Abstract
The influence of neonatal experiences upon later‐life affective behavior is increasingly recognized, but the reported effects on anxiety are often contradictory. The observed effect may depend upon the type of anxiety (state or trait) affected. The current study aims to investigate whether neonatal repetitive needle pricking alters anxiety behavior in adulthood, by assessing both state and trait anxiety in rats. Sprague–Dawley rat pups received four unilateral needle pricks per day, while controls received four tactile stimuli or were left completely undisturbed during the first postnatal week. Mechanical sensitivity was assessed in the neonatal phase and throughout the development. State anxiety was assessed in the open field test and trait anxiety in the elevated zero maze. The results show that repetitive needle pricking leads to acute mechanical hypersensitivity, but does not affect baseline mechanical sensitivity throughout development. In adulthood, animals previously exposed to neonatal procedural pain (including repetitive handling and removal from litter) showed lower state anxiety but did not differ in trait anxiety, as compared with the undisturbed controls. These findings indicate that early‐life procedural pain decreases state but not trait anxiety behavior in later life in a rodent model of repetitive needle pricking.
Keywords: anxiety, long‐term consequences, needle prick, neonate, procedural pain
1. INTRODUCTION
Painful and invasive procedures are common for neonates hospitalized in the neonatal intensive care unit (NICU), with up to 14 painful procedures experienced each day (Cruz et al., 2016). Early exposure to painful procedures has been shown to contribute to long‐term negative consequences including alterations in somatosensation, anxiety, and depression (Grunau et al., 2001; Peters et al., 2005; Ranger et al., 2014; Walker et al., 2018; Williams & Lascelles, 2020). Indeed, early exposure to painful procedures affects neurodevelopment, leading to a reduction in amygdala and thalamus volumes, which consequently can impair affective functioning (Brummelte et al., 2012; Chau et al., 2019; Cismaru et al., 2016; Duerden et al., 2018; Walker et al., 2018). Interestingly, survivors of neonatal repetitive pain show higher rates of internalizing behaviors, and higher anxiety and pain catastrophizing in childhood, infancy and early adulthood (Botting et al., 1997; Hayes & Sharif, 2009; Johnson et al., 2010; Ranger et al., 2014; Walker et al., 2018). Although clinical evidence shows that affective behavior in infants and children is altered by early‐life pain exposure, clinical data on the impact of early life pain on anxiety in adulthood remain limited.
Preclinical evidence suggests that anxiety in adolescence and adulthood is affected by neonatal procedural pain, but findings are heterogeneous (Anand et al., 1999; Chen et al., 2016; Page et al., 2005; Schellinck et al., 2003; Zuke et al., 2019). In more detail, neonatal repetitive procedural pain has been shown to increase (Davis et al., 2018; Schellinck et al., 2003), decrease (Anand et al., 1999; Chen et al., 2016; Page et al., 2005; Zuke et al., 2019), or fail to show an effect on adult anxiety behavior in rodents (Anand et al., 1999; Mooney‐Leber & Brummelte, 2020; Ranger et al., 2018). In both humans and laboratory animals, anxiety is not a unitary phenomenon and includes both innate (trait) and situation‐evoked (state) anxiety. Trait anxiety is an enduring feature that is consistent across time and context, whereas state anxiety is anxiety experienced at one particular time and can be contextual and conditioned (Ohl, 2003). Behavioral paradigms designed to measure trait and state anxiety have been developed in rodents. The open field test (OFT) represents normal or trait anxiety, as rodent behavior in this test reflects a natural balance between exploratory and escaping drives (Hawley et al., 2011; Prut & Belzung, 2003). On the other hand, the elevated zero maze (EZM) models situation evoked (state) anxiety that is contextual to the anxiogenic stimuli present (including openessness and elevation). Previous studies on the long‐term effect of neonatal pain used the term “anxiety” without an a priori assumption of whether or not trait or state anxiety was represented. The type of behavioral essay used, and thus the type of anxiety (trait vs. state) measured, might explain some of the contradictory findings as most often state but not trait anxiety seems affected by neonatal procedural pain. The aim of the present study is to investigate the long‐term effects of repetitive neonatal procedural pain on trait and state anxiety behavior in adulthood, in a well‐established animal model of repetitive needle pricks (NPs) (Knaepen et al., 2013; van den Hoogen et al., 2020). Trait anxiety is measured in the OFT, whereas state anxiety is measured in the EZM.
2. METHODS
2.1. Animals
Sprague–Dawley males and females were purchased from Charles River Laboratory and were mated at Maastricht University to produce eight litters. Dams were transferred to the experimental room at gestational day (G) 16, and all pups were born on G21. Litters were culled to a maximum of N = 10 to ensure equal caretaking by the dam. Each experimental litter included a balanced representation of neonatal condition, and equal distribution by sex per group (Table 1). A maximum of one or two male and/or female pups were taken from each litter for each condition to control for any litter effects (Chapman & Stern, 1979). Pups were weaned at postnatal day (P)21 and randomly housed in same‐sex groups of two or three in individually ventilated cages, in temperature (19–24°C) and humidity (55 ± 15%) controlled rooms with a reversed 12:12‐h day‐night cycle (lights on 7 p.m.–7 a.m.). Ad libitum water and food was available for the duration of the study.
TABLE 1.
Distribution of sex and condition in experimental litters
| NP | TC | UC | ||||
|---|---|---|---|---|---|---|
| Litter | f | m | f | m | f | m |
| 1 | 1 | 2 | 2 | 1 | ||
| 2 | 2 | 1 | 1 | 2 | ||
| 3 | 2 | 2 | ||||
| 4 | 2 | 2 | 0 | 1 | ||
| 5 | 2 | 2 | ||||
| 6 | 2 | 2 | 2 | 2 | ||
| 7 | 1 | 2 | 2 | 1 | ||
| 8 | 3 | 5 |
Abbreviations: f, female; m, male; NP, needle prick; TC, tactile control; UC, undisturbed control.
All experiments were performed in accordance with the European Directive for the Protection of Vertebrate Animal Use for Experimental and Other Scientific Purposes (2010/63/EU) and were approved by the Committee for Experiments on Animals, Maastricht, The Netherlands (DEC 2017‐017).
2.2. Neonatal procedures and mechanical sensitivity
A neonatal repetitive NP model was used as previously described (Knaepen et al., 2013; van den Hoogen et al., 2020). All pups were randomly assigned to neonatal conditions at birth (using a computer‐generated randomization list). Pups received either noxious NPs (n = 9 males, n = 8 females) or tactile stimulations (tactile control (TC); n = 7 males, n = 7 females) in the midplantar surface of the left hind paw four times a day (at 08:00, 09:00, 10:00, and 11:00 a.m.), from day of birth (postnatal day (P0) to P7. For each procedure, the nest was briefly separated from the dam. Paw withdrawal thresholds (PWT) of the ipsilateral and contralateral hind paws were assessed before (baseline, BL) and 1, 3, and 5 h after the last noxious or tactile stimulation using a dorsal von Frey design (Marsh et al., 1999). Ascending Von Frey filaments (bending force 0.407, 0.692, 1.202, 2.041, 3.63 (from P4 onwards) and 5.495 (from P6 onwards)) were applied five times to the dorsal surface of the hind paws. The number of positive responses (withdrawal or flinching behavior evoked by the filaments) were recorded, and behavioral testing was discontinued when five positive responses were observed. A 50% PWT was calculated using sigmoidal curve fitting in GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA). Separate nests were left undisturbed during the first postnatal week (undisturbed control (UC); n = 8 males, n = 8 females).
2.3. Assessment of mechanical sensitivity throughout development
Mechanical sensitivity of the hind paws was assessed weekly from weaning to adulthood (3–8 weeks of age; P21–P56). Animals were placed in individual Plexiglas cages on an elevated mesh floor and were allowed to acclimatize to the behavioral set‐up before testing. Von Frey filaments with logarithmically increasing stiffness (bending forces 1.202, 2.041, 3.63, 5.495, 8.511, 15.136 and 28.84 g; Stoelting, USA) were applied to the mid‐plantar surface of the hind paws for 5 s. Mechanical sensitivity was assessed using the up‐down method, and the mechanical force resulting in a 50% withdrawal frequency was assigned as the PWT (Chaplan et al., 1994).
2.4. Adult anxiety behavior testing
Anxiety‐like behavior was assessed in adulthood (8 weeks of age) under dim light conditions (2 lux, 0.02 W/m3 infrared light). Animals were allowed to acclimatize to the experimental room before testing. To control for possible carry‐over effects from the OFT to the EZM, the animals were allowed a recovery period of 3 days. The animal order of testing was randomized, and researchers were blinded to conditions during testing.
2.5. Trait anxiety in the OFT
To assess trait anxiety, animals were introduced into the center of an open arena (100 × 100 × 40 cm) and allowed to freely explore for 5 min. In‐between each session, the arena was thoroughly cleaned with 70% ethanol. Behavior of the rats was automatically tracked and analyzed using a video tracking system (Ethovision Pro, Noldus, the Netherlands). While in the arena, time spend in the center (%), center crossings (number of entries from and to the center), and total distance travelled (locomotor path in whole arena in cm) were recorded and analyzed using Ethovision XT10 (Noldus).
2.6. State anxiety in the EZM
Adult animals were exposed to the EZM (Shepherd et al., 1994) to assess state anxiety. The EZM is an elevated black annular arena (100 cm diameter, 10 cm path width and 70 cm above floor level) divided into four equal quadrants. Two opposite quadrants are “closed” by black Perspex walls (closed arms), while the remaining two quadrants are “open” (open arms). Animals were placed in the middle of an open arm facing one of the closed arms and allowed to explore the maze for 5 min. Between each session, the arena was thoroughly cleaned with 70% ethanol. Movement of the rats was recorded and scored using an infrared video camera connected to a video tracking system (Ethovision Pro, Noldus). Behavioral measures recorded included: time spend in open and closed arms, latency to enter each area, and open arm entries. Anxiety‐like behavior was classified as the time in the open arms as a percentage of total (corrected) trial time (= total trial duration – latency to enter first closed arm). In addition, head dips (paws in closed arm, head stretched forward into open arm) were hand scored by an experimenter, blinded to the animal's condition.
2.7. Statistical analysis
All data is presented as mean ± standard error of the mean (SEM). For the neonatal period, an area under the curve (AUC) analysis was performed. The AUC was calculated based on the PWTs for each group over the whole neonatal period (P0–7) and TC and NP animals were compared with an unpaired t‐test. Differences in mechanical sensitivity between TC, NP, and UC throughout development were analyzed using a repeated measures analysis of variance (ANOVA) with Tukey post‐hoc correction (to control for multiple testing). As litters can affect anxiety‐like behavioral measures, litter was used as unit of analysis, averaging data from pups of the same condition per sex for each litter (Chapman & Stern, 1979; Lazic, 2012). An additional analysis was performed using pups as unit of analysis (see supplemental data). A two‐way ANOVA was performed to compare the effects of condition and sex on anxiety‐like behavioral measurements. If sex effects were not observed, data were pooled by condition to increase power. All statistical analysis were conducted using Graphpad Prism 8.0 (GraphPad Software) and results were considered significant at p < 0.05.
3. RESULTS
3.1. Mechanical sensitivity throughout development
NP animals showed a significantly decreased AUC for ipsilateral PWTs during the entire neonatal period compared with TC animals, indicating the development of acute mechanical hypersensitivity in pups who received repetitive needle pricks (t (29) = 8.038; p < 0.001; Figure 1(a)). During development from weaning (P21) to adulthood (P56), ipsilateral PWT significantly increased over time (F (5,220) = 52.66; p < 0.0001), but did not differ between neonatal conditions (F(2,44) = 0.254; p = 0.777); Figure 1(b)). Contralateral PWTs significantly increased over time (F(5, 220) = 52.58; p < 0.01), but were not significantly different between conditions at any time point during the neonatal phase (t(29) = 0.8311; p = 0.4127) or development (F(2,44) = 0.2538; p = 0.777; Figure 2). No effect of sex on mechanical sensitivity was observed (p > 0.05).
FIGURE 1.
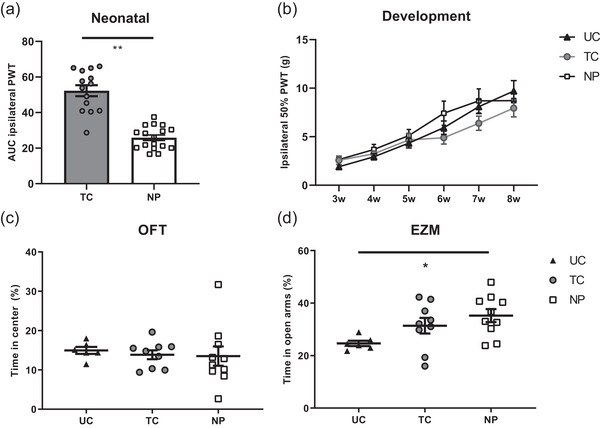
Mechanical sensitivity (a and b) and anxiety behavior (c and d) after repetitive neonatal needle pricking. (a) Repetitive neonatal needle pricks (NP; n = 17) result in a decreased paw‐withdrawal threshold (PWT) in the ipsilateral paw as compared with the repetitive tactile stimulation (TC; n = 14), shown by a lower area under the curve (AUC) over the whole neonatal period for NP animals compared with the TC animals (t(29) = 8.038; p < 0.001). (b) Ipsilateral PWTs increase over time for all groups (F(2,220) = 52.66; p < 0.01), did not significantly differ between NP, TC, and undisturbed control (UC; n = 16) animals (F(2,44) = 0.254; p = 0.777). (c) The percentage of time spend in the anxio‐genic (center) region of the open field test (OFT) in 8‐week‐old animals did not significantly differ between neonatal conditions (F(2,19) = 0.1206; p = 0.8871) or sex (F(1,19) = 0.2284; p = 0.6382). (d) The percentage of time spend in the anxio‐genic (open arms) region of the elevated zero maze (EZM) differs significantly between neonatal conditions (F(2,19) = 3.966; p = 0.0364) but not sex (F(1,19) = 0.0001; p = 0.9997). NP animals spend more time in the open arms of the elevated zero maze as compared with the UC animals (NP 35.21 ± 2.47; UD 24.63 ± 1.02; p = 0.0286). Data are presented as mean ± SEM, *p < 0.05; **p < 0.01
FIGURE 2.
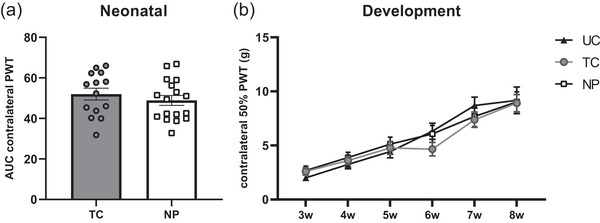
Contralateral mechanical sensitivity during the neonatal week and throughout development. (a) Area under the curve analysis (AUC) over the whole neonatal period does not show significant differences in contralateral paw‐withdrawal thresholds (PWT) between repetitive needle pricking (NP; n = 17) and tactile control animals (TCs; n = 14) (t(29) = 0.831; p = 0.4127). (b) Contralateral PWTs increase over time for all groups (F(5,220) = 52,58; p < 0.01) but was not significantly different between conditions at any time point (F(2,44) = 0.2538; p = 0.777). Data presented as mean ± SEM, *p < 0.05; **p < 0.01
3.2. Adult anxiety behavior
Trait anxiety as assessed in the OFT in adulthood showed no significant effect of condition (F(2, 19) = 0.3218; p = 0.7287; Figure 1(c)) or sex (F(1,19) = 0.2284; p = 0.6382; Figure 1(c)) on percentage of time spend in the center of the arena. The frequency of center crossings in the OFT showed a significant interaction effect for sex × condition (F(2,19) = 4.194; p = 0.0310; Figure 3), with females showing a trend to more center crossings in the TC group only (p = 0.060). Locomotor activity was affected by sex (F(1,19) = 6.701; p = 0.0180) but not condition (F(2,19) = 0.8271; p = 0.4625), with females showing higher locomotor activity as compared with the males (Figure 3).
FIGURE 3.
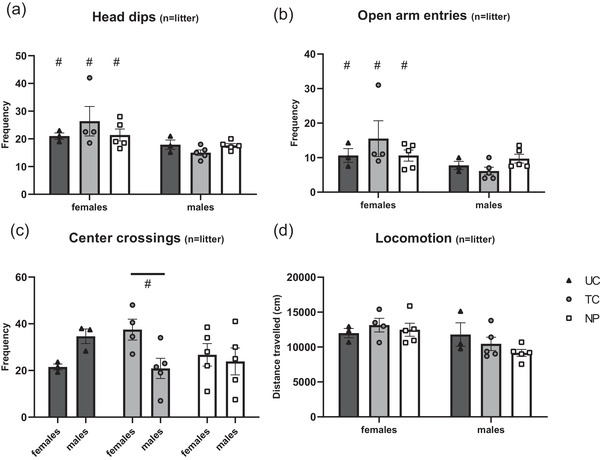
Exploratory behavior (head dips, open arm entries, and center crossings) and locomotor behavior of adult rats exposed to repetitive needle pricking, tactile stimulation, or left undisturbed as neonates. Females show significantly more exploratory behavior as compared with the males, measured by the frequency of head dips (a; F(1,19) = 8.597; p < 0.01) and open arm entries (b; F(1,19) = 4.683; p = 0.0434). (c) A significant interaction effect is observed in the frequency of center crossings (F(2,19) = 4,194; p = 0.0310), with females tending to cross the center more frequently as compared with the males in the TC group only (p = 0.06). (d) Locomotor activity in the open field test (OFT) was affected by sex (F(1,19) = 6.701; p = 0.0180) but not condition (F(2,19) = 0.8271; p = 0.4525), with females show significantly higher total distance travelled as compared with the males. NP, needle prick animals; TC, tactile controls; UC, undisturbed controls. Data presented as mean ± SEM, *p < 0.05; **p < 0.01; # sign. effect of males versus females
State anxiety as assessed in EZM demonstrates that the percentage of time spend in the open arms significantly differed between groups (F(2,19) = 3.966; p = 0.0364; Figure 1(d)) but was unaffected by sex (F(1,19) = 0.0001; p = 0.9997). When pooling data by condition, the significant difference between neonatal conditions in the time in open arms of the EZM persisted (F(2,22) = 3.777; p = 0.0389). Posthoc analysis revealed that 8‐week‐old NP animals spend significantly more time in the open arms of the EZM, compared with the UC (NP 35.21 ± 2.47; UC 24.63 ± 1.02; p = 0.0303; Figure 1(d)). Neonatal condition did not influence exploratory behavior (head dips and open arm entries) in the EZM (p > 0.05). However, females showed significantly more exploratory behavior, measured by the number of head dips (F(1,19) = 8.597; p < 0.01) and open arm entries (F(1,19) = 4.683; p = 0.0434; Figure 3). Using pups as unit of analysis (i.e., not averaging data of pups from the same condition per sex for each litter) did not influence behavioral outcome in the OFT and EZM (see Figure S1).
4. DISCUSSION
This study investigates the long‐term effect of neonatal procedural pain on adult state and trait anxiety behavior in a well‐established animal model of neonatal repetitive needle pricking. Our data show that a combination of repetitive neonatal procedural pain and handling decreases state, but not trait anxiety behavior in adult rats. At the same time, neonatal needle pricking results in acute mechanical hypersensitivity but does not produce long‐term changes in baseline mechanical sensitivity.
Consistent with previous studies, limited numbers of needle pricks from P0 to P7 leads to robust acute hypersensitivity without altering mechanical sensitivity throughout development (Knaepen et al., 2012; Knaepen et al., 2013; Ranger et al., 2018; van den Hoogen et al., 2020; Van den Hoogen et al., 2016). However, more widespread (all paws), prolonged (14 vs. 7 days), or more intense (paw incision or 10 needle pricks per day) tissue breaking procedures lead to altered baseline sensitivity (Carmo et al., 2016; Chen et al., 2016; Nuseir et al., 2017).
Whether neonatal procedural pain (“first‐hit”) affects anxiety in later life has been topic of debate. Previously, neonatal repetitive procedural pain has been shown to increase (Davis et al., 2018; Schellinck et al., 2003), decrease (Anand et al., 1999; Chen et al., 2016; Page et al., 2005; Zuke et al., 2019) or fail to show an effect on adult anxiety behavior in rodents (Anand et al., 1999; Mooney‐Leber & Brummelte, 2020; Ranger et al., 2018). The effect of neonatal repetitive procedural pain on anxiety seems to be dependent on the behavioral essay used, pointing at a distinct profile of anxiety (trait or state) affected. Rodent anxiety behavior in the OFT reflects the natural balance between exploratory and escaping drives, and therefore more likely represents inherent or trait anxiety (Hawley et al., 2011; Prut & Belzung, 2003). Our study shows that four needle pricks a day between P0 and P7 as a model of procedural neonatal pain does not influence trait anxiety, including time in the center of the arena and number of center crossings in the OFT, consistent with previous studies (Mooney‐Leber & Brummelte, 2020; Ranger et al., 2018). The open‐field entries from PVC tubes, or the time in the center of a circular arena was also unaffected by neonatal procedural pain (Anand et al., 1999; Low & Fitzgerald, 2012), suggesting that neonatal procedural pain does not influence trait anxiety in adulthood. Reduced exploratory behavior might confound measures of anxiety, as they depend on general locomotor activity (i.e., movement) (Ohl, 2003). However, locomotor activity was unaffected by neonatal condition in our study and did therefore not influence overall exploratory behavior and thus state anxiety in the OFT.
In contrast to the OFT, the EZM confronts the animal with an anxiety‐provoking situation such as height and avoidance of open spaces, modeling state‐induced anxiety (Goes et al., 2009). Adult animals exposed to a combination of repetitive neonatal needle pricking and handling showed lower state anxiety levels, as they spend more time in the open arms of the EZM as compared with their UCs in this study. Previous behavioral testing paradigms and handling can influence behavior in the EZM (Bailey & Crawley, 2009). As all animals were exposed to similar testing protocols and handling, this effect should be similar across all groups. Previous studies using the same neonatal procedural pain model have also reported decreased state anxiety in the elevated plus maze (EPM) as compared with the touched controls or irregularly stimulated animals (Page et al., 2005; Zuke et al., 2019). One study that increased the number of needle pricks per day did not show a long‐term effect on anxiety in the EPM, compared with the touched or handled controls (Ranger et al., 2018). In addition, repetitive needle pricking led to an increase in anxiety behavior when tested during adolescence (Schellinck et al., 2003). Therefore, inconsistencies in the manipulation procedure (4 vs. 10 NP daily), the developmental stage during testing (adolescent vs. adult), control groups used (touch, handling or undisturbed) as well as differences in behavioral essay used might account for the different outcomes (Table 2). Neonatal procedural pain has also been shown to reduce later‐life fear conditioning (Davis et al., 2018), suggesting that our findings are most consistent with a reduction in state anxiety in adult rodents.
TABLE 2.
Summary of literature on the effect of neonatal procedural pain on adult anxiety
| Model | Controls | Species | Time of modulation | Time of testing | Behavioral test | Outcome a | References |
|---|---|---|---|---|---|---|---|
| RNP (4 NPs in left hind paw) |
TC, UC |
Rats | P0–P7 | Adult (P56) | OFT, EZM |
↑ time in open arms No effect on open arm entries, head dips, time in center and center crossings |
This study |
| RNP (4 NPs balanced over all paws) | TC, UC | Rats | P0–P7 | Adult (P100) | OFT, EPM |
No effect on time in center or center entries ↑ time in open arms |
Page et al. (2005) |
| RNP (10 NPs balanced over all paws) | TC, handling | Mice | P1–P6 | Adult (P60) | OFT, EPM | No effect on time spend in center or time in open arms | Ranger et al. (2014) |
| RNP (4 NPs in left hind paw) | TC, UC | Rats | P1–P7 | P24–P25 | EPM |
↑ time in open arms No effect on (open) arm entries |
Zuke et al. (2019) |
| RNP (2 NPs on front and hind paw) | Sham | Mice | P8–P14 | P30 | EPM |
↓ open arm entries ↓ time in open arms ↓ head dips ↑ stretch attend postures |
Schellinck et al. (2003) |
| RNP (4 NPs balanced over all paws) | TC | Rats | P0–P7 | P24, P87 | OFT |
↑ distance in center at P24 and P86 ↑ center entries at P24 ↑ time in center at P24 and P87 ↑ total entries at P87 |
Chen et al. (2016) |
| RNP (8 NPs on P1, 6 NPs on P2, 4 NPs on P3, 2 pokes on P4, balanced over all paws) | TC, touch & isolation, UC | Rats | P1–P4 | Adult (P80) | OFT | No effect on distance travelled, time in center or center entries | Mooney‐Leber and Brummelte (2020) |
| RNP (4 NPs in left hind paw) | TC, UC | Rats | P1–P7 | P24, P45 and P66 | Fear conditioning | ↓ Auditory fear conditioning | Davis et al. (2018) |
| RNP (4 NPs, balanced over all paws) | TC | Rats | P0–P7 | Adult (P65) | DWT |
↑ latency to exit ↑ time in PVC tube No effect on open field entries |
Anand et al. (1999) |
NP animals versus control animals.
Abbreviations: DWT, defensive withdrawal testing; EPM, elevated plus maze; EZM, elevated zero maze; NPs, needle pricks; OFT, open field test; P, postnatal day; RNP, repetitive needle pricking; SD, Sprague‐Dawley; TC, tactile and handled control; UC, undisturbed or unhandled controls.
The lack of concordance between tests for trait and state anxiety has previously been shown, where animals presenting high levels of anxiety in one test did not necessarily present the same levels in another test (Goes et al., 2009). The combination of neonatal repetitive pain with a second “hit” by adding the additional factors of height and unprotected areas in the EZM, may unmask the lower anxiety levels in NP animals as compared with the UCs in this test. It is important to highlight that although the combination of repetitive neonatal procedural pain and handling reduces state anxiety as compared with the undisturbed animals in our study, no differences with TC animals are observed. This suggests that the effect is driven by handling and/or removal as well as pain rather than pain alone, and the combination results in differential state anxiety levels. Daily postnatal handling during the first two postnatal weeks also reduced anxiety behavior in adulthood as compared with the UC (Li et al., 2018). In addition, differences in rearing conditions between UC and NP animals may also play a role (Reshetnikov et al., 2020). In preclinical studies, a second “hit” is often necessary for the long‐term effects of neonatal pain to emerge (Williams & Lascelles, 2020). The impact of a subsequent injury in adulthood on anxiety level of rodents previously exposed to neonatal pain has not been evaluated yet. Contrary to preclinical findings showing lower state anxiety after repetitive needle pricking, the incidence of anxiety disorder is higher in children and adolescents born premature (Botting et al., 1997; Indredavik et al., 2004; Johnson et al., 2010; Walker et al., 2018). Patients with clinical anxiety tend to possess greater anxious traits in comparison with the healthy subjects, whereas state anxiety is not related to anxiety disorders (Kennedy et al., 2001). Hence, the effects of neonatal procedural pain may therefore not always emerge in clinical context. NICU admittance alone is already stressful, but the added neonatal pain can strengthen these effects. Overall, NICU infants appear to be surprisingly robust and adaptable; effects of neonatal pain exposure on later‐life behavioral adversity are subtle rather than extreme, although the effects of pain remain difficult to discriminate from anxiety or fear in a clinical setting. In this context, follow‐up studies should elucidate whether differences in trait and state anxiety exist in ex‐preterm patients in later life. In conclusion, our study shows that in a rodent model of repetitive needle pricking, early‐life procedural pain (including repetitive handling and removal) decreases state but not trait anxiety behavior in later life. On top of adequate analgesia, treatment of neonatal procedural pain should therefore take these adverse effects into account.
AUTHOR CONTRIBUTION
A. R. d. K., E. A. J., and N. v. d. H. designed and conceptualized the study and contributed to its structure and content. A. R. d. K. collected and analyzed the data. A. R. d. K. drafted the manuscript and figures. All authors commented on previous versions of the manuscript, critically revised, and quality assessed the manuscript. All authors have read and approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors have no conflict of interests to declare.
FUNDING
A. R. d. K. is financially supported by the Pain Knowledge Center from Maastricht and an institutional grant from University Maastricht, School Mental Health and Neuroscience.
Supporting information
Supporting Information
de Kort, A. R. , Joosten, E. A. , Patijn, J. , Tibboel, D. , & van den Hoogen, N. J. (2021). Neonatal procedural pain affects state, but not trait anxiety behavior in adult rats. Developmental Psychobiology, 63, e22210. 10.1002/dev.22210
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Anand, K. , Coskun, V. , Thrivikraman, K. , Nemeroff, C. , & Plotsky, P. (1999). Long‐term behavioral effects of repetitive pain in neonatal rat pups. Physiology & Behavior, 66(4), 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, K. R. , & Crawley, J. N. (2009). Anxiety‐related behaviors in mice. Boca Raton FL: CRC Press/ Taylor & Francis. [PubMed] [Google Scholar]
- Botting, N. , Powls, A. , Cooke, R. W. , & Marlow, N. (1997). Attention deficit hyperactivity disorders and other psychiatric outcomes in very low birthweight children at 12 years. Journal of Child Psychology and Psychiatry, 38(8), 931–941. [DOI] [PubMed] [Google Scholar]
- Brummelte, S. , Grunau, R. E. , Chau, V. , Poskitt, K. J. , Brant, R. , Vinall, J. , Gover, A. , Synnes, A. R. , , & Miller, S. P. (2012). Procedural pain and brain development in premature newborns. Annals of Neurology, 71(3), 385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo, E. d. C. d. , Sanada, L. S. , Machado, N. L. B. , & Fazan, V. P. S. (2016). Does pain in the neonatal period influence motor and sensory functions in a similar way for males and females during post‐natal development in rats? Pain Medicine, 17(8), 1520–1529. [DOI] [PubMed] [Google Scholar]
- Chaplan, S. R. , Bach, F. , Pogrel, J. , Chung, J. , & Yaksh, T. (1994). Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods, 53(1), 55–63. [DOI] [PubMed] [Google Scholar]
- Chapman, R. H. , & Stern, J. M. (1979). Failure of severe maternal stress or ACTH during pregnancy to affect emotionality of male rat offspring: Implications of litter effects for prenatal studies. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology, 12(3), 255–267. [DOI] [PubMed] [Google Scholar]
- Chau, C. M. , Ranger, M. , Bichin, M. , Park, M. T. M. , Amaral, R. S. , Chakravarty, M. , Poskitt, K. , Synnes, A. R. , Miller, S. P. , & Grunau, R. E. (2019). Hippocampus, amygdala, and thalamus volumes in very preterm children at 8 years: Neonatal pain and genetic variation. Frontiers in Behavioral Neuroscience, 13, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, M. , Xia, D. , Min, C. , Zhao, X. , Chen, Y. , Liu, L. , & Li, X. (2016). Neonatal repetitive pain in rats leads to impaired spatial learning and dysregulated hypothalamic‐pituitary‐adrenal axis function in later life. Scientific Reports, 6, 39159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cismaru, A. L. , Gui, L. , Vasung, L. , Lejeune, F. , Barisnikov, K. , Truttmann, A. , Tolsa, T. B. , & Hüppi, P. S. (2016). Altered amygdala development and fear processing in prematurely born infants. Frontiers in Neuroanatomy, 10, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz, M. , Fernandes, A. , & Oliveira, C. (2016). Epidemiology of painful procedures performed in neonates: A systematic review of observational studies. European Journal of Pain, 20(4), 489–498. [DOI] [PubMed] [Google Scholar]
- Davis, S. M. , Rice, M. , Rudlong, J. , Eaton, V. , King, T. , & Burman, M. A. (2018). Neonatal pain and stress disrupts later‐life Pavlovian fear conditioning and sensory function in rats: Evidence for a two‐hit model. Developmental Psychobiology, 60(5), 520–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden, E. G. , Grunau, R. E. , Guo, T. , Foong, J. , Pearson, A. , Au‐Young, S. , Lavoie, R. , Chakravarty, M. M. , Chau, V. , Synnes, A. , & Synnes, A. (2018). Early procedural pain is associated with regionally‐specific alterations in thalamic development in preterm neonates. Journal of Neuroscience, 38(4), 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goes, T. C. , Antunes, F. D. , & Teixeira‐Silva, F. (2009). Trait and state anxiety in animal models: Is there correlation? Neuroscience Letters, 450(3), 266–269. [DOI] [PubMed] [Google Scholar]
- Grunau, R. E. , Oberlander, T. F. , Whitfield, M. F. , Fitzgerald, C. , Morison, S. J. , & Saul, J. P. (2001). Pain reactivity in former extremely low birth weight infants at corrected age 8 months compared with term born controls. Infant Behavior and Development, 24(1), 41–55. [Google Scholar]
- Hawley, W. R. , Grissom, E. M. , & Dohanich, G. P. (2011). The relationships between trait anxiety, place recognition memory, and learning strategy. Behavioural Brain Research, 216(2), 525–530. [DOI] [PubMed] [Google Scholar]
- Hayes, B. , & Sharif, F. (2009). Behavioural and emotional outcome of very low birth weight infants–literature review. The Journal of Maternal‐Fetal & Neonatal Medicine, 22(10), 849–856. [DOI] [PubMed] [Google Scholar]
- Indredavik, M. , Vik, T. , Heyerdahl, S. , Kulseng, S. , Fayers, P. , & Brubakk, A. (2004). Psychiatric symptoms and disorders in adolescents with low birth weight. Archives of Disease in Childhood‐Fetal and Neonatal Edition, 89(5), F445‐F450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, S. , Hollis, C. , Kochhar, P. , Hennessy, E. , Wolke, D. , & Marlow, N. (2010). Psychiatric disorders in extremely preterm children: Longitudinal finding at age 11 years in the EPICure study. Journal of the American Academy of Child & Adolescent Psychiatry, 49(5), 453–463. e451. [PubMed] [Google Scholar]
- Kennedy, B. L. , Schwab, J. J. , Morris, R. L. , & Beldia, G. (2001). Assessment of state and trait anxiety in subjects with anxiety and depressive disorders. Psychiatric Quarterly, 72(3), 263–276. [DOI] [PubMed] [Google Scholar]
- Knaepen, L. , Patijn, J. , Tibboel, D. , & Joosten, E. (2012). Sex differences in inflammatory mechanical hypersensitivity in later life of rats exposed to repetitive needle pricking as neonates. Neuroscience Letters, 516(2), 285–289. [DOI] [PubMed] [Google Scholar]
- Knaepen, L. , Patijn, J. , van Kleef, M. , Mulder, M. , Tibboel, D. , & Joosten, E. A. (2013). Neonatal repetitive needle pricking: Plasticity of the spinal nociceptive circuit and extended postoperative pain in later life. Developmental Neurobiology, 73(1), 85–97. [DOI] [PubMed] [Google Scholar]
- Lazic, S. E. (2012). Translational neuroscience requires better design and analysis of preclinical studies. Nature Precedings, 1‐13. [Google Scholar]
- Li, H. , Ishikawa, C. , & Shiga, T. (2018). Effects of postnatal handling on adult behavior and brain mRNA expression of serotonin receptor, brain‐derived neurotrophic factor and GABA‐A receptor subunit. International Journal of Developmental Neuroscience, 68, 17–25. [DOI] [PubMed] [Google Scholar]
- Low, L. A. , & Fitzgerald, M. (2012). Acute pain and a motivational pathway in adult rats: Influence of early life pain experience. PLoS One, 7(3), e34316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, D. , Dickenson, A. , Hatch, D. , & Fitzgerald, M. (1999). Epidural opioid analgesia in infant rats I: Mechanical and heat responses. Pain, 82(1), 23–32. [DOI] [PubMed] [Google Scholar]
- Mooney‐Leber, S. M. , & Brummelte, S. (2020). Neonatal pain and reduced maternal care alter adult behavior and hypothalamic–pituitary–adrenal axis reactivity in a sex‐specific manner. Developmental Psychobiology, 62(5), 631–643. [DOI] [PubMed] [Google Scholar]
- Nuseir, K. Q. , Alzoubi, K. H. , Alhusban, A. , Bawaane, A. , Al‐Azzani, M. , & Khabour, O. F. (2017). Sucrose and naltrexone prevent increased pain sensitivity and impaired long‐term memory induced by repetitive neonatal noxious stimulation: Role of BDNF and β‐endorphin. Physiology & Behavior, 179, 213–219. [DOI] [PubMed] [Google Scholar]
- Ohl, F. (2003). Testing for anxiety. Clinical Neuroscience Research, 3(4‐5), 233–238. [Google Scholar]
- Page, G. G. , Blakely, W. P. , & Kim, M. (2005). The impact of early repeated pain experiences on stress responsiveness and emotionality at maturity in rats. Brain, Behavior, and Immunity, 19(1), 78–87. [DOI] [PubMed] [Google Scholar]
- Peters, J. W. , Schouw, R. , Anand, K. J. , van Dijk, M. , Duivenvoorden, H. J. , & Tibboel, D. (2005). Does neonatal surgery lead to increased pain sensitivity in later childhood? Pain, 114(3), 444–454. [DOI] [PubMed] [Google Scholar]
- Prut, L. , & Belzung, C. (2003). The open field as a paradigm to measure the effects of drugs on anxiety‐like behaviors: A review. European Journal of Pharmacology, 463(1‐3), 3–33. [DOI] [PubMed] [Google Scholar]
- Ranger, M. , Synnes, A. , Vinall, J. , & Grunau, R. (2014). Internalizing behaviours in school‐age children born very preterm are predicted by neonatal pain and morphine exposure. European Journal of Pain, 18(6), 844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranger, M. , Tremblay, S. , Chau, C. M. Y. , Holsti, L. , Grunau, R. E. , & Goldowitz, D. (2018). Adverse behavioral changes in adult mice following neonatal repeated exposure to pain and sucrose. Frontiers in Psychology, 9, 2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshetnikov, V. V. , Ryabushkina, Y. A. , & Bondar, N. P. (2020). Impact of mothers’ experience and early‐life stress on aggression and cognition in adult male mice. Developmental Psychobiology, 62(1), 36–49. [DOI] [PubMed] [Google Scholar]
- Schellinck, H. M. , Stanford, L. , & Darrah, M. (2003). Repetitive acute pain in infancy increases anxiety but does not alter spatial learning ability in juvenile mice. Behavioural Brain Research, 142(1‐2), 157–165. [DOI] [PubMed] [Google Scholar]
- Shepherd, J. K. , Grewal, S. S. , Fletcher, A. , Bill, D. J. , & Dourish, C. T. (1994). Behavioural and pharmacological characterisation of the elevated “zero‐maze” as an animal model of anxiety. Psychopharmacology, 116(1), 56–64. [DOI] [PubMed] [Google Scholar]
- van den Hoogen, N. J. , Patijn, J. , Tibboel, D. , & Joosten, E. A. (2020). Repetitive noxious stimuli during early development affect acute and long‐term mechanical sensitivity in rats. Pediatric Research, 87(1), 26–31. [DOI] [PubMed] [Google Scholar]
- Van den Hoogen, N. , Tibboel, D. , Honig, W. , Hermes, D. , Patijn, J. , & Joosten, E. (2016). Neonatal paracetamol treatment reduces long‐term nociceptive behaviour after neonatal procedural pain in rats. European Journal of Pain, 20(8), 1309–1318. [DOI] [PubMed] [Google Scholar]
- Walker, S. , Melbourne, A. , O'Reilly, H. , Beckmann, J. , Eaton‐Rosen, Z. , Ourselin, S. , & Marlow, N. (2018). Somatosensory function and pain in extremely preterm young adults from the UK EPICure cohort: Sex‐dependent differences and impact of neonatal surgery. British Journal of Anaesthesia, 121(3), 623–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, M. D. , & Lascelles, B. D. X. (2020). Early neonatal pain—A review of clinical and experimental implications on painful conditions later in life. Frontiers in Pediatrics, 8, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuke, J. T. , Rice, M. , Rudlong, J. , Paquin, T. , Russo, E. , & Burman, M. A. (2019). The effects of acute neonatal pain on expression of corticotropin‐releasing hormone and juvenile anxiety in a rodent model. Eneuro, 6(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


