Abstract
A reliable, selective and rapid multiclass method has been developed for the simultaneous determination of 55 antibacterial drug residues in shrimp muscle samples by ultra high performance liquid chromatography-tandem mass spectrometry. The investigated compounds comprise of eight different classes, namely fluoroquinolones, sulfonamides and synergistic agents, tetracyclines, macrolides, lincosamides, penicillins, nitroimidazole and amphenicols. A simple liquid extraction procedure was developed consisting of extraction with a mixture of acetonitrile and ethylenediaminetetraacetic acid (EDTA), followed by a defatting step with n-hexane. Chromatographic conditions were optimized, obtaining a running time <10 min. Mean recoveries ranged from 74.3% to 113.3%. For precision test, relative standard deviations (RSD, %) were lower than 15.0% and 24.0% for repeatability and reproducibility, respectively. Limits of detection and quantification ranged from 1.0 to 5.0 ng/g and 3.0–10.0 ng/g, respectively. Finally, the method was applied to real samples and the results demonstrated that enrofloxacin, ciprofloxacin, pefloxacin and doxycycline were quantifiable in shrimp samples.
Keywords: Antimicrobial drugs, Multiclass analysis, Shrimp, UHPLC-MS/MS
1. Introduction
Shrimp farming has grown significantly over recent decades, production now exceeds the global catch of all marine shrimps [1]. In 2010, the value of shrimp production accounted for approximately 15% of the total value of globally traded fishery products which made shrimps the largest single commodity. In terms of economic value, shrimp markets continue to show positive growth [2]. However, shrimp farming has faced serious disease outbreaks from viral, bacterial and parasitic infections causing significant economic loss [3]. For this reason, antimicrobial drugs have been widely employed to prevent and treat infectious disease. The use of these agents in aquatic animal production has been an issue in the past, and is of increasing concern for consumers in many countries. The misuse of these compounds including extra label and inappropriate use, can promote the persistence of drugs in animal products. The presence of antimicrobial drug residues in animal products can cause severe adverse health problems for consumers, such as allergic reactions in hypersensitive individuals. Moreover, prolonged exposure of low level doses of antimicrobial drugs may result in development of resistance in pathogenic bacteria, which can transfer from foods to humans, and initiate gene mutations [4–7]. There are several groups of antimicrobial drugs commonly applied in current veterinary practice, such as fluoroquinolones (FQs), sulfonamides (SAs), tetracyclines (TCs), amphenicols (APs), penicillins (PENs), macrolides (MCs), lincosamides (LINs), nitroimidazoles (NIs), and synergistic agents [8]. In addition, the usage of multiple compounds or multiple classes of drug has been noted in food-producing animals. Due to perceived economic benefit, farmers freely use antimicrobial drugs despite their limited knowledge about these agents [5,9,10]. To ensure food safety and protect consumer health, European Union (EU) has defined maximum residue limits (MRLs) for residues of veterinary drugs in food [11]. These limits require the development of sensitive, specific, accurate and precise methods for analysis of the trace residues in food. There have been a number of reports on the variety of chemical analysis techniques used for detecting multiple classes of antimicrobial residues in food of animal origin. Microbiological assays, based on growth inhibition in microorganisms of interest, are commonly used to determine the presence of antimicrobial residues because these methods are somewhat simple, fast and inexpensive. However, these methods generally cannot separate between members of a class of drug, lack sensitivity, and can only provide semi-quantitative measurements of the total amount of residues. These methods sometimes give false-positive results by detecting drug residues at a level far below the officially mandated safe levels [12–14].
Given these limitations, high performance liquid chromatography (HPLC) operated with tandem mass spectrometry (MS/MS) has become the predominant technique for detecting multiple drug classes. It combines analyte separation with structural information, for monitoring antibacterial residues in food matrices such as shrimp. The method requires simple sample pretreatment, provides rapid sample throughput, unambiguous identification and reliable confirmation. Interference is reduced, especially when multiple reaction monitoring (MRM) mode is used [15,16]. Nowadays, the use of ultra high performance liquid chromatography (UHPLC) shows a variety of advantages in relation to HPLC in terms of selectivity, sensitivity and resolution. In addition, it has features such as reduced time required for analysis, this is likely to be attractive for laboratories running large numbers of routine samples [17–20]. Despite this, the method has limitations for the simultaneous determination of antibacterial drugs from different classes in complex biological matrices, such as shrimp muscle, mainly due to differences in physicochemical properties of the drugs [17,21]. Hence, it is a challenge to develop an UHPLC-MS/MS method, which can simultaneously detect multiple residues, and multiple classes of antimicrobial drugs in shrimp products. To date, only a small number of methods for detecting and quantifying multiple classes of drug in shrimp muscle are available. Villar–Pulido et al. developed a fast liquid chromatography time-of-flight mass spectrometry (LC-TOFMS) method for simultaneous quantification of 7 residues of selected antibiotics and other veterinary drugs (benzalkonium chloride, ethoxyquin, leucomalachite green, malachite green, mebendazole, sulfathiazole and trimethoprim) in shrimp. The selected extraction method was QuEChERS. Recovery rates were in the range of 58–133%. Although it can be applied for screening and quantification purposes, it cannot be used as a confirmatory method because of the requirements of legislation [15] positive findings need to be confirmed using a MS/MS detector [22]. Storey et al. proposed the HPLC-MS/MS screening method for the detection and identification of 26 veterinary drugs in fish and shrimp. The analytes included 13 sulfonamides, trimethoprim, three fluoroquinolones, three quinolones, three triphenylmethane dyes, two leuco dye metabolites, and one hormone. In this method, tissue is mixed with ethylenediaminetetraacetic acid (EDTA)-Mcllvaine buffer, double-extracted with acetonitrile, p-toluenesulfonic acid (p-TSA acid) and N,N,N′,N′-tetramethyl-p-phenylenediamine dihydrochloride (TMPD) [23]. Kim et al. described a multi-class, multi-residue analytical method based on UHPLC-MS/MS detection, this was developed for the screening and confirmation of 28 veterinary drugs and metabolite residues in fish and shrimp. To achieve fast and simultaneous extraction of various analytes, a simple and generic liquid extraction procedure using EDTA-ammonium acetate buffer and acetonitrile, without clean-up step, was applied to sample preparation. Mostly, the recoveries were in the range of 60–110%, and precision, expressed as the relative standard deviation (RSD), was in the range of 5–15% [24]. To the best of our knowledge, this work is the first UHPLC-ESI-MS/MS method for the simultaneous determination of drugs belonging to different physiochemical classes in trace amounts in shrimp muscle.
This research aimed to develop and validate a rapid, effective UHPLC-MS/MS and simple liquid-extraction method for detection of 55 compounds in shrimp muscle. These compounds consisted of 52 antimicrobial drugs from 8 different families, namely Fluoroquinolones (FQs), Sulfonamides (SAs), Tetracyclines (TCs), Macrolides (MCs), Lincosamides (LINs), Penicillins (PENs), Nitroimidazoles (NIs) and Amphenicols (APs), and included 3 synergistic agents, namely, trimethoprim, ormetoprim and dapsone.
2. Materials and methods
2.1. Chemicals and reagents
All antimicrobial standards were purchased from Sigma–Aldrich (St. Louis, MO, USA). The investigated analytes are listed in Table 1. Stock standard solutions of each compound at concentration 100 μg/mL were prepared in the appropriate solvents. Sulfonamides, synergistic agents, macrolides and amphenicols were prepared in acetonitrile. Penicillins were prepared in acetonitrile:water (1:1, v/v), and the other classes of antimicrobial drugs were dissolved in methanol. The solutions were stored in the dark at −20 °C away from light for three months (except penicillins, these were kept at 4 °C). Cocktail working standard solutions of each group were prepared by combining each of individual stock solution and diluting with acetonitrile to a final concentration of 1 μg/mL, and they were stored at 4 °C in the dark. These solutions were re-prepared weekly. Suitable dilutions were also prepared to facilitate spiking for both the validation process and the routine analysis. All reagents were of analytical grade with the exception of chemicals used for the mobile phase, which were of HPLC grade. Acetonitrile, methanol and formic acid were HPLC grade, and were purchased from RCI Labscan (Bangkok, Thailand), Honey well (New Jersey, USA) and Merck (New Jersey, USA), respectively. Ethyl acetate and n-hexane were purchased from Avantor (Seoul, Korea). Ethylenediamine- tetra-acetic acid disodium salt (Na2EDTA) and sodium sulfate (Na2SO4) were purchased from Merck (Schiphol- Rijk, Netherlands). Ultrapure water with 18.2 MΩ/cm was obtained from a Millipore Milli-Q system (Bedford, MA, USA).
Table 1.
The MS/MS parameters of the selected antibacterial drugs.
| Compound | Abbreviated name | Precursor ion | Product ion | Fragmentator (V) | Collision energy (eV) | Retention time (min) |
|---|---|---|---|---|---|---|
| Fluoroquinolones | FQs | |||||
| Enrofloxacin | ENR | 360.2 | 316.2 | 156 | 16 | 3.68 |
| 286.1 | 156 | 36 | ||||
| Ciprofloxacin | CIP | 332.1 | 231.1 | 150 | 40 | 3.49 |
| 288.2 | 150 | 20 | ||||
| Difloxacin | DIF | 400.1 | 356.2 | 156 | 16 | 3.97 |
| 299.1 | 156 | 32 | ||||
| Sarafloxacin | SAR | 386.1 | 368.1 | 150 | 20 | 3.92 |
| 299.1 | 150 | 40 | ||||
| Norfloxacin | NOR | 320.0 | 302.1 | 150 | 20 | 3.42 |
| 231.1 | 150 | 40 | ||||
| Danofloxacin | DAN | 358.2 | 340.1 | 151 | 20 | 3.58 |
| 82.1 | 151 | 48 | ||||
| Ofloxacin | OFL | 362.2 | 318.2 | 150 | 20 | 3.44 |
| 261.1 | 150 | 40 | ||||
| Gatifloxacin | GAT | 376.3 | 261.0 | 130 | 36 | 3.82 |
| 289.0 | 130 | 23 | ||||
| Moxifloxacin | MOX | 402.2 | 384.2 | 150 | 20 | 4.09 |
| 96.1 | 150 | 40 | ||||
| Pefloxacin | PEF | 334.0 | 316.1 | 132 | 19 | 3.46 |
| 290.1 | 132 | 19 | ||||
| Marbofloxacin | MAR | 363.2 | 72.2 | 144 | 28 | 3.35 |
| 320.1 | 144 | 12 | ||||
| Fleroxacin | FLE | 370.1 | 326.1 | 150 | 20 | 3.45 |
| 58.1 | 150 | 40 | ||||
| Sparfloxacin | SPA | 393.1 | 349.0 | 140 | 20 | 3.96 |
| 292.0 | 140 | 23 | ||||
| Orbifloxacin | ORB | 396.0 | 352.0 | 120 | 15 | 3.76 |
| 295.0 | 120 | 22 | ||||
| Oxolinic acid | OXO | 262.1 | 202.0 | 114 | 32 | 6.59 |
| 215.9 | 114 | 29 | ||||
| Flumequine | FLU | 262.1 | 202.0 | 108 | 32 | 6.59 |
| 244.1 | 108 | 12 | ||||
| Nalidixic acid | NAL | 233.1 | 215.1 | 150 | 20 | 6.29 |
| 104.0 | 150 | 40 | ||||
| Sulfonamides | SAs | |||||
|
| ||||||
| Sulfadiazine | SDZ | 251.1 | 92.1 | 96 | 28 | 3.16 |
| 156.0 | 96 | 8 | ||||
| Sulfadimethoxine | SDMX | 311.1 | 156.0 | 128 | 16 | 5.27 |
| 92.1 | 128 | 36 | ||||
| Sulfamerazine | SMR | 265.1 | 92.1 | 114 | 28 | 3.60 |
| 156.0 | 114 | 12 | ||||
| Sulfamethazine | SMZ | 279.1 | 186.1 | 132 | 12 | 3.92 |
| 92.1 | 132 | 32 | ||||
| Sulfamethoxazole | SMXZ | 254.1 | 92.1 | 108 | 24 | 4.58 |
| 156.0 | 108 | 12 | ||||
| Sulfaquinoxaline | SQX | 301.1 | 156.0 | 118 | 16 | 5.27 |
| 92.0 | 118 | 32 | ||||
| Sulfachloropyridazine | SCP | 285.0 | 156.0 | 108 | 12 | 4.39 |
| 92.1 | 108 | 24 | ||||
| Sulfadoxine | SDX | 311.1 | 156.0 | 126 | 16 | 4.58 |
| 92.1 | 126 | 32 | ||||
| Sulfamethoxypyridazine | SMPZ | 281.1 | 156.0 | 120 | 12 | 3.94 |
| 92.1 | 120 | 32 | ||||
| Sulfamoxole | SMX | 268.1 | 92.0 | 150 | 20 | 3.74 |
| 108.0 | 150 | 20 | ||||
| Sulfonamides | SAs | |||||
|
| ||||||
| Sulfamethizole | SMTZ | 271.0 | 156.0 | 150 | 10 | 3.89 |
| 108.0 | 150 | 20 | ||||
| Sulphisoxazole | SFX | 268.1 | 92.0 | 150 | 40 | 4.77 |
| 108.0 | 150 | 20 | ||||
| Sulfathiazole | STZ | 256.0 | 92.1 | 102 | 28 | 3.31 |
| Sulfaphenazole | SPZ | 315.1 | 158.1 | 150 | 40 | 5.42 |
| 92.0 | 150 | 40 | ||||
| Sulfapyridine | SPD | 250.1 | 92.0 | 150 | 20 | 3.43 |
| 108.0 | 150 | 20 | ||||
| Sulfamonomethoxine | SMM | 281.0 | 155.9 | 120 | 17 | 4.22 |
| 126.0 | 120 | 21 | ||||
| Synergistic agents | SYNs | |||||
|
| ||||||
| Trimethoprim | TMP | 291.2 | 230.1 | 151 | 20 | 3.38 |
| 123.1 | 151 | 24 | ||||
| Ormetoprim | OMP | 275.2 | 123.0 | 122 | 25 | 3.54 |
| 259.0 | 122 | 25 | ||||
| Dapsone | DAP | 249.1 | 92.0 | 151 | 28 | 4.29 |
| 156.0 | 151 | 10 | ||||
| Tetracyclines | TCs | |||||
|
| ||||||
| Oxytetracycline | OTC | 461.0 | 426.0 | 120 | 20 | 3.50 |
| 444.0 | 120 | 10 | ||||
| Tetracycline | TC | 445.2 | 410.1 | 150 | 20 | 3.67 |
| 98.1 | 150 | 40 | ||||
| Chlortetracycline | CTC | 479.1 | 444.0 | 120 | 20 | 4.22 |
| 462.0 | 120 | 12 | ||||
| Doxycycline | DC | 445.2 | 428.1 | 113 | 16 | 4.36 |
| 321.1 | 113 | 28 | ||||
| Macrolides | MCs | |||||
| Erythromycin | ERY | 734.5 | 158.1 | 171 | 32 | 5.13 |
| 576.3 | 171 | 16 | ||||
| Tilmicosin | TIL | 869.6 | 174.1 | 294 | 48 | 4.43 |
| 696.4 | 294 | 44 | ||||
| Tylosin | TYL | 916.5 | 174.1 | 228 | 44 | 5.63 |
| 101.0 | 228 | 56 | ||||
| Josamycin | JOS | 828.5 | 174.1 | 228 | 36 | 6.91 |
| 109.0 | 228 | 56 | ||||
| Spiramycin I | SPI | 843.5 | 101.0 | 150 | 52 | 3.97 |
| 43.2 | 150 | 68 | ||||
| Lincosamides | LINs | |||||
|
| ||||||
| Lincomycin | LIN | 407.2 | 126.1 | 150 | 24 | 3.10 |
| 359.2 | 150 | 16 | ||||
| Clindamycin | CLI | 425.2 | 126.1 | 150 | 20 | 4.25 |
| 377.2 | 150 | 20 | ||||
| Penicillins | PENs | |||||
|
| ||||||
| Ampicillin | AMP | 350.0 | 106.0 | 113 | 16 | 3.26 |
| 160.0 | 113 | 4 | ||||
| Oxacillin | OXA | 402.0 | 160.0 | 113 | 8 | 6.91 |
| 243.0 | 113 | 8 | ||||
| Dicloxacillin | DIC | 470.0 | 160.0 | 113 | 10 | 7.36 |
| 311.0 | 113 | 6 | ||||
| Penicillin G | PEN G | 335.1 | 160.0 | 65 | 6 | 5.82 |
| 176.1 | 65 | 6 | ||||
| Nitroimidazoles | NIs | |||||
|
| ||||||
| Dimetridazole | DMZ | 142.1 | 96.1 | 100 | 16 | 3.10 |
| 81.1 | 100 | 28 | ||||
| Metronidazole | MNZ | 172.1 | 128.0 | 98 | 12 | 2.74 |
| 82.1 | 98 | 24 | ||||
| Ronidazole | RNZ | 201.1 | 140.1 | 80 | 8 | 3.08 |
| 55.2 | 80 | 20 | ||||
| Amphenicols | APs | |||||
|
| ||||||
| Florfenicol | FF | 356.0 | 185.1 | 141 | 12 | 4.65 |
| 336.0 | 141 | 4 | ||||
2.2. Samples and sample preparation
Shrimp samples were collected during October–December 2017 from local market in Thailand, and they were confirmed to be free of selected drug residues by UHPLC-MS/MS. The head, shell, tail and intestinal organs of the shrimp were removed prior to analysis. Muscle tissues were blended using a Moulinex mixer, and stored at −20 °C until analysis. Then, the targeted residues were extracted from shrimp muscle using simple liquid extraction modified from Freitas A. et al. [25]. Briefly, the optimized procedure was as follows; 1.0 g of homogenized sample was weighed into a 50 mL plastic centrifuge tube followed by addition of 1 g of Na2SO4 anhydrous, 800 μL of 0.05 M EDTA solution, 3 mL of n-hexane and 4 mL of acetonitrile, respectively. Then, the mixture was homogenized using a homogenizer until it was homogeneous sample. It was centrifuged at 4000 rpm (2688 × g), 4 °C for 10 min. After centrifugation, the acetonitrile layer was transferred to a 15mL glass tube, and the pellet was re-extracted by addition of 3 mL of n-hexane, and 4 mL of acetonitrile. The sample was shaken for 5 min, and centrifuged again, as described above. The acetonitrile layer was then transferred to the glass tube before the total acetonitrile layer was evaporated to dryness at 40 °C under a nitrogen stream. Finally, the residue was dissolved with 0.5 mL of a mixture of 0.1% formic acid solution and acetonitrile (1:1, v/v). The extract was filtered by passing through a 0.22 μm Nylon membrane filter (Sartorius AG, Goettingen, Germany) and then injected into the unit for UHPLC-MS/MS.
2.3. Instrumental and chromatographic conditions
UHPLC analysis was performed using an Agilent 1290 infinity (Agilent Technologies, Waldbronn, Germany) consisting of a binary pump, a degasser, a column oven and an auto sampler. The chromatographic separation was achieved on a ZORBAX RRHD Eclipse plus C18 column (100 × 2.1 mm, 1.8 μm particle size). The column was maintained at 40 °C. The mobile phase consisted of 0.1% formic acid in water (mobile phase A) and acetonitrile (mobile phase B). The gradient program of the mobile phase was as follows: 5% B (initial for 0.5 min), 5–35% B (5 min) and 95% B (10 min). The column was re-equilibrated for 5 min between injections. The flow rate was 0.3 mL/min while the injection volume was 5 μL. The chromatographic run time was 10 min.
The mass spectrometer used was a triple quadrupole analyzer (6460 triple, Agilent Technologies, Waldbronn, Germany) equipped with an electrospray ionization source run in both positive and negative ion modes under the multiple reaction monitoring (MRM) mode with two separate chromatographs analyzed per sample. The ionization source parameters were optimized as follows: capillary voltage 3500 V, gas temperature 300 °C, gas flow rate 8 L/min and nebulizer pressure 45 psi. The parameters (for the mass spectrometer) were optimized as shown in Table 1.
2.4. Validation procedure
Performance characteristics of the optimized method was determined to assess the efficiency of this analytical method by investigating the selectivity, matrix effect, linearity, limit of detection, limit of quantitation, accuracy, precision and applicability in accordance with the European Commission regulation for the performance of analytical methods [15].
3. Results and discussion
3.1. Optimization of UHPLC-MS/MS conditions
For multi-class analytical method, UHPLC-MS/MS was developed for simultaneous analysis in the single run with positive and negative electrospray ionization mode. For both ionization modes, the best sensitivity for all drugs was determined to provide the highest signals for quantification and confirmation. It was difficult to simultaneously evaluate 55 antimicrobial drugs with a short dwell time. Thus, dynamic multiple reaction monitoring (d-MRM) was used. The advantage of d-MRM is that it allows the MS/MS system to be focused directly on the expected retention time for an analyte in a defined retention time window. The sensitivity can be enhanced since optimal dwell times can be automatically achieved under d-MRM by reducing the number of concurrent ion transitions [26].
To achieve maximum response, standard solutions (0.5 μg/mL) for individual analytes were injected directly into the quadrupole mass spectrometer without the analytical column connected and detected in full scan ion detection mode to obtain two transitions per compound as precursor and product ions. The deprotonated molecular ion [M–H]− was selected as the precursor ion for amphenicols whereas the rest of the compounds were detected as the protonated molecular ion [M+H]+. Proposed mass fragmentation patterns of investigated antimicrobial drugs using the method described below and the results obtained in this work concur with previous works that have already been discussed [27–31].
For FQs, the most intense fragment ion observed is [MH–H2O]. The loss of CO2 and the further loss of piperazinic ring fragments (C2H5N for SAR, C3H7N for DIF and C4H9N for ENR) are other product ions for FQs with a piperazinyl ring. Oxolinic acid (OXO) and flumequine (FLU), which have no piperazinyl ring, showed less fragmentation. Besides the [MH–H2O] ions at m/z 216 and 202 are the most intense fragments observed for OXO and FLU, respectively. For SAs, two characteristic fragment ions with m/z 156 and 92 were observed. The former corresponds to the common molecular fragment for all SAs, the p-sulfoaniline group, and the ion with m/z 92 was the result of the loss of the sulfonyl group from this structure. TCs have a structure formed by an octahydrotetracene-2-carboxamide skeleton. Characteristic fragmentation patterns for the tetracyclines studied are the loss of water or ammonia. MCs are 12-, 14- or 16-membered macrocyclic lactone with sugar moieties attached to them by a glycosidic bond. In general, the loss of sugar moiety is the most intense fragment ion observed. Also, the sugar specific group can form product ions, which is demonstrated by the presence of ions at m/z 174 and 158 corresponding to the two sugar moiety [orthodesosamine + H]+ and [desosamine + H]+, respectively. LCs are a group of monoglycosides containing an amino acid-like side chain. Both LINs, lincomycin (LIN) and clindamycin (CLI) shared a fragment of m/z 126 corresponding to the loss of the monoglycoside with amino acid-like side chain ring. Characteristics fragmentation ions for the LINs studied are the loss of thiomethyl group [M-SCH3]+ on monoglycoside ring. The basic structure of PENs consists of a thiazolidinic ring condensed on a β-lactam ring with a lateral chain. All PENs studied share a fragment of m/z 160 corresponding to the thiazolidinic ring. The characteristic fragment is the result of the presence of the ion formed due to the loss of this fragment [M + H−159]. Another characteristic fragment is the result of the loss of the carboxylic group from the 160 fragment to obtain a fragment of m/z 114. NIs are a group with a 5-nitroimidazole cyclic ring. Characteristic fragmentation patterns for the NIs studied are due to the loss of the chemical group at the side-chain on the imidazole ring. The characteristic is the presence of the ion formed due to the loss of nitro group. FF is a representative of the amphenicol group as it contains a p-sulfomethyl phenyl ring bonded with a fluoropropaneidiol moiety and a dichloroacetamide side chain. The product ion was dominated by neutral loss of HF to give [M−HF]– ions and another characteristic fragment is due to the loss of dichloroacetamide side chain.
Different mobile phases consisting of methanol or acetonitrile, and acidified water with different concentrations of formic acid (0.01% and 0.1%, v/v) were tested to provide the best chromatographic results. The final result showed 0.1% formic acid in water (as aqueous phase) and acetonitrile (as organic phase) provided the best overall sensitivity. In order to shorten the analysis time, UHPLC technique was performed using conditions as described in section 2.3. Due to the differences in physiochemical properties of antimicrobial drugs, a gradient program from 95% aqueous phase to 95% acetonitrile was applied to elute 55 compounds within 10 min.
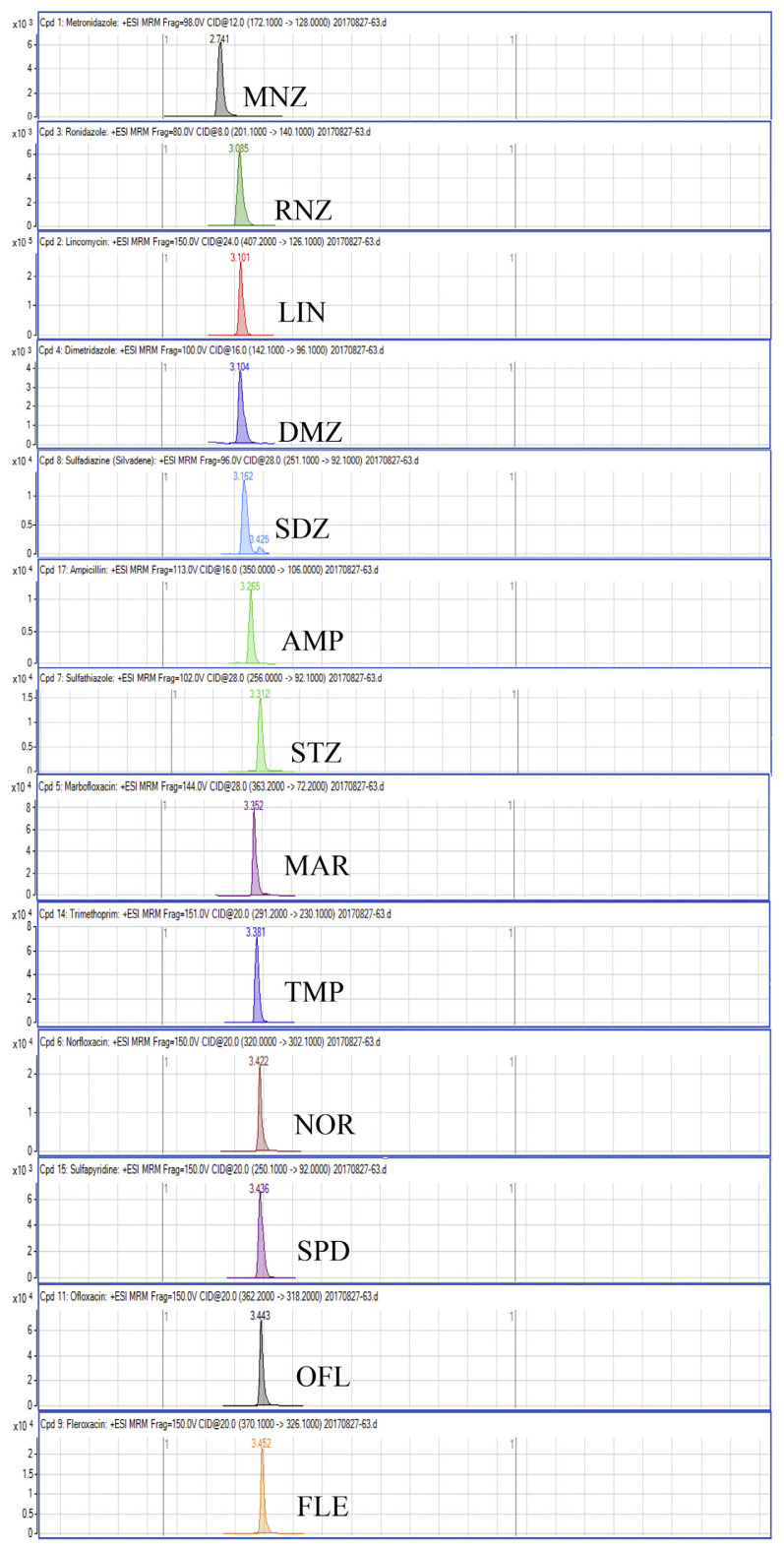
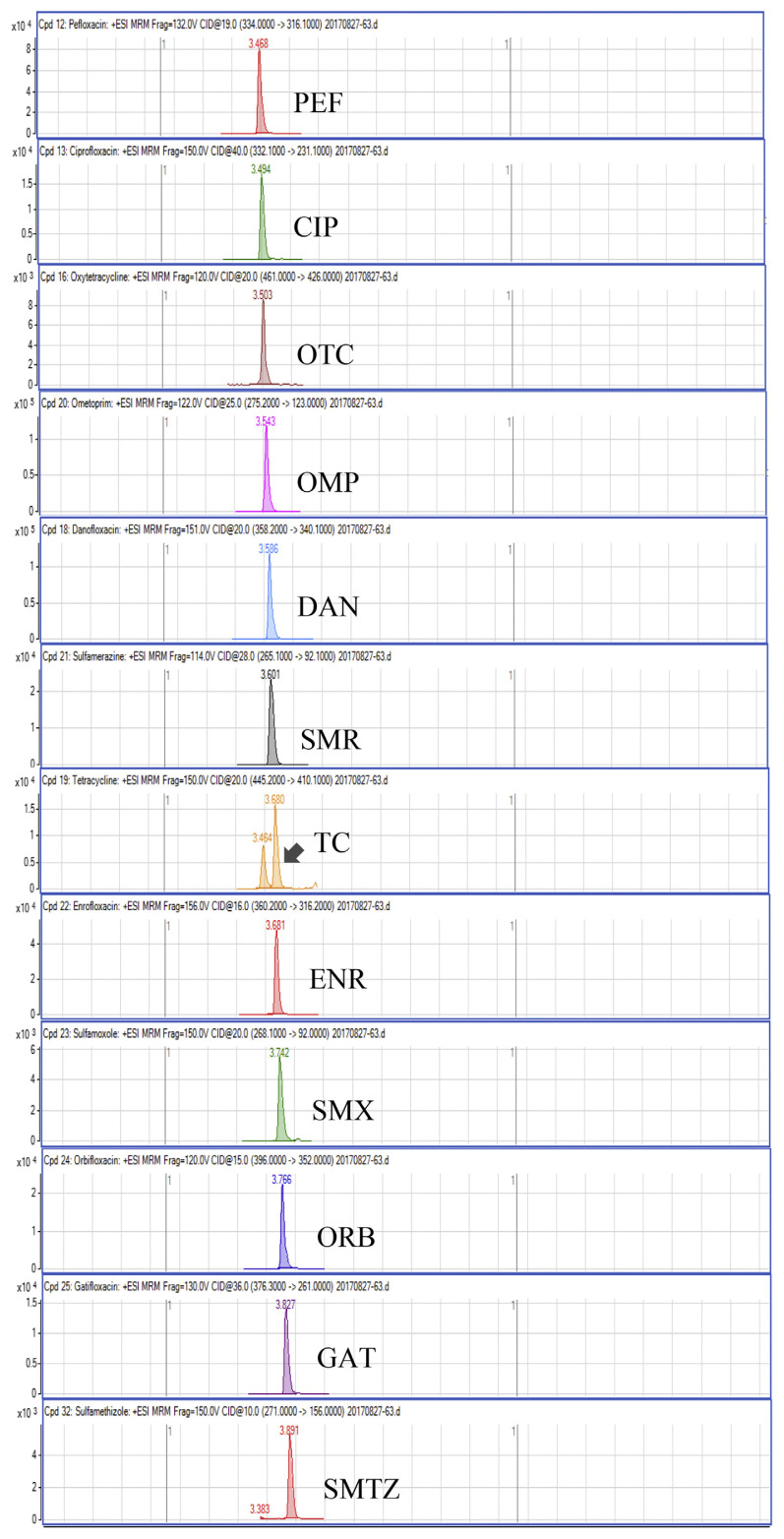
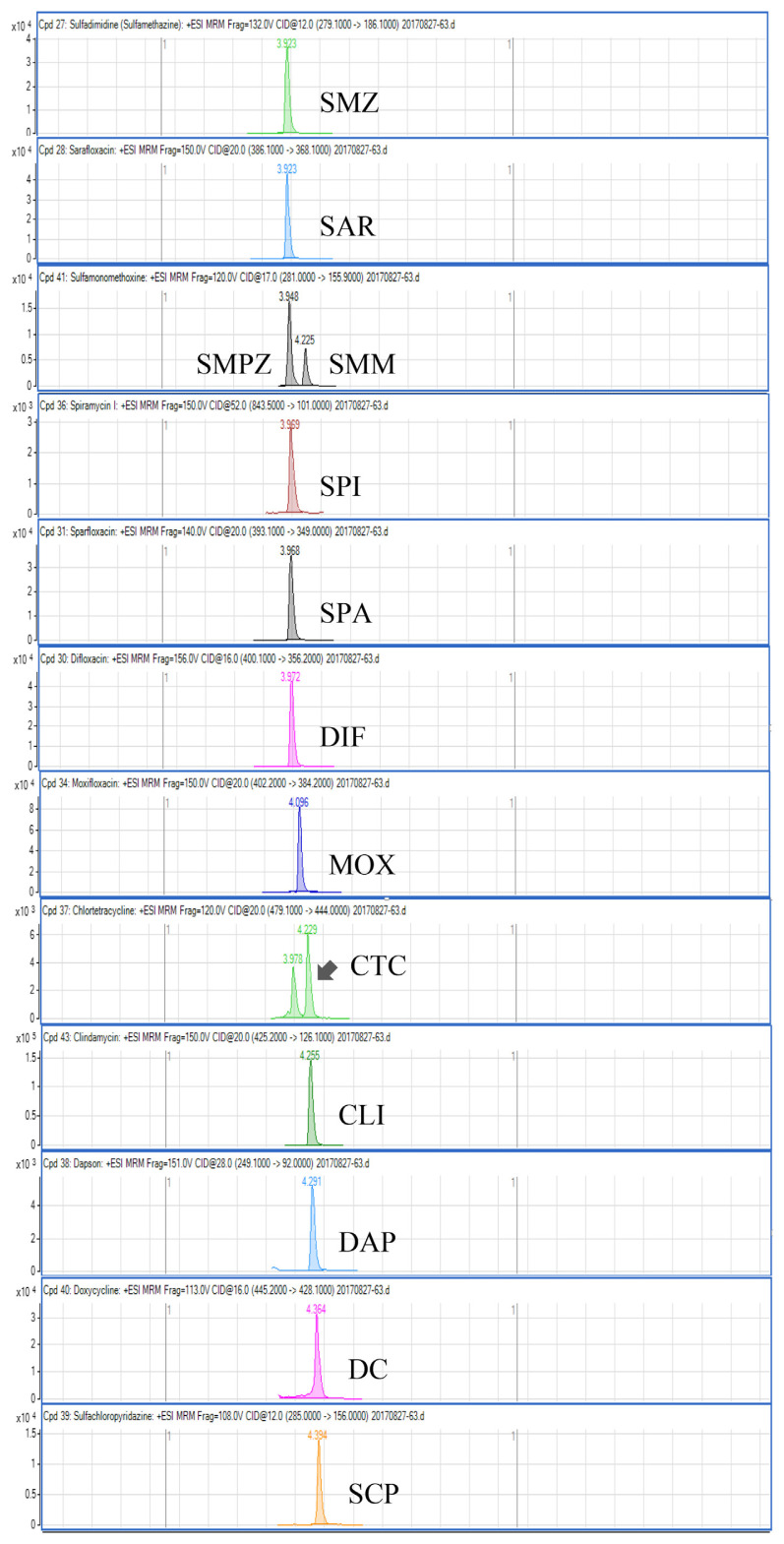
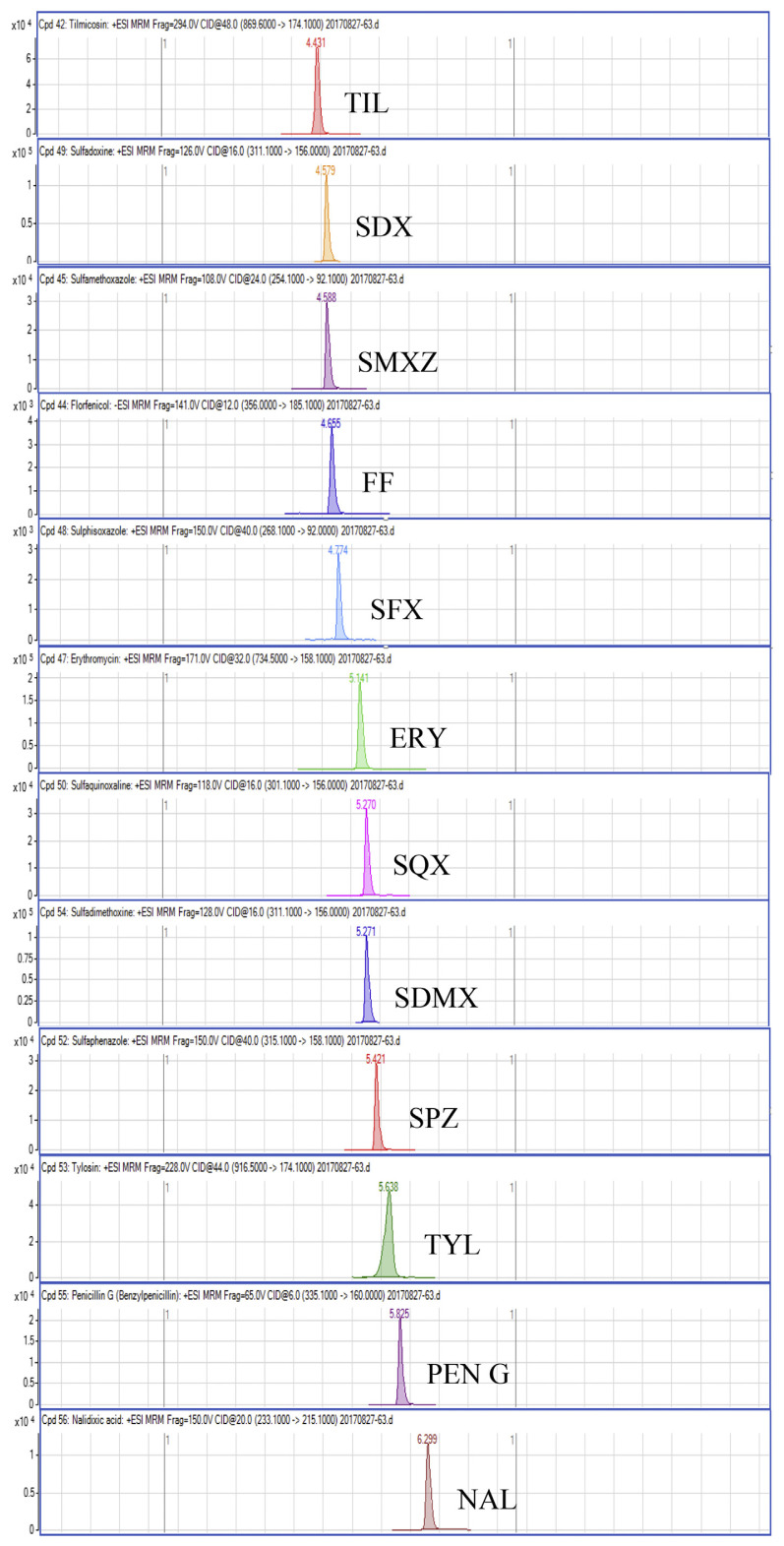
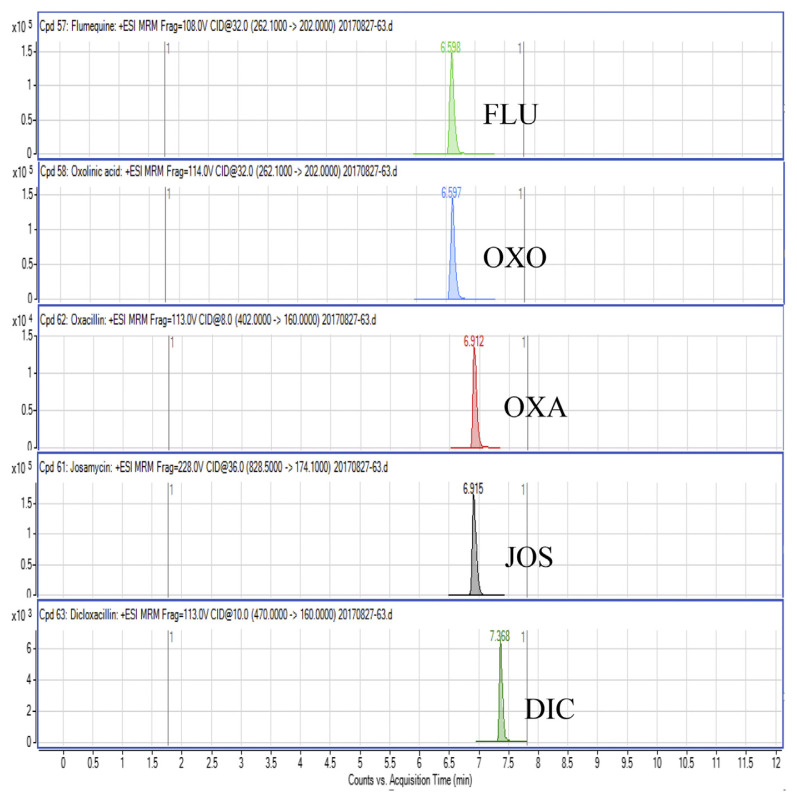
Regarding the results obtained from the optimization of UHPLC-MS/MS conditions, there are five isomeric pairs of compounds that have the mass transitions in common (OXO/FLU, tetracycline (TC)/doxycycline (DC), sulfadimethoxine (SDMX)/sulfadoxine (SDX), sulfamonomethoxine (SMM)/sulfamethoxypyridiazine (SMPZ), sulfamoxole (SMX)/sulphisoxazole (SFX). For OXO/FLU, they were eluted at the same time, but they could be differentiated by specific confirmation ions in the MRM mode. The remaining isomeric compounds could be separated by retention time, and the separation of these compounds was excellent under the developed conditions. Fig. 1 shows a chromatogram of the investigated antimicrobial drugs (quantifier transition ion only, is shown), obtained by spiking a blank sample at 15 ng/g for NIs and 50 ng/g for the other compounds.
Fig. 1.
UHPLC-MS/MS chromatograms of investigated antibacterial drugs spiked at 15 ng/g for nitroimidazoles and 50 ng/g for the other compounds in a blank shrimp muscle sample.
3.2. Optimization of the extraction procedure
Sample preparation is an important part of a multiclass analysis because of the different properties of the antimicrobial drugs that must be extracted simultaneously. A small number of methods for detecting and quantifying multi classes of drug in shrimp are available. Villar–Pulido et al. developed a fast liquid chromatography time-of-flight mass spectrometry (LC-TOFMS) method for simultaneous quantification of 7 residues of selected antibiotics and other veterinary drugs (benzalkonium chloride, ethoxyquin, leucomalachite green, malacite green, mebendazole, sulfathiazole and trimethoprim) in shrimp and used acetonitrile as solvent followed by a clean-up step with primary secondary amine (QuEChERS). Recovery rates were in the range of 58–133% [22]. Storey et al. proposed the HPLC-MS/MS screening method for the detection and identification of 26 veterinary drugs in fish and shrimp. The analytes included 13 sulfonamides, trimethoprim, three fluoroquinolones, three quinolones, three triphenylmethane dyes, two leuco dye metabolites, and one hormone. In this method, tissue is mixed with ethylenediaminetetraacetic acid (EDTA)-Mcllvaine buffer, double-extracted with acetonitrile, p-toluenesulfonic acid (p-TSA acid) and N,N,N′,N′-tetramethyl-p-phenylenediamine dihydrochloride (TMPD) [23]. Kim et al. described a multi-class, multi-residue analytical method based on UHPLC-MS/MS detection, this was developed for the screening and confirmation of 28 veterinary drugs and metabolite residues in fish and shrimp. To achieve fast and simultaneous extraction of various analytes, a simple and generic liquid extraction procedure using EDTA-ammonium acetate buffer and acetonitrile, without clean-up step, was applied to sample preparation. Mostly, the recoveries were in the range of 60–110%, and precision, expressed as the relative standard deviation (RSD), was in the range of 5–15% [24]. It is difficult to obtain good recoveries, and minimize the loss of all analytes during the extraction step. Multi-step and complex sample clean-ups can result in loss of some target compounds. Therefore a simple extraction method, described previously in section 2.2, was employed as the extraction method in this work and the recovery values of this work were higher than the previous studies [22–24].
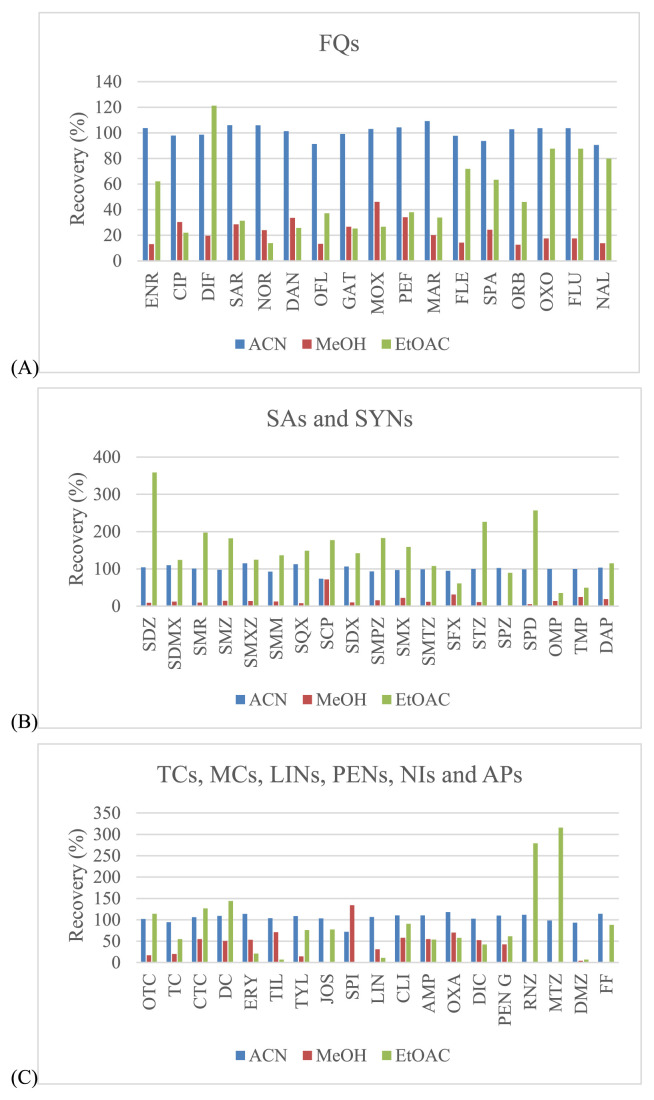
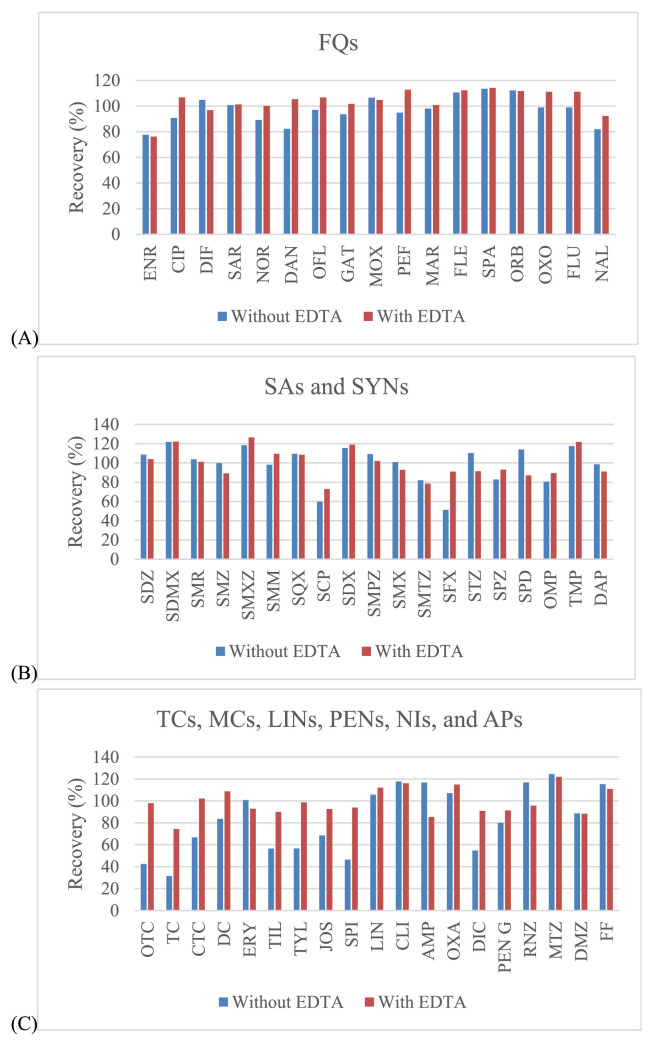
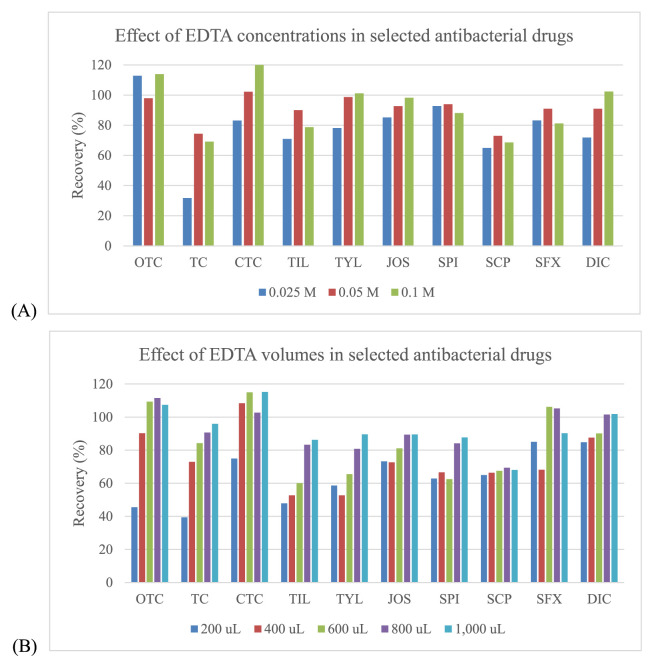
Three organic solvents including acetonitrile, methanol and ethyl acetate were evaluated for use in sample extraction. Acetonitrile showed the best extraction efficiency, and provided satisfied recovery values that were better than those for methanol and ethyl acetate for all drugs (Fig. 2). EDTA can be used in addition to improve the recovery of some antimicrobial drugs since these drugs can form chelation complexes with cations present in solution. However, these complexes can be lost during extraction procedure [32,33]. The EDTA, a quelating agent, was added, especially to compete with antibacterial drugs as tetracyclines and macrolides. It is known that these compounds can form complexes with the bivalent and trivalent cations present in the sample extraction solution which can lead to significant losses of those compounds during the procedure. Thus, EDTA can be used to improve the recovery of some antimicrobial drugs. From our experiments, the recovery was somewhat low for tetracyclines (oxytetracycline (OTC), TC and chlortetracycline (CTC)), macrolides (tilmicosin (TIL), tylosin (TYL), josamysin (JOS) and spiramycin I (SPI)), sulfonamides (sulfachlorpyridazine (SCP) and sulphisoxazole (SFX)) and penicillins (dicloxacillin (DIC)). To avoid this situation, EDTA was also added during the extraction step, whereas the other antimicrobial drugs had satisfactory recovery (Fig. 3). Given this finding, several concentrations (from 0.025 to 0.1 M) and volumes (from 200 to 1000 μL) of EDTA were examined. The results showed that the concentrations at 0.05 M and 0.1 M included the volumes at 800 μL and 1000 μL demonstrated the acceptable recovery for effecting antibacterial drugs. However, the volume of 800 μL (0.05 M) found to be the most appropriate for this procedure (Fig. 4).
Fig. 2.
Effect of different extraction solvents in the solid–liquid extraction procedure for shrimp muscle samples: (A) – fluoroquinolones; (B) – sulfonamides; (C) – the rest of other groups.
Fig. 3.
Effect of EDTA addition on extraction recovery of selected antibacterial drugs from shrimp muscle sample: (A) – fluoroquinolones; (B) – sulfonamides; (C) – the rest of other groups.
Fig. 4.
Effect of concentrations and volumes of EDTA in the selected antibacterial drugs: (A) – Effect of EDTA concentrations; (B) – Effect of EDTA volumes.
3.3. Method validation
The method was validated in shrimp muscle matrix based on European Commission Decision 2002/657/EC [15].
3.3.1. Selectivity
The selectivity was evaluated by analyzing 20 blank shrimp muscle samples using the developed method. The selectivity of this method was good with no interferences, above a signal to noise ratio of 3, at the retention time of each compound.
3.3.2. Matrix effect
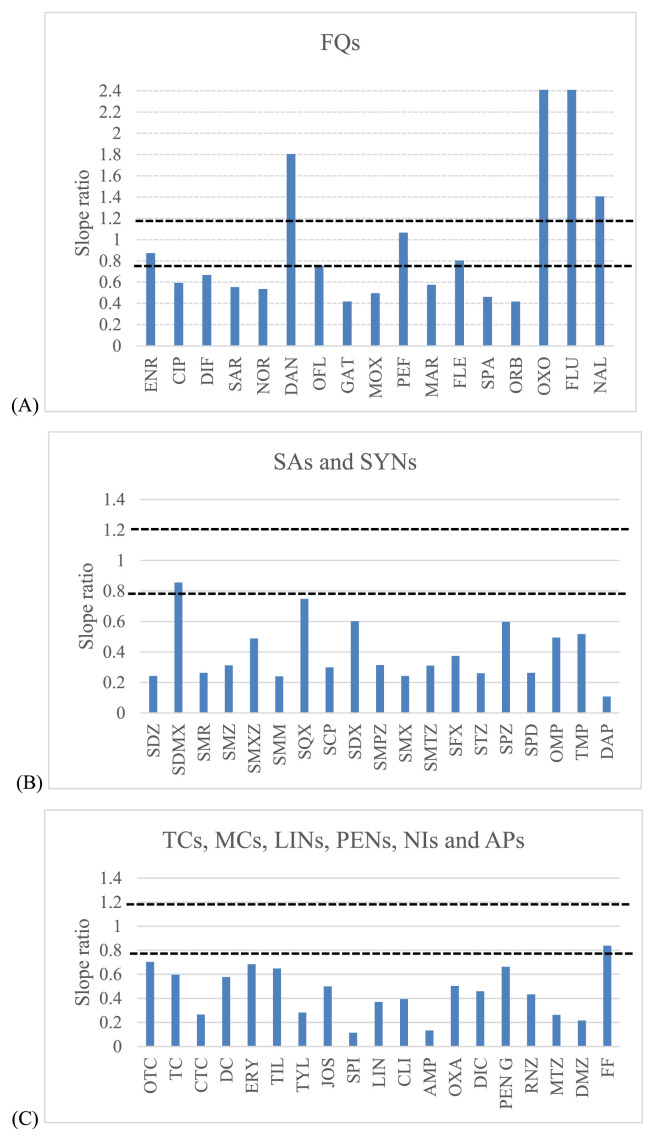
A major factor to avoid when ESI is used as the ionization technique in mass spectrometry, is the signal suppression or enhancement of the targeted drugs due to the interferences present in the matrix (matrix effect). To evaluate matrix effect, several standards in pure solvents and in the blank shrimp muscle matrix from the investigated range (3–15 ng/g for NIs, 10–50 ng/g for APs and 5–50 ng/g for the rest of antimicrobial drugs) were analyzed. The slopes obtained in the calibration with the post extracted shrimp muscle were compared with those obtained with solvent standard. Then, matrix/solvent slope ratios for each compound were obtained (Fig. 5) considering a signal enhancement or suppression effect as acceptable if the slope ratio ranged from 0.8 to 1.2. Slope ratios with values higher than 1.2 or lower than 0.8 indicate a strong matrix effect [34]. A matrix effect was demonstrated for most of the investigated compounds, 4 and 47 compounds showed signal enhancement and suppression, respectively, except for enrofloxacin (ENR), pefloxacin (PEF), sulfadimethoxine (SDMX) and florfenicol (FF). Therefore, the matrix-matched calibration (MMC) curves were used for quantitative analysis and compensated this phenomenon.
Fig. 5.
Slope ratios for all antibacterial drugs between matrix-matched and solvent calibration. The acceptable range of slope ratios between 0.8 and 1.2 has been plotted: (A) – fluoroquinolones; (B) – sulfonamides; (C) – the rest of other groups.
3.3.3. Linearity
Linearity was evaluated using MMC at five concentration levels ranging from 3 to 15 ng/g for NIs, 10–50 ng/g for APs and 5–50 ng/g for the rest of the antimicrobial drugs. The linearity of an analytical procedure is its ability (within a given range) to obtain test results that are directly proportional to the concentration (amount) of analytes in the sample. Five-point calibration curves were plotted as the peak area (y) of each antimicrobial drug against its concentration (y = mx ± c) at the concentration ranges mentioned above. The internal standard was not used for this procedure due to the differences in physiochemical properties of antibacterial drugs in multi-residues method. Therefore, it was difficult to find the suitable internal standard for all analytes. The calibration curves showed good linearity with determination coefficient (r2) higher than 0.99 in all the cases.
3.3.4. Limits of detection (LODs) and limits of quantification (LOQs)
LODs and LOQs were calculated by analyzing the spiked samples at low level concentrations. LODs were determined as the lowest concentration of the analyte for which a signal-to-noise (S/N) ratio was 3:1 whereas, S/N ratio was 10:1 for LOQs. The results are shown in Table 2. The LODs and LOQs of the compounds of interest ranged from 1 to 5 ng/g and 3–10 ng/g, respectively. It indicated that the proposed method showed good sensitivity because the LODs and LOQs of all compounds were lower than the MRL established by EU [11].
Table 2.
Maximum residue limit (MRL), limit of detection (LOD) and limit of quantification (LOQ) of the optimized UHPLC-MS/MS method for simultaneous determination of antimicrobial drugs.
| Compound | MRL (ng/g) | LOD (ng/g) | LOQ (ng/g) |
|---|---|---|---|
| Fluoroquinolones | |||
| ENR | 100a | 2.0 | 5.0 |
| CIP | 100a | 2.0 | 5.0 |
| DIF | 300 | 2.0 | 5.0 |
| SAR | -b | 2.0 | 5.0 |
| NOR | -b | 2.0 | 5.0 |
| DAN | 100 | 2.0 | 5.0 |
| OFL | -b | 2.0 | 5.0 |
| GAT | -b | 2.0 | 5.0 |
| MOX | -b | 2.0 | 5.0 |
| PEF | -b | 2.0 | 5.0 |
| MAR | -b | 2.0 | 5.0 |
| FLE | -b | 2.0 | 5.0 |
| SPA | -b | 2.0 | 5.0 |
| ORB | -b | 2.0 | 5.0 |
| OXO | 100 | 2.0 | 5.0 |
| FLU | 200 | 2.0 | 5.0 |
| NAL | -b | 2.0 | 5.0 |
| Sulfonamides | |||
| SDZ | 100c | 2.0 | 5.0 |
| SDMX | 100c | 2.0 | 5.0 |
| SMR | 100c | 2.0 | 5.0 |
| SMZ | 100c | 2.0 | 5.0 |
| SMXZ | 100c | 2.0 | 5.0 |
| SQX | 100c | 2.0 | 5.0 |
| SCP | 100c | 2.0 | 5.0 |
| SDX | 100c | 2.0 | 5.0 |
| SMPZ | 100c | 2.0 | 5.0 |
| SMX | 100c | 2.0 | 5.0 |
| SMTZ | 100c | 2.0 | 5.0 |
| SFX | 100c | 2.0 | 5.0 |
| STZ | 100c | 2.0 | 5.0 |
| SPZ | 100c | 2.0 | 5.0 |
| SPD | 100c | 2.0 | 5.0 |
| SMM | 100c | 2.0 | 5.0 |
| Synergistic agents | |||
| TMP | 50 | 2.0 | 5.0 |
| OMP | -b | 2.0 | 5.0 |
| DAP | -d | 2.0 | 5.0 |
| Tetracyclines | |||
| OTC | 100 | 2.0 | 5.0 |
| TC | 100 | 2.0 | 5.0 |
| CTC | 100 | 2.0 | 5.0 |
| DC | -b | 2.0 | 5.0 |
| Macrolides | |||
| ERY | 200 | 2.0 | 5.0 |
| TIL | 50 | 2.0 | 5.0 |
| TYL | 100 | 2.0 | 5.0 |
| JOS | -b | 2.0 | 5.0 |
| SPI | -b | 2.0 | 5.0 |
| Lincosamides | |||
| LIN | 100 | 2.0 | 5.0 |
| CLI | -b | 2.0 | 5.0 |
| Penicillins | |||
| AMP | 50 | 2.0 | 5.0 |
| OXA | -b | 2.0 | 5.0 |
| DIC | 300 | 2.0 | 5.0 |
| PEN G | 50 | 2.0 | 5.0 |
| Nitroimidazoles | |||
| DMZ | -d | 1.0 | 3.0 |
| MNZ | -d | 1.0 | 3.0 |
| RNZ | -d | 1.0 | 3.0 |
| Amphenicols | |||
| FF | 100 | 5.0 | 10.0 |
MRL expressed as sum of ENR and CIP.
MRL not established for this compound in the matrix evaluated.
MRL expressed as sum of all sulfonamides.
MRL cannot be established (prohibited drug).
3.3.5. Recovery and precision
Recovery and intraday precision (repeatability) were performed by preparing spiked samples at three different concentration levels (3, 7.5, 15 ng/g for NIs, 10, 30, 50 ng/g for APs and 5, 20, 50 ng/g for the rest of the compounds), and analyzed using ten replicates for each concentration level in one day. Inter-day precision (reproducibility) was evaluated by testing spiked samples at the same concentration levels on five different days (six spiked samples per day and per concentration level). Table 3 shows that good results were obtained from all tested compounds at the three different concentration levels with recoveries ranging from 74.3% (TYL at 20 ng/g) to 113.3% (LIN at 5 ng/g). For precision evaluation, we found that the relative standard deviations (RSDs) for all the investigated levels were lower than 15.0% and 24.0% for repeatability and reproducibility, respectively. All these results were in accordance with the acceptance criteria, according to the Commission Decision 2002/657/EC [15]. It can be concluded that the developed method was accurate and precise for the multiclass analysis of antimicrobial drugs in shrimp muscle.
Table 3.
Accuracy and precision for antimicrobials determination in optimal UHPLC-MS/MS conditions for spiked shrimp muscle samples.
| Compound | Recovery (%) | Interday precision (RSD %) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| 5 ng/g | 20 ng/g | 50 ng/g | 5 ng/g | 20 ng/g | 50 ng/g | |
| Fluoroquinolones | ||||||
| ENR | 94.8 (7.9)a | 107.6 (6.0) | 98.9 (4.8) | 11.5 | 5.8 | 6.4 |
| CIP | 111.8 (8.2) | 97.3 (4.4) | 88.35 (5.4) | 16.0 | 11.5 | 11.4 |
| DIF | 106.9 (8.6) | 107.2 (5.7) | 96.2 (3.2) | 13.9 | 9.8 | 8.3 |
| SAR | 103.9 (10.0) | 93.2 (4.5) | 89.6 (4.8) | 18.1 | 6.3 | 6.8 |
| NOR | 107.1 (7.9) | 97.4 (4.6) | 91.3 (5.3) | 17.4 | 8.7 | 8.9 |
| DAN | 105.1 (10.1) | 98.1 (4.0) | 89.4 (4.5) | 18.5 | 12.7 | 14.2 |
| OFL | 109.5 (6.4) | 99.8 (4.9) | 87.3 (4.4) | 18.5 | 7.8 | 11.3 |
| GAT | 109.8 (7.4) | 103.7 (4.7) | 97.0 (6.2) | 22.7 | 8.9 | 10.6 |
| MOX | 108.9 (8.0) | 105.4 (3.5) | 97.9 (7.1) | 18.7 | 14.8 | 11.0 |
| PEF | 94.2 (10.7) | 100.1 (3.2) | 96.6 (6.7) | 20.4 | 10.6 | 17.2 |
| MAR | 104.9 (8.7) | 104.6 (3.1) | 99.8 (5.2) | 14.7 | 12.8 | 11.2 |
| FLE | 96.9 (11.2) | 103.7 (4.5) | 92.5 (5.0) | 23.0 | 8.4 | 9.7 |
| SPA | 107.8 (11.7) | 97.7 (9.5) | 87.1 (4.0) | 12.0 | 13.7 | 11.3 |
| ORB | 104.7 (9.6) | 99.8 (9.2) | 90.0 (7.3) | 19.7 | 6.6 | 7.5 |
| OXO | 113.1 (5.1) | 97.2 (9.2) | 84.7 (6.7) | 20.6 | 12.7 | 10.4 |
| FLU | 102.5 (11.3) | 106.9 (10.1) | 98.4 (6.8) | 21.1 | 12.8 | 11.0 |
| NAL | 97.6 (5.8) | 92.3 (8.3) | 82.2 (4.4) | 21.6 | 14.1 | 12.2 |
| Sulfonamides | ||||||
| SDZ | 87.5 (5.2) | 97.0 (7.5) | 94.0 (10.8) | 10.1 | 8.1 | 10.0 |
| SDMX | 77.9 (5.9) | 79.0 (4.1) | 77.8 (5.3) | 14.3 | 11.8 | 10.2 |
| SMR | 87.7 (5.1) | 103.8 (8.1) | 95.9 (11.2) | 19.7 | 7.8 | 10.0 |
| SMZ | 84.0 (6.9) | 90.5 (8.0) | 83.7 (12.7) | 14.2 | 7.6 | 8.9 |
| SMXZ | 93.3 (8.9) | 92.9 (6.4) | 90.1 (5.9) | 22.5 | 6.1 | 7.0 |
| SQX | 78.6 (5.2) | 76.7 (5.7) | 77.7 (4.5) | 17.6 | 9.9 | 10.0 |
| SCP | 84.2 (12.7) | 97.4 (4.1) | 95.3 (9.1) | 16.0 | 8.0 | 13.8 |
| SDX | 82.4 (9.0) | 89.6 (3.7) | 83.7 (5.5) | 17.4 | 8.8 | 8.5 |
| SMPZ | 74.9 (4.7) | 88.4 (7.0) | 83.1 (11.5) | 17.8 | 10.4 | 7.2 |
| SMX | 81.4 (10.0) | 86.1 (6.1) | 82.1 (11.6) | 17.4 | 7.9 | 10.7 |
| SMTZ | 83.2 (13.2) | 106.4 (8.2) | 101.9 (10.0) | 21.8 | 10.5 | 9.1 |
| SFX | 90.6 (9.7) | 99.9 (5.6) | 101.0 (3.8) | 18.1 | 16.7 | 18.9 |
| STZ | 85.6 (4.8) | 94.3 (5.3) | 93.6 (9.1) | 13.6 | 5.0 | 5.5 |
| SPZ | 85.2 (9.8) | 77.6 (5.8) | 77.8 (6.2) | 23.2 | 11.1 | 7.9 |
| SPD | 80.1 (6.1) | 93.0 (7.0) | 91.6 (10.8) | 18.1 | 8.0 | 7.8 |
| SMM | 103.3 (7.7) | 99.8 (4.4) | 95.4 (9.8) | 10.7 | 5.9 | 6.6 |
| Tetracyclines | ||||||
| OTC | 95.3 (9.6) | 91.4 (5.4) | 87.6 (5.3) | 20.4 | 14.3 | 10.3 |
| TC | 101.1 (8.3) | 102.0 (7.5) | 91.8 (6.3) | 19.9 | 8.8 | 7.3 |
| CTC | 111.0 (7.7) | 108.4 (6.7) | 104.1 (7.4) | 10.9 | 8.5 | 7.6 |
| DC | 102.1 (5.4) | 99.0 (5.1) | 94.7 (9.8) | 17.4 | 7.5 | 9.6 |
| Macrolides | ||||||
| ERY | 104.1 (10.2) | 110.6 (7.0) | 111.1 (6.3) | 15.6 | 13.4 | 14.0 |
| TIL | 105.9 (11.2) | 106.0 (4.2) | 102.1 (2.7) | 13.5 | 12.2 | 16.3 |
| TYL | 77.0 (10.4) | 74.3 (6.5) | 78.9 (8.5) | 12.0 | 11.9 | 7.9 |
| JOS | 86.2 (14.5) | 86.8 (10.4) | 89.6 (7.2) | 16.6 | 10.0 | 9.1 |
| SPI | 84.0 (11.6) | 83.2 (7.6) | 89.7 (10.6) | 18.9 | 20.8 | 12.3 |
| Lincosamides | ||||||
| LIN | 113.3 (5.0) | 112.9 (3.4) | 107.3 (4.6) | 12.4 | 7.5 | 7.7 |
| CLI | 99.2 (5.8) | 112.5 (2.9) | 108.4 (9.7) | 9.7 | 5.9 | 7.7 |
| Penicillins | ||||||
| AMP | 98.3 (7.5) | 83.0 (5.7) | 80.1 (6.8) | 11.1 | 11.0 | 10.4 |
| OXA | 93.4 (7.5) | 94.6 (4.2) | 94.9 (4.7) | 22.7 | 9.2 | 9.4 |
| DIC | 82.6 (8.6) | 88.6 (5.7) | 86.8 (3.3) | 22.0 | 16.1 | 14.8 |
| PEN G | 96.9 (5.8) | 94.8 (7.3) | 97.8 (8.4) | 18.9 | 16.4 | 14.6 |
| Nitroimidazoles b | ||||||
| DMZ | 90.8 (7.0) | 101.3 (10.5) | 96.6 (11.5) | 20.2 | 11.9 | 11.8 |
| MNZ | 86.3 (2.8) | 110.0 (4.4) | 105.3 (3.5) | 12.4 | 8.2 | 8.5 |
| RNZ | 96.8 (8.2) | 110.3 (4.9) | 104.5 (3.0) | 8.6 | 4.8 | 6.2 |
| Amphenicols c | ||||||
| FF | 110.8 (7.0) | 111.3 (6.7) | 105.5 (6.3) | 16.6 | 12.5 | 13.7 |
Intraday precision expressed as % RSD.
Evaluated at 3, 7.5 and 15 ng/g for low, medium and high concentration levels, respectively.
Evaluated at 10, 30 and 50 ng/g for low, medium and high concentration levels, respectively.
3.3.6. Applicability
The validated method was used to determine the presence and to quantify the concentrations of antimicrobial drug residues in shrimp muscle samples obtained from local supermarkets in Thailand during October–December 2017. To ensure the quality of the results when the proposed method was applied, an internal quality control was tested in every batch of samples. This quality control consisted of a matrix-matched calibration, a reagent blank, and a spiked blank sample at 7.5 ng/g for NIs, 30 ng/g for APs and 20 ng/g for the rest of compounds. Furthermore, the retention time and the ion ratio of detected antimicrobial drug residues in real samples were compared to those of corresponding calibration standards in the same batch to confirm the identity of the detected compounds using the criteria established by Commission Decision 2002/657/EC [15]. The 30 shrimp muscle samples were analyzed for the 55 antimicrobial drugs studied by simultaneous determination using UHPLC-MS/MS. Four of 30 shrimp muscle samples were greater than LOQs. Most antimicrobial residues were detected at levels lower than the LOQ, except for ENR, CIP, PEF and DC which were higher than the LOQ in some shrimp muscle samples. Our results of antimicrobial drug residues in shrimp muscle samples are in line with the previous study [35]. In addition, multiple antimicrobials were detected in the shrimp samples, CIP/PEF being the most common mixture. Nevertheless, only CIP was detected in two samples at levels higher than the MRL (100 ng/g) established by EU [11]. The detected compounds as well as the residue concentrations in four positive samples are summarized in Table 4.
Table 4.
Concentration of antimicrobial drug residues (ng/g) found in real samples (total of 30 samples).
| Antibacterial drugs | Sample 8 | Sample 11 | Sample 16 | Sample 17 | Sample 18 | Sample 19 | Sample 30 |
|---|---|---|---|---|---|---|---|
| Fluoroquinolones- | |||||||
| ENR | – | – | <LOQ | <LOQ | <LOQ | <LOQ | 9.8 |
| CIP | – | – | 1169.2 | 1426.9 | – | – | – |
| OFL | – | – | – | – | – | <LOQ | – |
| PEF | – | – | 11.0 | 12.7 | – | – | |
| Sulfonamides- | |||||||
| SMXZ | – | – | <LOQ | <LOQ | – | – | |
| Tetracyclines- | |||||||
| DC | – | 83.1 | – | – | – | ||
| Lincosamides | |||||||
| CLI | <LOQ | – | |||||
4. Conclusions
A simple, cheap, fast, reliable and sensitive multiclass analytical method was developed for the simultaneous determination of antimicrobial drugs (55 compounds from eight different families) in shrimp muscle samples using simple liquid extraction with UHPLC-MS/MS. This method can detect antimicrobial drugs within a single run and was demonstrated to be an excellent tool for unambiguous identification of selected compounds using the retention time of quantification and confirmation ions. The method was validated according to international guidelines and satisfactory validation data were obtained for linearity, LODs, LOQs, recovery and precision. In addition the developed method was successfully applied to real samples, and the results of antimicrobial drug residues showed that ENR, CIP, PEF and DC were quantifiable in shrimp samples. CIP residue concentration was higher than the MRL established by EU, indicating inappropriate treatment and lack of adequate withdrawal times might be an issue in some Thai shrimp production facilities. However, further studies with a larger sample size are needed to confirm antimicrobial residues in shrimp muscles for assuring food safety.
Acknowledgements
The authors gratefully acknowledge the Graduate School, Kasetsart University for providing research funds and we would like to thank our colleagues at the Department of Pharmacology in Faculty of Veterinary Medicine, Kasetsart University for their helpful recommendations for this work. Authors acknowledge the English editing of the manuscript to Dr. H. Owen (University of Queensland, Australia).
References
- 1.Valderrama Diego Anderson, James L.2011. [Accessed 19 July 2017]. Available at: https://www.aquaculturealliance.org/wp-content/uploads/2015/04/goal11-diegovalderrama.pdf.
- 2.Food and Agriculture Organization. 2017. [Accessed 19 July 2017]. Available at: http://www.fao.org/in-action/globefish/market-reports/resource-detail/en/c/989543/
- 3. Chou Hy HCY, Wang CH, Chiang HC, Lo CF. Pathogenicity of a baculovirus infection causing white spot syndrome in cultured penaeid shrimp in Taiwan. Dis Aquat Org. 1995;23:165–73. [Google Scholar]
- 4. Herz U, Lacy P, Renz H, Erb K. The influence of infections on the development and severity of allergic disorders. Curr Opin Immunol. 2000;12:632–40. doi: 10.1016/s0952-7915(00)00155-2. [DOI] [PubMed] [Google Scholar]
- 5. Kemper N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol Indic. 2008;8:1–13. [Google Scholar]
- 6. Martinez JL. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ Pollut. 2009;157:2893–902. doi: 10.1016/j.envpol.2009.05.051. [DOI] [PubMed] [Google Scholar]
- 7. Wallmann J. Monitoring of antimicrobial resistance in pathogenic bacteria from livestock animals. Int J Med Microbiol: IJMM. 2006;296(Suppl 41):81–6. doi: 10.1016/j.ijmm.2006.01.064. [DOI] [PubMed] [Google Scholar]
- 8. Lee H-C, Chen C-M, Wei J-T, Chiu H-Y. Analysis of veterinary drug residue monitoring results for commercial livestock products in Taiwan between 2011 and 2015. J Food Drug Anal. 2018;26:565–71. doi: 10.1016/j.jfda.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pham DK, Chu J, Do NT, Brose F, Degand G, Delahaut P, et al. Monitoring antibiotic use and residue in freshwater aquaculture for domestic use in Vietnam. EcoHealth. 2015;12:480–9. doi: 10.1007/s10393-014-1006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stolker AA, Brinkman UA. Analytical strategies for residue analysis of veterinary drugs and growth-promoting agents in food-producing animals–a review. J Chromatogr A. 2005;1067:15–53. doi: 10.1016/j.chroma.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 11. Commission Decision. On pharmacologically active substance and their classification regarding maximum residue limits in foodstuffs of animal origin. Off J Eur Comm. 2009 No. L15/1. [Google Scholar]
- 12. Chafer-Pericas C, Maquieira A, Puchades R. Fast screening methods to detect antibiotic residues in food samples. Trac Trends Anal Chem. 2010;29:1038–49. [In English] [Google Scholar]
- 13. Pikkemaat MG, Dlik SOV, Schouten J, Rapallini M, Kortenhoeven L, van Egmond HJ. Nouws antibiotic test: validation of a post-screening method for antibiotic residues in kidney. Food Control. 2009;20:771–7. [In English] [Google Scholar]
- 14. Pikkemaat MG, Rapallini ML, Dijk SO, Elferink JW. Comparison of three microbial screening methods for antibiotics using routine monitoring samples. Anal Chim Acta. 2009;637:298–304. doi: 10.1016/j.aca.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 15. Commission Decision 2002/657/EC. Implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off J Eur Comm. 2002 No L221/8. [Google Scholar]
- 16. Vinci F, Guadagnuolo G, Danese V, Salini M, Serpe L, Gallo P. In-house validation of a liquid chromatography/electrospray tandem mass spectrometry method for confirmation of chloramphenicol residues in muscle according to decision 2002/657/EC. Rapid Commun Mass Spectrom: RCM. 2005;19:3349–55. doi: 10.1002/rcm.2200. [DOI] [PubMed] [Google Scholar]
- 17. De Brabander HF, Noppe H, Verheyden K, Vanden Bussche J, Wille K, Okerman L, et al. Residue analysis: future trends from a historical perspective. J Chromatogr A. 2009;1216:7964–76. doi: 10.1016/j.chroma.2009.02.027. [In eng] [DOI] [PubMed] [Google Scholar]
- 18. Geis-Asteggiante L, Lehotay SJ, Lightfield AR, Dutko T, Ng C, Bluhm L. Ruggedness testing and validation of a practical analytical method for >100 veterinary drug residues in bovine muscle by ultrahigh performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2012;1258:43–54. doi: 10.1016/j.chroma.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 19. Lehotay SJ, Lightfield AR, Geis-Asteggiante L, Schneider MJ, Dutko T, Ng C, et al. Development and validation of a streamlined method designed to detect residues of 62 veterinary drugs in bovine kidney using ultra-high performance liquid chromatography–tandem mass spectrometry. Drug Test Anal. 2012;4(Suppl 1):75–90. doi: 10.1002/dta.1363. [DOI] [PubMed] [Google Scholar]
- 20. Malik AK, Blasco C, Pico Y. Liquid chromatography-mass spectrometry in food safety. J Chromatogr A. 2010;1217:4018–40. doi: 10.1016/j.chroma.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 21. Kinsella B, O’Mahony J, Malone E, Moloney M, Cantwell H, Furey A, et al. Current trends in sample preparation for growth promoter and veterinary drug residue analysis. J Chromatogr A. 2009;1216:7977–8015. doi: 10.1016/j.chroma.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 22. Villar-Pulido M, Gilbert-Lopez B, Garcia-Reyes JF, Martos NR, Molina-Diaz A. Multiclass detection and quantitation of antibiotics and veterinary drugs in shrimps by fast liquid chromatography time-of-flight mass spectrometry. Talanta. 2011;85:1419–27. doi: 10.1016/j.talanta.2011.06.036. [In eng] [DOI] [PubMed] [Google Scholar]
- 23. Storey JM, Clark SB, Johnson AS, Andersen WC, Turnipseed SB, Lohne JJ, et al. Analysis of sulfonamides, trimethoprim, fluoroquinolones, quinolones, triphenylmethane dyes and methyltestosterone in fish and shrimp using liquid chromatography-mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2014;972:38–47. doi: 10.1016/j.jchromb.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 24. Kim J, Suh JH, Cho HD, Kang W, Choi YS, Han SB. Analytical method for fast screening and confirmation of multi-class veterinary drug residues in fish and shrimp by LC-MS/MS. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2016;33:420–32. doi: 10.1080/19440049.2016.1139752. [DOI] [PubMed] [Google Scholar]
- 25. Freitas A, Barbosa J, Ramos F. Multi-residue and multi-class method for the determination of antibiotics in bovine muscle by ultra-high-performance liquid chromatography tandem mass spectrometry. Meat Sci. 2014;98:58–64. doi: 10.1016/j.meatsci.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 26. Jin Y, Zhang J, Zhao W, Zhang W, Wang L, Zhou J, et al. Development and validation of a multiclass method for the quantification of veterinary drug residues in honey and royal jelly by liquid chromatography-tandem mass spectrometry. Food Chem. 2017;221:1298–307. doi: 10.1016/j.foodchem.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 27. Chico J, Rubies A, Centrich F, Companyo R, Prat MD, Granados M. High-throughput multiclass method for antibiotic residue analysis by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2008;1213:189–99. doi: 10.1016/j.chroma.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 28. Di Corcia A, Nazzari M. Liquid chromatographic-mass spectrometric methods for analyzing antibiotic and antibacterial agents in animal food products. J Chromatogr A. 2002;974:53–89. doi: 10.1016/s0021-9673(02)00905-6. [DOI] [PubMed] [Google Scholar]
- 29. Diaz-Cruz MS, Barcelo D. Determination of antimicrobial residues and metabolites in the aquatic environment by liquid chromatography tandem mass spectrometry. Anal Bioanal Chem. 2006;386:973–85. doi: 10.1007/s00216-006-0444-z. [DOI] [PubMed] [Google Scholar]
- 30. Kim SC, Carlson K. Quantification of human and veterinary antibiotics in water and sediment using SPE/LC/MS/MS. Anal Bioanal Chem. 2007;387:1301–15. doi: 10.1007/s00216-006-0613-0. [DOI] [PubMed] [Google Scholar]
- 31. Volmer DA, Mansoori B, Locke SJ. Study of 4-quinolone antibiotics in biological samples by short-column liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Anal Chem. 1997;69:4143–55. doi: 10.1021/ac970425c. [DOI] [PubMed] [Google Scholar]
- 32. Garrido Frenich A, Aguilera-Luiz Mdel M, Martinez Vidal JL, Romero-Gonzalez R. Comparison of several extraction techniques for multiclass analysis of veterinary drugs in eggs using ultra-high pressure liquid chromatography-tandem mass spectrometry. Anal Chim Acta. 2010;661:150–60. doi: 10.1016/j.aca.2009.12.016. [In eng] [DOI] [PubMed] [Google Scholar]
- 33. Yang S, Cha J, Carlson K. Trace analysis and occurrence of anhydroerythromycin and tylosin in influent and effluent wastewater by liquid chromatography combined with electrospray tandem mass spectrometry. Anal Bioanal Chem. 2006;385:623–36. doi: 10.1007/s00216-006-0416-3. [DOI] [PubMed] [Google Scholar]
- 34. Lopes RP, Reyes RC, Romero-Gonzalez R, Frenich AG, Vidal JL. Development and validation of a multiclass method for the determination of veterinary drug residues in chicken by ultra high performance liquid chromatography-tandem mass spectrometry. Talanta. 2012;89:201–8. doi: 10.1016/j.talanta.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 35. Uchida K, Konishi Y, Harada K, Okihashi M, Yamaguchi T, Do MHN, et al. Monitoring of antibiotic residues in aquatic products in urban and rural areas of Vietnam. J Agric Food Chem. 2016;64:6133–8. doi: 10.1021/acs.jafc.6b00091. [DOI] [PubMed] [Google Scholar]