Abstract
Peroxisome proliferator-activated receptor α (PPARα) is a nuclear hormone receptor that transcriptionally regulates lipid metabolism and inflammation; therefore, PPARα agonists are promising agents to treat dyslipidemia and metabolic disorders. PPARα full agonists, such as fibrates, are effective anti-hypertriglyceride agents, but their use is limited by adverse side effects. Hence, the aim of this study was to identify small molecules that can activate PPARα while minimizing the adverse effects. Antrodia cinnamomea, a rare medical mushroom, has been used widely in Asian countries for the treatment of various diseases, including liver diseases. Antcin B, H and K (antcins) and ergostatrien-3β-ol (EK100) are bioactive compounds isolated from A. cinnamomea with anti-inflammatory actions. Antcins, ergostane-type triterpenoids, contain the polar head with carboxylate group and the sterol-based body. Here, we showed at the first time that sterol-based compounds, antcins, but not EK100, activate PPARα in a cell-based transactivation study. The in silico docking studies presented several significant molecular interactions of antcins, including Tyr314, and His440 in the ligand-binding domain of PPARα, and these interactions are required for helix 12 (H12) stabilization. We propose that PPARα activation activity of antcins is related to their binding mode which requires conventional H12 stabilization, and that antcins can be developed as safe selective PPARα modulators.
Keywords: Antrodia cinnamomea, Drug discovery, Metabolism, Nuclear receptor, Peroxisome proliferator-activated receptor α
1. Introduction
Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear receptor superfamily of ligand-activated transcription factors. There are three PPAR isotypes, PPARα (NR1C1, encoded by PPARA), PPARβ/δ (NR1C2, encoded by PPARD), and PPARγ (NR1C3, encoded by PPARG) [1]. In general, all three members of the PPAR subfamily function as lipid sensors that transcriptionally modulate metabolic programs involved in lipid and energy metabolism. PPAR has been proposed as an important therapeutic target for metabolic complications, such as dyslipidemia, diabetes and fatty liver disease.
Specifically, each member has its tissue-specific distribution patterns and ligand specificities, which contribute to their distinct biological functions. PPARα is highly expressed in liver, where it has crucial roles in regulation of adaptive response to fasting by controlling fatty acid transport, β-oxidation and ketogenesis [2]. Mice lacking PPARα are prone to develop to liver steatosis, inflammation, and hepatocellular carcinoma [3–5]. Various fatty acids and their metabolites are considered as biological ligands for PPARα. It also can be activated by the fibrate class of anti-lipidemic drugs, which includes fenofibrate and clofibrate [6]. PPARγ is highly expressed in adipose tissues, where it governs adipogenesis and adipocyte function [7]. PPARγ is activated by thiazolidinediones (TZDs), such as rosiglitazone and pioglitazone. It has been suggested that TZDs function as insulin-sensitizers and are widely used for the treatment of type 2 diabetes mellitus (T2DM) [8,9].
Antrodia cinnamomea is a rare and precious medical mushroom found in Taiwan and has been reported to have anti-inflammatory, anticancer, hepatoprotective and immune-modulating properties [10]. Thus, it is used widely in Asian countries for the treatment of various diseases, including liver disease. A few bioactive compounds have been identified in fruiting bodies and mycelia of A. cinnamomea, including triterpenoids, polysaccharide, succinic and maleic acid derivatives, and ubiquinone derivatives [10,11]. Antcins are the typical triterpenoid compounds of A. cinnamomea. Among them, both antcin B and H possess anti-inflammatory, anti-cancer, insecticidal and hepatoprotective activities [12–17]. Similarly, antcin C and H have anti-inflammatory and anti-cancer activities in vitro [16,18–20]. Ergostatrien-3β-ol (EK100), a major component of the submerged whole broth of A. cinnamomea, was reported to have anti-inflammatory and anti-photodamaging activities in mice [21]. In addition to anti-inflammatory effects, a recent study showed that antcin K and EK100 displayed antihyperlipidemic and antidiabetic effects in a diet-induced obesity mouse model [22,23]. This prompts us to investigate if antcins and EK100 mediate lipid and glucose-lowering effects presumably through activation of PPARs.
In the present study, we examined the effects of antcin B, H, K and EK100 on PPARα and PPARγ activation using a cell-based transactivation assay, and predicted the potential nuclear receptor–ligands interactions using in silico docking approaches. As modest PPARα agonists, antcin B, H, and K selectively activated PPARα activity. Computational studies revealed that antcins occupied the ligand-binding site of PPARα like PPARα agonist Wy-14,643, which requires AF2 helix stabilization. Together, our findings suggest that natural compounds antcins are novel PPARα activators, and would be potential chemicals for the improvement of current anti-dyslipidemic agents.
2. Methods
2.1. Chemicals and reagents
The freeze-dried mycelium of A. cinnamomea of the submerged whole broth was extracted with methanol. Antcin B, H, and K were isolated from the fruiting body of A. cinnamomea. The detailed purification process of EK100, antcin B, H, and K were described previously [24,25].
2.2. Cell culture and treatment
Chinese hamster ovary cells (CHO–K1) (ATCC CRL-9618) were maintained in Ham’s F12 Nutrient medium (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco) at 37 °C in a humidified environment containing 5% CO2. Cells were subcultured every 3 days in 25- or 75-cm2 flasks. All chemicals were dissolved in absolute ethanol (Sigma, St. Louis, MO) and were then added at various concentrations to cells for 48 h. The final ethanol concentration was less than 0.1%.
2.3. Expression and reporter vectors
The chimeric receptor constructs used were pBK-CMV Gal4-rPPARα ligand binding domain (Gal4-rPPARα LBD) and the pBK-CMV Gal4-rPPARγ ligand binding domain (Gal4-rPPARγ LBD), respectively. The reporter gene was pBK-CMV-(UAS)4-tk-alkaline phosphatase (ALP) [26].
2.4. Transient transfection and alkaline phosphatase (ALP) activity assay
CHO–K1 cells were seeded in a 96-well plate (2.5 × 104 cells per well) in Ham’s F12 Nutrient medium supplemented with 10% charcoal-stripped FBS and incubated overnight before transfection. Then, nearly confluent cells were transfected with 0.1 μg the chimeric receptor constructs containing PPAR LBD and 0.04 μg of the reporter plasmid using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s instructions. Five hours after transfection, the supernatants were removed. The transfected cells were then treated for 48 h with Wy-14,643 (Cayman Chemicals, Ann Arbor, MI), BRL 49653 (rosiglitazone) (Sigma, St. Louis, MO), and other reagents at the indicated concentrations. All tested compounds were prepared in Ham’s F12 Nutrient medium supplemented with 10% TCM (Protide Pharmaceuticals, Inc, Lake Zurich, IL). The supernatants were collected, and ALP activity was measured by adding SEAP buffer, and p-Nitrophenyl Phosphate (pNPP) (Sigma, St. Louis, MO) was used as the substrate. Absorbance at 405 nm was measured using a Multiskan GO Microplate Reader (Thermo Fisher Scientific, Waltham, MA). Relative PPARα or PPARγ activity is expressed as a fold change relative to the vehicle control.
2.5. MTT assay
To determine the effect of the chemicals on cell viability, cells were applied with 55 μL of 0.45 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma, St. Louis, MO) dissolved in Ham’s F12 Nutrient medium in the dark, and then cells were incubated for 3 h at 37 °C, 5% CO2. To solubilize the product of MTT cleavage, 100 μL of isopropanol containing 0.04 N HCl was added to each well and thoroughly mixed using a microplate shaker (Scientific Industries, Inc., Bohemia, NY). After 20 min of HCl-isopropanol addition, absorbance at 540 nm was measured using a Multiskan GO Microplate Reader. Relative cell viability is expressed as percentage change relative to the vehicle control.
2.6. Docking simulation
Crystal structures of the ligand-bound LBD of human PPARα (PDB ID: 4BCR) in complex with Wy-14,643 were employed as receptor for docking study, which was edited using PyMOL (Schrödinger, NY) to select a single polypeptide chain and remove all water molecules and co-crystalized ligands. To perform docking with AutoDock4 [27], the structures of receptor and ligands were transformed to the pdbqt file format by using AutoDock Tools package. A grid with dimensions of 25 × 25 × 25 points was centered to ensure coverage of the binding pocket of LBD. The structures containing selected pose of ligands upon LBD were applied a procedure of solvation and following energy minimization to obtain the final structures. 2-Dimensional (2D) diagrams of interacting residues were depicted by default procedures and tools in Discovery Studio 3.5 software (Accelrys Inc., San Diego, CA).
2.7. Statistical analysis
Data are presented as the means ± SD. Each experiment was performed in triplicate. Statistical analyses were carried out using IBM SPSS Statistics 20 (IBM Corporation, USA). One-way analysis of variance (ANOVA) was used to determine differences among control and experimental groups. P < 0.05 was considered statistical significant. EC50 values were calculated with GraphPad Prism (GraphPad Software, San Diego, CA).
3. Results
3.1. Activation of PPARα ligand binding domains (LBD) by Wy-14,643 and antcins in CHO–K1 cells
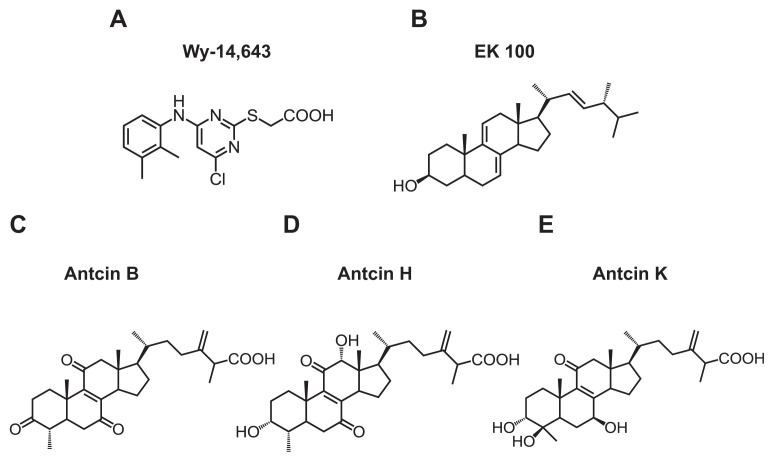
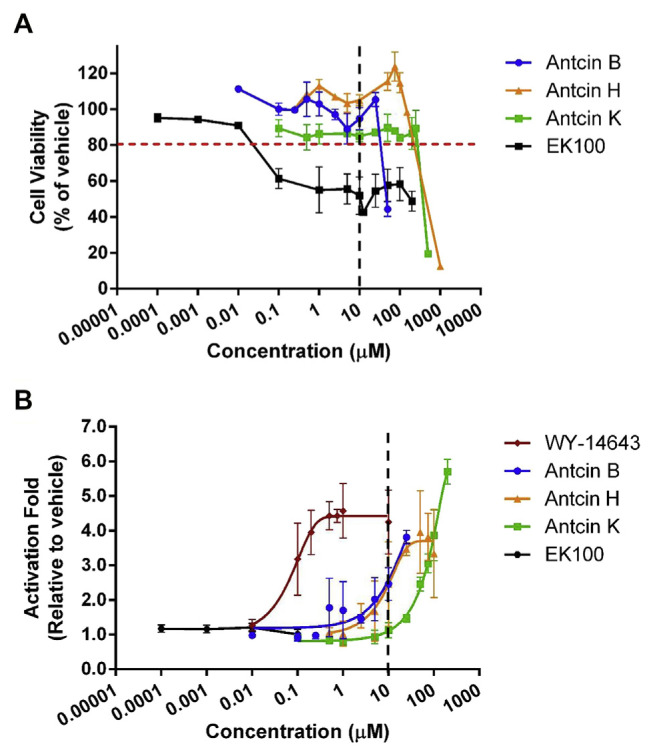
Antcin B, H, K and EK100 contain steroid-like four rings structure, and a side chain. All antcins and Wy-14,643, but not EK100 contain a carboxylic acid moiety on the side chain (Fig. 1). As all test compounds were dissolved in ethanol, we first examined the cytotoxic effects of the organic solvent on CHO–K1 cell by MTT assay. There were little or no effects on cell viability when the cells were treated with 1% (v/v) of ethanol, or lower than this concentration (Fig. S1) (Supplementary material). 10% ethanol significantly decreased cell survival to 15.7%. Next, we measured the effects of test compounds at various concentrations (0.1–200 μM) on the viability of CHO–K1 cells using MTT assay. None of the compounds caused a reduction in cell viability to 20% of the control when applied up to a level of 10 μM, except for 10 μM of EK100, which caused a 45% reduction in cell viability (Fig. 2A).
Fig. 1.
Chemical structure of (A) Wy-14,643, (B) EK100, (C) antcin B, (D) antcin H, and (E) antcin K.
Fig. 2.
Antcins are PPARα agonist. (A) Cell viability and (B) PPARα transactivation by different concentrations of Wy-14,643, antcin B, H, and K, and EK100. The CHO–K1 cells were co-transfected with the plasmids encoding chimeric receptor constructs containing PPARα LBD and their cognate ALP reporters (see “Methods”). After transfection, cells were treated with vehicle (ethanol) or ligands with various concentrations. Data are means ± SD of at least three replicates.
To understand whether the test compounds activate PPARα, PPARα activities were determined by the ALP reporter assay using co-transfection of a plasmid carrying chimera genes encoding the Gal4 PPARα ligand-binding domain (LBD), and a plasmid carrying UAS ALP reporter gene as described previously [28]. Cells were treated with ethanol (vehicle), Wy-14,643 (PPARα agonist), or test compounds for 48 h. The levels of PPARα activation were expressed as a fold change relative to the vehicle control. As shown in Fig. 2B, the strongest activation was observed following the application of Wy-14,643, which had an EC50 value of 0.08 μM. In this system, antcin B, H and K activated PPARα activity. As estimated from concentration–response curves, the EC50 for antcin H was
found to be 9.3 μM. The EC50 values for antcin B and K in this system could not be calculated since activation could not reach top plateau at the range of tested concentrations of these substances.
All of the examined antcins activated PPARα in a dose-dependent manner (Fig. 2B). Expression of the results of the application of 10 μM of each compound as a percentage of the control revealed a trend, in which the order of activation capacity was antcin B = antcin H>antcin K, with calculated folds of 2.5, 2.5 and 1.1, respectively (Fig. 2B). As concentrations were increased, maximal activation (5.7-fold) was achieved by application of 200 μM of antcin K. This activation was better than that observed for Wy-14,643, a well-recognized specific activator of PPARα (Fig. 2B).
3.2. Little or no activation of PPARγ LBD by antcin B and H in CHO–K1 cells
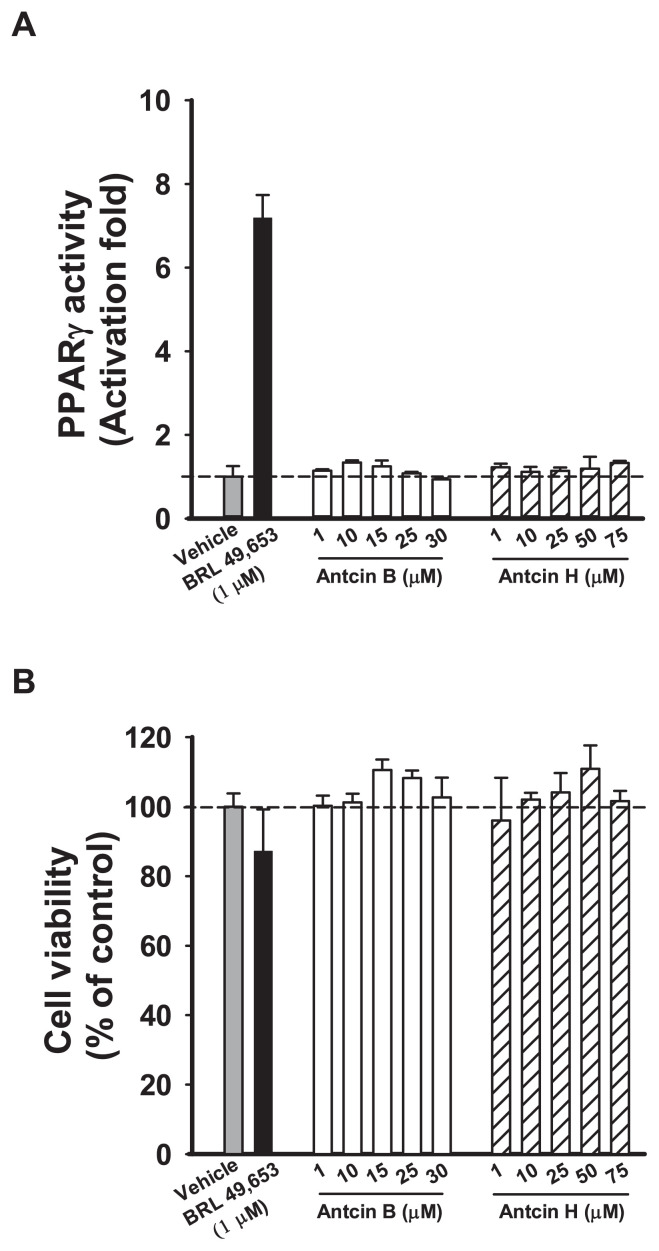
To understand whether antcin B and H activate PPARγ in CHO–K1 cells, cells were transfected with the PPARγ LBD plasmid construct and reporter construct and treated with BRL 49,653 (PPARγ agonist) for 48 h. 1 μM BRL49653 activated PPARγ more than 7 folds compared with vehicle in CHO–K1 cells, whereas none of antcins activated PPARγ activity at concentrations up to 30 μM (Fig. 3A). Cell viability was unaffected upon antcins treatment (Fig. 3B). These results suggest that antcins are less likely to act as PPARγ agonists.
Fig. 3.
Antcin B and H are not PPARγ agonists. (A) PPARγ transactivation and (B) Cell viability by different concentrations of antcin B and H. The CHO–K1 cells were co-transfected with the plasmids encoding chimeric receptor constructs containing PPARγ LBD and their cognate ALP reporters (see “ Methods”). After transfection, cells were treated with vehicle (ethanol) or ligands with various concentrations. Data are means ± SD of at least three replicates.
3.3. Computational analyses of bound ligands
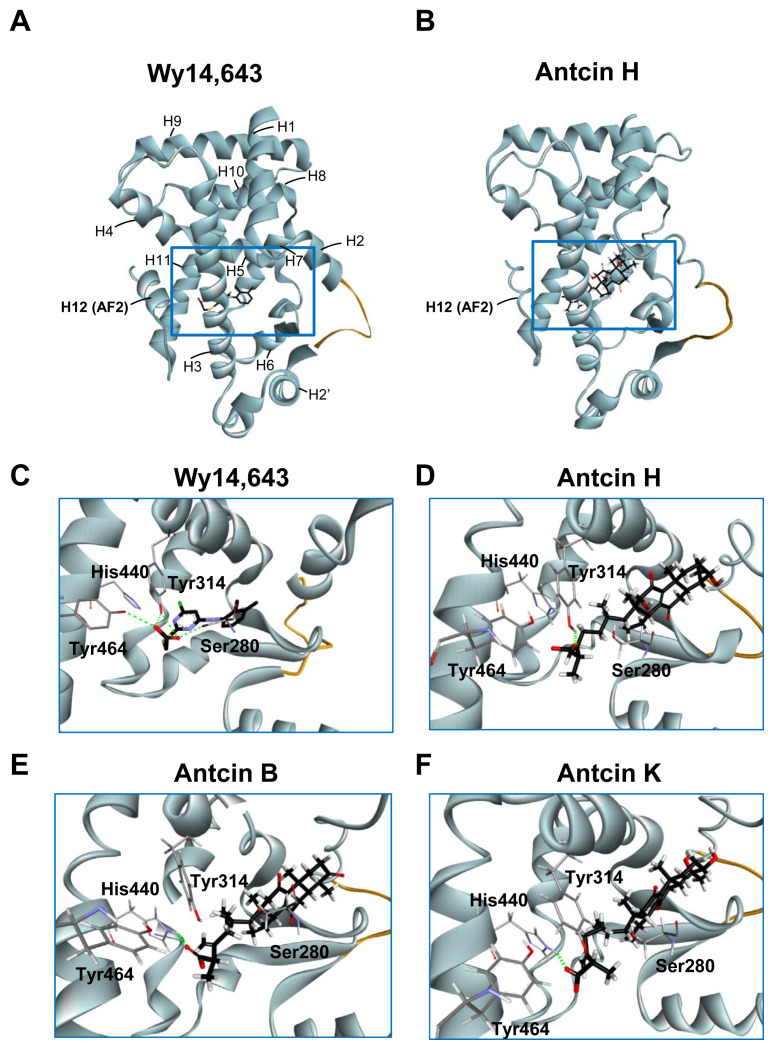
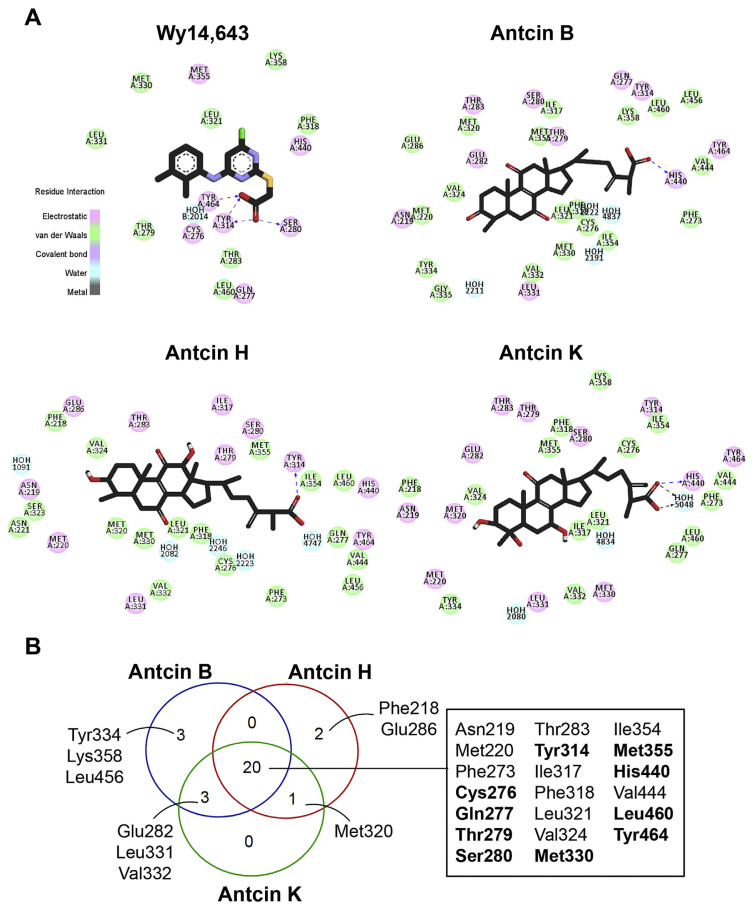
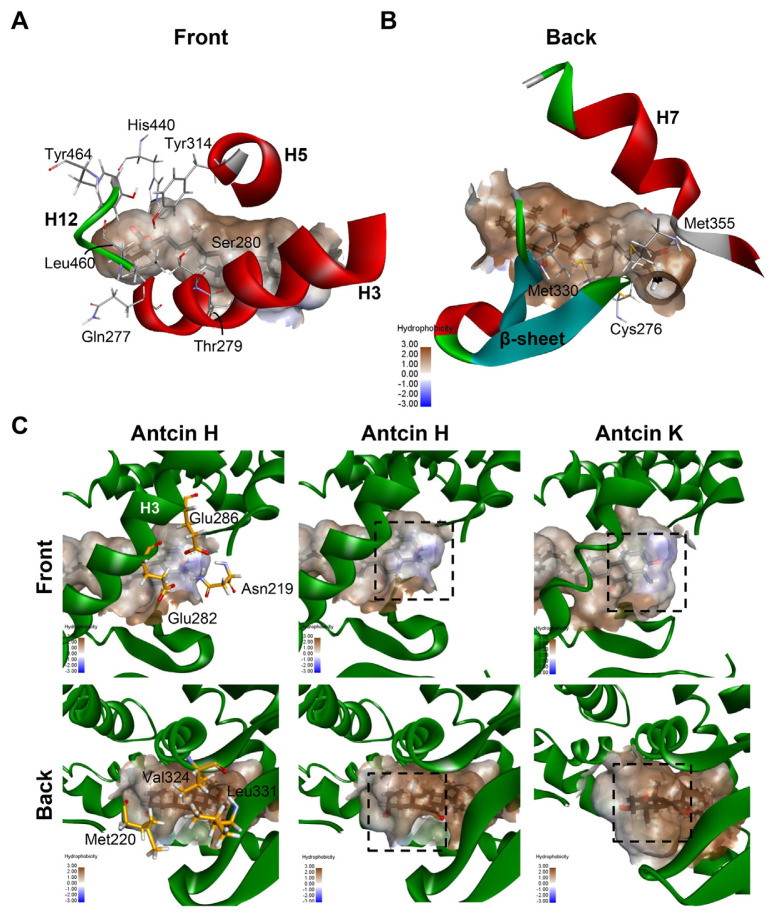
The orientation and residue bonding of Wy-14,643 inferred from the X-ray structure of human PPARα (PDB code 4BCR) served as a model for computing the interactions of Wy-14,643 and antcins. The overall structure of LBD of human PPARα (here denoted hPPARα _LBD) encompasses resides 196–467, which consists of 12 helices and a 3-stranded antiparallel β-sheet (Fig. 4A). C-terminal helix (helix 12 or AF-2 helix) which is packed onto the core of LBD is crucial for maintaining the active conformation of PPARα [29]. The region between helix 3 and the 3-stranded antiparallel β-sheet is proposed to be ligand entry site [29]. Based on the structural analysis, there are three main features can be described at the binding site, including hydrogen bonds, a hydrophobic region, and electrostatic interactions. Residues Ser280 (H3), Tyr314 (H5), and Tyr464 (H12) form a hydrogen bond network with the carboxylic acid group of the Wy-14,643 molecule (Figs. 4C and 5A). In addition, Wy-14,643 also makes hydrophobic contacts with residues Phe318, Leu321, Met330, Leu331, and Leu460 (Fig. 5A).
Fig. 4.
Three-dimensional structures of PPARα-LBD stabilized by the ligands. (A, B) Overall structure of Wy-14,643 (A) and antcin H (B) bound with PPARα in ribbon representation. The bound ligands are shown in stick representation. The H12 (AF2) helix is labeled. Missing residues in the H2–H2′ loop are depicted as an orange curve. (C–F) Close-up view of the Wy-14,643 (C), antcin H (D), antcin B (E), and antcin K (F) at the binding site of PPARα showing the interacting residues. Ligands are shown as black sticks, and receptor residues are shown as grey sticks. The bound ligands and receptor residues are shown in stick representation with oxygen, nitrogen, chloride, sulfur and hydrogen atoms depicted in red, blue, green, yellow, and white, respectively. Hydrogen bonds are shown as dotted green lines.
Fig. 5.
Major interactions established by key residues were identified using docking analysis. (A) Two-dimensional interaction diagram of the predicted binding mode of the ligands (Wy-14,643, antcin B, H, and K), PPARα, and bound water molecules (blue). Ligands are shown as black sticks with oxygen, nitrogen, chloride, sulfur and hydrogen atoms depicted in red, blue, green, yellow, and white, respectively. Hydrophobic and electrostatic interactions are indicated in green and pick, respectively. Hydrogen bonds are shown as dashed blue lines. (B) Venn diagram showing the overlapping numbers of key residues by antcin B, H and K. Twenty residues shared by antcins were listed in the box, and ten of them commonly interacted by Wy-14,643 were highlighted in bold.
Next, we compared the in silico docking results of antcins and Wy-14,643. Intriguingly, antcins interact with the hPPARα_LBD like Wy-14,643 did (Fig. 4B, D, E and F). The carboxylic acid moiety of antcin H forms a hydrogen bond with residues Tyr314 on helix 5 (Fig. 4D), while the carboxylic acid moiety of antcin B and K form hydrogen bonds with residues His440 on helix 10 of hPPARα_LBD (Fig. 4E and F). As identified for other PPARα agonist, the hydrogen bonds linking agonist and Ser280, Tyr314, His440 and Tyr464 of the receptor are crucial for stabilizing AF2 helix in the active conformational state [29].
The antcins-nuclear receptor interaction involved additional interactions at the binding site. Since antcins are composed of steroid-like four rings and are much bulkier than Wy-14,643, it is capable of carrying out a significant part of the hydrophobic interactions, such as those between antcins and residues Asn219, Met220, Phe273, Val324, and Val444 (Fig. 5A). Due to the structure similarity, antcin B, H, and K shared interactions with 20 surrounding residues of hPPARα_LBD (Fig. 5A and B). Among them, antcins and Wy-14,643 commonly interacted with 10 PPARα residues, including Cys276, Gln277, Thr279, Ser280, Tyr314, Met330, Met355, His440, Leu460, and Tyr464 (Fig. 5B, highlighted in bold in the box).
Combining the hydrophobicity analysis plot of PPARα ligand binding pocket and the common residues shared by antcins and Wy-14,643, we then divided these 10 residues into two groups. One group includes the seven residues Gln277, Thr279, Ser280, Tyr314, His440, Leu460 and Tyr464, which contributes to the hydrophilic center near the H12 of the pocket (Fig. 6A). The other group contains the rest residues Cys276, Met330 and Met355, which comprise the hydrophobic surface between H7 and β sheets (Fig. 6B).
Fig. 6.
Maps of the hydrophobicity at the binding site of PPARα docked with the antcin H and K. (A, B) Ten residues shared by antcins and Wy-14,643 are showed as grey sticks. The bound ligands are shown as black sticks. H3, H5 and H7 of PPARα are shown in ribbon representation. (C) Maps of the hydrophobicity at the binding site of PPARα docked with the antcin H and K. Ligands and receptor residues surrounding ring A of antcins are shown as black and yellow sticks, respectively. Regions surrounding ring A are highlighted in dotted rectangles.
Among the tested antcins, antcin H had the lowest EC50 value. We further examined the structure-activity relationships of antcins. Antcin B has a carbonyl group on A ring, while antcin H and K contain one and two hydroxyl groups on A ring, respectively (Fig. 1). We found that among the residues of ligand-binding pocket surrounding A ring, Asn219, Glu282, and Glu286 contribute to a local polar center, whereas Met220, Val324, and Leu331 make up the other non-polar region (Fig. 6C). Antcin K has two hydroxyl groups on ring A. One of them was pointed to the direction of the polar center, while the other was headed toward the non-polar hemisphere (Fig. 6C). The possible repulsion may lead to the decreased potency of antcin K. In contrast, the hydroxyl group on A ring of antcin H was predicted to pointed to the direction of the polar center, it may provide the stabilization energy for binding of antcin H to the ligand-binding site (Fig. 6C). This may account for the lowest EC50 of antcin H among antcins. In addition to interactions around ring A, the hydroxyl or carbonyl groups on the others rings of antcins can engage in additional hydrophobic interactions at the binding site. For instance, antcins were predicted to make hydrophobic interactions with Leu321 through their carbonyl group or hydroxyl group on B ring (Fig. 5A).
4. Discussion
Increasing evidence has shown that PPARα plays a crucial role in fatty acid metabolism, as it controls the expression of genes mediated peroxisomal and mitochondrial β-oxidation, ω-hydroxylation, and ketogenesis [30]. PPARα has been considered as an important therapeutic target for metabolic disorders, such as dyslipidemia, and fatty liver diseases. Hence, identification of PPARα agonists has a major medical interest. This is the first study showing that natural triterpenoid compounds antcins activate PPARα.
One of the common structural features among the activators is a carboxylate group, which is not found in the non-activator, EK100. These findings highlight the importance of the carboxylate moieties of these ligands in the activation of PPARα. Indeed, most known PPAR agonists share features including a carboxylate head group, a central hydrophobic part, and a flexible linker to the tail. The results of the computational analysis suggest that antcins are inserted in a similar orientation as Wy-14,643, in which the carboxylic group faces the hydrophilic region of the binding site and is close to a key switch helix, H12 (AF2 helix) [31]. In the absence of ligand, H12 is highly dynamic and is mobile along with other portions of the LBD [32]. Ligands stabilize the receptor folding and lower the conformational dynamics [32]. Like Wy-14,643, antcins form hydrogen bonds with Tyr314 on helix 5 and His440 on the helix 10 by its carboxylic group. Several studies showed that hydrogen bond network between the carboxyl group of ligands and the residues surrounding AF2 helix can stabilize the dynamics of AF2 helix, which permits coactivator recruitment [33,34]. These specific interactions enhance the transactivation activity of the PPARs [34]. As expected, antcins exhibit PPARα activating ability in the cell-based transactivation assay. These data suggest that formation of hydrogen bonds between residues near H12 of PPARα and its acidic ligands is crucial for ligand activation ability.
A recent study reported a bipartite mechanism of PPARα activation by Wy-14,643, which involves two ligands in the pocket simultaneously [31]. The first Wy-14,643 occupies standard PPARα agonist position, and requires conventional direct H12 stabilization [31]. The second Wy-14,643 molecule binds to a site positioned between H2′ and H3 (hereafter denoted site two), and is primarily stabilized by nonpolar interactions [31]. Site two is located in the region called omega loop. Activity assay showed that site two is required for full Wy-14,643 activity [31]. According to our results of docking
simulation, all antcins and EK100 can be docked to the standard PPARα agonist position (Fig. 4 and data not shown), but not all of them induce PPARα activation in vitro. One possibility is that EK100 is lack of carboxylate group, and the other is that the conventional docked position is not sufficient to induce PPARα activation. As antcins, which contain a carboxylate head group, have higher EC50 values than Wy-14,643, it is tempting to speculate that site two and/or simultaneous insertion of two ligands into the pocket might be also essential for full activity of the ligands. Further studies with site mutation analysis need to be performed to decipher this possibility.
In the present study, we showed that antcins activated PPARα not PPARγ. PPARα and PPARγ share 60–70% sequence identity in their LBDs. Although both subtypes bind to naturally occurring polyunsaturated fatty acids, they exhibit ligand selectivity. For instance, only PPARα binds to a wide range of saturated fatty acids [35]. This is supported by structural analyses revealing that PPARα ligand binding pocket has the more hydrophobic nature compared to that of PPARγ [34]. As expected, antcins containing the steroid core ring structure were detected to make hydrophobic interactions with PPARα LBD in the software simulation. Furthermore, one of the determinants of selectivity between PPARα and PPARγ is the substitution of Ala454 on the hinge (loop) of H12 in PPARα for Met463 in PPARγ [36]. H12 dynamic stabilization is crucial for activation of PPARs by ligands [37]. The larger volume of the Met463 side chain in PPARγ may act as a lid to limit the accessibility of ligands to ligand binding pocket. Thus, not only hydrophobic nature of PPARα ligand binding pocket, but the amino acids on the hinge (loop) of H12 may be involved in the ligand selectivity.
Several studies have demonstrated that PPARα activation ameliorates metabolic disorder and non-alcoholic fatty liver disease (NAFLD) in diet-induced obesity [1]. In this study, we showed that triterpenoid compounds antcins activate PPARα in vitro and in silico. Recent studies showed that the crude extract of A. cinnamomea, or purified antcin K prevent obesity and display antihyperlipidemic effects through regulating AMPK signaling in high fat diet-induced obese mice [22,38]. One could speculate that the protective effects of antcins against obesity and hepatic steatosis could be also attributed to their abilities to activate PPARα. In addition to synthetic agonists, several natural compounds, including triterpenoids, have been identified as PPARα modulators [39]. For example, the pentacyclic triterpene oleanolic acid was found to stimulate PPARα activation by a reporter gene assay using PPAR response element activity in CV-1 keratinocytes cells [40]. Cucurbitane-type triterpenoids isolated from wild bitter melon (Momordica charantia L.) were shown to activate PPARα and PPARγ in a transactivation assay [26]. In addition, wild bitter gourd has been shown to ameliorate metabolic syndrome (MetS) in animal models and subjects with MetS [26,41]. Thus, natural PPARα agonists may have therapeutic potential for treating metabolic disorders.
5. Conclusion
In summary, the present study is the first to elucidate molecular aspects of the nuclear receptor activation effects induced by antcins using both in vitro and in silico approaches. Our findings identified potential PPARα agonists from herbs, and provided insight into the interaction of antcins with PPARα pathways.
Acknowledgements
We thank Dr. Ching-Jang Huang for helpful discussion. This work was supported by grants MOST 105-2320-B-002-011 (F-J. L.), MOST 106-2311-B-002-038 (F-J.L.), MOST 106-2113-M-002-020 (C-H.H.), MOST 105-2313-B-002-050-MY3 (C-C.Y.), and MOST 107-2321-B-002-047 (C-C.Y.) from the Ministry of Science and Technology, Taiwan, and by grant MOHW107-TDU-B-212-123004 (Y-H.K.) from Taiwan Ministry of Health and Welfare Clinical Trial Center. This work was also supported in part by the “Chinese Medicine Research Center, China Medical University” from the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan (CMRC-CHM-4).
Abbreviations
- A. cinnamomea
Antrodia cinnamomea
- ALP
alkaline phosphatase
- EK100
ergostatrien-3β-ol
- LBD
Ligand Binding Domain
- MetS
metabolic syndrome
- PPARs
peroxisome proliferator-activated receptors
- TZDs
thiazolidinediones
- T2DM
type 2 diabetes mellitus
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jfda.2018.11.004.
Funding Statement
This work was supported by grants MOST 105-2320-B-002-011 (F-J. L.), MOST 106-2311-B-002-038 (F-J.L.), MOST 106-2113-M-002-020 (C-H.H.), MOST 105-2313-B-002-050-MY3 (C-C.Y.), and MOST 107-2321-B-002-047 (C-C.Y.) from the Ministry of Science and Technology, Taiwan, and by grant MOHW107-TDU-B-212-123004 (Y-H.K.) from Taiwan Ministry of Health and Welfare Clinical Trial Center.
Footnotes
Conflicts of interest statement
The authors declared that there are no conflicts of interest.
Authors’ contributions
Y-J.W. and S-C.L. performed the experiments, analyzed and interpreted the data. C-C.Y. interpreted the data, and provided advice. Y-H.K. provided the reagents and advice. C-H.H. and F-J. L. designed and performed the experiments, interpreted the data, and supervised the study. F-J.L. wrote the manuscript.
REFERENCES
- 1. Gross B, Pawlak M, Lefebvre P, Staels B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat Rev Endocrinol. 2017;13:36–49. doi: 10.1038/nrendo.2016.135. [DOI] [PubMed] [Google Scholar]
- 2. Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62:720–33. doi: 10.1016/j.jhep.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 3. Zhang N, Chu ES, Zhang J, Li X, Liang Q, Chen J, et al. Peroxisome proliferator activated receptor alpha inhibits hepatocarcinogenesis through mediating NF-kappaB signaling pathway. Oncotarget. 2014;5:8330–40. doi: 10.18632/oncotarget.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abdelmegeed MA, Yoo SH, Henderson LE, Gonzalez FJ, Woodcroft KJ, Song BJ. PPARalpha expression protects male mice from high fat-induced nonalcoholic fatty liver. J Nutr. 2011;141:603–10. doi: 10.3945/jn.110.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stienstra R, Mandard S, Patsouris D, Maass C, Kersten S, Muller M. Peroxisome proliferator-activated receptor alpha protects against obesity-induced hepatic inflammation. Endocrinology. 2007;148:2753–63. doi: 10.1210/en.2007-0014. [DOI] [PubMed] [Google Scholar]
- 6. Staels B, Maes M, Zambon A. Fibrates and future PPARalpha agonists in the treatment of cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2008;5:542–53. doi: 10.1038/ncpcardio1278. [DOI] [PubMed] [Google Scholar]
- 7. Willson TM, Lambert MH, Kliewer SA. Peroxisome proliferator-activated receptor gamma and metabolic disease. Annu Rev Biochem. 2001;70:341–67. doi: 10.1146/annurev.biochem.70.1.341. [DOI] [PubMed] [Google Scholar]
- 8. Soccio RE, Chen ER, Lazar MA. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014;20:573–91. doi: 10.1016/j.cmet.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chana RS, Lewington AJ, Brunskill NJ. Differential effects of peroxisome proliferator activated receptor-gamma (PPAR gamma) ligands in proximal tubular cells: thiazolidinediones are partial PPAR gamma agonists. Kidney Int. 2004;65:2081–90. doi: 10.1111/j.1523-1755.2004.00624.x. [DOI] [PubMed] [Google Scholar]
- 10. Geethangili M, Tzeng YM. Review of pharmacological effects of Antrodia camphorata and its bioactive compounds. Evid Based Complement Alternat Med. 2011;2011:212641. doi: 10.1093/ecam/nep108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang BB, Hu PF, Huang J, Hu YD, Chen L, Xu GR. Current advances on the structure, bioactivity, synthesis, and metabolic regulation of novel ubiquinone derivatives in the edible and medicinal mushroom Antrodia cinnamomea. J Agric Food Chem. 2017;65:10395–405. doi: 10.1021/acs.jafc.7b04206. [DOI] [PubMed] [Google Scholar]
- 12. Huo Y, Win S, Than TA, Yin S, Ye M, Hu H, et al. Antcin H protects against acute liver injury through disruption of the interaction of c-Jun-N-terminal kinase with mitochondria. Antioxid Redox Signal. 2017;26:207–20. doi: 10.1089/ars.2016.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiu KY, Chen TH, Wen CL, Lai JM, Cheng CC, Liu HC, et al. Antcin-H isolated from Antrodia cinnamomea inhibits renal cancer cell invasion partly through inactivation of FAK-ERK-C/EBP-beta/c-Fos-MMP-7 pathways. Evid Based Complement Alternat Med. 2017;2017:5052870. doi: 10.1155/2017/5052870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yeh CT, Rao YK, Yao CJ, Yeh CF, Li CH, Chuang SE, et al. Cytotoxic triterpenes from Antrodia camphorata and their mode of action in HT-29 human colon cancer cells. Cancer Lett. 2009;285:73–9. doi: 10.1016/j.canlet.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 15. Male KB, Rao YK, Tzeng YM, Montes J, Kamen A, Luong JH. Probing inhibitory effects of Antrodia camphorata isolates using insect cell-based impedance spectroscopy: inhibition vs chemical structure. Chem Res Toxicol. 2008;21:2127–33. doi: 10.1021/tx800202a. [DOI] [PubMed] [Google Scholar]
- 16. Shen YC, Wang YH, Chou YC, Chen CF, Lin LC, Chang TT, et al. Evaluation of the anti-inflammatory activity of zhankuic acids isolated from the fruiting bodies of Antrodia camphorata. Planta Med. 2004;70:310–4. doi: 10.1055/s-2004-818941. [DOI] [PubMed] [Google Scholar]
- 17. Chen CH, Yang SW, Shen YC. New steroid acids from Antrodia cinnamomea, a fungal parasite of Cinnamomum micranthum. J Nat Prod. 1995;58:1655–61. doi: 10.1021/np50125a002. [DOI] [PubMed] [Google Scholar]
- 18. Tien AJ, Chien CY, Chen YH, Lin LC, Chien CT. Fruiting bodies of Antrodia cinnamomea and its active triterpenoid, antcin K, ameliorates N-Nitrosodiethylamine-Induced hepatic inflammation, fibrosis and carcinogenesis in rats. Am J Chin Med. 2017;45:173–98. doi: 10.1142/S0192415X17500124. [DOI] [PubMed] [Google Scholar]
- 19. Huang YL, Chu YL, Ho CT, Chung JG, Lai CI, Su YC, et al. Antcin K, an active triterpenoid from the fruiting bodies of basswood-cultivated Antrodia cinnamomea, inhibits metastasis via suppression of integrin-mediated adhesion, migration, and invasion in human hepatoma cells. J Agric Food Chem. 2015;63:4561–9. doi: 10.1021/jf5059304. [DOI] [PubMed] [Google Scholar]
- 20. Gokila Vani M, Kumar KJ, Liao JW, Chien SC, Mau JL, Chiang SS, et al. Antcin C from Antrodia cinnamomea protects liver cells against free radical-induced oxidative stress and apoptosis in vitro and in vivo through Nrf2-dependent mechanism. Evid Based Complement Alternat Med. 2013;2013:296082. doi: 10.1155/2013/296082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuo YH, Lin TY, You YJ, Wen KC, Sung PJ, Chiang HM. Antiinflammatory and antiphotodamaging effects of ergostatrien-3beta-ol, isolated from Antrodia camphorata, on hairless mouse skin. Molecules. 2016;21 doi: 10.3390/molecules21091213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuo YH, Lin CH, Shih CC, Yang CS. Antcin K, a triterpenoid compound from Antrodia camphorata, displays antidiabetic and antihyperlipidemic effects via glucose transporter 4 and AMP-activated protein kinase phosphorylation in muscles. Evid Based Complement Alternat Med. 2016;2016:4867092. doi: 10.1155/2016/4867092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kuo YH, Lin CH, Shih CC. Ergostatrien-3beta-ol from Antrodia camphorata inhibits diabetes and hyperlipidemia in high-fat-diet treated mice via regulation of hepatic related genes, glucose transporter 4, and AMP-activated protein kinase phosphorylation. J Agric Food Chem. 2015;63:2479–89. doi: 10.1021/acs.jafc.5b00073. [DOI] [PubMed] [Google Scholar]
- 24. Shao YY, Chen CC, Wang HY, Chiu HL, Hseu TH, Kuo YH. Chemical constituents of Antrodia camphorata submerged whole broth. Nat Prod Res. 2008;22:1151–7. doi: 10.1080/14786410601132410. [DOI] [PubMed] [Google Scholar]
- 25. Shen CC, Kuo YC, HRL, Lin LC, Don MJ, Chang TT, et al. New ergostane and lanostane from Antrodia camphorata. J Chin Med. 2003;14:247–58. [Google Scholar]
- 26. Lu KN, Hsu C, Chang ML, Huang CJ. Wild bitter gourd increased metabolic rate and up-regulated genes related to mitochondria biogenesis and UCP-1 in mice. J Funct Foods. 2013;5:668–78. [Google Scholar]
- 27. Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–91. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chao CY, Huang CJ. Bitter gourd (Momordica charantia) extract activates peroxisome proliferator-activated receptors and upregulates the expression of the acyl CoA oxidase gene in H4IIEC3 hepatoma cells. J Biomed Sci. 2003;10:782–91. doi: 10.1159/000073966. [DOI] [PubMed] [Google Scholar]
- 29. Cronet P, Petersen JF, Folmer R, Blomberg N, Sjoblom K, Karlsson U, et al. Structure of the PPARalpha and -gamma ligand binding domain in complex with AZ 242; ligand selectivity and agonist activation in the PPAR family. Structure. 2001;9:699–706. doi: 10.1016/s0969-2126(01)00634-7. [DOI] [PubMed] [Google Scholar]
- 30. Schoonjans K, Staels B, Auwerx J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res. 1996;37:907–25. [PubMed] [Google Scholar]
- 31. Bernardes A, Souza PC, Muniz JR, Ricci CG, Ayers SD, Parekh NM, et al. Molecular mechanism of peroxisome proliferator-activated receptor alpha activation by WY14643: a new mode of ligand recognition and receptor stabilization. J Mol Biol. 2013;425:2878–93. doi: 10.1016/j.jmb.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 32. Rastinejad F, Huang P, Chandra V, Khorasanizadeh S. Understanding nuclear receptor form and function using structural biology. J Mol Endocrinol. 2013;51:T1–21. doi: 10.1530/JME-13-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gampe RT, Jr, Montana VG, Lambert MH, Miller AB, Bledsoe RK, Milburn MV, et al. Asymmetry in the PPARgamma/RXRalpha crystal structure reveals the molecular basis of heterodimerization among nuclear receptors. Mol Cell. 2000;5:545–55. doi: 10.1016/s1097-2765(00)80448-7. [DOI] [PubMed] [Google Scholar]
- 34. Xu HE, Lambert MH, Montana VG, Plunket KD, Moore LB, Collins JL, et al. Structural determinants of ligand binding selectivity between the peroxisome proliferator-activated receptors. Proc Natl Acad Sci U S A. 2001;98:13919–24. doi: 10.1073/pnas.241410198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, et al. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell. 1999;3:397–403. doi: 10.1016/s1097-2765(00)80467-0. [DOI] [PubMed] [Google Scholar]
- 36. Carrieri A, Giudici M, Parente M, De Rosas M, Piemontese L, Fracchiolla G, et al. Molecular determinants for nuclear receptors selectivity: chemometric analysis, dockings and site-directed mutagenesis of dual peroxisome proliferator-activated receptors alpha/gamma agonists. Eur J Med Chem. 2013;63:321–32. doi: 10.1016/j.ejmech.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 37. Rastinejad F, Ollendorff V, Polikarpov I. Nuclear receptor full-length architectures: confronting myth and illusion with high resolution. Trends Biochem Sci. 2015;40:16–24. doi: 10.1016/j.tibs.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peng CH, Yang MY, Yang YS, Yu CC, Wang CJ. Antrodia cinnamomea prevents obesity, dyslipidemia, and the derived fatty liver via regulating AMPK and SREBP signaling. Am J Chin Med. 2017;45:67–83. doi: 10.1142/S0192415X17500069. [DOI] [PubMed] [Google Scholar]
- 39. Rigano D, Sirignano C, Taglialatela-Scafati O. The potential of natural products for targeting PPARalpha. Acta Pharm Sin B. 2017;7:427–38. doi: 10.1016/j.apsb.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee HK, Nam GW, Kim SH, Lee SH. Phytocomponents of triterpenoids, oleanolic acid and ursolic acid, regulated differently the processing of epidermal keratinocytes via PPAR-alpha pathway. Exp Dermatol. 2006;15:66–73. doi: 10.1111/j.0906-6705.2005.00386.x. [DOI] [PubMed] [Google Scholar]
- 41. Tsai CH, Chen EC, Tsay HS, Huang CJ. Wild bitter gourd improves metabolic syndrome: a preliminary dietary supplementation trial. Nutr J. 2012;11:4. doi: 10.1186/1475-2891-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]