Abstract
Abelmoschus esculentus (AE) has been used in traditional medicine to ameliorate hyperglycemia, but its mucilage increased bioassay difficulties. We have obtained a series of AE subfractions. Among them F1 and F2 regulated dipeptidyl peptidase-4 (DPP-4) and type 1 glucagon-like peptide receptor (GLP-1R), the treatment targets for type 2 diabetes. F1, F2 and fraction residues (FR) showed advantage on different aspects, which attenuates insulin resistance and metabolic disorder in vivo, and prevents renal-tubular change in vitro. In the present study, using type 2 diabetes model induced by high fat diet (HFD) and streptozotocin (STZ), we aim to investigate whether AE prevent diabetic nephropathy by regulating the putative markers. The results showed that all the subfractions ameliorated albuminuria and renal hyperfiltration (measured by creatinine clearance rate; CCr) accompanied with diabetes, while F2 acted most promptly and consistently. Histologically AE reduced renal tubular change, fibrosis and fat deposition. F2 and FR exerted significant effects to decrease DPP-4 while increase GLP-1R. Although all the subfractions were effective to reduce oxidative stress, only F2 acted on kidneys specifically. In conclusion, we have demonstrated AE has benefits to regulate DPP-4 and GLP-1R, to reduce oxidative stress and renal fibrosis, with resultant to improve renal function and prevent diabetic renal damage. Taken together, F2 could be more promising to be developed as adjuvant for diabetic nephropathy.
Keywords: Abelmoschus esculentus, Diabetic nephropathy, Dipeptidyl peptidase-4, Type 1 glucagon-like peptide receptor
1. Introduction
Diabetes mellitus is increasing worldwide and has a profound impact on human health. Among the diabetic complications, nephropathy is thought to have the greatest socioeconomic impact [1–3]. Diabetic nephropathy is characterized by glomerulosclerosis and tubular matrix deposition, and renal fibrosis [4,5]. In addition to histological changes, the measurement of creatinine clearance rate (CCr) and urinary albumin excretion is widely used for detection of developing diabetic nephropathy [6]. The pathogenesis leads to diabetic nephropathy remains delicate and unmodified by the current therapy [7]. Once renal function begins to deteriorate, the metabolic and pharmacological controls become difficult and complicated. Hence new treatment paradigms for preventing diabetic nephropathy are needed urgently [8].
Type 2 diabetes is characterized by obesity, insulin resistance and insulin secretion defect. The insulin action is initiated by binding to its receptor, eliciting tyrosine phosphorylation of insulin receptor substrates (IRS), and activation of downstream mediators [9]. Recently, dipeptidyl peptidase-4 (DPP-4, a serine protease) inhibitors have become a useful remedy for treatment of type 2 diabetes. The mode of its action is based on inhibiting the degradation of type 1 glucagon-like peptide 1 (GLP-1), an incretin which stimulates glucose-dependent insulin secretion. Besides the action on pancreas, GLP-1 and its receptor (GLP-1R) have direct effects on various organs including heart, blood vessels and kidney [10]. It was also reported DPP-4 inhibitor lowers albuminuria in patients with type 2 diabetes and renal dysfunction, and could prevent diabetic patients from renal impairment [11]. Since type 2 diabetes is indeed the most prevalent type of diabetes, feasible in vitro or in vivo type 2 diabetes models would more precisely reflect the pharma-pathological changes. In our previous report, the renal damage of type 2 diabetic rat induced by high fat diet (HFD) and streptozotocin (STZ) is observed especially surround the tubular region, consistent with the high oxidative stress level [12]. In addition, the hyperfiltration indexed by high CCr, was also observed in type 2 diabetic rats [12].
The fruit of Abelmoschus esculentus (AE; also known as Okra), with edible green seed pods, is commonly consumed as vegetable in many countries [13]. Being a healthy food owing to its high fiber, vitamins, and content of trace elements, AE is used in traditional medicine to relieve fever, prevent liver damage, abate gastric inflammation, improve kidney function, and in particular, exert the anti-hyperglycemia effect [14]. Although AE is generally deemed beneficial for diabetic patients, there are few scientific reports regarding the action targets of AE. In addition to the lipophilic components, AE is abundant of mucilage presumed to be glycoprotein and polysaccharide [15]. The mucilage increases difficulties for analysis and test in bio-models. By a succession of extraction steps, we obtained a series of subfractions from AE. Among them subfraction 1 (F1, rich of flavonoid and triterpene) and subfraction 2 (F2, composed of carbohydrates and polysaccharides) showed effective in vitro to inhibit DPP-4 and diabetic renal-tubular change [16]. In palmitate-stimulated cells, F1 and F2 decreased the level of DPP-4 but increased GLP-1R [17]. By using type 2 diabetic rat, we recently reported that F2 was most effective in attenuating hyperglycemia and insulin resistance; F1 possessed an anti-obese effect; and the fraction residues (FR) significantly improved the lipoprotein profiles [18]. These results implicate AE could be an adjuvant for treatment of diabetes and its complications, and the subfractions with different targets merit further test respectively.
In the present study, we aim to investigate whether AE subfractions prevent diabetic nephropathy on the basis of type 2 diabetes model. The renal function during the experiment course will be consecutively observed. The histological changes and the putative regulation of biomarkers will also be analyzed.
2. Material and methods
2.1. Preparation of AE subfractions and chemical analysis
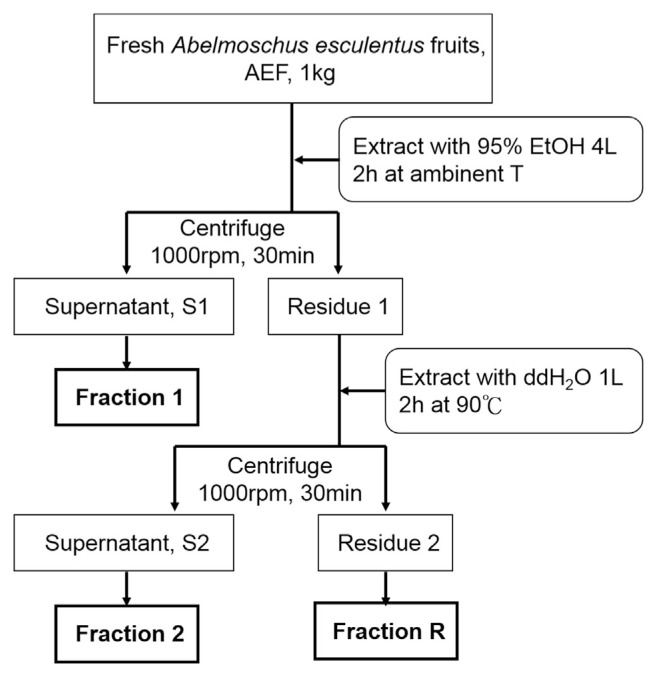
AE was purchased from Chuchi (Chiayi, Taiwan). The subfractions of AE (F1, F2 and the residue FR) were prepared according to the procedures shown in Fig. 1. The yields of dry base of F1, F2 and FR were 1.08%, 12.59%, and 48.27%, respectively. F1, the alcohol-extracted subfraction of AE, was analyzed by HPLC and LC-MS/MS in our previous report [16]. The quantification was based on each area under individual peak, divided by sum of the area of all peaks. At least 10 compounds were identified from F1, including quercetin glucosides (quercetin 3-O-glucosyl (1 → 6)glucoside, quercetin 3-O-xylosyl (1 → 2) glucoside, and quercetin 3-O-glucoside were estimated 1.848, 0.628, and 2.425 mg/g DW, respectively) and pentacyclic triterpene ester (4.301 mg/g DW). The F2 portion of AE, which was also analyzed in our previous study, contained a large amount of carbohydrates and polysaccharides [16]. Briefly, for determination of the composed monosaccharides, hydrolysis was performed according to Albersheim et al. [19], and the liberated sugars were transformed into alditol acetates according to Blakeney et al. [20]. Extracted by Water (5 mL) and dichloromethane (1 mL), the derivatives were then separated, dried, and dissolved in 1 mL of acetone. One μL of the aliquot was subjected to gas chromatographic analysis using Gas Chromatograph HP5890A series II (Hewlett–Packard, Palo Alto, CA, USA) equipped with a flame ionization detector (FID). The identification and quantification of monosaccharides were made in comparison with authentic samples. Peak areas of all the identified sugar compounds were calculated and expressed as percentage. The content of uronic acid was determined with the carbazole–H2SO4 method of Cesaretti et al. [21]. These analyses revealed that F2 was rich in uronic acid (23.14%; including galacturonic acid 16.26%), galactose (18.92%), glucose (18.26%) and myo-inositol (14.21%); rhamnose (9.79%), glucosamine (8.86%), and fucose (7.30%) were also found to be quite abundant. By gel permeation chromatography (GPC), the mean molecular weight of F2 was estimated to be 671 kDa [16].
Fig. 1.
Extraction procedure of AE subfractions.
2.2. Animal experiments
The animal experimental project was approved by the Animal Model Experimental Ethics Committee of Chung-Shan Medical University, and was conducted in accordance with the recommendation of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Briefly, male Sprague–Dawley rats (weight 250 ± 20 g, age 7 weeks) were obtained from LuxBiotech Co., Taiwan. The rats, 8 in each group and 4 in each cage, were acclimated and fed basic chow consisting of 12% fat for the first week before the experiments. The animal room was maintained at a 12 h light/ dark cycle, 25 °C, and 55 ± 5% relative humidity. All animals had free access to food and water. The protocol described by Yang et al. was used to induce type 2 diabetes of the rats [12]. Using the formulation described in AIN-76, normal and high-fat diets (HFD) were prepared and rationed according to the formula listed on Table 1. After 8 weeks, when the average body weight was 475 ± 15 g, the HFD-fed rats were injected intraperitoneally (ip) with 35 mg/kgbw of STZ. The other rats received only the same amount of 0.1 M citric acid buffer (pH 4.5). About 2 weeks later, when the hyperglycemic status was confirmed (indicated as the time point of start), the rats were tube-fed with or without different doses of AE subfractions. Briefly, the rats were divided into the following groups: control (normal diet), C1 (normal diet with 0.45 mg/kg F1), C2 (normal diet with 0.45 mg/kg F2), CR (normal diet with 0.45 mg/kg FR), HFD + STZ (HFD with STZ injection; diabetes model), HFD + STZ + F1 (L) (diabetes with 0.23 mg/kg F1), HFD + STZ + F1 (H) (diabetes with 0.45 mg/kg F1), HFD + STZ + F2 (L) (diabetes with 0.23 mg/kg F2), HFD + STZ + F2 (H) (diabetes with 0.45 mg/kg F2), HFD + STZ + FR (L) (diabetes with 0.23 mg/kg FR), and HFD + STZ + FR (H) (diabetes with 0.45 mg/kg FR). The body weight, serum glucose and insulin were measured every 2 weeks. Twelve weeks later, at the end of the experiment, the animals were sacrificed under 4–8 psi of CO2. Although unconsciousness often occurred within 1 min, the rats were left in the container for at least 3–5 min to ensure death, which was verified no cardiac pulse. During the processing of the whole experiment, the serum and urine samples were collected every 2 weeks.
Table 1.
Diet for the animal experiment.
| Ingredient (%) | C | HFD |
|---|---|---|
| Casein | 20.0 | 20.0 |
| Sucrose | 6.0 | 6.0 |
| Corn Starch | 51.8 | 37.8 |
| Corn Oil | 12.0 | 25.9 |
| Cholesterol | 0.1 | |
| Mineral premix | 4 | 4 |
| Vitamin premix | 1 | 1 |
| Choline | 0.2 | 0.2 |
| Cellulose | 5 | 5 |
| Total | 100 | 100 |
2.3. Serum biochemical assays
With ethylenediaminetetraacetates (EDTA) tubes, serum sample was collected and centrifuged at 3000 rpm for 10 min at 4 °C. The serum glucose and insulin levels were reported in our initial work [18], and briefly described as follows: the glucose levels (mg/dL) at 0, 6th and 12th wk for controls were 83.50, 83.75, 77.25; for HFD + STZ were 373.00, 444.14, 445.25; for DM treated with F1(H) were 412.50, 387.40, 396.40; for DM treated with F2(H) were 442.83, 315.00, 209.33; for DM treated with FR(H) were 397.00, 448.67, 411.50, respectively. The insulin levels (mU/L) at 0, 6th and 12th wk for controls were 13.01, 11.71, 11.84; for HFD + STZ were 13.07, 11.59, 8.58; for DM treated with F1(H) were 13.53, 6.15, 6.69; for DM treated with F2(H) were 9.03, 8.28, 9.85; for DM treated with FR(H) were 11.50, 6.22, 6.94, respectively. Treatment of AE has effectively improved these parameters [data set] [18]. In the present study, creatine phosphate kinase (CPK) and ketone bodies were evaluated (Beckman Synchron CX9 Clinical System, Beckman Coulter Inc.) to analyze the heart and metabolic situation. The index of GOT and BUN were evaluated in our previous report [data set] [18].
2.4. Renal function index
Creatinine was measured by auto-creatinine liquicolor/10052 (Human, Wiesbade n, Germany). Urinary albumin was measured by a rat albumin ELISA kit/1000-2 (Life Diagnostics, PA, USA).
2.5. Histological finding
After rats were sacrificed, the kidneys were fixed in 4% para-formaldehyde, embedded in paraffin, sectioned into 4 μm, and processed for histological examination with hematoxylin and eosin (H&E) stain.
2.6. Sirius Red stain
Slides were baked at 60 °C for 1 h then taken through xylene and graded ethanols (100%, 95%, 85%, 75%, 60%, and 50%) into distilled water. The samples were stained overnight (minimum 14 h) in saturated picric acid with 0.1% Sirius Red F3BA (Aldrich Chemicals), then washed with 0.01 N hydrochloric acid for 2 min. Slides were rapidly dehydrated through graded alcohols starting at 70%, then to xylene, and finally covered-slipped in Permount.
2.7. Oil Red O stain
Frozen tissue sections were prepared on a LEICA CM3050 S cryostat (Leica Microsystems, Germany). The 5 μm thick sections were stained 10 min with 1% Oil Red O (Sigma–Aldrich, O0625) in propylene glycol, counterstained with hematoxylin, and mounted in glycerin jelly.
2.8. Western blotting
Kidney chops were added to radioimmunoprecipitation assay (RIPA) buffer with protease and phosphatase inhibitors, and homogenized at 4 °C. The tissue homogenate was centrifuged (10,000 g for 20 min at 4 °C), and then resulting supernatant was collected. After quantification, equal amounts of protein samples (50 μg) were subjected to 10% SDS-PAGE electrophoresis and transferred to nitrocellulose membranes (Millipore, Bedford, MA, USA). The membranes were blocked with 5% nonfat milk powder with 0.1% Tween-20 in TBS and then incubated at 4 °C overnight with the primary antibodies to DPP-4 (1:1000), and GLP-1R (1:1000). Antibodies of GLP-1R and DPP-4 were from Abcam (Cambridge, U.K.). The membranes were then washed three times with 0.1% Tween-20 in TBS and incubated with the secondary antibodies (1:5000) conjugated to horseradish peroxidase (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Band detection was performed using enhanced chemiluminescence using ECL Western blot detection reagents and exposure to FUJFILM Las-3000 (Tokyo, Japan). The protein quantity was determined by densitometry using FUJFILM-Multi Gauge V2.2 software.
2.9. Thiobarbituric acid reacting substances (TBARS)
0.2 mL of serum or kidney homogenate was mixed with 0.2 mL of 25% TCA then centrifuged (10,000 g for 30 min at 10 °C). 0.3 mL of supernatant was added to 0.3 mL of TBA (1% thiobarbituric acid) to react for 40 min at 95 °C in the dark. Samples were analyzed in a Hitachi F2000 fluorescence spectrophotometer with excitation at 532 nm and emission at 600 nm, for measuring malondialdehyde (MDA), the lipid peroxidation product. The calibration curve was pre pared with MDA standards (1,1,3,3-tetramethoxypropane; TEP) of 0–50 nmol (r2 = 0.9927).
2.10. Statistical analysis
The statistica l soft ware SPSS v.12.0 was used to analyze the data. One-way ANOVA was performed (P < 0.05), while Bonferroni’s Multiple Comparison was used for the post-test.
3. Results
3.1. Dynamic change of urine albumin excretion
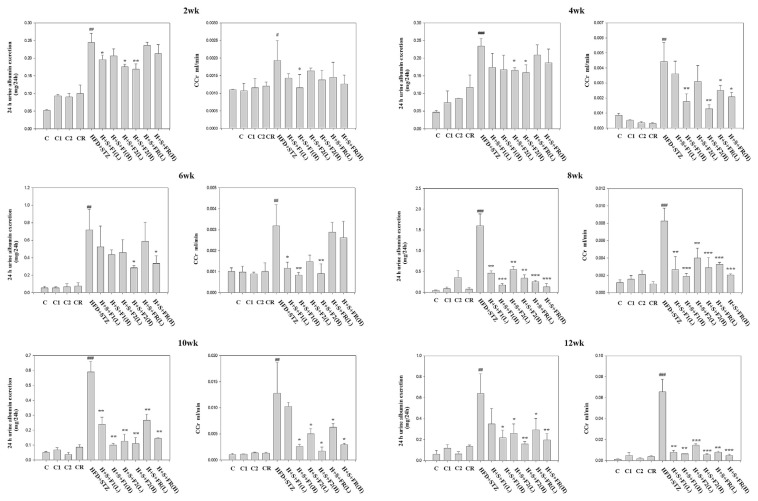
In type 2 diabetic rats, the 24 h urine albumin excretion was substantially increased nearly 5-folds, suggesting the alteration of kidney function at 2 wk (Fig. 2, 2 wk). The high dose administration of F2 reduced 30% of the excretion, and consistently effective at 4 wk (Fig. 2, 4 wk). F1 trended to lower the albumin excretion yet without statistical significance. FR showed non-effective at this time point.
Fig. 2.
Effect of AE on the dynamic change of urine albumin and CCr. Urine and serum of the experimental animals were collected and analyzed. Top to bottom: 2, 4, 6, 8, 10 and 12 wks, respectively, with or without the administration of AE subfractions. Data are presented as mean ± SD (n = 8 per group) and analyzed with ANOVA. #p < 0.05, ##p < 0.01, ###p < 0.001 compared with the control. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the HFD + STZ group.
At 6 wk, the albumin excretion of diabetic rats was 14-folds compared to the control. Although F1 still had no significance, F2(H) and FR(H) reduced 60% and 50% of the albumin excretion, respectively (Fig. 2, 6 wk).
At 8 wk, the albumin excretion of diabetic rats reached 30-folds. F1(H) was first time observed to lower the excretion significantly. In summary, all the AE subfractions, low or high dose, effectively improve the renal function at this time point (Fig. 2, 8 wk).
Near to the end of experiment, the urine albumin excretion of diabetic rats was about 10 to 12-fold higher. All the AE subfractions consistently acted to lower the albumin excretion (Fig. 2, 10 wk and 12 wk).
3.2. Dynamic change of CCr
At 2 wk, CCr was slightly increased in type 2 diabetic rats, viewed as indicative of hyperfiltration stage of early diabetic nephropathy. Most AE subfractions did not significantly reduce CCr (Fig. 2, 2 wk). Two weeks later, progressing with diabetes, CCr was consistently increased nearly 5-folds (Fig. 2, 4 wk). All the AE subfractions, especially F2 (H), were effective to ameliorate the hyperfiltraton. CCr reduction by F1(H), F2 (H) and FR (H) were 61%, 72% and 55%, respectively.
At 6 wk, the hyperfiltration still remained higher in diabetic group. F1 and F2 showed effective and reversed CCr even below the control group. However, the effect of FR diminished as the level of CCr rebounded (Fig. 2, 6 wk).
At 8 wk, all the AE subfractions significantly lower the hyperfiltration induced by diabetes. Treatment of F1 (H) and F2 (H) reduced CCr about 78% and 65% respectively. Noticeably, FR became effective at this time point (Fig. 2, 8 wk).
The phenomenon of hyperfiltration continued. In the end of the experiment, CCr reached above 50-folds (Fig. 2, 10 wk and 12 wk). All the AE subfractions significantly decreased the level of CCr.
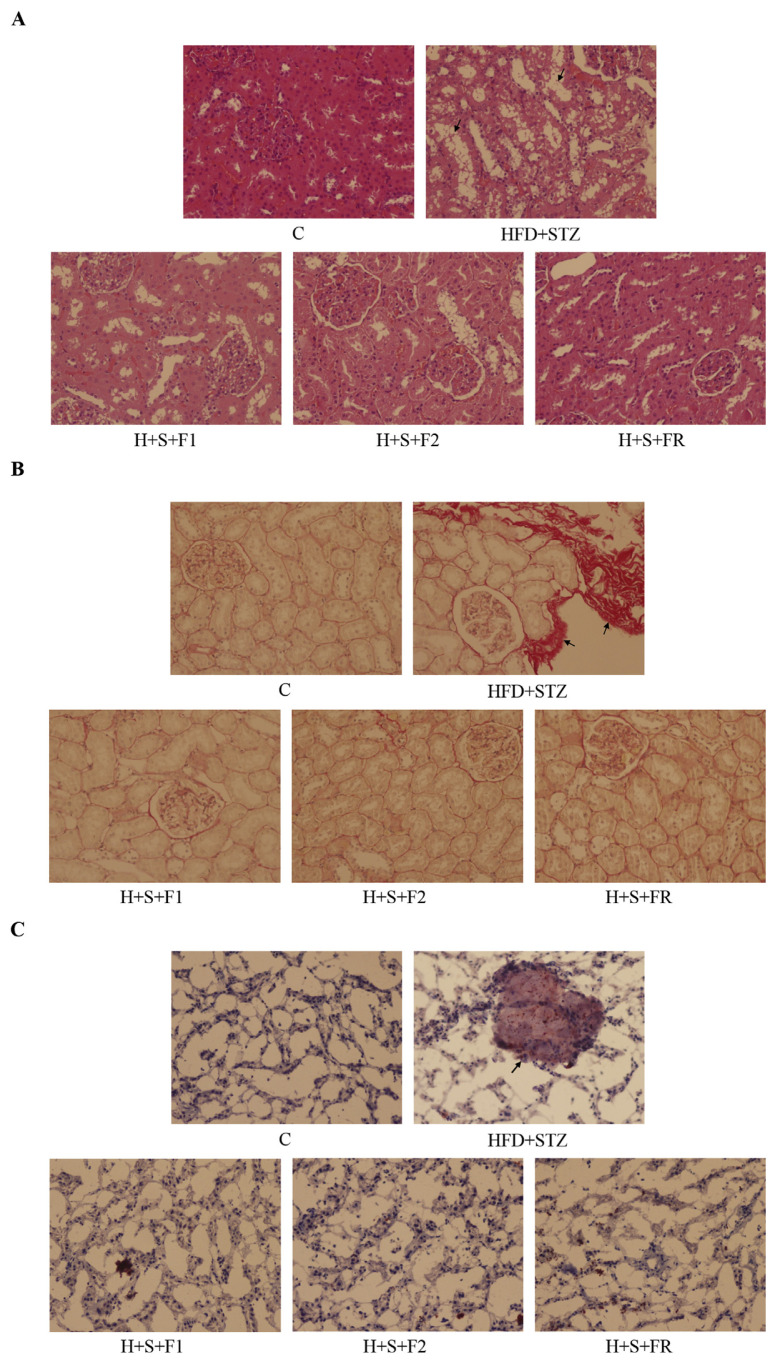
3.3. AE subfractions ameliorate the histological change of diabetic kidneys
The H-E staining results showed that slight glomerular contraction disjuncted from the capsules, and numerous vacuole fusions surround the tubular area of type 2 diabetic kidneys. Treatment of 0.45 mg/kg of AE subfractions reversed the nephritic change significantly (Fig. 3A).
Fig. 3.
Effect of AE on the histological change. Kidneys of the control, type 2 diabetes and 0.45 mg/kg of AE subfraction-treated diabetic rats were collected, fixed, sectioned, and stained for microscopic observation (200X). (A) H–E stain (B) Sirius red stain, collagen accumulation in the red area (C) Oil red O stain, fat deposits in the red-stained area. Arrows indicate the change in diabetic kidneys.
Sirius red staining was used to analyze the extracellular matrix deposition. Fig. 3B showed that the diabetic kidneys were strongly stained, which indicated severe collagen accumulation and fibrosis. The presentation was also observed by TEM (data not shown). AE subfractions ameliorated the nephropathy and matrix deposition, and hence reversed the fibrosis of diabetic kidney.
Oil Red O staining revealed that the fat mass deposited in diabetic kidneys, whereas such lipid accumulation was not found in the control or AE-treated groups (Fig. 3C).
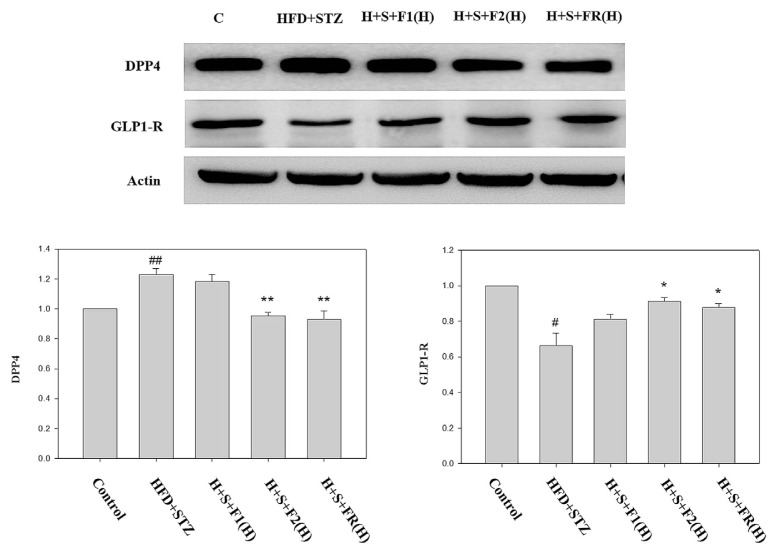
3.4. AE subfractions regulate the putative biomarkers involved in diabetic nephropathy
The expressions of renal DPP-4 and GLP-1R were analyzed by Western blotting. The protein level of DPP-4 was significantly increased above 20% in type 2 diabetic rats. Although F1 did not exert the effect, 0.45 mg/kg of F2 and FR completely abolished the DPP-4 expression (Fig. 4).
Fig. 4.
Effect of AE on the expressions of DPP-4 and GLP-1R. Kidneys of the experimental animals were collected. The proteins were then extracted and detected with Western blot for DPP-4 and GLP-1R. Data are presented as mean ± SD and analyzed with ANOVA. #p < 0.05, ##p < 0.01 compared with the control. *p < 0.05, **p < 0.01 compared with the HFD + STZ group.
On the contrary, GLP-1R was reduced about 30% in diabetic rats. F1 slightly increase GLP-1R but has no statistical significance, while 0.45 mg/kg of F2 and FR showed effective to reverse the level of GLP-1R (Fig. 4).
3.5. AE subfractions possess good anti-oxidant ability
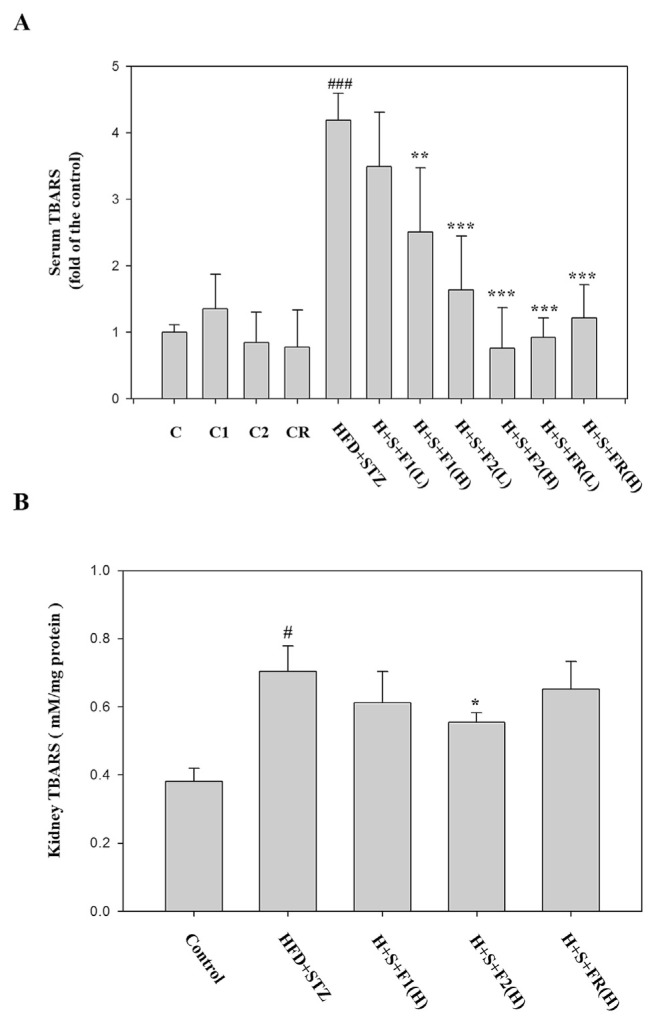
The serum TBARS analysis showed that diabetes increased the lipid peroxidation more than 4-folds. All the AE subfractions substantially lowered the peroxidation except F1(L). F2(H) and FR exhibited the superior effect (Fig. 5A). The lipid peroxidation was also increased in diabetic kidneys about 1.8-folds. F2 is the only one having significant anti-oxidative effect (Fig. 5B).
Fig. 5.
Effect of AE on the expression of lipid peroxidation. Serum and kidneys of the experimental animals were collected, and then analyzed with TBARS. (A) seum (B) kidney. Data are presented as mean ± SD (n = 8 per group) and analyzed with ANOVA. #p < 0.05, ##p < 0.01, ###p < 0.001 compared with the control. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the HFD + STZ group.
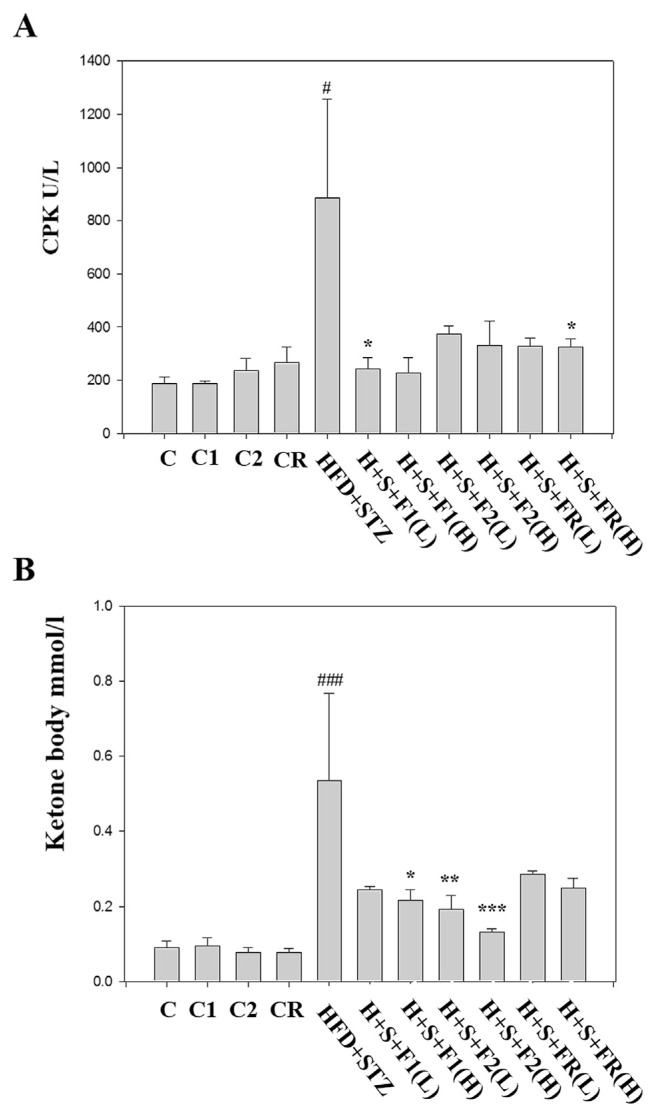
3.6. Safety valuation
All the AE subfractions per se did not affect the level CPK. Instead, AE substantially reversed the diabetes-increased CPK (Fig. 6A). Similar result was found in the ketone body analysis (Fig. 6B). According to our previous report, GOT increased in diabetic serum was significantly attenuated by AE subfractions. However, only F2(H) significantly reduced the diabetes-increased BUN [dataset] [18]. Noticeably, there was nearly no alteration of the groups C1, C2, and CR, which were the healthy rats treated with F1, F2, and FR alone, respectively. Therefore, AE treatment did not harm the liver and heart, and was beneficial for metabolism.
Fig. 6.
Effect of AE on the safety evaluation. Serum of the experimental animals was collected. Safety markers were then analyzed respectively. (A) CPK (B) ketone bodies. Data are presented as mean ± SD (n = 8 per group) and analyzed with ANOVA. #p < 0.05, ##p < 0.01, ###p < 0.001 compared with the control. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the HFD + STZ group.
4. Discussion
In the present study, we demonstrated the effects of AE against diabetic nephropathy. While all the subfractions ameliorated albuminuria and hyperfiltration accompanied with type 2 diabetes, the consecutive observation revealed that F2 acted most promptly and consistently. Histological finding showed that AE reduced the tubular change, renal fibrosis, and fat deposition. F2 and FR exerted significant effects to decrease DPP-4 but increase GLP-1R. All the AE subfractions were effective to reduce oxidative stress. Nevertheless, when considering the specific activity on kidneys, only F2 had good potential.
During the whole experiment course, the hyperglycemia and insulin resistance was consistent in type 2 diabetic animals [data set] [18]. Hyperglycemia promotes the formation of advanced glycation end product (AGE). Interacting with its specific receptor, AGE induces the generation of reactive oxygen species (ROS), mediates its action via oxidative stress [22], and accelerates lipid peroxidation [23]. Our data showed that oxidative stress increased in diabetic serum and kidney. Since the kidneys of type 2 diabetic rats contain a lot of fat, it could further produce lipid peroxidation and oxidative stress, thus mediating the related downstream signals.
Fibrosis is a common feature found in chronic kidney disease [24]. Although the expanded interstitial fibroblasts lead to fibrosis, emerging evidences suggest that epithelial mesenchymal transition (EMT) of renal tubular epithelial cells plays a critical role in renal fibrosis [25]. It has been found that by decreasing E-cadherin and increasing vimentin, tubular epithelial cells undergo a phenotypic transformation into matrix-producing fibroblasts [24]. We have reported the potential of AE subfractions on preventing renal EMT. F1 and F2 alleviated high glucose-induced vimentin and recoverd E-cadherin in tubular cells, with regulation of DPP-4 [16]. High glucose activated DPP-4, which played the pivotal role in signal transduction, and thus mediated the downstream cascades of renal EMT [26]. In addition to the observation in vitro, our previous reports had demonstrated the prominent renal tubular change in type 2 diabetic model. The polyphenols of Hibiscus sabdariffa (HPE) ameliorated diabetes-induced albuminuria, hyperfiltration, and the collagen accumulation in rat kidneys [12]. In diabetic rats, HPE reversed the expressions of vimentin, and then inhibited the renal-tubular change of EMT [27]. In the present study, AE was shown to improve the renal function and prevent kidney fibrosis, implicating that the regulation via EMT could not be excluded.
Since the renal function was observed consecutively, the mode of action by AE subfractions were discovered respectively, in which the effects could be attributed to the different chemical composition.
The chemical analysis revealed that F2 is mainly composed of carbohydrates and polysaccharides abundant in various monosaccharides or derivatives, including glucosamine, myoinositol, and rhamnose. An in vitro experiment demonstrated that at relatively low concentrations (between 10 and 100 μg mL–1), rhamnose-rich oligo- or polysaccharides provided significant protection against cytotoxicity induced by AGE [21]. The in vitro and in vivo experiments showed that glucosamine inhibited the EMT of retinal pigment cells by attenuating the related signals [28]. Glucosamine hydrochloride also suppressed renal fibrosis by inhibiting N-linked glycosylation of the TGF-β receptor, which led to inefficient membrane trafficking of the receptor, and interfered binding with TGF-β [29]. A clinical trial in patients with gestational diabetes revealed that fasting glucose, insulin, and homeostasis model assessment of insulin resistance decreased significantly greater in patients supplemented with myoinositol. Moreover, adiponectin was only increased in the myoinositol-treated group [30]. These evidences imply that myoinositol, glucosamine and rhamnose contained in AE could be potent to prevent diabetes induced damage like renal fibrosis and tubular EMT. Taken together, F2 is hypothesized having potential to improve hyperglycemia and insulin resistance, and to attenuate the related renal damage by suppressing renal fibrosis. In fact, according to the results of the GPC analysis, the mean molecular of F2 was estimated to be 671 kDa. Although the composition of monosaccharides was analyzed, the isomerization, sequence and linkage in the polymer could not be accomplished. The chemical analysis and bio-test of the polymer needs future investigation.
Our previous analysis showed that F1 is rich of flavonoid glycosides, among which, quercetin glucosides are prominent. In a previous report, the extract of Averrhoa bilimbi attenuated hyperglycemia-mediated oxidative, and the antidiabetic effect might be related to quercetin [31]. Noticeably, quercetin has been suggested to attenuate diabetic nephropathy. For diabetic rats exhibiting reduced creatinine and urea clearance, proteinuria, as well as a marked increase in oxidative stress, treatment with quercetin significantly attenuated renal dysfunction and oxidative stress [32]. In STZ-induced diabetic rats, quercetin improved renal function parameters with the exception of blood glucose. The overexpressions of the pathogenic markers TGF-β1 and CTGF in diabetic kidneys were attenuated by the administration of quercetin [33]. We had tested the mixture of quercetin compounds in vitro, and found that it can effectively inhibit not only the expressions of vimentin and AT-1, but also the activity of DPP-4 (data not shown). Therefore, the effects of F1 could be attributed to these quercetin glycosides, while the influence of minor components still cannot be excluded.
The residue FR is viewed as the portion of AE deducting F1 and F2. In comparison with our previous study, FR would be the mixture yields F3, F4 and F5 [16]. Although F3 and F4 cannot effectively reduce vimentin, the inhibition on DPP-4 is still considered valuable for the prevention of diabetic renal damage. The activity of F4 should be somewhat attributed to glycoproteins, with their protein portion being speculated rich in leucine, alanine, and glycine [16].
Both DPP-4 inhibitors and the GLP-1 agonist are useful remedies for treatment of type 2 diabetes. The DPP-4 inhibitor improved renal function parameters including albuminuria and CCr, or histological changes of tubular-interstitial volume and glomerular basement membrane thickness. However, the effects appear to be independent of blood glucose lowering [11,28,34]. In a clinical trial, one of the DPP-4 inhibitors linagliptin lowered the albuminuria in type 2 diabetic patients independent of the HbA1C level [29]. Our previous report demonstrated DPP-4 per se mediated the downstream cascades of IRS-1(Ser 307) and vimentin in the pathogenesis of renal EMT [29]. While palmitate brought about insulin resistance in tubular cells, linagliptin completely recovered insulin sensitivity and reduce vimentin, suggesting the critical role of DPP-4 in insulin resistance and the related tubular change [35]. Recently, it was reported GLP-1 treatment protected kidneys from saturated fatty acid-induced endoplasmic reticulum stress and apoptosis in tubular cells [36]. Hence the regulation of DPP-4 and GLP-1R by AE may directly act and mediate the renal-protective signals.
To our best knowledge, this is the first study systemically exploring the actions of AE subfractions based on the in vivo insulin resistance, diabetes model. In conclusion, using the type 2 diabetic rats, we have successfully shown that AE has benefits to regulate DPP-4 and GLP-1R, reduce oxidative stress and renal fibrosis, and thus improve the renal function and prevent diabetic renal damage. Among all the AE subfractions, F2 could be more promising to be developed as adjuvant for diabetic nephropathy.
Acknowledgement
This work was supported by the grant MOST 104-2320-B-241-001, and MOST 1052320-B-241-004-MY2 of Ministry of Science and Technology, Taiwan.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jfda.2018.07.004.
Funding Statement
This work was supported by the grant MOST 104-2320-B-241-001, and MOST 1052320-B-241-004-MY2 of Ministry of Science and Technology, Taiwan.
REFERENCES
- 1.A CDC. National diabetes fact sheet. Publications, Diabetes DDT; 2005. [Google Scholar]
- 2. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21(9):1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 3. Rodbard H, Blonde L, Braithwaite S, Brett E, Cobin R, Handelsman Y, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(Suppl 1):1–68. doi: 10.4158/EP.13.S1.1. [DOI] [PubMed] [Google Scholar]
- 4. Nakagawa T. A new mouse model resembling human diabetic nephropathy: uncoupling of VEGF with eNOS as a novel pathogenic mechanism. Clin Nephrol. 2009;71(2):103. doi: 10.5414/cnp71103. [DOI] [PubMed] [Google Scholar]
- 5.Wang S, Mitu GM, Hirschberg R. Osmotic polyuria: an overlooked mechanism in diabetic nephropathy. Oxford University Press; 2008. [DOI] [PubMed] [Google Scholar]
- 6. Levey AS, Coresh J, Bolton K, Culleton B, Harvey KS, Ikizler TA, et al. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1) [PubMed] [Google Scholar]
- 7. Balakumar P, Arora MK, Reddy J, Anand-Srivastava MB. Pathophysiology of diabetic nephropathy: involvement of multifaceted signalling mechanism. J Cardiovasc Pharmacol. 2009;54(2):129–38. doi: 10.1097/FJC.0b013e3181ad2190. [DOI] [PubMed] [Google Scholar]
- 8. Chaykovska L, von Websky K, Rahnenführer J, Alter M, Heiden S, Fuchs H, et al. Effects of DPP-4 inhibitors on the heart in a rat model of uremic cardiomyopathy. PLoS One. 2011;6(11):e27861. doi: 10.1371/journal.pone.0027861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choi K, Kim Y-B. Molecular mechanism of insulin resistance in obesity and type 2 diabetes. Kor J Intern Med. 2010;25(2):119. doi: 10.3904/kjim.2010.25.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hocher B, Reichetzeder C, Alter ML. Renal and cardiac effects of DPP4 inhibitors–from preclinical development to clinical research. Kidney Blood Press Res. 2012;36(1):65–84. doi: 10.1159/000339028. [DOI] [PubMed] [Google Scholar]
- 11. Groop P-H, Cooper ME, Perkovic V, Emser A, Woerle H-J, von Eynatten M. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care. 2013;36(11):3460–8. doi: 10.2337/dc13-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang Y-S, Huang C-N, Wang C-J, Lee Y-J, Chen M-L, Peng C-H. Polyphenols of Hibiscus sabdariffa improved diabetic nephropathy via regulating the pathogenic markers and kidney functions of type 2 diabetic rats. J Funct Foods. 2013;5(2):810–9. [Google Scholar]
- 13.S Development, and cooperation, policy and global affairs. Vol. 2. Washington, DC: National Research Council Lost Crops of Africa, National Academies Press; 2006. pp. 286–301. [Google Scholar]
- 14.Chang CNAK. The illustrated medical plant of Taiwan. Vol. 3. Taipei, Taiwan: Southern Materials Center; 1995. p. 107. [Google Scholar]
- 15. Lengsfeld C, Titgemeyer F, Faller G, Hensel A. Glycosylated compounds from okra inhibit adhesion of Helicobacter pylori to human gastric mucosa. J Agric Food Chem. 2004;52(6):1495–503. doi: 10.1021/jf030666n. [DOI] [PubMed] [Google Scholar]
- 16. Peng C-H, Chyau C-C, Wang C-J, Lin H-T, Huang C-N, Ker Y-B. Abelmoschus esculentus fractions potently inhibited the pathogenic targets associated with diabetic renal epithelial to mesenchymal transition. Food Funct. 2016;7(2):728–40. doi: 10.1039/c5fo01214g. [DOI] [PubMed] [Google Scholar]
- 17. Huang C-N, Wang C-J, Lee Y-J, Peng C-H. Active subfractions of Abelmoschus esculentus substantially prevent free fatty acid-induced β cell apoptosis via inhibiting dipeptidyl peptidase-4. PLoS One. 2017;12(7):e0180285. doi: 10.1371/journal.pone.0180285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang C-N, Wang C-J, Lin C-L, Lin H-T, Peng C-H. The nutraceutical benefits of subfractions of Abelmoschus esculentus in treating type 2 diabetes mellitus. PLoS One. 2017;12(12):e0189065. doi: 10.1371/journal.pone.0189065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adler AI, Stratton IM, Neil HAW, Yudkin JS, Matthews DR, Cull CA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. Bmj. 2000;321(7258):412–9. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ye J. Role of insulin in the pathogenesis of free fatty acid-induced insulin resistance in skeletal muscle, endocrine, metabolic & immune disorders-drug targets (formerly current drug targets-immune. Endocr Metab Disord. 2007;7(1):65–74. doi: 10.2174/187153007780059423. [DOI] [PubMed] [Google Scholar]
- 21. Ravelojaona V, Molinari J, Robert L. Protection by rhamnose-rich polysaccharides against the cytotoxicity of Maillard reaction products. Biomed Pharmacother. 2006;60(7):359–62. doi: 10.1016/j.biopha.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 22. Chen SC, Guh JY, Hwang CC, Chiou SJ, Lin TD, Ko YM, et al. Advanced glycation end-products activate extracellular signal-regulated kinase via the oxidative stress-EGF receptor pathway in renal fibroblasts. J Cell Biochem. 2010;109(1):38–48. doi: 10.1002/jcb.22376. [DOI] [PubMed] [Google Scholar]
- 23. Gasic-Milenkovic J, Loske C, Münch G. Advanced glycation endproducts cause lipid peroxidation in the human neuronal cell line SH-SY5Y. J Alzheim Dis. 2003;5(1):25–30. doi: 10.3233/jad-2003-5104. [DOI] [PubMed] [Google Scholar]
- 24. Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116(2):288–96. doi: 10.1172/JCI27699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li J, Qu X, Bertram JF. Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am J Pathol. 2009;175(4):1380–8. doi: 10.2353/ajpath.2009.090096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peng C-H, Yang Y-S, Chan K-C, Wang C-J, Chen M-L, Huang C-N. Hibiscus sabdariffa polyphenols alleviate insulin resistance and renal epithelial to mesenchymal transition: a novel action mechanism mediated by type 4 dipeptidyl peptidase. J Agric Food Chem. 2014;62(40):9736–43. doi: 10.1021/jf5024092. [DOI] [PubMed] [Google Scholar]
- 27. Yang Y-S, Wang C-J, Huang C-N, Chen M-L, Chen M-J, Peng C-H. Polyphenols of Hibiscus sabdariffa improved diabetic nephropathy via attenuating renal epithelial mesenchymal transition. J Agric Food Chem. 2013;61(31):7545–51. doi: 10.1021/jf4020735. [DOI] [PubMed] [Google Scholar]
- 28. Liang CM, Tai MC, Chang YH, Chen YH, Chen CL, Lu DW, et al. Glucosamine inhibits epithelial-to-mesenchymal transition and migration of retinal pigment epithelium cells in culture and morphologic changes in a mouse model of proliferative vitreoretinopathy. Acta Ophthalmol. 2011;89(6) doi: 10.1111/j.1755-3768.2011.02147.x. [DOI] [PubMed] [Google Scholar]
- 29. Park J, Lee S-Y, Ooshima A, Yang K-M, Kang JM, Kim Y-W, et al. Glucosamine hydrochloride exerts a protective effect against unilateral ureteral obstruction-induced renal fibrosis by attenuating TGF-β signaling. J Mol Med. 2013;91(11):1273–84. doi: 10.1007/s00109-013-1086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Corrado F, D’Anna R, Di Vieste G, Giordano D, Pintaudi B, Santamaria A, et al. The effect of myoinositol supplementation on insulin resistance in patients with gestational diabetes. Diabet Med. 2011;28(8):972–5. doi: 10.1111/j.1464-5491.2011.03284.x. [DOI] [PubMed] [Google Scholar]
- 31. Kurup SB, Mini S. Averrhoa bilimbi fruits attenuate hyperglycemia-mediated oxidative stress in streptozotocin-induced diabetic rats. J Food Drug Anal. 2017;25(2):360–8. doi: 10.1016/j.jfda.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anjaneyulu M, Chopra K. Quercetin, an anti-oxidant bioflavonoid, attenuates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2004;31(4):244–8. doi: 10.1111/j.1440-1681.2004.03982.x. [DOI] [PubMed] [Google Scholar]
- 33. Lai P-B, Zhang L, Yang L-Y. Quercetin ameliorates diabetic nephropathy by reducing the expressions of transforming growth factor-β1 and connective tissue growth factor in streptozotocin-induced diabetic rats. Ren Fail. 2012;34(1):83–7. doi: 10.3109/0886022X.2011.623564. [DOI] [PubMed] [Google Scholar]
- 34. Li Y, Zhao S, Zhang W, Zhao P, He B, Wu N, et al. Epigallocatechin-3-O-gallate (EGCG) attenuates FFAs-induced peripheral insulin resistance through AMPK pathway and insulin signaling pathway in vivo. Diabetes Res Clin Pract. 2011;93(2):205–14. doi: 10.1016/j.diabres.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 35. Huang C-N, Wang C-J, Yang Y-S, Lin C-L, Peng C-H. Hibiscus sabdariffa polyphenols prevent palmitate-induced renal epithelial mesenchymal transition by alleviating dipeptidyl peptidase-4-mediated insulin resistance. Food Funct. 2016;7(1):475–82. doi: 10.1039/c5fo00464k. [DOI] [PubMed] [Google Scholar]
- 36. Guo H, Li H, Wang B, Ding W, Ling L, Yang M, et al. Protective effects of glucagon-like peptide-1 analog on renal tubular injury in mice on high-fat diet. Cell Physiol Biochem. 2017;41(3):1113–24. doi: 10.1159/000464118. [DOI] [PubMed] [Google Scholar]