI] [PubMed] [Google Scholar]
- 2. Moreira APL, Motta MJ, Dal Molin TR, Viana C, Carvalho LMd. Determination of diuretics and laxatives as adulterants in herbal formulations for weight loss. Food Addit Contam. 2013;30:1230–7. doi: 10.1080/19440049.2013.800649. [DOI] [PubMed] [Google Scholar]
- 3. Guo C, Shi F, Jiang S, Gong L, Zhao Y, Zhang J, et al. Simultaneous identification, confirmation and quantitation of illegal adulterated antidiabetics in herbal medicines and dietary supplements using high-resolution benchtop quadrupole-Orbitrap mass spectrometry. J Chromatogr B. 2014;967:174–82. doi: 10.1016/j.jchromb.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 4. Rebiere H, Guinot P, Civade C, Bonnet PA, Nicolas A. Detection of harzardous weight-loss substances in adulterated slimming formulations using ultra-high-pressure liquid chromatography with diode-array detection. Food Addit Contam. 2012;29:161–71. doi: 10.1080/19440049.2011.638676. [DOI] [PubMed] [Google Scholar]
- 5. Petroczi A, Taylor G, Naughton DP. Mission impossible? Regulatory and enforcement issues to ensure safety of dietary supplements. Food Chem Toxicol. 2011;49:393–402. doi: 10.1016/j.fct.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 6. Woo H, Kim JW, Han KM, Lee JH, Hwang IS, Lee JH, et al. Simultaneous analysis of 17 diuretics in dietary supplements by HPLC and LC-MS/MS. Food Addit Contam. 2013;30:209–17. doi: 10.1080/19440049.2012.738939. [DOI] [PubMed] [Google Scholar]
- 7. Moreira APL, Gobo LA, Viana C, Carvalho LMd. Simultaneous analysis of antihypertensive drugs and diuretics as adulterants in herbal-based products by ultra-high performance liquid chromatography-electrospray tandem mass spectrometry. Anal Meth. 2016;8:1881–8. [Google Scholar]
- 8. Zhou C, Tang B, Xi C, Zhang L, Wang G, Xi J, et al. Simultaneous Determination of seven sulfonylurea-type oral anti-diabetic agents in adulterated dietary supplements and traditional chinesmedicines by ultraperformance liquid chromatography-tandem mass spectrometry. Spectrosc Lett. 2015;48:163–9. [Google Scholar]
- 9. Cheng Q, Shou L, Chen C, Shi S, Zhou M. Application of ultra-high-performance liquid chromatography coupled with LTQ-Orbitrap mass spectrometry for determination, conformation and quantitation of illegal adulterated weight-loss drugs in plant dietary supplements. J Chromatogr B. 2017;1064:92–9. doi: 10.1016/j.jchromb.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 10. Wang X-B, Zheng J, Yu H-Y, Li Q-Y, Xu L-H, Liu M-J, et al. Simultaneous analysis of 23 illegal adulterated aphrodisiac chemical ingredients in helath foods and Chinese traditional patent medicines by ultrahigh performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J Food Drug Anal. 2018;26:1138–53. doi: 10.1016/j.jfda.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo B, Wang M, Liu Y, Zhou J, Dai H, Huang Z, et al. Wide- Scope screening of illegal adulterants in dietary and herbal supplements via rapid polarity-switching and multistage accurate mass confirmation using an LC-IT/TOF hybrid instrument. J Agric Food Chem. 2015;63:6954–67. doi: 10.1021/acs.jafc.5b02222. [DOI] [PubMed] [Google Scholar]
- 12. Meng X, Bai H, Guo T, Niu Z, Ma Q. Broad screening of illicit ingredients in cosmetics using ultra-high-performance liquid chromatography-hybrid quadrupole-Orbitrap mass spectrometry with customized accurate-mass database and mass spectral library. J Chromatogr A. 2017;1528:61–74. doi: 10.1016/j.chroma.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 13. Jin R, Li L, Guo L, Li W, Shen Q. A graphene tip coupled with liquid chromatography tandem mass spectrometry for the determination of four synthetic adulterants in slimming supplements. Food Chem. 2017;224:329–34. doi: 10.1016/j.foodchem.2016.12.091. [DOI] [PubMed] [Google Scholar]
- 14. Wu X, Zhu B, Lu L, Huang W, Pang D. Optimization of a solid phase extraction and hydrophilic interaction liquid chromatography-tandem mass spectrometry method for the determination of metformin in dietary supplements and herbal medicines. Food Chem. 2012;133:482–8. doi: 10.1016/j.foodchem.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 15. Lin Y-P, Lee Y-L, Hung C-Y, Huang W-J, Lin S-C. Determination of multiresidue analysis of β-agonists in muscle and viscera using liquid chromatograph/tandem mass spectrometry with Quick, Easy, Cheap, Effective, Rugged, and Safe methodologies. J Food Drug Anal. 2017;25:275–84. doi: 10.1016/j.jfda.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chemicalize : ChemAxon Ltd. 1998. [Accessed May 1, 2018]. Available at: https://chemicalize.com/#/calulation.
- 17.ICH Harmonized Tripartite Guidelines - ICH harmonized tripartite guideline, validation of analytical procedures: text and methodology Q2 (R1) Nov, 2005. http://www.ich.org/LOB/media/MEDIA417.pdf .
- 18. Hoff R, Pizzolato TM, Diaz-Cruz MS. Trends in sulfonamides and their by-products analysis in environmental samples using mass spectrometry techniques. Trends Anal Chem. 2016;9:24–36. [Google Scholar]
- 19. Herrera-Herrera AV, Hernández-Borges J, Borges-Miquel TM, Rodríguez-Delgado MÁ. Dispersive liquid-liquid microextraction combined with ultra-high performance liquid chromatography for the simultaneous determination of 25 sulfonamide and quinolone antibiotics in water samples. J Pharm Biomed Anal. 2013;75:130–7. doi: 10.1016/j.jpba.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 20. Paíga P, Rodrigues MJE, Correia M, Amaral JS, Oliveira MBPP, Delerue-Matos C. Analysis of pharmaceutical adulterants in plant food supplements by UHPLC-MS/MS. Eur J Pharm Sci. 2017;99:219–27. doi: 10.1016/j.ejps.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 21. Martín J, Buchberger W, Santos JL, Alonso E, Aparicio I. High-performance liquid chromatography quadrupole time-of flight mass spectrometry method for the analysis of antidiabetics drugs in aqueous environmental samples. J Chromatogr B. 2012;895–6:94–101. doi: 10.1016/j.jchromb.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 22. Marchi I, Rudaz S, Veuthey JL. Sample preparation development and matrix effects evaluation for multianalyte determination in urine. J Pharm Biomed Anal. 2009;49:459–67. doi: 10.1016/j.jpba.2008.11.040. [DOI] [PubMed] [Google Scholar]
- 23. Bourdat-Deschamps M, Leang S, Bernet N, Daudin JJ, Nélieu S. Multi-residue analysis of pharmaceuticals in aqueous environmental samples by online solid-phase-extraction-ultra-high-performance liquid chromatography-tandem mass spectrometry: optimisation and matrix effects reduction by quick, easy, cheap, effective, rugged and safe extraction. J Chromatogr A. 2014;1349:11–23. doi: 10.1016/j.chroma.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 24. Kung TA, Tsai CW, Ku BC, Wang WH. A generic and rapid strategy for determining trace multiresidues of sulfonamides in aquatic products by using an improved QuEChERS method and liquid chromatography-electrospray quadrupole tandem mass spectrometry. Food Chem. 2015;175:189–96. doi: 10.1016/j.foodchem.2014.11.133. [DOI] [PubMed] [Google Scholar]
- 25. Dasenaki ME, Thomaidis NS. Multi-residue determination of seventeen sulfonamides and five tetracyclines in fish tissue using a multistage LC-ESI-MS/MS approach based on advanced mass spectrometric techniques. Anal Chim Acta. 2010;672:93–102. doi: 10.1016/j.aca.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 26. Dmitrienko SG, Kochuk EV, Apyari VV, Tolmacheva VV, Zolotov YA. Recent advances in sample preparation techniques and methods of sulfonamides detection-A review. Anal Chim Acta. 2014;850:6–25. doi: 10.1016/j.aca.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 27. Vijlder TD, Valkenborg D, Lemière F, Romijn EP, Laukens K, Cuyckens F. A tutorial in small molecule identification via electrospray ionization mass spectrometry: the practical art of structural elucidation. Mass Spectrom Rev. 2018;3:1–23. doi: 10.1002/mas.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Niessen WMA. Fragmentation of toxicologically relevant drugs in negative-ion liquid chromatography-tandem mass spectrometry. Mass Spectrom Rev. 2012;31:626–65. doi: 10.1002/mas.20359. [DOI] [PubMed] [Google Scholar]
- 29. Thevis M, Schanzer W, Schmickler H. Effect of the location of hydrogen abstraction on the fragmentation of diuretics in negative electrospray ionization mass spectrometry. J Am Soc Mass Spectrom. 2003;14:658–70. doi: 10.1016/S1044-0305(03)00213-7. [DOI] [PubMed] [Google Scholar]