Abstract
Background
Factor XI (FXI) inhibition offers the promise of hemostasis‐sparing anticoagulation for the prevention and treatment of thromboembolic events. Abelacimab (MAA868) is a novel fully human monoclonal antibody that targets the catalytic domain and has dual activity against the inactive zymogen Factor XI and the activated FXI.
Objectives
To investigate the safety, pharmacokinetics (PK), and pharmacodynamics (PD) of single dose intravenous and multiple dose subcutaneous administration of abelacimab in healthy volunteers and patients with atrial fibrillation, respectively.
Patients/Methods
In study ANT‐003, healthy volunteers were administered single intravenous doses of abelacimab (30–150 mg) or placebo. The ANT‐003 study also included a cohort of obese but otherwise healthy subjects. In study ANT‐004, patients with atrial fibrillation were administered monthly subcutaneous doses of abelacimab (120 mg and 180 mg), or placebo, for 3 months. Key PK and PD parameters, including activated partial thromboplastin time (aPTT) and free FXI levels, as well as anti‐drug antibodies (ADA) were assessed.
Results
Following intravenous administration of abelacimab, the terminal elimination half‐life ranged from 25 to 30 days. One hour after the start of the intravenous infusion greater than 99% reductions in free FXI levels were observed. Following once monthly subcutaneous administration, marked reductions from baseline in free FXI levels were sustained. Parenteral administration of abelacimab demonstrated a favorable safety profile with no clinically relevant bleeding events.
Conclusions
Intravenous and multiple subcutaneous dose administration of abelacimab were safe and well tolerated. The safety, PK, and PD data from these studies support the clinical development of abelacimab.
Keywords: antibodies, monoclonal; blood; Factor XI; pharmacodynamics; pharmacokinetics
Essentials.
Abelacimab is a monoclonal antibody that inhibits Factor XI and the activated Factor XI (FXIa).
The pharmacokinetics of abelacimab were studied in healthy subjects and patients with atrial fibrillation.
Abelacimab IV/SC was well‐tolerated and profoundly suppressed FXI for 4 weeks.
Abelacimab produces rapid FXI inhibition and can be given monthly to maintain FXI inhibition.
1. INTRODUCTION
Despite the introduction of direct oral anticoagulants (DOACs), bleeding remains a clinical concern. There remains an unmet medical need for novel anticoagulants with a superior safety profile over existing agents, in particular for patients at risk for thrombosis who may be undertreated or not treated at all because of an increased risk of bleeding due to advanced age, severe kidney disease, or other chronic medical conditions. 1
Targeting Factor XI (FXI), a blood coagulation factor that is part of the intrinsic pathway, may offer the potential for effective anticoagulation that spares hemostasis and thus carries a lower risk of bleeding. 2 Existing anticoagulants, such as DOACs and warfarin, exert their effects by targeting components of the common coagulation pathway. These components, however, are also important for physiologic hemostasis. Consequently, these agents have all been associated with an increased risk of bleeding events, some of which can be severe and sometimes fatal. 3
There is a growing appreciation, however, that coagulation factors of the intrinsic pathway, such as FXI, play a non‐essential role in physiologic hemostasis even though they strongly contribute to pathologic thrombosis 4 suggesting that the pathways involved in hemostasis and thrombosis are distinct and separable. The uncoupling of physiologic hemostasis from pathologic thrombosis is illustrated by individuals with severe FXI deficiency. Individuals with severe FXI deficiency appear to be protected from developing venous thromboembolism (VTE) and ischemic stroke compared to the general population. 5 Spontaneous bleeding in FXI deficiency, however, has been reported to be uncommon and not clearly related to the FXI level. Bleeding in FXI deficient patients has been reported following injury or in association with surgery and more frequently at anatomic regions with increased fibrinolytic activity such as the nasopharynx or genitourinary system. 6 Furthermore, population studies have shown that elevated levels of FXI are associated with an increased risk of VTE, stroke, and myocardial infarction 7 , 8 suggesting that FXI plays an important role in pathologic thrombosis.
Abelacimab (MAA868) is a fully human monoclonal antibody that binds to the catalytic domain of Factor XI and locks it in a zymogen conformation, thereby preventing its activation by Factor XIIa or thrombin. 9 In a first‐in‐human Phase 1 study, single subcutaneous administration of abelacimab up to the 240 mg dose was demonstrated to be safe and well‐tolerated. In addition, abelacimab led to dose dependent reductions in free FXI levels and prolonged the aPTT with abelacimab doses 150 mg and higher leading to a robust and sustained PD effects for at least 4 weeks.
In order to explore the PK and PD properties of abelacimab following different routes of administration, a Phase 1 study of single dose intravenous abelacimab in healthy volunteers (ANT‐003) and a Phase 2a study of multiple once‐monthly subcutaneous administration of abelacimab in patients with atrial fibrillation (ANT‐004) were conducted. The results from these clinical studies contribute to a detailed pharmacokinetic and pharmacodynamic assessment of abelacimab and support the future clinical development of abelacimab as a novel once monthly agent for the prevention and treatment of arterial and venous thromboembolic events.
2. METHODS
2.1. Study design and participants
ANT‐003 was a randomized, subject‐ and investigator‐blinded, placebo‐controlled study conducted in healthy subjects and in obese, but otherwise healthy, subjects aged 18 to 60 years of age (Figure 1A). A total of four cohorts of eight subjects each were enrolled and randomized 3:1 to single intravenous administration of abelacimab or matching placebo. Cohorts 1, 2, and 3 evaluated the safety, tolerability, PK, and PD of abelacimab 30, 50, and 150 mg, respectively. Cohort 4 evaluated the safety, tolerability, PK, and PD of abelacimab 150 mg in obese (BMI ≥ 35 kg/m2), but otherwise healthy, subjects. All eligible patients presented to the clinical site for baseline assessments and then received study drug on day 1. Abelacimab was administered diluted in 100 ml of 5% dextrose solution, and 100 ml bags of D5W were administered for subjects randomized to the placebo group. Subjects were domiciled for 3 days for safety, PK, and PD monitoring. After discharge from the clinical site, the subjects were then followed for an outpatient observation period of 106 days (Figure 1B).
FIGURE 1.
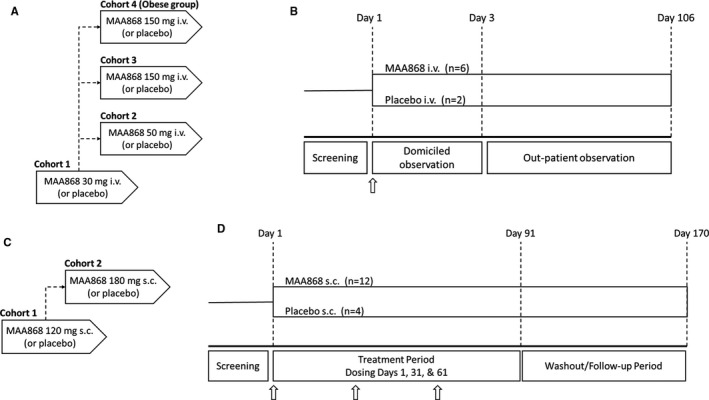
(A) Flowchart of study ANT‐003. Cohorts 1–3 enrolled healthy subjects, while cohort 4 enrolled healthy subjects with a BMI ≥ 35 kg/m2. (B) In study ANT‐003, healthy subjects in each cohort were randomized to receive a single intravenous dose of 30, 50, and 150 mg abelacimab, or matching placebo. Following a domicile period of 3 days, subjects were monitored as an outpatient out to day 106. Arrows indicate dose administration. (C) Flowchart of study ANT‐004. (D) Patients with low‐risk atrial fibrillation were randomized to receive 3 monthly subcutaneous doses of abelacimab at doses of 120 and 180 mg, or matching placebo and then followed in a washout period out to day 170. Arrows indicate dose administration
ANT‐004 was a randomized, patient‐ and investigator‐blinded, placebo‐controlled, multiple ascending dose study in patients with atrial fibrillation (AF) or atrial flutter aged 18–85 years old with a CHA2DS2‐VASc risk score of 0–1 for men and 1–2 for women and in whom the use of an anticoagulant for stroke prevention was not planned for the duration of the trial. The study was designed to enroll up to three cohorts of approximately 16 patients randomized 3:1 to receive once monthly subcutaneous abelacimab, or matching placebo, for 3 months (Figure 1C). All eligible patients presented to the clinic on Day 1 for baseline efficacy and safety assessments and were then randomized to abelacimab or matching placebo. Study drug was prepared by an unblinded pharmacist and appropriate measures were taken to ensure that the treatment assignments are concealed from the rest of the site staff. During the treatment period, patients were scheduled to return to the clinical site on days 11, 31, 41, 61, 71, and 91 for safety assessments such as vital signs, adverse event assessments, laboratory tests, PK, PD, biomarkers, and other study assessments (Figure 1D). The second and third monthly subcutaneous doses were administered on days 31 and 61, respectively. After day 91, the patients entered the follow‐up period where they were followed for safety, PK, PD, and other study assessments until day 170 (end of study visit).
All subjects/patients provided written informed consent before any study procedures were performed. ANT‐003 was conducted at Covance Clinical Research Unit Daytona Beach, Florida, while ANT‐004 was conducted at six clinical sites in the United States. All study procedures were reviewed and approved by a central institutional review board (Advarra). Both studies were conducted in accordance with the ICH Harmonized Tripartite Guidelines for Good Clinical Practice and with the ethical principles laid down in the Declaration of Helsinki.
2.2. Procedures
Adverse events were assessed at each study visit. Vital signs, 12‐lead electrocardiogram, physical examination, and clinical laboratory tests were assessed throughout the study. In ANT‐004 study, all suspected bleeding events were adjudicated by a central independent adjudication committee blinded to treatment assignment. Bleeding events were adjudicated as a major bleeding event or a clinically relevant non‐major (CRNM) bleeding event using definitions based on International Society of Thrombosis and Haemostasis (ISTH) criteria. 10
Concentrations of plasma total abelacimab were measured by blood collection in 3.2% sodium citrate tubes and analyzed by a validated liquid chromatography/tandem mass spectrometry (LC‐MS/MS) method. Briefly, abelacimab measurement was performed after denaturation, alkylation and tryptic cleavage of a reference standard. As an internal standard, stable isotope labeled‐MAA868 was spiked prior to denaturation, alkylation, and tryptic cleavage steps. After digestion and solid phase extraction, the surrogate peptide and the internal standard were analyzed in Multi Reaction Monitoring mode by LC‐MS/MS using electrospray ionization in positive mode.
Free FXI was centrally measured using LC/MS‐MS by LGC (Fordham). 9 Briefly, FXI levels were tested after immunoprecipitation of samples or reference standard with a monoclonal biotinylated abelacimab antibody coupled to streptavidin magnetic beads, denaturation, alkylation and tryptic cleavage on the beads. After digestion and quenching of the samples, the surrogate peptide and the internal standard are analyzed in Multi Reaction Monitoring mode by LC‐MS/MS using electrospray ionization in positive mode. Unknown samples are quantitated by comparison to a standard curve generated with a Wagner (log‐Log quadratic) regression. LC‐MS/MS methods for quantitation of plasma total abelacimab and free FXI in plasma concentrations were validated in accordance with global health authority guidelines on validation of bioanalytical methods.
The activated partial thromboplastin time (aPTT) were centrally measured using Actin FSL as a reagent at various time‐points by Covance Central Laboratory, Inc. Indianapolis, IN. Factor XI coagulation activity (FXI:C) was performed based on a modified one‐stage aPTT assay in citrated poor platelets plasma samples at Esoterix Coagulation, Inc. using Actin FS (Siemens Healthcare Diagnostics) as a reagent. 9
Anti‐abelacimab antibodies were measured in serum samples using an electrochemiluminescence‐based immunoassay validated in accordance with global health authority guidance on validation of antidrug antibody (ADA) assays. Briefly, 96‐well multi‐array standard plates were coated with abelacimab and then blocked while simultaneously incubating controls and samples in an acidic dissociation buffer to dissociate complexes on a separate assay plate. After blocking, plates were washed and a Tris buffer was added to the wells, followed by the addition of the acidified samples and controls. After an incubation step, plates were washed followed by the addition of ruthenylated anti‐immunoglobulin detector antibodies. After incubation and wash, a read buffer was added, and the plates were read on an electrochemiluminescence reader (MSD QuickPlexSQ120, Meso Scale Discovery). For the confirmatory assay, a predetermined amount of abelacimab is added to samples at the stage of neutralization with Tris buffer. During assay validation the screening cut point (tier 1) was determined to be 1.56, the confirmatory cut point (tier 2) was determined to be 23.75%, and the titration cut point (tier 3) was determined to be 1.80. The screening sensitivity was determined to be 2.06 ng/ml and the confirmatory sensitivity was determined to be 1.48 ng/ml.
2.3. Pharmacokinetic and pharmacodynamic analyses and modeling
Pharmacokinetic analysis was performed with non‐compartmental methods and population PK/PD modeling by Certara Consulting. For the population PK/PD analyses, data from both ANT‐003 and ANT‐004 studies were combined with previous Phase 1 studies conducted in healthy volunteers and healthy Japanese males. 9 Population pharmacokinetic parameters were estimated by nonlinear mixed‐effects modeling using NONMEM version 7.4.3 software. The selection of population PK/PD models was based on pre‐determined model selection criterion and statistical model comparison procedures. The final population PK/PD model was verified through visual predictive check and goodness‐of‐fit diagnostics plots.
2.4. Immunogenicity
In the ANT‐003 study, samples to determine the presence of anti‐drug antibodies (ADAs) against abelacimab were taken at baseline (day −3 to day −1), on day 7, and day 10 after intravenous abelacimab administration. In the ANT‐004 study, ADA samples were taken before subcutaneous abelacimab administration on days 1, 31, and 61 and also on days 71, 91, 121 and 170 (end of study visit). The ADA assay followed a three‐tiered approach of screening, confirmation, and titer determination as described. Positive screening samples were subjected to a confirmatory step which demonstrated the specificity of the response for abelacimab. Any sample that confirmed positive was then diluted to determine the anti‐drug antibody titer.
3. RESULTS
3.1. Subject disposition in ANT‐003 and 004
In ANT‐003, 32 subjects were randomized, dosed, and completed the study in accordance with the protocol (and protocol amendment; Figure 1B). All subjects completed the study, and there were no discontinuations. The baseline demographics of subjects in ANT‐003 are shown in Table S1. The median age of subjects across the different dose cohorts (including pooled placebo subjects) ranged from 40.0 to 50.0 years. The median BMI of subjects in the 30, 50, and 150 mg abelacimab treatment groups ranged from 26.1 to 27.9 kg/m2, while the median BMI of subjects in the obese cohort was 44.3 kg/m2.
In ANT‐004, eight patients were randomized in Cohort 1 (abelacimab 120 mg dose cohort), and 10 patients were randomized in Cohort 2 (abelacimab 180 mg dose cohort) between December 2019 and September 2020. Between March 2020 and May 2020, dosing in ANT‐004 was paused due to the COVID‐19 pandemic. In May 2020, sites were permitted to resume dosing on a case‐by‐case basis if the Investigator felt it was safe for patients to resume study participation. Patients who received only one dose of study drug before the study pause were given the option of either resuming study drug with the second dose or restarting with the first dose of study drug if a sufficient amount of time had elapsed such that the expected PD effect had returned to baseline. Most of the patients affected by the pause in the study were in Cohort 1, where 7 out of 8 patients either had a disruption in the planned dosing schedule or a missed a study visit. In September 2020, the Sponsor elected to close the study to further enrollment due to operational challenges presented by the COVID‐19 pandemic. The baseline demographics of subjects in ANT‐004 are shown in Table S2. The median age of patients across the different treatment groups (including pooled placebo) ranged from 40.0 to 50.0 years, and the median BMI across the different treatment groups were similar (Table S2).
3.2. Safety
In the ANT‐003 study, single intravenous doses of 30, 50, and 150 mg abelacimab in healthy subjects, and 150 mg in subjects with a BMI ≥ 35 kg/m2 were safe and well tolerated. There were no discontinuations due to treatment emergent adverse events (TEAEs) during the study. There were no deaths. One serious adverse event of left gluteus cellulitis with abscess was reported in a subject in the abelacimab 150 mg obese cohort on day 110 after their dose administration (and after their End of Study Visit). This event was considered by the investigator to be not related to study drug and resolved with drainage and medication. No major or clinically relevant non‐major bleeding events were reported. There were no reports of any hypersensitivity reactions. One subject in the BMI ≥ 35 kg/m2 cohort who received abelacimab 150 mg experienced an infusion site reaction of bruising that started approximately 1 h after the start of the infusion and induration that was noted at 5, 7, and 13 h after the start of the infusion. This reaction was considered mild in severity and resolved spontaneously after approximately 20–22 h. The reaction was considered not related to the study drug.
Overall, the number of subjects reporting TEAEs across all abelacimab doses was similar or less than subjects receiving placebo (Table S3). The number of subjects with treatment related TEAEs was similar between abelacimab doses, and the majority of AEs were mild in severity. All AEs resolved by the end of the study.
In the ANT‐004 study, once monthly subcutaneous administration of abelacimab doses of 120 mg and 180 mg was safe and well‐tolerated in patients with AF. There were no serious adverse events or deaths. The rate of TEAEs was similar across all treatment groups (Table S4). In the abelacimab 120 mg dose group, 5 out of 6 patients (83.3%) reported at total of 16 treatment‐emergent adverse events (TEAEs), and in the abelacimab 180 mg dose group, 3 out of 7 patients (42.9%) reported a total of 7 TEAEs. Meanwhile, in the pooled placebo groups, 4 out of 5 patients (80.0%) reported a total of 8 TEAEs. The majority of AEs were mild in severity, and there were no trends in the pattern of AEs. There were no reports of injection site reactions or hypersensitivity reactions in any patients. There were no discontinuations due to TEAEs during the study. One patient in Cohort 2 receiving the abelacimab 180 mg dose was discontinued from the study by the investigator after the second dose in order to undergo a non‐urgent invasive cardiac catheterization. The patient underwent the procedure approximately a month after their last dose of abelacimab without any complications.
There were no major or CRNM bleeding events in ANT‐004. Five suspected bleeding events were reported in four patients—three events in three abelacimab treated patients and two events in a placebo treated patient—all of which were adjudicated by a central independent adjudication committee blinded to treatment assignment as “nuisance bleeding” events or no bleeding event.
In both studies, the administration of abelacimab was not associated with any apparent treatment related data trends in vital signs or ECG parameters. In addition, abelacimab administration was not associated with any apparent trends in the serum chemistry, hematology, or urinalysis data over time.
3.3. Pharmacokinetics
Plasma total abelacimab concentration–time profiles after single intravenous administration are presented in Figure 2A. In healthy subjects, the median time‐to‐peak abelacimab plasma concentrations ranged from 1.75 to 2.00 h (after start of the 1‐h infusion) and were dose independent. The increase in exposure over the dose range of 30–150 mg appeared to be dose proportional for the initial concentration, C0, and more than dose proportional for AUCinf and AUClast. The mean terminal elimination half‐life (T 1/2) ranged from 595 and 709 h (25–30 days). In healthy obese subjects, severe obesity was associated with a moderate decrease (30%–45%) in abelacimab exposure (C0 and AUC). A summary of PK parameters for each dose cohort is given in Table 1. Plasma total abelacimab concentration‐time profiles after once monthly subcutaneous administration of abelacimab are presented in Figure 2B.
FIGURE 2.
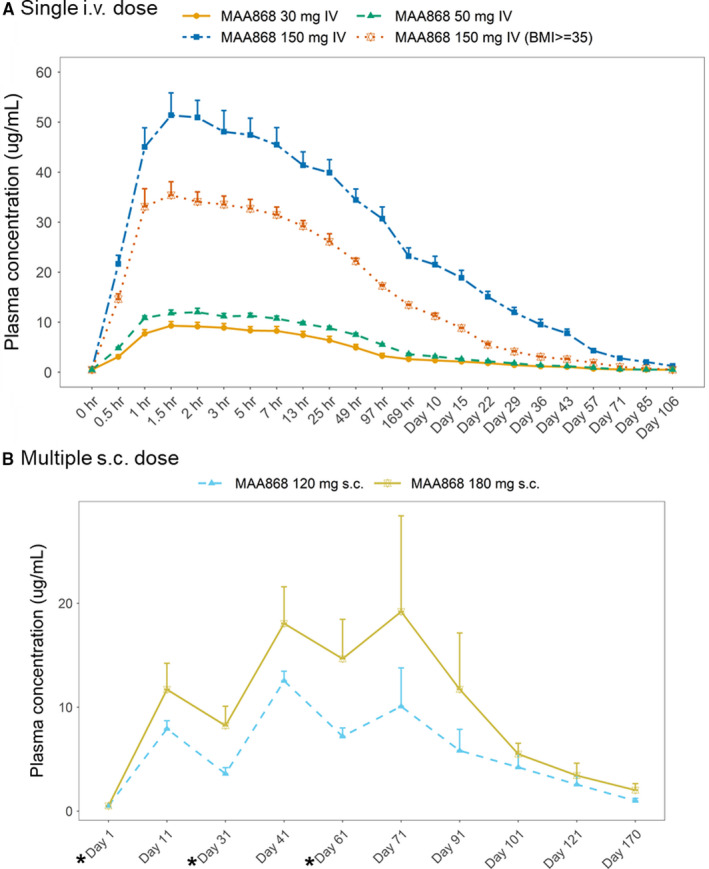
(A) Arithmetic mean (±SEM) plasma concentration‐time profiles of abelacimab following a 1‐hr intravenous infusion (ANT‐003). (B) Arithmetic mean (±SEM) plasma concentration‐time profiles of abelacimab with once monthly subcutaneous administration (ANT‐004). (*) indicates study visit days with abelacimab subcutaneous administration
TABLE 1.
Summary of pharmacokinetic parameters in ANT‐003.
|
PK parameters n = 6 |
MAA868 30 mg n = 6 |
MAA868 50 mg n = 6 |
MAA868 150 mg n = 6 |
MAA868 150 mg (BMI ≥ 35) n = 6 |
|---|---|---|---|---|
| C0 (μg/ml) | 7.50 (26.7) | 10.8 (9.2) | 44.2 (21.0) | 31.0 (13.7) |
| Cmax (μg/ml) | 9.43 (21.0) | 12.2 (12.8) | 52.3 (17.7) | 36.7 (16.8) |
| T 1/2 (h) | 621 (12.6) | 709 (38.0) | 595 (29.7) | 621 (17.1) |
| t max (h) | 1.75 (1.50–2.00) | 2.03 (1.50–5.00) | 2.00 (1.50–3.03) | 1.75 (1.20–2.02) |
| AUClast (h × μg/ml) | 2235 (44.5) | 3379 (19.6) | 21782 (18.3) | 11785 (26.8) |
| AUCinf (h × μg/ml) | 2940 (34.2) | 4020 (20.3) | 22969 (17.6) | 12543 (26.6) |
| Vss (L) | 8.48 (32.1) | 10.5 (17.0) | 5.00 (24.1) | 8.29 (20.8) |
| CL (L/h) | 0.0102 (34.2) | 0.0124 (20.3) | 0.00653 (17.6) | 0.0120 (26.6) |
Geometric Mean (Geometric Mean CV%) Abelacimab PK Parameters.
3.4. Pharmacodynamics
In ANT‐003, the intravenous infusion of abelacimab led to a rapid and >99% reduction in the concentration of free FXI across all abelacimab doses from 1 h post start of infusion until Day 5 (Figure 3A). The reduction of free FXI concentration remained sustained and returned to baseline occurred in a dose and time dependent manner. By Day 106, mean concentrations of free FXI at all abelacimab doses returned to levels consistent with those observed at baseline.
FIGURE 3.
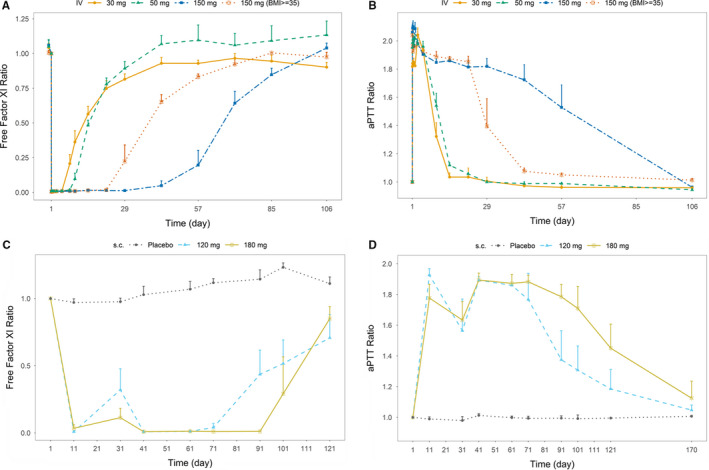
(A) Arithmetic mean effect‐time profiles of free Factor XI following intravenous abelacimab administration. (B) Arithmetic mean effect‐time profiles of aPTT following intravenous abelacimab administration. (C) Arithmetic mean effect‐time profiles of free Factor XI following monthly subcutaneous administration. (D) Arithmetic mean effect‐time profiles of aPTT following monthly subcutaneous abelacimab administration. Ratios were calculated by dividing by the subject's baseline values
Reductions in free FXI levels were associated with a rapid and sustained prolongation of aPTT with approximately a 2‐fold prolongation reached between 1 h post start of infusion to Day 5 (Figure 3B). The abelacimab 150 intravenous dose produced a peak aPTT prolongation of approximately 2‐fold at 48 h. The aPTT returned to baseline in a dose and time dependent manner. In subjects receiving placebo, there was no trend in the mean change from baseline in aPTT. No changes were observed in prothrombin or thrombin time (data not shown).
In ANT‐004, the repeat monthly subcutaneous administration of abelacimab led to a robust and sustained reduction in free FXI concentrations from baseline (Figure 3C) while free FXI concentrations remained relatively constant in pooled placebo patients. The reduction of free FXI concentration recovered somewhat at trough prior to the second dose in the abelacimab 120 mg arm. With repeat monthly administration of abelacimab, the reductions in free FXI were sustained. During the washout/follow‐up period, concentrations of free FXI in both the abelacimab 120 and 180 mg dose groups returned to near baseline levels up to day 110 (end of study visit) in a dose and time dependent manner.
Reductions in free FXI levels was associated with a sustained prolongation of aPTT from baseline (Figure 3D). There was an approximately 1.8 to 2‐fold prolongation of aPTT observed at the first post‐dose time point on day 11 for both the abelacimab 120 and 180 mg dose groups. Before the second dose, the aPTT returned to approximately 1.6‐fold increase relative to baseline. Both the 120 and 180 mg dose groups produced an approximately 1.9‐fold prolongation in aPTT before the third and final dose before returning to baseline in a dose and time dependent manner. In patients receiving placebo, there was no trend in the mean change from baseline in aPTT.
Inhibition of FXI coagulation activity (FXI:C) in both studies trended similarly to free FXI concentrations (Figure S1). No changes were observed in prothrombin time (PT) consistent with the mode of action of abelacimab (data not shown).
3.5. Immunogenicity
None of the subjects and patients in either the ANT‐003 and ANT‐004 studies had a confirmed, treatment‐emergent ADA response. The screen positive rate from the two studies combined was approximately 5%. However, none of the screen positive samples were confirmed positive. The false positive rate is in accordance with global health authority recommendations for ADA assays and confirms that the assay cut point was appropriately set to yield a sufficiently sensitive assay.
4. DISCUSSION
Targeting Factor XI offers the potential for anticoagulation that carries a lower risk of bleeding. Abelacimab binds to the catalytic domain of Factor XI with high affinity and locks it in a zymogen conformation, thereby preventing its activation by Factor XIIa or thrombin. 9 This novel mechanism of action differentiates abelacimab from other agents that only target the activated FXI. Abelacimab should prevent the activation of circulating (zymogen) FXI, thereby preventing this coagulation factor from entering the thrombogenic process, as well as inhibit any FXIa that has already been formed.
The results of the ANT‐003 and ANT‐004 studies demonstrate that intravenous and once monthly subcutaneous administration of abelacimab is safe and well‐tolerated and produce marked reduction of free Factor XI from the first time point assessed (1 h after start of intravenous infusion) that can be sustained throughout the monthly dosing interval. Anticipating that a large percentage of patients in need of a safer anticoagulation therapy will be overweight, we also investigated the pharmacokinetics of abelacimab in a cohort of obese subjects. PK exposure of abelacimab and duration of FXI inhibition were moderately lower in this cohort, possibly due to the observed larger volume of distribution and slightly faster clearance of abelacimab. Added to prior studies of abelacimab in healthy volunteers, the PK and PD data of abelacimab presented here contribute to a comprehensive PK/PD profile of abelacimab administered via the intravenous or subcutaneous route. Taken together, these results demonstrate the feasibility of achieving rapid suppression of FXI activity with intravenous administration of abelacimab and the ability to maintain FXI inhibition with once monthly subcutaneous dosing for chronic indications.
Besides the mild effect of body weight on PK parameters, it is expected that abelacimab as a monoclonal antibody will have a predictable in vivo profile with an absence of renal clearance and a low risk for drug–drug interactions. For these reasons as well as the potential for reduced bleeding, abelacimab should also be suitable for use in an elderly population with multiple comorbidities and/or underlying chronic kidney disease, a patient population that often does not receive sufficient antithrombotic therapy due to the limitations of the currently available treatment options. Furthermore, the ease of use associated with a once‐monthly subcutaneous administration of abelacimab may result in enhanced adherence and improved efficacy compared with small‐molecule‐based anticoagulation therapies that may lose efficacy when trough concentrations are not maintained. One patient in the ANT‐004 study was discontinued from the study and underwent an invasive procedure without any bleeding complications. The optimal management of patients on abelacimab who need to undergo an invasive procedure or surgery remains to be defined.
The limitations of these studies include, by design, the single dose exposure in ANT‐003 which limits the ability to detect clinically relevant safety signals. In addition, due to the COVID‐19 pandemic, enrollment in the ANT‐004 study was stopped early and thus the fully planned data set of PK, PD, and immunogenicity data for monthly subcutaneous administration of abelacimab could not be collected.
Emerging clinical trials suggest that targeting FXI with either an antisense oligonucleotide–mediated approach or a monoclonal antibody directed against activated FXI can provide highly effective antithrombotic efficacy without an increased risk of bleeding. 11 , 12 Abelacimab has the benefit of achieving rapid and sustained Factor XI inhibition after an intravenous dose. In a recent Phase 2 study, a single intravenous infusion of abelacimab administered in patients after elective total knee arthroplasty was effective for the prevention of venous thromboembolism and was associated with a low risk of bleeding. 13 Coupled with subsequent monthly subcutaneous administration, abelacimab could be used in the acute setting for immediate FXI inhibition followed by monthly subcutaneous administration for chronic indications. Once monthly dosing may achieve better adherence compared to existing oral anticoagulant medications which require once or twice daily dosing that result in frequent peak‐trough excursions in plasma concentrations balancing the need for efficacy with bleeding risk. Abelacimab has the potential to introduce a paradigm shift in anticoagulation therapy as a long‐acting FXI inhibitor that is able to target maximal efficacy with minimal bleeding.
In conclusion, abelacimab, a fully human FXI antibody, is expected to effectively inhibit FXI activity leading to reductions in free FXI levels associated with a rapid and sustained prolongation of aPTT. The safety and PD efficacy data in ANT‐003 and ANT‐004 warrant further investigation of abelacimab in Phase 2/3 clinical studies to evaluate safety and efficacy compared with current anticoagulant agents. Further evaluation of the safety and efficacy of abelacimab in the acute setting following total knee arthroplasty (EudraCT 2019‐003756‐37) and in the chronic setting in patients with atrial fibrillation (NCT 04755283) is underway. The data collected here contribute to our understanding of the PK and PD properties of intravenous and subcutaneous administered abelacimab and enable the design of longer duration clinical trials to assess the efficacy and safety of abelacimab in the prevention and treatment of arterial and venous thromboembolism.
CONFLICT OF INTEREST
The author(s) declared the following potential conflict of interest with respect to the research, authorship, and/or publication of this article: B. A. Yi, D. Freedholm, N. Widener, S. Coulter, and D. Bloomfield are employees or affiliates of Anthos Therapeutics. X. Wang, E. Simard, C. Cullen, N. M. Al‐Saady, N. E. Lepor, and M. Lovern declare no conflicts of interest.
AUTHOR CONTRIBUTION
BA Yi, D Freedholm, N Widener, and D Bloomfield participated in the study design. BA Yi, D Freedholm, S Coulter, N Widener, N Al‐Saady, C Cullen, X Wang, E Simard, N Lepor, M Lovern, and D Bloomfield participated in data analysis, data interpretation, and manuscript preparation, review, and revisions. All authors granted final approval of the manuscript for submission.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the volunteers who participated in this study. The authors acknowledge M. Gussie, L. Rystrom, M. Sanderson and other members of the Covance clinical study team for their work in executing this study. The authors also acknowledge the individuals at Anthos Therapeutics who contributed to the research and development of abelacimab. These studies were sponsored by Anthos Therapeutics.
Yi BA, Freedholm D, Widener N, et al. Pharmacokinetics and pharmacodynamics of Abelacimab (MAA868), a novel dual inhibitor of Factor XI and Factor XIa. J Thromb Haemost. 2022;20:307–315. doi: 10.1111/jth.15577
Manuscript handled by: Saskia Middeldorp
REFERENCES
- 1. Weitz JI, Fredenburgh JC. Factors XI and XII as targets for new anticoagulants. Front Med. 2017;4:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gailani D, Gruber A. Factor XI as a therapeutic target. Arterioscler Thromb Vasc Biol. 2016;36:1316‐1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tomaselli GF, Mahaffey KW, Cuker A, et al. 2017 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70:3042‐3067. [DOI] [PubMed] [Google Scholar]
- 4. Deloughery E, Olson SR, Puy C, Shatzel JJ. The safety and efficacy of novel agents targeting Factors XI and XII in early phase human trials. Semin Thromb Hemost. 2019;45:502‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fredenburgh JC, Weitz JI. Factor XI as a target for new anticoagulants. Hamostaseologie. 2021;41:104‐110. [DOI] [PubMed] [Google Scholar]
- 6. Bolton‐maggs PHB. Factor XI deficiency—resolving the enigma? Hematol Am Soc Hematol Educ Progr. 2009;2009:97‐105. [DOI] [PubMed] [Google Scholar]
- 7. Meijers J, Tekelenburg W, Bouma B, Bertina R, Rosendaal F. High levels of coagulation factor XI as a risk factor for venous thrombosis. N Engl J Med. 2000;342:696‐701. [DOI] [PubMed] [Google Scholar]
- 8. Yang DT, Flanders MM, Kim H, Rodgers GM. Elevated factor XI activity levels are associated with an increased odds ratio for cerebrovascular events. Am J Clin Pathol. 2006;126:411‐415. [DOI] [PubMed] [Google Scholar]
- 9. Koch AW, Schiering N, Melkko S, et al. MAA868, a novel FXI antibody with a unique binding mode, shows durable effects on markers of anticoagulation in humans. Blood. 2019;133:1507‐1516. [DOI] [PubMed] [Google Scholar]
- 10. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S. Definition of clinically relevant non‐major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non‐surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:2119‐2126. [DOI] [PubMed] [Google Scholar]
- 11. Büller HR, Bethune C, Bhanot S, et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372:232‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weitz JI, Bauersachs R, Becker B, et al. Effect of osocimab in preventing venous thromboembolism among patients undergoing knee arthroplasty: the FOXTROT randomized clinical trial. JAMA. 2020;323:130‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Verhamme P, Yi BA, Segers A, et al. Abelacimab for prevention of venous thromboembolism. N Engl J Med. 2021;385:609‐617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


