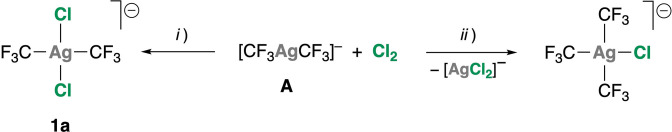Abstract
By using suitable synthetic procedures, we have first isolated the square‐planar organosilver(III) compounds [PPh4][trans‐(CF3)2AgX2] [X=Cl (1 a), Br (2 a)]. The geometry and stereochemistry of the chloro‐derivative 1 a have been unambiguously established by single‐crystal X‐ray diffraction (SC‐XRD) methods. Following our calculations on the relative stability of the cis‐/trans‐[(CF3)2AgX2]− couples (X=F, Cl, Br, I), the experimentally obtained compounds 1 a and 2 a appear to be kinetically favored stereoisomers. They display some tendency to associate an additional X− ligand affording rare five‐coordinate AgIII species [(CF3)2AgX3]2−. Interestingly, compound [PPh4]2[(CF3)2AgBr3] (3) has been identified by SC‐XRD methods as the first AgIII derivative with trigonal symmetry in general and trigonal bipyramidal geometry in particular. This unusual five‐coordinate species also exhibits inverted ligand field.
Keywords: axial acidity, five-coordination, highest oxidation states, inverted ligand field, silver(III)
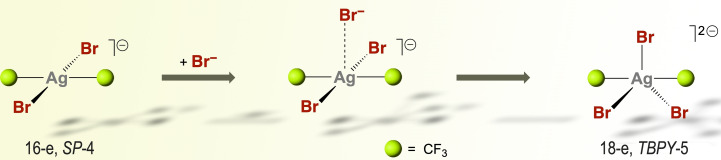
The square‐planar organosilver(III) complex [trans‐(CF3)2AgBr2]− (16 electron) undergoes a marked stereochemical change upon coordination of an additional Br− ligand. The resulting five‐coordinate [(CF3)2AgBr3]2− complex (18 electron) exhibits trigonal bipyramidal geometry, which is unprecedented for AgIII. This five‐coordinate compound also exhibits inverted ligand field.
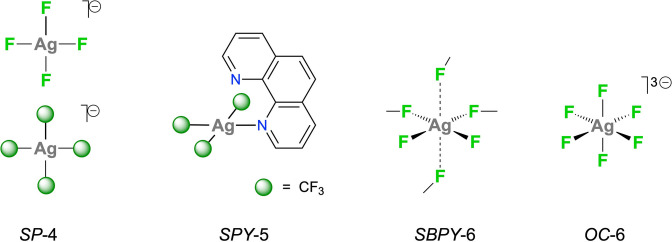
Oxidation state III is the highest currently available for silver.[ 1 , 2 ] Nearly all Ag iii compounds are diamagnetic and show square‐planar (SP‐4) geometry, as exemplified (Scheme 1) by the fluoro‐complex [3] [AgF4]− and by the homoleptic organometallic complexes [AgR4]− (R=CHF2, [4] CF3). [5] These prototypical compounds are stabilized by small monodentate ligands with no steric constraints that might bias the preferred geometry. [6] Different geometries are hardly ever found for this 4d8 ion, and the few departing cases are invariably based on the tetragonal symmetry (Scheme 1). In the square pyramidal (SPY‐5: τ<0.1)[ 7 , 8 ] structure of the neutral complex (CF3)3Ag(phen), the fairly long apical Ag⋅⋅⋅N separation (>240 pm) induces little distortion in the nearly SP‐4 basal plane; [9] the observed overall arrangement might well be favored by the rigid bidentate phen ligand. In the chain‐like structure of AgF3, the loose axial Ag⋅⋅⋅F interactions (254.0(4) pm) established between adjacent chains result in an elongated octahedral geometry, which can also be described as square bipyramidal (SBPY‐6). [10] Finally, a regular octahedral environment (OC‐6) for Ag iii is most certainly attained in the paramagnetic double perovskite Cs2K[AgF6], [11] which is isomorphous with the Cs2K[CuF6] homologue. [12]
Scheme 1.
Stereochemical patterns currently established for the Ag iii center.
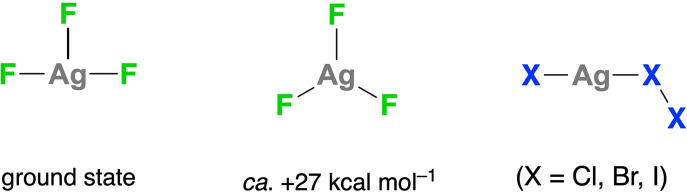
The structure of mononuclear AgF3 (Scheme 2) both in inert matrixes (IR spectroscopy) [13] and in the gas phase (calculated)[ 13 , 14 ] is again a square with a vacant site (T shape, C 2v ). A symmetric trigonal arrangement (D 3h ) is prevented by Jahn–Teller distortion. [14b] The structures calculated for the heavier‐halide AgX3 homologues (X=Cl, Br, I) are better described as XAg⋅X2 adducts (Scheme 2) involving reduction to Ag i . [14b] This tendency to undergo reduction explains why the vast majority of Ag iii compounds currently isolated are stabilized by hard ligands with first‐row donor atoms (C, N, F) [15] and why none of the heavier binary halides AgX3 or related [AgX4]− complexes have been prepared to date.
Scheme 2.
Calculated structures of the mononuclear AgF3 and AgX3 halides in the gas phase (X=Cl, Br, I), with the trigonal structure of AgF3 (D 3h ) lying +23 kcal mol−1 above the T‐shaped ground state (C 2v ). [14b]
In our effort to assay the stabilizing ability of the CF3 ligand, [16] we recently isolated the whole series of halide complexes [PPh4][(CF3)3AgX] (X=F, Cl, Br, I). [17] Now, we report on the remarkable tendency of the related [trans‐(CF3)2AgX2]− anions to associate an additional X− ligand to afford [(CF3)2AgX3]2− complexes, with the bromo‐derivative [PPh4]2[(CF3)2AgBr3] showing unprecedented trigonal geometry. The analysis of its electronic structure reveals this novel compound as a singular five‐coordinate species with inverted ligand field.
The slow addition of the homoleptic organosilver(i) compound [PPh4][CF3AgCF3] (A) [5c] dissolved in CH2Cl2 to a solution of Cl2 in CCl4/CH2Cl2 at −78 °C affords the oxidized compound [PPh4][trans‐(CF3)2AgCl2] (1 a) in nearly quantitative spectroscopic yield (19F NMR). In this process (Scheme 3), the order of addition of the reagents is crucial to avoid ligand rearrangement, which takes readily place if the oxidant is added onto A. [17] No such rearrangement processes were observed working with the homologous gold system. [18] Upon chlorination under the indicated conditions, the 19F NMR signal of the starting product A (δF=−25.6 ppm) [5a] is downfield shifted to δF=−24.54 ppm (Figure S4). More importantly, the 2 J(109Ag,19F) coupling constant undergoes a dramatic reduction from 100.7 Hz in A to 14.0 Hz in 1 a. [19] The very small value denotes both oxidation of the metal centre and a trans arrangement of the CF3 groups. Our spectroscopic parameters are in agreement with those reported by Eujen, Hoge and Brauer, who first observed complex 1 a in solution, formed upon reaction of [PPh4][trans‐(CF3)2Ag(CN)2] with AcCl. [20] Unfortunately, this reaction was so slow that decomposition processes and competing side‐reactions producing undesired by‐products could not be avoided. In turn, our simple and efficient procedure has enabled us to isolate compound 1 a as a thermally unstable orange solid. According to its colour, compound 1 a in Me2CO solution at −50 °C shows a characteristic absorption at λ=369 nm in the visible region of the optical spectrum (Figure S3). The composition of the anion is determined by the appropriate isotopic distribution of the nominal peak in MS and confirmed by high‐resolution mass spectrometry (HRMS): 314.8339 Da.
Scheme 3.
Different outcome of the reaction of A with Cl2 working under local excess of either Cl2 (i) or A (ii).
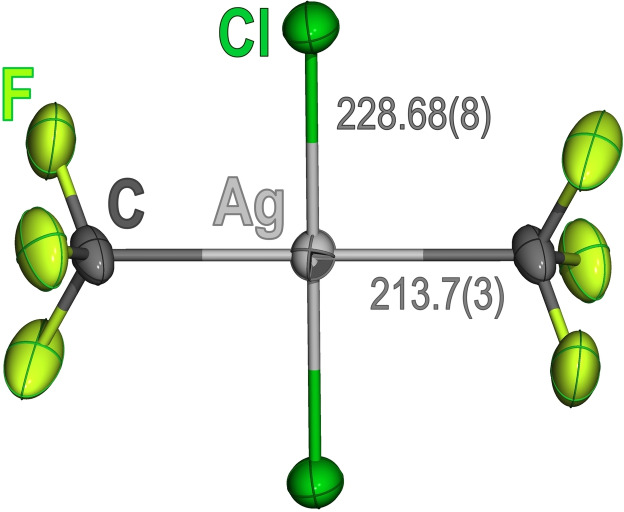
The stereochemistry of 1 a (Figure 1) was unambiguously established by single‐crystal X‐ray diffraction (SC‐XRD). [21] Since the Ag atom is located at an inversion center, the Cl‐Ag‐Cl and CF3‐Ag‐CF3 units are perfectly linear (imposed by symmetry). The Ag‐C distance, 213.7(3) pm, is in the longest edge found in organosilver(III) compounds, [17] being comparable to that found in the highly distorted porphyrinoid macrocyclic complex Ag{N3C}: 212.6(2) pm. [22] In line with this elongated Ag−C bond is the low value of 2 J(109Ag,19F) observed in solution. In contrast, the Ag‐Cl distance, 228.68(8) pm, is significantly shorter than in the only precedent described to date, namely [PPh4][(CF3)3AgCl]: 232.03(4) pm. [17] This difference evidences the marked trans influence of the CF3 ligand [23] operating in the latter compound. Accordingly, the ν(Ag‐Cl) band observed in the IR spectrum of 1 a (B1u: 386 cm−1) appears at higher frequency than found for [PPh4][(CF3)3AgCl] (A1: 348 cm−1). [17]
Figure 1.
Displacement‐ellipsoid diagram (50 % probability) of the [trans‐(CF3)2AgCl2]− anion as found in crystals of 1 a with interatomic Ag‐Cl and Ag‐C distances [pm] indicated. Only one set of the rotationally‐disordered F atoms is shown. [21]
The bromo‐derivative [PPh4][trans‐(CF3)2AgBr2] (2 a) was obtained by reaction of A with Br2 following a similar procedure as indicated above. It was isolated as a thermally unstable, dark orange solid characterized by an absorption at λ=405 nm in the visible region of the electronic absorption spectrum in Me2CO solution at −50 °C (Figure S3). The substantial red‐shift observed with respect to 1 a suggests that these absorptions are ligand‐to‐metal charge‐transfer (LMCT) bands associated with lone pairs (np) on the halide X ligands. The 19F NMR signal of 2 a (δF=−16.14 ppm) appears significantly deshielded with respect to 1 a. The similar coupling constant to the metal center, 2 J(109Ag,19F)=18.1 Hz, also points to a trans stereochemistry. [20] As in the previous case, the composition of the anion 2 a is determined by the appropriate isotopic distribution of the nominal peak in MS and confirmed by HRMS: 402.7322 Da.
No oxidation is observed by reaction of A with I2 under similar conditions and all our attempts to obtain the fluoro‐derivative [PPh4][trans‐(CF3)2AgF2] failed: By treating 1 a with AgF, massive reduction to silver metal occurred, whereas treatment of A with XeF2 in the solid state invariably resulted in explosion even at low temperatures. Nevertheless, the whole series of stereoisomers [trans‐(CF3)2AgX2]− (X=F, Cl, Br, I) were identified as local minima by DFT calculation (Figure S13). We also found that the isomeric species [cis‐(CF3)2AgX2]− were invariably more stable than their corresponding trans stereoisomers (Figure S14). The electronic structures of the [trans‐(CF3)2AgX2]− stereoisomers reveal ligand‐field inversion in all cases (Figure S15).[ 24 , 25 ] According to our calculations, our essays have led to the kinetically favored trans stereoisomers. Hence, we sought to promote isomerization to the thermodynamically favored cis stereoisomers. Owing to the low stability of compounds 1 a and 2 a, thermal activation was pointless. However, it was noticed that by redissolving freshly prepared solid samples of 2 a in Me2CO at −80 °C, a new signal appears in the 19F NMR spectra at δF=−18.25 ppm in minor ratio (1:20) with an associated 2 J(109Ag,19F)=52.96 Hz (Figure S5), which we tentatively assign to the stereoisomer [cis‐(CF3)2AgBr2]− (2 b). Both isomers decompose into BrCF3 and [CF3AgBr]− (Figure S10). Compound 1 a decomposed in a similar way (Figure S9), but in this case, we were not able to identify the corresponding cis stereoisomer 1 b. The decomposition of 1 a and 2 a/2 b in solution [Eq. 1] coincides with the main unimolecular fragmentation path observed in the gas phase by tandem mass spectrometry under collision‐induced (CID) conditions (Figures S11 and S12).
Isomerization in d8 square‐planar X2ML2 complexes is a thoroughly studied process. [26] In general, it occurs more readily with the heavier halides and is favored by the presence of Lewis bases. [26] However, the addition of Br− to solutions of 2 a did not result in the desired isomerization. In turn, a significant broadening of the 19F NMR signal suggested some kind of dynamic association (Figures S7 and S8). The effect is also observed, but less noticeable, on addition of Cl− to the chloro‐derivative 1 a in solution (Figure S6). Association of an additional ligand had been suggested for some AgIII complexes in solution, [27] and the only two structural evidences contain the tetradentate ethylenedibiguanide frame and are again based on a tetragonal symmetry. [28]
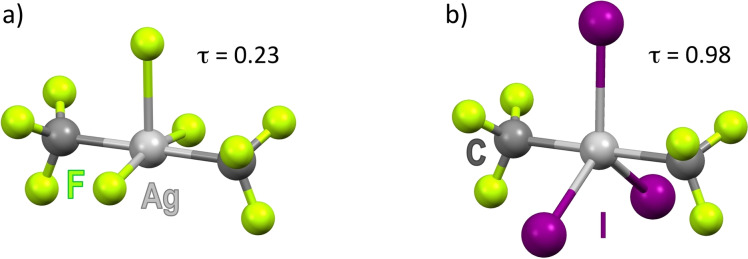
Aiming to find out the generality of the process, we have calculated the interaction of the whole series of [trans‐(CF3)2AgX2]− complexes (X=F, Cl, Br, I) with an additional X− ion by theoretical methods. Well‐defined five‐coordinate [(CF3)2AgX3]2− minima were located in all cases. The interaction is enthalpy‐favored but is roughly balanced by the adverse entropic factor implied in every association process (Table 1). The optimized geometry for the fluoro‐complex [(CF3)2AgF3]2− can be described as SPY‐5 (Figure 2 a), whereas the structures of the heavier homologues are all trigonal bipyramidal (TBPY‐5; Figures 2 b and S16). In order to ascertain the reasons underlying this structural duality, the energy impact of X‐Ag‐X bending to 120° and subsequent X− association were separately analyzed (Scheme S1). We found that the bending energy follows the sequence F> Cl > Br > I (Figure S17), [29] and that the Br ligand occupies a privileged position among the halogens (Table 1 and S1).
Table 1.
Energy involved in the interaction of [trans‐(CF3)2AgX2]− with an additional X− ligand in the indicated solvent.[a]
|
|
X=F |
X=Cl |
X=Br |
X=I |
||||
|---|---|---|---|---|---|---|---|---|
|
solvent |
ΔG |
ΔH |
ΔG |
ΔH |
ΔG |
ΔH |
ΔG |
ΔH |
|
Me2CO |
4.4 |
−3.2 |
1.6 |
−7.5 |
−1.2 |
−9.5 |
−0.4 |
−8.0 |
|
MeCN |
4.1 |
−4.8 |
0.3 |
−8.7 |
−2.4 |
−10.7 |
−1.0 |
−9.2 |
[a] Values [kcal mol−1] calculated at the DFT/M06/Def2‐TZVPD level of theory.
Figure 2.
Geometry of the anions [(CF3)2AgF3]2− (a) and [(CF3)2AgI3]2− (b) calculated in MeCN solution at the DFT/M06/Def2‐TZVPD level. The whole set of [(CF3)2AgX3]2− anions is shown in Figure S16.
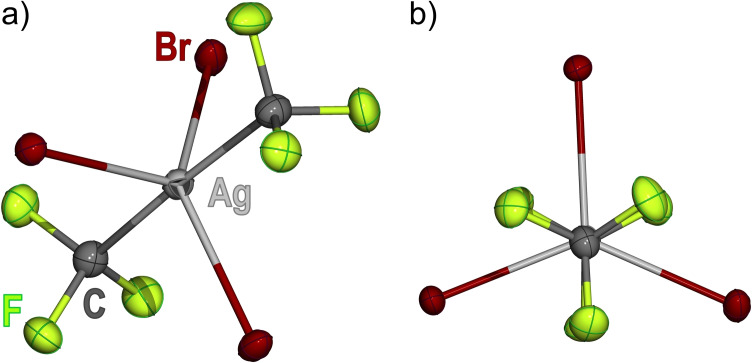
We were fortunate enough to obtain good crystals of the addition compound [PPh4]2[(CF3)2AgBr3] (3), which was unambiguously characterized by SC‐XRD methods. [21] The anion of 3 (Figure 3) is gratifyingly similar to our calculation (Figure S16), although less regular. The CF3 groups are located in the axial sites with virtual linear arrangement: C‐Ag‐C 176.72(16)°. The equatorial sites are occupied by the three Br ligands in a nearly perfect planar disposition together with the metal center (highest deviation: 1.07(3) pm). The axial C‐Ag‐C axis deviates <1° from the normal to the equatorial plane. One of the Br‐Ag‐Br angles is wider (134.277(16)°) than the other two: 109.379(15)° and 116.331(15)°. Although this deviation results in lowering of the τ geometric descriptor from the ideal 1 value to τ=0.71,[ 7 , 8 ] the trigonal arrangement around the metal is undeniable. The Ag‐C distances, 2.077(4) and 2.092(4) pm, are comparable to those observed in the homoleptic compound [PPh4][Ag(CF3)4]: 209.8(2) pm. [5c] The Ag−C bonds in 3 are actually shorter than in the square‐planar complex 1 a. The Ag‐Br distances (255.86(4), 256.69(5) and 265.16(4) pm) are all longer than that found in the square‐planar complex [PPh4][(CF3)3AgBr]: 246.25(2) pm. [17] We would like to stress that five‐coordination in 3 is not sterically forced, since every ligand around the metal is monodentate. The overall geometry is surprisingly similar to that reported for the neutral gold(III) compound (Me3P)2AuI3, which exhibits a nearly regular TBPY‐5 geometry (τ=0.94). [30] To the best of our knowledge compound 3 is the first AgIII derivative with trigonal symmetry described to date.
Figure 3.
Displacement‐ellipsoid diagram (50 % probability) of the [(CF3)2AgBr3]2− anion as found in crystals of 3 (a) and its projection along the C‐Ag‐C axis (b). [21]
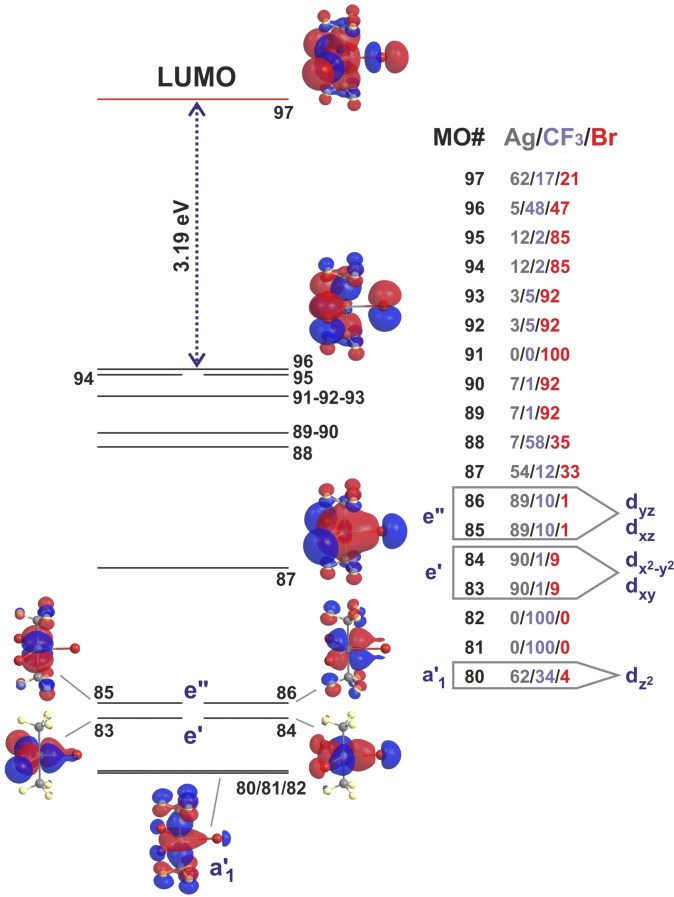
A detailed analysis of the electronic structure of the [(CF3)2AgBr3]2− anion under imposed D 3h symmetry (Figure 4) [31] reveals that the MOs with major metal contribution are well below the HOMO and inverted in order with respect to the standard arrangement derived from D 3h ligand‐field splitting. [32] Thus, the a1′ MO with mainly d character, which is usually the highest lying orbital of the d set, is here greatly stabilized. The significant contribution from the CF3 groups (34 %) indicates an important degree of covalency in the Ag‐CF3 bond. Slightly above lie the degenerate e′ (dxy, d ) and e′′ (dxz,dyz) pairs with roughly 10 % contribution from the ligands. The HOMO is, in turn, mainly contributed by the ligands. The observed electronic structure is characteristic of an inverted ligand field, which is extremely rare in five‐coordinate compounds.[ 24 , 33 ]
Figure 4.
Energy levels calculated for the symmetrised D 3h ‐[(CF3)2AgBr3]2− anion in the gas phase with indication of each moiety contribution (%) to the most relevant valence MOs. Depicted are only the frontier orbitals and those with a significant metal contribution. A full version is shown in Figure S19.
In summary, the thermally unstable organosilver(III) dihalide complexes [PPh4][trans‐(CF3)2AgX2] [X=Cl (1 a), Br (2 a)] exhibit substantial acidic (electrophilic) behavior, as they interact with additional X− ligands. The interaction is dynamic in solution (19F NMR). The structural characterization of [PPh4]2[(CF3)2AgBr3] (3) in the solid state (SC‐XRD) gives unambiguous experimental proof of direct Ag‐Br interaction. The trigonal structure of the [(CF3)2AgBr3]2− anion in compound 3 illustrates an unanticipated plasticity of the AgIII coordination environment, which was hitherto entirely based on the tetragonal symmetry. This five‐coordinate compound also exhibits inverted ligand field. The unusual electronic structure associated with an unprecedented structural change will certainly have important implications in the reactivity of silver(III), which is still underdeveloped.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information
Acknowledgements
This work was supported by the Spanish MICIU/FEDER (Project PGC2018‐094749‐B‐I00) and the Gobierno de Aragón (Grupo E17_20R). We are indebted to INMA researcher Dr. Rafael Cases for the use of valuable equipment. BIFI (Instituto de Biocomputación y Física de Sistemas Complejos) and CESGA (Centro de Supercomputación de Galicia) are acknowledged for allocation of computational resources. D. J.‐S. also thanks the Spanish MICIU for a grant (BES‐2016–078732).
D. Joven-Sancho, M. Baya, A. Martín, J. Orduna, B. Menjón, Angew. Chem. Int. Ed. 2021, 60, 26545.
Dedicated to Dr. F. Martínez‐Buenaga on the occasion of his 75th birthday
References
- 1.
- 1a. Malischewski M., in Comprehensive Organometallic Chemistry IV, Vol. 1 (Ed.: Holland P. L.), Elsevier, 2021, in press, 10.1016/B978-0-12-820206-7.00004-4; [DOI] [Google Scholar]
- 1b. Higelin A., Riedel S., in Modern Synthesis Processes and Reactivity of Fluorinated Compounds, Vol. 3 (Eds.: Groult H., Leroux F., Tressaud A.); Elsevier: Amsterdam, 2017, Ch. 19, pp. 561–586; [Google Scholar]
- 1c. Riedel S., in Comprehensive Inorganic Chemistry II, (Eds.: Antipov E. V., Abakumov A. M., Shevelkov A. V.), Elsevier, Amsterdam, 2013, Ch. 2.08, pp. 187–221; [Google Scholar]
- 1d. Riedel S., Kaupp M., Coord. Chem. Rev. 2009, 253, 606. [Google Scholar]
- 2.Previous reports on silver in oxidation states higher than III, would need confirmation:
- 2a.A. I. Popov, Y. M. Kiselev, Russ. J. Inorg. Chem. 1988, 33, 541; Zh. Neorg. Khim. 1988, 33, 965;
- 2b.A. I. Popov, Y. M. Kiselev, V. F. Sukhoverkhov, V. I. Spitsyn, Dokl. Chem. 1988, 296, 424; Dokl. Akad. Nauk SSSR 1987, 296, 615;
- 2c. Hoppe R., Isr. J. Chem. 1978, 17, 48; [Google Scholar]
- 2d. Sorbe P., Grannec J., Portier J., Hagenmuller P., J. Fluorine Chem. 1978, 11, 243; [Google Scholar]
- 2e. Grannec J., Sorbe P., Portier J., Hagenmuller P., C. R. Acad. Sci. Ser. C 1977, 284, 231. [Google Scholar]
- 3.
- 3a. Grochala W., Hoffmann R., Angew. Chem. Int. Ed. 2001, 40, 2742; [PubMed] [Google Scholar]; Angew. Chem. 2001, 113, 2816; [Google Scholar]
- 3b. Lutar K., Milićev S., Žemva B., Müller B. G., Bachmann B., Hoppe R., Eur. J. Solid State Inorg. Chem. 1991, 28, 1335; [Google Scholar]
- 3c. Müller B. G., Angew. Chem. Int. Ed. Engl. 1987, 26, 1081; [Google Scholar]; Angew. Chem. 1987, 99, 1120. [Google Scholar]
- 4. Eujen R., Hoge B., Brauer D. J., Inorg. Chem. 1997, 36, 3160. [DOI] [PubMed] [Google Scholar]
- 5.Various salts of the homoleptic [Ag(CF3)4]− anion are currently known:
- 5a. Demonti L., Saffon-Merceron N., Mézailles N., Nebra N., Chem. Eur. J. 2021, 10.1002/chem.202102836; [DOI] [PubMed] [Google Scholar]
- 5b. Lu Z., Liu S., Lan Y., Leng X., Shen Q., Organometallics 2021, 40, 1713; [Google Scholar]
- 5c. Joven-Sancho D., Baya M., Martín A., Menjón B., Chem. Eur. J. 2018, 24, 13098; [DOI] [PubMed] [Google Scholar]
- 5d. Dukat W., Naumann D., Rev. Chim. Miner. 1986, 23, 589. [Google Scholar]
- 6.Silver(III) compounds stabilized by polydentate or macrocyclic ligands are clearly affected by severe geometric constraints, but are still based on tetragonal frames:
- 6a. Lash T. D., Chem. Rev. 2017, 117, 2313; [DOI] [PubMed] [Google Scholar]
- 6b. Font M., Ribas X., Top. Organomet. Chem. 2016, 54, 269; [Google Scholar]
- 6c. Lash T. D., Chem. Asian J. 2014, 9, 682; [DOI] [PubMed] [Google Scholar]
- 6d. Brückner C., J. Chem. Educ. 2004, 81, 1665; [Google Scholar]
- 6e. Po H. N., Coord. Chem. Rev. 1976, 20, 171. [Google Scholar]
- 7.The angular structural parameter τ is used as a criterion of trigonality in five-coordinate species; it takes values from τ=0 in an ideal SPY-5 to τ=1 in a regular TBPY-5: Addison A. W., Rao T. N., Reedijk J., van Rijn J., Verschoor G. C., J. Chem. Soc. Dalton Trans. 1984, 1349. [Google Scholar]
- 8.Continuous shape measures (CShM) have also been successfully used to map the geometry of five-coordinate species: Alvarez S., Llunell M., J. Chem. Soc. Dalton Trans. 2000, 3288. [Google Scholar]
- 9.Two polymorphs of (CF3)3Ag(phen) are currently known: orthorhombic (Ref. [5a]) and triclinic (Ref. [5b]). The related compound (CF3)3Ag(bpy) exhibits a similar SPY-5 arrangement (Ref. [5a]).
- 10. Žemva B., Lutar K., Jesih A., W. J. Casteel Jr. , Wilkinson A. P., Cox D. E., von Dreele R. B., Borrmann H., Bartlett N., J. Am. Chem. Soc. 1991, 113, 4192. [Google Scholar]
- 11.
- 11a. Jia T., Zhang X., Liu T., Fan F., Zeng Z., Li X. G., Khomskii D. I., Wu H., Phys. Rev. B 2014, 89, 245117; [Google Scholar]
- 11b. Hoppe R., Homann R., Naturwissenschaften 1966, 53, 501. [Google Scholar]
- 12. Kissel D., Hoppe R., Z. Anorg. Allg. Chem. 1986, 532, 17. [Google Scholar]
- 13. Wang X., Andrews L., Brosi F., Riedel S., Chem. Eur. J. 2013, 19, 1397. [DOI] [PubMed] [Google Scholar]
- 14.
- 14a. Koirala P., Willis M., Kiran B., Kandalam A. K., Jena P., J. Phys. Chem. C 2010, 114, 16018; [Google Scholar]
- 14b. Müller-Rösing H.-C., Schulz A., Hargittai M., J. Am. Chem. Soc. 2005, 127, 8133. [DOI] [PubMed] [Google Scholar]
- 15.Compound (CF3)2Ag(S2CNEt2) containing the bidentate S-donor N,N-diethyldithiocarbamato ligand has also been isolated: Naumann D., Tyrra W., Trinius F., Wessel W., Roy T., J. Fluorine Chem. 2000, 101, 131. [Google Scholar]
- 16. García-Monforte M. A., Martínez-Salvador S., Menjón B., Eur. J. Inorg. Chem. 2012, 4945. [Google Scholar]
- 17. Joven-Sancho D., Baya M., Falvello L. R., Martín A., Orduna J., Menjón B., Chem. Eur. J. 2021, 27, 12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martínez-Salvador S., Falvello L. R., Martín A., Menjón B., Chem. Eur. J. 2013, 19, 14540. [DOI] [PubMed] [Google Scholar]
- 19.The ratio of the couplings to the 109Ag and 107Ag nuclei, both with I= , conforms to the ratio of their respective gyromagnetic constants: γ(109Ag)/γ(107Ag)≈1.15.
- 20. Eujen R., Hoge B., Brauer D. J., Inorg. Chem. 1997, 36, 1464. [DOI] [PubMed] [Google Scholar]
- 21. Deposition Numbers 2096147 (for 1 a) and 2096148 (for 3) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 22.{N3C}3−=10,19-diethyl-9,20-dimethyl-14,15-diphenyltropiporphyrinato(3−): Bergman K. M., Ferrence G. M., Lash T. D., J. Org. Chem. 2004, 69, 7888. [DOI] [PubMed] [Google Scholar]
- 23.
- 23a. Algarra A. G., Grushin V. V., Macgregor S. A., Organometallics 2012, 31, 1467; [Google Scholar]
- 23b. Sgarbossa P., Scarso A., Strukul G., Michelin R. A., Organometallics 2012, 31, 1257. [Google Scholar]
- 24. Hoffmann R., Alvarez S., Mealli C., Falceto A., T. J. Cahill III , Zeng T., Manca G., Chem. Rev. 2016, 116, 8173. [DOI] [PubMed] [Google Scholar]
- 25.For previous examples of inverted ligand field in the related SP-4 silver(iii) complexes [AgR4]− (R=F, CF3), see:
- 25a. Grochala W., Egdell R. G., Edwards P. P., Mazej Z., Žemva B., ChemPhysChem 2003, 4, 997; [DOI] [PubMed] [Google Scholar]
- 25b.Ref. [5c].
- 26. Anderson G. K., Cross R. J., Chem. Soc. Rev. 1980, 9, 185. [Google Scholar]
- 27.
- 27a. Sun Y., Kirschenbaum L. J., J. Coord. Chem. 2018, 71, 1863; [Google Scholar]
- 27b. Grzegorzek N., Latos-Grażyński L., Szterenberg L., Org. Biomol. Chem. 2012, 10, 8064; [DOI] [PubMed] [Google Scholar]
- 27c. Stépień M., Latos-Grażyński L., Org. Lett. 2003, 5, 3379; [DOI] [PubMed] [Google Scholar]
- 27d. Banerjee R., Das A., Dasgupta S., J. Chem. Soc. Dalton Trans. 1990, 1207; [Google Scholar]
- 27e. Iqbal J., Sharp D. W. A., Winfield J. M., J. Chem. Soc. Dalton Trans. 1989, 461; [Google Scholar]
- 27f. Pesavento M., Profumo A., Soldi T., Fabbrizzi L., Inorg. Chem. 1985, 24, 3873; [Google Scholar]
- 27g. Kirschenbaum L. J., Ambrus J. H., Atkinson G., Inorg. Chem. 1973, 12, 2832. [Google Scholar]
- 28.
- 28a. Coghi L., Pelizzi G., Acta Crystallogr. Sect. B 1975, 31, 131; [Google Scholar]
- 28b. Simms M. L., Atwood J. L., Zatko D. A., J. Chem. Soc. Chem. Commun. 1973, 46. [Google Scholar]
- 29.Similar trends were also observed in the d10 silver(I) system [XAgX]− (X=Cl, Br, I): Carvajal M. A., Novoa J. J., Alvarez S., J. Am. Chem. Soc. 2004, 126, 1465. [DOI] [PubMed] [Google Scholar]
- 30. Godfrey S. M., Ho N., McAuliffe C. A., Pritchard R. G., Angew. Chem. Int. Ed. Engl. 1996, 35, 2344; [Google Scholar]; Angew. Chem. 1996, 108, 2496. [Google Scholar]
- 31.Symmetrization greatly simplifies the analysis of the electronic structures at virtually no energy cost in this case: ΔE≈0.0 kcal mol−1.
- 32.
- 32a. Albright T. A., Burdett J. K., Whangbo M.-H., Orbital Interactions in Chemistry , 2nd ed., Wiley, Hoboken, NJ, 2013, Ch. 17, pp. 465–502; [Google Scholar]
- 32b. Rossi A. R., Hoffmann R., Inorg. Chem. 1975, 14, 365. [Google Scholar]
- 33.Non-standard electronic configurations tending to an ILF state were identified by Lancaster and their co-workers in the five-coordinate copper(III) compounds (CF3)3Cu(bpy) and [(tptm)CuCl]+ [tptm=tris(2-pyridylthio)methane]:
- 33a. DiMucci I. M., Lukens J. T., Chatterjee S., Carsch K. M., Titus C. J., Lee S. J., Nordlund D., Betley T. A., MacMillan S. N., Lancaster K. M., J. Am. Chem. Soc. 2019, 141, 18508; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33b. Walroth R. C., Lukens J. T., MacMillan S. N., Finkelstein K. D., Lancaster K. M., J. Am. Chem. Soc. 2016, 138, 1922. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information