Abstract
Acacia catechu L., (Fabaceae) named as “catechu” is a plant, the decoction of heartwood of which is daily consumed as thirst quencher by a good percentage of the population in South India. The plant is mainly distributed in India and other Asian countries. It has been used in Indian traditional medicine for the treatment of asthma, bronchitis, colic, diarrhea, boils, skin afflictions, sores and stomatitis. The present investigation was aimed to study the immunomodulatory effects of different fractions of ethanol extract of A. catechu heartwood and HPLC analysis of the active fraction. Three fractions namely, butanol, chloroform and ethyl acetate were prepared from ethanol extract of A. catechu heartwood. Each of these fractions was assessed for its immunomodulatory activity. In vivo immunomodulatory activity was analyzed by sheep red blood cells (SRBC) specific hemagglutinating antibody titer, plaque-forming cell assay and delayed type hypersensitivity (DTH) reaction in Swiss albino mice. In vitro immunomodulating potential of the fractions was studied using murine peritoneal macrophages and splenocytes. Non-specific immune functions such as phagocytosis (nitroblue tetrazolium reduction assay and cellular lysosomal enzyme assay), nitric oxide (NO) production and cytokine release (TNF-α and IL-10) were studied in macrophages. In addition, splenocyte proliferation was also studied. In the in vivo experiments, butanol and chloroform fractions showed an increase in antibody titer dose-dependently. At higher dose (400 mg/kg b. w.) treatment the butanol fraction produced an enhancement in the number of plaque-forming cells (antibody producing cells) in the spleen. SRBC induced DTH reaction was significantly increased with butanol fraction in a dose-dependent manner. Peritoneal macrophages showed an increased phagocytic response on treatment with butanol fraction (100 μg/mL) as evidenced by its effect on nitroblue tetrazolium reduction and cellular lysosomal enzyme activity. All three fractions inhibited the production of NO and the release of TNF-α. Interleukin-10 production was significantly increased after treatment with butanol fraction. High-performance liquid chromatography analysis of the butanol fraction showed the presence of high concentration of catechin. The results suggested that butanol fraction of ethanol extract of A. catechu heartwood had immunomodulatory effects on non-specific, humoral, and cell-mediated immune functions. This study may be useful in validating the rationality of daily consumption of decoction of A. catechu and also its use in traditional medicine system. The study also suggests the possible use of A. catechu in the immunostimulatory herbal preparations.
Keywords: Acacia catechu L., DTH, IL-10, Immunomodulatory, TNF-α
1. Introduction
Acacia catechu, (family: Fabaceae) generally known as black cutch, catechu, and cachou is a deciduous tree indigenous in India, other Asian countries like Nepal, Bangladesh, Pakistan, Myanmar, Thailand, Indonesia, and East Africa. The decoction prepared from the heartwood of this plant is used as a health drink in the southern part of India. It is believed as a blood purifying and can improve skin texture and enhance body’s defense mechanism [1]. In the traditional system of medicine, A. catechu has been used as an antimicrobial, anti-inflammatory, antidiarrheal, astringent, coagulant, and vermifuge, and has also been employed to treat diabetes and obesity, heal wounds and to maintain dental hygiene [2]. The heartwood extract called black catechu is used for asthma, bronchitis, colic, diarrhea, boils, skin afflictions, sores and stomatitis. The bark shows anthelmintic, antipyretic and anti-inflammatory properties. The immunomodulating potential of aqueous extract of the bark of A. catechu has been reported [1]. The sap of A. catechu is commonly used for the treatment of diarrhea and wounds in ruminants. In veterinary folk medicine the extracts of bark and heartwood are used for broken horn [3]. A traditional Chinese medicine “Ercha” prepared from the heartwood extract of catechu used in the medication of dysentery, cough, skin ulcerations and lesions [4].
The primary constituents of heartwood extract of A. catechu are catechins; gallic acid derivatives and polymers [2]. Catechin, epicatechin, and its derivatives such as epigallocatechin-3-O-gallate, epicatechin-3-O-gallate is the main catechins present in A. catechu [4]. Secondary metabolites like flavonol glycosides, flavonal dimers, and caffeine are also present [2]. Fisetinidol, 4-hydroxyphenol, rhamnetin, 5-hydroxy-2-[2-(4-hydroxyphenyl)acetyl]-3-methoxybenzoic acid, 3,3′,5,5′,7-pentahydroxyflavane, and (2S, 3S)-3,7,8,3′,4′-pentahydroxyflavane are other components identified in A. catechu [5]. Hiraganahalli et al. [6]reported that 90% of the composition of heartwood extract of A. catechu contain catechin (66.9%) and epicatechin (23.1%). These compounds largely present in the heartwood of A. catechu may contribute to the biopotency of A. catechu [7].
The human immune system is a versatile defense system involved in protecting the host from invading pathogenic microorganisms; maintaining a surveillance system that continuously monitors the integrity of host tissues [8]. The immune system also influences pathophysiological conditions as well as etiology of many diseases. Such diseases can be alleviated by the modulation of immune responses. The stimulation of immune system for the development of immunity and the suppression of undesired immune responses are the two purposes of immunomodulation. Any substance, biological or synthetic origin, which can stimulate or suppress the immune system is known as immunomodulators [9]. The traditional system of medicines plays a pivotal role in stimulating and repressing the host immune responses. In this regard, Ayurveda gives more attention towards promotion of health by enhancing host defenses against different diseases [10]. Rasayana herbs are especially suggested for the treatment of many immune system disorders [11]. Ayurvedic medicine plays a significant role in modern health care, especially where adequate treatments are not available. Ayurvedic remedies can act as adjuvants to neutralize aftereffects of modern therapy and are cost-effective [12]. Many medicinal plants have been reported with their possible effects on immune response to finding out their therapeutic applications in immune-related illness. Of these many have been subjected to the isolation, purification and characterization of the immunomodulatory phytochemicals [13]. These phytochemicals have shown immunomodulatory effects on non-specific immune functions of granulocytes, macrophages, lymphocytes and NK cells and complement system [9]. Most of the research has been concentrate on identifying natural substances of plant origin that can provide as immunomodulators to regulate specific immune responses [14].
In the present investigation, we have studied the immunomodulatory potential of different fractions of ethanol extract of A. catechu heartwood using in vivo and in vitro models. The phagocytic activity, nitric oxide production, TNF-α and IL-10 production by LPS stimulated macrophages and splenocyte proliferation were studied using in vitro models. The effect of fractions on antibody production and antibody-producing cells in spleen and cell-mediated immune response as shown by the effect on delayed-type hypersensitivity were studied using in vivo models.
2. Materials and methods
2.1. Plant material collection and extraction
A. catechu heartwood was collected from Kannur District, Kerala, India and was correctly identified and validated as A. catechu by the taxonomist from Department of Botany, St. Thomas College, Palai, Kerala, India (vide Sunil MA 1504). A voucher specimen has been collected and retained at the herbarium of Department of Botany, St. Thomas College. Dried, powdered heartwood was subjected to Soxhlet extraction using ethanol as a solvent. The ethanol extract was dried using a rotary evaporator and stored at 4 °C, till used.
2.2. HPTLC analysis
HPTLC was performed using CAMG HPTLC system (Switzerland). Prior to the analysis TLC silica gel 60 F254 plates (Merck Millipore, USA) were prewashed using methanol and activated in an oven at 100 °C for 10 min. Dried ethanol extract was dissolved in methanol (1 mg/mL) and spotted (5 μL) using a Linomat 5 sample applicator. Catechin was used as a standard. The ethanol extract was separated using a solvent system consisting of chloroform, ethyl acetate, glacial acetic acid at the ratio of 5:4:1. The spots were developed and visualized under CAMAG UV cabinet at 254 nm.
2.3. Fractionation of crude extract by column chromatography
The crude ethanol extract of A. catechu was subjected to fractionation on a glass column (450 × 30 mm) with silica gel (60–120 mesh, Merck) packing. A methanol solution of the extract was prepared by dissolving 4 g of the extract in a minimal quantity of methanol and mixed with silica gel and gently layered on the top of 1-butanol equilibrated silica gel column and subjected to chromatography. The flow-rate was fixed as 2 mL per minute and the column was sequentially eluted with 200 mL of solvents of varying polarity; butanol, chloroform, ethyl acetate. Eluted fractions were collected and concentrated to dryness, lyophilized and yield noted.
2.4. Animals
Swiss albino male mice (20–30 g) were purchased from Kerala Veterinary University, Thrissur, Kerala. The animals were maintained in an air-conditioned and pathogen-free standard condition and provide with standard pellet diet (Sai Durga feeds and foods, Banglore) and purified water ad libitum. The experiments got clearance from Institutional Animal Ethics Committee (No: B21032014-08).
2.5. Antigen
Fresh sheep blood was collected from a slaughterhouse in Alsever’s solution (Sigma–Aldrich, USA) and was kept in the refrigerator. Red blood cells were washed thrice with sterile normal saline. The washed blood cells were then suspended in phosphate buffered saline (PBS) (pH 7.2) at the required concentrations for the experiments [14].
2.6. Selection of doses for in vivo experiments
Irwin test was performed using different concentrations of the fractions (500, 1000, 2000 and 4000 mg/kg body weight) for fixing the doses for animal experiments [15]. Butanol, chloroform and ethyl acetate fractions were dissolved in the vehicle, phosphate-buffered saline (pH 7.2). For in vivo experiments, 1/10th–1/20th of the dose at which behavioral variations were observed was considered as safe dose [16]. The fractions did not produce any harmful effect up to 4 g/kg b. w. concentration of fractions. The doses for further studies were set between 200 and 400 mg/kg b. w. Nine groups of Swiss albino mice (6 number/group) were used in the present study. Group, I was normal control which received PBS. Group II and III were treated with Levamisole (Klab, Pvt. Ltd., Maharashtra) at a dose of 2.5 mg/kg b. w. as positive control and Dexamethasone (Weimer Pharma GmbH, Germany) at a dose of 1.25 mg/kg b. w. as negative control respectively. Animals of group IV, V, VI, VII, VIII and IX received 200 and 400 mg/kg b. w. of the three fractions orally in volumes of 0.2 mL/day for 30 days [17].
2.7. Isolation of mouse macrophages
Murine peritoneal macrophages were evoked in mice by intraperitoneal injection with one milliliter of 3% Brewer thioglycollate medium (Himedia, India) as a stimulant. The peritoneal effusion was collected after five days by washing the peritoneum with 10 mL of RPMI-1640 medium (Himedia, India). The collected effusion was centrifuged at 2000 rpm for 10 min at 4 °C and the supernatant was discarded. The cell pellets were resuspended in complete RPMI-1640 medium and erythrocytes were lysed using erythrocyte lysis buffer. The cell number was estimated and viability was checked by the trypan blue dye exclusion method [18].
2.8. Isolation of mouse splenocytes
Spleens were collected from mice after euthanasia under aseptic conditions; homogenized in RPMI-1640 medium. After centrifugation at 1000 rpm for 10 min at 4 °C, red blood cells were lysed by erythrocyte lysis buffer. Cells were washed twice using phosphate buffered saline (pH 7.2) and re-suspended in a complete RPMI-1640 medium. Splenocytes viability was determined by the trypan-blue dye exclusion test and the cell number was fixed to the desired concentration [19].
2.9. Hemagglutinating antibody titer
Hemagglutinin titer was analyzed using the procedure of Bin-Hafeez et al. [20]. Experimental animals were immunized with 0.2 mL of SRBCs (10%) through intraperitoneal route on the last day of treatment with the fractions. The blood samples of each mouse were collected from retro-orbital plexus on the fifth day after immunization and serum were separated. The complement was inactivated by incubating serum at 56 °C for 15 min. Serum was serially diluted (two-fold dilutions) in PBS (pH 7.2); 50 μL of diluted serum was mixed with 50 μL of 1% SRBC suspension in a 96-well plate. After mixing, 96-well plates were incubated at room temperature for 2 h and visually screened for agglutination. The antibody titer was confirmed by considering the highest serum dilution which showed visible hemagglutination.
2.10. Plaque forming cell assay
The effect of fractions of ethanol extract on antibody-producing cells in the spleen was carried out by a method described by Davis and Kuttan [21]. The experimental animals were immunized with 2.5 × 108 SRBC, intraperitoneally on the day of completion of treatment with fractions. Then animals were euthanized on the 5th day after immunization. Splenocytes were prepared as mentioned earlier. Fifty microliters of spleen cell suspension (1 × 106 cells/mL) and 50 μL of sheep red blood cells (7%)were added to 0.5mL ofmolten agarose (45 °C),mixed and spread on the surface of sterile slides. Allowed the agarose to solidify and the gels were incubated at 37 °C in the presence of rabbit serum (Himedia, India) as a complement system for 1 h. The number of antibody-producing cells (plaque forming cells) from the spleen was calculated by the Jerne’s Plaque assay [22].
2.11. Delayed-type hypersensitivity
The delayed type hypersensitivity reaction was examined by a previously described method [23]. On the last day of treatment with the fractions, experimenting animals were immunized with sheep red blood cells (1 × 109 cells), subcutaneously. The animals were again challenged with 1 × 108 sheep red blood cells in the left hind footpad on the 5th day after the first immunization. Phosphate buffered saline (pH 7.2) was injected into the right footpad and served as a control for nonspecific inflammation. After 24 h, raise in footpad thickness was evaluated using an engineering micrometer (Digimatic micrometer – Mitutoyo South Asia).
2.12. Nitroblue tetrazolium reduction test
The nitroblue tetrazolium reduction assay was performed by the method reported by Rainard [24]. In this experiment, macrophage cell suspension was seeded in a 96-well plate and incubated in 5% CO2 humidified incubator for 2 h; then non-adherent cells were eliminated by washing in RPMI-1640 medium. After washing the adherent cells (1 × 105 cells/well) were cultured in 100 μL RPMI-1640 medium with fractions of ethanol extract prepared in 0.1% dimethyl sulfoxide in PBS so that their final concentrations were 25 μg/mL, 50 μg/mL, and 100 μg/mL. The plate was incubated for 24 h at 37 °C in 5% CO2 humidified atmosphere. The 0.1% DMSO alone served as control. After incubation, 20 μL of nitroblue tetrazolium (Sigma, USA) solution (1.5 mg/mL in PBS) and 20 μL of the heat-inactivated yeast suspension (5 × 106 cells/mL) were added to the wells. The well plate was further incubated at abovementioned conditions for 1 h. The macrophages were first gently washed with RPMI-1640 medium and then washed four times with methanol (200 μL). The cells were air dried. Finally, 140 μL of DMSO and 120 μL of 2MKOH were added to each well. The absorbance of the well plate was measured at 570 nm by a microplate reader (Thermo Fisher Scientific, USA) and the percentage of nitroblue tetrazolium reduction was determined by the following equation:
2.13. Cellular lysosomal enzyme assay
Cellular lysosomal enzyme (CLE) activity was measured as previously described by Suzuki et al. [25]. Macrophage suspension was added to each well of a 96-well plate and incubated for 2 h in 5% CO2 humidified incubator; then unattached cells were discarded by washing in RPMI-1640 medium. The remaining adherent cells (1 × 105 cells/well) were cultured in 100 μL RPMI-1640 medium with fractions of ethanol extract prepared in 0.1% DMSO (diluted with PBS) so that their final concentrations were 25 μg/mL, 50 μg/mL, and 100 μg/mL. The plate was incubated for 24 h at 37 °C in 5% CO2 humidified atmosphere. The 0.1% DMSO alone was used as a control. The medium was discarded after incubation and 100 μL of 10 mM p-nitrophenyl phosphate (Himedia, India) solution, 20 μL of 0.1% Triton X-100 (Himedia, India), and 50 μL of 0.1 M citrate buffer (pH 5.0) were added to each well. The plate was again incubated at above-mentioned conditions for 30 min and after incubation 150 μL of borate buffer (0.2 M) at a pH of 9.8 was added to terminate the reaction. The absorbance was measured at 405 nm using a microplate reader. The percentage of lysosomal enzyme activity was calculated by the following equation:
2.14. Nitric oxide assay
The peritoneal macrophages were seeded in 24-well plate and incubated in 5% CO2 humidified incubator for 2 h; then washed with RPMI-1640 medium. The remaining adherent cells (2 × 106 cells/well) were cultured in 1 mL RPMI complete medium containing 10% fetal calf serum and incubated for 24 h with different concentrations of the fractions (prepared in 0.1% DMSO in PBS) ranging from 25 to 100 μg/mL both in the absence and presence of lipopolysaccharide (1 μg/mL). The level of nitric oxide production was determined by measuring concentration in culture media by Griess method [26]. After incubation, 100 μL cell culture supernatant was mixed with 100 μL Griess reagent in a 96-well plate and incubated for 10 min at room temperature. The absorbance of the reaction mixture was measured at 540 nm in a multi well plate reader. Sodium nitrite dissolved and diluted in the culture medium was used as a standard.
2.15. Assessment of cytokine secretion in macrophages
The macrophage cells were seeded in a 96-well microplate and incubated at 37 °C in 5% CO2 humidified incubator for 2 h; then non-adherent cells were discarded by washing in RPMI-1640 medium. The adherent cells (1 × 106 cells/well)were cultured at 37 °C in 5% CO2 for 24 h in RPMI-1640 complete medium without or with LPS (1 μg/mL) in the presence of different concentrations (25–100 μg/mL) of the fractions. Lipopolysaccharide-treated macrophage cells were served as a positive control and cells without lipopolysaccharide treatment as a negative control. After incubation, cell culture supernatants were collected by centrifugation at 2000 rpm for 20 min at 18 °C and assayed for IL-10 and TNF-α using ELISA kits (BioLegend, USA) according to the manufacturer’s protocol.
2.16. Splenocyte proliferation assay
The splenocyte proliferation assay was carried out according to MTT method [27]. Briefly, twenty microliters of different concentrations (25–100 μg/mL) of the fractions were added to 20 μL of spleen cell suspension (1 × 105 cells/mL) and 40 μL of RPMI-1640 medium in a 96-well microplate. The proliferation of spleen cells in the presence and absence of mitogens was studied. Concanavalin-A (ConA) and lipopolysaccharide (LPS) were used at 5 μg/mL as T and B cell mitogens, respectively. After 48 h incubation at 37 °C in humidified 5% CO2 incubator, 20 μL of MTT (Himedia, India) prepared in PBS (5 mg/mL) and 40 μL of RPMI-1640 medium were added to the wells and incubated for additional 4 h under the same conditions. The culturemedium was discarded and 100 μL of acidified isopropyl alcohol was added to the wells. Then, the solution was diluted by adding 100 μL of distilled water. The absorbance of the solution was measured at 570 nm. The percentage of splenocyte proliferation was calculated by the following equation:
2.17. HPLC and UV-VIS analysis of butanol fraction
Sample (1 mg/mL) and standard (0.5 mg/mL) were prepared in methanol and filtered through a membrane filter (pore size 0.22 μm) prior to the analysis. HPLC analysis was performed on Shimadzu LC 20AP HPLC system with UV detector. The LC column was a C18 column with column dimension 250mm × 4.6mm × 5 μm. Two mobile phases designated as A and B were used at the flow rate of 1.1 mL/min. Water with 0.1% trifluoroacetic acid was used as mobile phase A and mobile phase B consisted of acetonitrile. The mobile phase was filtered through a filter with pore size 0.22 μm, and degassed by vacuum, followed by sonication. A gradient starting at 90% A to 50% A was run for 35 min. The HPLC profile of butanol fraction of A. catechu heartwood was compared with that of catechin, a standard compound at 280 nm. Catechin was run at five concentrations (500–31.25 μg/mL) found to be linear with an R2 value of 0.98. Catechin content in the fraction was estimated by performing a linear regression analysis. The presence of catechin in butanol fraction was further confirmed by UV spectrometry. The butanol fraction and standard compound, catechin were dissolved in methanol, centrifuged at 3000 rpm for 10 min and filtered. The absorbance was measured at 250–300 nm using a UV–visible spectrophotometer (Shimadzu, Japan) [28].
2.18. Statistical analysis
All the in vivo experiment values were expressed as mean ± SEM for six animals. Values for the in vitro experiments were expressed as mean ± SEM for triplicate independent experiments. Statistical significance was evaluated using one-way ANOVA followed by Tukey’s HSD using SPSS program (version 20). The differences were considered significant at a p-value of less than 0.05.
3. Results
3.1. HPTLC analysis and column chromatography of ethanol extract
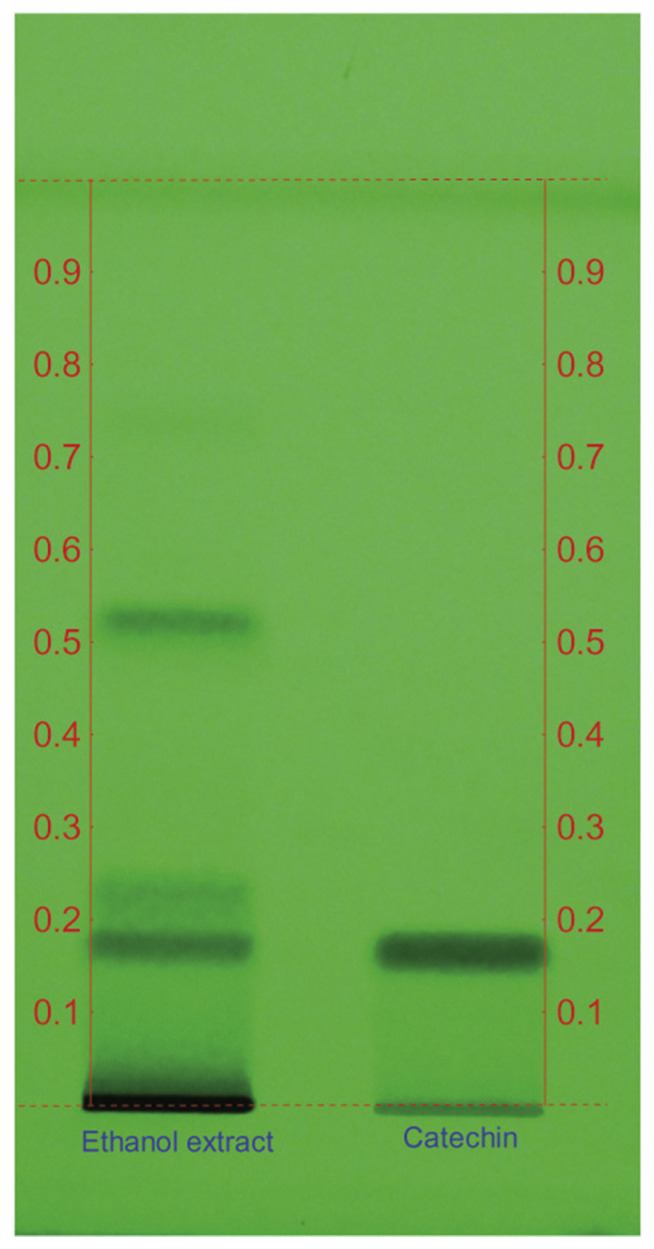
In HPTLC analysis a retardation factor (Rf) value of 0.18 was obtained for catechin and the compounds in A. catechu ethanol extract was separated and a compound with similar Rf (0.18) was obtained (Fig. 1). Fractions (butanol, chloroform and ethyl acetate) eluted from column chromatography of ethanol extract were further analyzed for their immunomodulatory properties.
Fig. 1.
HPTLC chromatogram of catechin standard and ethanol extract of Acacia catechu. An Rf of 0.18 was observed for A. catechu ethanol extract which corresponds to the Rf of standard catechin. HPTLC = high-performance thin-layer chromatography; Rf = retardation factor.
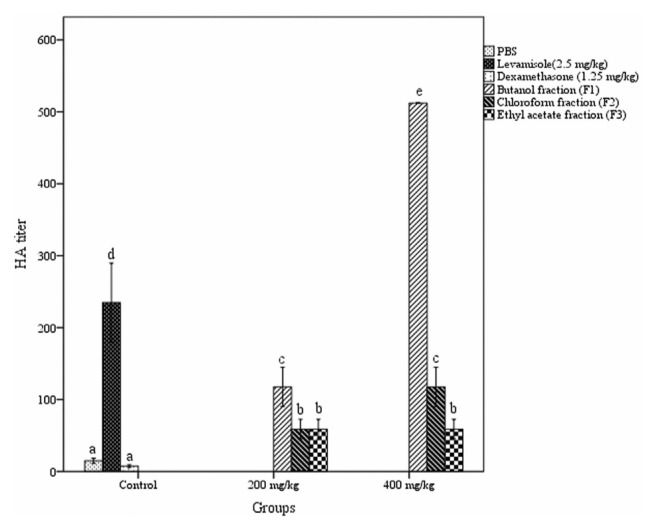
3.2. Hemagglutinating antibody titer
The hemagglutinating antibody titer assay was used to determine the effect of different fractions of A. catechu heartwood on humoral immunity. A dose-dependent rise in antibody titer was detected with butanol and chloroform fraction treated groups compared to control groups (Fig. 2). The butanol fraction when administered orally at a dosage of 400 mg/kg b. w. produced a significant rise in antibody titer (512 ± 0.00) in the blood of mice.
Fig. 2.
Effect of different fractions of Acacia catechu heartwood on HA titer. Values represent mean ± SEM for six animals; values carrying the same alphabet did not vary significantly from each other (Tukey’s HSD; p ≤ 0.05). HA = Hemagglutination; PBS = phosphate buffered saline; SEM = standard error of the mean.
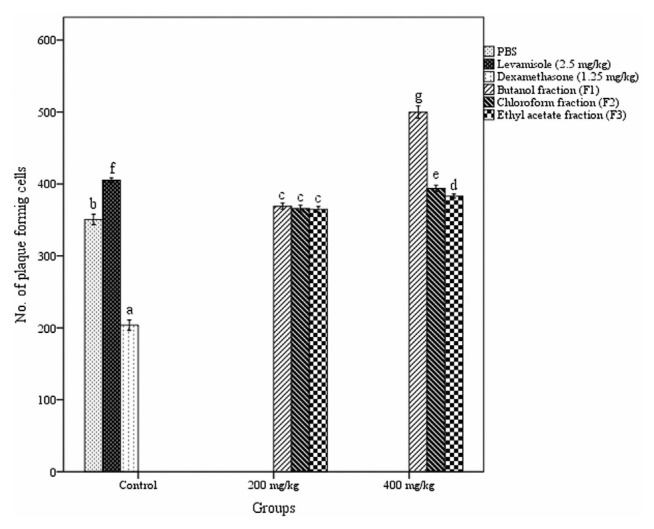
3.3. Effect of fractions on antibody-producing cells in spleen
The humoral immune response was also assessed by plaque-forming cell assay as demonstrated in Fig. 3. The treatment with fractions of A. catechu heartwood significantly augmented the number of antibody-producing cells in the spleen. All three fractions produce a similar effect at a dosage of 200 mg/kg b. w. But at the higher dose (400 mg/kg b. w.) there was a significant variation in the number of plaque forming cells produced by different fractions treated group. The maximal number of plaque forming cells was noticed in butanol fraction administered group (499.67 ± 3.31 PFC/106spleen cells) while the Levamisole administered group had only 405.17 ± 1.30 PFC/106 spleen cells (p < 0.05).
Fig. 3.
Effect of different fractions of Acacia catechu heartwood on antibody-producing cells. Values represent mean ± SEM for six animals; values carrying the same alphabet did not vary significantly from each other (Tukey’s HSD; p ≤ 0.05). PBS = phosphate buffered saline; SEM = standard error of the mean.
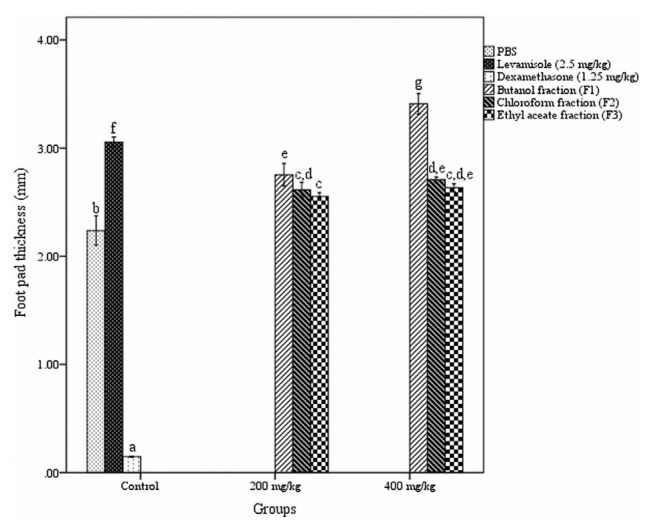
3.4. Delayed-type hypersensitivity reaction
The response of the fractions on T-cell mediated delayed-type hypersensitivity reaction is shown in Fig. 4. DTH response was significantly increased in fractions treated group in comparison to PBS treated (normal control) group. Among the three fractions, only butanol fraction produced a significant, dose-dependent increment in DTH reactivity compared to the other two fractions. Butanol fraction produced a maximum increase in paw thickness (3.41 ± 0.036 mm) at a dosage of 400 mg/kg b.w., after 24 h of induction.
Fig. 4.
Effect of different fractions of Acacia catechu heartwood on DTH assay. Values represent mean ± SEM for six animals; values carrying the same alphabet did not vary significantly from each other (Tukey’s HSD; p ≤ 0.05). DTH = delayed-type hypersensitivity; PBS = phosphate buffered saline; SEM = standard error of the mean.
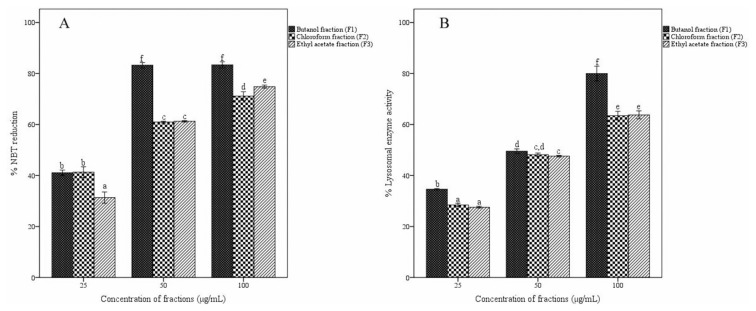
3.5. Effect of fractions on phagocytosis
Effects of different fractions of A. catechu heartwood on the reduction of nitroblue tetrazolium dye and cellular lysosomal enzyme activity in macrophages were established (Fig. 5A and B). The treatment with butanol fraction at 50 and 100 μg/mL showed maximum activity in NBT dye reduction (83%) without a dose–response relationship. The other two fractions also reduced NBT dye but in a dose-dependent manner. All three fractions activated the lysosomal activity. The butanol fraction at a concentration of 100 μg/mL exhibited the maximum activity of 79%, higher than those of chloroform and ethyl acetate fractions.
Fig. 5.
Effect of fractions of Acacia catechu heartwood on in vitro phagocytosis assay. Effect of fractions on (A) NBT reduction and (B) cellular lysosomal enzyme activity. Values represent the mean ± SEM of triplicate independent experiments; and values carrying the same alphabet did not vary significantly from each other (Tukey’s HSD; p ≤ 0.05). NBT = nitro blue tetrazolium; SEM = standard error of the mean.
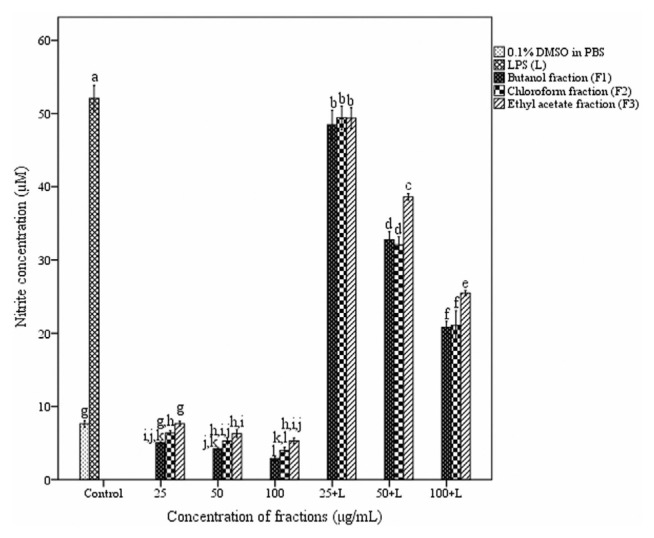
3.6. Assay for NO production
Nitric oxide production by peritoneal macrophages was inhibited by fractions of A. catechu heartwood in a dose-dependent manner (Fig. 6). Lipopolysaccharide (LPS) stimulated macrophages produced nitric oxide up to 52.08 ± 0.41 μM whereas untreated macrophages produced only 7.63 ± 0.11 μM. Nitric oxide production was significantly decreased both in LPS stimulated and unstimulated macrophages treated with fractions. Among the three fractions, butanol and chloroform fractions showed similar inhibitory effect.
Fig. 6.
Effect of different fractions of Acacia catechu heartwood on NO production. Values represent the mean ± SEM of triplicate independent experiments; and values carrying the same alphabet did not vary significantly from each other (Tukey’s HSD; p ≤ 0.05). DMSO = dimethyl sulfoxide; LPS = lipopolysaccharide; NO = nitric oxide; PBS = phosphate buffered saline; SEM = standard error of the mean.
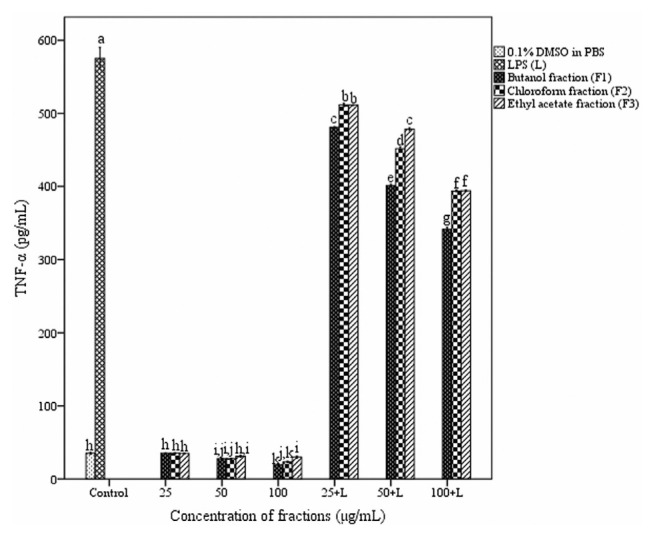
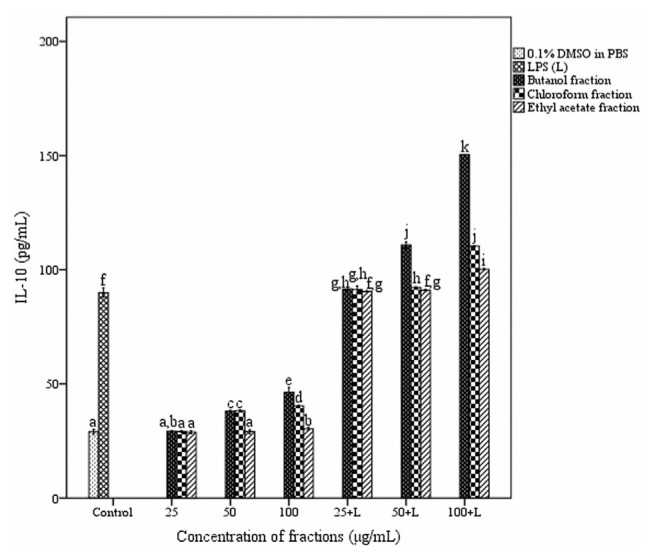
3.7. Assessment of cytokine secretion in macrophages
The effect of fractions of A. catechu heartwood on cytokine release in peritoneal macrophages is depicted in Figs. 7 and 8. Macrophages produced 35.09 ± 0.33 and 575.33 ± 3.5 pg/mL of TNF-α in the absence and presence of LPS respectively. TNF-α secretion by stimulated macrophages was significantly decreased in the presence of all three fractions. Butanol fraction showed more inhibition (341.45 ± 0.63 pg/mL) compared to chloroform and ethyl acetate fractions at a concentration of 100 μg/mL. The treatment with the fractions resulted in an enhancement in the level of IL-10 production in macrophages. But a dose-dependent increase in the IL-10 release was observed only with butanol fraction. At a concentration of 100 μg/mL in the presence of lipopolysaccharide (1 μg/mL) butanol fraction produced 150.35 ± 0.04 pg/mL of IL-10.
Fig. 7.
Effect of different fractions of Acacia catechu heartwood on the secretion of TNF-α in peritoneal macrophages. Values represent the mean ± SEM of triplicate independent experiments; and values carrying the same alphabet did not vary significantly from each other (Tukey’s HSD; p ≤ 0.05). DMSO = dimethyl sulfoxide; LPS = lipopolysaccharide; PBS = phosphate buffered saline; SEM = standard error of the mean; TNF = tumor necrosis factor.
Fig. 8.
Effect of different fractions of Acacia catechu heartwood on the secretion of IL-10 in peritoneal macrophages. Values represent the mean ± SEM of triplicate independent experiments; and values carrying the same alphabet did not vary significantly from each other (Tukey’s HSD; p ≤ 0.05). DMSO = dimethyl sulfoxide; IL = interleukin; LPS = lipopolysaccharide; PBS = phosphate buffered saline; SEM = standard mean of the error.
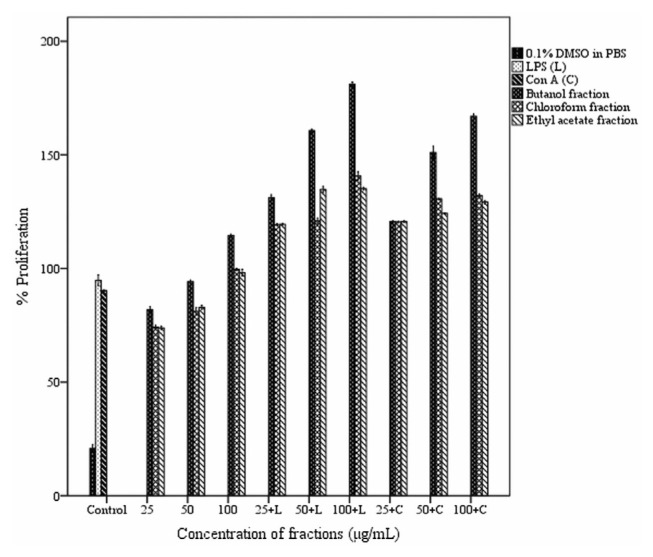
3.8. Effect of fractions on mouse splenocyte proliferation
Effect of fractions of ethanol extract on splenocyte proliferation with or without mitogens is shown in Fig. 9. The fractions exhibited a stimulatory effect on cell proliferation with a dose–response relationship. The butanol fraction gave the maximum proliferation enhancement both in the absence and presence of mitogens. In the absence of mitogen, butanol fraction (100 μg/mL) increased proliferation response by 114% (p< 0.05) compared to the normal control. In the presence of the mitogens, LPS and Con A, butanol fraction at a concentration of 100 μg/mL stimulated splenocyte proliferation by 181% and 167% respectively (p < 0.05).
Fig. 9.
Effect of different fractions of Acacia catechu heartwood on in vitro lymphocyte proliferation. Values represent the mean ± SEM of triplicate independent experiments; and values carrying the same alphabet did not vary significantly from each other (Tukey’s HSD; p ≤ 0.05). ConA = concanavalin A; DMSO = dimethyl sulfoxide; LPS = lipopolysaccharide; PBS = phosphate buffered saline SEM = standard mean of the error.
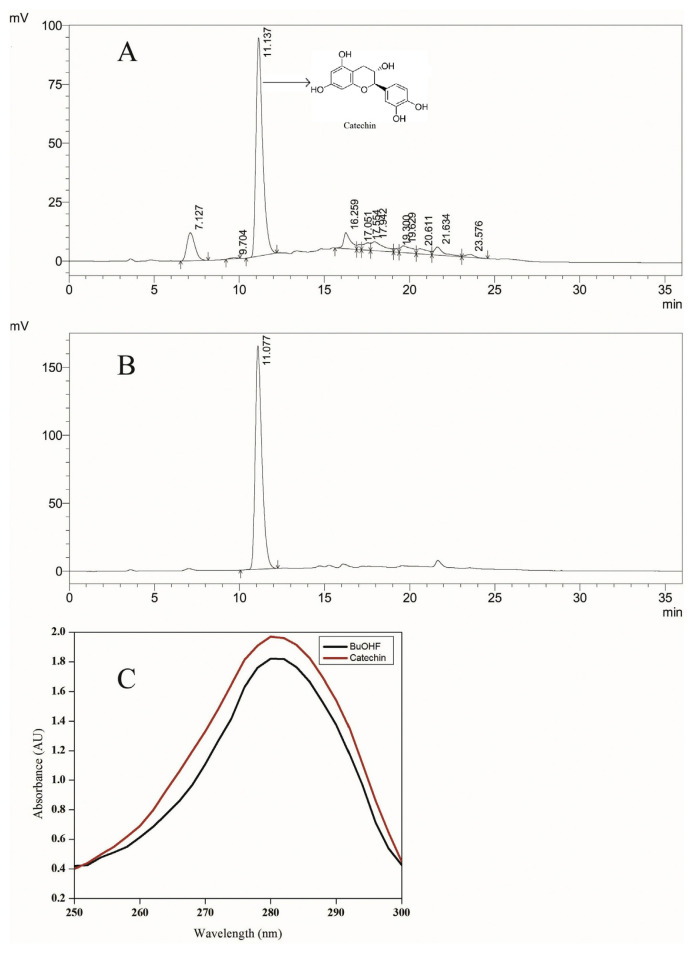
3.9. HPLC and UV-VIS analysis of butanol fraction
The standard compound used for HPLC analysis, catechin has been previously reported in the heartwood of A. catechu [29]. Therefore, it was used for standardization of the butanol fraction of heartwood [7]. The retention time (RT) of the standard compound, catechin with UV detection at 280 nm was 11.077. The identical HPLC condition was used to run the butanol fraction and compared with the standard peak of catechin. The HPLC chromatogram of the butanol fraction exhibited a peak with RT of 11.12 (64.89% area) (Fig. 10A). The retention time of 11.1 min was identical to that of the standard compound, catechin (Fig. 10B). The presence of catechin in the butanol fraction was corroborated by UV spectrometry (250–300 nm) (Fig. 10C). The UV spectra of both butanol fraction and catechin showed maxima at 280 nm, suggesting the existence of catechin in butanol fraction. The linear regression analysis demonstrated that the butanol fraction of ethanol extract of A. catechu heartwood contained 51% of catechin.
Fig. 10.
Standardization of butanol fraction of ethanol extract of Acacia catechu heartwood with catechin. (A) HPLC chromatogram of butanol fraction (BuOH F) of ethanol extract of A. catechu heartwood. (B) HPLC chromatogram of catechin. (C) UV spectrum of catechin and butanol fraction of A. catechu heartwood. AU = arbitrary unit; HPLC = high-performance liquid chromatography; nm = nanometer; UV = ultraviolet.
4. Discussion
Several plants used for food and drink purposes possess medicinal properties and may have a broader physiological role than as simple nutrient sources. Daily consumption of such plants may have some effect on our immunity. Medicinal plants are used as health supplements or alternative therapies for the treatment of diseases. They are often considered safer and are of lower cost than conventional chemotherapeutics [30]. A healthy state is always maintained by the modulation of the immune system through either stimulation or suppression of immune responses. Immunomodulatory herbal medicines that activate host defense system in the presence of a debilitated immune response can serve as a supportive therapy to conventional chemotherapy [9]. They can also be used as immunosuppressive drugs under conditions where immunosuppression is necessary for survival like autoimmune diseases and transplant rejection.
Antibody molecules are the product of B lymphocytes and play a key role in humoral immune responses. IgM and IgG are the major antibodies associated with opsonization, neutralization of toxins, complement activation, etc. The ability of antibody production reflects the capability of immunity in the body. The stimulation of the humoral immune response by fractions of A. catechu heartwood, as indicated by an increase in the antibody titer in mice demonstrated the augmented responsiveness of B and T lymphocyte subsets, involved in the antibody production [9]. The spleen plays a crucial role in developing immune responses to antigens in the bloodstream. It functions as a secondary lymphoid tissue and is responsible for the removal of aging erythrocytes, platelets and some bloodborne pathogens by phagocytosis [8]. Plaque forming cell (PFC) assay was used to demonstrate the number of antibody-producing cells in the spleen. The higher PFC and hemagglutination titer indicated the stimulatory response of butanol fraction of A. catechu heartwood on the humoral immune response.
Cell-mediated immune responses are important in defense against infectious microorganisms, delayed-type hypersensitivity reactions, organ transplantation and tumor immunity. Cell-mediated immunity involves effector mechanisms accomplished by T lymphocytes and their products, lymphokines [9]. DTH response consists of two phases: (a) sensitization phase and (b) effector phase. In sensitization phase, T cells are exposed to an antigen and undergo proliferation and differentiation. The sensitized T cells on second exposure to the same antigen secrete a variety of cytokines and chemokines in the effector phase. These factors recruit macrophages and other inflammatory cells to the site of reaction. Activated macrophages and other cells are involved in developing a defensive inflammatory response [31]. Hence delayed-type hypersensitivity reaction was used to assess the cell-mediated immune response. Enhancement of delayed-type hypersensitivity reaction in mice in response to T cell-dependent antigen explained the stimulatory effect of different fractions of A. catechu heartwood on T cells.
Phagocytosis is a mechanism by which certain body cells, known as phagocytes, ingest and removes pathogenic microorganisms, tissue debris, malignant cells, and inorganic particles from the body [9]. Macrophage-mediated phagocytosis is considered as one of the major indicators in the body’s innate immunity. Phagocytosis and the killing of invading pathogenic microorganisms by macrophages establish the body’s fundamental line of defense against infectious diseases [32,33]. Macrophages act as phagocytic, microbicidal and tumoricidal effector cells through interaction with lymphocytes and play a significant role in the induction and regulation of immune response. Many herbal extracts have been reported to exhibit an important effect on nonspecific immunity especially macrophages function [34]. Phagocytosis of microorganisms by macrophage is generally accompanied by respiratory burst, a burst of the oxidative metabolism leading to the rapid release of reactive oxygen species (ROS). These reactive oxygen species especially superoxide anion can be detected through nitroblue tetrazolium reduction assay and thus confirming the intracellular killing property of phagocytosing macrophage [35]. Reduction of NBT in nitroblue tetrazolium reduction assay represents the activity of NADPH oxidase enzyme thereby displaying the stimulation of phagocytes [24]. NADPH oxidase enzyme plays an essential role in the generation of ROS such as superoxide radical which are involved in the intracellular killing of pathogens by phagocytes. Lysosomal enzymes secreted by macrophages may be crucial in their ability to kill bacteria and tumor cells. There is a gradual increase in lysosomes and their hydrolytic content as macrophage matures [36]. The cellular lysosomal enzyme (CLE) assay is used to analyze acid phosphatase secretion in macrophages. The acid phosphatase associated with the membrane of treated macrophages transforms p-nitrophenyl phosphate to a colored compound, indicating the effect of fractions on degranulation process of macrophages [25]. In the present investigation lysosomal enzyme activity significantly increased after treatment with different fractions. This also represents a stimulatory effect on phagocytes. The treatment with fractions of A. catechu heartwood increased the phagocytic activity of macrophages and this might be attributed to the nonspecific stimulatory effect of fractions on macrophage [37].
Nitric oxide (NO) is an important inflammatory mediator. A small amount of NO is required for many physiological functions. Overproduction of NO may lead to inflammatory diseases. The reduction of nitric oxide production is important in three conditions. First, the increased amounts of nitric oxide produced by stimulated macrophages may be responsible not only for immune defense against pathogenic microorganisms and tumor cells but also may induce tissue damage. A higher level of NO and TNF-α is involved in endotoxic shock and sepsis during an inflammatory response [38,39]. Second, the simultaneous production of NO and superoxide by macrophages resulted in the formation of peroxynitrite. It is a potent oxidant, causes varied chemical reactions in biological systems such as lipid peroxidation and develops oxidative damage in macromolecules and tissues. Third, the nitric oxide or their derivatives and free radical are the key molecules involved in carcinogenesis. Unique properties exhibited by peroxynitrite such as DNA-strand breakage and guanine nitration, protein nitration, may produce a cytotoxic and mutagenic effect. The events involved in chronic inflammation may lead to carcinogenesis. Thus, mediation against nitric oxide overproduction in chronic inflammation could prevent the development of tumors [40]. The release of NO was significantly inhibited in LPS stimulated macrophages treated with different fractions of A. catechu heartwood. This indicates the anti-inflammatory effect of the plant.
Mediators, such as pro-inflammatory cytokines, including interleukins IL-1, IL-6, IL-12, IL-18, tumor necrosis factor (TNF)-α, interferon (INF)-γ and the granulocyte–macrophage colony-stimulating factor (GM-CSF), are produced during an inflammatory response which is opposed by anti-inflammatory cytokines, such as IFN-α, IL-4, IL-10, IL-13 and the transforming growth factor (TGF) [41]. Tumor necrosis factor-α, a pleiotropic cytokine and a strong mediator of inflammatory response, is secreted by monocytes and macrophages. It is involved in many of the clinical dilemma related to autoimmune disorders such as rheumatoid arthritis, inflammatory bowel diseases and psoriasis [42]. The fundamental mechanism of anti-inflammatory activity of several herbal extracts and their role in immunomodulation involve inhibition of TNF-α production and scavenging activity of free radicals [43]. Interleukin-10 plays a significant role in immunoregulation and inflammation. IL-10 enhances proliferation of B lymphocytes, thymocytes and mast cells and downregulates the production of IL-1β, IL-6, IL-12, TNF-α, IFN-γ, and NO [44,45]. It can block the activity of NF-kB, a major transcription factor that regulates genes involved in inflammation. The lipopolysaccharide-stimulated macrophage is a standard model for examining the effect of anti-inflammatory drugs or herbs [41]. Lipopolysaccharide induces progressive secretion of pro-inflammatory cytokines including IL-1β, TNF-α, IL-6, IL-12, and NO; at the same time, it also triggers the production of anti-inflammatory cytokines such as IL-10 [45]. In this study, the IL-10 level was increased by treatment with butanol fraction. All three fractions inhibited the secretion of TNF-α both in the presence and absence of LPS. Several herbal extracts and compounds exhibit anti-inflammatory activity by augmenting anti-inflammatory IL-10 production and decreasing pro-inflammatory TNF-α production [41]. Kuo et al. [46] reported the anti-inflammatory effect of water extracts from Formosan sambar deer and red deer by inhibiting TNF-α secretion and increasing IL-10 in LPS-stimulated RAW 264.7 macrophages. The IL-10 augmenting activity of butanol fraction as observed in the study may be responsible for the down-regulating effect of the fraction on TNF-α and NO production.
The MTT assay is a method used for studying cell viability and proliferation. All living, metabolically active cells convert MTT into insoluble, colored formazan crystals. The amount of formazan produced is directly proportional to the cell number [27]. In the MTT assay for splenocyte proliferation LPS was used as a mitogen for the proliferation of B cell whereas Con A was used for T cell proliferation. The type of mitogen present in the system can suggest the probable activation pathway of the fractions [47]. In the present study, all three fractions stimulated splenocyte proliferation both in the absence and presence of mitogens. The maximum proliferative effect was observed in the presence of LPS which is involved in B cell proliferation. The result obtained in this investigation showed the stimulatory effect of different fractions of A. catechu heartwood on splenocyte viability and proliferation.
Catechin is a natural phenolic compound and antioxidant. It exists in different forms such as (+)-catechin, (−)-epicatechin, (−)-epigallocatechin, (+)-gallocatechin; and their gallate derivatives. They are a universal constituent of vascular plants and normal ingredient of traditional herbal medicine. The primary constituents of A. catechu heartwood are catechin. Catechins are belonging to flavan-3-ol subclass of flavonoids. Flavan-3-ol compounds have been reported to exhibit anti-inflammatory properties. The levels of proinflammatory cytokines and biomarkers of oxidative stress are decreased with higher intakes of polymeric flavan-3-ols in humans [48]. Catechins may have a stimulatory effect on endothelium-dependent vasodilation in humans contributing towards the normal blood flow control [49]. Shen et al. [4] used liquid chromatography coupled with electrospray ionization mass spectrometry for the determination of predominant catechins in A. catechu. Recently Tung et al. [50] used HPLC-UV-ESI-MS for the qualitative and quantitative analysis of a fungal immunomodulatory protein, FIP-fve from Flammunlina velutipes. In the present study, the total catechin content in butanol fraction of A. catechu heartwood was quantified by HPLC analysis. The results obtained from HPLC quantification showed that fifty-one percent of butanol fraction contained catechin and also revealed the possible role of catechin in the immunostimulatory potential of the plant.
The present study reveals the immunostimulatory potential of catechin-rich butanol fraction extracted from A. catechu heartwood. To the best of our knowledge, this is the first of such a report. Though A. catechu is a plant well-known for antipyretic, anti-inflammatory, antidiarrheal, hypoglycemic, hepatoprotective, and antimicrobial activities [51] the immunostimulatory potential of this plant has not been studied in detail earlier. Hence the results of the present study are novel and will find application in the studies related to natural immunostimulatory agents.
5. Conclusions
In conclusion, the present investigations with three different fractions of ethanol extract of A. catechu heartwood have proven this plant to be an effective immunostimulant. Therefore daily consumption of decoction of this plant as a thirst quencher has a beneficial effect on the immune system. The present investigation ratifies the practice of traditional use of A. catechu as a thirst quencher in some parts of India, which can be popularized in other parts of the world also. We found that catechin-rich butanol fraction displayed various modulating effects on the functions of the immune system in comparison with control drug in mice. It stimulated different cells of the immune system. Though catechin can be supposed to be the active compound responsible for immunomodulatory effects, we are in the process of determination of other unidentified compounds in the fraction and their effects on the immune system. Further purification and characterization of the active compound may lead to the discovery of an immunomodulatory drug which can replace conventional chemotherapeutic agents. The present results also indicate its possible use as an ingredient in the immunostimulatory herbal preparations.
Acknowledgement
This study was supported financially by University Grants Commission (UGC), New Delhi, India (F. No.41-78/2012).
Funding Statement
This study was supported financially by University Grants Commission (UGC), New Delhi, India (F. No.41-78/2012).
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
REFERENCES
- 1. Ismail S, Asad M. Immunomodulatory activity of Acacia catechu. Indian J Physiol Pharmacol. 2009;53:25–33. [PubMed] [Google Scholar]
- 2. Stohs SJ, Bagchi D. Antioxidant, anti-inflammatory, and chemoprotective properties of Acacia catechu heartwood extracts. Phyther Res. 2015;29:1–7. doi: 10.1002/ptr.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williamson EM. Major herbs of Ayurveda. London: Churchill Livingstone; 2002. pp. 13–5. [Google Scholar]
- 4. Shen D, Wu Q, Wang M, Yang Y, Lavoie EJ, Simon JE. Determination of the predominant catechins in Acacia catechu by liquid chromatography/electrospray ionization-mass spectrometry. J Agric Food Chem. 2006;54:3219–24. doi: 10.1021/jf0531499. [DOI] [PubMed] [Google Scholar]
- 5. Li X-C, Liu C, Yang L-X, Chen R-Y. Phenolic compounds from the aqueous extract of Acacia catechu. J Asian Nat Prod Res. 2011;13:826–30. doi: 10.1080/10286020.2011.597384. [DOI] [PubMed] [Google Scholar]
- 6. Deepak Hiraganahalli Bhaskarmurthy, Chandrasekaran Chinampudur Velusami, Dethe Shekhar, Deepak Mundkinajeddu, Pandre Manoj Kumar, Balachandran Jaya AA. Hepatoprotective and antioxidant activity of standardized herbal extracts. Pharmacogn Mag. 2012;8:116. doi: 10.4103/0973-1296.96553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Devi VG, John A, Devi RS, Prabhakaran VA. Pharmacognostical studies on Acacia catechu Willd and identification of antioxidant principles. Int J Pharm Pharm Sci. 2011;3:108–11. [Google Scholar]
- 8.Delves PJ, Martin SJ, Burton DR, Roitt IM. Roitt’s essential immunology. 12th ed. 2011. pp. 4–5. [Google Scholar]
- 9. Ghule BV, Murugananthan G, Nakhat PD, Yeole PG. Immunostimulant effects of Capparis zeylanica Linn. leaves. J Ethnopharmacol. 2006;108:311–5. doi: 10.1016/j.jep.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 10. Thatte UM, Dahanukar SA. Ayurveda and contemporary scientific thought. Trends Pharmacol Sci. 1986;7:247–51. doi: 10.1016/0165-6147(86)90336-6. [DOI] [Google Scholar]
- 11.Wagner H, editor. Immunomodulatory agents from plants. Basel AG: Springer; 1999. [DOI] [Google Scholar]
- 12. Dahanukar SA, Thatte UM. Current status of ayurveda in phytomedicine. Phytomedicine. 1997;4:359–68. doi: 10.1016/S0944-7113(97)80048-7. [DOI] [PubMed] [Google Scholar]
- 13.Wagner H, Hikino H, Farnsworth NR, editors. Economic and medicinal plant research. Vol. 3. Academic Press; 1989. pp. 15–6. [Google Scholar]
- 14. Saravanan S, Prakash Babu N, Pandikumar P, Karunai Raj M, Gabriel Paulraj M, Ignacimuthu S. Immunomodulatory potential of Enicostema axillare (Lam.) A. Raynal, a traditional medicinal plant. J Ethnopharmacol. 2012;140:239–46. doi: 10.1016/j.jep.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 15. Roux S, Sablé E, Porsolt RD. Primary observation (Irwin) test in rodents for assessing acute toxicity of a test agent and its effects on behavior and physiological function. Curr Protoc Pharmacol. 2004:10.10.1–10.10.23. doi: 10.1002/0471141755.ph1010s27. [DOI] [PubMed] [Google Scholar]
- 16. Oliveira HC, dos Santos MP, Grigulo R, Lima LL, Martins DTO, Lima JCS, et al. Antidiabetic activity of Vatairea macrocarpa extract in rats. J Ethnopharmacol. 2008;115:515–9. doi: 10.1016/j.jep.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 17. George A, Chinnappan S, Choudhary Y, Bommu P, Sridhar M. Immunomodulatory activity of an aqueous extract of Polygonum minus Huds on Swiss albino mice using carbon clearance assay. Asian Pacific J Trop Dis. 2014;4:398–400. doi: 10.1016/S2222-1808(14)60594-6. [DOI] [Google Scholar]
- 18. Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008:14.1.1–14.1.14. doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gautam M, Saha S, Bani S, Kaul A, Mishra S, Patil D, et al. Immunomodulatory activity of Asparagus racemosus on systemic Th1/Th2 immunity: implications for immunoadjuvant potential. J Ethnopharmacol. 2009;121:241–7. doi: 10.1016/j.jep.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 20. Bin-Hafeez B, Ahmad I, Haque R, Raisuddin S. Protective effect of Cassia occidentalis L. on cyclophosphamide-induced suppression of humoral immunity in mice. J Ethnopharmacol. 2001;75:13–8. doi: 10.1016/s0378-8741(00)00382-2. doi:S0378-8741(00)00382-2. [DOI] [PubMed] [Google Scholar]
- 21. Davis L, Kuttan G. Immunomodulatory activity of Withania somnifera. J Ethnopharmacol. 2000;71:193–200. doi: 10.1016/s0378-8741(99)00206-8. doi:S0378-8741(99)00206-8. [DOI] [PubMed] [Google Scholar]
- 22. Jerne NK, Nordin AA. Plaque formation in agar by single antibody-producing cells. Science. 1963;140:405. [PubMed] [Google Scholar]
- 23. Raisuddin S, Zaidi SI, Singh KP, Ray PK. Effect of subchronic aflatoxin exposure on growth and progression of Ehrlich’s ascites tumor in mice. Drug Chem Toxicol. 1991;14:185–206. doi: 10.3109/01480549109017876. [DOI] [PubMed] [Google Scholar]
- 24. Rainard P. A colorimetric microassay for opsonins by reduction of NBT in phagocytosing bovine polymorphs. J Immunol Meth. 1986;90:197–201. doi: 10.1016/0022-1759(86)90076-1. [DOI] [PubMed] [Google Scholar]
- 25. Suzuki I, Tanaka H, Yoshiyuki A, Yadomae T. Rapid Measurement of phagocytosis by macrophages. Chem Pharm Bull. 1988;36:4871–5. doi: 10.1248/cpb.36.4871. [DOI] [PubMed] [Google Scholar]
- 26. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 27. Mosmann T. Rapid colorimetric assay for cellular growth and Survival: application to proliferation and cytotoxicity assays. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. 0022-1759/83/$03.00. [DOI] [PubMed] [Google Scholar]
- 28. Jain PK, Soni A, Jain P, Bhawsar J. Research Article Phytochemical analysis of Mentha spicata plant extract using UV-VIS, FTIR and GC/MS technique. J Chem Pharm Res. 2016;8:1–6. [Google Scholar]
- 29. Liu L, Castonguay A. Inhibition of the metabolism and genotoxicity of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in rat hepatocytes by (+)-catechin. Carcinogenesis. 1991;12:1203–8. doi: 10.1093/carcin/12.7.1203. [DOI] [PubMed] [Google Scholar]
- 30.Ahmad I, Aqil F, Owais M. Modern phytomedicine. Weinheim: WILEY-VCH Verlag GmbH & Co. KGaA; 2006. pp. 3–4. [Google Scholar]
- 31. Bhalerao BM, Kasote DM, Nagarkar BE, Jagtap SD, Vishwakarma KS, Pawar PK, et al. Comparative analysis of radical scavenging and immunomodulatory activities of Tinospora cordifolia growing with different supporting trees. Acta Biol Szeged. 2012;56:65–71. [Google Scholar]
- 32. Hawiger J. Innate immunity and inflammation: a transcriptional paradigm. Immunol Res. 2001;23:99–109. doi: 10.1385/IR:23:2-3:099. [DOI] [PubMed] [Google Scholar]
- 33. Hume DA, Ross IL, Himes SR, Sasmono RT, Wells CA, Ravasi T. The mononuclear phagocyte system revisited. J Leukoc Biol. 2002;72:621–7. [PubMed] [Google Scholar]
- 34. Song X, Li CY, Zeng Y, Wu HQ, Huang Z, Zhang J, et al. Immunomodulatory effects of crude phenylethanoid glycosides from Ligustrum purpurascens. J Ethnopharmacol. 2012;144:584–91. doi: 10.1016/j.jep.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 35. Suja RS, Nair AMC, Sujith S, Preethy J, Deepa AK. Evaluation of immunomodulatory potential’ of Emblica officinalis fruit pulp extract in mice. Indian J Anim Res. 2009;43:103–6. [Google Scholar]
- 36. Bishayi B, Roychowdhury S, Ghosh S, Sengupta M. Hepatoprotective and immunomodulatory properties of Tinospora cordifolia in CCl4 intoxicated mature albino rats. J Toxicol Sci. 2002;27:139–46. doi: 10.2131/jts.27.139. [DOI] [PubMed] [Google Scholar]
- 37. Yu KW, Shin KS, Choi YM, Suh HJ. Macrophage stimulating activity of exo-biopolymer from submerged culture of Lentinus edodes with rice bran. J Microbiol Biotechnol. 2004;14:658–64. [Google Scholar]
- 38. Chi DS, Qui M, Krishnaswamy G, Li C, Stone W. Regulation of nitric oxide production from macrophages by lipopolysaccharide and catecholamines. Nitric Oxide. 2003;8:127–32. doi: 10.1016/S1089-8603(02)00148-9. [DOI] [PubMed] [Google Scholar]
- 39. Ryu JH, Ahn H, Lee HJ. Inhibition of nitric oxide production on LPS-activated macrophages by kazinol B from Broussonetia kazinoki. Fitoterapia. 2003;74:350–4. doi: 10.1016/S0367-326X(03)00062-5. [DOI] [PubMed] [Google Scholar]
- 40. Napolitano DR, Mineo JR, De Souza MA, De Paula JE, Espindola LS, Espindola FS. Down-modulation of nitric oxide production in murine macrophages treated with crude plant extracts from the Brazilian Cerrado. J Ethnopharmacol. 2005;99:37–41. doi: 10.1016/j.jep.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 41. Mueller M, Hobiger S, Jungbauer A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010;122:987–96. doi: 10.1016/j.foodchem.2010.03.041. [DOI] [Google Scholar]
- 42. Pandey R, Maurya R, Singh G, Sathiamoorthy B, Naik S. Immunosuppressive properties of flavonoids isolated from Boerhaavia diffusa Linn. Int Immunopharmacol. 2005;5:541–53. doi: 10.1016/j.intimp.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 43. Sandoval M, Charbonnet RM, Okuhama NN, Roberts J, Krenova Z, Trentacosti AM, et al. Cat’S claw inhibits TNFα production and scavenges free radicals: role in cytoprotection. Free Radic Biol Med. 2000;29:71–8. doi: 10.1016/s0891-5849(00)00327-0. [DOI] [PubMed] [Google Scholar]
- 44. Gru G. New insights into the molecular mechanism of interleukin-10-mediated immunosuppression. J Leukoc Biol. 2005;77:3–15. doi: 10.1189/jlb.0904484.Journal. [DOI] [PubMed] [Google Scholar]
- 45. Li F, Wang HD, Lu DX, Wang YP, Qi RB, Fu YM, et al. Neutral sulfate berberine modulates cytokine secretion and increases survival in endotoxemic mice. Acta Pharmacol Sin. 2006;27:1199–205. doi: 10.1111/j.1745-7254.2006.00368.x. [DOI] [PubMed] [Google Scholar]
- 46. Kuo CY, Cheng YT, Ho ST, Yu CC, Chen MJ. Comparison of anti-inflammatory effect and protein profile between the water extracts from Formosan sambar deer and red deer. J Food Drug Anal. 2018:4–11. doi: 10.1016/j.jfda.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nakamura A, Nagai K, Suzuki S, Ando K, Tamura G. A novel method of screening for immunomodulating substances, establishment of an assay system and its application to culture broths of microorganisms. J Antibiot (Tokyo) 1986;39:1148–54. doi: 10.7164/antibiotics.39.1148. [DOI] [PubMed] [Google Scholar]
- 48. Cassidy A, Rogers G, Peterson JJ, Dwyer JT, Lin H, Jacques PF. Higher dietary anthocyanin and flavonol intakes are associated with anti-inflammatory effects in a population of US adults. Am J Clin Nutr. 2015;102:172–81. doi: 10.3945/ajcn.115.108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, et al. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials 1 – 3. Am J Clin Nutr. 2012;95:740–51. doi: 10.3945/ajcn.111.023457. [DOI] [PubMed] [Google Scholar]
- 50. Tung CH, Lin CC, Wang HJ, Chen SF, Sheu F, Lu TJ. Application of thermal stability difference to remove flammutoxin in fungal immunomodulatory protein, FIP-fve, extract from Flammulina velutipes. J Food Drug Anal. 2018:1–10. doi: 10.1016/j.jfda.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lakshmi TL, Aravind kumar S. Preliminary phytochemical analysis & in vitro antibacterial activity of Acacia catechu willd bark against streptococcus mitis, streptococcus sanguis & Lactobacillus acidophilus. Int J Phytomed. 2011;3:579–84. [Google Scholar]