Abstract
The rapid development of nanotechnology has been facilitating the transformations of traditional food and agriculture sectors, particularly the invention of smart and active packaging, nanosensors, nanopesticides and nanofertilizers. Numerous novel nanomaterials have been developed for improving food quality and safety, crop growth, and monitoring environmental conditions. In this review the most recent trends in nanotechnology are discussed and the most challenging tasks and promising opportunities in the food and agriculture sectors from selected recent studies are addressed. The toxicological fundamentals and risk assessment of nanomaterials in these new food and agriculture products are also discussed. We highlighted the potential application of biosynthesized and bio-inspired nanomaterial for sustainable development. However, fundamental questions with regard to high performance, low toxic nanomaterials need to be addressed to fuel active development and application of nanotechnology. Regulation and legislation are also paramount to regulating the manufacturing, processing, application, as well as disposal of nanomaterials. Efforts are still needed to strengthen public awareness and acceptance of the novel nano-enabled food and agriculture products. We conclude that nanotechnology offers a plethora of opportunities, by providing a novel and sustainable alternative in the food and agriculture sectors.
Keywords: Nanotechnology, Food, Agriculture, Bio-synthesized nanomaterial, Bio-inspired nanomaterial, Public acceptance, Regulation
1. Introduction
Nanotechnology is widely applied in our everyday life and is changing the entire society. It has begun marching into the agriculture and food industry since 2003 when United States Department of Agriculture published the first roadmap in September 9, 2003 [1]. Research on this topic has skyrocketed over the last decade. It almost covers every aspect in the food and agriculture industry, including agriculture, irrigation/water filtration, food processing and packaging, animal feed, and aquiculture [2–6].
The food and beverage sector is a global multi trillion dollar industry [7]. A recent estimate of the global economical impact of nanotechnology is projected to be at least $3 trillion by 2020, which may employee 6 million labors in the rising nanotechnology industries worldwide [8]. This is very attractive and has driven many food enterprises involved in development and marketing of novel nanomaterial based products, and improving production efficiency, food characteristics, taste and safety. Incredibly, there are hundreds of products that have already been marketed and used in the food business over the past decade. Majority of these products are designed “out-of-food” but “inside” food industry, i.e. food contacting materials but not directly consumed by people. No novel nanomaterials containing products have been directly put into human food yet, except titanium dioxide and iron oxide that have been used as food pigment and colorant respectively already. The fundamental reason is that regulation and legislation is very limited regarding nano food, especially due to complexity of nanomaterials and case-bycase legislating procedures [9–11].
A deeper cause for the limited regulation is the poor knowledge of toxicity and risk which novel nanomaterials could bring [5,12–14]. Many studies focus on in vitro toxicity of nanomaterials while very little in vivo toxicity data is available, not to mention chronic effect of nanomaterials (especially metal nanoparticles, NPs) [15]. At least several gaps have to be filled: toxicity of nanomaterial to mammal cells, tissues/organs and chronic effect to human body; migration of nanomaterials to food; degradation or environmental fate of nanomaterials; bioaccumulation of nanomaterials and their impact on ecosystems.
Another important aspect is public acceptance which is often ignored by researchers, manufacturers, as well as authorities [16,17]. It ultimately determines whether or not nanotechnology could be actually applied and/or accepted by customers. Nanomaterials can and have been widely applied in every aspect of food industry, from food agriculture, processing, storage and transportation to our plate. Regardless of the population of those who accept these novel nano foods, the waste will ultimately be disposed into the environment and elicit specific impact on flora, fauna and ecosystems. Unfortunately, this piece of information is very little. To make it worse, the proper disposal method has not been mentioned yet by either researcher, food company or government agency. In addition the in vivo toxicity data of nanomaterials is very lacking, especially the potential chronic effect on human organs.
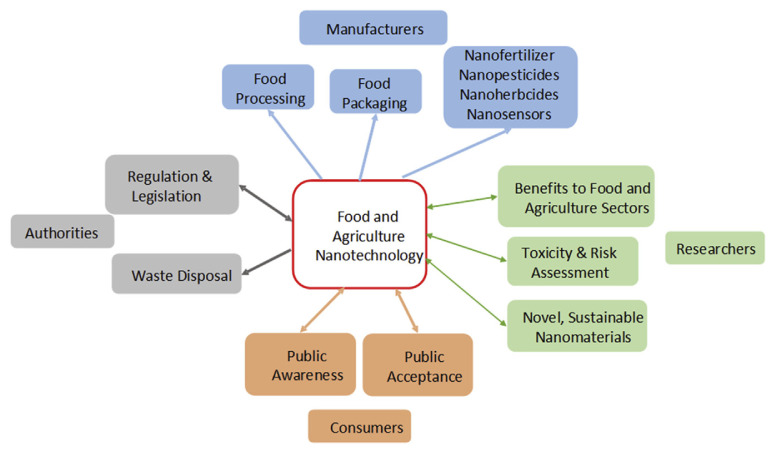
As shown in Fig. 1, from food nanotechnology to marketed product, or from fundamental aspects of nanotoxicity to regulation and legislation, or from knowledge of food nanotechnology to public awareness and acceptance, a huge amount of information and effort is needed and all these aspects are strictly related to each other. Each section has abundant reports and reviews are available for every single one of them. This chapter briefly covers recent progress on all these aspects and emphasizes the urgent necessity to get insight into risk assessment and toxicity evaluation of novel nanomaterials for the purpose of legislation as well as the public acceptance. Under this circumstance, bio-synthesized (or “green synthesized”) nanomaterials may provide an alternative solution to apply novel nanomaterials in food industry with relatively “acceptable” negative impact.
Fig. 1.
Schematic illustration of food nanotechnology from scientific research to marketed product, and to consumer’s plate. Scientific research is a one-way output providing guidance for both food manufacturer to develop product, and for agencies to make regulation and legislation. Regulation and legislation play a central and core role to control marketing product and proper disposal of the waste, which, unfortunately is currently very limited globally. Public awareness and acceptance are often ignored by scientists and manufacturers, since government agency (regulation/law) and manufacturer (product/advertisement) serve as the main information source for the general public. But it is the end user who decides whether or not food nanotechnology can actually appear in consumer’s plates.
2. Current status on food and agriculture nanotechnology
Nanotechnology deals with nanomaterials which have at least one dimension ranging from 1 to 100 nm. Tested nanomaterials in food industry include inorganic (metal and metal oxide NPs), organic (mainly natural product NPs) and combined (i.e. clay). Among all metal NPs, silver NP is the one most commercially made and applied due to its antimicrobial activity while gold NP is widely studied as a sensor/detector. Titanium dioxide NPs are also well studied as disinfecting agent as well as food additive (white color pigment) and flavor enhancer. Natural product NPs are normally designed as a delivery system, and also involved as ingredients or supplements in food industry.
As shown in Fig. 1, various nanomaterials have shown exciting potential in every aspect of food industry ranging from agriculture to plates, while much information remains unknown. The recent progress will be briefly discussed in the following parts, using representatives in the past 5 years.
2.1. Current status on food nanotechnology
Food nanotechnology has infiltrated into many aspects of customer products, such as food packaging, additives, and food preservation. The recognition of this novel technology has advanced the food processing and storage in ensuring food safety. Many conventional chemicals added as food additives or packaging materials have also been found partially existing at nanometer scale. For example, food-grade TiO2 NPs now have been found up to approximately 40% in the nanometer range [18,19]. Although nanomaterials like TiO2 NPs are generally recognized low toxic at ambient conditions, long-term exposure to such nanomaterials may cause adverse damages [20]. The application of novel food nanotechnology, together with the presence of nanoscale chemicals, has also attracted public attention regarding the potential risks. We carefully review current progress on the application of food nanotechnology in this section. Selected nanomaterials used in food products are listed in Table 1. United State Food and Drug Administration (U.S. FDA) and European Commission (EC) are themain sources for legislation and regulation on food nanotechnology. Some authorizations made by the U.S. FDA and EC in Table 1 are based on the risk assessment of the conventional particle size of a substance; therefore, a case-by-case basis by the authority may be required for engineered NPs. A few applications under research and development (R&D) are also included in Table 1 to indicate potential future applications.
Table 1.
Current status of nanotechnology-enabled food products.
| Sector | Application | Nanomaterials | Manufacturer | Current status | Note | Reference |
|---|---|---|---|---|---|---|
| Food processing | Color additives | TiO2 | Exempt from certification | <1% by weight of the food | [41] | |
| Synthetic iron oxide | Exempt from certification | <0.25% (for dogs and cats) and 0.1 (for human) % by weight of the finished food | [41,42] | |||
| Additive or polymer production aid | ZnO Iron oxide Aluminium oxide Silicon dioxide Cobalt oxide Manganese oxide (E530) |
Authorized by EC 10/2011 | Authorization based on conventional particle size | [43] | ||
| Titanium nitride | No migration reported. Only to be used in PET bottles up to 20 mg/kg | |||||
| Carbon black | Authorized by EC 10/2011; no longer authorized by the U.S. FDA as additives | <2.5% w/w in the polymer | ||||
| Preservatives | Silver-silica | Nanox Intelligent Materials | FCS Inventorya | FCN No. 1235. <4 ppm by weight of silver as an antimicrobial agent blended into polymers | [44] | |
| Flavor carrier | Silicon dioxide (E551d) | Authorized by EC 1334/2008 | <10,000 mg/kg, excluding foods for infants and young children | [45] | ||
| Marking fruit and vegetables | Silicon dioxide (E551) | Exempt from certification | <2% of the ink solids | [41] | ||
| Anticaking agents | Silicon dioxide (E551) | REGb | <2% by weight of the food | [46] | ||
| Nutritional dietary supplement | Copper oxide Iron oxide |
Approved for animal feed | [47] | |||
| ZnO | GRASc | |||||
| Food contact packaging | Pesticides detection | Zinc Oxide QDs | R&D | [33] | ||
| Pathogens detection | Magnetic nanosensors | R&D | [34,35] | |||
| Plasmonic nanosensors | [36] | |||||
| Fluorescent nanosensors | [37] | |||||
| Toxins detection | Fluorescent nanosensors | R&D | [38] | |||
| Plasmonic nanosensors | [39] | |||||
| Phosphorescent QDs | [40] | |||||
| Edible film/coating | Chitosan/Nano-Silica Coating | Tested on Longan fruit | [48] | |||
| Poly-ɛ-caprolactone | Tested on fresh-cut “Red Delicious” apples | [49] | ||||
| Nanoemulsion/Quinoa Protein/Chitosan | Tested on fresh strawberries | [32] | ||||
| Bio-nano-hybrid pectins and LDH-salicylate | Tested on fresh apricots | [30] | ||||
| Nanoemulsion with lemongrass essential oil | R&D | Tested on fresh-cut Fuji apples | [31] | |||
| Bentonite (Al2O34SiO2nH2O) | GRAS | U.S. FDA 21CFR184.1155 | [50] | |||
| Flame Retardation Additives, gas barrier, etc. Prevent abrasive wear |
Montmorillonite | PolyOne Corporation Nanocor® Inc. | FCS Inventory | FCN No. 1163. | [44] | |
| Montmorillonite Chromium (III) oxide | Toyo Seikan Kaisha | FCN No. 932. | [30] | |||
| Limited and Nanocor Incorporated | ||||||
| Nanoemulsion with lemongrass essential oil | Oerlikon Balzers Coating AG, Oerlikon Surface Solutions AG | FCN No. 1839. For use at a thickness not to exceed 200 nm, not for use in contact with infant formula and human milk. | [31] | |||
| Prevent abrasive wear Heating enhancer in polyethylene terephthalate (PET) polymers |
Titanium aluminum nitride | Balzers Aktiengesellschaft |
GRAS | FCN No. 302. The maximum thickness of the surface coating shall not exceed 5 μm. | [50] | |
| Tin antimony oxide | Nyacol Nano Technologies, Inc. |
FCS Inventory | FCN No. 1437. <0.05% by weight of the polymer. | [44] |
FCS: Effective Food Contact Substance (FCS) Notifications.
REG: Food additives for which a petition has been filed and a regulation issued.
GRAS: Generally Recognized as Safe.
E numbers are codes of specific substances used as food additives approved by the European Food Safety Authority (EFSA).
2.1.1. Food processing
Nanomaterials are well designed as color or flavor additives, preservatives, or carriers for food supplement (i.e. nanoencapsulation and nanoemulsion), including animal feed products. The unique properties of engineered nanomaterials offer great advantages for food processing as ingredients or supplement. Additionally, inorganic oxide chemicals such as SiO2 (E551), MgO (E530), and TiO2 (E171) are permitted by the U.S. FDA as anti-caking agent, food flavor carrier, and food color additives (see Table 1). For instance, TiO2 is widely used as additive in foods such as gum, white sauces, cake icing, candy and puddings [19]. Current authorizations on the chemicals listed in Table 1 for food processing are all based on conventional particle size, except carbon black (authorized by EC 10/2011 but no longer authorized by the U.S. FDA) and titanium nitride. However, it is common to detect those chemicals used in foods in nanometer scale.
In addition to food products directly serving human beings, animal feeds play a significant role in the global food industry, ensuring economic and safe production of animal products throughout the world. Copper oxide, iron oxide, and zinc oxide now have been categorized as “generally recognized as safe” (GRAS) by the U.S. FDA as nutritional dietary supplement in animal feeds (Table 1). The European Food Safety Authority (EFSA) Panel on Additives and Products or Substances used in Animal Feed (FEEDAP Panel) also concludes the use of dicopper oxide (Cu2O or copper(I) oxide) as nutritional supply for all animal species is of no concern for consumer safety [21].
2.1.2. Food packaging
Food contact materials are intended to directly contact food products during manufacturing, transportation, and storage. Nanotechnology as novel solution now has been widely studied and developed for food packaging in food industry [22]. Nanomaterials designed for food packaging possess many advantages when compared to conventional packaging materials. Among many novel nanomaterials, nanoclay is one of the most widely used and studied for food packaging due to their mechanical, thermal, and barrier properties, and low cost. For instance, 1 wt % bentonite clay/poly (vinyl alcohol) loaded nanocomposite membrane significantly enhanced permeance with a water permeance of 6500 gpu and a selectivity value of 46 [23]. Another study from Gabr et al. (2015) showed 3% nanoclay loaded woven carbon fiber/compatibilized polypropylene nanocomposites significantly improved interlaminar fracture toughness and elevated glass transition temperature increased by about 6 °C [24]. Additionally, significant enhancement of corrosion resistance was reported for epoxy/clay nanocomposites [25]. Nanoclays are developed into several subclasses including montmorillonite, bentonite, kaolinite, hectorite, and halloysite, depending on the physiochemical properties of the nanomaterials. Montmorillonite and bentonite are now listed as GRAS and in Effective Food Contact Substance (FCS) notifications by the U.S. FDA (see Table 2). FCS acts as the U.S. FDA effective premarket notifications for food contact substances that have been demonstrated to be safe for their intended use. However, recent reports indicate potential migration risks associated with nanoclay packaging [26–28]. Case studies for migration risks are provided in section 3.
Table 2.
Current status of nanotechnology-enabled agriculture products.
| Application | Commercial names | Manufacturer | Current status and legislation compliance | Nanomaterial compositions | Function of nanomaterials | Reference |
|---|---|---|---|---|---|---|
| Nanofertilizer | Nano-Ag Answer® | Urth Agriculture | Commercialized. | Unknown nanomaterials | Fertilizer | a |
| NanoPro™, NanoRise™, NanoGro™, NanoPhos™, NanoK™, NanoPack™, NanoStress™, NanoZn™. | Aqua-Yield® Operations, LLC | Commercialized. Compliance with OSHA HCS (29CFR 1910.1200) and WHMIS 2015 Regulations |
Unknown nanomaterials | Fertilizer | a | |
| pH5® | Bio Nano Technology, Giza, Egypt | Commercialized. | Unknown nanomaterials | Increase permeability | a | |
| Saula Drip, Saula Solocross, Saula Motawazen | Commercialized. | Minor elements (Iron, Zinc, Manganese, Copper, Boron) NPs | Fertilizer | a | ||
| Ready to Use Spray, Plus (Concentrate) | GreenEarth-NanoPlant, FL, USA | Commercialized. US patents (US 15/290,257, US 15/429,380) | Biohumus in size range 100–700 nm | Fertilizer | a | |
| NovaLand-Nano | Land Green & Technology Co., Ltd., Taiwan | Commercialized. | Microelements as Mn, Cu, Fe, Zn, Mo, N NPs | Fertilizer | a | |
| Nanopesticides | N/A | N/A | R&D | Cu(OH)2 NPs | Fungicide | [69] |
| N/A | N/A | R&D | Silica NPs | Controlled release | [52] | |
| N/A | N/A | R&D | Polymeric NPs | Controlled release | [53] | |
| N/A | N/A | R&D | ZnO NPs | Bactericide | [70] | |
| NANOCU® | Bio Nano Technology, Giza, Egypt | Commercialized. | Copper NPs | Fungicide and bactericide | a | |
| Nanoherbicides | N/A | N/A | R&D | Poly (epsilon-caprolactone) (PCL) nanocapsules | Controlled release | [51] |
| Nanosensors | N/A | N/A | R&D | copper doped montmorillonite | Propineb fungicide detection in aquatic environment | [62] |
| N/A | N/A | R&D | Graphene | Pathogen detection in wastewater | [63] |
Note: Information is available online through company’s website. The authors do not certify the company’s claims of nano-ingrediens.
Edible coating with nanomaterials encoded has also shown its potential for food preserving and storage. Coated fresh food products such as vegetables and fruits remain active during transportation and storage processes. The active respiration processes may cause significant postharvest losses and poor quality cosmetically and nutritionally in products as the transportation and storage time extends. The control over such weight and nutrition loss is paramount to extend shelf life of fresh food products. Relative humidity and temperature are of the utmost concerns. They act together to alter fresh food respiration, as well as the microbial activities in the products. A thin layer of hybrid nano-edible films (normally < 100 um, see examples listed in Flores-López et al., 2016 [29]) can be used as gas and moisture barrier, enhance mechanical properties and sensory perceptions, prevent microbial spoilage, and improve the storage life of fresh food products. Unlike the metal-based nanomaterials discussed in section 2.1.1, most edible coatings are based on organic chemicals from natural extracts (see Table 2). For instance, pectin from apples [30], lemongrass essential oil [31], and quinoa protein/chitosan [32] have been developed as edible coating to increase the shelf life of highly perishable products.
Other applications in food contact packaging include pesticides detection [33], pathogens detection [34–37], and toxins detection [38–40] are also under active research and development due to the ultra-sensitive properties of nanomaterials. A recent report from Saho et al. (2018) found that ZnO quantum dots (QD) can be used to detect various pesticides, including aldrin, tetradifon, glyphosate, and atrazine, due to the fact that the pesticide containing strong leaving groups (e.g. –Cl) interact with QD quickly with high binding affinity at 107 M−1. Additionally, ZnO QD could also photocatalyze pesticides during the interaction. “Active packaging” and “smart packing” now have become a popular trend to utilize nanomaterials based sensor for monitoring the quality of the food.
2.2. Current status on agriculture nanotechnology
In agriculture, nanotechnology is employed to increase food production, with equivalent or even higher nutritional value, quality and safety. Efficient use of fertilizers, pesticides, herbicides and plant growth factors/regulators are the most important ways to improve crop production. Controlled release of pesticides, herbicides and plant growth regulators can be achieved via the usage of nanocarriers. For instance, poly (epsilon-caprolactone) nanocapsules have been recently developed as herbicide carrier for atrazine [51]. The treatment of mustard plants (Brassica juncea) with atrazine loaded poly (epsilon-caprolactone) nanocapsules enhanced the herbicidal activity compared to commercial atrazine, showing a drastic decrease in net photosynthetic rates and stomatal conductance, a significant increase of oxidative stresses, and ultimately weight loss and growth reduction of tested plants [51]. Similarly, other nanocarriers like silica NPs [52] and polymeric NPs [53] have also been developed as modified release system to deliver pesticides in a controlled manner. Nanoscale carriers can be utilized to perfectly achieve the delivery and slow release of these species. Such strategies are known as “precision farming” that improves crop yields but not damage soil and water [54]. Most importantly, application of nanoencapsulation can lower dosage of the herbicide, without any loss of efficiency, which benefits environment. In addition to nanocarriers, nanoparticle-mediated gene or DNA transfer in plants was used to develop insect-resistant varieties. More details can be found in previously published reviews [55,56]. Moreover, certain nanomaterials per se can act as pesticides with enhanced toxicity and sensitivity (see examples listed in Table 2). Metal oxide nanomaterials like ZnO, TiO2, and CuO are widely studied to protect plant from pathogen infections owing to their intrinsic toxicity. We take ZnO NPs as an example. It has been demonstrated that ZnO NPs can effectively inhibit growth of microbes such as Fusarium graminearum [57], Aspergillus flavus [58], Aspergillus niger [58], Aspergillus fumigatus [58], F. culmorum [58] and F. oxysporium [58], showing strong antifungal and antibacterial activity.
Conventional mineral fertilizers suffer substantially from low nutrient uptake efficiencies and high losses. The development of nanofertilizers brings forward the novel solution for such economic losses. Nanofertilizers are capable of reducing nutrient loss and enhancing nutrient incorporation by crops and soil microorganisms [59]. Commercialized nanofertilizers are mainly the micro-nutrients at nanoscale (e.g., Mn, Cu, Fe, Zn, Mo, N, B, see Table 2). It is noted that the use of other nanomaterials (instead of the typical conventional crop fertilizers), such as carbon nano-onions [60] and chitosan NPs [61], could also increase crop growth and quality. It is anticipated that the novel nanofertilizers will motivate and transform current fertilizer production industries in the next decade.
Owing to many beneficial aspects of nanomaterials, nanosensors, particularly wireless nanosensors, have also been developed to monitor crop diseases and growth, nutrient efficiency, and environmental conditions in field. Notably, engineered nanosensors can detect chemicals such as pesticides and herbicides, as well as pathogens at trace amount in food and agricultural systems. Such in situ and real-time monitoring system helps to remediate potential crop losses and improve crop production, together with the proper use of nanofertilizer, nanopesticide, and nanoherbicides. A recent report showed that copper doped montmorillonite can be used for on-line monitoring of propineb fungicide in aquatic environment (both in fresh and salty water), with a low detection limit of about 1 μM [62]. Another study showed that nanomaterials like graphene can be developed to detect pathogen in wastewater [63] and purify it for use as drinking water [64], indicating potential application in aquaculture. Many other nanomaterials such as copper NPs [65], carbon nanotube [66], gold NPs [67] and silver NPs [68] have also been under developing as nanosensors for real-time monitoring of environmental conditions and crop health and growth.
3. Toxicological fundamentals and risk assessment
3.1. Exposure routes and interactions
The increasing application of nanotechnology in the food and agriculture sectors has attracted public attentions over the past decade. Nanomaterials are either intentionally added as food additives or unintentionally introduced via migration [71] in many food and agriculture products. Consequently, concerns over environmental and human health arise as the spread of nano-products expands, owing to the unique physiochemical properties of nanomaterials [72–77]. The concerns over environmental health are a direct consequence from the interaction of nanomaterials used as nanofertilizers, nanopesticides, nanoherbicides, and less likely the immobilized nanosensors. The behavior and fate of nanomaterials in environments largely depend on the physiochemical properties of the nanomaterials per se. Additionally, the complexity of environmental conditions limits the predictability of the behavior and fate of nanomaterials. It is difficult to trace and monitor the distribution of nanomaterials as a result of the complicated nano-bio-eco interactions [74,77,78]. Although holistic approach has been recommended for understanding of the nano-bio-eco interactions between the nanomaterials and biotic and abiotic environments in a connected ecosystem [73], case-by-case studies are needed for a conclusive assessment of environmental nanotoxicity.
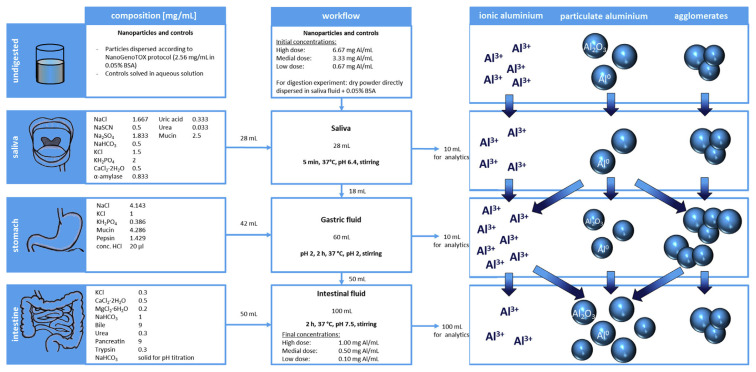
We here take the exposure routes of aluminum NPs in human digestion system as an example [79] to illustrate the complexity and possible experimental workflow to assess nano-products associated risks (Fig. 2). At the initial stage of digestion, samples remain unchanged in artificial saliva. Aluminum NPs begin to partially dissolve and release aluminum ions in stomach fluid. Particle agglomeration may occur any time during the digestions process, but mostly significantly in stomach fluid. In intestinal fluid, agglomerates tend to deagglomerate into primary particles. In addition, nanoparticulate structures are formed de novo from free ions [79]. The same routine applies to silver NPs. Silver colloids exist in any medium as complicated mixtures with many different species absorbed on the surface [13,80,81]. This triggers release of Ag+ ions as both culprit for toxicity [82–84] and as antimicrobial agents for drug-resistant bacteria [85–87]. Gold NPs can also release Au (I and III) ions in ambient conditions and contribute to downstream interactions [88].
Fig. 2.
The fate of engineered aluminum nanoparticles in digestion system as an example to illustrate the complexity and possible experimental workflow to assess nano-products associated risks. Reproduced with permission from Sieg et al., 2017, American Chemical Society [79].
3.2. Nanotoxicology mechanisms
Human health upon exposure to nanomaterials as food additives and other functional ingredients of food and agriculture products is also of major concerns. The direct contact of nanomaterials used as food additives/functional/nutritional ingredients may pose threats to human health. The production of reactive oxidative species (ROS) acts as one of the main toxicological mechanisms causing cellular damage and death [73]. Overproduction of ROS can lead to autophagy [89], neuron damage [90], and severe damage to DNA [78,91], and potentially mutagenesis, carcinogenesis, and aging-related diseases in humans. Allergic reactions and damage from metal ion release from nanomaterials are also possible adverse outcomes upon the exposure to food nano-products [76]. Additionally, the accumulation of nanomaterials in edible parts (seeds) of plants [60] and human body [92] may cause severer problems at a higher concentration and long-term interactions.
3.3. Data generating and analysis
Thorough and accurate assessment of nanotoxicology thus becomes paramount to the safe engineering, handling and use of nanomaterials in food and agriculture products. Moreover, current methodologies typically used for toxicology provide little information that is useful for chemists to improve their sustainable design for large scale use [93]. Aside from many research progresses on cellular damage in vitro and in vivo, toxicological data is still largely limited to reach any conclusive statement for the general pattern of nanomaterial exposure and toxic impact on human health. The limit at large is due to the complexity of nano-bio-eco interactions as we have discussed elsewhere [74,77,78]. The cost is high and the generated data is limited for traditional in vitro and in vivo analyses focusing on limited endpoints and processes, such as ROS production, DNA damage, immune responses, and many others. Using model organisms and cell lines such as Escherichia coli [94] and human A549 lung adenocarcinoma cell line [95], respectively, for generating omics data is probably the future trend for the study of nanotoxicity. Machine learning approach should be adapted to explore the growing data at the same time.
4. Frontier topics
Although some studies showed the low toxic impact of nanomaterials in food and agriculture products [96,97], the toxicity may be altered as a result from long-term exposure. It is better to take precautions, as we know little about the bioavailability and biodistribution of nanomaterials therein, and the ultimate acute and chronic toxicity upon exposure to them. Recently France has led the global trend to re-evaluate the safety of TiO2 (E171) as food additives at the legislation level. In 2017, the French Agricultural Research Institute (INRA)-led research group reported the non-malicious, pretumorous damages in the colon of rats fed with TiO2 NPs over 100 days of treatment [98]. Later, the French Food safety agency (ANSES) evaluated the French INRA study and made recommendations on the carcinogen potential of TiO2 to European Chemicals Agency (ECHA). In the mid of 2018, one of the French Farm and Food bill amendments that passed the National Assembly, yet not final, is targeted to ban on the import and marketing of TiO2 additives in food products by 2020 [99]. Many French food manufacturers, like Mars Chocolat France, the French confectionary subsidiary of Mars Inc., have already announced to follow the ban on TiO2. Dunkin’ Brands, a U.S. company, has also announced that they will be removing the alleged TiO2 NPs from Dunkin’ Donuts’ powdered sugar donuts. The progress taken on banning the use of E171 gives an example of how legislation would impact the application and marketing of nano-food products. Many other factors such as public awareness and acceptance, together with research progress and governmental legislation, make the future of nanotechnology in food industry uncertain, in spite of all the exciting marketed applications.
The potential risks of traditional nanomaterials are an ongoing debate and are under active research. More data on risk assessment is definitely required. Additionally, many approaches have been utilized to reduce the toxicity of engineered nanomaterials, and meanwhile, improve the target selection and performing reliability. For example, controlled tailoring on surface functionalization, doping, and morphological (i.e. size and shape) control has been demonstrated as effective approaches to make engineered nanomaterials more sustainable and less toxic. In the following section we highlight possible solutions including bio-synthesized (or “green synthesized”) and bioinspired nanomaterials for the future guidance and consideration.
4.1. Perspectives on biosynthesized and bioinspired nanomaterials
4.1.1. Biosynthesized nanomaterials
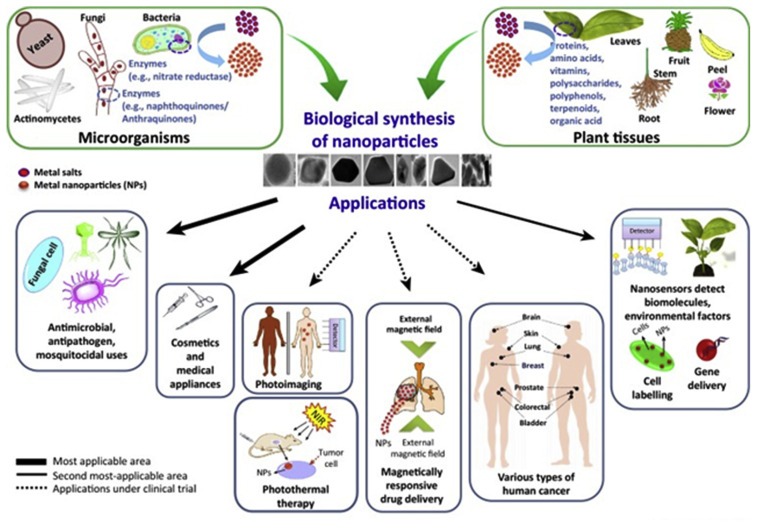
Biosynthesis has become a hot topic for the design and development of many nanomaterials in searching for sustainable and eco-friendly green chemistry method (Fig. 3). Many biological systems, including bacteria, fungi, yeast, actinomycetes, enzymes, and various plant parts (e.g. leaves, fruits, roots, and their extracts), have shown promising suitability for the nanomaterials biosynthesis, as listed in Table 3. Three main advantages have been noted by many researchers including: 1) biological system as manufacturing host can act as capping, stabilizing, and reducing agent so that less hazardous materials will be used during the engineering processes; 2) biosynthesis often takes in ambient temperature and pressure, and neutral pH that can help alleviate the use of energy resources and hazardous chemicals; and 3) most biosynthesized nanomaterials are biocompatible and low toxic due to the surface functionalization during the biosynthesis process. In this section we will illustrate briefly on those three aspects of the biosynthesis application on designing and engineering nanomaterials.
Fig. 3.
Biological snthesis and applications of nnoparticles. Reproduced with permission from Singh et al., 2016, Elsevier [100].
Table 3.
Selected examples of biosynthesized nanomaterials in recent 5 years (2014–2018).
| Biological system | Biogenic nanoparticles (NPs) | Characterization | Features | Note | References | |
|---|---|---|---|---|---|---|
| Bacteria | Pichia fermentans JA2 | Silver and zinc oxide NPs | UV–vis, XRD, and FE–SEM–EDX analysis | Silver NPs inhibited most of the G− clinical pathogens; ZnO NPs inhibited only Pseudomonas aeruginosa. | Showed synergistic effect with antibiotics | [112] |
| Bacillus cereus strain HMH1 | Magnetic iron oxide NPs | 29.3 nm. FE-SEM, DLS, VSM, UV–vis, FT-IR and EDS | Low cytotoxicity: IC50, MCF-7 > 5 mg/ml and IC50, 3T3 > 7.5 mg/ml | capping and stabilizing agents | [113] | |
| Serratia sp. BHU–S4 | Silver NPs | TEM (10–20 nm), XRD, EDXA, FTIR | As fungicide against phytopathogen Bipolaris sorokiniana causing spot blotch disease in wheat | reduction and stabilization | [114] | |
| Fungi | Saccharomyces cerevisiae | Silver NPs | UV–vis, XRD, TEM, FTIR | Photocatalytic degradation of methylene blue | Biomolecules as reducing and capping agent | [115] |
| Aspergillus flavus TFR 7 | TiO2 NPs | TEM (12–15 nm), EDX, DLS | Stimulate plant growth: shoot length (+17%), root length (+49.6%), root area (+43%) and root nodule (+67.5%). Promote rhizospheric microbes | Fungi directly isolated from rhizosphere soil | [116] | |
| Aspergillus flavus and Emericella nidulans | Silver NPs | Hexagonal- and triangular-shaped. DLS (36–531 nm, 37–340 nm), XRD, TEM (30–150 nm, 10–450 nm), FTIR, EDX | Synergistic antibacterial and antibiofilm activity | reducing and capping agent | [117] | |
| Yeast | Candida lusitaniae | Silver/silver chloride NPs | UV–vis, XRD, TEM (13.4 ± 14.5 nm and 6.9 ± 4.5 nm), FIB/SEM, SEM-EDS | Antimicrobial activity | Yeast isolated from termite gut | [118] |
| Magnusiomyces ingens LH-F1 | Gold NPs | UV–vis, DLS (137.8 ± 4.6 nm), TEM (80.1 ± 9.8 nm), SEM, SDS-PAGE, FTIR | Catalytic reduction of nitrophenols | reducing, stabilizing/capping agent. | [101] | |
| Cryptococcus laurentii and Rhodotorula glutinis | Silver NPs | UV–vis, TEM (15–220 nm), XRD, FTIR | Antifungal activity against phytopathogenic fungi (Botrytis cinerea, Penicillium expansum, Aspergillus niger, Alternaria sp., and Rhizopus sp.) | Yeast isolated from apple peel | [119] | |
| Actinomycetes | isolate VITBN4 | CuO NPs | UV–vis, TEM (61.7 nm), DLS (198 nm), SEM, EDX, FTIR, XRD (61.7 nm) | Antibacterial activity against human and fish bacterial pathogens | Isolated from soil samples. reduction, capping and stabilization | [120] |
| Streptomyces sp. strain NH21 | Silver and gold NPs | UV–vis, TEM (44 ±9 nm for supernatant and 8.4 ± 12 nm for biomass synthesized particles), AFM, FTIR | Antibacterial activity | Isolated from acidic soil. Capping agent | [121] | |
| Enzyme | alpha amylase | TiO2 NPs | XRD, TEM, FTIR | MIC of 62.50 μg/ml on Staphylococcus aureus and Escherichia coli | Enzyme as reducing and capping agent | [122] |
| Plant extracts | Coffea arabica seed | Silver NPs | DLS (20–30 nm), UV–vis, XRD, TEM, SEM–EDXA, FTIR | MIC ≤ 0.2675 mg/L on E. coli and S. aureus | [123] | |
| Red ginseng root | Silver and gold NPs | UV–vis, TEM (10–30 nm), EDX | Antimicrobial activity | Reduction and stabilization | [124] | |
| Aloe vera plant | Nanoscale zero-valent iron | FESEM, EDS, XRD, FT-IR and TGA | Removal of arsenic (As) and selenium (Se) from water | Plant extract as reducing agent | [125] | |
| Cassia tora leaf | Silver NPs | XRD, FTIR, SEM and EDAX | antioxidant and antibacterial activities | Plant extract as reducing agent | [126] | |
| Nigella sativa leaf | Silver NPs | 15 nm, UV–vis, FTIR, SEM | Lower cytotoxicity and phytotoxicity than wet-chemistry synthesized ones (30 nm) | Plant extract as reducing and capping agent | [127] | |
| Atrocarpus altilis leaf | Silver NPs | SEM (34 nm), TEM (38 nm) and DLS (162.3 nm), FTIR, XRD and EDX | Antimicrobial and antioxidant activity | Phyto constituents as capping agent | [128] | |
| Butea monosperma leaf | Gold and silver NPs | DLS, UV–vis, XRD, TEM, XPS, FTIR | inhibition of cancer cell proliferation | Plant extract as reducing, stabilizing/capping agent. | [129] | |
| Pineapples and oranges fruits | Silver NPs | UV–vis, SEM (10–300 nm) | N/A | Reducing agent | [110] | |
| Longan fruit | Silver NPs | UV–vis, TEM (4–10 nm), XRD, EDX, FTIR | Enzymatic browning reduction on white cabbage. MIC 31.25 μg/ml against Staphylococcus aureus and Basillus subtilus, 62.5 μg/ml against Escherichia coli. | Reducing, stabilizing/capping agent. | [130] | |
| Butea monosperma bark | Silver NPs | DLS (98.28 nm), TEM, FTIR, XRD and EDX | Cytotoxic effect on human myeloid leukemia cell line and antibacterial activity | Reducing and capping agent | [131] | |
| Marine algae | Macroalga Sargassum muticum | ZnO NPs | 30–57 nm. FESEM, UV–vis, XRD, FTIR | N/A | [132] | |
| Brown alga Cystoseira trinodis | CuO NPs | XRD, AFM, EDX, FE-SEM (6–7.8 nm), TEM (7–10 nm), Raman | Catalytic, antioxidant and antibacterial properties | Reducing, stabilizing | [133] |
Instead of using chemical capping, stabilizing, and reducing agents, biological systems per se act as those functioning factors. Studies have shown that some macrobiomolecules such as proteins and lipids with functional amide and carboxyl groups can be adsorbed on nanoparticles surface, for example, which could be involved in the reduction of Au3+ and the stabilization of Au NPs [101]. Jain et al. (2011) showed that SDS-PAGE profiles of the extracellular proteins are responsible for stabilizing silver NPs during the biosynthesis [102]. Additionally, many reports have found the role of biomolecules as capping agent in nanomaterials synthesis (see examples listed in Table 3). Moreover, ambient temperature and pressure, and neutral pH are the common requirements for biological systems. Chemical synthesis of nanomaterials often is carried out at high temperature, pressure, and extreme pH. For instance, to engineer TiO2 NPs final solution was maintained at 100 °C-400 °C for hours [103], and extreme pH (NaOH solution at pH 11 and HCl at pH 1) to engineer ZnO [104]. Lastly, surface functionalization of biosynthesized nanomaterials can be easily confirmed by using FT-IR. For instance, the results of FT-IR showed the existence of various functional groups such as hydroxyl, carboxyl, carbonyl and amine as well as iron and oxygen bounds in the biosynthesized iron NPs. Moreover, surface functionalization is a common approach to engineer biocompatible nanomaterials and to simulate many bioprocesses in nature [105–108]. Therefore, biological system frequently acts as a natural environment to provide surface functionalization on nanomaterials.
However, biosynthesis of nanomaterials suffers drawbacks such as low production and less controllable engineering processes. This is largely owing to the fact that the exact mechanism and the biological processes responsible for nanoparticles biosynthesis remain to be elucidated. Though it has been proposed that many biomolecules and metabolites such as proteins, amino acids, vitamins, flavonoids, alkaloids, and polysaccharides are involved in metal reduction, capping and stabilizing, the systematic pathways remain unclear. Additionally, it is extremely difficult to design nanomaterials with exact features, such as shape and size, in biological systems. This is particularly important for engineering nanomaterials in biomedical uses, in which a desirable morphological feature may be required to act as nanocarriers. A few studies have attempted to control the morphology of nanomaterials by using different biological systems [109,110]. For instance, orange extract resulted in round and almost spherical silver NPs, whereas pineapple extract produced nanoparticles with sharp corners [110]. Although the results indicated a certain level of controllable manufacturing, the processes need to be studied further in detail for the underlying mechanisms. Nevertheless, future research in this direction involving biosynthesized nanomaterials is strongly recommended [111].
4.1.2. Bioinspired nanomaterials
It is not uncommon to misuse the concept of bioinspired approach with biosynthesis approach (for example, reports from Refs. [134,135] misuse biosynthesis as bioinspired approach, and sometimes bio-inspired synthesis is used for biosynthesis [136]). In general, bioinspired approach often associates with the design of novel nanomaterials with similar morphologies and functions with a biological template, e.g. mussel, cilia, and insect tentacles, etc. Bioinspired approach has been widely studied in biomedical researches [137] and many other fields [138,139] owing to the intrinsic nature of bioinspired approach [140] (see Figure 4). With biosynthesis approach one uses biological systems (e.g. cells, plant extracts as we discussed in section 4.1.1) to directly synthesize green and low toxic nanomaterials. The evolved optimal biological structures have enlightened many modern inventions in human history, by mimicking the well-ordered multiscale interfaces. Those innovations include, to name a few, Gecko-inspired adhesives [141,142], spider-web membrane [143], and artificial compound eyes [144]. In nature, taking Gecko as an example, the complex hierarchical adhesion system utilizes nanoscale fibers to produce adhesion through van der Waals forces and to attach and detach by controlling the loading angle [145]. Gecko-inspired adhesives are inspired from the particular nanoscale structures evolved over million years [142].
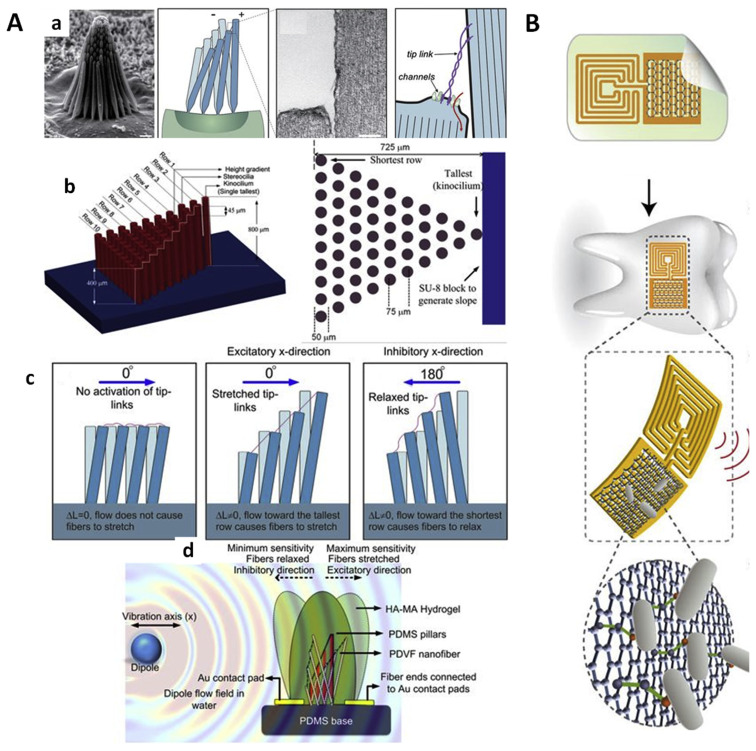
Fig. 4.
(A). Microelectromechanical system flow sensors that mimic the anatomy and function of hair cells. a). Morphology of actual hair bundles and the schematic design of microelectromechanical system flow sensors that mimic hair cells. b). Schematic illustration of pillars that mimic the function of hair cells. c). Illustration of how pillars respond to the flow with different designing features. d). Illustration of how the nanofiber sensor generates electric charge readings in response to flow disturbances. Reproduced with permission from Asadnia et al., 2016, licensed under a Creative Commons Attribution 4.0 International License [148] (B). Schematic illustration of silk inspired, graphene based wireless pathogen sensor on tooth enamel. Reproduced with permission from Mannoor et al., 2012, Springer Nature [149].
Analogous to naturally existed biofunctions, many novel bioinspired nanomaterials are able to dynamically adjust their physicochemical properties in a controlled manner, with the changes of interfacial environmental conditions. The precision to control the nanomaterials at such high-resolution in response to immediate environmental changes is very difficult to achieve. It has been a challenging task to mimic the dynamic structure of naturally occurred biological systems. The study of bio-inspired nanomaterials in food and agriculture sectors is very limited currently. However, some artificial bio-inspired devices have been successfully studied for pesticide delivery, chemical and pathogen detection, and environmental sensing, as listed in Table 4.
Table 4.
Selected examples of bioinspired nanomaterials in food and agriculture sectors (2012–2018).
| Bioinspired template | Nanomaterials | Characterization | Features | Note | Reference |
|---|---|---|---|---|---|
| Mussel | Mussel avermectin NPs [P(St–MAA)–Av–Cat] | 120 nm in diameter. | As nanocarrier for controlled release of avermectin and protection against UV light. Higher toxicity towards aphids. | Show potential to enhance folia retention | [146] |
| Cactus | ZnSnO3 Nanostructures | BET (29.2 m2 g−1), TEM (30–50 nm in diameter) | High-performance humidity nanosensors | Show potential in humidity monitoring | [147] |
| Silk | Graphene | N/A | Battery-free sensors for remote monitoring of pathogenic bacteria at single cell level | Shed light on wireless nanosensors for food pathogen detection | [149] |
| Biological cilia | Polyvinylidenefluoride piezoelectric nanofiber | N/A | Flow velocity and flow direction | May assist in taste sensors or real-time sensing in food safety [150], such as food pathogen [151], allergens [152], and food quality monitoring [153] | [148] |
| Mussel | Polydopamine (PDA)-coated molecularly imprinted SiO2 NPs | TEM (~85 nm) | Specific recognition of the trace quantities of papain with low detection limit of 0.63 nM | Show potential in bioanalaysis in nutritional and dietary supplement | [154] |
| Zwitterion | Fluorescent biomimetic carbon quantum dots | DLS (4.65 nm) | Detection limit for vitamin B12 at 81 nM; highly biocompatible | Show potential in bioanalaysis in nutritional and dietary supplement | [155] |
| Insect tentacles | Nanoporous Prussian blue (PB) nanocube heads/TiO2 nanowire (NW) arms | TEM (diameter and interspacing between adjacent NWs are ~100 and 150 nm) | Sensitive detection of H2O2 at a low detection limit (~20 nM), broad detection range (10−8 to 10–5 M), short response time (~5 s) and long-term biocatalytic activity (up to 6 months). | Show potential for biomolecule detection in food safety | [156] |
Here we take three interesting topics as examples to illustrate the application of bioinspired nanostructures in food and agriculture systems. 1). Pesticide delivery. Liang et al. (2018) [146] recently reported bioinspired mussel avermectin NPs [P(St–MAA)–Av–Cat] with strong adhesion to crop foliage to reduce the loss and possible further contamination to soils of pesticide. The bioinspired nanostructure showed remarkably high retention of avermectin, excellent storage stability, as well as sustained release. As a result, the indoor toxicity of P(St–MAA)–Av–Cat was highly enhanced. 2). Environmental sensing. Zhang et al. (2015) [147] developed cactus bioinspired ZnSnO3 nanoneedles that can adsorb and desorb water molecules depending on environmental humidity due to the small area of the tips (1.5–2.5 nm). The device showed good sensitivity with response and recovery times of ~2.5 s and ~3 s, and can be applied to detect tiny fluctuations in moisture. Asadnia et al. (2016) [148] reported a novel microelectromechanical system flow sensors that mimic the anatomy and function of hair cells (Fig. 4Aa). The individual bundle has a tall pillar and another 54 short pillars that are analogous to stereocilia (Fig. 4Ab). These pillars are designed to detect and respond to the flow disturbances (Fig. 4Ac). The voltage output as a result of tensile stress applied to nanofibers at both contact pads can be collected and corrected to flow velocity and direction (Fig. 4Ad). The system is highly biocompatible and does not need external power supply, showing high sensitivity to flow velocity and directions. 3). Pathogen monitoring. In 2012, Mannoor and coworkers successfully developed a silk inspired, graphene based wireless pathogen sensor on tooth enamel [149]. Graphene was printed onto bioresorbable silk and formed containing a wireless coil on the surface of a tooth (Fig. 4B). The device showed bioselective detection of bacteria at single-cell levels. Though it is not a direct application in food and agriculture sectors, it shows promising results for real-time pathogen monitoring in food and agriculture systems. Nevertheless, one can anticipate that more complex and advanced natural biofunctions await to be discovered and fully understood, and bioinspired nanomaterials that fully mimic such biofunctions remain to be developed with low-cost methodologies and large-scale production.
4.2. Regulation and legislation
Regulation and legislation play a fundamental and key role for the implication of nanotechnology as well as marketing of products. They also serve as official sources and references for public knowledge and awareness. There are normally three main steps to legislate. 1) Suggestions are firstly made by academic parties (can be research institute, organization, government agency or even individuals). 2) Reasonable suggestions are taken by a government agency (sometimes named guidance) and are proposed (by head of a state/country or a legislative body) as a directive, known as legislation. Legislation is then passed by a parliament of a country or other legislative arm of a government and becomes laws. 3) Legislation (or law) usually covers broad and general aspect pertaining to an industry. Thus, when it is enforced by regulators, regulations are generated, referring to the detailed and specific requirement that can take on various forms. Regulations are much broader in the scope for a particular industry.
4.2.1. Recent updates
It takes the same procedures to legislate for the nanotechnology in food industry. Back to 2003 when nanotechnology in food industry was first proposed, governing agencies were formed or appointed by the relevant governments all over the world. Some are listed in Ref. [157]. Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), as the main regulation for Europe Union (EU), is the most active agency concerning legislation of nanotechnology in food industry, followed by Food and Drug Administration (FDA) of USA. Their up to date documents are available at https://europa.eu/european-union/index_en and https://www.fda.gov/default.htm, respectively.
In the past decades, much effort has been put into legislation, which has been extensively reviewed in previous publications [11,158–163]. Identification and expression by different agencies may vary but they share the common basic concepts, for instance, nanotechnology deals with materials with at one dimension lying within 100 nm. Some key updates are listed in Table 5.
Table 5.
Several key regulation and legislation.
| Year | Policy (change/update) | Reference |
|---|---|---|
| 2011 | “Official” definition of nanomaterial. | EU Commission Recommendation [164], available at https://ec.europa.eu/research/industrial_technologies/pdf/policy/commission-recommendation-on-the-definition-of-nanomater-18102011_en.pdf |
| 2011 | Provide data on the stability of the nanomaterials in the proposed formulations and under the proposed conditions of use (in feed). | FDA final guidance [165], available at https://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM401508.pdf |
| 2012 | Mandatory labelling for nano-ingredients in food introduced in labelling Regulation, Labelling applicable from December 2014 | Second Regulatory Review on Nanomaterials (by EU) [166], available at https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52012DC0572&from=EN |
| 2012 | Evaluation of REACH registration dossiers concerning nanomaterials prioritised by ECHA for compliance check. Substance of the “CoRAP” list includes silicon dioxide (NL 2012), silver (NL 2013) and titanium dioxide (F 2014). | Second Regulatory Review on Nanomaterials (by EU) [166] |
| 2012 | Assess the coverage of nanomaterials in environmental legislation such as waste, water and air legislation. | EU MEMO [167], available at http://europa.eu/rapid/press-release_MEMO-12-732_en.htm?locale=en |
| 2012 | Case-by-case safety evaluation for nanomaterials. | EU Press Release [168], available at http://europa.eu/rapid/press-release_IP-12-1050_en.htm?locale=en |
| 2014 | FDA does not categorically judge all products containing nanomaterials (or involving application of nanotechnology) as intrinsically benign or harmful. | Final guidance for industry, by FDA [169], https://www.fda.gov/downloads/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/UCM616225.pdf |
| 2017 | Taiwan FDA considers nanomaterials as new food contact substances and enforces food packaging nanomaterials to go through safety assessment and obtain pre-market approval. | Taiwan FDA guidelines (in Chinese), available at https://www.fda.gov.tw/TC/newsContent.aspx?id=21901&chk=8b47bc84-e1c1-4c36-b410-4ff637bf7f05¶m=pn&cid=3&cchk=46552e96-810a-42c3-83e1-bd5e42344633#.WMqh0zuLS71 |
4.2.2. Limitation and urgent need for legislation
In the nano world, materials behave distinctly from bulks in a conventional world. Nanomaterials have different “standard protocol” to follow, which is of very little we now understand. This includes both understanding and control of nanoscale interactions/properties/functions/toxicities, which further determine beneficial application, adverse effect, transition of nanomaterials, and ultimately their environmental fate and impact to ecosystem. As a result, it is extremely difficult to make suggestions as the first step stated above, since suggestions are based on a set of database containing scientific findings and sufficient evidences. Thus, regulation and legislation has no foundation to be developed and to stand on. To make it worse, each type of nanomaterials has its unique properties, leading to a “case-by-case” or “product-by-product ” evaluation regarding regulation and legislation [167,168].
Given these limitations, current legislation is still at initial stage of development. It might share a common framework globally to address all aspects of applications, risks, food safety and disposal. Currently, it covers an extremely broad and general aspect regarding nanomaterials and nanotechnology. It needs evaluation or re-evaluation when it comes to a specific nanomaterial. However, in most cases, it is unclear of the detailed criteria for the specific nanomaterials to be put into the food market. Even in the final guidance for industry proposed by FDA, it states that “FDA does not categorically judge all products containing nanomaterials or involving application of nanotechnology as intrinsically benign or harmful”, with no further details of evaluation standards/protocols [169].
On the other hand, food manufacturers have invested a lot of money to develop novel nanotechnology based products in food industry. Their efforts stimulate and promote implication of nanotechnology and many products are marketed in the food industry [3]. Most of them are in the field of food contacting materials, i.e. food package, sensors and detectors. No product has been approved yet as food additive or pigment, directly consumed by human being, except titanium dioxide and iron oxide that have been used as food pigment and colorant already. This is determined by food safety code. Food directly consumed by human has the highest safety level and is under strictly control/evaluation while safety level of “side” product (like food contacting materials) is relatively lower and easier to be approved and marketed [3,170–172]. Safety to human being (mainly evaluated from toxicity data) is the first concern for all these new products. However, the concern of their potential impact to environment and natural ecosystems should be raised as well. So far, data of environmental fate of nanomaterials and the impact on natural ecosystems is not a focus of a food manufacturer or a novel product developer. This piece of information is more likely contributed by interested academic research groups and thus very limited. This could be improved if such data is required to market a specific product, as proposed by EU commission [167]. Otherwise, it is highly risky that wide applications of nanomaterials could bring new disaster, like plastics to the marine, and ultimately tragedy for the earth.
Since nowadays our main focus is to implement nanotechnology and develop novel product in food industry, the number of marketing products has been increasing in folders every year. This makes regulation and legislation more urgent. Whether or not these novel products can have a stand-point in the food market also depends on public attitude and consumers’ acceptance.
4.3. Public awareness and acceptance
There were cases that silver NPs were added into package materials due to their antimicrobial activity and these materials had been widely used in many food (like milk) packages. General public consumed these products without knowing the addition of nanoparticles. That could be a both ethic and legislative issue. Now proper labeling becomes mandatory so that public is aware of what they consume. It’s manufacturer’s responsibility to keep this information transparent and available to the public.
Public awareness and acceptance is an important part but often ignored by a food manufacturer. In fact, most food manufacturers keep their new product development “underground ” and would not like to share with the public (maybe partly due to the competition and trade secret) [173]. This can be a conflict with the fact that the public intends to know what and why the food manufacturer is marketing a new product. The case study in Singapore already proved that unawareness of nanotechnology and its adverse effects of nanotechnology increases negative perception of the public [174]. It’s even worse that agri-food organisations (stakeholders) also have a very low awareness with regards to nanotechnology, as reported by a survey on the island of Ireland [175].
Public voice is twofold, assent and dissent (or altruism and skepticism) with nanotechnology in food industry [176]. Public attitude greatly depends on the specific applications. Nanotechnology in food received 49% support in 2005 and 32% in 2008 (which is one of the lowest among all nanotechnology application) while applications of food packing that monitors condition was supported by 73% of respondents in 2008. The highest concern was expressed regarding the usage of nanotechnology, particular in food (28%) [16]. Using canola oil as an example, according to a nationwide online survey in the U.S., consumers are only willing to pay less for canola oil that is processed or packaged with nanotechnology modified seeds or techniques. No significant difference was found for canola oil with health-enhancing nano-engineered oil drops [177]. It feels that public hold a neutral attitude toward nanoengineered canola oil whereas nano-engineered canola oil does not present all nano food.
So far “Organic” is likely the most commonly accepted standard for healthy food. Many public prefer traditional and “organic” food, especially when they compare it with genetically modified food. Sometimes public are even confused by genetically modified food with nano-engineered food [178]. The main cause is the poor access to information and resources of food nanotechnology for the public. Nanotechnology is not yet a matured technique to be used in food industry. Limited evidence from scientific standpoint makes it hard or even unethical to advertise this uncertain technology to the public.
5. Perspective
Nanotechnology exhibits promising potentials to be widely utilized in every aspect of food industry. This is based on limited knowledge obtained mainly from labs. The practical application of nanotechnology and marketing nanomaterial based product remains uncertain, considering the poor capability to control properties and interaction of materials at nanoscale, as well as the unclear environmental effect and almost vacant toxicity database. This also limits development of the body of regulation and legislation, further turning as obstacles for marketing of novel products.
Public shows low awareness to food nanotechnology while their attitude is tunable depending on the way nanotechnology is used and advocated. The conflict seems to be that public wants to be informed on the status of food nanotechnology (especially development of related novel products) while food manufacturers prefer the opposite since their technology is confidential. For the information of both public and food manufacturers, sufficient database and evidence should be built up and serve as logistic support, which is of urgent demands.
Since implementing environmentally friendly practices has become more and more essential for success in today’s biotechnology business, bioinspired approach is becoming popular in biological researches and many other relevant fields. However, by comparison to biomedical field currently the research and development of bio-inspired nanomaterials for usages in food and agriculture sectors are rather limited.
Driven by the food industry that is a trillion-dollar business, many products involving novel nanotechnology have been marketed all over the world, particularly in the field of food contact materials/technologies (like packaging materials/monitors). This will continue to be a battlefield for the manufacturer due to safety code control by legislative branch of a government.
Acknowledgement
This study was supported by NSF-CREST program [The National Science Foundation-Centers of Research Excellence in Science and Technology (NSF-CREST)] with grant #HRD-1547754 to Jackson State University.
Funding Statement
This study was supported by NSF-CREST program [The National Science Foundation-Centers of Research Excellence in Science and Technology (NSF-CREST)] with grant #HRD-1547754 to Jackson State University.
REFERENCES
- 1.US DOA. Nanoscale science and engineering for agriculture and food systems: a report submitted to cooperative state research, education and extension service. Washington, DC: Department of Agriculture; 2003. the United States Department of Agriculture: National Planning Workshop. [Google Scholar]
- 2.Dasgupta N, Ranjan S. Nanotechnology in food sector An Introduction to food grade nanoemulsions. Springer; 2018. pp. 1–18. [Google Scholar]
- 3. Peters RJ, Bouwmeester H, Gottardo S, Amenta V, Arena M, Brandhoff P, et al. Nanomaterials for products and application in agriculture, feed and food. Trends Food Sci Technol. 2016;54:155–64. [Google Scholar]
- 4. Finglas PM, Yada RY, Toldrá F. Nanotechnology in foods: science behind and future perspectives. Trends Food Sci Technol. 2014;40:125–6. [Google Scholar]
- 5. Bryksa BC, Yada RY. Challenges in food nanoscale science and technology. J Food Drug Anal. 2012;20:418–21. [Google Scholar]
- 6. Sozer N, Kokini JL. Nanotechnology and its applications in the food sector. Trends Biotechnol. 2009;27:82–9. doi: 10.1016/j.tibtech.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 7. Cushen M, Kerry J, Morris M, Cruz-Romero M, Cummins E. Nanotechnologies in the food industry–Recent developments, risks and regulation. Trends Food Sci Technol. 2012;24:30–46. [Google Scholar]
- 8.Roco MC, Mirkin CA, Hersam MC. Nanotechnology research directions for societal needs in 2020 retrospective and outlook. Springer Science & Business Media; 2011. [Google Scholar]
- 9.Kavitha P, Manjunath M, Huey-min H. Nanotechnology applications for environmental industry. In: Mustansar HC, editor. Handbook of nanomaterials for industrial applications. Elsevier; 2018. [Google Scholar]
- 10.Xiaojia H, Hua D, AWG, Huey-min H. Regulation and safety of nanotechnology in the food and agriculture industry. In: Molina G, Inamuddin FMP, Asiri AM, editors. Food applications of nanotechnology. CRC Press, Taylor & Francis Group; 2018. [Google Scholar]
- 11. Marrani D. Nanotechnologies and novel foods in European law. NanoEthics. 2013;7:177–88. [Google Scholar]
- 12.Dasari T, Deng H, McShan D, Yu H. Nanosilver-based antibacterial agents for food safety. In: RPC, editor. Food poisoning: outbreaks, bacterial sources and adverse health effects. NOVA Science Publishers; 2014. pp. 35–62. [Google Scholar]
- 13. Deng H, Zhang Y, Yu H. Nanoparticles considered as mixtures for toxicological research. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2018;36:1–20. doi: 10.1080/10590501.2018.1418792. [DOI] [PubMed] [Google Scholar]
- 14. Senjen R. Nanotechnology and patents - how can potential risks be assessed? Recent Pat Food, Nutr Agric. 2012;4:245–9. doi: 10.2174/2212798411204030245. [DOI] [PubMed] [Google Scholar]
- 15. Duncan TV. Applications of nanotechnology in food packaging and food safety: barrier materials, antimicrobials and sensors. J Colloid Interface Sci. 2011;363:1–24. doi: 10.1016/j.jcis.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cormick C. Why do we need to know what the public thinks about nanotechnology? NanoEthics. 2009;3:167–73. doi: 10.1007/s11569-009-0065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arnaldi S, Muratorio A. Nanotechnology, uncertainty and regulation. A guest editorial. NanoEthics. 2013;7:173–5. [Google Scholar]
- 18. Dudefoi W, Terrisse H, Richard-Plouet M, Gautron E, Popa F, Humbert B, et al. Criteria to define a more relevant reference sample of titanium dioxide in the context of food: a multiscale approach. Food Addit Contam A. 2017;34:653–65. doi: 10.1080/19440049.2017.1284346. [DOI] [PubMed] [Google Scholar]
- 19. Weir A, Westerhoff P, Fabricius L, Hristovski K, Von Goetz N. Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol. 2012;46:2242–50. doi: 10.1021/es204168d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dorier M, Béal D, Marie-Desvergne C, Dubosson M, Barreau F, Houdeau E, et al. Continuous in vitro exposure of intestinal epithelial cells to E171 food additive causes oxidative stress, inducing oxidation of DNA bases but no endoplasmic reticulum stress. Nanotoxicology. 2017;11:751–61. doi: 10.1080/17435390.2017.1349203. [DOI] [PubMed] [Google Scholar]
- 21. EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) Safety and efficacy of dicopper oxide as feed additive for all animal specie. ESFA J. 2016;14:e04509. [Google Scholar]
- 22.Pereda M, Marcovich NE, Ansorena MR. Nanotechnology in food packaging applications: barrier materials, antimicrobial agents, sensors, and safety assessment. In: Martínez L, Kharissova O, Kharisov B, editors. Handbook of ecomaterials. Springer; 2018. [Google Scholar]
- 23. Jose T, George SC, Maria HJ, Wilson R, Thomas S. Effect of bentonite clay on the mechanical, thermal, and pervaporation performance of the poly (vinyl alcohol) nanocomposite membranes. Ind Eng Chem Res. 2014;53:16820–31. [Google Scholar]
- 24. Gabr MH, Okumura W, Ueda H, Kuriyama W, Uzawa K, Kimpara I. Mechanical and thermal properties of carbon fiber/polypropylene composite filled with nano-clay. Compos B Eng. 2015;69:94–100. [Google Scholar]
- 25. Sari MG, Ramezanzadeh B, Shahbazi M, Pakdel AS. Influence of nanoclay particles modification by polyesteramide hyperbranched polymer on the corrosion protective performance of the epoxy nanocomposite. Corros Sci. 2015;92:162–72. [Google Scholar]
- 26. Echegoyen Y, Rodríguez S, Nerín C. Nanoclay migration from food packaging materials. Food Addit Contam A. 2016;33:530–9. doi: 10.1080/19440049.2015.1136844. [DOI] [PubMed] [Google Scholar]
- 27. Huang JY, Li X, Zhou W. Safety assessment of nanocomposite for food packaging application. Trends Food Sci Technol. 2015;45:187–99. [Google Scholar]
- 28. Störmer A, Bott J, Kemmer D, Franz R. Critical review of the migration potential of nanoparticles in food contact plastics. Trends Food Sci Technol. 2017;63:39–50. [Google Scholar]
- 29. Flores-López ML, Cerqueira MA, de Rodríguez DJ, Vicente AA. Perspectives on utilization of edible coatings and nano-laminate coatings for extension of postharvest storage of fruits and vegetables. Food Eng Rev. 2016;8:292–305. [Google Scholar]
- 30. Gorrasi G, Bugatti V. Edible bio-nano-hybrid coatings for food protection based on pectins and LDH-salicylate: preparation and analysis of physical properties. LWT-Food Sci Technol. 2016;69:139–45. [Google Scholar]
- 31. Salvia-Trujillo L, Rojas-Graü MA, Soliva-Fortuny R, Martín-Belloso O. Use of antimicrobial nanoemulsions as edible coatings: impact on safety and quality attributes of fresh-cut Fuji apples. Postharvest Biol Technol. 2015;105:8–16. [Google Scholar]
- 32. Robledo N, López L, Bunger A, Tapia C, Abugoch L. Effects of antimicrobial edible coating of thymol nanoemulsion/quinoa protein/chitosan on the safety, sensorial properties, and quality of refrigerated strawberries (Fragaria×ananassa) under commercial storage environment. Food Bioprocess Technol. 2018;11:1566–74. [Google Scholar]
- 33. Sahoo D, Mandal A, Mitra T, Chakraborty K, Bardhan M, Dasgupta AK. Nanosensing of pesticides by zinc oxide quantum dot: an optical and electrochemical approach for the detection of pesticides in water. J Agric Food Chem. 2018;66:414–23. doi: 10.1021/acs.jafc.7b04188. [DOI] [PubMed] [Google Scholar]
- 34. Sun Y, Fang L, Wan Y, Gu Z. Pathogenic detection and phenotype using magnetic nanoparticle-urease nanosensor. Sens Actuators, B. 2018;259:428–32. [Google Scholar]
- 35. Kearns H, Goodacre R, Jamieson LE, Graham D, Faulds K. SERS detection of multiple antimicrobial-resistant pathogens using nanosensors. Anal Chem. 2017;89:12666–73. doi: 10.1021/acs.analchem.7b02653. [DOI] [PubMed] [Google Scholar]
- 36. Perçin I, Idil N, Bakhshpour M, Yılmaz E, Mattiasson B, Denizli A. Microcontact imprinted plasmonic nanosensors: powerful tools in the detection of Salmonella paratyphi. Sens Actuators, B. 2017;17:1375. doi: 10.3390/s17061375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Banerjee T, Sulthana S, Shelby T, Heckert B, Jewell J, Woody K, et al. Multiparametric magneto-fluorescent nanosensors for the ultrasensitive detection of Escherichia coli O157: H7. ACS Infect Dis. 2016;2:667–73. doi: 10.1021/acsinfecdis.6b00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun A, Chai J, Xiao T, Shi X, Li X, Zhao Q, et al. Development of a selective fluorescence nanosensor based on molecularly imprinted-quantum dot optosensing materials for saxitoxin detection in shellfish samples. Sens Actuators, B. 2018;258:408–14. [Google Scholar]
- 39. Zhang CH, Liu LW, Liang P, Tang LJ, Yu RQ, Jiang JH. Plasmon coupling enhanced Raman scattering nanobeacon for single-step, ultrasensitive detection of cholera toxin. Anal Chem. 2016;88:447–52. doi: 10.1021/acs.analchem.6b00944. [DOI] [PubMed] [Google Scholar]
- 40. Zhang W, Han Y, Chen X, Luo X, Wang J, Yue T, et al. Surface molecularly imprinted polymer capped Mn-doped ZnS quantum dots as a phosphorescent nanosensor for detecting patulin in apple juice. Food Chem. 2017;232:145–54. doi: 10.1016/j.foodchem.2017.03.156. [DOI] [PubMed] [Google Scholar]
- 41.Code of Federal Regulations (CFR) Electronic code of federal regulations. Title 21 food and drugs. PART 73 —LISTING OF COLOR ADDITIVES EXEMPT FROM CERTIFICATION. The United States office of the federal register (OFR) and the United States. Government Publishing Office; 2018. https://www.ecfr.gov/cgi-bin/text-idx?SID=79a76b1d7e7a98ae9459d88005ab7058&mc=true&node=pt21.1.73&rgn=div5 . [Google Scholar]
- 42.US FDA. Color additive status list. United States Food & Drug Administration; 2015. [Accessed 7 August 2018]. [Google Scholar]
- 43. The European Commission. Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food Text with EEA relevance. Official J Eur Union. 2011;12:1–89. [Google Scholar]
- 44.US FDA. Inventory of effective food contact substance (FCS) notifications. Administration USFaD; 2018. [Accessed 8 August 2018]. Accessed on, https://www.accessdata.fda.gov/scripts/fdcc/?set=FCN. [Google Scholar]
- 45.Euroapen Commision. Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives. The European Parliament and The Council of The European Union; 2008. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008R1333 . [Google Scholar]
- 46.Code of Federal Regulations (CFR) Title 21 –food and drugs Chapter i–food and drug administration Department of health and human services Subchapter B–food for human consumption (continued). Part 172 – food additives permitted for direct addition to food for human consumption. Subpart E–anticakingagents. Sec. 172, 480 silicon dioxide. United State Food and Drug Administration; 2017. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=172.480 . [Google Scholar]
- 47.US FDA. Food additive status list. US FDA/CFSAN Office of Food Additive Safety. 2018. [Accessed 7 August 2018]. Accessed on, https://www.fda.gov/Food/IngredientsPackagingLabeling/FoodAdditivesIngredients/ucm091048.htm.
- 48. Shi S, Wang W, Liu L, Wu S, Wei Y, Li W. Effect of on the physicochemical characteristics of longan fruit under ambient temperature. J Food Eng. 2013;118:125–31. [Google Scholar]
- 49. Zambrano-Zaragoza ML, Mercado-Silva E, Gutiérrez-Cortez E, Cornejo-Villegas MA, Quintanar-Guerrero D. The effect of nano-coatings with A-tocopherol and xanthan gum on shelf-life and browning index of fresh-cut “red delicious” apples. Innovat Food Sci Emerg Technol. 2014;22:188–96. [Google Scholar]
- 50.Code of Federal Regulations (CFR) Electronic code of federal regulations. title 21 food and drugs. part 184—direct food substances affirmed as generally recognized as safe. subpart b—listing of specific substances affirmed as gras. the united states office of the federal register (ofr) and the united states. Government Publishing Office; 2018. https://www.ecfr.gov/cgi-bin/text-idx?SID=79a76b1d7e7a98ae9459d88005ab7058&mc=true&node=pt21.1.73&rgn=div5 . [Google Scholar]
- 51. Oliveira HC, Stolf-Moreira R, Martinez CBR, Grillo R, de Jesus MB, Fraceto LF. Nanoencapsulation enhances the post-emergence herbicidal activity of atrazine against mustard plants. PLoS One. 2015;10:e0132971. doi: 10.1371/journal.pone.0132971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cao L, Zhou Z, Niu S, Cao C, Li X, Shan Y, et al. Positive-charge functionalized mesoporous silica nanoparticles as nanocarriers for controlled 2, 4-dichlorophenoxy acetic acid sodium salt release. J Agric Food Chem. 2018;66:6594–603. doi: 10.1021/acs.jafc.7b01957. [DOI] [PubMed] [Google Scholar]
- 53. Kumar S, Kumar D, Dilbaghi N. Preparation, characterization, and bio-efficacy evaluation of controlled release carbendazim-loaded polymeric nanoparticles. Environ Sci Pollut Res. 2017;24:926–37. doi: 10.1007/s11356-016-7774-y. [DOI] [PubMed] [Google Scholar]
- 54. Duhan JS, Kumar R, Kumar N, Kaur P, Nehra K, Duhan S. Nanotechnology: the new perspective in precision agriculture. Biotechnol Rep. 2017;15:11–23. doi: 10.1016/j.btre.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sekhon BS. Nanotechnology in agri-food production: an overview. Nanotechnol Sci Appl. 2014;7:31. doi: 10.2147/NSA.S39406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Khot LR, Sankaran S, Maja JM, Ehsani R, Schuster EW. Applications of nanomaterials in agricultural production and crop protection: a review. Crop Protect. 2012;35:64–70. [Google Scholar]
- 57. Dimkpa CO, McLean JE, Britt DW, Anderson AJ. Antifungal activity of ZnO nanoparticles and their interactive effect with a biocontrol bacterium on growth antagonism of the plant pathogen Fusarium graminearum. Biometals. 2013;26:913–24. doi: 10.1007/s10534-013-9667-6. [DOI] [PubMed] [Google Scholar]
- 58. Rajiv P, Rajeshwari S, Venckatesh R. Bio-Fabrication of zinc oxide nanoparticles using leaf extract of Parthenium hysterophorus L. and its size-dependent antifungal activity against plant fungal pathogens. Spectrochim Acta A. 2013;112:384–7. doi: 10.1016/j.saa.2013.04.072. [DOI] [PubMed] [Google Scholar]
- 59. Dimkpa CO, Bindraban PS. Nanofertilizers: new products for the industry? J Agric Food Chem. 2018;66:6462–73. doi: 10.1021/acs.jafc.7b02150. [DOI] [PubMed] [Google Scholar]
- 60. Tripathi KM, Bhati A, Singh A, Sonker AK, Sarkar S, Sonkar SK. Sustainable changes in the contents of metallic micronutrients in first generation gram seeds imposed by carbon nano-onions: life cycle seed to seed study. ACS Sustainable Chem Eng. 2017;5:2906–16. [Google Scholar]
- 61. Khalifa NS, Hasaneen MN. The effect of chitosan–PMAA–NPK nanofertilizer on Pisum sativum plants. 3 Biotech. 2018;8:193. doi: 10.1007/s13205-018-1221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Abbacia A, Azzouz N, Bouznit Y. A new copper doped montmorillonite modified carbon paste electrode for propineb detection. Appl Clay Sci. 2014;90:130–4. [Google Scholar]
- 63.Wibowo KM, Sahdan MZ, Ramli NI, Muslihati A, Rosni N, Tsen VH, et al. J Phy: Conf Series. IOP Publishing; 2018. Detection of Escherichia coli bacteria in wastewater by using graphene as a sensing material; p. 012063. [Google Scholar]
- 64. Deng H, Gao Y, Dasari TPS, Ray PC, Yu H. A facile 3D construct of graphene oxide embedded with silver nanoparticles and its potential application as water filter. J Miss Acad Sci. 2016;61:190–7. [Google Scholar]
- 65. Geszke-Moritz M, Clavier G, Lulek J, Schneider R. Copper-or manganese-doped ZnS quantum dots as fluorescent probes for detecting folic acid in aqueous media. J Lumin. 2012;132:987–91. [Google Scholar]
- 66. Esser B, Schnorr JM, Swager TM. Selective detection of ethylene gas using carbon nanotube-based devices: utility in determination of fruit ripeness. Angew Chem Int Ed. 2012;51:5752–6. doi: 10.1002/anie.201201042. [DOI] [PubMed] [Google Scholar]
- 67. Lin YW, Huang CC, Chang HT. Gold nanoparticle probes for the detection of mercury, lead and copper ions. Analyst. 2011;136:863–71. doi: 10.1039/c0an00652a. [DOI] [PubMed] [Google Scholar]
- 68. Jokar M, Safaralizadeh MH, Hadizadeh F, Rahmani F, Kalani MR. Design and evaluation of an apta-nano-sensor to detect Acetamiprid in vitro and in silico. J Biomol Struct Dyn. 2016;34:2505–17. doi: 10.1080/07391102.2015.1123188. [DOI] [PubMed] [Google Scholar]
- 69. Zhao L, Ortiz C, Adeleye AS, Hu Q, Zhou H, Huang Y, et al. Metabolomics to detect response of lettuce (Lactuca sativa) to Cu(OH)2 nanopesticides: oxidative stress response and detoxification mechanisms. Environ Sci Technol. 2016;50:9697–707. doi: 10.1021/acs.est.6b02763. [DOI] [PubMed] [Google Scholar]
- 70. Graham JH, Johnson EG, Myers ME, Young M, Rajasekaran P, Das S, et al. Potential of nano-formulated zinc oxide for control of citrus canker on grapefruit trees. Plant Dis. 2016;100:2442–7. doi: 10.1094/PDIS-05-16-0598-RE. [DOI] [PubMed] [Google Scholar]
- 71. Hannon JC, Kerry JP, Cruz-Romero M, Azlin-Hasim S, Morris M, Cummins E. Assessment of the migration potential of nanosilver from nanoparticle-coated low-density polyethylene food packaging into food simulants. Food Addit Contam A. 2016;33:167–78. doi: 10.1080/19440049.2015.1114184. [DOI] [PubMed] [Google Scholar]
- 72.Hwang HM, Ray PC, Yu H, He X. Toxicology of designer/engineered metallic nanoparticles. In: Luque R, Varma R, editors. Sustainable preparation of metal nanoparticles: methods and applications. Cambridge, United Kingdom: Royal Society of Chemistry; 2012. [Google Scholar]
- 73. He X, Aker WG, Leszczynski J, Hwang H-M. Using a holistic approach to assess the impact of engineered nanomaterials inducing toxicity in aquatic systems. J Food Drug Anal. 2014;22:128–46. doi: 10.1016/j.jfda.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. He X, Aker WG, Fu PP, Hwang H-M. Toxicity of engineered metal oxide nanomaterials mediated by nano–bio–eco–interactions: a review and perspective. Environ Sci: Nano. 2015;2:564–82. doi: 10.1080/10590501.2017.1418793. [DOI] [PubMed] [Google Scholar]
- 75. He X, Aker WG, Huang M-J, Watts DJ, Hwang H-M. Metal oxide nanomaterials in nanomedicine: applications in photodynamic therapy and potential toxicity. Curr Top Med Chem. 2015;15:1887–900. doi: 10.2174/1568026615666150506145251. [DOI] [PubMed] [Google Scholar]
- 76. He X, Hwang H-M. Nanotechnology in food science: functionality, applicability, and safety assessment. J Food Drug Anal. 2016;24:671–81. doi: 10.1016/j.jfda.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. He X, Fu P, Aker WG, Hwang H-m. Toxicity of engineered nanomaterials mediated by nano-bio-eco interactions. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2018;36:21–42. doi: 10.1080/10590501.2017.1418793. [DOI] [PubMed] [Google Scholar]
- 78. He X, Aker WG, Hwang H-M. An in vivo study on the photo-enhanced toxicities of S-doped TiO2 nanoparticles to zebrafish embryos (Danio rerio) in terms of malformation, mortality, rheotaxis dysfunction, and DNA damage. Nanotoxicology. 2014;8:185–95. doi: 10.3109/17435390.2013.874050. [DOI] [PubMed] [Google Scholar]
- 79. Sieg H, Kästner C, Krause B, Meyer T, Burel A, Böhmert L, et al. Impact of an artificial digestion procedure on aluminum-containing nanomaterials. Langmuir. 2017;33:10726–35. doi: 10.1021/acs.langmuir.7b02729. [DOI] [PubMed] [Google Scholar]
- 80. Deng H, Yu H. Self-assembly of rhodamine 6G on silver nanoparticles. Chem Phys Lett. 2018;692:75–80. [Google Scholar]
- 81. Deng H, Yu H. A mini review on controlling the size of Ag nanoclusters by changing the stabilizer to Ag ratio and by changing DNA sequence. Adv Nat Sci. 2015;8:1–9. [Google Scholar]
- 82. Ray PC, Yu H, Fu PP. Toxicity and environmental risks of nanomaterials: challenges and future needs. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:1–35. doi: 10.1080/10590500802708267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. McShan D, Ray PC, Yu H. Molecular toxicity mechanism of nanosilver. J Food Drug Anal. 2014;22:116–27. doi: 10.1016/j.jfda.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fu PP, Xia Q, Hwang H-M, Ray PC, Yu H. Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal. 2014;22:64–75. doi: 10.1016/j.jfda.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. McShan D, Zhang Y, Deng H, Ray PC, Yu H. Synergistic antibacterial effect of silver nanoparticles combined with ineffective antibiotics on drug resistant Salmonella typhimurium DT104. J Environ Sci Health C. 2015;33:369–84. doi: 10.1080/10590501.2015.1055165. [DOI] [PubMed] [Google Scholar]
- 86. Deng H, McShan D, Zhang Y, Sinha SS, Arslan Z, Ray PC, et al. Mechanistic study of the synergistic antibacterial activity of combined silver nanoparticles and common antibiotics. Environ Sci Technol. 2016;50:8840–8. doi: 10.1021/acs.est.6b00998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dasari T, Deng H, McShan D, Yu H. Nanosilver-based antibacterial agents for food safety. food poisoning: outbreaks, bacterial sources and adverse health effects. 2014:35–62. [Google Scholar]
- 88. Zhang Y, Dasari TPS, Deng H, Yu H. Antimicrobial activity of gold nanoparticles and ionic gold. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2015;33:286–327. doi: 10.1080/10590501.2015.1055161. [DOI] [PubMed] [Google Scholar]
- 89. Khan MI, Mohammad A, Patil G, Naqvi SAH, Chauhan LKS, Ahmad I. Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials. 2012;33:1477–88. doi: 10.1016/j.biomaterials.2011.10.080. [DOI] [PubMed] [Google Scholar]
- 90. Long TC, Tajuba J, Sama P, Saleh N, Swartz C, Parker J, et al. Nanosize titanium dioxide stimulates reactive oxygen species in brain microglia and damages neurons in vitro. Environ Health Perspect. 2007;115:1631. doi: 10.1289/ehp.10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Singh N, Manshian B, Jenkins GJ, Griffiths SM, Williams PM, Maffeis TG, et al. NanoGenotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials. 2009;30:3891–914. doi: 10.1016/j.biomaterials.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 92. Chen XX, Cheng B, Yang YX, Cao A, Liu JH, Du LJ, et al. Characterization and preliminary toxicity assay of nanotitanium dioxide additive in sugar-coated chewing gum. Small. 2013;9:1765–74. doi: 10.1002/smll.201201506. [DOI] [PubMed] [Google Scholar]
- 93. Maertens A, Plugge H. Better metrics for “sustainable by design”: toward an in silico green toxicology for green(er) chemistry. ACS Sustainable Chem Eng. 2018;6:1999–2003. [Google Scholar]
- 94. Gou N, Onnis-Hayden A, Gu AZ. Mechanistic toxicity assessment of nanomaterials by whole-cell-array stress genes expression analysis. Environ Sci Technol. 2010;44:5964–70. doi: 10.1021/es100679f. [DOI] [PubMed] [Google Scholar]
- 95. Li X, Zhang C, Bian Q, Gao N, Zhang X, Meng Q, et al. Integrative functional transcriptomic analyses implicate specific molecular pathways in pulmonary toxicity from exposure to aluminum oxide nanoparticles. Nanotoxicology. 2016;10:957–69. doi: 10.3109/17435390.2016.1149632. [DOI] [PubMed] [Google Scholar]
- 96. Liu R, Zhang H, Lal R. Effects of stabilized nanoparticles of copper, zinc, manganese, and iron oxides in low concentrations on lettuce (Lactuca sativa) seed germination: nanotoxicants or nanonutrients? Water. Air Soil Pollution. 2016;227:42. [Google Scholar]
- 97. Dudefoi W, Moniz K, Allen-Vercoe E, Ropers MH, Walker VK. Impact of food grade and nano-TiO2 particles on a human intestinal community. Food Chem Toxicol. 2017;106:242–9. doi: 10.1016/j.fct.2017.05.050. [DOI] [PubMed] [Google Scholar]
- 98. Bettini S, Boutet-Robinet E, Cartier C, Coméra C, Gaultier E, Dupuy J, et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci Rep. 2017;7:4037. doi: 10.1038/srep40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.USDA France. Plans to ban titanium dioxide in food products. Information Network (GAIN) Report. Global Agriculture: USDA Foreign Agriculture Service; 2018. [Google Scholar]
- 100. Singh P, Kim YJ, Zhang D, Yang DC. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016;34:588–99. doi: 10.1016/j.tibtech.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 101. Zhang X, Qu Y, Shen W, Wang J, Li H, Zhang Z, et al. Biogenic synthesis of gold nanoparticles by yeast Magnusiomyces ingens LH-F1 for catalytic reduction of nitrophenols. Colloids Surf, A. 2016;497:280–5. [Google Scholar]
- 102. Jain N, Bhargava A, Majumdar S, Tarafdar J, Panwar J. Extracellular biosynthesis and characterization of silver nanoparticles using Aspergillus flavus NJP08: a mechanism perspective. Nanoscale. 2011;3:635–41. doi: 10.1039/c0nr00656d. [DOI] [PubMed] [Google Scholar]
- 103. He X, Aker WG, Pelaez M, Lin Y, Dionysiou DD, Hwang H-m. Assessment of nitrogen–fluorine-codoped TiO2 under visible light for degradation of BPA: implication for field remediation. J Photochem Photobiol, A. 2016;314:81–92. [Google Scholar]
- 104. Sharma D, Rajput J, Kaith BS, Kaur M, Sharma S. Synthesis of ZnO nanoparticles and study of their antibacterial and antifungal properties. Thin Solid Films. 2010;519:1224–9. [Google Scholar]
- 105. Zhou Q, Albert O, Deng H, Yu X-L, Cao Y, Li J-B, et al. Effect of functional groups on the crystallization of ferric oxides/oxyhydroxides in suspension environment. Front Mater Sci. 2012;6:297–303. [Google Scholar]
- 106. Deng H, Wang X-M, Du C, Shen X-C, Cui F-Z. Combined effect of ion concentration and functional groups on surface chemistry modulated CaCO 3 crystallization. CrystEngComm. 2012;14:6647–53. [Google Scholar]
- 107. Deng H, Wang S, Wang X, Du C, Shen X, Wang Y, et al. Two competitive nucleation mechanisms of calcium carbonate biomineralization in response to surface functionality in low calcium ion concentration solution. Regen Biomater. 2015;2:187–95. doi: 10.1093/rb/rbv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Deng H, Shen X-C, Wang X-M, Du C. Calcium carbonate crystallization controlled by functional groups: a mini-review. Front Mater Sci. 2013;7:62–8. [Google Scholar]
- 109. Soni N, Prakash S. Factors affecting the geometry of silver nanoparticles synthesis in Chrysosporium tropicum and Fusarium oxysporum. Am J Nanotechnol. 2011;2:112–21. [Google Scholar]
- 110. Hyllested JÆ, Palanco ME, Hagen N, Mogensen KB, Kneipp K. Green preparation and spectroscopic characterization of plasmonic silver nanoparticles using fruits as reducing agents. Beilstein J Nanotechnol. 2015;6:293. doi: 10.3762/bjnano.6.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mishra S, Singh HB. Biosynthesized silver nanoparticles as a nanoweapon against phytopathogens: exploring their scope and potential in agriculture. Appl Microbiol Biotechnol. 2015;99:1097–107. doi: 10.1007/s00253-014-6296-0. [DOI] [PubMed] [Google Scholar]
- 112. Chauhan R, Reddy A, Abraham J. Biosynthesis of silver and zinc oxide nanoparticles using Pichia fermentans JA2 and their antimicrobial property. Appl Nanosci. 2015;5:63–71. [Google Scholar]
- 113. Fatemi M, Mollania N, Momeni-Moghaddam M, Sadeghifar F. Extracellular biosynthesis of magnetic iron oxide nanoparticles by Bacillus cereus strain HMH1: characterization and in vitro cytotoxicity analysis on MCF-7 and 3T3 cell lines. J Biotechnol. 2018;270:1–11. doi: 10.1016/j.jbiotec.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 114. Mishra S, Singh BR, Singh A, Keswani C, Naqvi AH, Singh HB. Biofabricated silver nanoparticles act as a strong fungicide against Bipolaris sorokiniana causing spot blotch disease in wheat. PLoS One. 2014;9:e97881. doi: 10.1371/journal.pone.0097881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Roy K, Sarkar CK, Ghosh CK. Photocatalytic activity of biogenic silver nanoparticles synthesized using yeast (Saccharomyces cerevisiae) extract. Appl Nanosci. 2015;5:953–9. doi: 10.1016/j.saa.2015.02.058. [DOI] [PubMed] [Google Scholar]
- 116. Raliya R, Biswas P, Tarafdar JC. TiO2 nanoparticle biosynthesis and its physiological effect on mung bean (Vigna radiata L.) Biotechnol Rep. 2015;5:22–6. doi: 10.1016/j.btre.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Barapatre A, Aadil KR, Jha H. Synergistic antibacterial and antibiofilm activity of silver nanoparticles biosynthesized by lignin-degrading fungus. Bioresour Bioprocess. 2016;3:8. [Google Scholar]
- 118. Eugenio M, Müller N, Frasés S, Almeida-Paes R, Lima LMT, Lemgruber L, et al. Yeast-derived biosynthesis of silver/silver chloride nanoparticles and their antiproliferative activity against bacteria. RSC Adv. 2016;6:9893–904. [Google Scholar]
- 119. Fernández JG, Fernández-Baldo MA, Berni E, Camí G, Durán N, Raba J, et al. Production of silver nanoparticles using yeasts and evaluation of their antifungal activity against phytopathogenic fungi. Process Biochem. 2016;51:1306–13. [Google Scholar]
- 120. Nabila MI, Kannabiran K. Biosynthesis, characterization and antibacterial activity of copper oxide nanoparticles (CuO NPs) from actinomycetes. Biocatal Agric Biotechnol. 2018;15:56–62. [Google Scholar]
- 121. Składanowski M, Wypij M, Laskowski D, Golinska P, Dahm H, Rai M. Silver and gold nanoparticles synthesised from Streptomyces sp. isolated from with special reference to its antibacterial activity against pathogens. J Cluster Sci. 2017;28:59–79. [Google Scholar]
- 122. Ahmad R, Mohsin M, Ahmad T, Sardar M. Alpha amylase assisted synthesis of TiO2 nanoparticles: structural characterization and application as antibacterial agents. J Hazard Mater. 2015;283:171–7. doi: 10.1016/j.jhazmat.2014.08.073. [DOI] [PubMed] [Google Scholar]
- 123. Dhand V, Soumya L, Bharadwaj S, Chakra S, Bhatt D, Sreedhar B. Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater Sci Eng C. 2016;58:36–43. doi: 10.1016/j.msec.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 124. Singh P, Kim YJ, Wang C, Mathiyalagan R, El-Agamy Farh M, Yang DC. Biogenic silver and gold nanoparticles synthesized using red ginseng root extract, and their applications. Artif Cells Nanomed Biotechnol. 2016;44:811–6. doi: 10.3109/21691401.2015.1008514. [DOI] [PubMed] [Google Scholar]
- 125. Adio SO, Omar MH, Asif M, Saleh TA. Arsenic and selenium removal from water using biosynthesized nanoscale zero-valent iron: a factorial design analysis. Process Safe Environ. 2017;107:518–27. [Google Scholar]
- 126. Saravanakumar A, Ganesh M, Jayaprakash J, Jang HT. Biosynthesis of silver nanoparticles using Cassia tora leaf extract and its antioxidant and antibacterial activities. J Ind Eng Chem. 2015;28:277–81. [Google Scholar]
- 127. Amooaghaie R, Saeri MR, Azizi M. Synthesis, characterization and biocompatibility of silver nanoparticles synthesized from Nigella sativa leaf extract in comparison with chemical silver nanoparticles. Ecotox Environ Safe. 2015;120:400–8. doi: 10.1016/j.ecoenv.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 128. Ravichandran V, Vasanthi S, Shalini S, Shah SAA, Harish R. Green synthesis of silver nanoparticles using Atrocarpus altilis leaf extract and the study of their antimicrobial and antioxidant activity. Mater Lett. 2016;180:264–7. [Google Scholar]
- 129. Patra S, Mukherjee S, Barui AK, Ganguly A, Sreedhar B, Patra CR. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater Sci Eng C. 2015;53:298–309. doi: 10.1016/j.msec.2015.04.048. [DOI] [PubMed] [Google Scholar]
- 130. Khan AU, Wei Y, Ahmad A, Khan ZUH, Tahir K, Khan SU, et al. Enzymatic browning reduction in white cabbage, potent antibacterial and antioxidant activities of biogenic silver nanoparticles. J Mol Liq. 2016;215:39–46. [Google Scholar]
- 131. Pattanayak S, Mollick MMR, Maity D, Chakraborty S, Dash SK, Chattopadhyay S, et al. Butea monosperma bark extract mediated green synthesis of silver nanoparticles: characterization and biomedical applications. J Saudi Chem Soc. 2017;21:673–84. [Google Scholar]
- 132. Azizi S, Ahmad MB, Mamvar F, Mohamad R. Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater Lett. 2014;116:275–7. doi: 10.3390/ma6125942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Gu H, Chen X, Chen F, Zhou X, Parsaee Z. Ultrasound-assisted biosynthesis of CuO-NPs using brown alga Cystoseira trinodis: characterization, photocatalytic AOP, DPPH scavenging and antibacterial investigations. Ultrason Sonochem. 2018;41:109–19. doi: 10.1016/j.ultsonch.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 134. Kumar D, Nair M, Painuli R. Highly responsive bioinspired AgNPs probe for the precise colorimetric detection of the Mn (II) in aqueous systems. Plasmonics. 2018:1–9. doi: 10.1016/j.micpath.2018.07.013. [DOI] [Google Scholar]
- 135. Chaudhry N, Dwivedi S, Chaudhry V, Singh A, Saquib Q, Azam A, et al. Bio-inspired nanomaterials in agriculture and food: current status, foreseen applications and challenges. Microb Pathog. 2018;123:196–200. doi: 10.1016/j.micpath.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 136. Huang J, Lin L, Sun D, Chen H, Yang D, Li Q. Bio-inspired synthesis of metal nanomaterials and applications. Chem Soc Rev. 2015;44:6330–74. doi: 10.1039/c5cs00133a. [DOI] [PubMed] [Google Scholar]
- 137. Yoo JW, Irvine DJ, Discher DE, Mitragotri S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov. 2011;10:521. doi: 10.1038/nrd3499. [DOI] [PubMed] [Google Scholar]
- 138. Zong L, Li M, Li C. Bioinspired coupling of inorganic layered nanomaterials with marine polysaccharides for efficient aqueous exfoliation and smart actuating hybrids. Adv Mater. 2017;29:1604691. doi: 10.1002/adma.201604691. [DOI] [PubMed] [Google Scholar]
- 139. Feng Y, Zhu W, Guo W, Jiang L. Bioinspired energy conversion in nanofluidics: a paradigm of material evolution. Adv Mater. 2017;29:1702773. doi: 10.1002/adma.201702773. [DOI] [PubMed] [Google Scholar]
- 140. Zan G, Wu Q. Biomimetic and bioinspired synthesis of nanomaterials/nanostructures. Adv Mater. 2016;28:2099–147. doi: 10.1002/adma.201503215. [DOI] [PubMed] [Google Scholar]
- 141. Hawkes EW, Eason EV, Christensen DL, Cutkosky MR. Human climbing with efficiently scaled gecko-inspired dry adhesives. J R Soc Interface. 2015;12:20140675. doi: 10.1098/rsif.2014.0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Hu S, Xia Z, Dai L. Advanced gecko-foot-mimetic dry adhesives based on carbon nanotubes. Nanoscale. 2013;5:475–86. doi: 10.1039/c2nr33027j. [DOI] [PubMed] [Google Scholar]
- 143. Pant HR, Kim HJ, Joshi MK, Pant B, Park CH, Kim JI, et al. One-step fabrication of multifunctional composite polyurethane spider-web-like nanofibrous membrane for water purification. J Hazard Mater. 2014;264:25–33. doi: 10.1016/j.jhazmat.2013.10.066. [DOI] [PubMed] [Google Scholar]
- 144. Jeong K-H, Kim J, Lee LP. Biologically inspired artificial compound eyes. Science. 2006;312:557–61. doi: 10.1126/science.1123053. [DOI] [PubMed] [Google Scholar]
- 145. Autumn K, Sitti M, Liang YA, Peattie AM, Hansen WR, Sponberg S, et al. Evidence for van der Waals adhesion in gecko setae. Proc Natl Acad Sci USA. 2002;99:12252–6. doi: 10.1073/pnas.192252799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Liang J, Yu M, Guo L, Cui B, Zhao X, Sun C, et al. Bioinspired development of P (St–MAA)–avermectin nanoparticles with high affinity for foliage to enhance folia retention. J Agric Food Chem. 2018;66:6578–84. doi: 10.1021/acs.jafc.7b01998. [DOI] [PubMed] [Google Scholar]
- 147. Zhang Z, Huang J, Dong B, Yuan Q, He Y, Wolfbeis OS. Rational tailoring of ZnSnO3/TiO2 heterojunctions with bioinspired surface wettability for high-performance humidity nanosensors. Nanoscale. 2015;7:4149–55. doi: 10.1039/c4nr07559e. [DOI] [PubMed] [Google Scholar]
- 148. Asadnia M, Kottapalli AGP, Karavitaki KD, Warkiani ME, Miao J, Corey DP, et al. From biological cilia to artificial flow sensors: biomimetic soft polymer nanosensors with high sensing performance. Sci Rep. 2016;6:32955. doi: 10.1038/srep32955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Mannoor MS, Tao H, Clayton JD, Sengupta A, Kaplan DL, Naik RR, et al. Graphene-based wireless bacteria detection on tooth enamel. Nat Commun. 2012;3 doi: 10.1038/ncomms1767. [DOI] [PubMed] [Google Scholar]
- 150. Weng X, Neethirajan S. Ensuring food safety: quality monitoring using microfluidics. Trends Food Sci Technol. 2017;65:10–22. [Google Scholar]
- 151. Ikeda M, Yamaguchi N, Tani K, Nasu M. Rapid and simple detection of food poisoning bacteria by bead assay with a microfluidic chip-based system. J Microbiol Methods. 2006;67:241–7. doi: 10.1016/j.mimet.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 152. Weng X, Gaur G, Neethirajan S. Rapid detection of food allergens by microfluidics ELISA-based optical sensor. Biosensors. 2016;6:24. doi: 10.3390/bios6020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wu SY, Yang C, Hsu W, Lin L. 3D-printed microelectronics for integrated circuitry and passive wireless sensors. Microsyst Nanoeng. 2015;1:15013. [Google Scholar]
- 154. Yang B, Lv S, Chen F, Liu C, Cai C, Chen C, et al. A resonance light scattering sensor based on bioinspired molecularly imprinted polymers for selective detection of papain at trace levels. Anal Chim Acta. 2016;912:125–32. doi: 10.1016/j.aca.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 155. Wang M, Liu Y, Ren G, Wang W, Wu S, Shen J. Bioinspired carbon quantum dots for sensitive fluorescent detection of vitamin B12 in cell system. In Press Anal Chim Acta. 2018 doi: 10.1016/j.aca.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 156. Kong B, Tang J, Wu Z, Selomulya C, Wang H, Wei J, et al. Bioinspired porous antenna-like nanocube/nanowire heterostructure as ultra-sensitive cellular interfaces. NPG Asia Mater. 2014;6:e117. [Google Scholar]
- 157. Chau C-F, Wu S-H, Yen G-C. The development of regulations for food nanotechnology. Trends Food Sci Technol. 2007;18:269–80. [Google Scholar]
- 158. Wacker MG, Proykova A, Santos GML. Dealing with nanosafety around the globe - regulation vs. innovation. Int J Pharm. 2016;509:95–106. doi: 10.1016/j.ijpharm.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 159. Kaphle A, Navya PN, Umapathi A, Daima HK. Nanomaterials for agriculture, food and environment: applications, toxicity and regulation. Environ Chem Lett. 2018;16:43–58. [Google Scholar]
- 160. Jain A, Ranjan S, Dasgupta N, Ramalingam C. Nanomaterials in food and agriculture: an overview on their safety concerns and regulatory issues. Crit Rev Food Sci Nutr. 2018;58:297–317. doi: 10.1080/10408398.2016.1160363. [DOI] [PubMed] [Google Scholar]
- 161. Azamat A, Kunal S. Risks of nanotechnology in the food industry: a review of current regulation. Nanotechnol Percept. 2015;11:27–30. [Google Scholar]
- 162.Arnaldi S, Muratorio A. Nanotechnology, uncertainty and regulation A guest editorial. Springer; 2013. [Google Scholar]
- 163.Xiaojia H, Hua D, Winfred AG, Huey-min H. Regulation and safety of nanotechnology in the food and agriculture industry. In: Molina G, editor. Food applications of nanotechnology. CRC Press, Taylor & Francis Group; 2018. [Google Scholar]
- 164.Commission EU. Recommendation on the definition of nanomaterial, 2011/696//EU. Official Journal of the European Union; 2011. [Google Scholar]
- 165.FDA. Guidance for Industry use of nanomaterials in food for animals. Rockville, MD: Center for Veterinary Medicine, Division of Animal Feeds (HFV-226), Food and Drug Administration; 2011. [Google Scholar]
- 166.EU. Second regulatory review on nanomaterials. COM; final European Commission; 2012. 2012. p. 572. [Google Scholar]
- 167.Nanomaterials EU. Commission proposes case by case approach to assessment MEMO-12-732_EN. Brussels: European Commission; 2012. [Google Scholar]
- 168.Nanomaterials EU. Case by case safety approach for breakthrough technology IP-12-1050_EN. Brussels: European Commission; 2012. [Google Scholar]
- 169.FDA. Guidance for Industry, assessing the effects of significant manufacturing process changes, Including emerging technologies, on the safety and regulatory status of food Ingredients and food contact substances, Including food Ingredients that are color additives. College Park, MD: Office Of food additive safety, HFS-205 center for food safety and applied nutrition Food and Drug Administration; 2014. [Google Scholar]
- 170. Zhang Y, Leu Y-R, Aitken RJ, Riediker M. Inventory of engineered nanoparticle-containing consumer products available in the Singapore retail market and likelihood of release into the aquatic environment. Int J Environ Res Publ Health. 2015;12:8717–43. doi: 10.3390/ijerph120808717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Quadros ME, Pierson R, Tulve NS, Willis R, Rogers K, Thomas TA, et al. Release of silver from nanotechnology-based consumer products for children. Environ Sci Technol. 2013;47:8894–901. doi: 10.1021/es4015844. [DOI] [PubMed] [Google Scholar]
- 172. Benn T, Cavanagh B, Hristovski K, Posner JD, Westerhoff P. The release of nanosilver from consumer products used in the home. J Environ Qual. 2010;39:1875–82. doi: 10.2134/jeq2009.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Chun AL. Will the public swallow nanofood? Nat Nanotechnol. 2009;4:790–1. doi: 10.1038/nnano.2009.359. [DOI] [PubMed] [Google Scholar]
- 174. George S, Kaptan G, Lee J, Frewer L. Awareness on adverse effects of nanotechnology increases negative perception among public: survey study from Singapore. J Nanopart Res. 2014;16 [Google Scholar]
- 175. Handford CE, Dean M, Spence M, Henchion M, Elliott CT, Campbell K. Awareness and attitudes towards the emerging use of nanotechnology in the agri-food sector. Food Contr. 2015;57:24–34. [Google Scholar]
- 176. Brown J, Fatehi L, Kuzma J. Altruism and skepticism in public attitudes toward food nanotechnologies. J Nanopart Res. 2015;17 [Google Scholar]
- 177. Zhou G, Hu W. Public acceptance of and willingness-to-pay for nanofoods in the U.S. Food Contr. 2018;89:219–26. [Google Scholar]
- 178.Bennett D, Radford T. Public perceptions of nanotechnologies: lessons from genetically modified foods. In: Chaudhry Q, Castle L, Watkins R, editors. Nanotechnologies in food. Editon 2. Royal Society of Chemistry; 2017. pp. 60–80. [Google Scholar]