Abstract
Objective
To describe the real‐world treatment persistence (defined as the continuation of medication for the prescribed treatment duration), demographics and clinical characteristics, and treatment patterns for patients prescribed erenumab for migraine prevention in Canada.
Background
The effectiveness of prophylactic migraine treatments is often undermined by poor treatment persistence. In clinical trials, erenumab has demonstrated efficacy and tolerability as a preventive treatment, but less is known about the longer term treatment persistence with erenumab.
Methods
This is a real‐world retrospective cohort study where a descriptive analysis of secondary patient data was conducted. Enrollment and prescription data were extracted from a patient support program for a cohort of patients prescribed erenumab in Canada between September 2018 and December 2019 and analyzed for persistence, baseline demographics, clinical characteristics, and treatment patterns. Descriptive analyses and unadjusted Kaplan–Meier (KM) curves were used to summarize the persistence and dose escalation/de‐escalation at different timepoints.
Results
Data were analyzed for 14,282 patients. Median patient age was 47 years, 11,852 (83.0%) of patients were female, and 9443 (66.1%) had chronic migraine at treatment initiation. Based on KM methods, 71.0% of patients overall were persistent to erenumab 360 days after treatment initiation. Within 360 days of treatment initiation, it is estimated that 59.3% (KM‐derived) of patients who initiated erenumab at 70 mg escalated to 140 mg, and 4.4% (KM‐derived) of patients who initiated at 140 mg de‐escalated to 70 mg.
Conclusions
The majority of patients prescribed erenumab remained persistent for at least a year after treatment initiation, and most patients initiated or escalated to a 140 mg dose. These results suggest that erenumab is well tolerated, and its uptake as a new class of prophylactic treatment for migraine in real‐world clinical practice is not likely to be undermined by poor persistence when coverage for erenumab is easily available.
Keywords: dose, erenumab, migraine, persistence, prophylactic
Abbreviations
- CGRP
calcitonin gene‐related peptide
- CM
chronic migraine
- EM
episodic migraine
- IQR
interquartile range
- KM
Kaplan–Meier
- MMDs
monthly migraine days
INTRODUCTION
Migraine is a common and important neurological disorder, which can produce substantial disability and negatively affect health‐related quality of life. 1 , 2 Canadian studies have shown migraine prevalence rates of 23.0%–26.0% in women, and 7.8%–10.0% in men. 2 , 3 , 4 The World Health Organization ranks migraine as the third most prevalent and second most debilitating neurological disorder, globally. 5 With over 4,000,000 people with migraine in Canada, migraine is associated with substantial social and economic impact. 6 Migraine is most common among those in their prime working years. 3
Chronic migraine (CM) is defined as at least 15 headache days per month of which 8 days are migraine days. 7 Migraine management consists of education on lifestyle and nondrug strategies, acute migraine management strategies and, where appropriate, prophylactic therapy. 8 All patients with CM should be, by definition of their migraine frequency, candidates for prophylactic therapy. Patients with episodic migraine (EM) may also be candidates for migraine prophylaxis based on the severity of their migraine and the associated disability, and lack of benefits from, or contraindications to, standard acute migraine therapies. 8
Despite comprehensive guidelines by the Canadian Headache Society for the prophylactic management of migraine, the vast majority of Canadian patients with migraine remain undertreated or inadequately treated, with meaningful migraine‐related disability. 9 The most comprehensive study of migraine prophylaxis in Canada was the CHORD Study in 2006, which found that, at the time of initial consultation to a Headache Medicine specialist in Canada, only 30.9% of patients were on a prophylactic therapy. 9 Unfortunately, neurologists have continued to observe ongoing underutilization of prophylactic therapy. In US studies, 1‐year persistence (defined as the duration of time a patient remains on a prescribed medication after initiating therapy) on preventive therapy has been reported between 7% and 55%, 10 with approximately 50% of patients discontinuing preventive therapy in less than 60 days. 11 Lack of tolerability, side effects, and poor efficacy remain very common reasons for medication discontinuation. Unfortunately, non–migraine‐specific, standard‐of‐care prophylactic therapies (e.g., amitriptyline, topiramate, and propranolol) are fraught with potential side effects including sedation, weight gain, exercise intolerance, and cognitive difficulties. Accordingly, there has been substantial unmet individual, societal, and economic need for safe, effective, and tolerable prophylactic treatments for the prevention of migraine.
Calcitonin gene‐related peptide (CGRP) is a proinflammatory, vasodilating neuropeptide that contributes to migraine pathophysiology and presents a promising new target for prophylactic migraine treatment. 12 Erenumab is a first‐in‐class, fully human monoclonal antibody that binds to the CGRP receptor to interrupt the CGRP signaling pathway associated with migraine pathophysiology. 13 In Phase II and III clinical trials with patients with episodic and chronic migraine, erenumab demonstrated statistically significant efficacy over placebo in reducing the number of monthly migraine days (MMDs), improving functional scores, and reducing the need for acute migraine medications, with a tolerability profile similar to that of placebo. 13 , 14 , 15 Erenumab is self‐administered as a monthly subcutaneous injection available as a 70 or 140 mg dose. It received Health Canada approval for the prevention of migraine in August 2018 and became available in September 2018 through the Novartis‐sponsored patient support program (the “Go Program®”). 16
Despite significant sustained persistence to erenumab treatment observed in open‐label extension arms of the randomized clinical trials, the extent of long‐term treatment persistence to erenumab in Canada in a real‐world setting is unknown. This current study is an analysis of real‐world data available through the Go Program®. The objective of this study was to evaluate the demographic and clinical characteristics of patients prescribed erenumab following its launch in Canada and to describe the real‐world treatment persistence and dose change patterns for patients prescribed erenumab in Canada.
METHODS
Study design and data sources
This was a real‐world retrospective cohort study using de‐identified secondary patient data. The data were collected through Novartis’ Go Program® for a cohort of Canadian patients who initiated erenumab between September 2018 and December 31, 2019. The Go Program® provides injection training, reimbursement support, and coordination of erenumab access including a free‐of‐charge access to erenumab until patients have access to public or private reimbursement. To enroll in the Go Program®, patients must have been prescribed erenumab for the prevention of migraine in accordance with the Health Canada–approved product monograph. 16 At enrollment, patients provided written informed consent to participate in the program and to the use of their de‐identified data for research purposes, enabling post hoc observational reporting of aggregate data. Data used for this study were collected from two main sources: enrollment data and prescription data.
Physician‐recorded enrollment data contained information on patients’ baseline demographics and clinical characteristics, including mean MMDs. Information was also collected on previous prophylactic medications the patient had tried and the duration of use. The Go Program® recorded the start date of erenumab treatment and the date of erenumab discontinuation for patients who discontinued during the analysis period. Prescription data were reported longitudinally by the pharmacy dispensing the erenumab prescription and contained fill date, days of supply, and initiating drug dose (70 or 140 mg).
All the data used for this analysis, including treatment start date, discontinuation date, and dose of each prescription, were either physician‐recorded or pharmacy‐reported. The data were initially reported in the Go Program® database and subsequently extracted for analysis purposes. As this was a retrospective post hoc analysis, the data collection process was not specified by a research protocol nor externally validated or monitored. However, a data management plan was implemented, and the data collected from both sources mentioned above were crosschecked and cleaned to ensure data validity and accuracy. A descriptive analysis method was used for this study given there were no planned comparative analyses nor an intent to establish relationships between the analyzed variables.
Inclusion criteria
Patients in the Go Program® with documented consent were included in the analysis if they met the following criteria:
Status in the Go Program database was either “In Treatment” or “Discontinued”
Treatment start date was available
Age was ≥18 years old at treatment start date
Treatment start date was ≤ December 31, 2019
In addition, all patients reported having at least four MMDs at enrollment. At the beginning of the study, access to erenumab was either through enrollment in the Go Program® or purchased directly by the patient. Only a small number of Canadians using erenumab are anticipated to have paid out of pocket given the cost and availability of free medication trial through the Go Program®. With the exception of the small number of patients who covered the cost out of pocket and those excluded based on the eligibility criteria, the study population is assumed to be a highly representative sample of the entire patient population taking erenumab in Canada from September 2018 to December 2019.
Variables
Baseline characteristics
Baseline demographics and clinical characteristics were collected at enrollment, including age at treatment initiation, sex, province, insurance coverage type (private or public), number of MMDs, and previous preventive therapies. At enrollment, physicians were asked to report at least two previously discontinued prophylactic therapies along with the associated start (month/year) and end (month/year) estimates. Previous therapies were counted by class; if the Go Program® documented prior use of multiple molecules falling within a single class, this was recorded as one previous therapy. The duration of use was calculated as the number of months between the start and end date (month/year) reported for the therapy class. It is important to note that physicians were only provided space to report up to three previous therapies on the patients’ paper enrollment form; therefore, the reported information on previous therapies was likely incomplete for many patients and the true number of previous therapies is likely higher than reported here. Therefore, this analysis does not report summary statistics on the number of previous therapies used prior to initiation of erenumab.
Primary outcome
Persistence
The primary outcome was persistence to erenumab, which was derived from the erenumab treatment start date and discontinuation date. Days persistent were calculated as the number of days between the treatment start date and discontinuation date or the end of study follow‐up, whichever occurred first, and persistence was reported for 90, 180, 270, 360, and 450 days after the treatment start date.
Secondary outcomes
Treatment patterns (dose change)
This analysis described two patient journeys in relation to dose changes: those who initiated on 70 mg of erenumab and those who initiated on 140 mg. Patients could either be escalated or de‐escalated depending on their initial dose and patient–physician decision during treatment. Dose escalation and de‐escalation data were derived from longitudinal pharmacy prescription data. Patients had to have at least two recorded prescriptions (of any dose) to be included in the dose change analysis. Time‐to‐dose escalation was calculated as the number of days between a patient's first 70 mg fill date and the fill date of the first subsequent 140 mg dose. Time‐to‐dose de‐escalation was the number of days between a patient's first 140 mg fill date and the fill date of the first subsequent 70 mg dose.
Patients could have both a dose escalation and a dose de‐escalation within the analysis period if the dose was changed more than once. For example, a patient could initiate erenumab at 70 mg, escalate to 140 mg, then de‐escalate back to 70 mg (Figure S1). For this patient, time‐to‐dose escalation would be the number of days between the first 70 mg and the first 140 mg dose, and time‐to‐dose de‐escalation would be the number of days between the first 140 mg and their second initiation of the 70 mg dose. Only a patient's first dose escalation event and first dose de‐escalation event were included in the analysis.
Data analysis
All analyses conducted were descriptive. No formal sample size calculation was carried out for this study given the retrospective, descriptive, and post hoc nature of the analysis conducted using the data collected by the Go Program®. Continuous variables were summarized using mean, standard deviation, median and interquartile range (IQR), and categorical variables were summarized by counts and proportions (%). Time‐to‐event outcomes (i.e., persistence and dose change) were analyzed using Kaplan–Meier (KM) survival methods and the associated life table based on product limit estimates 17 to estimate the survival rate for persistence and cumulative incidence for dose change at specified timepoints. Patients were censored administratively at the time of data extraction or at 450 days, whichever came first. All KM analyses assume that the probability of the outcome (i.e., discontinuation, dose escalation, or dose de‐escalation) and the probability of censoring are the same for censored and uncensored patients. All analyses are reported for overall patients and stratified by baseline migraine status (i.e., EM and CM). Persistence was also reported with age, sex, and baseline MMD stratifications. Results based on fewer than six patients were masked for patient privacy. All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Baseline characteristics
Between September 2018 and December 2019, 14,282 patients were enrolled in the program and met eligibility criteria (Figure 1). Table 1 describes the baseline characteristics of the study population. 83.0% of patients were female, and the median age at treatment start was 47 years, with the majority of patients residing in Ontario or Québec (36.0% and 24.6%, respectively). Although 72.3% of patients had private insurance available at enrollment, erenumab was acquired initially directly through the Go Program® while waiting for private insurance coverage. Most patients (99.7%) reported at least eight MMDs at baseline, with 66.1% experiencing CM (i.e., ≥15 MMDs). Baseline characteristics were mostly similar between patients with EM and CM. A slightly larger proportion of patients with EM initiated a 70 mg dose relative to patients with CM (51.6% vs. 45.2%, respectively).
FIGURE 1.
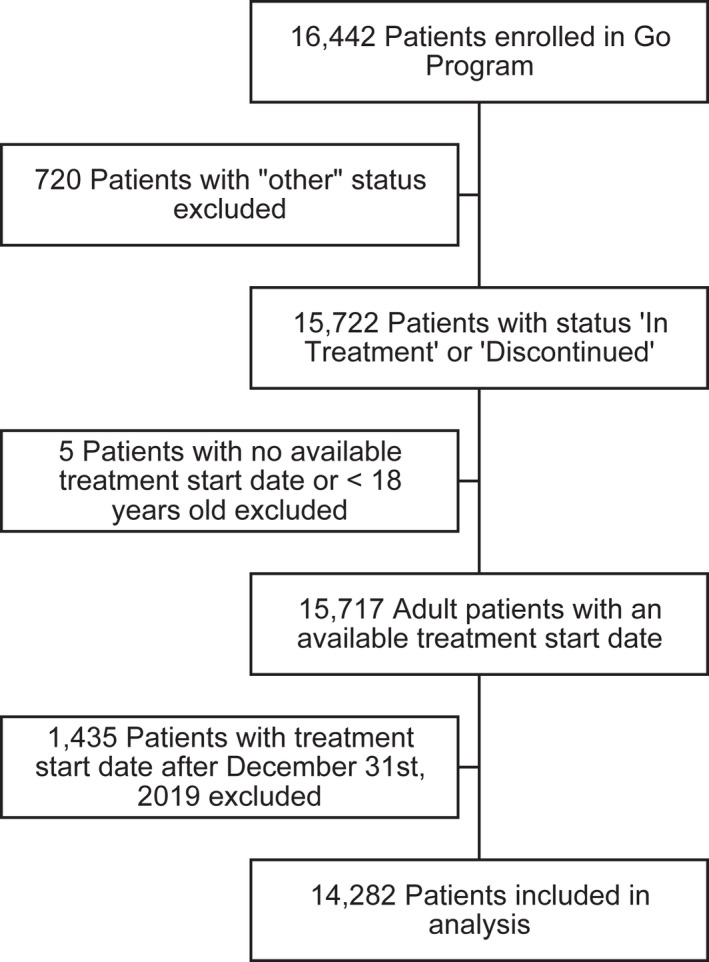
Patient selection flow chart
TABLE 1.
Patient baseline characteristics
| Overall (N = 14,282) | EM at baseline, <15 MMD (N = 4839) | CM at baseline, ≥15 MMD (N = 9443) | |
|---|---|---|---|
| Age (years), median (IQR) | 47 (37, 56) | 46 (38, 55) | 47 (37, 56) |
| Age (years), mean (SD) | 46.3 (13.0) | 46.1 (12.5) | 46.4 (13.2) |
| Age category | |||
| 18–29, n (%) | 1649 (11.5) | 527 (10.9) | 1122 (11.9) |
| 30–49, n (%) | 6772 (47.4) | 2372 (49.0) | 4400 (46.6) |
| 50–64, n (%) | 4742 (33.2) | 1633 (33.7) | 3109 (32.9) |
| 65+, n (%) | 1119 (7.8) | 307 (6.3) | 812 (8.6) |
| Sex | |||
| Female, n (%) | 11,852 (83.0) | 4142 (85.6) | 7710 (81.6) |
| Male, n (%) | 2426–2430 a | 697 (14.4) | 1726–1730 a |
| Other, n (%) | 1–5* | – | 1–5* |
| Province | |||
| Ontario, n (%) | 5138 (36.0) | 1810 (37.4) | 3328 (35.2) |
| Quebec, n (%) | 3517 (24.6) | 1320 (27.3) | 2197 (23.3) |
| Alberta, n (%) | 2326 (16.3) | 650 (13.4) | 1676 (17.7) |
| British Columbia, n (%) | 1364 (9.6) | 369 (7.6) | 995 (10.5) |
| Manitoba, n (%) | 559 (3.9) | 137 (2.8) | 422 (4.5) |
| Saskatchewan, n (%) | 521 (3.6) | 126–130 a | 391–395 a |
| Atlantic Canada, n (%) | 840 (5.9) | 423 (8.7) | 417 (4.4) |
| Territories, n (%) | 17 (0.1) | 1–5* | 11–15 a |
| Insurance b | |||
| Private, n (%) | 10,332 (72.3) | 3672 (75.9) | 6660 (70.5) |
| Public, n (%) | 3868 (27.1) | 1145 (23.7) | 2723 (28.8) |
| Unknown, n (%) | 82 (0.6) | 22 (0.5) | 60 (0.6) |
| Treatment start year c | |||
| 2018, n (%) | 1304 (9.1) | 458 (9.5) | 846 (9.0) |
| 2019, n (%) | 12,978 (90.9) | 4381 (90.5) | 8597 (91.0) |
| Baseline MMD | |||
| 4–7, n (%) | 40 (0.3) | 40 (0.8) | – |
| 8–14, n (%) | 4799 (33.6) | 4799 (99.2) | – |
| 15–21, n (%) | 5316 (37.2) | – | 5316 (56.3) |
| ≥22, n (%) | 4127 (28.9) | – | 4127 (43.7) |
| Initiation dosage | |||
| 70 mg, n (%) | 6768 (47.4) | 2499 (51.6) | 4269 (45.2) |
| 140 mg, n (%) | 7504 (52.5) | 2331–2335 a | 5165–5170 a |
| Unknown, n (%) | 10 (0.1) | 6–10 a | 1–5* |
Results with patient counts fewer than 6 were masked as “1–5*” for privacy purposes. Additional cells were masked wherever necessary to avoid back calculation.
The insurance variable indicates the insurance plan that was available to the patients at baseline. It does not indicate the insurance plan that reimbursed erenumab.
The minimum treatment start date for patients included in the analysis was September 24, 2018; the maximum treatment start date for patients included in the analysis was December 31, 2019.
Given the limited information on previous therapies described in the Methods section, summary statistics on number of previous therapies are not reported and the analysis, instead, focused on patterns of most frequently reported therapy classes and average duration of use. The majority of patients reported previously using anti‐seizures (77.4%) and anti‐depressants (70.1%) as prophylactic treatment for migraine. The next most frequently reported previous therapies were onabotulinumtoxinA and beta‐blockers, which were reported among 41.2% and 40.2% of patients, respectively. The median duration of use ranged from 4 months for antihypertensives to 9 months for onabotulinumtoxinA (Table 2).
TABLE 2.
Previous therapies and duration of use
| Previous prophylactic therapies | Overall a (N = 13,732) | EM at baseline, <15 MMD (N = 4653) | CM at baseline, ≥15 MMD (N = 9079) |
|---|---|---|---|
| Anti‐seizures, n (%) | 10,628 (77.4) | 3504 (75.3) | 7124 (78.5) |
| Duration of use (months), median (IQR) | 5 (3, 12) | – | – |
| Antidepressants, n (%) | 9622 (70.1) | 3281 (70.5) | 6341 (69.8) |
| Duration of use (months), median (IQR) | 6 (3, 12) | – | – |
| Beta‐blockers, n (%) | 5521 (40.2) | 2051 (44.1) | 3470 (38.2) |
| Duration of use (months), median (IQR) | 4 (3, 12) | – | – |
| Calcium channel blockers, n (%) | 1317 (9.6) | 378 (8.1) | 939 (10.3) |
| Duration of use (months), median (IQR) | 5 (3, 9) | – | – |
| ACEIs/ARBs, n (%) | 1609 (11.7) | 538 (11.6) | 1071 (11.8) |
| Duration of use (months), median (IQR) | 4 (2, 8) | – | – |
| Serotonin antagonists, n (%) | 313 (2.3) | 99 (2.1) | 214 (2.4) |
| Duration of use (months), median (IQR) | 4 (3, 9) | – | – |
| OnabotulinumtoxinA, n (%) | 5660 (41.2) | 1675 (36.0) | 3985 (43.9) |
| Duration of use (months), median (IQR) | 9 (6, 18) | – | – |
Abbreviations: ACEI, angiotensin‐converting‐enzyme inhibitors; ARB, angiotensin II receptor blockers.
There were 13,732 patients for whom previous therapy information was available.
Persistence
Treatment persistence was measured up to a maximum of 450 days from treatment initiation, with a median follow‐up time of 323 days. Based on the KM analysis in the overall population, an estimated 95.7% and 87.3% of patients who initiated erenumab were still persistent to therapy 90 days and 180 days, respectively, after their treatment start date. After 360 and 450 days, the KM‐derived persistence was 71.0% and 63.4%, respectively. Stratification of the results by EM and CM shows a KM‐derived persistence of 74.7% for EM versus 69.1% for CM at Day 360 and 68.0% versus 61.1%, respectively, at Day 450. Among patients with the most severe monthly migraine burden (i.e., ≥22 MMDs), KM‐derived 64.6% and 56.9% of patients were still persistent to erenumab 360 and 450 days, respectively, from treatment initiation (Table 3; Figure 2).
TABLE 3.
Persistence to erenumab up to 450 days from treatment initiation
| Persistence b | Number of patients at treatment start, N | Persistence at 90 days, % (n at risk) | Persistence at 180 days, % (n at risk) | Persistence at 270 days, % (n at risk) | Persistence at 360 days, % (n at risk) | Persistence at 450 days, % (n at risk) |
|---|---|---|---|---|---|---|
| Overall | 14,282 | 95.7% (13,704) | 87.3% (11,218) | 79.3% (8385) | 71.0% (5433) | 63.4% (2075) |
| Baseline migraine category | ||||||
| Episodic (<15 MMD) | 4839 | 96.3% (4670) | 89.6% (3919) | 82.5% (2982) | 74.7% (1968) | 68.0% (728) |
| Chronic (≥15 MMD) | 9443 | 95.5% (9034) | 86.2% (7299) | 77.6% (5403) | 69.1% (3465) | 61.1% (1347) |
| Baseline MMD category | ||||||
| 4–7 | 40 | 100.0% (40) | 97.5% (37) | 97.5% (25) | 87.6% (13) | 87.6% (1–5*) a |
| 8–14 | 4799 | 96.3% (4630) | 89.5% (3882) | 82.3% (2957) | 74.6% (1955) | 67.8% (726) |
| 15–21 | 5316 | 96.2% (5122) | 88.2% (4211) | 80.3% (3158) | 72.5% (2002) | 64.4% (747) |
| ≥22 | 4127 | 94.5% (3912) | 83.5% (3088) | 74.1% (2245) | 64.6% (1463) | 56.9% (600) |
| Age at treatment start (years) | ||||||
| 18–29 | 1649 | 97.0% (1603) | 87.8% (1296) | 78.9% (919) | 69.7% (579) | 60.6% (172) |
| 30–49 | 6772 | 96.1% (6516) | 88.3% (5368) | 80.4% (4019) | 71.8% (2619) | 64.6% (1002) |
| 50–64 | 4742 | 95.1% (4524) | 86.9% (3719) | 78.7% (2832) | 70.8% (1841) | 63.0% (759) |
| 65+ | 1119 | 94.5% (1061) | 82.4% (835) | 75.0% (615) | 69.0% (394) | 62.3% (142) |
| Sex | ||||||
| Female | 11,852 | 95.8% (11,377) | 87.4% (9342) | 79.7% (7017) | 71.3% (4569) | 63.9% (1754) |
| Male | 2427 | 95.5% (2324) | 87.0% (1874) | 77.1% (1368) | 69.6% (864) | 60.9% (321) |
Results with patient counts less than 6 were masked as “1–5*” for privacy purposes. Additional cells were masked wherever necessary to avoid back calculation.
All persistence estimates are KM‐derived. The number of patients at risk included patients who remained persistent at each timepoint and excluded patients who had discontinued erenumab or had been censored prior to that timepoint. Censoring date is April 24, 2020 or 450 days after treatment start, whichever happened first. The maximum observable persistence was 450 days.
FIGURE 2.
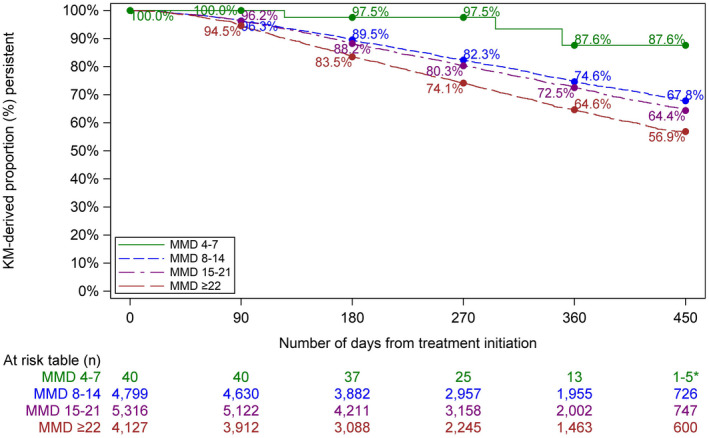
Time to erenumab discontinuation by monthly migraine day category [Color figure can be viewed at wileyonlinelibrary.com]
Treatment patterns
13,325 patients who had at least two recorded prescriptions of any dose were included in the dose change analysis. 6357 (47.7%) initiated erenumab treatment at 70 mg, whereas 6968 (52.3%) patients initiated on the 140 mg dose (Figure S1).
Patients initiating erenumab at 70 mg dose
6357 patients who initiated on 70 mg erenumab were included in the dose change analysis (Table 4). Based on the KM analysis, 12.6% escalated within 90 days, 59.3% within 360 days, and 63.6% within 450 days (Table 4; Figure 3). Of those patients who escalated, 1.9% returned to their original 70 mg dose within 90 days of escalation, and 6.0% had returned within 450 days of escalation (KM‐derived; Figure S2).
TABLE 4.
Treatment patterns for patients initiating erenumab at 70 mg
| First dose escalation a , b i.e., 70 to 140 mg | Number of patients at treatment start, N | Escalation by 90 days, % (n at risk) | Escalation by 180 days, % (n at risk) | Escalation by 270 days, % (n at risk) | Escalation by 360 days, % (n at risk) | Escalation by 450 days, % (n at risk) |
|---|---|---|---|---|---|---|
| Overall | 6357 | 12.6% (5309) | 37.8% (2987) | 51.1% (1658) | 59.3% (865) | 63.6% (233) |
| Episodic (<15 MMD) | 2322 (36.5%) | 9.3% (2023) | 31.9% (1245) | 45.6% (701) | 53.5% (376) | 57.0% (104) |
| Chronic (≥15 MMD) | 4035 (63.5%) | 14.5% (3286) | 41.2% (1742) | 54.3% (957) | 62.8% (489) | 67.6% (129) |
| Subsequent de‐escalation i.e., 70 to 140 to 70 mg c | Number of patients at treatment start, N | De‐escalation by 90 days, % (n at risk) | De‐escalation by 180 days, % (n at risk) | De‐escalation by 270 days, % (n at risk) | De‐escalation by 360 days, % (n at risk) | De‐escalation by 450 days, % (n at risk) |
|---|---|---|---|---|---|---|
| Overall | 3016 | 1.9% (2237) | 3.9% (1340) | 5.0% (656) | 6.0% (109) | 6.0% (0) |
| Episodic (<15 MMD) | 999 (33.1%) | 1.5% (762) | 4.2% (457) | 6.2% (212) | 9.0% (31) | 9.0% (0) |
| Chronic (≥15 MMD) | 2017 (66.9%) | 2.0% (1475) | 3.8% (883) | 4.4% (444) | 4.4% (78) | 4.4% (0) |
All dose change estimates are KM‐derived. Number of patients at risk included patients who remained on their prescribed dose at each timepoint, and excluded patients who had changed dose or had been censored prior to that timepoint. Censoring date is April 24, 2020 or 450 days after treatment start, whichever happened first. The maximum observable persistence was 450 days.
For a patient to be included in the dose change analysis, the patient needed to have at least two recorded prescriptions.
Time to the first dose de‐escalation was computed from the first 140 mg fill date.
FIGURE 3.
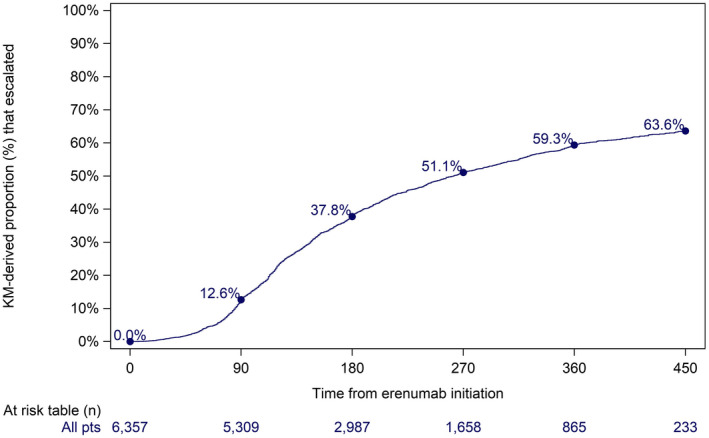
Time from erenumab initiation to first dose escalation for patients who initiated erenumab at 70 mg [Color figure can be viewed at wileyonlinelibrary.com]
Patients initiating erenumab at 140 mg dose
6968 patients who initiated erenumab at 140 mg were included in the dose change analysis (Table 5). Based on the KM analysis, 1.4% had de‐escalated within 90 days, 4.4% within 360 days, and 4.7% within 450 days (Figure 4). Of those patients who de‐escalated, 45.7% had re‐escalated to 140 mg within 90 days of de‐escalation, and 62.7% had re‐escalated within 360 days of de‐escalation (KM‐derived; Figure S3).
TABLE 5.
Treatment patterns for patients initiating erenumab at 140 mg
| First dose de‐escalation b , c i.e., 140 to 70 mg | Number of patients at treatment start, N | De‐escalation by 90 days, % (n at risk) | De‐escalation by 180 days, % (n at risk) | De‐escalation by 270 days, % (n at risk) | De‐escalation by 360 days, % (n at risk) | De‐escalation by 450 days, % (n at risk) |
|---|---|---|---|---|---|---|
| Overall | 6968 | 1.4% (6439) | 2.6% (4887) | 3.8% (3459) | 4.4% (2212) | 4.7% (919) |
| Episodic (<15 MMD) | 2158 (31.0%) | 0.9% (2013) | 2.3% (1558) | 3.4% (1128) | 4.1% (747) | 4.1% (289) |
| Chronic (≥15 MMD) | 4810 (69.0%) | 1.6% (4426) | 2.8% (3329) | 3.9% (2331) | 4.6% (1465) | 5.0% (630) |
| Subsequent escalation i.e., 140 to 70 to 140 mg d | Number of patients at treatment start, N | Escalation by 90 days, % (n at risk) | Escalation by 180 days, % (n at risk) | Escalation by 270 days, % (n at risk) | Escalation by 360 days, % (n at risk) | Escalation by 450 days, % (n at risk) |
|---|---|---|---|---|---|---|
| Overall | 242 | 45.7% (80) | 56.7% (33) | 62.7% (7) | 62.7% (0) | – |
| Episodic (<15 MMD) | 67 (27.7%) | 33.9% (28) | 52.3% (12) | 60.2% (1–5*) a | 60.2% (0) | – |
| Chronic (≥15 MMD) | 175 (72.3%) | 50.3% (52) | 58.3% (21) | 64.2% (1–5*) a | 64.2% (0) | – |
Results with patient counts fewer than 6 were masked as “1–5*” for privacy purposes. Additional cells were masked where necessary to avoid back calculation.
All dose change estimates are KM‐derived. The number of patients at risk included patients who remained on their prescribed dose at each timepoint, and excluded patients who had changed dose or had been censored prior to that timepoint. Censoring date is April 24, 2020 or 450 days after treatment start, whichever happened first. The maximum observable persistence was 450 days.
For a patient to be included in the dose change analysis, the patient needed to have at least two recorded prescriptions.
Time to first dose escalation was computed from the first 70 mg fill date.
FIGURE 4.
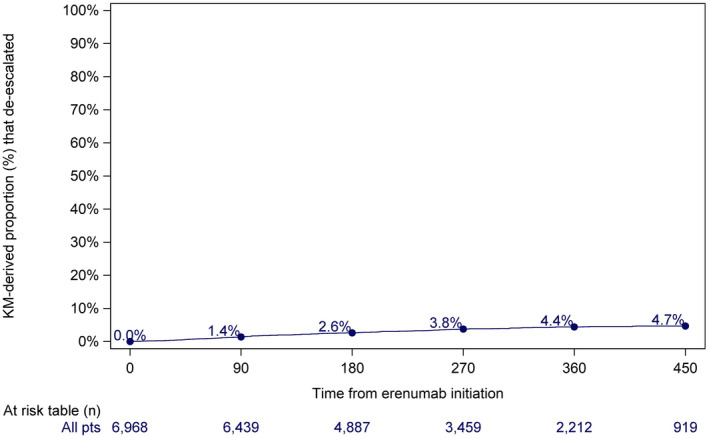
Time from erenumab initiation to first dose de‐escalation for patients who initiated erenumab at 140 mg [Color figure can be viewed at wileyonlinelibrary.com]
DISCUSSION
Migraine is a leading cause of years lived with disability, negatively affecting health‐related quality of life, family relationships, social relationships, and workplace attendance and productivity. 1 , 4 Without effective and well‐tolerated preventive treatment, patients are at risk of acute medication overuse (and associated rebound headache), medication related side effects (e.g., excessive consumption of nonsteroidal anti‐inflammatory drugs leading to gastrointestinal or renal impairment, or excessive consumption of acetaminophen leading to hepatic impairment), opioid overuse and associated sequelae, and progression to escalating migraine frequencies and their associated disability. 18 Despite the availability of multiple classes of prophylactic migraine treatments, the majority of patients with migraine who are appropriate candidates for migraine prophylactic therapy are not being treated prophylactically and, if they have, the overwhelming majority have discontinued therapy due to limited success with available preventive treatments secondary to poor tolerance, side effects, and/or lack of efficacy. 11 , 19
The results of this study showed that approximately 71% of patients across all groups continued treatment with erenumab 360 days after treatment initiation. These results are in line with the findings of another recent real‐world retrospective study that showed that 62.7% of the patients prescribed erenumab reported their intent to continue using this treatment for their migraine. 20 Persistence in the current cohort was numerically higher for patients with EM than those with CM. Notably, even the most affected patients with CM (i.e., >22 MMDs) showed persistence of 64.6% by Day 360. These persistence results for erenumab are considerably higher than those reported for other prophylactic therapies for migraine. Indeed, a previously published meta‐analysis of persistence to propranolol (a beta‐blocker), amitriptyline (an antidepressant), and topiramate (an antiepileptic) reported 12‐month persistence estimates ranging from 7% to 55%. 10 A more recent study looking at current oral migraine prophylactics, including antidepressants, beta‐blockers, and anticonvulsants, found only 14% of patients were persistent 12 months after treatment initiation, and roughly 50% of patients discontinued after only 60 days. 11 These persistence data are all the more remarkable given that the patients enrolled in the Go Program® during the first year of the program represented and included the majority of the most highly disabled and patients with refractory disease from Canadian tertiary care migraine clinics, as reflected by the baseline MMDs reported herein. Notably, as the first‐available CGRP medication in Canada, it was anticipated that the most highly disabled and refractory patients would be among the first patients to be prescribed erenumab in Canada. These results do echo several discussions within the Headache Medicine physician community in Canada indicating that many of the initial patients enrolled by Headache Medicine specialists during the first year of the Go Program® included patients with numerous (i.e., 5–10 or more) prior migraine prophylactic failures and complex migraine prophylactic polypharmacy.
With respect to treatment patterns, the current results suggest that in a real‐world setting, clinicians tended to prescribe a 140 mg erenumab dose over a 70 mg dose. Roughly half of both patients with EM and CM initiated erenumab on a 140 mg dose, although a slightly higher proportion of patients with EM were initiated on 70 mg (51.6%). Among patients who initiated erenumab at 70 mg, a considerable proportion escalated to 140 mg (59.3% by Day 360). In contrast, very few patients who initiated erenumab at 140 mg de‐escalated to 70 mg (4.4% by Day 360). Furthermore, patients who did de‐escalate were likely to return to 140 mg (62.7% by Day 360), although these results should be interpreted with caution due to limited patient count (242 in total). These findings suggest that, overall, the 140 mg dose is well tolerated.
Overall, the persistence and dose change results reported here show that most patients persist on erenumab for at least 360 days from treatment initiation and either initiate or escalate to a 140 mg dose. These results build on the positive randomized clinical trial results for erenumab 13 , 14 , 15 and on more recent real‐world positive efficacy results, 21 , 22 suggesting that erenumab is effective and well tolerated by patients in the Canadian real‐world setting. Given the historically low adherence to prophylactic therapies (due to poor efficacy and/or tolerability) 10 , 11 , 19 and considering the significant burden of migraine, these results offer valuable evidence on a better and more sustainable uptake of erenumab in a real‐world clinical practice context and its potential to alleviate the significant burden associated with migraine. 5
The strengths of this analysis included a sizable study population that was not restricted by geography, prescriber, nor insurance and should be broadly representative of the migraine population across the Canadian healthcare system. Furthermore, this is the first study to report on longer term use and tolerability of erenumab in a real‐world context. However, observations reported herein were made in the context of routine Canadian clinical practice and insurance coverage and may not be generalizable outside of Canada.
Use of routinely collected patient support program data allowed for the analysis of persistence and treatment patterns in a naturalistic setting, unlikely to be affected by the biases associated with invasive follow‐up. However, the secondary use of data in this study may have conferred some limitations and potential sources of bias. Because the primary use of these data was not for research purposes, data collection was not specifically designed for this analysis and was not externally validated. Enrollment data were collected via a standard enrollment form for each patient and may have been prone to the biases of the physician and/or patient. For example, information on previous therapies may have been dependent on physician estimates, patient recall, and may not have referenced a medical chart, resulting in incomplete or inaccurate information. In particular, the paper enrollment form only provided space to report three previous therapies, even if the patient had previously tried more than three therapies. As a result, no summary measures on the number of previous therapies were reported. Although the analysis does summarize how frequently therapy classes were reported and their duration of use, it is acknowledged that these results are still prone to recall bias. However, the consistency between these results and those of previous studies 10 , 11 , 19 increases confidence in the validity of these findings in the context of a real‐world study. Second, although prescription data were reported directly by the pharmacy, it only captured what was prescribed and dispensed to the patient. Because erenumab is administered through self‐injection, as opposed to an infusion clinic where administration is monitored and can be verified, it is impossible to know whether patients were truly taking erenumab as prescribed. This limitation is difficult to overcome for most research using real‐world data to study persistence or adherence, where the objective to gather data in a naturalistic and minimally invasive manner competes with methodological rigor. Finally, since attrition from the Go Program was deemed to be indicative of discontinuation of erenumab, it is still technically possible that a patient could discontinue from the Go Program and then access erenumab by paying out of pocket. However, this is very unlikely given the ease of free access to erenumab through the Go Program.
CONCLUSION
In summary, the results of this analysis demonstrated that the majority of patients prescribed erenumab in Canada during the postlaunch study period remained persistent to the therapy up to 450 days following treatment initiation. Additionally, treatment patterns suggest that the vast majority of patients are either initiated on, or titrate up to, the 140 mg erenumab dosage with positive persistence outcomes. These findings suggest that erenumab (both 70 and 140 mg) has been generally well tolerated by patients, increasing confidence that erenumab presents a promising new treatment option for the Canadian population with migraine that has experienced limited success with previous prophylactic therapies.
CONFLICT OF INTEREST
This study was conducted by Jagdeep Minhas, Calum S. Neish, G. Sarah Power, and Zhiyi Lan, who are employees of IQVIA and have provided consulting services to Novartis Pharmaceuticals Inc. Dr. Jonathan Gladstone and Dr. Sameer Chhibber received consultation fees from Novartis Pharmaceuticals Inc.
INSTITUTIONAL REVIEW BOARD APPROVAL
Institutional Review Board approval was granted by Advarra (Protocol # PRO00037279).
AUTHOR CONTRIBUTIONS
Study concept and design: Jagdeep Minhas, Calum S. Neish, G. Sarah Power, Zhiyi Lan, Driss Rochdi, Natacha Bastien. Acquisition of data: Jessica Lanthier‐Martel. Analysis and interpretation of data: Sameer Chhibber, Jonathan Gladstone, Jagdeep Minhas, Calum S. Neish, G. Sarah Power, Jessica Lanthier‐Martel, Zhiyi Lan, Driss Rochdi, Natacha Bastien. Drafting of the manuscript: Driss Rochdi, Natacha Bastien. Revising it for intellectual content: Jonathan Gladstone, Sameer Chhibber. Final approval of the completed manuscript: Driss Rochdi, Natacha Bastien.
Supporting information
Supplementary Material
Gladstone J, Chhibber S, Minhas J, et al. Real‐world persistence of erenumab for preventive treatment of chronic and episodic migraine: retrospective real‐world study. Headache. 2022;62:78–88. 10.1111/head.14218
Funding information
This study was sponsored by Novartis Pharmaceuticals Inc.
REFERENCES
- 1. Becker WJ, Gladstone JP, Aubé M. Migraine prevalence, diagnosis, and disability. Can J Neurol Sci. 2007;34(4):S3‐S9. [PubMed] [Google Scholar]
- 2. Cooke LJ, Becker WJ. Migraine prevalence, treatment and impact: the Canadian women and migraine study. Can J Neurol Sci. 2010;37(5):580‐587. doi: 10.1017/s0317167100010738 [DOI] [PubMed] [Google Scholar]
- 3. O’Brien B, Goeree R, Streiner D. Prevalence of migraine headache in Canada: a population‐based survey. Int J Epidemiol. 1994;23(5):1020‐1026. doi: 10.1093/ije/23.5.1020 [DOI] [PubMed] [Google Scholar]
- 4. Pryse‐Phillips W, Findlay H, Tugwell P, Edmeads J, Murray TJ, Nelson RF. A Canadian population survey on the clinical, epidemiologic and societal impact of migraine and tension‐type headache. Can J Neurol Sci. 1992;19(3):333‐339 [PubMed] [Google Scholar]
- 5. Stovner LJ, Nichols E, Steiner TJ, et al. Global, regional, and national burden of migraine and tension‐type headache, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2018;17(11):954‐976. doi: 10.1016/S1474-4422(18)30322-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Becker WJ, Christie SN, Mackie G, Cooper P, Canadian Headache Society Migraine Strategy Task Force . Consensus statement: the development of a national Canadian migraine strategy. Can J Neurol Sci. 2010;37(4):449‐456. doi: 10.1017/s0317167100010453 [DOI] [PubMed] [Google Scholar]
- 7. Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1‐211. doi: 10.1177/0333102417738202 [DOI] [PubMed] [Google Scholar]
- 8. Pringsheim T, Davenport WJ, Mackie G, et al. Canadian Headache Society guideline for migraine prophylaxis. Can J Neurol Sci. 2012;39:S1‐S59. [PubMed] [Google Scholar]
- 9. Jelinski SE, Becker WJ, Christie SN, et al. Clinical features and pharmacological treatment of migraine patients referred to headache specialists in Canada. Cephalalgia. 2006;26(5):578‐588. doi: 10.1111/j.1468-2982.2005.01077.x [DOI] [PubMed] [Google Scholar]
- 10. Hepp Z, Bloudek LM, Varon SF. Systematic review of migraine prophylaxis adherence and persistence. J Manag Care Pharm. 2014;20(1):22‐33. doi: 10.18553/jmcp.2014.20.1.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hepp Z, Dodick DW, Varon SF, et al. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: a retrospective claims analysis. Cephalalgia. 2017;37(5):470‐485. doi: 10.1177/0333102416678382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lattanzi S, Brigo F, Trinka E, et al. Erenumab for preventive treatment of migraine: a systematic review and meta‐analysis of efficacy and safety. Drugs. 2019;79(4):417‐431. doi: 10.1007/s40265-019-01069-1 [DOI] [PubMed] [Google Scholar]
- 13. Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double‐blind, placebo‐controlled phase 2 trial. Lancet Neurol. 2017;16(6):425‐434. doi: 10.1016/S1474-4422(17)30083-2 [DOI] [PubMed] [Google Scholar]
- 14. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123‐2132. doi: 10.1056/NEJMoa1705848 [DOI] [PubMed] [Google Scholar]
- 15. Dodick D, Ashina M, Brandes JL, et al. ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38(6):1026‐1037. doi: 10.1177/0333102418759786 [DOI] [PubMed] [Google Scholar]
- 16. Novartis Pharmaceuticals Canada Inc . Aimovig (erenumab) [product monograph]. Published online 2019. [Google Scholar]
- 17. Fink SA, Brown RS, Jr . Survival analysis. Gastroenterol Hepatol. 2006;2(5):380. [PMC free article] [PubMed] [Google Scholar]
- 18. Dodick DW. Migraine. Lancet. 2018;391:1315‐1330. doi: 10.1016/S0140-6736(18)30478-1 [DOI] [PubMed] [Google Scholar]
- 19. Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS‐II). Headache. 2013;53(4):644‐655. doi: 10.1111/head.12055 [DOI] [PubMed] [Google Scholar]
- 20. Kanaan S, Hettie G, Loder E, Burch R. Real‐world effectiveness and tolerability of erenumab: a retrospective cohort study. Cephalalgia. 2020;40(13):1511‐1522. doi: 10.1177/0333102420946725 [DOI] [PubMed] [Google Scholar]
- 21. Robblee J, Devick KL, Mendez N, Potter J, Slonaker J, Starling AJ. Real‐world patient experience with erenumab for the preventive treatment of migraine. Headache. 2020;60(9):2014‐2025. doi: 10.1111/head.13951 [DOI] [PubMed] [Google Scholar]
- 22. Lambru G, Hill B, Murphy M, Tylova I, Andreou AP. A prospective real‐world analysis of erenumab in refractory chronic migraine. J Headache Pain. 2020;21(1):61. doi: 10.1186/s10194-020-01127-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


