Abstract
Background
The use of mechanical thrombectomy to restore intracranial blood flow after proximal large artery occlusion by a thrombus has increased over time and led to better outcomes than intravenous thrombolytic therapy alone. Currently, the type of anaesthetic technique during mechanical thrombectomy is under debate as having a relevant impact on neurological outcomes.
Objectives
To assess the effects of different types of anaesthesia for endovascular interventions in people with acute ischaemic stroke.
Search methods
We searched the Cochrane Stroke Group Specialised Register of Trials on 5 July 2022, and CENTRAL, MEDLINE, and seven other databases on 21 March 2022. We performed searches of reference lists of included trials, grey literature sources, and other systematic reviews.
Selection criteria
We included all randomised controlled trials with a parallel design that compared general anaesthesia versus local anaesthesia, conscious sedation anaesthesia, or monitored care anaesthesia for mechanical thrombectomy in acute ischaemic stroke. We also included studies reported as full‐text, those published as abstract only, and unpublished data. We excluded quasi‐randomised trials, studies without a comparator group, and studies with a retrospective design.
Data collection and analysis
Two review authors independently applied the inclusion criteria, extracted data, and assessed the risk of bias and the certainty of the evidence using the GRADE approach. The outcomes were assessed at different time periods, ranging from the onset of the stroke symptoms to 90 days after the start of the intervention. The main outcomes were functional outcome, neurological impairment, stroke‐related mortality, all intracranial haemorrhage, target artery revascularisation status, time to revascularisation, adverse events, and quality of life. All included studies reported data for early (up to 30 days) and long‐term (above 30 days) time points.
Main results
We included seven trials with 982 participants, which investigated the type of anaesthesia for endovascular treatment in large vessel occlusion in the intracranial circulation. The outcomes were assessed at different time periods, ranging from the onset of stroke symptoms to 90 days after the procedure. Therefore, all included studies reported data for early (up to 30 days) and long‐term (above 30 up to 90 days) time points.
General anaesthesia versus non‐general anaesthesia(early)
We are uncertain about the effect of general anaesthesia on functional outcomes compared to non‐general anaesthesia (mean difference (MD) 0, 95% confidence interval (CI) –0.31 to 0.31; P = 1.0; 1 study, 90 participants; very low‐certainty evidence) and in time to revascularisation from groin puncture until the arterial reperfusion (MD 2.91 minutes, 95% CI –5.11 to 10.92; P = 0.48; I² = 48%; 5 studies, 498 participants; very low‐certainty evidence). General anaesthesia may lead to no difference in neurological impairment up to 48 hours after the procedure (MD –0.29, 95% CI –1.18 to 0.59; P = 0.52; I² = 0%; 7 studies, 982 participants; low‐certainty evidence), and in stroke‐related mortality (risk ratio (RR) 0.98, 95% CI 0.52 to 1.84; P = 0.94; I² = 0%; 3 studies, 330 participants; low‐certainty evidence), all intracranial haemorrhages (RR 0.92, 95% CI 0.65 to 1.29; P = 0.63; I² = 0%; 5 studies, 693 participants; low‐certainty evidence) compared to non‐general anaesthesia. General anaesthesia may improve adverse events (haemodynamic instability) compared to non‐general anaesthesia (RR 0.21, 95% CI 0.05 to 0.79; P = 0.02; I² = 71%; 2 studies, 229 participants; low‐certainty evidence). General anaesthesia improves target artery revascularisation compared to non‐general anaesthesia (RR 1.10, 95% CI 1.02 to 1.18; P = 0.02; I² = 29%; 7 studies, 982 participants; moderate‐certainty evidence). There were no available data for quality of life.
General anaesthesia versus non‐general anaesthesia (long‐term)
There is no difference in general anaesthesia compared to non‐general anaesthesia for dichotomous and continuous functional outcomes (dichotomous: RR 1.21, 95% CI 0.93 to 1.58; P = 0.16; I² = 29%; 4 studies, 625 participants; low‐certainty evidence; continuous: MD –0.14, 95% CI –0.34 to 0.06; P = 0.17; I² = 0%; 7 studies, 978 participants; low‐certainty evidence). General anaesthesia showed no changes in stroke‐related mortality compared to non‐general anaesthesia (RR 0.88, 95% CI 0.64 to 1.22; P = 0.44; I² = 12%; 6 studies, 843 participants; low‐certainty evidence). There were no available data for neurological impairment, all intracranial haemorrhages, target artery revascularisation status, time to revascularisation from groin puncture until the arterial reperfusion, adverse events (haemodynamic instability), or quality of life.
Ongoing studies
We identified eight ongoing studies. Five studies compared general anaesthesia versus conscious sedation anaesthesia, one study compared general anaesthesia versus conscious sedation anaesthesia plus local anaesthesia, and two studies compared general anaesthesia versus local anaesthesia. Of these studies, seven plan to report data on functional outcomes using the modified Rankin Scale, five studies on neurological impairment, six studies on stroke‐related mortality, two studies on all intracranial haemorrhage, five studies on target artery revascularisation status, four studies on time to revascularisation, and four studies on adverse events. One ongoing study plans to report data on quality of life. One study did not plan to report any outcome of interest for this review.
Authors' conclusions
In early outcomes, general anaesthesia improves target artery revascularisation compared to non‐general anaesthesia with moderate‐certainty evidence. General anaesthesia may improve adverse events (haemodynamic instability) compared to non‐general anaesthesia with low‐certainty evidence. We found no evidence of a difference in neurological impairment, stroke‐related mortality, all intracranial haemorrhage and haemodynamic instability adverse events between groups with low‐certainty evidence. We are uncertain whether general anaesthesia improves functional outcomes and time to revascularisation because the certainty of the evidence is very low.
However, regarding long‐term outcomes, general anaesthesia makes no difference to functional outcomes compared to non‐general anaesthesia with low‐certainty evidence. General anaesthesia did not change stroke‐related mortality when compared to non‐general anaesthesia with low‐certainty evidence. There were no reported data for other outcomes.
In view of the limited evidence of effect, more randomised controlled trials with a large number of participants and good protocol design with a low risk of bias should be performed to reduce our uncertainty and to aid decision‐making in the choice of anaesthesia.
Keywords: Humans; Anesthesia, General; Brain Ischemia; Brain Ischemia/surgery; Intracranial Hemorrhages; Ischemic Stroke; Quality of Life; Randomized Controlled Trials as Topic; Retrospective Studies; Stroke; Stroke/surgery
Plain language summary
Does the type of anaesthesia for recanalisation therapies for acute ischaemic stroke affect patient outcomes?
What was the review about?
Acute ischaemic stroke is a sudden loss of blood circulation in a specific brain area, caused by a blockage in one of the blood vessels, promoting neurological damage. Urgent (recanalisation) treatment to remove the blockage can be beneficial. We wanted to know whether the type of anaesthesia used for this procedure influences treatment to restore blood flow after blood vessels are blocked (recanalisation therapies).
What are recanalisation therapies and anaesthesia types?
Recanalisation therapies use different approaches to restore blood flow. This can be done by using different devices to remove the blockage from the large arteries that supply the brain. The procedure can be performed under different types of anaesthesia. General anaesthesia – complete medicine‐induced anaesthesia followed by supporting breathing (where the person is 'put to sleep'); local anaesthesia – the medicine is directly applied only to a small specific area, providing pain relief; conscious sedation anaesthesia – medicines are given to make the person feel drowsy and relaxed and then carefully monitored, and monitored anaesthesia care – a specific type of anaesthesia service requested by the anaesthesiologist for the care of a patient undergoing a procedure that may fluctuate between the different levels of sedation anaesthesia (i.e. minimal, moderate, and deep).
What did we want to find out?
We wanted to know what type of anaesthesia approach promotes better patient outcomes during recanalisation therapies for acute ischaemic stroke.
What did we do?
We searched for studies that compared different types of anaesthesia for endovascular interventions (where catheters are inserted in small incisions in the groin or arms, and are guided through the blood vessels) in people with acute ischaemic stroke. We compared and summarised their results, and rated our confidence in the evidence, based on factors such as study methods and group size. We included trials that compared general anaesthesia with any other anaesthesia type in people who received recanalisation therapies in acute ischaemic stroke. Studies could have taken place anywhere in the world and participants could have been of any age as long as they received an endovascular recanalisation therapy for acute ischaemic stroke under any anaesthesia type.
Search date: 21 March 2022
What we found?
We found six trials, involving 982 people, in hospitals in high‐income countries including China (three), Denmark (one), France (one), Germany (one), and Sweden (one). We pooled the results when appropriate.
People treated with general anaesthesia had more artery recanalisation compared to non‐general anaesthesia in the short term. General anaesthesia did not change functional wellness and death compared to non‐general anaesthesia in the long term.
Reliability of evidence
We have either little or moderate confidence in these results because, in most studies, it was possible that researchers collecting information about the outcomes of surgery knew which type of anaesthetic people had been given. This could have influenced their assessments. Also, a small number of trials were included with a small population. Furthermore, the variability between included studies, management and anaesthetic type, type of recanalisation therapy, and the experience of the healthcare provider involved in the procedure may have had a significant influence on outcomes.
What happens next?
Our search found eight ongoing studies with 2578 participants. We plan to add the results of these studies to update the review.
Summary of findings
Summary of findings 1. General anaesthesia compared to non‐general anaesthesia for acute ischaemic stroke endovascular treatment (early).
| General anaesthesia compared to non‐general anaesthesia for acute ischaemic stroke endovascular treatment (early) | ||||||
| Patient or population: acute ischaemic stroke endovascular treatment Setting: hospital Intervention: general anaesthesia Comparison: non‐general anaesthesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with non‐general anaesthesia | Risk with general anaesthesia | |||||
|
Functional outcome (continuous; mRS) Follow‐up: at discharge |
The mean functional outcome (continuous; mRS ≤ 2) was 3 | MD 0 (0.31 lower to 0.31 higher) | — | 90 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | — |
|
Neurological impairment (NIHSS) Follow‐up: from 24 to 48 hours |
The mean neurological impairment (NIHSS) was 11.3 | MD 0.29 lower (1.18 lower to 0.59 higher) | — | 982 (7 RCTs) | ⊕⊕⊝⊝ Lowb,d | — |
|
Stroke‐related mortality Follow‐up: in hospital |
104 per 1000 | 102 per 1000 (54 to 191) | RR 0.98 (0.52 to 1.84) | 330 (3 RCTs) | ⊕⊕⊝⊝ Lowb,d | — |
|
All intracranial haemorrhage Follow‐up: in hospital |
165 per 1000 | 152 per 1000 (107 to 213) | RR 0.92 (0.65 to 1.29) | 693 (5 RCTs) | ⊕⊕⊝⊝ Lowb,d | — |
|
Target artery revascularisation (dichotomous; mTICI 2b–3) Follow‐up: 1 day after procedure |
757 per 1000 | 833 per 1000 (772 to 893) | RR 1.10 (1.02 to 1.18) | 982 (7 RCTs) | ⊕⊕⊕⊝ Moderated | — |
|
Time to revascularisation from groin puncture until arterial reperfusion (minutes) Follow‐up: 1 day after procedure |
The mean time to revascularisation from the groin puncture until the arterial reperfusion (minutes) was 71.4 | MD 2.91 higher (5.11 lower to 10.92 higher) | — | 498 (5 RCTs) | ⊕⊝⊝⊝ Very lowb,c,d | — |
|
Adverse events (haemodynamic instability) Follow‐up: 1 day after procedure |
98 per 1000 | 21 per 1000 (5 to 78) | RR 0.21 (0.05 to 0.79) | 229 (2 RCTs) | ⊕⊕⊝⊝ Lowe,f | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; mRS: modified Rankin Scale; mTICI: modified Thrombolysis in Cerebral Infarction; NIHSS: National Institutes of Health Stroke Scale; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to high risk of reporting and other bias. bDowngraded one level due to imprecision: 95% CI consistent with possible benefit and harm. cDowngraded one level due to indirectness: population. dDowngraded one level due to high risk of performance, attrition, reporting and other bias. eDowngraded one level due to high risk of performance and attrition bias. fDowngraded one level due to inconsistency: substantial heterogeneity.
Summary of findings 2. General anaesthesia compared to non‐general anaesthesia for acute ischaemic stroke endovascular treatment (long‐term).
| General anaesthesia compared to non‐general anaesthesia for acute ischaemic stroke endovascular treatment (long‐term) | ||||||
| Patient or population: acute ischaemic stroke endovascular treatment (long‐term) Setting: – Intervention: general anaesthesia Comparison: non‐general anaesthesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with non‐general anaesthesia | Risk with general anaesthesia | |||||
| Functional outcome (dichotomous; mRS ≤ 2) | Study population | RR 1.21 (0.93 to 1.58) | 625 (4 RCTs) | ⊕⊕⊝⊝ Lowa,b | — | |
| 330 per 1000 | 400 per 1000 (307 to 522) | |||||
| Functional outcome (continuous; mRS ≤ 2) | The mean functional outcome (continuous; mRS ≤ 2) was 0 | MD 0.14 lower (0.34 lower to 0.06 higher) | — | 978 (7 RCTs) | ⊕⊕⊝⊝ Lowb,c | — |
| Neurological impairment (NIHSS) | — | — | — | — | — | Not reported |
| Stroke‐related mortality | Study population | RR 0.88 (0.64 to 1.22) | 843 (6 RCTs) | ⊕⊕⊝⊝ Lowb,c | — | |
| 191 per 1000 | 169 per 1000 (123 to 234) | |||||
| All intracranial haemorrhage | — | — | — | — | — | Not reported |
| Target artery revascularisation status | — | — | — | — | — | Not reported |
| Time to revascularisation | — | — | — | — | — | Not reported |
| Adverse events | — | — | — | — | — | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; mRS: modified Rankin Scale; mTICI: modified Thrombolysis in Cerebral Infarction; NIHSS: National Institutes of Health Stroke Scale; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to high risk of performance and attrition bias. bDowngraded one level due to imprecision: 95% CI consistent with possible benefit and harm. cDowngraded one level due to high risk of performance, attrition, reporting and other bias.
Background
See Table 3 for a glossary of terms.
1. Glossary of terms.
| Term | Definition |
| Acute ischaemic stroke (AIS) | A sudden loss of blood circulation to an area of the brain, caused by the thrombotic or embolic occlusion of a cerebral artery, resulting in a corresponding loss of neurological function from the onset of symptoms to 1 week. |
| Alberta Stroke Program Early Computed Tomography Score (ASPECTS) | A 10‐point quantitative score used to assess early ischaemic changes on non‐contrast CT head. |
| American Heart Association (AHA) | A non‐profit organisation in the USA that funds cardiovascular medical research, educates consumers on healthy living, and fosters appropriate cardiac care in an effort to reduce disability and deaths caused by cardiovascular disease and stroke. |
| Angioplasty | A minimally invasive, endovascular procedure to widen narrowed or obstructed arteries or veins. |
| Atherosclerosis | A disease characterised by a build‐up of abnormal fat, cholesterol, and platelet deposits on the inner wall of the arteries. |
| Computed tomography (CT) | A computerised X‐ray imaging procedure in which a narrow beam of X‐rays is aimed at a patient and quickly rotated around the body, producing signals that are processed by the machine's computer to generate cross‐sectional images or 'slices' of the body. |
| Computed tomography angiography (CTA) | Computed tomography scanning that uses an injection of contrast material into the blood vessels to help diagnose and evaluate blood vessel disease or related conditions. |
| Computed tomography perfusion (CTP) | Uses special X‐ray equipment to show which areas of the brain are adequately supplied with blood (perfused) and provides detailed information about blood flow to the brain. |
| Digital subtraction angiography (DSA) | Fluoroscopy technique used in interventional radiology to clearly visualise blood vessels in a bony or dense soft tissue environment. |
| Diffusion‐weighted imaging (DWI) | MR imaging based upon measuring the random Brownian motion of water molecules within a voxel of tissue, particularly useful in tumour characterisation and acute cerebral ischaemia. |
| Direct thrombin inhibitors | A drug that acts as anticoagulant by directly inhibiting the enzyme thrombin (factor IIa). |
| Arterial dissection | A blister‐like delamination between the outer and inner walls of a blood vessel, generally originating with a partial leak in the inner lining. |
| Dolichoectasia | Arteries throughout the human body that have shown significant deterioration of their tunica intima (and occasionally the tunica media), weakened the vessel walls, and caused the artery to elongate and distend. |
| Duplex ultrasound (DUS) | Non‐invasive evaluation of blood flow through the arteries and veins by ultrasound devices. |
| Embolism | Obstruction of an artery or vein, typically by a clot of blood or an air bubble. |
| Fibromuscular dysplasia | A non‐atherosclerotic, non‐inflammatory disease of the blood vessels that causes abnormal growth within the wall of an artery. |
| Magnetic resonance imaging (MRI) | A test that uses powerful magnets, radio waves, and a computer to make detailed pictures inside the body. |
| Magnetic resonance angiography (MRA) | A group of techniques based on magnetic resonance imaging (MRI) to image blood vessels. |
| Placebo | Substance or treatment with no active effect, such as a sugar tablet. |
| Randomised controlled trial (RCT) | A study in which the participants are divided randomly into separate groups to compare different treatments. |
| Recombinant tissue plasminogen activator (r‐tPA) | A protein involved in the breakdown of blood clots. |
| Stent | A metal or plastic tube inserted into the lumen of an anatomic vessel or duct to keep the passageway open. |
| Stent retriever | A self‐expanding stent used to retrieve the thromboembolism and restore blood flow. |
| Stroke | Neurological deficit attributed to an acute focal injury of the central nervous system by a vascular cause, persisting ≥ 24 hours or until death. |
| Thrombectomy | Interventional procedure of removing a blood clot (thrombus) from a blood vessel. |
| Thromboaspiration | Aspiration of occlusive thrombi with suction devices to restore blood flow. |
| Thrombolysis | Breakdown (lysis) of blood clots formed in blood vessels. |
| Thrombosis | Local coagulation of blood (clot) in a part of the circulatory system. |
| Transient ischaemic attack (TIA) | A transient episode (less than 24 hours) of neurological dysfunction caused by focal brain, spinal cord, or retinal ischaemia without acute infarction. |
| Vascular | Relating to blood vessels (arteries and veins). |
Description of the condition
Stroke is an important cause of neurological disability and death worldwide, producing a negative socioeconomic impact. About 30% of ischaemic strokes are related to an acute proximal large vessel occlusion (LVO) by a thrombus, and early interventions have substantial impingement over good neurological outcomes (Benjamin 2019; Flumignan 2017a; Goyal 2014; Lakomkin 2019; Meretoja 2017; Norrving 2013; Wilson 2002).
Restoration of blood flow after a major cerebral artery blockage by a thrombus can be performed by two different interventions: chemical or mechanical. Chemical thrombolysis is achieved by intravenous (IV) or intra‐arterial administration of a thrombolytic agent, or both, in order to dissolve the thrombus, while mechanical thrombectomy (MT) uses intra‐arterial devices to fragment or remove (or both) the thrombus. These two techniques (i.e. thrombolysis or thrombectomy) can be used together as pharmacomechanical thrombolysis (Goyal 2016; Wardlaw 2014).
MT may have some benefits over IV thrombolysis for the treatment of cerebral LVO. The American Heart Association (AHA) recommends IV recombinant tissue plasminogen activator (r‐tPA) within 4.5 hours and MT within six hours with an Alberta Stroke Program Early Computed Tomography Score (ASPECTS) greater than 6, or six to 24 hours with a good clinical‐radiological mismatch, after the onset stroke (Albers 2018; Goyal 2016; Lindekleiv 2018; Nogueira 2018; Powers 2018; Powers 2019a).
Among ischaemic strokes, there are some special causes of cervicocerebral artery injury, such as dissection, atherosclerosis, fibromuscular dysplasia, web vessels, and dolichoectasia, which might produce pseudo‐occlusions and embolic events. Such lesions may have stenting or balloon angioplasty as an alternative intervention (Bang 2018; Flumignan 2017b; Kim 2016; Luo 2018; Naylor 2018; Pereira 2018).
In order to diagnose and classify LVO following a stroke, there are some complementary imaging tests: duplex ultrasound (DUS), magnetic resonance image (MRI), computed tomography (CT), or digital subtraction angiography (DSA). The AHA recommends CT and MRI and their multimodal protocols (non‐contrast, angiography, and perfusion) for acute ischaemic stroke (AIS) to predict risk‐benefit, plan any therapeutic intervention, and also exclude stroke mimics. In the stroke setting, CT is the main imaging method used due to its speed, cost‐effectiveness, and availability in most stroke centres (Cassola 2018; Powers 2019a).
Description of the intervention
In addition to different endovascular approaches for AIS, the type of anaesthesia technique has been debated as having a relevant impact on neurological outcomes. Anaesthetic interventions can be performed by administering inhaled, IV, or percutaneous agents to reduce pain, anxiety, and patient mobility, thereby reducing the procedural time and complications; this might make the procedure safer and achieve better clinical results. General anaesthesia (GA) is normally used in people with worse neurological symptoms in the endovascular treatment (EVT) of acute LVO stroke. Local anaesthesia (LA), conscious sedation anaesthesia (CSA), and monitored anaesthesia care (MAC) have the potential for faster recovery, use smaller amounts of medication, and enable the conscious monitoring of neurological intervention effects (ASA 2019).
Local anaesthesia
LA is a percutaneous approach drug that numbs a small specific area, disrupting the sensations of pain in the body. The patient will remain conscious during the procedure and may feel some pressure without pain in this specific anaesthetised area (ASA 2019).
Conscious sedation anaesthesia
CSA is considered a moderate sedation/analgesia, defined as a drug‐induced slightly deeper depression of consciousness after IV administration of sedative and analgesic agents. The patient responds purposefully to verbal commands, either alone or accompanied by light tactile stimulation. The physician provider must be prepared to recognise 'deep' sedation, manage its consequences, and adjust the level of sedation to a 'moderate' or lesser level. Usually, spontaneous ventilation and cardiovascular function are maintained and no intervention is required to keep a patent airway (ASA 2019).
Monitored anaesthesia care
MAC is defined as a specific type of anaesthesia service requested by the anaesthesiologist for the care of a patient undergoing a procedure that may fluctuate between the different levels of sedation anaesthesia (i.e. minimal (anxiolysis), moderate (CSA), and deep (MAC)). Anaesthesia care includes a preprocedure evaluation, intraprocedure care, and postprocedure management, as well as the flexibility to match sedation levels to patient needs and procedural requirements. MAC is considered to be deep sedation/analgesia, defined as a drug‐induced depression of consciousness after IV administration of sedative, analgesic, amnesic, and anxiolytic agents, or other medications as necessary for patient safety. Normally, it is associated with LA. The patient cannot be easily aroused, but responds purposefully after repeated or painful stimulation. The ability to independently maintain ventilatory function may be impaired. Patients may require assistance in maintaining a patent airway, and spontaneous ventilation may be inadequate. Cardiovascular function is usually maintained. The presence of a qualified anaesthesiologist is essential and patient oxygenation, ventilation, circulation, and temperature must be monitored continuously. MAC may lead to conversion to GA at any time and must assure a return to full consciousness, pain relief, and management of adverse effects from medications administered during the procedure. In some cases, the anaesthesiologist may provide only monitored care without any anaesthetic administration (ASA 2019).
General anaesthesia
GA is a complete drug‐induced loss of consciousness after administration of inhalation or IV agents, or both. The patient cannot be aroused, even after pain stimulation. Significant respiratory and cardiovascular depression occurs and the airway patency is lost, which normally requires insertion of a laryngeal mask airway or endotracheal tube. Positive pressure ventilation is often necessary due to hypoventilation and drug‐induced depression of neuromuscular function. Cardiovascular function may be affected (ASA 2019).
How the intervention might work
The anaesthetic team is increasingly involved in patient care during the EVT of AIS. They have to monitor the heart rhythm, haemodynamic changes, temperature, blood glucose, oxygen saturation, and level of consciousness, as well as neuromuscular blockade during anaesthesia management, which has been correlated with a better neurological outcome (Talke 2014).
Most professionals prefer performing MT under non‐GA (LA, CSA, or MAC) rather than GA (Peng 2018; Rasmussen 2017; Steinberg 2019).
GA keeps the patient immobile, lowering the risk of vascular injuries, such as perforation or dissection, protecting the airways against broncho‐aspiration, and promoting pain and anxiety control. While MT under GA may be more effective and safer, it may also be faster than non‐GA with regard to revascularisation time. The major disadvantages of GA are the delay to the start of the procedure and blood pressure hypotension, which can increase the ischaemic area of the brain, leading to a poor functional outcome. Usually, GA is performed in those patients with worse neurological symptoms of AIS (McDonald 2015; Molina 2010; Takahashi 2014).
Non‐GA enables the patient to remain awake, permitting the monitoring of neurological status and haemodynamic stability, and decreasing procedural time but does not protect the airways. Nevertheless, not controlling patient movement during the procedure might prolong the revascularisation time and increase the incidence of intraprocedural complications. During MT, the patient sometimes shows a decrease in their level of consciousness and develops agitation, vomiting, or swallowing difficulties, making it necessary to convert the non‐GA to GA, further delaying the procedure time. Any delay to the procedure might result in impaired neurological outcomes. Indeed, anaesthetic intervention can be faster and more feasible in non‐GA than GA, with fewer haemodynamic changes, and may result in better neurological outcomes. The effects of the type of anaesthesia for endovascular interventions in AIS remains unclear (McDonald 2015; Molina 2010; Takahashi 2014; Talke 2014).
Why it is important to do this review
Currently, the number of endovascular interventions for AIS is increasing, and, regardless of the device or technique used, the type of anaesthesia has been shown to be one of the main factors impacting neurological outcomes. Among the anaesthesia types, there are GA and non‐GA (LA, CSA, or MAC), both of which have several advantages and disadvantages. There is no consensus on the best anaesthesia type for AIS EVT (Rusy 2021).
A direct comparison is required at this time and may help the neurointerventionalist to make the procedure safer and promote the best neurological outcomes for the patient.
There have been some randomised controlled trials (RCTs) attempting to establish which anaesthesia type promotes better patient‐centred outcomes with fewer complications. To date, none has shown a robust difference in clinical outcomes between the GA and non‐GA groups. Two systematic reviews reported significantly less disability for GA at three months (Bai 2021; Schonenberger 2019); however, the effect of the type of anaesthesia for the treatment of AIS is still under debate (Löwhagen Hendén 2017; Schonenberger 2016; Simonsen 2018).
Objectives
To assess the effects of different types of anaesthesia for endovascular interventions in people with acute ischaemic stroke.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs with a parallel (e.g. cluster or individual) design. We included studies reported as full‐text, those published as abstract only, and unpublished data. We excluded quasi‐randomised trials (i.e. studies in which participants were allocated to intervention groups based on methods that were not truly random, such as hospital number or date of birth).
Types of participants
We considered the inclusion of participants of any gender and any age with AIS defined by any related extracranial or intracranial artery occlusion, irrespective of the time at which the participant underwent any type of endovascular intervention. All participants who experienced the onset of stroke symptoms were included, and grouped into those with an unknown length of symptom onset and those with symptoms for less or more than six hours. We only considered participants with LVO undergoing EVT under anaesthesia for inclusion (i.e. the anaesthesia type was the only difference between the control and experimental groups). AIS was defined as an occlusion of the internal or common carotid artery (extracranial) or any intracranial artery occlusion diagnosed by at least one valid objective test (e.g. DUS or angiography by tomography, magnetic resonance, or digital subtraction). All trials involving people with LVO who underwent an endovascular procedure were considered, irrespective of the degree or the method used to determine the degree of the brain ischaemic injury. In studies with mixed populations (e.g. haemorrhagic and ischaemic stroke), in which only a subset of the participants met our inclusion criteria (i.e. ischaemic stroke with LVO), we planned to request data for the subgroup of interest from the triallists for inclusion in our review. For studies with mixed populations, such as haemorrhagic and ischaemic stroke, in which we could not get data from the subgroup of interest, but for which at least 50% of the study population were of interest, we planned to include all participants in our analysis. Moreover, we planned to explore the effect of this decision in a sensitivity analysis.
Types of interventions
We included trials comparing one type of anaesthesia versus another with any combination of interventions, providing that the cotreatments were balanced between the experimental and control arms. We also included studies that compared different types and doses of anaesthetic drugs. We did not foresee identifying any study comparing placebo anaesthesia, but we planned to consider them if we did.
We considered the following interventions.
Local anaesthesia (LA).
Conscious sedation anaesthesia (CSA).
Monitored anaesthesia care (MAC).
General anaesthesia (GA).
Possible comparisons included:
GA versus CSA;
GA versus LA;
GA versus MAC;
GA versus CSA plus LA;
any combination of the above interventions versus any combination.
Types of outcome measures
We presented all outcomes at two time points after the start of the intervention if data were available.
Early outcomes (up to one month after the start of the intervention).
Long‐term outcomes (more than one month after the start of the intervention).
Primary outcomes
Functional outcome at the end of the scheduled follow‐up period, categorised by the modified Rankin Scale (mRS): good outcome: scores 0 to 2 (i.e. functional independence); poor outcome: scores 3 to 6 (i.e. functional dependency or death). If the mRS score was not reported, we used the trial's own definition of functional outcome. If more than one functional outcome score was reported, we used the mRS as our main score of interest. If we identified both dichotomous and continuous variables related to independence, we reported them separately as independent outcomes (Wilson 2002).
Neurological impairment assessed using clinical outcome measures or any validated international scales (e.g. the National Institutes of Health Stroke Scale (NIHSS)). If we identified both dichotomous and continuous variables related to neurological impairment, we reported them separately as independent outcomes (Brott 1989).
Secondary outcomes
Stroke‐related mortality.
All intracranial haemorrhage: asymptomatic and symptomatic, as classified in the third European Cooperative Acute Stroke Study (Hacke 2008), reported as the proportion of participants with intracranial haemorrhage.
Target artery revascularisation status: revascularised or not revascularised or assessed by any validated scale (e.g. the modified Thrombolysis In Cerebral Infarction (mTICI) scale (Fugate 2013), cerebral infarction perfusion categories (Higashida 2003)). If we identified both dichotomous and continuous variables related to neurological impairment, we reported them separately as independent outcomes.
Time to revascularisation: time (in minutes) from groin puncture or the start of the EVT until arterial reperfusion.
Adverse events: any reported adverse events (excluding death), reported separately as independent outcomes.
Quality of life (QoL): participant's subjective perception of improvement (yes or no) as reported by the study authors or using any validated scoring system such as the Short Form‐36 Health Survey (SF‐36) (Ware 1992).
Search methods for identification of studies
See the 'Specialised register' information available at the Cochrane Stroke Group's website (stroke.cochrane.org). We searched for trials in all languages and arranged for the translation of relevant articles where necessary.
Electronic searches
Cochrane Stroke Group Specialised Register of Trials (last searched 5 July 2022) (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL; Issue 3, 2022) in the Cochrane Library (Appendix 2);
MEDLINE Ovid (from 1946 to 14 August 2020) (last searched 21 March 2022) (Appendix 3);
Embase Ovid (from 1980 to week 33 2020) (last searched 21 March 2022) (Appendix 4);
Literatura Latino‐Americana e do Caribe em Ciências da Saúde (LILACS) (from 1982) (last searched 21 March 2022), via Virtual Health Library (Appendix 5);
Indice Bibliográfico Español de Ciencias de la Salud (IBECS) (searched 21 March 2022), via Virtual Health Library (Appendix 5).
We modelled the subject strategies for databases on the search strategy designed for MEDLINE by the Cochrane Stroke Group's Information Specialist (Appendix 3). We combined all search strategies deployed with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCT and controlled clinical trials, as described in Chapter 4 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2021).
We searched the following ongoing trial registers:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov/) (Appendix 6);
World Health Organization (WHO) International Clinical Trials Registry Platform (who.int/ictrp/en/) (Appendix 7).
The most recent searches were carried out on 21 March 2022.
Searching other resources
In an effort to identify further published, unpublished and ongoing trials, we:
checked the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials and searched Google Scholar to forward track relevant references (scholar.google.co.uk/);
contacted the original trial authors for clarification and further data if trial reports were unclear;
where necessary, contacted experts/trialists/organisations in the field to obtain additional information on relevant trials using a standard letter template (Appendix 8); and
-
conducted a search of various grey literature sources, dissertation and theses databases, and databases of conference abstracts, including:
British Library EThOS (UK E‐Theses Online Service) (Appendix 9);
ProQuest Dissertation and Theses Global (Appendix 10).
Data collection and analysis
Selection of studies
Two review authors (RT, CNBC) independently screened titles and abstracts of the references obtained as a result of our searching activities and excluded obviously irrelevant reports using the Covidence tool (Covidence). We retrieved the full‐text articles for the remaining references and two review authors (RT, CNBC) independently screened the full‐text articles and identified studies to determine and record reasons for exclusion of the ineligible studies. We resolved any disagreements through discussion or, if required, we consulted a third review author (RLGF). We collated multiple reports of the same study so that each study, rather than each reference, was the unit of interest in the review. We recorded the selection process and completed a PRISMA flow diagram (Page 2021a).
Data extraction and management
We used a data collection form for study characteristics and outcome data, which we piloted on at least one study in the review. Two review authors (RT, CNBC) independently extracted data from the included studies. We extracted the following study characteristics.
Methods: study design, total duration of the study, details of any 'run in' period, number of study centres, and the location, study setting, and date of the study.
Participants: number randomised, the number lost to follow‐up/withdrawn, number analysed, mean age, age range, gender, the severity of the condition, diagnostic criteria, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for the trial, and notable conflicts of interest of trial authors.
We resolved disagreements by consensus or by involving a third review author (RLGF). One review author (RT) transferred data into Review Manager 5 (Review Manager 2014). We double‐checked that the data were entered correctly by comparing the data presented in the systematic review with the data extraction form. A second review author (CNBC) spot‐checked study characteristics for accuracy against the trial reports.
Assessment of risk of bias in included studies
Two review authors (RT, CNBC) independently assessed the risk of bias for each study using the criteria outlined in Chapter 8 of the CochraneHandbook(Higgins 2017). We resolved any disagreements by discussion or by involving another review author (RLGF). We assessed the risk of bias according to the following domains.
Random sequence generation. Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data. Selective outcome reporting.
Other bias.
In cluster‐randomised trials, we planned to consider particular biases, as recommended in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions: recruitment bias; baseline imbalance; loss of clusters; incorrect analysis; and comparability with individually randomised trials (Higgins 2017). We graded each potential source of bias as high, low, or unclear, and provided a quote from the study report, together with a justification for our judgement in the risk of bias table. We summarised the risk of bias judgements across different studies for each of the domains listed. Where information on the risk of bias related to unpublished data or correspondence with a trialist, we noted this in the risk of bias table. When considering treatment effects, we considered the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol (Tosello 2020), and reported any deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs). We analysed continuous data using the mean difference (MD) when studies used the same scale/score, or the standardised mean difference (SMD) when studies used different scales/scores, with 95% CIs. We entered data presented as a scale with a consistent direction of effect. We narratively described skewed data reported as medians and interquartile ranges.
Unit of analysis issues
Individuals were the unit of analysis. If trials included multiple intervention arms, we planned to consider only the arms relevant to the scope of our review, but list the remaining arms in the Characteristics of included studies table. Where a study included multiple intervention groups, we planned to combine groups to create a single pair‐wise comparison.
Cluster‐randomised trials
We did not identify any cluster‐RCTs. However, if we had identified any such studies, we planned to include them in the analyses along with individually randomised trials. We planned to adjust their sample sizes using the methods described in Chapter 23 of the CochraneHandbook (Higgins 2021), using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population. If we used ICCs from other sources, we planned to report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identified both cluster‐randomised trials and individually randomised trials, we planned to synthesise the relevant information. We planned to consider it reasonable to combine the results from both types of trials if there was little heterogeneity between the study designs, and the interaction between the effect of the intervention and the choice of randomisation unit was considered to be unlikely. We also planned to acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as an abstract only). Where possible, we used the Review Manager 5 calculator to calculate missing standard deviations using other data from the trial, such as CIs. Where this was not possible, and the missing data were thought to introduce serious bias, we planned to explore the impact of including such studies in the overall assessment of results by a sensitivity analysis. For all outcomes, we followed intention‐to‐treat (ITT) principles to the greatest degree possible, where we analysed participants in their randomised group regardless of the intervention received. We used available‐case data for the denominator if ITT data were not available.
We presented study‐level data so that missing and unclear data were clearly indicated and to make any unpublished data acquired from investigators available.
Assessment of heterogeneity
We inspected forest plots visually to consider the direction and magnitude of effects and the degree of overlap between CIs. We used the I² statistic to measure heterogeneity among the trials in each analysis; we acknowledge that there is substantial uncertainty in the value of the I² statistic when there is only a small number of studies. If we identified substantial heterogeneity, we reported it and explored possible causes by prespecified subgroup analysis. We considered an I² statistic greater than 50% as substantial heterogeneity and explored the individual trial characteristics to identify potential sources of heterogeneity (Deeks 2019).
Assessment of reporting biases
We planned to use funnel plots to investigate reporting biases if we identified 10 or more studies for only the primary outcomes, as recommended in Chapter 13 by the CochraneHandbook (Page 2021b).
Data synthesis
We synthesised the data using Review Manager 5 (Review Manager 2014). We undertook meta‐analyses only where this was meaningful (i.e. if the treatments, participants, and the underlying clinical question were similar enough for pooling to be appropriate).
If we were confident that trials were estimating the same underlying treatment effect (i.e. the included studies were homogeneous (considering population, interventions, comparators, and outcome characteristics)), we used a fixed‐effect meta‐analysis. If clinical heterogeneity was sufficient to expect that underlying treatment effects differed between trials, or if there was at least substantial heterogeneity, we used a random‐effects meta‐analysis. If there was substantial clinical, methodological, or statistical heterogeneity across trials that prevented the pooling of data, we used a narrative approach to data synthesis (Deeks 2019).
We addressed all outcomes listed in the Types of outcome measures subsection in the Results section of the review under the heading Effects of interventions, with outcomes addressed in the order in which they are shown in Types of outcome measures.
We included the results of individual studies and any statistical summary of the in Data and analyses tables in the review.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses when there were five or more studies included in a single analysis, all with sufficient information to determine the subgroups.
Participant characteristics:
age: for example, adults (18 years to 74 years) and elderly people (75 years and over);
comorbidities: for example, diabetes, tobacco addiction;
artery occlusion site: for example, common or internal carotid artery; anterior, medial, or posterior cerebral artery; vertebrobasilar system and hemisphere side;
ASPECTS score (to 6 versus more than 6) (Barber 2000).
Intervention characteristics:
types of drugs: for example, analgesic, anti‐muscarinic, anxiolytic, barbiturates, benzodiazepines, dissociative, hypnotic, inhaled anaesthetics, opioids, muscle relaxants, vasoactive;
doses of drugs;
time from stroke onset until the start of the revascularisation (in minutes);
anaesthesia duration (in minutes);
blood pressure during the intervention.
After the inspection of forest plots, and to investigate heterogeneity, we also performed a subgroup analysis for more extracted time points of outcome assessment (at 24 hours after the intervention versus more than 24 hours; Analysis 1.2). We used the following outcomes (i.e. the primary outcomes) in subgroup analyses.
1.2. Analysis.

Comparison 1: General anaesthesia versus non‐general anaesthesia (early time point), Outcome 2: Neurological impairment
Functional outcome at the end of the scheduled follow‐up.
Neurologic impairment.
We used the formal test for subgroup differences in Review Manager 5 (Review Manager 2014), and base our interpretation on this.
Sensitivity analysis
We planned to carry out the following sensitivity analyses, to test whether key methodological factors or decisions affected the main result. We planned to group these analyses according to study design (individual or cluster), if data were available. However, the data for sensitivity analysis were only related to the risk of bias of included studies. We excluded studies where less than 50% of the population were of interest and the subgroup of interest data were not available.
Only studies with a low risk of bias were included. We considered a study to have a low risk of bias overall if there was no high‐risk judgement in any of the four main domains (i.e. random sequence generation, allocation concealment, incomplete outcome data, and selective reporting).
We planned to examine both the fixed‐effect model and the random‐effects model meta‐analyses and will explore the differences between the two estimates.
If we identified studies with missing data that were unobtainable, we planned to repeat analyses excluding these studies to determine their impact on the primary analyses.
We used the following outcomes (i.e. the primary outcomes) in the sensitivity analyses.
Functional outcome at the end of the scheduled follow‐up.
Neurological impairment.
Reaching conclusions
We based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this review. We avoided making recommendations for practice and our implications for research suggested priorities for future research and outlined the remaining uncertainties in the area.
Summary of findings and assessment of the certainty of the evidence
We created a separate summary of findings table for the early and long‐term time points using the following outcomes: functional outcome at the end of the scheduled follow‐up; neurological impairment; stroke‐related mortality; all intracranial haemorrhage; target artery revascularisation status; time to revascularisation; and adverse events.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of a body of evidence as it relates to the studies that contributed data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used methods and recommendations described in Chapter 14 of the CochraneHandbook(Schünemann 2019) using GRADEpro GDT software (GRADEpro GDT). We justified all decisions to downgrade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
Two review authors (RT, CNBC) independently made judgements about evidence certainty, with disagreements resolved by discussion or involving a third review author (RLGF). We justified, documented, and incorporated judgements into the reporting of results for each outcome.
We extracted study data, formatted our comparisons in data tables, and prepared summary of findings tables before writing the results and conclusions of our review.
Results
Description of studies
We presented the details of studies included in this review in the Characteristics of included studies table, and reasons for exclusion in the Characteristics of excluded studies table. We have detailed the status of ongoing trials in the Characteristics of ongoing studies table.
Results of the search
We completed the search on 5 June 2022. We retrieved 11,339 records from electronic databases and identified no additional records through other sources. After the exclusion of 1413 duplicate records, we screened titles and abstracts of 9926 unique records. We considered 9801 records not relevant at this stage and we selected 125 records for full‐text reading. We included seven studies (29 reports). We excluded 36 studies with reasons and assessed another 52 as not relevant at this stage (see Characteristics of excluded studies table). Eight trials are ongoing (see Characteristics of ongoing studies table). The flowchart for the results of the search is presented in Figure 1.
1.
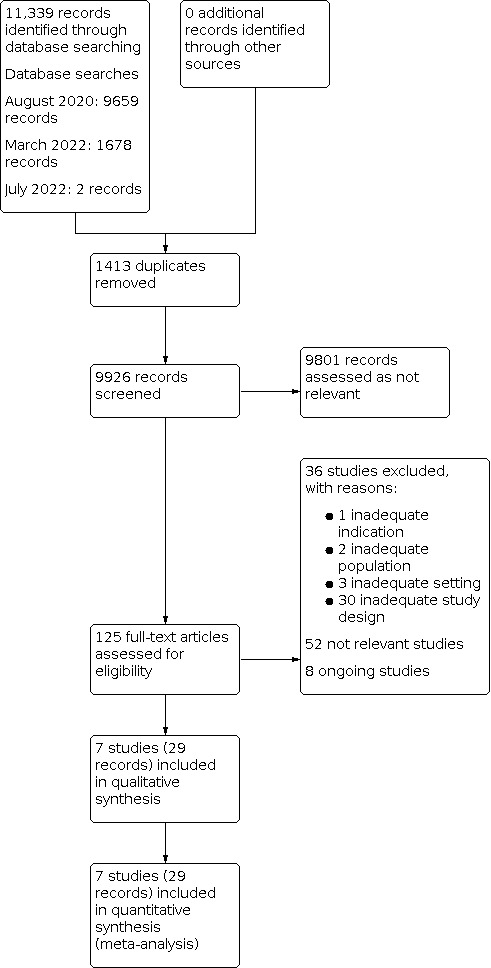
Study flow diagram.
Included studies
The seven included studies (982 participants) tested only one comparison: GA versus non‐GA (AnStroke 2017; CANVAS 2020; GOLIATH 2018; Maurice 2022; Ren 2020; SIESTA 2016). The studies were carried out from 2016 to 2022.
For details of the included studies, see the Characteristics of included studies table.
Design
We classified all seven included studies as randomised trials, but one did not provide clear details of the method used for randomisation (GOLIATH 2018). Four studies did not provide clear details about the allocation concealment (CANVAS 2020; GOLIATH 2018; Hu 2020; Ren 2020). We identified no cross‐over or cluster RCT.
No study was triple‐blinded because the nature of the intervention did not allow for blinding of personnel. Four were single‐blinded because the outcome assessment was blinded (AnStroke 2017; CANVAS 2020; Hu 2020; Ren 2020; SIESTA 2016), and two were unclear about blinding (GOLIATH 2018; Maurice 2022).
Settings
All seven studies were conducted in hospital settings in the following countries: Sweden (AnStroke 2017), Denmark (GOLIATH 2018), France (Maurice 2022), Germany (SIESTA 2016), and China (CANVAS 2020; Hu 2020; Ren 2020).
Participants
All seven studies provided data of participants with a large arterial vessel occlusion intracranial circulation submitted to EVT under any anaesthesia type; six studies provided data for anterior intracranial circulation (AnStroke 2017; CANVAS 2020; GOLIATH 2018; Maurice 2022; Ren 2020; SIESTA 2016), and one study provided data for posterior intracranial circulation (Hu 2020). Of those 1009 participants, 10 had withdrawn (one in AnStroke 2017, three in CANVAS 2020, and six in Maurice 2022); two were excluded due to missing informed consent (SIESTA 2016); and five were lost to follow‐up (four in Maurice 2022 and one in SIESTA 2016). The remaining 982 participants were analysed as ITT, 56.8% were men with a mean age of 71.2 years old. About 72% received IV r‐tPA before EVT (AnStroke 2017; GOLIATH 2018; Maurice 2022; Ren 2020; SIESTA 2016), 9% were converted from CSA to GA, and mean NIHSS was 16.1 (AnStroke 2017; CANVAS 2020; GOLIATH 2018; Maurice 2022; Ren 2020; SIESTA 2016). CANVAS 2020 did not report if the participants included received IV r‐tPA before EVT, and GOLIATH 2018 did not report any loss to follow‐up.
Sample size
The number of participants included in each of the six studies ranged from 40 in CANVAS 2020 to 345 in Maurice 2022. Most studies had small sample sizes.
Funding
Two trials reported that they had no funding sources (GOLIATH 2018; SIESTA 2016). One trial was declared as self‐funded (Ren 2020), two trials were declared as funded by government grants (AnStroke 2017; Maurice 2022), one trial was declared as funded by a private company (Hu 2020), and one trial was declared as host hospital funded (CANVAS 2020).
Conflict of interest
Six trials stated they had no conflicts of interest (AnStroke 2017; CANVAS 2020; GOLIATH 2018; Hu 2020; Ren 2020; SIESTA 2016), and one trial declared having a conflict of interest (Maurice 2022).
Interventions
All seven studies tested two different types of interventions: GA and non‐GA (LA, CSA, and MAC). Six trials reported their anaesthesia protocol (AnStroke 2017; CANVAS 2020; GOLIATH 2018; Hu 2020; Maurice 2022; Ren 2020), and SIESTA 2016 reported no details. The anaesthesia protocol for each trial is reported in the Characteristics of included studies table.
Outcomes
Most studies included in this review had similar outcomes, and study authors provided data for all outcomes relevant to this review. The main outcome measures were functional outcome, neurological impairment, stroke‐related mortality, all intracranial haemorrhage, target artery revascularisation status, time to revascularisation, adverse events, and QoL. These outcomes were assessed at different time periods, ranging from the onset of the stroke symptoms to 90 days after the start of the intervention. Therefore, all included studies reported data for early (up to 30 days) and long‐term (above 30 days) time points.
Primary outcomes
All studies reported our primary outcomes functional outcome and neurological impairment.
Secondary outcomes
All studies reported target artery revascularisation status. Six included studies reported stroke‐related mortality (AnStroke 2017; CANVAS 2020; GOLIATH 2018; Maurice 2022; Ren 2020; SIESTA 2016). Five studies reported time to revascularisation (AnStroke 2017; CANVAS 2020; GOLIATH 2018; Ren 2020; SIESTA 2016), and two studies reported haemodynamic instability adverse events (AnStroke 2017; Hu 2020). Five studies reported all intracranial haemorrhages (AnStroke 2017; CANVAS 2020; GOLIATH 2018; Maurice 2022; Ren 2020). None of the studies reported QoL.
Excluded studies
We excluded 36 studies for at least one reason (Characteristics of excluded studies table). Three studies had an inadequate population because they evaluated participants with unruptured intracranial aneurysms (ACTRN12618000509268), participants underwent sedation collateral support in EVT for AIS (NCT03737786), and participants were provided with laryngeal mask airway support during anaesthesia in stent‐assisted angioplasty for extracranial and intracranial artery stenosis (Yao 2009). Two studies had an inadequate comparator because the trial considered infarct growth after EVT for AIS in participants sedated with propofol and dexmedetomidine for six hours before extubation (NCT04517383), the trial compared the type of drug (dexmedetomidine versus propofol) in MAC for EVT in AIS (Wu 2019). One study had an inadequate indication comparing GA versus regional anaesthesia during carotid endarterectomy (Sindelic 2004).
All other thirty‐six excluded studies had inadequate study design or at least one of the following reasons:
retrospective analysis of the results for anaesthetic type in RCTs that compared EVT versus IV r‐tPA for AIS (Abou‐Chebl 2015; Berkhemer 2016; Bracard 2016; Crosby 2016; Goldhoorn 2020; Menon 2016; Powers 2019b; Simonsen 2017; Wong 2011);
non‐randomised studies (Campbell 2019; Chabanne 2020; Jovin 2009; Le 2020; Moritz 2010; Neimark 2010; Nichols 2010; Nii 2018; Pishjoo 2019; Rohde 2019; Schönenberger 2019; Shan 2018; Starke 2017; Thomas 2012; Wolf 2019; Zussman 2018);
literature review of studies comparing GA versus CSA for EVT in AIS (Rabinstein 2018);
observational case‐control study (Avitsian 2016; Bonafe 2016; Taqi 2019);
retrospective analysis of the results of an RCT that did not compare the type of anaesthesia (Tekle 2018).
Studies awaiting classification
There are no studies awaiting classification.
Ongoing studies
We identified eight ongoing studies evaluating the following interventions. Five studies are comparing GA versus CSA (Chabanne 2019; Chen 2020; DRKS00006801; DRKS00023679; NCT03247998); one study is comparing GA versus CSA plus LA (Liang 2020); and two studies are comparing GA versus LA (ChiCTR2000035282; Peng 2017).
Seven ongoing studies plan to report data on functional outcome using the mRS (Chabanne 2019; Chen 2020; ChiCTR2000035282; DRKS00006801; Liang 2020; NCT03247998; Peng 2017); five studies plan to report data on neurological impairment (Chabanne 2019; Chen 2020; DRKS00023679; Liang 2020; Peng 2017); five will use the NIHSS (Chabanne 2019; Chen 2020; DRKS00023679; Liang 2020; Peng 2017); and three studies did not report which score will be used (ChiCTR2000035282; DRKS00006801; NCT03247998). Six studies plan to report data on stroke‐related mortality (Chabanne 2019; Chen 2020; ChiCTR2000035282; DRKS00006801; Liang 2020; Peng 2017). Two studies plan to report data on all intracranial haemorrhages (Chabanne 2019; Chen 2020). Five studies plan to report data on target artery revascularisation status (Chabanne 2019; Chen 2020; DRKS00023679; Liang 2020; Peng 2017). Four studies plan to report data on time to revascularisation (Chabanne 2019; DRKS00006801; DRKS00023679; Liang 2020). Four studies plan to report data on adverse events (Chabanne 2019; Chen 2020; Liang 2020; Peng 2017). One study plans to report data on QoL (Chen 2020).
One study did not plan to report any outcome of interest for this review (NCT03247998).
Risk of bias in included studies
Risk of bias varied considerably across the included studies, and there was insufficient detail to inform judgement in several cases. Figure 2 and Figure 3 summarise the risk of bias in the included studies.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We judged the overall risk of bias in four included studies as high (AnStroke 2017; CANVAS 2020; Maurice 2022; Ren 2020). We judged two at unclear (GOLIATH 2018; Hu 2020), and one at low risk of bias overall (SIESTA 2016).
Allocation
Five studies had a low risk of bias for random sequence generation (AnStroke 2017; CANVAS 2020; Maurice 2022; Ren 2020; SIESTA 2016), and two had an unclear risk of bias (GOLIATH 2018; Hu 2020).
Three studies had a low risk of bias for allocation concealment (AnStroke 2017; Maurice 2022; SIESTA 2016), and four studies had an unclear risk of bias (CANVAS 2020; GOLIATH 2018; Hu 2020; Ren 2020).
Blinding
All included studies had a high risk of bias for blinding of participants and personnel due to the nature of the interventions.
We assessed five studies at low risk of bias for blinding of outcome assessment (AnStroke 2017; CANVAS 2020; Hu 2020; Ren 2020; SIESTA 2016), and two studies at unclear risk of bias (GOLIATH 2018; Maurice 2022).
Incomplete outcome data
Three studies had a high risk of attrition bias because they had cross‐over from CSA to GA in more than 10%, promoting a data imbalance between groups (AnStroke 2017; CANVAS 2020; Maurice 2022). The four other studies had a low risk of attrition bias (GOLIATH 2018; Hu 2020; Ren 2020; SIESTA 2016).
Selective reporting
Ren 2020 was at high risk of reporting bias due to several changes between the protocol and the trial reporting related to the inclusion and exclusion criteria, as well as primary and secondary outcomes. Maurice 2022 was at high risk due to a high number of participants who had their mRS score evaluated between two and six months, which might affect neurological outcomes. Hu 2020 was at unclear risk because we did not find the study protocol. The other four studies had a low risk of reporting bias.
Other potential sources of bias
Five studies had a low risk of other potential sources of bias (AnStroke 2017; CANVAS 2020; GOLIATH 2018; Maurice 2022; SIESTA 2016), and one study was at high risk due to change in the study objectives (Ren 2020). According to the protocol, the objective of Ren 2020 was to assess the effects of different concentrations and ways of administering dexmedetomidine with remifentanil for people receiving craniocerebral disease interventional therapy under GA, but in the published trial, the study objective was reported as the effect of CSA versus GA on outcomes in people undergoing MT for AIS. Hu 2020 was at unclear risk because we did not find the study protocol.
Effects of interventions
General anaesthesia versus non‐general anaesthesia (early time point)
See Table 1.
All seven studies compared GA versus non‐GA and reported all outcomes from at discharge up to 30 days (AnStroke 2017; CANVAS 2020; GOLIATH 2018; Hu 2020; Maurice 2022; Ren 2020; SIESTA 2016). We judged the overall risk of bias as low for SIESTA 2016; unclear for GOLIATH 2018 and Hu 2020, and high for the other four studies (AnStroke 2017; CANVAS 2020; Maurice 2022; Ren 2020).
Primary outcomes
Functional outcome (continuous; mRS)
One study reported functional outcome at discharge (Ren 2020). The evidence is very uncertain about the effect of GA on functional outcomes compared to CSA (MD 0, 95% CI –0.31 to 0.31; P = 1.0; 1 study, 90 participants; very low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: General anaesthesia versus non‐general anaesthesia (early time point), Outcome 1: Functional outcome (continuous; mRS)
Neurological impairment
All studies reported neurological impairment using the NIHSS with a follow‐up to 48 hours. Four studies reported this outcome between 24 and 48 hours (AnStroke 2017; CANVAS 2020; Ren 2020; SIESTA 2016), although three studies reported it to 24 hours (GOLIATH 2018; Hu 2020; Maurice 2022). GA may lead to no difference in neurological impairment compared to CSA up to 48 hours (MD –0.29, 95% CI –1.18 to 0.59; P = 0.52; I² = 0%; 7 studies, 982 participants; low‐certainty evidence; Analysis 1.2). The test for subgroup differences did not modify the effect on neurological impairment. The sensitivity analysis including only trials with a low risk of bias did not change the effect estimate substantially (MD –1.19, 95% CI –3.84 to 1.46; Analysis 1.3).
1.3. Analysis.

Comparison 1: General anaesthesia versus non‐general anaesthesia (early time point), Outcome 3: Neurological impairment (only low‐risk trials)
We performed separate analyses of two NIHSS subgroups (24 to 48 hours and at 24 hours). Four studies compared GA to non‐GA. There was no evidence of a difference in NIHSS (24 to 48 hours) between groups (MD –0.09, 95% CI –1.20 to 1.02; I² = 0%; 370 participants). For the three studies that reported NIHSS at 24 hours, there was no evidence of a difference between GA and non‐GA for neurological impairment (MD –0.79, 95% CI –2.48 to 0.89; I² = 10%; 612 participants).
Secondary outcomes
Stroke‐related mortality
Three studies reported stroke‐related mortality in hospitals (AnStroke 2017; Ren 2020; SIESTA 2016). GA may lead to no difference in stroke‐related mortality compared to non‐GA (RR 0.98, 95% CI 0.52 to 1.84; P = 0.94; I² = 0%; 330 participants; low‐certainty evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1: General anaesthesia versus non‐general anaesthesia (early time point), Outcome 4: Stroke‐related mortality
All intracranial haemorrhage
Five studies reported all intracranial haemorrhages in hospitals (AnStroke 2017; CANVAS 2020; GOLIATH 2018; Maurice 2022; Ren 2020). GA may lead to no difference in all intracranial haemorrhages compared to non‐GA (RR 0.92, 95% CI 0.65 to 1.29; P = 0.63; I² = 0%; 693 participants; low‐certainty evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: General anaesthesia versus non‐general anaesthesia (early time point), Outcome 5: All intracranial haemorrhage
Target artery revascularisation
All studies reported target artery revascularisation up to one day after the procedure (AnStroke 2017; CANVAS 2020; GOLIATH 2018; Hu 2020; Maurice 2022; Ren 2020; SIESTA 2016). GA improves to target artery revascularisation compared to non‐GA (RR 1.10, 95% CI 1.02 to 1.18; P = 0.02; I² = 29%; 982 participants; moderate‐certainty evidence; Analysis 1.6).
1.6. Analysis.

Comparison 1: General anaesthesia versus non‐general anaesthesia (early time point), Outcome 6: Target artery revascularisation (dichotomous; mTICI 2b‐3)
Time to revascularisation from groin puncture until arterial reperfusion (minutes)
Five studies reported time to revascularisation from groin puncture until arterial reperfusion up to one day after the procedure (AnStroke 2017; CANVAS 2020; GOLIATH 2018; Ren 2020; SIESTA 2016). The evidence is very uncertain about the effect of GA on time to revascularisation from groin puncture until arterial reperfusion compared to non‐GA (MD 2.91 minutes, 95% CI –5.11 to 10.92; P = 0.48; I² = 48%; 498 participants; very low‐certainty evidence; Analysis 1.7).
1.7. Analysis.

Comparison 1: General anaesthesia versus non‐general anaesthesia (early time point), Outcome 7: Time to revascularisation from groin puncture until arterial reperfusion (minutes)
Adverse events
Three studies reported 'substantial movement' with a follow‐up of up to one day after the procedure (AnStroke 2017; CANVAS 2020; SIESTA 2016). GA reduced adverse events (substantial movement) compared to non‐GA (RR 0.06, 95% CI 0.01 to 0.30; P = 0.0006; I² = 0%; 280 participants; Analysis 1.8).
1.8. Analysis.

Comparison 1: General anaesthesia versus non‐general anaesthesia (early time point), Outcome 8: Adverse events (substantial movement)
Two studies reported vomiting with a follow‐up of up to one day after the procedure (CANVAS 2020; GOLIATH 2018). There was no evidence of a difference between groups (RR 0.32, 95% CI 0.01 to 7.79; P = 0.49; 168 participants; Analysis 1.9).
1.9. Analysis.

Comparison 1: General anaesthesia versus non‐general anaesthesia (early time point), Outcome 9: Adverse events (vomiting)
Three studies reported aspiration with a follow‐up of up to one day after the procedure (AnStroke 2017; GOLIATH 2018; SIESTA 2016). There was no evidence of a difference between groups (RR 0.43, 95% CI 0.06 to 2.86; P = 0.38; I² = 0%; 368 participants; Analysis 1.10).
1.10. Analysis.

Comparison 1: General anaesthesia versus non‐general anaesthesia (early time point), Outcome 10: Adverse events (aspiration)
One study reported a loss of airway with a follow‐up of up to one day after the procedure (AnStroke 2017). There was no evidence of a difference between groups (RR 0.20, 95% CI 0.01 to 4.05; P = 0.29; 90 participants; Analysis 1.11).
1.11. Analysis.

Comparison 1: General anaesthesia versus non‐general anaesthesia (early time point), Outcome 11: Adverse events (loss of airway)
Two studies reported haemodynamic instability with a follow‐up of up to one day after the procedure (AnStroke 2017; Hu 2020). GA may improve haemodynamic instability compared to non‐GA (RR 0.21, 95% CI 0.05 to 0.79; P = 0.02; I² = 71%; 229 participants; low‐certainty evidence; Analysis 1.12).
1.12. Analysis.

Comparison 1: General anaesthesia versus non‐general anaesthesia (early time point), Outcome 12: Adverse events (haemodynamic instability)
Two studies reported delayed extubation with a follow‐up of up to one day after the procedure (AnStroke 2017; SIESTA 2016). There was no evidence of a difference between groups (RR 3.05, 95% CI 0.42 to 22.29; P = 0.27; I² = 80%; 240 participants; Analysis 1.13).
1.13. Analysis.

Comparison 1: General anaesthesia versus non‐general anaesthesia (early time point), Outcome 13: Adverse events (delayed extubation)
Two studies reported hypoxaemia with a follow‐up of up to one day after the procedure (CANVAS 2020; SIESTA 2016). There was no evidence of a difference between groups (RR 1.05, 95% CI 0.22 to 5.06; 190 participants; P = 0.95; Analysis 1.14).
1.14. Analysis.

Comparison 1: General anaesthesia versus non‐general anaesthesia (early time point), Outcome 14: Adverse events (hypoxaemia)
Four studies reported target vessel injury: perforation, dissection, or several vasospasms with a follow‐up of up to one day after the procedure (AnStroke 2017; CANVAS 2020; GOLIATH 2018; SIESTA 2016). There was no evidence of a difference between groups (RR 0.84, 95% CI 0.18 to 3.90; P = 0.82; I² = 13%; 408 participants; Analysis 1.15).
1.15. Analysis.

Comparison 1: General anaesthesia versus non‐general anaesthesia (early time point), Outcome 15: Adverse events (target vessel injury: perforation, dissection, or several vasospasm)
Five studies reported artery perforation with a follow‐up of up to one day after the procedure (AnStroke 2017; CANVAS 2020; GOLIATH 2018; Hu 2020; SIESTA 2016). There was no evidence of a difference between groups (RR 0.82, 95% CI 0.37 to 1.83; P = 0.63; I² = 0%; 752 participants; Analysis 1.16).
1.16. Analysis.

Comparison 1: General anaesthesia versus non‐general anaesthesia (early time point), Outcome 16: Adverse events (artery perforation)
Three studies reported clot migration to a previously unaffected area with a follow‐up of up to one day after the procedure (AnStroke 2017; GOLIATH 2018; Hu 2020). There was no evidence of a difference between groups (RR 1.74, 95% CI 0.75 to 4.01; P = 0.20; I² = 0%; 562 participants; Analysis 1.17).
1.17. Analysis.

Comparison 1: General anaesthesia versus non‐general anaesthesia (early time point), Outcome 17: Adverse events (clot migration to previously unaffected territory)
Five studies reported pneumonia with follow‐up at discharge (AnStroke 2017; CANVAS 2020; Hu 2020; Ren 2020; SIESTA 2016). There was no evidence of a difference between groups (RR 1.85, 95% CI 0.93 to 3.66; P = 0.08; I² = 35%; 509 participants; Analysis 1.18).
1.18. Analysis.

Comparison 1: General anaesthesia versus non‐general anaesthesia (early time point), Outcome 18: Adverse events (pneumonia)
Two studies reported perforation, dissection, and distal thrombus migration with a follow‐up of up to 1 day after the procedure (Hu 2020; Ren 2020). There was no evidence of a difference between groups (RR 0.82, 95% CI 0.45 to 1.49; P = 0.52; I² = 0%; 2 studies, 425 participants; Analysis 1.19).
1.19. Analysis.

Comparison 1: General anaesthesia versus non‐general anaesthesia (early time point), Outcome 19: Adverse events (perforation, dissection, distal thrombus migration)
Quality of life
None of the studies reported QoL.
General anaesthesia versus conscious sedation anaesthesia (long‐term time point)
See Table 2.
Primary outcomes
Functional outcome (dichotomous; mRS of 2 or less)
Four studies reported functional outcomes with a follow‐up of up to 90 days (AnStroke 2017; CANVAS 2020; Maurice 2022; SIESTA 2016). There was no evidence of a difference between groups (RR 1.21, 95% CI 0.93 to 1.58; P = 0.16; I² = 29%; 625 participants; low‐certainty evidence; Analysis 2.1). The test for subgroup differences was not applicable. The sensitivity analysis including only trials with a low risk of bias did not substantially change the effect estimate (RR 2.03, 95% CI 1.16 to 3.56; Analysis 2.2).
2.1. Analysis.

Comparison 2: General anaesthesia versus non‐general anaesthesia (long‐term time point), Outcome 1: Functional outcome (dichotomous; mRS ≤ 2)
2.2. Analysis.

Comparison 2: General anaesthesia versus non‐general anaesthesia (long‐term time point), Outcome 2: Functional outcome (dichotomous; mRS ≤ 2; only low risk trials)
Functional outcome (continuous; mRS)
All studies reported functional outcomes with a follow‐up of up to 90 days (AnStroke 2017; CANVAS 2020; GOLIATH 2018; Hu 2020; Maurice 2022; Ren 2020; SIESTA 2016). There was no evidence of a difference between groups (MD –0.14, 95% CI –0.34 to 0.06; P = 0.17; I² = 0%; 978 participants; low‐certainty evidence; Analysis 2.3). The test for subgroup differences was not applicable. The sensitivity analysis including only trials with a low risk of bias did not substantially change the effect estimate (MD –0.07, 95% CI –0.44 to 0.30; Analysis 2.4).
2.3. Analysis.

Comparison 2: General anaesthesia versus non‐general anaesthesia (long‐term time point), Outcome 3: Functional outcome (continuous; mRS)
2.4. Analysis.

Comparison 2: General anaesthesia versus non‐general anaesthesia (long‐term time point), Outcome 4: Functional outcome (continuous; mRS; only low risk trials)
Neurological impairment
None of the studies reported neurological impairment.
Secondary outcomes
Stroke‐related mortality
Six studies reported stroke‐related mortality with a follow‐up of up to 90 days (AnStroke 2017; CANVAS 2020; GOLIATH 2018; Maurice 2022; Ren 2020; SIESTA 2016). There was no evidence of a difference between groups (RR 0.88, 95% CI 0.64 to 1.22; P = 0.44; I² = 12%; 843 participants; low‐certainty evidence; Analysis 2.5). The test for subgroup differences was not applicable. The sensitivity analysis including only trials with a low risk of bias did not substantially change the effect estimate (RR 0.88, 95% CI 0.54 to 1.44; Analysis 2.6).
2.5. Analysis.

Comparison 2: General anaesthesia versus non‐general anaesthesia (long‐term time point), Outcome 5: Stroke‐related mortality
2.6. Analysis.

Comparison 2: General anaesthesia versus non‐general anaesthesia (long‐term time point), Outcome 6: Stroke‐related mortality (only low‐risk trials)
All intracranial haemorrhage
None of the studies reported all intracranial haemorrhage.
Target artery revascularisation status
None of the studies reported target artery revascularisation status.
Time to revascularisation from groin puncture until arterial reperfusion (minutes)
None of the studies reported time to revascularisation from the groin puncture until arterial reperfusion.
Adverse events
None of the studies reported adverse events.
Quality of life
None of the studies reported QoL.
Discussion
Summary of main results
This review aimed to assess the effects of the type of anaesthesia for AIS EVT. We included seven RCTs, which compared GA versus LA, CSA, or MAC in 982 participants submitted to an EVT for AIS. Four studies compared GA with CSA (AnStroke 2017; Maurice 2022; Ren 2020; SIESTA 2016), two compared GA with CSA plus LA (CANVAS 2020; GOLIATH 2018), and one study compared GA with MAC (Hu 2020). We analysed all included studies in two groups: GA and non‐GA. The non‐GA group included different types of anaesthesia (i.e. LA, CSA, and MAC). Six RCTs had an LVO in arterial intracranial circulation submitted to EVT since they provided data for anterior intracranial circulation (AnStroke 2017; CANVAS 2020; GOLIATH 2018; Maurice 2022; Ren 2020; SIESTA 2016), and one study provided data for posterior intracranial circulation (Hu 2020).
The seven RCTs reported some data for our analyses. Most of the outcome data were obtained in hospitalised participants and most of the early outcome data were obtained at the hospital. However, for long‐term outcomes, we had satisfactory data only on functional outcomes and stroke‐related mortality. No studies reported any data on QoL.
For the early outcomes, we found no evidence of a difference in neurological impairment, stroke‐related mortality, and all intracranial haemorrhage between the groups with low‐certainty evidence. GA may improve haemodynamic instability in adverse events (RR 0.21, 95% CI 0.05 to 0.79; P = 0.02; I² = 71%; 2 studies, 229 participants; low‐certainty evidence) and probably improves target artery revascularisation when compared to non‐GA (RR 1.10, 95% CI 1.02 to 1.18; P = 0.02; I² = 29%; 7 studies, 982 participants; moderate‐certainty evidence). We are uncertain whether GA improves functional outcomes and time to revascularisation from groin puncture until arterial reperfusion because the certainty of the evidence was very low. Substantial movement adverse events may favour GA, but in most of the adverse events, there was no difference between the groups. We found no substantial change in the effect estimate for sensitivity analysis in neurological impairment. The sensitivity analysis was not applicable to the functional outcomes. There were no available data for QoL (Table 1).
For the long‐term outcomes, we found no evidence of a difference in GA compared to non‐GA for functional outcomes (dichotomous: RR 1.21, 95% CI 0.93 to 1.58; P = 0.16; I² = 29%; 4 studies, 625 participants; low‐certainty evidence) and (continuous: MD –0.14, 95% CI –0.34 to 0.06; P = 0.17; I² = 0%; 7 studies, 978 participants; low‐certainty evidence). GA did not improve stroke‐related mortality compared to non‐GA (RR 0.88, 95% CI 0.64 to 1.22; P = 0.44; I² = 12%; 6 studies, 843 participants; low‐certainty evidence). There were no available data for neurological impairment, all intracranial haemorrhage, target artery revascularisation status, time to revascularisation from groin puncture until arterial reperfusion, adverse events, and QoL (Table 2).
We found eight ongoing studies that plan to evaluate 2578 participants in this setting (France: Chabanne 2019; NCT03247998; Germany: DRKS00006801; DRKS00023679; China: ChiCTR2000035282; Liang 2020; Peng 2017; USA: Chen 2020). Six studies plan to include participants with arterial LVO in the anterior intracranial circulation treated by EVT (Chabanne 2019; Chen 2020; DRKS00006801; DRKS00023679; NCT03247998; Peng 2017). One study plans to include participants with arterial LVO in the posterior intracranial circulation treated by EVT (Liang 2020). One study plans to include participants with arterial LVO but did not report which intracranial circulation (ChiCTR2000035282). Regarding the interventions, five studies plan to compare GA versus CSA (Chabanne 2019; Chen 2020; DRKS00006801; DRKS00023679; NCT03247998), two studies plan to compare GA versus LA (ChiCTR2000035282; Peng 2017), and one study plans to compare GA versus CSA plus LA (Liang 2020). As primary outcomes, seven studies plan to calculate data for functional outcomes at 90 days using the mRS (Chabanne 2019; Chen 2020; ChiCTR2000035282; DRKS00006801; DRKS00023679; Liang 2020; Peng 2017), and one has not reported plans for outcomes yet (NCT03247998).
Overall completeness and applicability of evidence
All included studies had a small sample size (from 40 to 345 participants). Most participants included in the RCTs had an arterial LVO in the anterior intracranial circulation treated by EVT. One study included participants with AIS with arterial LVO in the posterior intracranial circulation.
We obtained data for all our outcomes. Most outcomes were from people hospitalised with severe AIS submitted for EVT under any anaesthesia type, for which most data were reported in early outcomes. None of the included studies reported data on QoL. The subgroup analysis was not calculated in order to identify some cofounders variables that may reflect on the actual result, because we included only seven RCTs.
These studies did not report data for our primary long‐term outcome of neurological impairment, minimising the evaluation of clinical quantification and giving us less power to know the right direction of the effect.
The studies were performed in reference stroke centres of different countries (three in China; one each in Denmark, France, Germany, and Sweden) with good technical support, such as technology and specialised trained staff involved in stroke treatment, making us unable to predict if their results could be applied in other centres with less stroke experience. Therefore, the external validity of the overall evidence presented in this review should be considered with caution.
We know that designing and conducting an appropriate study with available data for this topic is difficult. However, there is now available evidence based on RCTs and systematic reviews that have shown a robust difference in clinical outcomes between the GA and non‐GA groups. This reinforces the importance of this review and serves as an incentive for further investigation.
Quality of the evidence
We are confident that the true effect lies close to that of the estimate of the effect.
The weaknesses of this review were the small number of included RCTs with small sample sizes, with different types of anaesthesia employing different anaesthetic drugs, different modalities of EVT in combination or not with endovenous r‐tPA, and the experience of the stroke personnel involved, mainly the anaesthesiologist and interventionist. Although is not possible to blind the anaesthesiologist and person performing the intervention, we judged all studies at high risk of performance bias because none described blinding of performance.
We judged four studies had a high risk of overall bias (AnStroke 2017; CANVAS 2020; Maurice 2022; Ren 2020), and three studies had a low risk of bias (GOLIATH 2018; Hu 2020; SIESTA 2016). However, we judged all almost included studies at low risk in selection bias (random sequence generation) (AnStroke 2017; CANVAS 2020; Maurice 2022; Ren 2020; SIESTA 2016); detection bias (AnStroke 2017; CANVAS 2020; Hu 2020; Ren 2020; SIESTA 2016); attrition bias (GOLIATH 2018; Hu 2020; Ren 2020; SIESTA 2016); reporting bias (AnStroke 2017; CANVAS 2020; GOLIATH 2018; SIESTA 2016); and other bias (AnStroke 2017; CANVAS 2020; GOLIATH 2018; Maurice 2022; SIESTA 2016). Four RCTs had an unclear risk of selection bias as well as one in random sequence generation (GOLIATH 2018; Hu 2020), and four in allocation concealment (CANVAS 2020; GOLIATH 2018; Hu 2020; Ren 2020); two trials had an unclear risk of blinding outcome assessment (GOLIATH 2018; Maurice 2022). Four studies were at high risk of bias, three studies in attrition bias (AnStroke 2017; CANVAS 2020; Maurice 2022), two studies in reporting bias (Maurice 2022; Ren 2020), and one study in other bias (Ren 2020). All studies had a high risk of bias for performance bias due to the impossibility of blinding the professionals involved in the procedure.
All studies reported data for the primary outcomes and this is, therefore, the strength of this review. The overall certainty of the evidence was very low to moderate in the early outcomes. We found moderate‐certainty evidence that GA probably improves target artery revascularisation compared to non‐GA. GA may improve to adverse events (haemodynamic instability) and there may be no difference in neurological impairment, stroke‐related mortality, and all intracranial haemorrhage when compared to non‐GA with low‐certainty evidence. We are uncertain about the effects on the functional outcome and time to revascularisation from groin puncture until artery reperfusion because the certainty of the evidence was very low. In the risk of bias, the certainty of the evidence was downgraded one level due to a high risk of bias, mainly due to the detection of performance bias in six RCTs, attrition bias in three RCTs, reporting bias in two RCTs, and other bias in one RCT. The certainty of the evidence was also downgraded due to study limitations (risk of bias), inconsistency (unexplained heterogeneity), and imprecision (large CI) (Table 1).
In the long‐term outcomes, the overall certainty of the evidence was low. We found low‐certainty evidence that GA did not improve functional outcome or change stroke‐related mortality compared to non‐GA. There was no difference in GA compared to non‐GA for both functional outcomes (dichotomous and continuous) with low‐certainty evidence. There were no data for all intracranial haemorrhages, target artery revascularisation, time to revascularisation from groin puncture until arterial reperfusion, and adverse events (haemodynamic instability) because almost all data were recorded in hospitalised participants. In the risk of bias analysis, the certainty of the evidence was downgraded one level due to a high risk of bias, mainly to detection of performance bias in six RCTs, attrition bias in three RCTs, reporting bias in two RCTs, and other bias in one RCT. The certainty of the evidence was also downgraded due to study limitations (risk of bias) and imprecision (large CI). There were no data available on neurological impairment, all intracranial haemorrhages, target artery revascularisation, time to revascularisation from groin puncture until arterial reperfusion, and adverse events (haemodynamic instability) (Table 2).
Potential biases in the review process
We minimised potential biases in the review process by searching for published and unpublished studies from several sources, including grey literature sources with no restriction on the date of publication or language. Two review authors independently extracted data and conducted the risk of bias assessment. Due to a small number of RCTs included in our review, a funnel plot was not produced and we were unable to detect publication bias. The conversion from CSA to GA might lead to additional delays in the non‐GA group and delay in the start of endovascular reperfusion, which has been estimated to worsen the clinical outcome. Thus, the high rate of conversion could have reduced the effect of EVT in the non‐GA group.
The variability between included studies such as eligibility criteria, type of anaesthesia and anaesthetic drugs, type of devices used for EVT, and the experience of the anaesthesiologist and interventionist involved in the procedure may have a significant influence on outcomes. However, we were unable to assess the effect of important variables because we found only seven RCTs to include in our review.
Agreements and disagreements with other studies or reviews
There are four systematic reviews and meta‐analyses that included only RCTs that compared the effects of the anaesthesia type in EVT for AIS.
Campbell 2021 searched in CENTRAL, MEDLINE, and Embase with no language limits. They found four of our included RCTs (AnStroke 2017; CANVAS 2020; GOLIATH 2018; SIESTA 2016), used the Cochrane RoB 2 tool to calculate risk of bias, and did not assess the certainty of the evidence. They concluded there was significantly less disability (mRS of 2 or less) for GA at three months and also better successful recanalisation (mTICI 2b‐3) for GA.
Schonenberger 2019 searched in MEDLINE and did not report on language limits to their search. They found three of our included RCTs (AnStroke 2017; GOLIATH 2018; SIESTA 2016), used the Cochrane RoB 2 tool to calculate risk of bias, and did not assess the certainty of the evidence. They concluded there was significantly less disability (mRS of 2 or less) for GA at three months.
Zhang 2019 searched in CENTRAL, MEDLINE, Embase, clinical trial registries (ClinicalTrials.gov, European Union Clinical Trials Register, World Health Organization International Clinical Trials Registry Platform, Stroke Trials Registry, and ISRCTN (International Standard Randomised Controlled Trial Number) Registry) with no language limits. They found three of our included RCTs (AnStroke 2017; GOLIATH 2018; SIESTA 2016), used the Cochrane RoB 1 tool to calculate risk of bias, and assessed the certainty of the evidence using the GRADE approach. They concluded there was significantly less disability (mRS of 2 or less) for GA at three months and also better successful recanalisation (mTICI 2b–3) for GA without reporting on certainty of the evidence.
Bai 2021 searched in CENTRAL, MEDLINE, Embase, and Web of Science for relevant RCTs. They found five of our included RCTs (AnStroke 2017; CANVAS 2020; GOLIATH 2018; Ren 2020; SIESTA 2016), and used the Cochrane RoB 1 tool to calculate risk of bias. They concluded there was significantly less disability (mRS of 2 or less) for GA at three months and also better successful recanalisation (mTICI 2b–3) for GA.
Our review seems to be more robust than the previous reviews identified here because we included seven RCTs (AnStroke 2017; CANVAS 2020; GOLIATH 2018; Hu 2020; Maurice 2022; Ren 2020; SIESTA 2016), following the Cochrane search strategies (Search methods for identification of studies; Electronic searches; Searching other resources). We also used the Cochrane RoB 1 tool to calculate risk of bias and assessed the certainty of the evidence using the GRADE approach. We added 484 participants (from Hu 2020 and Maurice 2022) more than the previous reviews, which is 50.3% more participants than Bai 2021; therefore, we believe that our conclusions are more robust and decisive for clinical practice than the previous reviews.
Authors' conclusions
Implications for practice.
In early outcomes, we are uncertain whether general anaesthesia compared to non‐general anaesthesia improves functional and neurological outcomes. General anaesthesia probably improves target artery revascularisation compared to conscious sedation non‐general anaesthesia with moderate‐certainty evidence. We found that general anaesthesia may improve haemodynamic instability adverse events compared with non‐general anaesthesia, and no evidence of a difference between the intervention groups in stroke‐related mortality, time to revascularisation from groin puncture until arterial reperfusion, and all intracranial haemorrhage.
Although in long‐term outcomes, there is no difference in general anaesthesia compared to non‐general anaesthesia for both functional outcomes (continuous and dichotomous), the certainty of the evidence is low. General anaesthesia did not improve stroke‐related mortality compared to conscious sedation non‐general anaesthesia with moderate‐certainty evidence. Neurological impairment (measured using the National Institutes of Health Stroke Scale), target artery revascularisation, time to revascularisation from groin puncture until arterial reperfusion, and adverse events were not reported. More evidence should soon be available from ongoing trials.
Implications for research.
We have no evidence effect and more randomised controlled trials (RCTs) with a large number of participants and good protocol design with a low risk of bias should be performed to dismiss the uncertainty of evidence to make the correct decision and guide us to detect the effects in terms of better clinical outcomes.
The small number of included RCTs afforded us insufficient ability to detect the presence of publication bias. We know that all included trials were conducted in high‐income countries, making us unable to predict if their results could be applied in low‐ and middle‐income countries. Further research in these countries would improve the generalisability. Due to the small number of RCTs included, we were unable to assess important variables in this review, and because of that, we are awaiting further publications.
Finally, none of the studies reported data on our primary long‐term outcome of neurological impairment, minimising the evaluation of clinical quantification and giving us less power to determine the correct direction of the effect. New RCTs would benefit from including neurological impairment as an outcome at 90 days.
History
Protocol first published: Issue 7, 2020
Acknowledgements
We thank Cochrane Stroke, Cochrane Brazil, Joshua David Cheyne (Cochrane Stroke's Information Specialist), and the Division of Vascular and Endovascular Surgery of Universidade Federal de Sao Paulo, Brazil, for their methodological support.
We also thank Peter Langhorne, Aryelly Rodriguez, and Daniel Bereczki (Cochrane Stroke Editors), and Deepak Sharma, for the peer review comments.
Appendices
Appendix 1. Cochrane Stroke Group Specialised Register of Trials
Strategy search
Review Title: Type of anaesthesia for acute ischaemic stroke endovascular treatment
Report Part One: Study / Reference Report
Each study in the Trials Register is coded to reflect the stage of the condition at which the intervention is given, the type of condition being treated, and the type of intervention and control group.
The codes we have used to search the Register for studies relevant to this review are given below:
Search method: 1
Stage: Acute treatment (< 30 days)
Disease: Ischaemic stroke
Condition: Not specified
Intervention type: Pharmacology
Intervention code: Anaesthesia
Search method: 2
Stage: Acute treatment (< 30 days)
Disease: Ischaemic stroke
Condition: Not specified
Intervention type: Pharmacology
Intervention code: Analgesic
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 MeSH descriptor: [Cerebrovascular Disorders] this term only 1428
#2 MeSH descriptor: [Basal Ganglia Cerebrovascular Disease] this term only 10
#3 MeSH descriptor: [Brain Ischemia] explode all trees 3559
#4 MeSH descriptor: [Carotid Artery Diseases] this term only 472
#5 MeSH descriptor: [Carotid Artery Thrombosis] this term only 18
#6 MeSH descriptor: [Intracranial Arterial Diseases] this term only 10
#7 MeSH descriptor: [Cerebral Arterial Diseases] this term only 26
#8 MeSH descriptor: [Intracranial Embolism and Thrombosis] explode all trees 310
#9 MeSH descriptor: [Stroke] explode all trees 9568
#10 (isch?emi* near/6 (stroke* or apoplex* or cerebral vasc* or cerebrovasc* or cva or attack*)):ti,ab,kw 15479
#11 ((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebr* or mca* or anterior circulation) near/5 (isch?emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*)):ti,ab,kw 16502
#12 {or #1‐#11} 31122
#13 MeSH descriptor: [Anesthesia] this term only 1562
#14 MeSH descriptor: [Anesthesia, Conduction] this term only 416
#15 MeSH descriptor: [Anesthesia, Epidural] explode all trees 1980
#16 MeSH descriptor: [Anesthesia, Local] this term only 2120
#17 MeSH descriptor: [Anesthesia, Spinal] this term only 2351
#18 MeSH descriptor: [Anesthesia, General] this term only 4835
#19 MeSH descriptor: [Anesthesia, Inhalation] explode all trees 1828
#20 MeSH descriptor: [Balanced Anesthesia] this term only 3
#21 MeSH descriptor: [Anesthesia, Intravenous] this term only 1908
#22 MeSH descriptor: [Conscious Sedation] this term only 1397
#23 MeSH descriptor: [Deep Sedation] this term only 155
#24 (an?esthe*):ti,ab,kw 71990
#25 MeSH descriptor: [Anesthetics] this term only 3039
#26 MeSH descriptor: [Anesthetics, Combined] explode all trees 843
#27 MeSH descriptor: [Anesthetics, General] this term only 124
#28 MeSH descriptor: [Anesthetics, Inhalation] explode all trees 2555
#29 MeSH descriptor: [Anesthetics, Local] explode all trees 8388
#30 (amydricaine or amylocaine or articaine or articainic acid or aslavital or benzocaine or benzofurocaine or benzyl alcohol or bucricaine or bumecaine or bupivacaine or butacaine or butanilicaine or butethamine or butoxycaine or butylcaine or carbisocaine or carcainium chloride or centbucridine or cetacaine or chloroprocaine or cinchocaine or cocaine or cyclomethycaine or dimethocaine or diperodon or dyclonine or etidocaine or eugenol or euprocin or fluress or fomocaine or guafecainol or heptacaine or hexathricin or hexylcaine or instillagel or ipravacaine or isobutamben or ketocaine or levobupivacaine or lidamidine or lidocaine or mepivacaine or meprylcaine or metabutethamine or myrtecaine or oxetacaine or oxybuprocaine or pentacaine or phenacaine or phenol or piperocaine or polidocanol or pramocaine or prilocaine or procaine or propanocaine or propipocaine or propoxycaine or propylcaine or proxymetacaine or pyrrocaine or quinisocaine or ropivacaine or tanax or tetracaine or tolycaine or tricaine or trimecaine or xyloproct or zolamine):ti,ab,kw 35424
#31 (alcohol or alfadolone or alfadolone acetate or alfaxalone or althesin or azd 3043 or betaxalone or chloralose or eltanolone or equithesin or esketamine or etomidate or flunitrazepam or flutomidate or fospropofol or hydroxydione or ketamine or methohexital or metomidate or midazolam or midazolam maleate or minaxolone or oxybate or phencyclidine or propanidid or propofol or remimazolam or renanolone or sameridine or thiamylal or thiobutabarbital or thiopental or tiletamine or trichloroethanol or xenon or xylazine):ti,ab,kw 50522
#32 (aliflurane or bromethol or chloroethane or chloroform or cyclopropane or desflurane or dichloromethane or enflurane or ether or fluroxene or halothane or isoflurane or methoxyflurane or nitrous oxide or sevoflurane or trichloroethylene):ti,ab,kw 13620
#33 MeSH descriptor: [Analgesia] this term only 2004
#34 MeSH descriptor: [Analgesia, Epidural] this term only 2004
#35 MeSH descriptor: [Analgesia, Patient‐Controlled] this term only 2032
#36 MeSH descriptor: [Conscious Sedation] this term only 1397
#37 MeSH descriptor: [Deep Sedation] explode all trees 155
#38 MeSH descriptor: [Analgesics] explode all trees 20802
#39 (sedat* or (pain near/3 (manag* or relief))):ti,ab,kw 48973
#40 (analges*):ti,ab,kw 61126
#41 {or #13‐#40} 173366
#42 MeSH descriptor: [Endarterectomy] explode all trees 585
#43 MeSH descriptor: [Endovascular Procedures] this term only 420
#44 MeSH descriptor: [Angioplasty] explode all trees 4415
#45 MeSH descriptor: [Vascular Surgical Procedures] this term only 646
#46 MeSH descriptor: [Cerebral Revascularization] this term only 56
#47 MeSH descriptor: [Blood Vessel Prosthesis] this term only 434
#48 MeSH descriptor: [Blood Vessel Prosthesis Implantation] this term only 446
#49 MeSH descriptor: [Stents] explode all trees 4127
#50 MeSH descriptor: [Dilatation] this term only 424
#51 MeSH descriptor: [Catheterization] this term only 1614
#52 (endarterect* or endovasc* or angioplasty or stent* or pta or revasculari?ation or catheter* or dilatation):ti,ab,kw 62001
#53 {or #42‐#52} 62745
#54 #12 AND #41 AND #53 356
Appendix 3. MEDLINE Ovid search strategy
1. cerebrovascular disease/ or cerebral artery disease/ or cerebrovascular accident/ or stroke/ or vertebrobasilar insufficiency/ or carotid artery disease/ or exp carotid artery obstruction/ or exp brain infarction/ or exp brain ischemia/ or exp occlusive cerebrovascular disease/ 2. (isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)).tw. 3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. or/1‐3 5. anesthesia/ or anesthesia, conduction/ or exp anesthesia, epidural/ or anesthesia, local/ or anesthesia, spinal/ or anesthesia, general/ or exp anesthesia, inhalation/ or balanced anesthesia/ or anesthesia, intravenous/ or conscious sedation/ or deep sedation/ 6. an?esthe$.tw. 7. anesthetics/ or exp anesthetics, combined/ or anesthetics, general/ or exp anesthetics, inhalation/ or exp anesthetics, local/ 8. (amydricaine or amylocaine or articaine or articainic acid or aslavital or benzocaine or benzofurocaine or benzyl alcohol or bucricaine or bumecaine or bupivacaine or butacaine or butanilicaine or butethamine or butoxycaine or butylcaine or carbisocaine or carcainium chloride or centbucridine or cetacaine or chloroprocaine or cinchocaine or cocaine or cyclomethycaine or dimethocaine or diperodon or dyclonine or etidocaine or eugenol or euprocin or fluress or fomocaine or guafecainol or heptacaine or hexathricin or hexylcaine or instillagel or ipravacaine or isobutamben or ketocaine or levobupivacaine or lidamidine or lidocaine or mepivacaine or meprylcaine or metabutethamine or myrtecaine or oxetacaine or oxybuprocaine or pentacaine or phenacaine or phenol or piperocaine or polidocanol or pramocaine or prilocaine or procaine or propanocaine or propipocaine or propoxycaine or propylcaine or proxymetacaine or pyrrocaine or quinisocaine or ropivacaine or tanax or tetracaine or tolycaine or tricaine or trimecaine or xyloproct or zolamine).tw. 9. (alcohol or alfadolone or alfadolone acetate or alfaxalone or althesin or azd 3043 or betaxalone or chloralose or eltanolone or equithesin or esketamine or etomidate or flunitrazepam or flutomidate or fospropofol or hydroxydione or ketamine or methohexital or metomidate or midazolam or midazolam maleate or minaxolone or oxybate or phencyclidine or propanidid or propofol or remimazolam or renanolone or sameridine or thiamylal or thiobutabarbital or thiopental or tiletamine or trichloroethanol or xenon or xylazine).tw. 10. (aliflurane or bromethol or chloroethane or chloroform or cyclopropane or desflurane or dichloromethane or enflurane or ether or fluroxene or halothane or isoflurane or methoxyflurane or nitrous oxide or sevoflurane or trichloroethylene).tw. 11. analgesia/ or analgesia, epidural/ or analgesia, patient‐controlled/ or conscious sedation/ or deep sedation/ 12. exp analgesics/ 13. (sedat$ or (pain adj3 (manag$ or relief))).tw. 14. analges$.tw. 15. or/5‐14 16. endarterectomy/ or endovascular procedures/ or exp angioplasty/ 17. vascular surgical procedures/ 18. cerebral revascularization/ or Blood Vessel Prosthesis/ or Blood Vessel Prosthesis Implantation/ 19. balloon dilatation/ or stents/ or dilatation/ or catheterization/ 20. (endarterect$ or endovasc$ or angioplasty or stent$ or pta or revasculari?ation or catheter$ or dilatation).tw. 21. or/16‐20 22. randomized controlled trial.pt. 23. controlled clinical trial.pt. 24. randomized.ab. 25. placebo.ab. 26. randomly.ab. 27. trial.ab. 28. groups.ab. 29. or/22‐28 30. 4 and 15 and 21 and 29
Appendix 4. Embase Ovid search strategy
TOSELLO Renato Embasev1 Type of anesthesia for acute ischemic stroke endovascular treatment_Draftv1 1. cerebrovascular disease/ or cerebral artery disease/ or cerebrovascular accident/ or stroke/ or vertebrobasilar insufficiency/ or carotid artery disease/ or exp carotid artery obstruction/ or exp brain infarction/ or exp brain ischemia/ or exp occlusive cerebrovascular disease/ 2. (isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)).tw. 3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. 1 or 2 or 3 5. anesthesia/ or anesthesiological procedure/ or exp epidural anesthesia/ or exp general anesthesia/ or exp intravenous anesthesia/ or exp local anesthesia/ or exp regional anesthesia/ or exp sedation/ 6. an?esthe$.tw. 7. anesthetic agent/ or exp inhalation anesthetic agent/ or intravenous anesthetic agent/ 8. (amydricaine or amylocaine or articaine or articainic acid or aslavital or benzocaine or benzofurocaine or benzyl alcohol or bucricaine or bumecaine or bupivacaine or butacaine or butanilicaine or butethamine or butoxycaine or butylcaine or carbisocaine or carcainium chloride or centbucridine or cetacaine or chloroprocaine or cinchocaine or cocaine or cyclomethycaine or dimethocaine or diperodon or dyclonine or etidocaine or eugenol or euprocin or fluress or fomocaine or guafecainol or heptacaine or hexathricin or hexylcaine or instillagel or ipravacaine or isobutamben or ketocaine or levobupivacaine or lidamidine or lidocaine or mepivacaine or meprylcaine or metabutethamine or myrtecaine or oxetacaine or oxybuprocaine or pentacaine or phenacaine or phenol or piperocaine or polidocanol or pramocaine or prilocaine or procaine or propanocaine or propipocaine or propoxycaine or propylcaine or proxymetacaine or pyrrocaine or quinisocaine or ropivacaine or tanax or tetracaine or tolycaine or tricaine or trimecaine or xyloproct or zolamine).tw. 9. (alcohol or alfadolone or alfadolone acetate or alfaxalone or althesin or azd 3043 or betaxalone or chloralose or eltanolone or equithesin or esketamine or etomidate or flunitrazepam or flutomidate or fospropofol or hydroxydione or ketamine or methohexital or metomidate or midazolam or midazolam maleate or minaxolone or oxybate or phencyclidine or propanidid or propofol or remimazolam or renanolone or sameridine or thiamylal or thiobutabarbital or thiopental or tiletamine or trichloroethanol or xenon or xylazine).tw. 10. (aliflurane or bromethol or chloroethane or chloroform or cyclopropane or desflurane or dichloromethane or enflurane or ether or fluroxene or halothane or isoflurane or methoxyflurane or nitrous oxide or sevoflurane or trichloroethylene).tw. 11. analgesia/ or epidural analgesia/ 12. exp analgesic agent/ 13. (sedat$ or (pain adj3 (manag$ or relief))).tw. 14. analges$.tw. 15. or/5‐14 16. artery surgery/ or exp carotid artery surgery/ or exp endarterectomy/ or exp endovascular surgery/ or exp angioplasty/ 17. vascular surgery/ or exp cerebrovascular surgery/ or exp endovascular surgery/ or exp thrombectomy/ 18. exp blood vessel prosthesis/ 19. nonsurgical invasive therapy/ or balloon dilatation/ or catheterization/ 20. stent/ or exp drug eluting stent/ or exp metal stent/ or plastic stent/ or exp self expanding stent/ 21. (endarterect$ or endovasc$ or angioplasty or stent$ or pta or revasculari?ation or catheter$ or dilatation).tw. 22. or/16‐21
This subject search was linked to an adapted version of the Cochrane Highly Sensitive Search Strategy for identifying controlled trials in Embase Ovid (2018 version) as referenced in Chapter 4.S1 of the Cochrane Handbook for Systematic Reviews of Interventions [Version 6] and detailed in box 3.e of the Technical Supplement to Chapter 4: Searching for and selecting studies (Lefebvre 2019). 23. Randomized Controlled Trial/ or "randomized controlled trial (topic)"/ 24. Randomization/ 25. Controlled clinical trial/ or "controlled clinical trial (topic)"/ 26. control group/ or controlled study/ 27. clinical trial/ or "clinical trial (topic)"/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ 28. Crossover Procedure/ 29. Double Blind Procedure/ 30. Single Blind Procedure/ or triple blind procedure/ 31. placebo/ or placebo effect/ 32. (random$ or RCT or RCTs).tw. 33. (controlled adj5 (trial$ or stud$)).tw. 34. (clinical$ adj5 trial$).tw. 35. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 36. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 37. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 38. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 39. (cross‐over or cross over or crossover).tw. 40. (placebo$ or sham).tw. 41. trial.ti. 42. (assign$ or allocat$).tw. 43. controls.tw. 44. or/23‐43 45. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 46. 4 and 15 and 22 and 44 47. 46 not 45
Appendix 5. LILACS and IBECS search strategy
Literatura Latino‐Americana e do Caribe em Ciências da Saúde (LILACS) (from 1982) and Indice Bibliográfico Español de Ciencias de la Salud (IBECS), via Virtual Health Library
Search 1. (mh:(cerebrovascular disease) OR mh:(cerebral artery disease) OR mh:(cerebrovascular accident) OR mh:(stroke) OR mh:(vertebrobasilar insufficiency) OR mh:(carotid artery disease) OR mh:(carotid artery obstruction) OR mh:(brain infarction) OR mh:(brain ischemia) OR mh:(occlusive cerebrovascular disease)) AND ( db:("LILACS" OR "IBECS") AND type_of_study:("clinical_trials"))
Search 2. tw:(((brain OR cerebr* OR cerebell* OR vertebrobasil* OR hemispher* OR intracran* OR intracerebral OR infratentorial OR supratentorial OR middle cerebr* OR mca* OR anterior circulation) AND (isch?emi* OR infarct* OR thrombo* OR emboli* OR occlus* OR hypoxi*))) AND ( db:("LILACS" OR "IBECS") AND type_of_study:("clinical_trials"))
Appendix 6. US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov search strategy
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) ( anaesthesia OR anaesthetics OR sedation OR analgesia OR analgesics ) AND AREA[StudyType] EXPAND[Term] COVER[FullMatch] "Interventional" AND AREA[ConditionSearch] ( ischemic stroke OR brain infarction OR brain ischemia OR carotid artery obstruction OR cerebral ischemia )
Appendix 7. World Health Organization (WHO) International Clinical Trials Registry Platform search strategy
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch)
Basic search: ischemic stroke AND anaesthesia OR ischemic stroke AND analgesia OR ischemic stroke AND sedation brain infarction AND anesthesia OR brain infarction AND analgesia OR brain infarction AND sedation Phases are: ALL
Appendix 8. Enquiry Letter
Dear Doctor
I am currently conducting a systematic review entitled 'Type of anaesthesia for acute ischaemic stroke endovascular treatment' with Cochrane Stroke Group based in the University of Edinburgh. To ensure that the results are valid, it is essential that all relevant trials are included.
Cochrane was established to ensure all forms of health care will be subject to critical evaluation using standard criteria and specialised software.
As a [expert/triallist] of [intervention name], it is possible that a trial of this has been conducted in patients with acute ischaemic stroke endovascular treatment. If so, we would be grateful if you could supply us with copies of any relevant protocols, reports or publications in the first instance; later it may become necessary to obtain the raw data. If the trial is eligible for inclusion in the review, [specialist name] will be cited in the final report which will be published electronically within the Cochrane Database of Systematic Reviews, and in standard medical journals.
I would be grateful if you could fill in the accompanying form, and forward any information which you feel may be appropriate.
Thank you for your help.
Yours faithfully
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Form for reply from Pharmaceutical Company/Triallist/Expert Trials that fulfil the following criteria will be eligible for inclusion in the review: ‐ Types of participants: ‐ Treatment regimen: ‐ A valid randomisation method:
for example: a centralised scheme, e.g. by telephone or scheme controlled by pharmacy, e.g. precoded or numbered containers or on‐site computer system where allocations are in a locked unreadable file or assignment envelopes ‐ sequentially numbered, sealed and opaque or other combinations which provide assurance of adequate concealment. ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Name of Pharmaceutical Company/Triallist/Expert Name (person to whom any future correspondence should be addressed): Trials fulfilling the above criteria: Have not been conducted ( ) Are currently underway * ( ) Have been conducted in the past * ( ) * Please enclose relevant protocols, citations, reports or other publications Thank you for your valuable help. Please complete and return to:
Dr Renato Tosello, MD
Department of Neurointerventional Radiology Hospital Beneficencia Portuguesa de Sao Paulo Sao Paulo Brazil E‐mail: retosello@hotmail.com
Appendix 9. British Library EThOS search strategy
British Library EThOS e‐theses online service
Advanced search (stroke OR ischemia OR infarction) AND (aneasthesia OR anaesthetic OR analgesic OR analgesia OR sedation)
Appendix 10. ProQuest Dissertation and Theses Global search strategy
ProQuest Dissertation and Theses Global via ProQuest
S1 noft(isch?emi* AND (stroke* or apoplex* or cerebral vasc* or cerebrovasc* or cva or attack*))
S2 noft((brain OR cerebr* OR cerebell* OR vertebrobasil* OR hemispher* OR intracran* OR intracerebral OR infratentorial OR supratentorial OR middle cerebr* OR mca* OR anterior circulation) AND (isch?emi* OR infarct* OR thrombo* OR emboli* OR occlus* OR hypoxi*))
S3 S1 OR S2
S4 noft(an?esthe* OR analges* OR sedat*)
S5 noft(endarterect* or endovasc* or angioplasty or stent* or pta or revasculari?ation or catheter* or dilatation)
S6 S3 AND S4 AND S5
Data and analyses
Comparison 1. General anaesthesia versus non‐general anaesthesia (early time point).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Functional outcome (continuous; mRS) | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.31, 0.31] |
| 1.2 Neurological impairment | 7 | 982 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐1.18, 0.59] |
| 1.2.1 NIHSS (24–48 hours) | 4 | 370 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐1.20, 1.02] |
| 1.2.2 NIHSS at 24 hours | 3 | 612 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐2.48, 0.89] |
| 1.3 Neurological impairment (only low‐risk trials) | 2 | 278 | Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐3.84, 1.46] |
| 1.4 Stroke‐related mortality | 3 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.52, 1.84] |
| 1.5 All intracranial haemorrhage | 5 | 693 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.65, 1.29] |
| 1.6 Target artery revascularisation (dichotomous; mTICI 2b‐3) | 7 | 982 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [1.02, 1.18] |
| 1.7 Time to revascularisation from groin puncture until arterial reperfusion (minutes) | 5 | 498 | Mean Difference (IV, Random, 95% CI) | 2.91 [‐5.11, 10.92] |
| 1.8 Adverse events (substantial movement) | 3 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.01, 0.30] |
| 1.9 Adverse events (vomiting) | 2 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.79] |
| 1.10 Adverse events (aspiration) | 3 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.06, 2.86] |
| 1.11 Adverse events (loss of airway) | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.05] |
| 1.12 Adverse events (haemodynamic instability) | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.05, 0.79] |
| 1.13 Adverse events (delayed extubation) | 2 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.42, 22.29] |
| 1.14 Adverse events (hypoxaemia) | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.22, 5.06] |
| 1.15 Adverse events (target vessel injury: perforation, dissection, or several vasospasm) | 4 | 408 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.18, 3.90] |
| 1.16 Adverse events (artery perforation) | 5 | 752 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.37, 1.83] |
| 1.17 Adverse events (clot migration to previously unaffected territory) | 3 | 562 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.75, 4.01] |
| 1.18 Adverse events (pneumonia) | 5 | 509 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [0.93, 3.66] |
| 1.19 Adverse events (perforation, dissection, distal thrombus migration) | 2 | 434 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.45, 1.49] |
Comparison 2. General anaesthesia versus non‐general anaesthesia (long‐term time point).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Functional outcome (dichotomous; mRS ≤ 2) | 4 | 625 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.93, 1.58] |
| 2.2 Functional outcome (dichotomous; mRS ≤ 2; only low risk trials) | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.16, 3.56] |
| 2.3 Functional outcome (continuous; mRS) | 7 | 978 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.34, 0.06] |
| 2.4 Functional outcome (continuous; mRS; only low risk trials) | 2 | 278 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.44, 0.30] |
| 2.5 Stroke‐related mortality | 6 | 843 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.64, 1.22] |
| 2.6 Stroke‐related mortality (only low‐risk trials) | 2 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.54, 1.44] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AnStroke 2017.
| Study characteristics | ||
| Methods | Setting: single‐centre, Sweden Design: RCT, 2 arms, parallel assignment, single‐blind Start date: 14 November 2013 (reported in protocol) Completion date: 30 September 2016 (reported in protocol) |
|
| Participants | 106 men and women randomised: experimental (GA) = 54 and comparator (CSA) = 52; procedure interrupted in GA = 8 and CSA = 7; 1 consent withdrawn in GA arm and 0 lost to follow‐up, 90 analysed Mean age: 72 years (range: 65–80 years) Gender (men/women): 49/51 Mean NIHSS score: 18 (score range: 15–22) Mean ASPECTS: 10 (score range: 8–10) 7 (15.6%) participants were converted from CSA to GA 66 (73.3%) participants received IV r‐tPA before EVT Diagnostic criteria: AIS with LVO in anterior cerebral circulation Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: GA
Comparator: CSA performed by remifentanil infusion Blood pressure monitoring
Excluded medications: not reported |
|
| Outcomes | Primary outcome (specified)
Primary outcome (collected)
Secondary outcomes (specified)
Secondary outcomes (collected)
|
|
| Notes | Conflicts of interest: (quote) "none". Funding: (quote) "The study was supported by Swedish State Support for Clinical Research (ALFGBG‐75130 and ALFGBG‐590861), The Gothenburg Medical Society, John and Britt Wennstroms/Per‐Olof Ahls Fund, Sahlgrenska University Hospital Foundations, and Swedish Stroke Association". Protocol: NCT01872884 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly allocated in blocks to either GA or CS". |
| Allocation concealment (selection bias) | Low risk | Quote: "1:1 ratio using sealed non‐transparent envelopes". |
| Blinding of participants and personnel (performance bias) | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The review of the neuroradiologic and angiographic data was done by experienced neuroradiologists, blinded to neurological outcome". Quote: "A vascular neurologist, blinded to treatment allocation and mTICI score, assessed mRS score by direct examination (n = 81, 90%) or by telephone interview (n = 9, 10%) 3 months after stroke". |
| Incomplete outcome data (attrition bias) | High risk | There were no losses. Crossover occurred in 7 (15.6%) participants who were converted from CSA to GA. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | No evidence of other bias related to this study. |
CANVAS 2020.
| Study characteristics | ||
| Methods | Setting: single‐centre, China Design: RCT, 2 arms, open‐label, blinded endpoint (PROBE) Start date: 5 February 2016 (reported in protocol) Completion date: December 2022 (reported in protocol) |
|
| Participants | 43 men and women randomised: experimental GA = 21, comparator CSA = 22; procedure interrupted in GA = 8 and CSA = 7; 1 GA and 2 CSA withdrawn due to large infarct and 0 lost to follow‐up was reported, 40 analysed Median age: 65 years (IQR 45–74 years) Gender (men/women): 26/14 Median NIHSS score 13.9 (score range: 10.2–16.0) 4 (18.2%) participants were converted from CSA to GA after randomisation because of significant agitation IV r‐tPA before EVT was not reported Diagnostic criteria: AIS with LVO in anterior cerebral circulation Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: GA
Comparator: CSA
Excluded medications: not reported |
|
| Outcomes | Primary outcome (specified)
Primary outcome (collected)
Secondary outcomes (specified)
Secondary outcomes (collected)
|
|
| Notes | Conflict of interest: (quote) "The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article". Funding: (quote) "The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The trial is funded by the Beijing Municipal Administration of Hospitals 'Youth Program' (reference number: QML20150508) and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (code number: ZYLX201708)". Protocol: NCT02677415 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation occurred when patients were sent to the interventional neuroradiology suite for EVT and were obtained through a purposely built web‐based program, stratified by the site of culprit's vessels (ICA or MCA) using permuted blocks". Comment: method for randomisation was described and seemed appropriate. |
| Allocation concealment (selection bias) | Unclear risk | Details were not fully described. Quote: "Patients were randomly allocated to receive either GA or CS in a 1 to 1 ratio". |
| Blinding of participants and personnel (performance bias) | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "we measured mRS at 90 days by the certified neurologists who were blinded to the group allocation". |
| Incomplete outcome data (attrition bias) | High risk | There were no losses. Crossover occurred in 4 (18.2%) participants from CSA to GA group. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | No evidence of other bias related to this study. |
GOLIATH 2018.
| Study characteristics | ||
| Methods | Setting: single‐centre, Denmark RCT, 2 arms, open‐label, blinded endpoint Start date: 12 March 2015 (reported in protocol) Completion date: 2 February 2017 (reported in protocol) |
|
| Participants | 128 men and women randomised: experimental GA group = 65, comparator CSA group (LA + CSA) = 63; lost to follow‐up (not reported); 128 analysed Mean age: 71.4 (SD 11.4) years Gender (men/women): 66/62 Mean NIHSS score: 18 (IQR 14–21) 4/63 participants allocated to CSA (6.3%) who crossed over from the CSA to the GA arm but remained in the CSA group for ITT analysis IV r‐tPA before EVT: GA = 50 and CSA = 46 96 (75%) participants received IV r‐tPA before EVT Diagnostic criteria: AIS with large vessels occlusions in the anterior circulation Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: GA
Comparator: CSA
Excluded medications: not reported |
|
| Outcomes | Primary outcome (specified)
Primary outcome (collected)
Secondary outcomes (specified)
Secondary outcomes measures (collected)
|
|
| Notes | Conflicts of interest: quote: "The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article". Funding: quote: "The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Albert J Yoo received a research grant from Penumbra Inc. and Neuravi Inc.". Protocol: NCT02317237 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Block randomisation (with sizes 4, 6, and 8) was performed after stratification". |
| Allocation concealment (selection bias) | Unclear risk | Details were not fully described Quote: "Allocation of block size was also random". |
| Blinding of participants and personnel (performance bias) | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The allocation to either GA or CS could not be blinded but was unknown by the imaging core laboratory that evaluated the primary outcome and by the nurse who evaluated the 90‐day mRS score". |
| Incomplete outcome data (attrition bias) | Low risk | There were 0 losses and crossover in 2 (6.3%) participants from CSA to the GA arm but remained in the CSA group for ITT analysis. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes and 2 additional outcomes were reported in the final article. |
| Other bias | Low risk | No evidence of other bias related to this study. |
Hu 2020.
| Study characteristics | ||
| Methods | Setting: single‐centre, China Design: RCT, 2 arms, parallel assignment, single‐blind Start date: February 2017 Completion date: 30 March 2021 |
|
| Participants | 139 men and women randomised: experimental (GA) = 72, comparator (monitored care anaesthesia (MAC)) = 67; 0 lost to follow‐up Mean age: 72 years Gender (men/women): 72/67 Mean NIHSS and mean ASPECTS not reported 2 (2.9%) participants converted from MAC to GA Participants received IV r‐tPA before EVT (not reported) Diagnostic criteria: AIS with LVO in posterior cerebral circulation (vertebrobasilar system) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: GA
Comparator: MAC
Excluded medications: not reported |
|
| Outcomes | Primary outcome (collected): not reported Primary outcome (collected)
Secondary outcomes (collected): not reported Secondary outcomes (collected)
|
|
| Notes | Conflicts of interest: (quote) "none". Funding: (quote) "This work was supported by the Guangzhou Science and Technology Project (201904010‐389) and National Natural Science Foundation of China (62076253)". Protocol available: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information on random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were randomized into GA group and MAC group (about 1:1 ratio)". Comment: not reported if sealed non‐transparent envelopes were used. |
| Blinding of participants and personnel (performance bias) | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "blinded end point cohort study, the primary outcome of mRS at 90 days (80–100 days)". |
| Incomplete outcome data (attrition bias) | Low risk | 0 losses. Crossover in 2 (2.9%) participants who were converted from MAC to GA. |
| Selective reporting (reporting bias) | Unclear risk | Comment: we did not find the study protocol to analyse. |
| Other bias | Unclear risk | Comment: we did not find the study protocol to analyse. |
Maurice 2022.
| Study characteristics | ||
| Methods | Setting: single‐centre, France Design: RCT, 2 arms, parallel assignment, open‐label single‐blind Start date: 27 July 2016 (reported in protocol) Completion date: August 2020 (reported in protocol) |
|
| Participants | 351 men and woman aged ≥ 18 years randomised: experimental (GA) = 174, comparator (CSA) = 177 Received allocated intervention:
345 participants were analysed: GA = 169 and CSA = 176 Mean age: 72 years (range: 60–85 years) Gender (men/women): 188/157 Mean NIHSS score: 16 (score range: 10–22) Localisation of stroke in left hemisphere: GA = 84 (50%) and CSA = 90 (51%) 8 (4%) participants converted from CSA to GA Received IV r‐tPA before EVT: GA = 111 (66%) and CSA = 114 (65%) Diagnostic criteria: AIS with LVO in anterior cerebral circulation Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: GA
Comparator: CSA
Conversion from CSA to GA was standardised and allowed in the following situations: agitation or restlessness not allowing the EVT; vomiting not allowing the EVT; GCS < 8; deglutition disorders, severe hypoxaemia with oxygen saturation measured by pulse oximetry at < 96% with oxygen being delivered via high‐concentration mask (maximum 10 L/minute), respiratory rate > 35/minute, clinical signs of respiratory exhaustion |
|
| Outcomes | Primary outcome (specified)
Primary outcome (collected)
Secondary outcomes (specified)
Secondary outcomes (collected)
|
|
| Notes | Conflicts of interest: quote "Dr. Beloeil received speaking fees from AbbVie (Chicago, Illinois) and Aspen Pharmacare (Durban, South Africa) and is a member of an expert board for Orion Pharma (Espoo, Finland). The other authors declare no competing interests". Funding: quote "The GASS trial was supported by funding from the French Ministry of Health (Paris, France; National Clinical Research Hospital Program, 2015). The funding sources had no role in the trial design, trial conduct, data handling, data analysis, or writing and publication of the manuscript". Protocol: NCT02822144 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was centralized and computer generated, and each patient was given a unique randomization number (patient code)". |
| Allocation concealment (selection bias) | Low risk | Quote: "patients underwent randomization in a 1:1 ratio to undergo either general anesthesia or conscious sedation". |
| Blinding of participants and personnel (performance bias) | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "the modified Rankin score was assessed by trained research nurses blinded to the randomization group". Comment: the author did not report blinding of secondary outcomes. |
| Incomplete outcome data (attrition bias) | High risk | 4 lost to follow‐up. Crossover in 3 participants from CSA to GA and 8 participants from GA to CSA but were analysed as ITT. Consent withdrawal occurred in 5 participants in GA arm and 1 participant in CSA arm and excluded from ITT. Comment: the number of participants lost to follow‐up and consent withdrawal was considerable and generated an imbalance between groups. |
| Selective reporting (reporting bias) | High risk | A high number of participants had the mRS score evaluated between 2 and 6 months (GA = 96% and CSA = 94%) and 6% of participants had the mRS were evaluated after 6 months. Comment: the variations in the time of mRS score evaluation might affect the neurological outcomes. |
| Other bias | Low risk | No evidence of other bias related to this study. |
Ren 2020.
| Study characteristics | ||
| Methods | Setting: single‐centre, China Design: RCT, 2 arms, open‐label, blind endpoint Start date: August 2017 Completion date: December 2018 |
|
| Participants | 90 men and women randomised: experimental GA = 48, comparator CSA = 42; procedure interrupted in GA = 8 and CSA = 7; 0 lost to follow‐up, 90 analysed Mean age: CSA = 69.19 (SD 6.46) years and GA = 69.21 (SD 5.78) years Gender (men/women): 50/40 Mean NIHSS score: 14 (score range: 11–16) Mean ASPECTS: 9 (score range: 8–10) ASA I/II/III: CSA = 5/15/22 and GA = 4/19/25 Mean BMI (kg/m²): CSA = 24.91 (SD 2.59) and GA = 23.84 (SD 2.02) 4 (9.52%) participants converted from CSA to GA 71 (78.8%) participants received IV r‐tPA before EVT Diagnostic criteria: AIS with LVO in anterior cerebral circulation Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: GA
Comparator: CSA
Blood pressure was routinely recorded non‐invasively at 3‐minute intervals The anaesthesiologist performed GA if the procedure was not possible due to the restlessness of participants in the CSA group At the end of the surgery, recanalisation was classified by the neuroradiologist according to the mTICI perfusion grade. After removal of the tracheal intubation, all participants were transferred to the SU or ICU for ≥ 24 hours and cared for by an expert neurologist Vasoactive drugs such as phenylephrine, ephedrine, atropine, urapidil, and nimodipine were used to keep blood pressure and heart rate fluctuation stable at the target values. Phenylephrine was the most commonly used vasopressor, and nimodipine was the most commonly used agent for hypotension Excluded medications: not reported |
|
| Outcomes | Primary outcome (specified)
Primary outcome (collected)
Secondary outcomes (specified)
Secondary outcomes (collected)
|
|
| Notes | Conflicts of interest: quote: "The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest". Funding: quote: "self' (describe at ChiCTR‐IPR‐16008494) Protocol: ChiCTR‐IPR‐16008494 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computer‐generated randomisation table was used by an independent anaesthesia assistant to allocate patients into two groups: the CSA group (n = 42) and the GA group (n = 48)". |
| Allocation concealment (selection bias) | Unclear risk | Details not fully described. Quote: "a computer‐generated randomisation table was used by an independent anaesthesia assistant to allocate patients into two groups: the CSA group (n = 42) and the GA group (n = 48)". |
| Blinding of participants and personnel (performance bias) | High risk | Although the authors described that personnel were blinded, we judged it as a high risk of bias due to the nature of the intervention. Quote: "our anaesthesia team included an attending anaesthesiologist and an anaesthesiologist assistant who were both blinded to group allocation". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "all of the investigators who assessed primary and secondary outcomes were blinded to group allocation". |
| Incomplete outcome data (attrition bias) | Low risk | 0 losses and crossovers occurred in 4 (9.52%) participants from CSA to GA, but they were analysed as ITT. |
| Selective reporting (reporting bias) | High risk | We noted several changes from what was reported in the protocol against the trial publication for the inclusion and exclusion criteria, and primary and secondary outcomes. |
| Other bias | High risk | Authors changed study objective. Protocol stated the objective was to observe the effect of different concentrations and ways of giving dexmedetomidine with remifentanil for people with craniocerebral disease interventional therapy under GA; however, the trial published results regarding the effect of CSA vs GA on outcomes in people undergoing mechanical thrombectomy for AIS. |
SIESTA 2016.
| Study characteristics | ||
| Methods | Setting: single‐centre, Germany Design: RCT, parallel‐group, open‐label, blind endpoint Start date: April 2014 Completion date: February 2016 |
|
| Participants | 152 men and women randomised: experimental GA = 73, comparator CSA = 77; 2 excluded (missing informed consent); 150 analysed Mean age: 71.5 years Gender (men/women): 90/60 Mean NIHSS score: 17 Mean ASPECTS: 8 1 lost to 24‐hour follow‐up in CSA arm IV r‐tPA before EVT: GA = 46 (63%) and CSA = 50 (65%) 1 participant who was randomised to GA was mistakenly treated under CSA representing the only major protocol violation 11 (9.2%) participants were intubated at time of evaluation of NIHSS due to:
Diagnostic: AIS with LVO in anterior cerebral circulation Inclusion criteria
Exclusion criteria
|
|
| Interventions | Comparator: GA
Experimental: CSA
Peri‐interventional management followed in‐house protocols for GA or CSA (drugs and dose used not specified in protocol). All randomised participants were non‐invasively monitored for the same haemodynamic and respiratory targets. Participants in the CSA group received IV, low‐dose, short‐acting analgesics and sedatives. Participants in the GA group received the same medication at higher doses or alternative or additional medications if necessary. In cases of interventional emergency or intolerable difficulty, respiratory failure, coma, or loss of airway protective reflexes, participants receiving CSA were immediately converted to GA Excluded medications: not reported |
|
| Outcomes | Primary outcome (specified)
Primary outcome (collected)
Secondary outcomes (specified)
Secondary outcomes (collected)
Time points reported: at discharge and 90 days after the procedure |
|
| Notes | Conflicts of interest: (quote) "There is no external steering committee for this monocentric trial". Funding: quote: "There is no funding of the trial". Protocol: NCT02126085 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly divided into two groups GA and CS … computer‐generated list". |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients selected for thrombectomy were preliminarily randomised 1:1 (using sealed, opaque envelopes based on a computer‐generated list not allowing for sequence guessing) to receive either conscious sedation or general anaesthesia, standardised according to institutional treatment protocols". |
| Blinding of participants and personnel (performance bias) | High risk | Not described, but due to the nature of the interventions, we assumed that blinding of personnel was not possible. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Investigators evaluating the primary (early neurological improvement) and certain secondary outcomes (long‐term functional outcome and causes of mortality) were blinded to allocation". |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "1 lost to 24‐hour follow‐up (primary endpoint)". There was 1 loss to 24‐hour follow‐up (primary outcome). Crossover occurred in 1 participant from CSA to GA group during the intervention because of respiratory insufficiency but was analysed as ITT. 1 participant who was randomised to the GA group was mistakenly treated under CSA representing the only major protocol violation. 11 (9.2%) participants were intubated at time of evaluation of NIHSS due to several clinical statuses and were primarily randomised to the GA group. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | No evidence of other bias related to this study. |
AIS: acute ischaemic stroke; ASA: American Society of Anaesthesiologists; ASPECT: Alberta Stroke Program Early Computed Tomography Score; BIS: Bispectral Index; BMI: body mass index; CAM: Confusion Assessment Method; CS: conscious sedation; CSA: conscious sedation anaesthesia; CT: computed tomography; CTA: computed tomographic angiogram; DBP: diastolic blood pressure; DSA: digital subtraction angiography; DWI: diffusion‐weighted imaging; EEG: electroencephalography; EST: endovascular stroke therapy; ETCO2: end‐tidal carbon dioxide; EVT: endovascular treatment; GA: general anaesthesia; GCS: Glasgow Coma Scale; GFAP: glial fibrillary acidic protein; ICU: intensive care unit; IQR: interquartile range; ITT: intention‐to‐treat; IV: intravenous; LA: local anaesthesia; LVO: large vessel occlusion; MAP: mean arterial pressure; MMSE: Mini‐Mental State Examination; MOCA: Montreal Cognitive Assessment; MRA: magnetic resonance angiography; MRI: magnetic resonance image; mRS: modified Rankin Scale; mTICI: modified Thrombolysis in Cerebral Infarction; NIHSS: National Institutes of Health Stroke Scale; PROBE: prospective, randomized, open, blinded endpoint; RCT: randomised clinical trial; r‐tPA: recombinant tissue plasminogen activator; SBP: systolic blood pressure; SD: standard deviation; SOP: standard operating procedure; SpO2: saturation of peripheral oxygen; SU: stroke unit; TICI: Thrombolysis In Cerebral Infarction.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abou‐Chebl 2015 | Non‐randomised study design. Retrospective analysis results for type of anaesthetic in RCT that compared EVT vs IV r‐tPA for AIS. |
| ACTRN12618000509268 | Inadequate population. RCT conducted in participants with unruptured intracranial aneurysm. |
| Avitsian 2016 | Non‐randomised study design. Retrospective analysis for type of anaesthetic technique in EVT for AIS. |
| Berkhemer 2016 | Non‐randomised study design. Retrospective analysis results for type of anaesthetic in RCT that compared EVT vs IV r‐tPA for AIS. |
| Bonafe 2016 | Non‐randomised study design. Retrospective analysis results for type of anaesthetic in RCT that compared EVT vs IV r‐tPA for AIS. |
| Bracard 2016 | Non‐randomised study design. Retrospective analysis results for type of anaesthetic in RCT that compared EVT vs IV r‐tPA for AIS. |
| Campbell 2019 | A systematic review of 3 RCTs (SIESTA, ANSTROKE, and GOLIATH) that compared GA vs CSA after mechanical thrombectomy for AIS. |
| Chabanne 2020 | Non‐randomised study design. Retrospective analysis results for type of anaesthetic in RCT that compared EVT vs IV r‐tPA for AIS. |
| Crosby 2016 | Non‐randomised study design. Retrospective analysis results for type of anaesthetic in RCT that compared EVT vs IV r‐tPA for AIS. |
| Goldhoorn 2020 | Non‐randomised study design. Retrospective analysis results for type of anaesthetic in RCT that compared EVT vs IV r‐tPA for AIS. |
| Jovin 2009 | Non‐randomised study design. Retrospective analysis results for type of anaesthetic in RCT that compared EVT vs IV r‐tPA for AIS. |
| Le 2020 | Non‐randomised study design. Retrospective analysis results for type of anaesthetic in RCT that compared EVT vs IV r‐tPA for AIS. |
| Menon 2016 | Non‐randomised study design. Retrospective analysis results for RCT that compared EVT vs IV r‐tPA for AIS. |
| Moritz 2010 | Inadequate population. RCT for carotid endarterectomy. |
| NCT03737786 | Inadequate population. RCT was conducted with participants to analyse sedation collateral support in EVT for AIS. |
| NCT04517383 | Inadequate comparator. RCT that considered infarct growth after EVT for AIS in participants sedated with propofol and dexmedetomidine for 6 hours before extubation. |
| Neimark 2010 | Inadequate population. Study was conducted with participants who underwent carotid endarterectomy. |
| Nichols 2010 | Non‐randomised study design. Retrospective study comparing GA vs non‐GA for EVT in AIS. |
| Nii 2018 | Non‐randomised study design. Retrospective analysis results for type of anaesthetic in RCT that compared EVT vs IV r‐tPA for AIS. |
| Pishjoo 2019 | Non‐randomised study design. |
| Powers 2019b | Non‐randomised study design. Retrospective analysis results for type of anaesthetic in RCT that compared EVT vs IV r‐tPA for AIS. |
| Rabinstein 2018 | Non‐randomised study design. Literature review of studies comparing GA vs non‐GA for EVT in AIS. |
| Rohde 2019 | Non‐randomised study design. Retrospective analysis results for type of anaesthetic in RCT that compared EVT vs IV r‐tPA for AIS. |
| Schönenberger 2019 | Non‐randomised study design. |
| Shan 2018 | Non‐randomised study design. Retrospective analysis results for type of anaesthetic in RCT that compared EVT vs IV r‐tPA for AIS. |
| Simonsen 2017 | Inadequate comparator. RCT analyses of population excluded for GOLIATH trial. |
| Sindelic 2004 | Inadequate population. Study conducted for carotid endarterectomy. |
| Starke 2017 | Non‐randomised trial. |
| Taqi 2019 | Non‐randomised study design. Observational case‐control study. |
| Tekle 2018 | Non‐randomised study design. Retrospective analysis results of an RCT that did not compare the type of anaesthesia. |
| Thomas 2012 | Non‐randomised study design. Retrospective analysis results for type of anaesthetic in carotid endarterectomy. |
| Wolf 2019 | Non‐randomised study design. Retrospective analysis results for type of anaesthetic in RCT that compared EVT vs IV r‐tPA for AIS. |
| Wong 2011 | Non‐randomised study design. Retrospective analysis results for type of anaesthetic in RCT that compared EVT vs IV r‐tPA for AIS. |
| Wu 2019 | Inadequate comparator. RCT compared the type of drugs (dexmedetomidine vs propofol) in MAC for EVT in AIS. |
| Yao 2009 | Inadequate population. RCT conducted with participants who used a laryngeal mask airway during anaesthesia in stent‐assisted angioplasty for extracranial and intracranial artery stenosis. |
| Zussman 2018 | Non‐randomised study design. |
AIS: acute ischaemic stroke; CSA: conscious sedation anaesthesia; EVT: endovascular treatment; GA: general anaesthesia; IV: intravenous; MAC: monitored anaesthesia care; RCT: randomised controlled trial; r‐tPA: recombinant tissue plasminogen activator.
Characteristics of ongoing studies [ordered by study ID]
Chabanne 2019.
| Study name | Sedation versus general anaesthesia in endovascular therapy for anterior circulation acute ischaemic stroke: the multicentre randomised controlled AMETIS trial study protocol |
| Methods | Setting: multicentre, France Design: RCT, 2 arms, parallel assignment, open‐label single‐blind Start date: 20 July 2017 (reported in protocol) Completion date: 30 June 2020 (reported in protocol) |
| Participants | 332 men and women aged ≥ 18 years Diagnostic criteria: AIS with LVO in anterior cerebral circulation Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: GA Comparator: CSA |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | 20 July 2017 |
| Contact information | Telephone number and email address not provided |
| Notes | NCT03229148 |
Chen 2020.
| Study name | SEGA – sedation versus general anaesthesia for endovascular therapy in acute ischemic stroke – a randomised comparative effectiveness trial |
| Methods | Setting: single‐centre, USA Design: RCT, 2 arms, parallel assignment, open‐label single‐blind Start date: 16 August 2017 (reported in protocol) Completion date: 31 December 2021 (reported in protocol) |
| Participants | 260 men and women aged 18–90 years Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: GA Comparator: CSA |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | 16 August 2017 |
| Contact information | Peng Roc Chen University of Texas Health Science Center, USA 713‐486‐8016; peng.r.chen@uth.tmc.edu |
| Notes | NCT03263117 |
ChiCTR2000035282.
| Study name | Effects of different anesthesia on hemodynamics and prognostic in patients with stroke having endovascular treatment: a multi‐centered, prospective, randomized controlled study |
| Methods | Setting: multicentre, China Design: RCT, 2 arms, parallel assignment, open‐label Start date: 26 June 2020 |
| Participants | 240 men and women aged 18–85 years Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: GA Comparator: LA |
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | 26 June 2020 |
| Contact information | Chen Jing Department of Anesthesiology, the First Affiliated Hospital of Guangxi Medical University, China +86 13607815280/352880721@qq.com |
| Notes | ChiCTR2000035282 |
DRKS00006801.
| Study name | Impact of anesthesia during endovascular treatment of acute ischemic stroke |
| Methods | Setting: single‐centre, Germany Design: RCT, 2 arms, parallel assignment, open‐label Start date: 13 October 2014 |
| Participants | 130 men and women aged 18–85 years Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: CSA Comparator: GA |
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | 13 October 2014 |
| Contact information | Dr Kathrin Waurick Universitätsklinikum Münster Klinik für Anästhesiologie, Germany +49‐251‐83‐47255; k.broeking at gmx.com |
| Notes | DRKS00006801 |
DRKS00023679.
| Study name | Anaesthesiological care for thrombectomy in stroke |
| Methods | Setting: multicentre, Germany Design: RCT, 2 arms, parallel assignment, blind Start date: 6 April 2021 |
| Participants | 868 men and women aged ≥ 18 years Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: GA Comparator: sedation anaesthesia |
| Outcomes | Primary outcome
Secondary outcome
|
| Starting date | 6 April 2021 |
| Contact information | Mr Dr med Andreas Ranft, Klinikum rechts der Isar der TU München, Ismaninger Str 22, 81675, München, Germany Telephone: 089 4140 9632; Fax: 089 4140 4886; E‐mail: andreas.ranft at mri.tum.de; URL: www.med.tu-muenchen.de |
| Notes | DRKS00023679 |
Liang 2020.
| Study name | Choice of anaesthesia for endovascular treatment of acute ischaemic stroke at posterior circulation (CANVAS II): protocol for an exploratory randomised controlled study |
| Methods | Setting: single‐centre, China Design: RCT, 2 arms, parallel assignment, open‐label single‐blind Start date: 15 October 2017 Completion date: 31 March 2021 |
| Participants | 88 men and women aged 18–85 years Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: GA Comparator: local/conscious anaesthesia |
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | 15 October 2017 |
| Contact information | Ruquan Han Beijing Tiantan Hospital, China 8610‐67096660; ruquan.han@gmail.com |
| Notes | NCT03317535 |
NCT03247998.
| Study name | GASTROKE – the effect of general anaesthesia versus sedation for patients with acute ischemic stroke undergoing endovascular treatment on three month morbidity and mortality: a feasibility study |
| Methods | Setting: single‐centre, France Design: RCT, 2 arms, parallel assignment, open‐label single‐blind Start date: 26 July 2017 (reported in protocol) Completion date: 1 August 2019 (reported in protocol) |
| Participants | 20 men and women aged 18–95 years Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: GA Comparator: CSA with remifentanil |
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | 26 July 2017 |
| Contact information | Miguel Arango Lawson Health Research Institute, Canada 519‐685‐8500 ext 35571; miguel.arango@lhsc.on.ca |
| Notes | NCT03247998 |
Peng 2017.
| Study name | Choice of anaesthesia for endovascular treatment of acute ischemic stroke: protocol for a randomised controlled (CANVAS) trial |
| Methods | Setting: single‐centre, China Design: RCT, 2 arms, parallel assignment, open‐label single‐blind Start date: 5 February 2016 (reported in protocol) Completion date: December 2022 (reported in protocol) |
| Participants | 640 men and women aged ≥ 18 years Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: GA Comparator: LA |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | 5 February 2016 |
| Contact information | Ruquan Han Beijing Tiantan Hospital, China 8610‐67096660; ruquan.han@gmail.com |
| Notes | NCT02677415 |
AIS: acute ischaemic stroke; ASPECTS: Alberta Stroke Program Early Computed Tomography Score; CAM: Confusion Assessment Method; CSA: conscious sedation anaesthesia; CT: computed tomography; CTA: computed tomography angiography; DSA: digital subtraction angiography; EVT: endovascular treatment; GA: general anaesthesia; GCS: Glasgow Coma Score; ICA: internal carotid artery; ICU: intensive care unit; LA: local anaesthesia; LVO: large vessel occlusion; MCA: middle cerebral artery; MMSE: Mini‐Mental State Examination; MOCA: Montreal Cognitive Assessment; MRA: magnetic resonance angiography; MRI: magnetic resonance imaging; mRS: modified Rankin Scale; mTICI: modified treatment in cerebral ischaemia; NIHSS: National Institutes of Health Stroke Scale; pc‐ASPECTS: post‐circulation Alberta Stroke Program Early Computed Tomography Score; RCT: randomised controlled trial; tPA: tissue plasminogen activator.
Differences between protocol and review
In our protocol (Tosello 2020), we planned to report the primary outcomes at the early time point established and the secondary outcomes at the early and long‐term time points, but we reported all outcomes for both time points (early and long‐term), when there were available data.
Although we did not specify this in our protocol (Tosello 2020), we performed subgroup analysis for more extracted time points of outcome assessment (at 24 hours after the intervention versus more than 24 hours; Analysis 1.2).
In our protocol (Tosello 2020), we planned to create a summary of findings table for the early time point, but in our review, we created summary of findings tables for both the early and long‐term time points, when there were available data.
Contributions of authors
RT: drafted the review, screened titles and abstracts, screened full‐text articles, identified studies, extracted data, transferred data into Review Manager 5, assessed risk of bias, and is the guarantor of the review.
RR: drafted the review.
GT: drafted the review.
CNBC: drafted the review, screened titles and abstracts, screened full‐text articles, identified studies, extracted data, spot‐checked study characteristics for accuracy, and assessed risk of bias.
JEA: drafted the review.
VV: drafted the review.
BBJ: drafted the review.
RLGF: drafted the review, acted as arbitrator.
All authors reviewed and approved the review content prior to submission.
Sources of support
Internal sources
-
Division of Vascular and Endovascular Surgery, Universidade Federal de São Paulo, Brazil
Non‐financial internal support
External sources
-
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil
This study was financed in part by CAPES, finance code 001
Declarations of interest
RT: none.
RR: none.
GT: none.
CNBC: none.
JEA: none.
VV: none.
BBJ: none.
RLGF: none.
New
References
References to studies included in this review
AnStroke 2017 {published data only}
- Hendén PL, Rentzos A, Karlsson JE, Rosengren L, Leiram B, Sundeman H, et al. General anesthesia versus conscious sedation for endovascular treatment of acute ischemic stroke: the AnStroke trial (anesthesia during stroke). Stroke 2017;48(6):1601-7. [DOI: 10.1161/STROKEAHA.117.016554.] [DOI] [PubMed] [Google Scholar]
- Hendén PL, Rentzos A. Response by Lowhagen Hendén and Rentzos to letter regarding article, "General anesthesia versus conscious sedation for endovascular treatment of acute ischemic stroke: the AnStroke trial (anesthesia during stroke)". Stroke 2017;48(9):e265. [DOI: 10.1161/strokeaha.117.018508] [DOI] [PubMed] [Google Scholar]
- NCT01872884. Sedation versus general anesthesia for endovascular therapy in acute stroke – impact on neurological outcome. clinicaltrials.gov/ct2/show/NCT01872884 (first received 4 June 2013).
CANVAS 2020 {published data only}
- NCT02677415. Impact of general vs local anesthesia on neurological function in patients with acute ischemic stroke undergoing endovascular treatment. clinicaltrials.gov/ct2/show/NCT02677415 (first received 5 February 2016).
- Peng Y, Li Y, Jian M, Liu X, Sun J, Jia B, et al. Choice of anesthesia for endovascular treatment of acute ischemic stroke: protocol for a randomized controlled (CANVAS) trial. International Journal of Stroke 2017;12(9):991-7. [DOI: 10.1177/1747493017706243] [DOI] [PubMed] [Google Scholar]
- Sun J, Liang F, Wu Y, Zhao Y, Miao Z, Zhang L, et al. Choice of anesthesia for endovascular treatment of acute ischemic stroke (CANVAS): results of the CANVAS pilot randomized controlled trial. Journal of Neurosurgical Anesthesiology 2020;32(1):41-7. [DOI: 10.1097/ANA.0000000000000567] [DOI] [PubMed] [Google Scholar]
GOLIATH 2018 {published data only}
- Compagne KC, de Graaf RA, Lugt A. Additional factors regarding clinical outcomes of general anesthesia and conscious sedation for acute ischemic stroke. JAMA 2018;75(9):1151. [DOI: 10.1001/jamaneurol.2018.1844] [DOI] [PubMed] [Google Scholar]
- NCT02317237. Anaesthetic strategy during endovascular therapy: general anaesthesia or conscious sedation? clinicaltrials.gov/ct2/show/NCT02317237 (first received 4 December 2014).
- Rasmussen M, Espelund US, Juul N, Yoo AJ, Sørensen LH, Sørensen KE, et al. The influence of blood pressure management on neurological outcome in endovascular therapy for acute ischaemic stroke. British Journal of Anaesthesia 2018;120(6):1287-94. [DOI: 10.1016/j.bja.2018.01.039] [DOI] [PubMed] [Google Scholar]
- Raychev R, Liebeskind D, Yoo A, Rasmussen M, Arnaudov D, Brown S, et al. Physiologic predictors of collateral circulation and infarct growth during anesthesia – detailed analyses of the GOLIATH trial. Journal of Cerebral Blood Flow & Metabolism 2019;40(6):1203-12. [DOI: 10.1177/0271678X19865219] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen LH, Speiser L, Karabegovic S, Yoo AJ, Rasmussen M, Sørensen KE, et al. Safety and quality of endovascular therapy under general anesthesia and conscious sedation are comparable: results from the GOLIATH trial. Journal of Neurointerventional Surgery 2019;11(11):1070-2. [DOI: 10.1136/neurintsurg-2019-014712] [DOI] [PubMed] [Google Scholar]
- Simonsen CZ, Sørensen LH, Juul N, Johnsen SP, Yoo AJ, Andersen G, et al. Anesthetic strategy during endovascular therapy: general anesthesia or conscious sedation? (GOLIATH – General Or Local anesthesia In intra-Arterial THerapy) – a single-center randomized trial. International Journal of Stroke 2016;11(9):1045-52. [DOI: 10.1177/1747493016660103] [DOI] [PubMed] [Google Scholar]
- Simonsen CZ, Yoo AJ, Sørensen LH, Juul N, Johnsen SP, Andersen G, et al. Effect of general anesthesia and conscious sedation during endovascular therapy on infarct growth and clinical outcomes in acute ischemic stroke: a randomized clinical trial. JAMA 2018;75(4):470-7. [DOI: 10.1001/jamaneurol.2017.4474] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen CZ, Yoo AJ, Sorensen LH, Juul N, Johnsen SP, Andersen G, et al. General or local anesthesia in intra arterial therapy ("GOLIATH"): a randomized trial. European Stroke Journal 2017;2(1 Suppl 1):477. [DOI] [PubMed] [Google Scholar]
Hu 2020 {published data only}
- Hu G, Shi Z, Li B, Shao W, Xu B. General anesthesia versus monitored anesthesia care during endovascular therapy for vertebrobasilar stroke. American Journal of Translational Research 2021;13(3):1558-67. [AJTR: 0123350] [PMC free article] [PubMed] [Google Scholar]
Maurice 2022 {published data only}
- Beloeil H. Data results: general anesthesia versus sedation, both with hemodynamic control, during intraarterial treatment for stroke: the GASS randomized trial [personal communication]. Email to: R Tosello 2 April 2022. [DOI] [PubMed]
- Maurice A, Eugène F, Ronzière T, Devys JM, Taylor G, Subileau A, et al. General anesthesia versus sedation, both with hemodynamic control, during intraarterial treatment for stroke: the GASS randomized trial. Anesthesiology 2022;136:567-76. [DOI: 10.1097/ALN.0000000000004142] [DOI] [PubMed] [Google Scholar]
- Maurice A, Ferré JC, Ronzière T, Devys JM, Subileau A, Laffon M, et al. GASS trial study protocol: a multicentre, single-blind, randomised clinical trial comparing general anaesthesia and sedation during intra-arterial treatment for stroke. BMJ 2019;9(5):e024249. [DOI: 10.1136/bmjopen-2018-024249] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02822144. General anesthesia versus sedation during intra-arterial treatment for stroke. clinicaltrials.gov/ct2/show/NCT02822144 (first received 27 June 2016).
Ren 2020 {published data only}16008494
- ChiCTR-IPR-16008494. The curative effect analysis of combined application of dexmedetomidine and remifentanil on neurosurgical intervention. chictr.org.cn/showprojen.aspx?proj=14373 (first received 1 May 2016).
- Ren C, Xu G, Liu Y, Liu G, Wang J, Gao J. Effect of conscious sedation vs general anesthesia on outcomes in patients undergoing mechanical thrombectomy for acute ischemic stroke: a prospective randomized clinical trial. Frontiers in Neurology 2020;11:170. [DOI: 10.3389/fneur.2020.00170] [DOI] [PMC free article] [PubMed] [Google Scholar]
SIESTA 2016 {published data only}
- NCT02126085. Sedation vs. intubation for endovascular stroke treatment. clinicaltrials.gov/ct2/show/NCT02126085 (first received 23 April 2014).
- Pfaff JA, Schönenberger S, Nagel S, Ringleb PA, Hacke W, Bendszus M, et al. Effect of general anesthesia versus conscious sedation for stroke thrombectomy on angiographic workflow in a randomized trial: a post hoc analysis of the SIESTA trial. Radiology 2018;286(3):1016-21. [DOI: 10.1148/radiol.2017171002] [DOI] [PubMed] [Google Scholar]
- Schönenberger S, Möhlenbruch M, Pfaff J, Mundiyanapurath S, Kieser M, Bendszus M, et al. Sedation vs. intubation for endovascular stroke treatment (SIESTA) – a randomized monocentric trial. International Journal of Stroke 2015;10(6):969-78. [DOI: 10.1111/ijs.12488] [DOI] [PubMed] [Google Scholar]
- Schönenberger S, Uhlmann L, Hacke W, Schieber S, Mundiyanapurath S, Purrucker JC, et al. Effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: a randomized clinical trial. JAMA 2016;316(19):1986-96. [DOI: 10.1001/jama.2016.16623] [DOI] [PubMed] [Google Scholar]
- Schönenberger S, Uhlmann L, Ungerer M, Pfaff J, Nagel S, Klose C, et al. Association of blood pressure with short- and long-term functional outcome after stroke thrombectomy: post hoc analysis of the SIESTA trial. Stroke 2018;49(6):1451-6. [DOI: 10.1161/STROKEAHA.117.019709] [DOI] [PubMed] [Google Scholar]
- Schonenberger S, Bosel J. Reply from Schonenberger et al. to the letter from Kofke and Sharma regarding "Sedation vs. intubation for endovascular stroke treatment (SIESTA) – a randomized monocentric trial". International Journal of Stroke 2016;11(7):NP73. [DOI: 10.1177/1747493016641117] [DOI] [PubMed] [Google Scholar]
- Schonenberger S, Uhlmann L, Hacke W, Schieber S, Mundiyanapurath S, Purrucker JC. [Correction] Incorrect information in table 3: effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: a randomized clinical trial. JAMA 2017;317(5):538. [DOI: 10.1001/jama.2016.20888] [DOI] [PubMed] [Google Scholar]
- Schonenberger S, Uhlmann L, Schieber S, Mundiyanapurath S, Purrucker J, Nagel S. Sedation and airway management during endovascular treatment of acute ischemic stroke: final results from the randomized trial SIESTA (sedation vs intubation for endovascular stroke treatment). International Journal of Stroke 2016;11(3):23. [DOI: 10.1177/1747493016670567] [DOI] [Google Scholar]
References to studies excluded from this review
Abou‐Chebl 2015 {published data only}
- Abou-Chebl A, Yeatts SD, Yan B, Cockroft K, Goyal M, Jovin T, et al. Impact of general anesthesia on safety and outcomes in the endovascular arm of interventional management of stroke (IMS) III trial. Stroke 2015;46(8):2142-8. [DOI: 10.1161/STROKEAHA.115.008761] [DOI] [PMC free article] [PubMed] [Google Scholar]
ACTRN12618000509268 {published data only}
- ACTRN12618000509268. Laryngeal mask airway versus tracheal tube for endovascular treatment. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373483 (first received 16 August 2017).
Avitsian 2016 {published data only}
- Avitsian R, Machado SB. Anesthesia for endovascular approaches to acute ischemic stroke. Anesthesiology Clinics 2016;34(3):497-509. [DOI: 10.1016/j.anclin.2016.04.004] [DOI] [PubMed] [Google Scholar]
Berkhemer 2016 {published data only}
- Berkhemer OA, den Berg LA, Fransen PS, Beumer D, Yoo AJ, Lingsma HF, et al. The effect of anesthetic management during intra-arterial therapy for acute stroke in MR CLEAN. American Academy of Neurology 2016;87(7):656-64. [DOI: 10.1212/WNL.0000000000002976] [DOI] [PubMed] [Google Scholar]
Bonafe 2016 {published data only}
- Bonafe A, Goyal M, Jahan R, Eker O, Levy EI, Nogueira RG, et al. General anesthesia effects on outcomes with mechanical thrombectomy: observations from the swift prime trial. European Stroke Journal 2016;1(1):36. [DOI: 10.1177/2396987316642909] [DOI] [Google Scholar]
Bracard 2016 {published data only}
- Bracard S, Guillemin F, Ducrocq X. Anesthesia type during endovascular acute ischemic stroke treatment and clinical outcomes in THRACE study. European Stroke Journal 2016;1(1):68-9. [DOI: 10.1177/2396987316642909] [DOI] [Google Scholar]
Campbell 2019 {published data only}
- Campbell D, Barber PA. Pooled-analysis of general anesthesia versus conscious sedation or local anesthesia in stroke endovascular thrombectomy. European Stroke Journal 2019;4(Suppl 1):81. [EMBASE: 10.1177/2396987319845560] [Google Scholar]
Chabanne 2020 {published data only}
- Chabanne R, Begard M, Mazighi M, Maier B. Anterior circulation acute ischemic stroke: endovascular mechanical thrombectomy indications and perioperative anaesthetic management. Anesthesie et Reanimation 2020;6(1):96-102. [DOI: 10.1016/j.anrea.2019.11.015] [DOI] [Google Scholar]
Crosby 2016 {published data only}
- Crosby G, Muir KW. Anesthesia and neurologic outcome of endovascular therapy in acute ischemic stroke: MR (not so) CLEAN. Neurology 2016;87(7):648-9. [DOI: 10.1212/WNL.0000000000002989] [DOI] [PubMed] [Google Scholar]
Goldhoorn 2020 {published data only}
- Goldhoorn RB, Bernsen ML, Hofmeijer J, Martens JM, Lingsma HF, Dippel DW, et al. Anesthetic management during endovascular treatment of acute ischemic stroke in the MR CLEAN registry. Neurology 2020;94(1):e97-106. [DOI: 10.1212/WNL.0000000000008674] [DOI] [PubMed] [Google Scholar]
Jovin 2009 {published data only}
- Jovin TG, Jumaa MA, Zhang F, Gupta R, Zaidi SF, Lin R, et al. Comparison of safety, radiographic and clinical outcome of intra-arterial thrombolysis under conscious sedation versus pre-procedural intubation. Stroke 2009;40(4):e251. [ID: 70014885] [Google Scholar]
Le 2020 {published data only}
- Le R, Hoefnagel A, Yao J, Rao D. Conscious sedation versus general anesthesia on outcomes in mechanical thrombectomy for patients with acute ischemic stroke. Journal of Vascular and Interventional Radiology 2020;31(3 Suppl):S309-S310. [DOI: 10.1016/j.jvir.2019.12.778] [DOI] [Google Scholar]
Menon 2016 {published data only}
- Menon BK, Sajobi TT, Zhang Y, Rempel JL, Shuaib A, Thornton J, et al. Analysis of workflow and time to treatment on thrombectomy outcome in the endovascular treatment for small core and proximal occlusion ischemic stroke (ESCAPE) randomized, controlled trial. Circulation 2016;133(23):2279-86. [DOI] [PubMed] [Google Scholar]
Moritz 2010 {published data only}
- Moritz S, Schmidt C, Bucher M, Wiesenack C, Zimmermann M, Schebesch KM, et al. Neuromonitoring in carotid surgery: are the results obtained in awake patients transferable to patients under sevoflurane/fentanyl anesthesia? Journal of Neurosurgical Anesthesiology 2010;22(4):228-95. [DOI: 10.1097/ANA.0b013e3181e16e14] [DOI] [PubMed] [Google Scholar]
NCT03737786 {published data only}
- NCT03737786. SEACOAST 1 sedation with collateral support in endovascular therapy for acute ischemic stroke 1: a randomized controlled phase 2B clinical trial. clinicaltrials.gov/ct2/show/NCT03737786 (first received 13 November 2018).
NCT04517383 {published data only}
- NCT04517383. Effects of post-operative sedation on infarct growth and clinical outcomes in patients receiving endovascular thrombectomy for acute ischemic stroke. clinicaltrials.gov/ct2/show/NCT04517383 (first received 12 August 2020).
Neimark 2010 {published data only}
- Neimark MI, Shmelev VV, Simagin VI. Comparative assessment of various general anesthesia methods during reconstructive operations on the carotid arteries. Anesteziologiia i Reanimatologiia 2010;2:19-23. [PMID: ] [PubMed] [Google Scholar]
Nichols 2010 {published data only}
- Nichols C, Carrozzella J, Yeatts S, Tomsick T, Broderick J, Khatri P. Is periprocedural sedation during acute stroke therapy associated with poorer functional outcomes? Journal of NeuroInterventional Surgery 2010;2(1):67-70. [DOI: 10.1136/jnis.2009.001768] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nii 2018 {published data only}
- Nii K, Hanada H, Hiraoka F, Eto A, Mitsutake T, Tsutsumi M. Usefulness of consciousness sedation with dexmedetomidine and pentazocine during endovascular treatment for acute stroke. Neurologia Medico-Chirurgica 2018;58(2):79-84. [DOI: 10.2176/nmc.oa.2017-0188] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pishjoo 2019 {published data only}
- Pishjoo M, Fazeli F, Hashemi M, Yekta MJ, Mashhadinejad M, Farzan M, et al. General anesthesia versus conscious sedation in mechanical thrombectomy for acute ischemic stroke. Journal of the Neurological Sciences 2019;405(Suppl):150-1. [DOI: 10.1016/j.jns.2019.10.726] [DOI] [Google Scholar]
Powers 2019b {published data only}
- Powers CJ, Dornbos D, Mlynash M, Gulati D, Torbey M, Nimjee SM, et al. Thrombectomy with conscious sedation compared with general anesthesia: a DEFUSE 3 Analysis. American Journal of Neuroradiology 2019;40(6):1001-5. [DOI: 10.3174/ajnr.A6059] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rabinstein 2018 {published data only}
- Rabinstein AA, Kallmes DF. Optimal anesthetic strategy for endovascular stroke therapy begging for a good trial. Neurology 2018;91(1):16-8. [DOI: 10.1212/WNL.0000000000005754] [DOI] [PubMed] [Google Scholar]
Rohde 2019 {published data only}
- Rohde S, Schwarz S, Alexandrou M, Reimann G, Ellerkmann RK, Politi M, et al. Effect of general anaesthesia versus conscious sedation on clinical and procedural outcome in patients undergoing endovascular stroke treatment: a matched-pair analysis. Cerebrovascular Diseases 2019;48(1-2):91-5. [DOI: 10.1159/000503779] [DOI] [PubMed] [Google Scholar]
Schönenberger 2019 {published data only}
- Schönenberger S, Weber D, Ungerer MN, Pfaff J, Schieber S, Uhlmann L, et al. The KEEP SIMPLEST study: improving in-house delays and periinterventional management in stroke thrombectomy – a matched pair analysis. Neurocritical Care 2019;31(1):46-55. [DOI: 10.1007/s12028-018-00667-3] [DOI] [PubMed] [Google Scholar]
Shan 2018 {published data only}
- Shan W, Yang D, Wang H, Xu L, Zhang M, Liu W, et al. General anesthesia may have similar outcomes with conscious sedation in thrombectomy patients with acute ischemic stroke: a real-world registry in China. European Neurology 2018;80(1-2):7-13. [DOI: 10.1159/000490901] [DOI] [PubMed] [Google Scholar]
Simonsen 2017 {published data only}
- Simonsen CZ, Rasmussen M, Yoo AJ, Sorensen LH. Patients treated with endovascular treatment outside guidelines. Stroke 2017;48(Suppl 1):ATP17. [EMBASE: 617461302] [Google Scholar]
Sindelic 2004 {published data only}
- Sindelic R, Vlajkovic G, Davidovic L, Vujanac B, Vjestica M. Comparison of the influence of general and regional anesthesia on basic haemodynamic parameters during carotid endarterectomy. Acta Chirurgica Iugoslavica 2004;51(3):37-43. [DOI: 10.2298/ACI0403037S] [DOI] [PubMed] [Google Scholar]
Starke 2017 {published data only}
- Starke RM, Peterson EC, Komotar RJ, Connolly ES. A randomized clinical trial of conscious sedation vs general anesthesia on outcomes in patients receiving thrombectomy for ischemic stroke. Clinical Neurosurgery 2017;80(5):N27-8. [DOI: 10.1093/neuros/nyx113] [DOI] [PubMed] [Google Scholar]
Taqi 2019 {published data only}
- Taqi MA, Suriya SS, Sodhi A, Quadri SA, Farooqui M, Zafar A, et al. Ideal sedation for stroke thrombectomy. Neurosurgical Focus 2019;46(2):e16. [DOI: 10.3171/2018.11.FOCUS18522] [DOI] [PubMed] [Google Scholar]
Tekle 2018 {published data only}
- Tekle W, Hassan A, Jadhav A, Liebeskind D, Jovin T, Nogueira R, et al. Impact of peri-procedural and technical factors and patient characteristics on revascularization and clinical outcome in DAWN trial. Stroke 2018;13(2 Suppl 1):10. [DOI: 10.1161/STROKEAHA.119.026437] [DOI] [Google Scholar]
Thomas 2012 {published data only}
- Thomas SD, Norman PE, Temple SE, Vijayan V, Mwipatayi BP. General versus local anesthesia for carotid endarterectomy: the West Australian experience. Chirurgia 2012;25(6):423-8. [ID: 368571734] [Google Scholar]
Wolf 2019 {published data only}
- Wolf A, Molders P, Yilmaz U. Stroke thrombectomy: anaesthesiologic aspects. Anasthesiologie Intensivmedizin Notfallmedizin Schmerztherapie 2019;54(4):283-93. [DOI: 10.1055/a-0816-4707] [DOI] [PubMed] [Google Scholar]
Wong 2011 {published data only}
- Wong C, Hills N, Walker G, Smith W. General anesthesia during mechanical thrombectomy negatively impacts functional independence and mortality in only less severe ischemic stroke. Stroke 2011;42(3):e233. [DOI: 10.1161/STR.0b013e3182074d9b] [DOI] [Google Scholar]
Wu 2019 {published data only}
- Wu B, Hu H, Cai A, Ren C, Liu S. The safety and efficacy of dexmedetomidine versus propofol for patients undergoing endovascular therapy for acute stroke: a prospective randomized control trial. Medicine 2019;98(21):e15709. [DOI: 10.1097/MD.0000000000015709] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yao 2009 {published data only}
- Yao DX, Lang Y, Wei LM, Wang KJ. Application of disposable laryngeal mask airway during anesthesia in stent-assisted angioplasty for extracranial and intracranial artery stenosis. Chinese Journal of Cerebrovascular Diseases 2009;6(4):194. [DOI: 10.3969/j.issn.1672-5921.2009.04.007] [DOI] [Google Scholar]
Zussman 2018 {published data only}
- Zussman B, Weiner G, Ducruet A. A second randomized trial comparing general anesthesia to conscious sedation in acute ischemic stroke patients undergoing endovascular treatment. Neurosurgery 2018;82(3):N27-8. [DOI: 10.1093/neuros/nyx589] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Chabanne 2019 {published data only}
- Chabanne R, Fernandez-Canal C, Degos V, Lukaszewicz C, Velly L, Mrozek S, et al. Sedation versus general anaesthesia in endovascular therapy for anterior circulation acute ischaemic stroke: the multicentre randomised controlled AMETIS trial study protocol. BMJ 2019;9:e027561. [DOI: 10.1136/bmjopen-2018-027561] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03229148. Anesthesia management in endovascular therapy for ischemic stroke: a multicenter randomised study. clinicaltrials.gov/ct2/show/NCT03229148 (first received 20 July 2017).
Chen 2020 {published data only}
- Chen PR, Sanzgiri A, Sheth S, Artime C, Inam M, Pedroza C et al. Is there clinical equipoise for a randomized trial of anesthesia (sedation vs. general) during endovascular therapy? Stroke 2020;50(Suppl 1):Abst.WP53. [DOI: 10.1161/str.50.suppl_1.WP53] [DOI] [Google Scholar]
- NCT03263117. SEGA – sedation versus general anesthesia for endovascular therapy in acute ischemic stroke – a randomized comparative effectiveness trial. clinicaltrials.gov/ct2/show/NCT03263117 (first received 16 August 2017).
ChiCTR2000035282 {published data only}2000035282
- ChiCTR2000035282. Effects of different anesthesia on hemodynamics and prognostic in patients with stroke having endovascular treatment: a multi-centered, prospective, randomized controlled study. www.medresman.org.cn (first received 26 June 2020). [CHICTR: 2000035282]
DRKS00006801 {published data only}DRKS00006801
- DRKS00006801. Impact of anesthesia during endovascular treatment of acute ischemic stroke. drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00006801 (first received 13 October 2014).
DRKS00023679 {published data only}00023679
- DRKS00023679. Anaesthesiological care for thrombectomy in stroke. www.actinstroke.de (first received 6 April 2021).
Liang 2020 {published data only}
- Liang F, Zhao Y, Yan X, Wu Y, Li X, Zhou Y, et al. Choice of anaesthesia for endovascular treatment of acute ischaemic stroke at posterior circulation (CANVAS II): protocol for an exploratory randomised controlled study. BMJ 2020;10(7):e036358. [DOI: 10.1136/bmjopen-2019-036358] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03317535. Choice of anesthesia for endovascular treatment of acute ischemic stroke in posterior circulation: a randomized controlled exploratory study (CANVAS-Ⅱ). clinicaltrials.gov/ct2/show/NCT03317535 (first received 15 October 2017).
NCT03247998 {published data only}
- NCT03247998. GASTROKE – the effect of general anesthesia versus sedation for patients with acute ischemic stroke undergoing endovascular treatment on three month morbidity and mortality: a feasibility study. clinicaltrials.gov/ct2/show/NCT03247998 (first received 26 July 2017).
Peng 2017 {published data only}
- NCT02677415. Impact of general vs local anesthesia on neurological function in patients with acute ischemic stroke undergoing endovascular treatment. clinicaltrials.gov/ct2/show/NCT02677415 (first received 5 February 2016).
- Peng Y, Li Y, Jian M, Liu X, Sun J, Jia B, et al. Choice of anesthesia for endovascular treatment of acute ischemic stroke: protocol for a randomized controlled (CANVAS) trial. International Journal of Stroke 2017;12(9):991-7. [DOI: 10.1177/1747493017706243] [DOI] [PubMed] [Google Scholar]
Additional references
Albers 2018
- Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. New England Journal of Medicine 2018;378(8):708-18. [DOI: 10.1056/NEJMoa1713973] [DOI] [PMC free article] [PubMed] [Google Scholar]
ASA 2019
- American Society of Anesthesiologists. Continuum of depth of sedation: definition of general anesthesia and levels of sedation/analgesia. Committee of origin: quality management and departmental administration, 2019. Available at www.asahq.org/standards-and-guidelines/continuum-of-depth-of-sedation-definition-of-general-anesthesia-and-levels-of-sedation analgesia (last accessed prior to 21 June 2022).
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. GRADE working group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI: 10.1136/bmj.328.7454.1490] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bai 2021
- Bai X, Zhang X, Wang T, Feng Y, Wang Y, Lyu X, et al. General anesthesia versus conscious sedation for endovascular therapy in acute ischemic stroke: a systematic review and meta-analysis. Journal of Clinical Neuroscience 2021;86:10-7. [DOI: 10.1016/j.jocn.2021.01.012] [DOI] [PubMed] [Google Scholar]
Bang 2018
- Bang OY, Toyoda K, Arenillas JF, Liu L, Kim JS. Intracranial large artery disease of non-atherosclerotic origin: recent progress and clinical implications. Journal of Stroke 2018;20(2):208-17. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barber 2000
- Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS study group. Alberta Stroke Programme Early CT Score. Lancet 2000;355(9216):1670-4. [DOI: 10.1016/s0140-6736(00)02237-6] [DOI] [PubMed] [Google Scholar]
Benjamin 2019
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics – 2019 update: a report from the American Heart Association. Circulation 2019;139(10):e56-e528. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brott 1989
- Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20(7):864-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Campbell 2021
- Campbell D, Diprose WK, Deng C, Barber PA. General anesthesia versus conscious sedation in endovascular thrombectomy for stroke: a meta-analysis of 4 randomized controlled trials. Journal of Neurosurgical Anesthesiology 2021;33(1):21-7. [DOI: 10.1097/ANA.0000000000000646] [DOI] [PubMed] [Google Scholar]
Cassola 2018
- Cassola N, Baptista-Silva JC, Flumignan CD, Sesso R, Vasconcelos V, Flumignan RL. Duplex ultrasound for diagnosing symptomatic carotid stenosis in the extracranial segments. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD013172. [DOI: 10.1002/14651858.CD013172] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, (accessed 25 March 2020). Available at www.covidence.org.
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, Li T, Page MJ, et al, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 6 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Flumignan 2017a
- Flumignan CD, Flumignan RL, Navarro TP. Extracranial carotid stenosis: evidence based review [Estenose de carotida extracraniana: revisao baseada em evidencias]. Revista do Colegio Brasileiro de Cirurgioes 2017;44(3):293-301. [PMID: ] [DOI] [PubMed] [Google Scholar]
Flumignan 2017b
- Flumignan CD, Flumignan RL, Nakano LC, Amorim JE. Spontaneous carotid dissection. Revista da Associacao Medica Brasileira 2017;63(5):397-400. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fugate 2013
- Fugate JE, Klunder AM, Kallmes DF. What is meant by "TICI"? American Journal of Neuroradiology 2013;34(9):1792-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goyal 2014
- Goyal M, Menon BK, Hill MD, Demchuk A. Consistently achieving computed tomography to endovascular recanalization < 90 minutes: solutions and innovations. Stroke 2014;45(12):e252-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Goyal 2016
- Goyal M, Menon BK, Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387(10029):1723-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 20 March 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Hacke 2008
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. New England Journal of Medicine 2008;359(13):1317-29. [DOI: 10.1056/NEJMoa0804656] [DOI] [PubMed] [Google Scholar]
Higashida 2003
- Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003;34(8):e109-37. [DOI: 10.1161/01.STR.0000082721.62796.09] [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2021
- Higgins JP, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Kim 2016
- Kim JS, Caplan LR. Non-atherosclerotic intracranial arterial diseases. Frontiers of Neurology and Neuroscience 2016;40:179-203. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lakomkin 2019
- Lakomkin N, Dhamoon M, Carroll K, Singh IP, Tuhrim S, Lee J, et al. Prevalence of large vessel occlusion in patients presenting with acute ischemic stroke: a 10-year systematic review of the literature. Journal of Neurointerventional Surgery 2019;11(3):241-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Lindekleiv 2018
- Lindekleiv H, Berge E, Bruins Slot KM, Wardlaw JM. Percutaneous vascular interventions versus intravenous thrombolytic treatment for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2018, Issue 10. Art. No: CD009292. [DOI: 10.1002/14651858.CD009292.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Löwhagen Hendén 2017
- Löwhagen Hendén P, Rentzos A, Karlsson JE, Rosengren L, Leiram B, Sundeman H, et al. General anesthesia versus conscious sedation for endovascular treatment of acute ischemic stroke: the AnStroke Trial (Anesthesia During Stroke). Stroke 2017;48(6):1601-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Luo 2018
- Luo J, Wang T, Gao P, Krings T, Jiao L. Endovascular treatment of intracranial atherosclerotic stenosis: current debates and future prospects. Frontiers in Neurology 2018;9:666. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2015
- McDonald JS, Brinjikji W, Rabinstein AA, Cloft HJ, Lanzino G, Kallmes DF. Conscious sedation versus general anaesthesia during mechanical thrombectomy for stroke: a propensity score analysis. Journal of Neurointerventional Surgery 2015;7(11):789-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Meretoja 2017
- Meretoja A, Keshtkaran M, Tatlisumak T, Donnan GA, Churilov L. Endovascular therapy for ischemic stroke: save a minute-save a week. Neurology 2017;88(22):2123-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Molina 2010
- Molina CA, Selim MH. General or local anesthesia during endovascular procedures: sailing quiet in the darkness or fast under a daylight storm. Stroke 2010;41(11):2720-1. [PMID: ] [DOI] [PubMed] [Google Scholar]
Naylor 2018
- Naylor AR, Ricco JB, Borst GJ, Debus S, Haro J, Halliday A, et al. Editor's choice – management of atherosclerotic carotid and vertebral artery disease: 2017 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). European Journal of Vascular and Endovascular Surgery 2018;55(1):3-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nogueira 2018
- Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. New England Journal of Medicine 2018;378(1):11-21. [DOI: 10.1056/NEJMoa1706442] [DOI] [PubMed] [Google Scholar]
Norrving 2013
- Norrving B, Kissela B. The global burden of stroke and need for a continuum of care. Neurology 2013;80(3 Suppl 2):S5-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Page 2021a
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:71. [DOI: 10.1136/bmj.n71] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2021b
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Peng 2018
- Peng Y, Wu Y, Huo X, Wu P, Zhou Y, Li J, Endovascular Therapy for Acute Ischemic Stroke Trial (EAST) group. Outcomes of anesthesia selection in endovascular treatment of acute ischemic stroke. Journal of Neurosurgical Anesthesiology 2018;31(1):43-9. [DOI: 10.1097/ANA.0000000000000500] [DOI] [PubMed]
Pereira 2018
- Pereira BJ, Batista UC, Tosello RT, Stroher IN, Baeta AM, Piske RL. Web vessels: literature review and neurointerventional management. World Neurosurgery 2018;110:907-16. [DOI: 10.1016/j.wneu.2017.11.115] [DOI] [PubMed] [Google Scholar]
Powers 2018
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018;49(3):e46-e110. [PMID: ] [DOI] [PubMed] [Google Scholar]
Powers 2019a
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 Guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019;50(12):e344-e418. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rasmussen 2017
Review Manager 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rusy 2021
- Rusy DA, Hofer A, Rasmussen M, Paisansathan C, Sharma D. Assessment of anesthesia practice patterns for endovascular therapy for acute ischemic stroke: a Society for Neuroscience in Anesthesiology and Critical Care (SNACC) member survey. Journal of Neurosurgical Anesthesiology 2021;33(4):343-6. [DOI: 10.1097/ANA.0000000000000661] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schonenberger 2016
- Schonenberger S, Uhlmann L, Hacke W, Schieber S, Mundiyanapurath S, Purrucker JC, et al. Effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: a randomized clinical trial. JAMA 2016;316(19):1986-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schonenberger 2019
- Schonenberger S, Henden PL, Simonsen CZ, Uhlmann L, Klose C, Pfaff JA, et al. Association of general anesthesia vs procedural sedation with functional outcome among patients with acute ischemic stroke undergoing thrombectomy: a systematic review and meta-analysis. JAMA 2019;322(13):1283-93. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Simonsen 2018
- Simonsen CZ, Yoo AJ, Sorensen LH, Juul N, Johnsen SP, Andersen G, et al. Effect of general anesthesia and conscious sedation during endovascular therapy on infarct growth and clinical outcomes in acute ischemic stroke: a randomized clinical trial. JAMA 2018;75(4):470-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Steinberg 2019
Takahashi 2014
- Takahashi C, Liang CW, Liebeskind DS, Hinman JD. To tube or not to tube? The role of intubation during stroke thrombectomy. Frontiers in Neurology 2014;5:170. [DOI: 10.3389/fneur.2014.00170] [DOI] [PMC free article] [PubMed] [Google Scholar]
Talke 2014
- Talke PO, Sharma D, Heyer EJ, Bergese SD, Blackham A, Stevens RD. Republished: Society for neuroscience in anesthesiology and critical care expert consensus statement: anesthetic management of endovascular treatment for acute ischemic stroke. Stroke 2014;45(8):e138-50. [DOI: 10.1161/STROKEAHA.113.003412] [DOI] [PubMed] [Google Scholar]
Wardlaw 2014
- Wardlaw JM, Murray V, Berge E, Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2014, Issue 7. Art. No: CD000213. [DOI: 10.1002/14651858.CD000213.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [PMID: ] [PubMed] [Google Scholar]
Wilson 2002
- Wilson JT, Hareendran A, Grant M, Baird T, Schulz UG, Muir KW, et al. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke 2002;33(9):2243-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhang 2019
- Zhang Y, Jia L, Fang F, Ma L, Cai B, Faramand A, et al. General anesthesia versus conscious sedation for intracranial mechanical thrombectomy: a systematic review and meta-analysis of randomized clinical trials. Journal of the American Heart Association 2019;8:e011754. [DOI: 10.1161/JAHA.118.011754] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Tosello 2020
- Tosello R, Riera R, Tosello G, Clezar CN, Amorim JE, Vasconcelos V, et al. Type of anaesthesia for acute ischaemic stroke endovascular treatment. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013690. [DOI: 10.1002/14651858.CD013690] [DOI] [PMC free article] [PubMed] [Google Scholar]


