Abstract
CCM111 is an aqueous extract of Antrodia cinnamomea (AC) that has exhibited anti-liver fibrosis functions. However, the detailed mechanisms of AC action against liver fibrosis have not been elucidated yet. The present research showed that CCM111 significantly lowered the levels of the hepatic enzyme markers glutamate oxaloacetate transaminase (GOT) and glutamic pyruvic transaminase (GPT), prevented liver damage and collagen deposition, and downregulated TGF-β/Smad signaling in a dose-dependent manner compared with CCl4 treatment alone. CCM111 markedly inhibited TGF-β, Wnt and STAT3 signaling pathway-regulated downstream genes in the liver by next-generation sequencing. The antifibrotic mechanisms of CCM111 were further demonstrated in HSC-T6 cells. Our data demonstrated for the first time that CCM111 can protect against CCl4-induced liver fibrosis by the cooperative inhibition of TGF-β-, Wnt- and STAT3-dependent proinflammatory and profibrotic mediators, suggesting that CCM111 might be a candidate for preventing and treating chronic fibrotic liver diseases.
Keywords: Antrodia cinnamomea, Liver fibrosis, STAT3 pathway, TGF-β pathway, Wnt pathway
1. Introduction
Liver fibrosis is a chronic liver disease caused by many stimulatory factors; the production of excessive extracellular matrix (ECM) proteins results in the formation of scar tissue that affects liver function and results in liver cirrhosis [1]. During the progression of liver fibrosis, the activation of hepatic stellate cells (HSCs) plays a critical role in the excessive production of ECMproteins, including type I collagen, type III collagen and alpha-smooth muscle actin (α-SMA); these proteins are recognized as fibrosis markers and are involved in a series of inflammatory and fibrotic processes [2]. Therefore, reducing the activation of HSCs, inducing the apoptosis of HSCs or decreasing the accumulation of ECM proteins are considered antifibrotic methods or methods that reverse liver fibrosis [3].
TGF-β is a key mediator in liver fibrosis through the activation of HSCs and fibroblasts to generate ECM proteins [3]. The TGF-β pathway regulates the activation of the MAPK pathway to promote the activation of HSCs [4]. In addition to TGF-β signaling, signal transducer and activator of transcription 3 (STAT3) also plays an important role in inflammatory responses through inducing a variety of proinflammatory cytokines and chemokines [5,6]. Furthermore, STAT3 inhibition ameliorates CCl4-induced liver fibrosis in vivo [7]. Recently, Wnt signaling has been implicated in liver fibrosis progression since the TGF-β pathway regulates the activation of the Wnt pathway through downregulating the Wnt pathway antagonist Dkk-1 and activating HSCs [8]. Reducing the activation of the Wnt pathway attenuated liver fibrosis progression in vitro and in vivo [9].
Carbon tetrachloride (CCl4), a hepatotoxin, is the most commonly used liver carcinogen inmodels that mimic human liver fibrosis and lesions in mice and rats [10]. Furthermore, CCl4 induces the activation of Kupffer cells, to produce the proinflammatory cytokines that also promote the activation of HSCs [11].
Antrodia cinnamomea (also known as Antrodia camphorata and Taiwanofungus camphorata; AC) is a Polyporaceae fungus indigenous to Taiwan. AC is currently used as a food supplement for hepatic protection [12]. Previous studies have indicated that AC from different preparations reduced CCl4- and TAA-induced liver fibrosis in rats [13–16]. Although a reduction in liver fibrosis markers affected by AC has been observed, the detailed mechanisms of AC action against liver fibrosis have not been elucidated yet. In our previous study, we analyzed the CCM111, an aqueous extract of AC, by HPLC-UV and LC/MS and found that CCM111 inhibited the inflammation response through reducing the activity of STAT3 and NF-κB pathway [17]. Inflammation is one of the important factor in liver fibrosis progression [18]. In this study, we investigated the effects of CCM111 on the progression of liver fibrosis in vitro and in vivo.
Our results indicated that CCM111 reduced the progression of CCl4-induced liver fibrosis through inhibition of the TGF-β1/Smad signaling pathway and ECM protein expression. Furthermore, we revealed by the next-generation sequencing (NGS) analysis that CCM111 decreased the expression of genes downstream of Wnt, TGF-β, and STAT3, the levels of several proinflammatory cytokines and the production of ECM proteins. CCM111 significantly inhibited the TGF-β1-mediated expression phosphorylated of Smad2, Smad3, and STAT3, α-SMA, MMP2 proteins and the translocation of β-catenin in HSC-T6 cells. Therefore, CCM111 has hepatoprotective effects in CCl4-induced liver fibrosis and has a potential application in clinical interventions.
2. Material and methods
2.1. Animals and treatments
Thirty-two male Bltw:CD1 (ICR) mice, weighing 20–22 g, were purchased from BioLASCO (Taipei, Taiwan). The ICR mice were randomly divided into five groups: Group 1: Normal (corn oil only); Group 2: CCl4 (CCl4-treated only); Group 3: SIL (CCl4 plus silymarin 200 mg/kg); Group 4: L-CCM111 (CCl4 plus silymarin 20 mg/kg); and Group 5: H-CCM111 (CCl4 plus CCM111 100 mg/kg). The CCl4-treated animals were administered 40% CCl4/corn oil (0.1 ml per 100 g body weight) via intraperitoneal (i.p.) injection twice a week for eight consecutive weeks. Animals in the normal group were given an equal volume of corn oil and ddH2O. The SIL group was given CCl4 plus silymarin at a dose of 200 mg/kg by oral gavage daily. Two of the CCl4 groups were given either CCM111 at a dose of either 20 mg or 100 mg per kg body weight by oral gavage daily (Fig. S1).
2.2. Biochemical analysis of liver function
Serum was separated from the animal blood samples by centrifugation at 3000 rpm for 10 min. Serum levels of aspartate transaminase (GOT), alanine transaminase (GPT), triacylglycerol (TG), and total cholesterol (TCHO) were measured on Fujifilm Dri-Chem slides (Fujifilm, Kanagawa, Japan), and the level of each biochemical indicator was determined using a blood biochemistry analyzer (Fujifilm Dri-Chem 3500s; Fujifilm, Kanagawa, Japan). The liver sections (3 μm) were stained with hematoxylin–eosin (H&E) stain and Sirius Red/Fast Green staining. All protocols for assessing liver function were conducted according to the manufacturer’s instructions.
2.3. Chemicals
Carbon tetrachloride (CCl4) was purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan). All other chemicals used in this study were of the highest grade of purity and were purchased from Sigma, Inc. (St. Louis, MO, USA).
2.4. Preparation of the crude water extract
The A. cinnamomea mycelial culture broth was concentrated under vacuum and freeze-dried to a powder form. The preparation protocol, HPLC-UV and LC/MS analysis were performed in accordance with that of a previous study [17].
2.5. Cells and toxicity assay
HSC-T6 cells were cultured in Waymouth’s medium with 10% fetal bovine serum, 1% penicillin/streptomycin and 1% l-glutamine in a humidified atmosphere at 5% CO2 and 37 °C. The cells were plated in 96-well plates and treated with different concentrations of CCM111 (0, 5, 10, 30, 60, 120, and 160 μg/ml). After 24 h, the cell toxicity was determined by Alamar Blue assays (Invitrogen Life Technologies, NY, USA) according to the manufacturer’s instructions. The fluorescence values were measured at excitation wavelengths of 530–560 nm and an emission wavelength of 590 nm. All values were measured using a Synergy HT microplate reader (BioTek, VT, USA).
2.6. Western blotting
Liver tissue from each rat was homogenized individually, and total protein was extracted from the tissue using ice-cold Gold lysis buffer. Protein on the gel was separated on the SDS-PAGE and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The α-SMA and matrix metalloprotease 2 (MMP2) antibodies were obtained from Abcam (Cambridge, UK). The β-actin antibody was from Oncogene Science (Oncogene Science Inc., Uniondale, NJ). TGF-β1 was purchased from Sigma, Inc. (Sigma–Aldrich, MO, USA). p-Smad2 (Ser465/467), p-Smad3 (Ser423/425), p-Stat3, Stat3, β-Catenin, Smad3, and TGF-β1 antibodies were purchased from Cell Signaling Technology (Cell Signaling, MA, USA). α-SMA and MMP2 antibodies were purchased from Abcam (Abcam. MA, USA).
2.7. Hematoxylin–eosin staining and Sirius Red/Fast Green staining
For histological examination, mouse liver sections were fixed in 10% formalin for 48 h and embedded in paraffin. The sections (4 mm) were stained with hematoxylin–eosin stain (H&E) and Sirius Red/Fast Green stain and analyzed under a microscope.
2.8. Next-generation sequencing (NGS)
mRNA profiling was performed by NGS. Total RNA was isolated from the mouse liver sections with TRIzol reagent (Invitrogen, USA). The total RNA was sent to Genomics BioSci & Tech. (Genomics, New Taipei City, Taiwan) for RNA-seq analysis. The criteria for the Gene Ontology (GO) analysis were reads per kilobase per million mapped reads (RPKM) > 3.5 and coverage > 50% [19]. Read counts obtained from NGS were normalized as RPKM to compare the gene expression levels of mRNA across samples. The relative fold change of the gene expression was determined by the normalized reads counts, RPKM. The pathways analysis was performed by the Kyoto Encyclopedia of Genes and Genomes (KEGG) [20].
2.9. Subcellular fractionation
The cytoplasmic and nuclear fractions were prepared by the Buffer A (1 mM DTT pH7.9, 0.1 mM EDTA, 10 mM KCL, 10 mM HEPES, and proteinase inhibitor), 9% NP-40, and Buffer B (400 mM NaCl pH7.9, 20 mM HEPES, 1 mM EDTA, 1 mM DTT, and proteinase inhibitor) as previous protocol [17]. The cytoplasmic and nuclear fractions concentration was determined by the BCA assay kit (Pierce). After collection, the fractions were performed for western blotting.
2.10. Statistical analysis
Data are expressed as the mean ± standard deviation. All statistical analyses were generated using SigmaPlot 10.0 (SYSTAT, CA, USA). Image analysis was performed using ImageJ 1.47 (NIH, Bethesda, MD, USA).
3. Results
3.1. Effects of CCM111 on CCl4-treated mice
This experiment was performed to investigate the effects of CCM111 on liver fibrosis progression. The body weights of the CCl4-treated mice were lower than those of the control animals at the end of the study (8 weeks), but there was no significant difference in body weight between the CCl4-treated and the control animals (Fig. S2). The kidney weights of the mice in the CCl4-treated alone group were significantly higher than those of the control animals. The oral administration of silymarin (SIL) at 200 mg/kg was the antifibrosis control for this experiment. Treatment with CCl4 plus SIL decreased the kidney and spleen weight compared with treatment with CCl4 only. The groups receiving oral administration of CCM111 at 20 (L-CCM111) or 100 (H-CCM111) mg/kg exhibited a slightly higher kidney weight than the control group, but the difference was not significant. The L-CCM111 group showed a significantly lower spleen weight compared with the CCl4-only group (Table 1). The GOT, GPT and TCHO levels in serum and the liver injury markers in the CCl4-only group were higher than those in the control group, but no difference was observed in the serum TG levels. The CCM111 and SIL treatment groups demonstrated significantly reduced serum GOT and GPT levels, but there were no effects on the serum TCHO or TG levels (Table 2). These data suggested that CCM111 could reduce CCl4-induced liver injury.
Table 1.
Relative organ weight of CCl4-treated rats with or without CCM111.
| Groups | Relative organ weight (g/of bw) | ||
|---|---|---|---|
|
| |||
| Liver | Kidney | Spleen | |
| Control | 5.09 ± 0.32 | 1.84 ± 0.27 | 0.27 ± 0.06 |
| CCl4 | 5.69 ± 0.76 | 2.10 ± 0.14# | 0.49 ± 0.12# |
| SIL | 5.53 ± 0.93 | 2.03 ± 0.24 | 0.39 ± 0.10 |
| L-CCM111 | 5.57 ± 0.35 | 2.17 ± 0.43 | 0.33 ± 0.05* |
| H-CCM111 | 5.49 ± 0.70 | 2.14 ± 0.35 | 0.38 ± 0.11 |
CCl4 was intraperitoneally given at a dose of 40% twice per week for 8 weeks to each group except control group. The data represent the mean ± SD of six mice.
Significantly different from the control group, P < 0.05.
Significantly different from the group treated with CCl4 alone, P < 0.05.
Table 2.
Effect of CCM111 on the serum parameters in the CCl4-treated mice.
| Groups | Activity | |||
|---|---|---|---|---|
|
| ||||
| GOT (U/L) | GPT (U/L) | TG (mg/dl) | TCHO (mg/dl) | |
| Control | 89.33 ± 47.9 | 26.83 ± 8.52* | 213.50 ± 46.37 | 199.17 ± 40.51 |
| CCl4 | 323.83 ± 188.09# | 262.50 ± 199.21# | 232.17 ± 74.99 | 133.83 ± 26.12# |
| SIL | 70.60 ± 37.87* | 54.60 ± 52.64* | 202.50 ± 43.54 | 125.50 ± 32.46 |
| L-CCM111 | 71.33 ± 9.93* | 71.33 ± 9.93* | 176.67 ± 57.69 | 109.83 ± 19.55 |
| H-CCM111 | 53.00 ± 13.88* | 53.00 ± 13.88* | 191.00 ± 39.22 | 125.20 ± 28.60 |
CCl4 was intraperitoneally given at a dose of 40% twice per week for 8 weeks to each group except control group. The data represent the mean ± SD of six mice.
Significantly different from the control group, P < 0.05.
Significantly different from the group treated with CCl4 alone, P < 0.05.
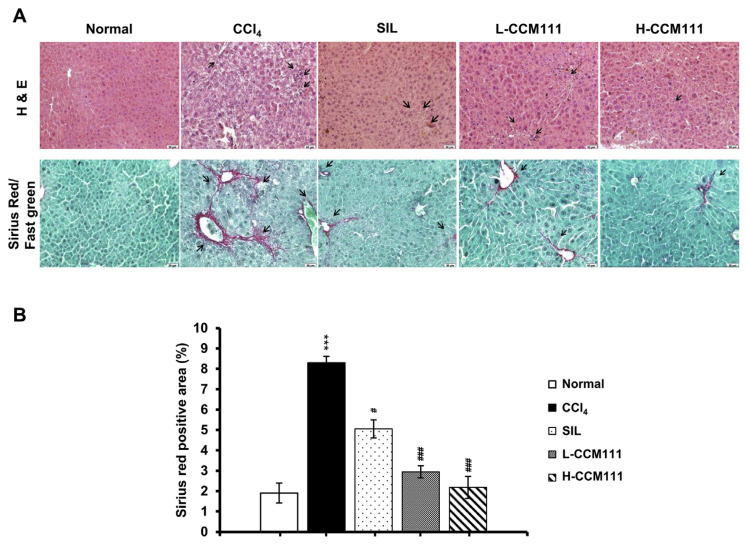
The effects of CCl4-treated liver injury were evaluated by histopathological examination of the liver sections. The liver samples were stained with Sirius Red/Fast Green stain to examine the collagen deposition and with hematoxylin–eosin (H&E) stain to examine the extent of tissue damage. Compared with the control group, the CCl4-only group showed substantial inflammation and collagen deposition. CCM111 or SIL administration markedly reduced collagen deposition (Fig. 1A and B). These results suggested that CCM111 alleviated liver inflammation and fibrosis progression.
Fig. 1.
CCM111 suppressed the liver morphology in CCl4-treated animals. (A) Images of liver tissue sections stained separately with H&E stain and Sirius Red/Fast Green stain. The sections were imaged at 200× magnification. (B) Quantification of Sirius Red/Fast Green staining. All values are expressed as the mean ± SD (n = 6). Student’s t-test: *P-value < 0.05, **P-value < 0.01, ***P-value < 0.001 for Normal vs. CCl4. #P-value < 0.05, ##P-value < 0.01, ###P-value < 0.001, for CCl4 vs. SIL, L-CCM111, or H-CCM111, respectively.
3.2. CCM111 represses the progression of liver fibrosis through reducing activation of the TGF-β pathway
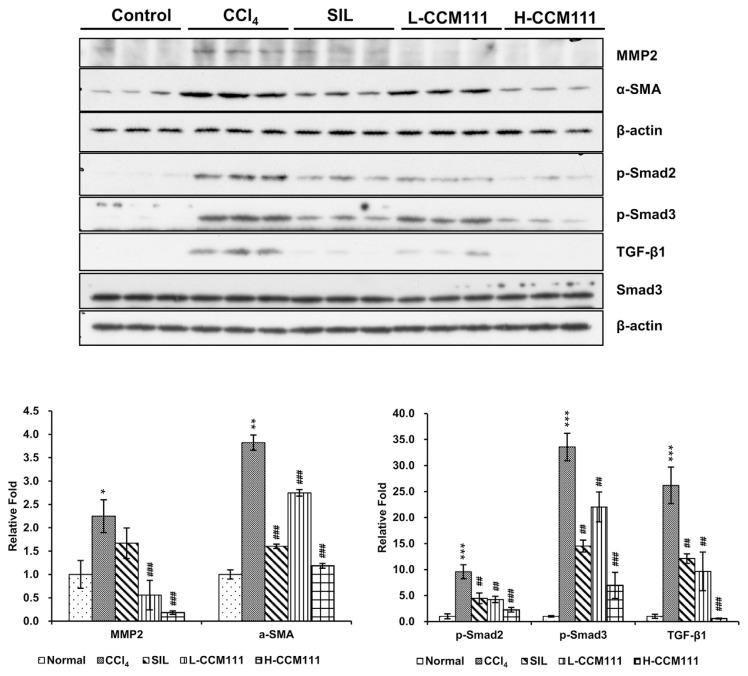
To further examine the mechanism of CCM111 on CCl4-induced liver fibrosis, we analyzed the expression of ECM proteins and proteins in several signaling pathways associated with liver fibrosis. The CCl4-only group had a significantly increased expression of α-SMA and MMP2 compared with the control group, whereas CCM111 treatment significantly reduced the expression of these proteins. These results indicated that CCM111 suppressed the expression of ECM proteins in a dose-dependent manner. CCl4 significantly induced the levels of phosphorylated Smad2, Smad3, and TGF-β1, whereas CCM111 significantly reduced these levels in a dose-dependent manner (Fig. 2). All these data indicated that CCM111 suppressed liver fibrosis progression through inhibiting the activity of the TGF-β pathway.
Fig. 2.
CCM111 reduced TGF-β1-induced expression of α-SMA and MMP2 and activation of the TGF-β signaling pathway in CCl4-treated animals. Total liver cell lysates were analyzed for α-SMA, MMP2, p-Smad2, p-Smad3, TGF-β1, and Smad3 protein expression by western blot. β-Actin was the loading control. The quantification data are reported as the mean ± standard deviation of three independent experiments. Student’s t-test: *P-value < 0.05, **P-value < 0.01, ***P-value < 0.001 for Normal vs. CCl4. #P-value < 0.05, ##P-value < 0.01, ###P-value < 0.001, for CCl4 vs. SIL, L-CCM111, or H-CCM111, respectively.
3.3. CCM111 inhibits the expression of inflammation-and fibrogenesis-related genes
To further investigate the mechanism of CCM111 in liver fibrosis, we analyzed liver sections (Normal, CCl4, H-CCM111) by next-generation sequencing (NGS). We analyzed the functions of genes below the cutoff point (coverage: 50, RPKM: 3.5) according to GO terms (Fig. S3). We found that the metabolic and immune responses of the CCl4 group were different from those of the normal group, which suggested that CCl4 affected these functions. The response of the H-CCM111 group was similar to that of the normal group, which indicated that CCM111 may reverse the liver function in terms of gene expression levels (Fig. S4A and B).
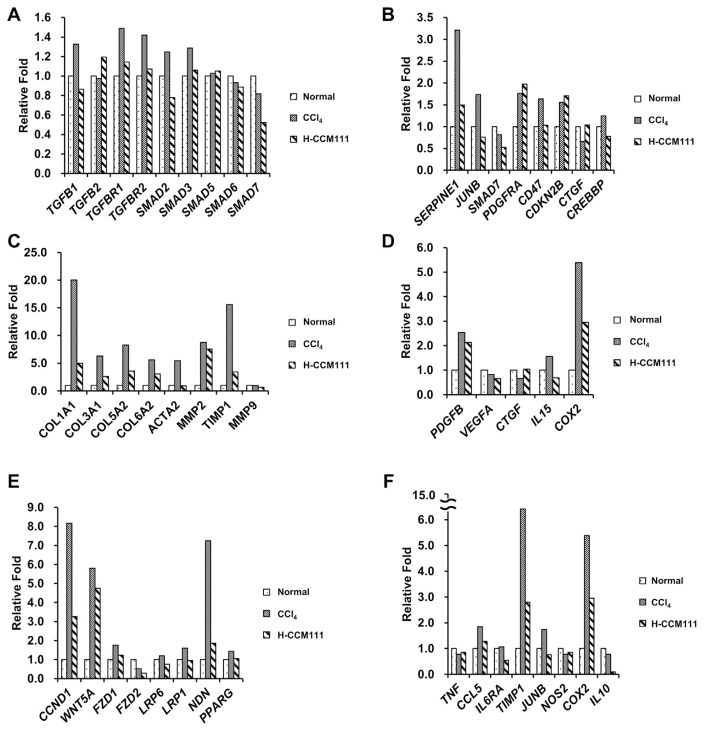
We further analyzed the effects of CCM111 on the pathways important to liver fibrosis progression. We found that CCl4 alone induced the some gene expressions of TGF-β, cytokines, and ECM proteins and that CCM111 reduced the expression of these genes (Fig. 3A–D). The Wnt pathway plays important roles in the development, regeneration and metabolism of the liver [21]. The Wnt pathway has also been reported to be involved in the activation of HSCs and the progression of fibrosis [22].We also found that CCM111 reduced the some gene expressions of several downstream genes in the Wnt pathway (Fig. 3E). Previous reports indicated that the STAT3 pathway is involved in the inflammatory response and the progression of liver fibrosis [23,24]. We also found that CCl4 induced the expressions of several downstreamgenes of STAT3 pathway such as TIMP1, which is an important factor for the survival of HSCs and has a positive correlation with liver fibrosis (Fig. 3F). These results suggested that CCM111 repressed the progression of liver fibrosis and inflammatory responses through downregulating the TGF-β, Wnt, and STAT3 pathways.
Fig. 3.
Assessment of genes differentially expressed in liver samples. Liver samples were analyzed by next-generation sequencing. The fold change was calculated by the RPKM of each group relative to the normal group. (A) TGF-β pathway genes. (B) Downstream genes of the TGF-β pathway. (C) ECM genes. (D) Cytokines. (E) Downstream genes of the Wnt pathway. (F) Downstream genes of the STAT3 pathway.
3.4. Effects of CCM111 on TGF-β1-induced MMP2, α-SMA and TGF-β pathway activation in HSC-T6
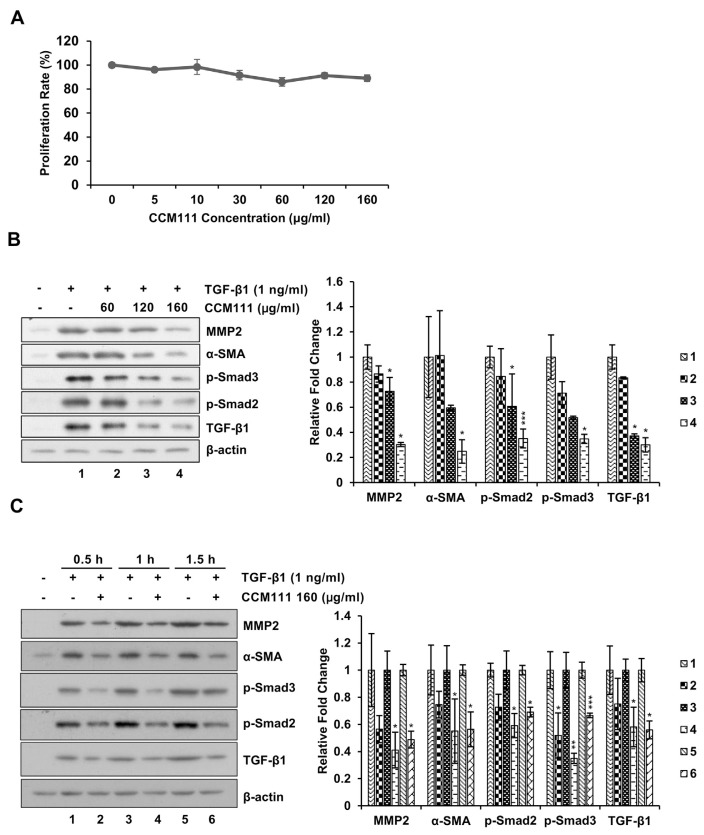
We used the HSC-T6 cell line to investigate the effects of CCM111 on the activation of HSCs. CCM111 did not induce toxicity in the HSC-T6 cells (Fig. 4A). We then tested the effects of CCM111 on the TGF-β1-induced TGF-β pathway and fibrosis markers α-SMA and MMP2. CCM111 significantly inhibited the TGF-β1-mediated expression of phosphorylated Smad2, Smad3, α-SMA, and MMP2 in a dose- and time-dependent manner (Fig. 4B and C).
Fig. 4.
CCM111 reduced TGF-β1-induced expression of α-SMA and MMP2 and activation of the TGF-β signaling pathway in HSCs. (A) The cells were treated with CCM111 (0, 5, 10, 30, 60, 120 or 160 μg/ml) for 24 h. Proliferation was detected by the Alamar Blue assay. (B) The cells were starved for 24 h and treated with TGF-β1 (1 ng/ml) and CCM111 (0, 60, 120 or 160 μg/ml) for 1 h. (C) The cells were starved for 24 h and treated with TGF-β1 (1 ng/ml) and CCM111 (160 μg/ml) for 0.5, 1 and 1.5 h. Total cell lysates were prepared for western blot analysis to detect protein levels. β-actin was the loading control. The quantification data are reported as the mean ± standard deviation of three independent experiments. Student’s t-test was performed to compare the CCM111 group with the TGF-β1-only group. *P-value < 0.05, **P-value < 0.01 and ***P-value < 0.001.
To further investigate the candidate compounds of CCM111, we chromatographed CCM111 into 7 fractions (F1F7). We examined the toxicity of these fractions by toxicity assay (Fig. S5A). We found that F4 and F5 fractions of CCM111 decreased the TGF-β1-mediated the expression of α-SMA (Fig. S5B). We have analyzed the compounds of F4 by LC/MS in the previous study [17]. The results suggested that F4 and F5 are the two major fractions for alleviation of liver fibrosis.
3.5. Effects of CCM111 on TGF-β1-induced STAT3 and Wnt pathways activation in HSC-T6
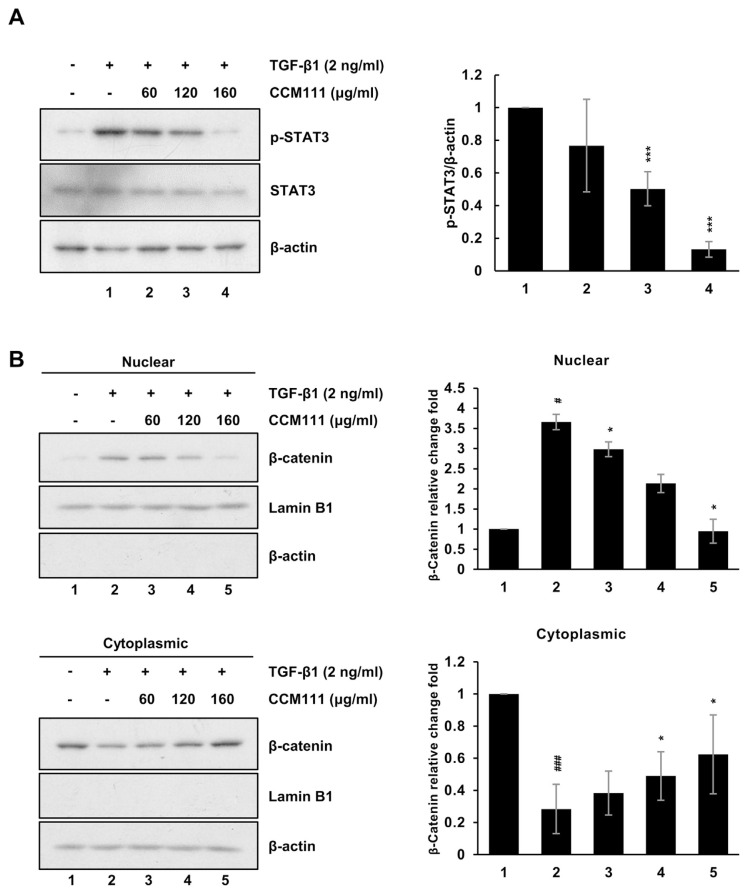
Our NGS analysis revealed that CCM111 reduced the expressions of some genes downstream of STAT3 and Wnt pathways (Fig. 3D and E). Therefore, we examined if CCM111 can affect TGF-β1-mediated the activity of STAT3 and Wnt pathways by western blots. As consistent with previous results, we found that CCM111 reduced the TGF-β1-mediated expression of phosphorylated STAT3 and the translocation of β-catenin in HSC-T6 cells (Fig. 5A and B). The results suggested that CCM111 reduced liver fibrosis progression through repressing the activation of HSCs.
Fig. 5.
CCM111 reduced TGF-β1-induced the activation of the Wnt and STAT3 signaling pathways in HSC cells. The cells were starved for 24 h and treated with TGF-β1 (2 ng/ml) and CCM111 (0, 60, 120, 160 μg/ml) for 24 h. (A) The protein levels of p-STAT3 and STAT3 were detected. (B) The protein levels of β-catenin expression were detected. β-actin was used as the cytosol internal control, and lamin B1 was used as the nuclear internal control. The quantification data are reported as the mean standard deviation of three independent experiments. Student’s t-test compared the CCM111 treatment with the TGFβ1 only group. *P-value < 0.05, **P-value < 0.01 and ***P-value < 0.001. The control group vs. TGF-β1 only group. #P-value < 0.05, and ###P-value < 0.001.
4. Discussion
CCM111 alleviated the liver damage in the CCl4-only group by increasing the relative spleen and body weights, decreasing the levels of the liver damage markers GOT and GPT, and alleviating the inflammatory responses and collagen deposition in the liver sections (Tables 1 and 2, Fig. 1, and Fig. S2). We also investigated the CCl4-treated liver sections and found that CCM111 also reversed the CCl4-induced gene expression profile; these results imply that CCM111 may reverse the progression of fibrosis (Fig. S4). We analyzed genes important in fibrogenesis and found that CCM111 markedly downregulated the expression of proinflammatory cytokines and chemokines and the expression of TGF-β, Wnt, and STAT3-regulated genes (Figs. 2 and 3). Furthermore, we performed the fractions of CCM111 by chromatography and found that F4 and F5 reduced the TGF-β expression of α-SMA (Fig. S5B). We suggested that CCM111 has potential as an antifibrotic agent and has potential applications in clinical interventions.
HSCs play an important role in the progression of fibrogenesis. However, activated HSCs are a major source of ECM proteins [25]. The TGF-β/Smad signaling pathway, including the Smad and TGF-β1 families, is one of the major pathways that activate HSCs and induce expression and deposition of collagen and the expression of fibrogenesis-related cytokines and chemokines [26]. We found that CCM111 reduced TGF-β pathway activity and the expression of genes downstream in the TGF-β pathway (Fig. 3B and C). However, CCM111 did not influence the expression of genes in the TGF-β pathway (Fig. 3A); rather, CCM111 specifically inhibits the expression of phosphorylated Smad proteins (Fig. 4B and C).
The major biological functions of matrix metalloproteinase (MMP) are degrading ECM proteins and promoting wound repair [27]. However, MMP-2 has been found to be involved in hepatic fibrogenesis [28]. Our study reported that CCM111 reduced the TGF-β1-induced expression of MMP2 and the CCl4-induced expression of MMP2 in ICR mice (Figs. 2 and 4B and C). Some potential antifibrotic targets may include the induction of HSCs apoptosis and the elimination of profibrogenic pathways; inducing apoptosis in activated HSCs appears to drive the regression of liver fibrosis. The inactivation of hepatic stellate cells to prevent further liver damage is a promising antifibrotic strategy.
Abnormal activation of the canonical Wnt pathway plays an important role in the activation of HSCs and the differentiation of myofibroblasts [29]. Treating with antagonists of Dickkopf1 (DKK1) or reducing the level of β-catenin can reduce the activation of HSCs [22]. In our study, we found that CCM111 reduced the expression of genes downstream of the Wnt pathway and the translocation of β-catenin (Figs. 3E and 5B). This result implied that CCM111 represses the progression of liver fibrosis through reduced Wnt pathway activation.
Activation of the STAT3 pathway has been reported in a variety of diseases such as cancer, inflammation, liver injury and liver fibrosis [30]. The inhibition of inflammation alleviates liver injury [31]. However, some reports indicated that STAT3 pathway activation reduced fibrosis through preventing hepatocellular damage [32]. In our study, we found that the expression of genes downstream of STAT3 was lower in the H-CCM111 group than in the CCl4 group, which implied that CCM111 may reduce the activation of the STAT3 pathway (Fig. 3F).We also found that CCM111 reduced the expression of phosphorylated STAT3 in HSC cells (Fig. 5A). This result was consistent with that of our previous study, in which we found that CCM111 reduced the activity of the STAT3 pathway in RAW264.7 macrophages [17].
F4 and F5 fractions of CCM111 could reduce the TGF-βmediated the expression of α-SMA (Fig. S5A and B). We perfumed the LC/MS to analysis the compounds of F4 in our previous studies [17]. The components of F4, antroquinonol B, 14-deoxy-11,12-didehydroandrographolide, and antrocinnamomin C, have been reported on the anti-inflammation functions [33–35]. In addition, 14-deoxy-11,12-didehydroandrographolide also has been indicated the antifibrosis ability in murine renal mesangial cell lines [36]. These studies supported our finding that compounds in F4 and F5 of CCM111 may play an important role to alleviate liver fibrosis.
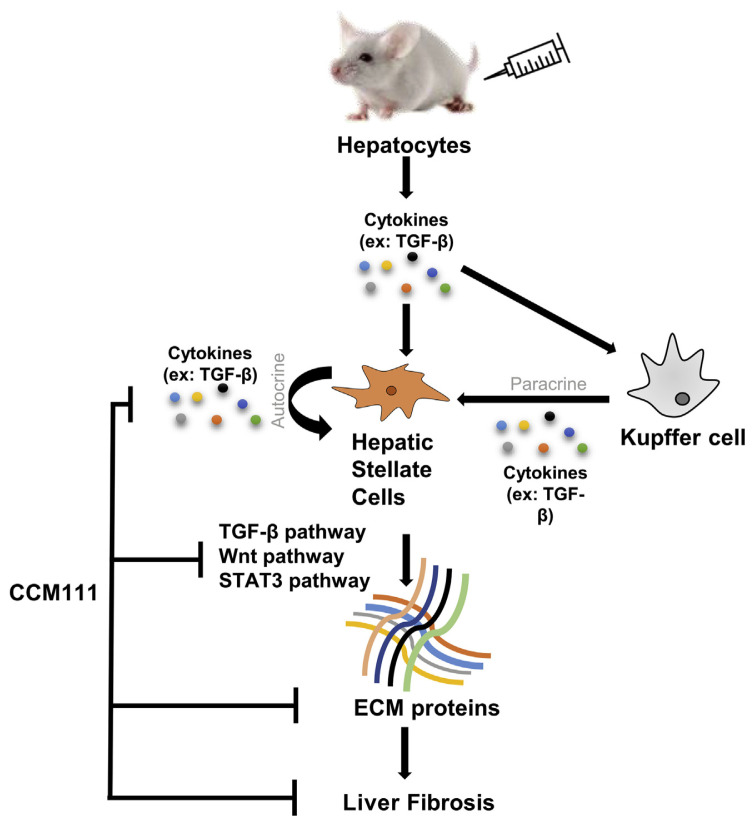
In summary, our study provided novel insight into the mechanism by which AC ameliorates liver fibrosis. Our results suggested that CCM111 reduces the activity of TGF-β signaling pathways through inhibiting the levels of phosphorylated Smad2 and Smad3 and reducing the expression of α-SMA, MMP2, and TGF-β1. CCM111 also decreased the activity of STAT3 and Wnt pathways through reducing the expression of phosphorylated STAT3 and the translocation of β-catenin in HSC-T6. CCM111 decreases CCl4-induced expression of ECM proteins by downregulating the activation of the TGF-β, Wnt, and STAT3 pathways and by reducing inflammatory responses (Fig. 6).
Fig. 6.
CCM111 remarkably attenuated the severity of CCl4-induced liver fibrosis through the inhibition of the TGF-β1/Smad, Wnt, and STAT3 pathways and the expression of ECM proteins and cytokines. CCM111 exhibited hepatoprotective effects in CCl4-induced experimental fibrosis, implying its potential application in clinical intervention.
Acknowledgments
This work was supported by the following programs: the Ministry of Science and Technology, Taiwan (MOST106-2320-B-008-005-MY3), the National Central University-Landseed Hospital United Research Center (NCU-LSH-105-A-006, NCU-LSH-106-A-004).
Appendix A. Supplementary material
Supplementary data related to this article can be found online at https://doi.org/10.1016/j.jfda.2018.09.008.
Funding Statement
This work was supported by the following programs: the Ministry of Science and Technology, Taiwan (MOST106-2320-B-008-005-MY3), the National Central University-Landseed Hospital United Research Center (NCU-LSH-105-A-006, NCU-LSH-106-A-004).
Footnotes
Conflicts of interest
The authors have declared no conflicts of interest.
REFERENCES
- 1. Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–18. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hellerbrand C. Hepatic stellate cells – the pericytes in the liver. Pflugers Arch. 2013;465:775–8. doi: 10.1007/s00424-012-1209-5. [DOI] [PubMed] [Google Scholar]
- 3. Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 4. Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–38. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 5. Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. He G, Karin M. NF-kappaB and STAT3 – key players in liver inflammation and cancer. Cell Res. 2011;21:159–68. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hafez MM, Al-Harbi NO, Al-Hoshani AR, Al-Hosaini KA, Al Shrari SD, Al Rejaie SS, et al. Hepato-protective effect of rutin via IL-6/STAT3 pathway in CCl4-induced hepatotoxicity in rats. Biol Res. 2015;48:30. doi: 10.1186/s40659-015-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, et al. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nat Commun. 2012;3:735. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li W, Zhu C, Li Y, Wu Q, Gao R. Mest attenuates CCl4-induced liver fibrosis in rats by inhibiting the Wnt/beta-catenin signaling pathway. Gut Liver. 2014;8:282–91. doi: 10.5009/gnl.2014.8.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dong S, Chen QL, Song YN, Sun Y, Wei B, Li XY, et al. Mechanisms of CCl4-induced liver fibrosis with combined transcriptomic and proteomic analysis. J Toxicol Sci. 2016;41:561–72. doi: 10.2131/jts.41.561. [DOI] [PubMed] [Google Scholar]
- 11. Liu C, Tao Q, Sun M, Wu JZ, Yang W, Jian P, et al. Kupffer cells are associated with apoptosis, inflammation and fibrotic effects in hepatic fibrosis in rats. Lab Invest. 2010;90:1805–16. doi: 10.1038/labinvest.2010.123. [DOI] [PubMed] [Google Scholar]
- 12. Geethangili M, Tzeng YM. Review of pharmacological effects of Antrodia camphorata and its bioactive compounds. Evid Based Complement Alternat Med. 2011;2011:212641. doi: 10.1093/ecam/nep108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin WC, Kuo SC, Lin WL, Fang HL, Wang BC. Filtrate of fermented mycelia from Antrodia camphorata reduces liver fibrosis induced by carbon tetrachloride in rats. World J Gastroenterol. 2006;12:2369–74. doi: 10.3748/wjg.v12.i15.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu MT, Tzang BS, Chang YY, Chiu CH, Kang WY, Huang CH, et al. Effects of Antrodia camphorata on alcohol clearance and antifibrosis in livers of rats continuously fed alcohol. J Agric Food Chem. 2011;59:4248–54. doi: 10.1021/jf104561h. [DOI] [PubMed] [Google Scholar]
- 15. Chen YR, Chang KT, Tsai MJ, Lee CH, Huang KJ, Cheng H, et al. Antrodia cinnamomea profoundly exalted the reversion of activated hepatic stellate cells by the alteration of cellular proteins. Food Chem Toxicol. 2014;69:150–62. doi: 10.1016/j.fct.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 16. Chiu HW, Hua KF. Hepatoprotective effect of wheat-based solid-state fermented Antrodia cinnamomea in carbon tetrachloride-induced liver injury in rat. PLoS One. 2016;11:e0153087. doi: 10.1371/journal.pone.0153087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin IY, Pan MH, Lai CS, Lin TT, Chen CT, Chung TS, et al. CCM111, the water extract of Antrodia cinnamomea, regulates immune-related activity through STAT3 and NF-kappaB pathways. Sci Rep. 2017;7:4862. doi: 10.1038/s41598-017-05072-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest. 2017;127:55–64. doi: 10.1172/JCI88881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gene Ontology Consortium: going forward. Nucleic Acids Res. 2015;43:D1049–56. doi: 10.1093/nar/gku1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353–61. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Behari J. The Wnt/beta-catenin signaling pathway in liver biology and disease. Expert Rev Gastroenterol Hepatol. 2010;4:745–56. doi: 10.1586/egh.10.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ge WS, Wang YJ, Wu JX, Fan JG, Chen YW, Zhu L. beta-catenin is over expressed in hepatic fibrosis and blockage of Wnt/beta-catenin signaling inhibits hepatic stellate cell activation. Mol Med Rep. 2014;9:2145–51. doi: 10.3892/mmr.2014.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu MY, Hu JJ, Shen J, Wang ML, Zhang QQ, Qu Y, et al. Stat3 signaling activation crosslinking of TGF-beta1 in hepatic stellate cell exacerbates liver injury and fibrosis. Biochim Biophys Acta. 2014;1842:2237–45. doi: 10.1016/j.bbadis.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 24. Wang H, Lafdil F, Kong X, Gao B. Signal transducer and activator of transcription 3 in liver diseases: a novel therapeutic target. Int J Biol Sci. 2011;7:536–50. doi: 10.7150/ijbs.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER, Brenner DA. The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo. J Hepatol. 1999;30:77–87. doi: 10.1016/s0168-8278(99)80010-5. [DOI] [PubMed] [Google Scholar]
- 27. Jablonska-Trypuc A, Matejczyk M, Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhib Med Chem. 2016;31:177–83. doi: 10.3109/14756366.2016.1161620. [DOI] [PubMed] [Google Scholar]
- 28. Preaux AM, Mallat A, Nhieu JT, D’Ortho MP, Hembry RM, Mavier P. Matrix metalloproteinase-2 activation in human hepatic fibrosis regulation by cell–matrix interactions. Hepatology. 1999;30:944–50. doi: 10.1002/hep.510300432. [DOI] [PubMed] [Google Scholar]
- 29. Miao CG, Yang YY, He X, Huang C, Huang Y, Zhang L, et al. Wnt signaling in liver fibrosis: progress, challenges and potential directions. Biochimie. 2013;95:2326–35. doi: 10.1016/j.biochi.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 30. Haga S, Terui K, Zhang HQ, Enosawa S, Ogawa W, Inoue H, et al. Stat3 protects against Fas-induced liver injury by redox-dependent and-independent mechanisms. J Clin Invest. 2003;112:989–98. doi: 10.1172/JCI17970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li J, Sapper TN, Mah E, Rudraiah S, Schill KE, Chitchumroonchokchai C, et al. Green tea extract provides extensive Nrf2-independent protection against lipid accumulation and NFkappaB pro-inflammatory responses during nonalcoholic steatohepatitis in mice fed a high-fat diet. Mol Nutr Food Res. 2016;60:858–70. doi: 10.1002/mnfr.201500814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kong X, Horiguchi N, Mori M, Gao B. Cytokines and STATs in liver fibrosis. Front Physiol. 2012;3:69. doi: 10.3389/fphys.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee WT, Lee TH, Cheng CH, Chen KC, Chen YC, Lin CW. Antroquinonol from Antrodia camphorata suppresses breast tumor migration/invasion through inhibiting ERK-AP-1- and AKT-NF-kappaB-dependent MMP-9 and epithelial-mesenchymal transition expressions. Food Chem Toxicol. 2015;78:33–41. doi: 10.1016/j.fct.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 34. Lim JC, Goh FY, Sagineedu SR, Yong AC, Sidik SM, Lajis NH, et al. A semisynthetic diterpenoid lactone inhibits NF-kappaB signalling to ameliorate inflammation and airway hyper responsiveness in a mouse asthma model. Toxicol Appl Pharmacol. 2016;302:10–22. doi: 10.1016/j.taap.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 35. Wu MD, Cheng MJ, Wang BC, Yech YJ, Lai JT, Kuo YH, et al. Maleimide and maleic anhydride derivatives from the mycelia of Antrodia cinnamomea and their nitric oxide inhibitory activities in macrophages. J Nat Prod. 2008;71:1258–61. doi: 10.1021/np070634k. [DOI] [PubMed] [Google Scholar]
- 36. Lee MJ, Rao YK, Chen K, Lee YC, Chung YS, Tzeng YM. Andrographolide and 14-deoxy-11,12-didehydroandrographolide from Andrographis paniculata attenuate high glucose-induced fibrosis and apoptosis in murine renal mesangeal cell lines. J Ethnopharmacol. 2010;132:497–505. doi: 10.1016/j.jep.2010.07.057. [DOI] [PubMed] [Google Scholar]