Abstract
In randomized clinical trials, the androgen‐receptor inhibitor enzalutamide has demonstrated efficacy and safety in metastatic castration‐resistant prostate cancer (mCRPC). This study captured efficacy, safety and patient‐reported outcomes (PROs) of enzalutamide in mCRPC patients in a real‐world European setting. PREMISE (NCT0249574) was a European, long‐term, prospective, observational study in mCRPC patients prescribed enzalutamide as part of standard clinical practice. Patients were categorized based on prior docetaxel and/or abiraterone use. The primary endpoint was time to treatment failure (TTF), defined as time from enzalutamide initiation to permanent treatment discontinuation for any reason. Secondary endpoints included prostate‐specific antigen (PSA) response, time to PSA progression, time to disease progression and safety. PROs included EuroQol 5‐Dimension, 5‐Level questionnaire, Functional Assessment of Cancer Therapy—Prostate and Brief Pain Inventory—Short Form. Overall, 1732 men were enrolled. Median TTF with enzalutamide was 12.9 months in the chemotherapy‐ and abiraterone‐naïve cohort (Cohort 1) and 8.4 months in the postchemotherapy and abiraterone‐naïve cohort (Cohort 2). Clinical outcomes based on secondary endpoints also varied between cohorts. Cohorts 1 and 2 showed small improvements in health‐related quality of life and pain status. The proportions of patients reporting treatment‐emergent adverse events (TEAEs) were 51.0% and 62.2% in Cohorts 1 and 2, respectively; enzalutamide‐related TEAEs were similar in both cohorts. The most frequent TEAE across cohorts was fatigue. These data from unselected mCRPC patients in European, real‐world, clinical‐practice settings confirmed the benefits of enzalutamide previously shown in clinical trial outcomes, with safety results consistent with enzalutamide's known safety profile.
Keywords: enzalutamide, Europe, metastatic castration‐resistant prostate cancer
Short abstract
What's new?
In clinical trials, the androgen‐receptor inhibitor enzalutamide has demonstrated efficacy and safety in metastatic castration‐resistant prostate cancer (mCRPC). However, results in the real world may differ from those in controlled studies. This large, prospective study thus assessed unselected mCRPC patients with different prior treatment histories, who were then treated with enzalutamide. The results confirm and validate the benefits of enzalutamide in real‐world, clinical‐practice settings that were previously seen in clinical‐trial outcomes. These include improved health‐related quality of life (HRQoL).
Abbreviations
- ADT
androgen deprivation therapy
- BPI‐SF
Brief Pain Inventory—Short Form
- CI
confidence interval
- EQ
EuroQol
- EQ‐5D‐5L
EuroQol 5‐Dimension, 5‐Level
- FACT‐G
Functional Assessment of Cancer Therapy—General
- FACT‐P
Functional Assessment of Cancer Therapy—Prostate
- FACT‐PCS
Functional Assessment of Cancer Therapy—Prostate Cancer Subscale
- FAS
full analysis set
- HRQoL
health‐related quality of life
- IQR
interquartile range
- IR
incidence rate
- mCRPC
metastatic castration‐resistant prostate cancer
- MID
minimally important difference
- PCWG
Prostate Cancer Working Group
- PRO
patient‐reported outcome
- PSA
prostate‐specific antigen
- QoL
quality of life
- SAF
safety analysis set
- TEAE
treatment‐emergent adverse event
- TTF
time to treatment failure
- VAS
visual analog scale
1. INTRODUCTION
Globally, prostate cancer is the second most frequent cancer and the fifth leading cause of death in men. 1 An estimated 449 800 new cases and 107 300 prostate cancer‐related deaths across the disease continuum occurred in Europe in 2018. 2 Advanced cases are usually treated with androgen deprivation therapy (ADT), but eventually they will progress to metastatic castration‐resistant prostate cancer (mCRPC), which is associated with reduced quality of life (QoL) and limited survival. 3 Therefore, effective treatments are needed to meaningfully extend mCRPC patients' survival and maintain or improve their QoL.
Enzalutamide is an oral androgen‐receptor inhibitor, either approved or under regulatory consideration for approval for castration‐resistant prostate cancer, irrespective of the presence of metastases and metastatic castration‐sensitive prostate cancer (also known as metastatic hormone‐sensitive prostate cancer) around the world. 4 , 5 , 6 The efficacy and safety of enzalutamide in chemotherapy‐naïve mCRPC patients was established in the phase III PREVAIL randomized clinical trial (NCT01212991), which showed significant improvements in radiographic progression‐free survival and overall survival with enzalutamide plus ADT vs placebo plus ADT. 7 Enzalutamide also demonstrated significant improvements in overall survival vs placebo in mCRPC patients previously treated with docetaxel in the phase III AFFIRM trial (NCT00974311). 8 Data from PREVAIL and AFFIRM also showed health‐related (HR) QoL benefits associated with enzalutamide vs placebo in mCRPC based on patient‐reported outcomes (PROs), 9 , 10 , 11 including delays in pain progression. 9 A further open‐label, single‐arm phase IV trial assessing the efficacy and safety of enzalutamide in mCRPC patients who previously progressed and were treated with abiraterone for at least 24 weeks (NCT02116582) showed antitumor activity in some patients with mCRPC regardless of chemotherapy treatment history. 12
While these clinical trials of enzalutamide demonstrated efficacy and safety in selected mCRPC patient populations, limited data from large‐scale studies are available that examine key clinical efficacy and health‐related quality of life (HRQoL) outcomes with enzalutamide in a real‐world setting. 13 , 14 , 15 Therefore, the objectives of our study were to capture efficacy, safety and PRO data in European patients with mCRPC who were prescribed enzalutamide in an observational clinical practice setting.
2. METHODS
2.1. Study design and conduct
PREMISE was a long‐term, prospective, observational study (NCT02495974) in Europe.
Eligible patients were men with mCRPC who were prescribed enzalutamide as a part of routine clinical practice (Figure S1). Abiraterone treatment history was based on prior concurrent abiraterone acetate treatment with prednisone/prednisolone. Patients with a history of abiraterone prior to chemotherapy were not included in the study; prior abiraterone use was only permitted if it followed chemotherapy, then directly preceded enzalutamide. Chemotherapy treatment history was based on prior docetaxel use only; patients who had received any other chemotherapeutic agents were not included in the study. Patients were free to discontinue enzalutamide treatment at any time for any reason, including treatment‐emergent adverse events (TEAEs), progressive disease, death and withdrawal of consent.
Patients were categorized into one of four cohorts based on their treatment history prior to enzalutamide initiation: Cohort 1 (chemotherapy naïve and abiraterone naïve); Cohort 2 (postchemotherapy and abiraterone naïve); Cohort 3 (chemotherapy naïve and postabiraterone); and Cohort 4 (postchemotherapy and postabiraterone [patients who received prior chemotherapy and then abiraterone consecutively]).
2.2. Data collection
Data were collected from September 8, 2015 (first informed consent) through February 8, 2019 (last end‐of‐study visit). A baseline visit occurred following the decision to prescribe enzalutamide but prior to starting enzalutamide. Data for key endpoints and for safety analysis were collected at baseline, at routine visits every 3 months (±4 weeks) for the duration of enzalutamide treatment (up to a maximum of 18 months), and at another visit 3 to 6 months following treatment discontinuation for patients who discontinued within 18 months of starting enzalutamide.
2.3. Study endpoints
The primary endpoint was time to treatment failure (TTF), defined as the time from initiation of enzalutamide to the date of permanent treatment discontinuation for any reason, including disease progression, skeletal‐related events, treatment toxicity, patient preference or death. Secondary endpoints included other efficacy assessments, PRO assessments and safety. Efficacy assessments included time to prostate‐specific antigen (PSA) progression, time to disease progression (including PSA progression, radiographic progression and clinical progression) and PSA response rate. PRO assessments included EuroQol (EQ) 5‐Dimension, 5‐Level (EQ‐5D‐5L) questionnaire, 16 Functional Assessment of Cancer Therapy—Prostate (FACT‐P), 17 and Brief Pain Inventory–Short Form (BPI‐SF). 18 Safety was also assessed. Detailed endpoint definitions can be found in Table S1.
2.4. Data analysis
The safety analysis set (SAF) consisted of patients who received at least one dose of enzalutamide. The full analysis set (FAS) consisted of all patients in the SAF who had at least one postbaseline efficacy assessment. The SAF was used to analyze demographic and baseline characteristics, PRO data and safety data. The FAS was used for primary and secondary efficacy endpoint data analyses.
TTF was estimated and presented graphically using Kaplan‐Meier methods. Median estimates with their corresponding 95% confidence intervals (CIs), estimated using the Brookmeyer and Crowley method and the number of patients with treatment failure, were summarized descriptively. Subgroup analysis for TTF was also performed for age; two subgroups were based on the median age of all patients in the population (ie, ≤75 years vs 75 years). Time to PSA progression and time to disease progression were analyzed in the same way as TTF, respectively, using PSA progression and disease progression as the event. The proportions of patients with a 30%, 50% or 90% PSA response were estimated, including their 95% CIs, using the Clopper‐Pearson method based on exact binomial distribution.
Mean EQ‐5D‐5L index and EQ visual analog scale (EQ‐VAS) scores per visit were summarized descriptively. EQ index scores were calculated using the 3L “crosswalk” algorithm as recommended at the time of analysis by the National Institute for Health and Care Excellence. 19 , 20 FACT‐P mean scores, which are a composite of scores from a core general scale (FACT‐G) and a FACT‐P Prostate Cancer Subscale (FACT‐PCS), were summarized descriptively per visit. For BPI‐SF, separate scores are presented for pain severity (mean of items 3‐6) and pain interference (mean of item 9); mean scores were summarized descriptively.
Proportions of patients showing a meaningful change (improvement or worsening) in EQ‐5D‐5L, FACT‐P and BPI‐SF scores at each visit were assessed using minimally important differences (MIDs). Details on MID thresholds used for PRO instruments can be found in Table S2.
Safety was assessed via the reporting of TEAEs or deaths that occurred from the time of consent until 30 days following enzalutamide discontinuation. Additionally, the incidence rate, defined as the number of TEAEs per 100 patient‐years, was also calculated in order to account for the difference in treatment duration across cohorts. The incidence rate is calculated as follows: the number of TEAEs x 100, divided by the sum of treatment‐emergent period duration of all patients treated in the corresponding cohort in years. Given the small patient numbers in Cohorts 3 (n = 42) and 4 (n = 96), the described clinical and PRO findings were limited to Cohorts 1 and 2. However, data from Cohorts 3 and 4 are still presented in Supporting Information.
3. RESULTS
3.1. Patient disposition, baseline demographics and disease history
A total of 1732 patients were enrolled and treated across 16 European countries (Figure S1). Baseline demographics and disease history characteristics varied between cohorts (Table S3). Patients tended to be older in Cohort 1 (77.0 years) and slightly younger in Cohort 2 (71.0 years). The median time from diagnosis of prostate cancer to enrollment varied slightly between Cohorts 1 (4.9 years) and 2 (4.0 years).
3.2. Primary endpoint: TTF
Median TTF (95% CI) was 12.9 months (12.0‐13.8) in Cohort 1 and 8.4 months (7.0‐9.8) in Cohort 2 (Table 1; Figure 1). The most frequently reported and primary reason for treatment failure was PSA progression in Cohort 1 (16.1%) and radiographic progression in Cohort 2 (23.9%). The proportions of patients with treatment failure due to TEAEs were 15.9% in Cohort 1 and 12.7% in Cohort 2. TTF was also measured for Cohorts 3 and 4, but sample size limitations prevented conclusive analyses (Figure S2; Table S4). Similar results were observed in age‐based subgroups. However, in Cohort 1, TTF was longer in the younger subgroup aged ≤75 years (n = 509; 15.1 months [13.7‐17.0]) than in the subgroup aged >75 years (n = 662; 11.2 months [10.2‐12.6]) [Table S5].
TABLE 1.
Primary endpoint: TTF
| Enzalutamide Cohort 1: chemotherapy naïve + abiraterone naïve (n = 1171) | Enzalutamide Cohort 2: postchemotherapy + abiraterone naïve (n = 418) | |
|---|---|---|
| TTF, months, median (95% CI) a | 12.9 (12.0‐13.8) | 8.4 (7.0‐9.8) |
| Patients with treatment failure | 650 (58.9) | 311 (74.4) |
| Primary reason for treatment failure b | ||
| PSA progression c | 188 (16.1) | 93 (22.2) |
| Radiographic progression | 144 (12.3) | 100 (23.9) |
| Clinical progression | 72 (6.1) | 40 (9.6) |
| Lack of efficacy | 10 (0.9) | 4 (1.0) |
| TEAEs | 186 (15.9) | 53 (12.7) |
| Withdrawal of consent by patient | 24 (2.0) | 5 (1.2) |
| Deaths d | 38 (3.2) | 9 (2.2) |
| Other | 28 (2.4) | 7 (1.7) |
Note: Data presented as n (%) unless otherwise stated. Data from FAS (n = 1727).
Defined as the time from initiation of enzalutamide to the date of treatment discontinuation for any reason, including disease progression, skeletal‐related events, treatment toxicity, patient preference or death.
No skeletal‐related events leading to treatment failure were observed across all cohorts.
Defined as a PSA rise of ≥25% and an absolute increase of ≥2 ng/mL above nadir.
Deaths that were the primary cause of treatment failure were those that occurred during the 18‐month study period.
FIGURE 1.
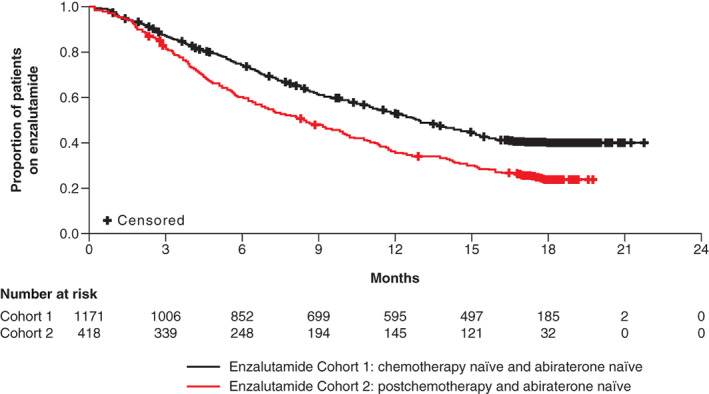
Kaplan‐Meier estimate of TTF: Cohorts 1 and 2
3.3. Secondary endpoints
3.3.1. Time to PSA progression and PSA response rate
Median time to PSA progression (95% CI) was 17.7 months (16.3‐18.7) in Cohort 1 and 9.8 months (8.5‐11.5) in Cohort 2 (Table 2). In Cohorts 1 and 2, 40.4% and 57.7% of patients experienced PSA progression, respectively. At least 76% and 61% of patients in Cohorts 1 and 2, respectively, had a PSA decline of ≥50% from baseline. Time to PSA progression and PSA response rate were measured in Cohorts 3 and 4, but sample size limitations prevented conclusive analyses (Table S6).
TABLE 2.
Secondary endpoint: efficacy
| Enzalutamide Cohort 1: chemotherapy naïve + abiraterone naïve (n = 1171) | Enzalutamide Cohort 2: postchemotherapy + abiraterone naïve (n = 418) | |
|---|---|---|
| PSA progression a | ||
| Time to PSA progression, months, median (95% CI) | 17.7 (16.3‐18.7) | 9.8 (8.5‐11.5) |
| Patients with PSA progression | 476 (40.6) | 241 (57.7) |
| PSA response rate b | ||
| 30% (95% CI) | 82.4 (80.1‐84.7) | 69.1 (64.5‐73.7) |
| 50% (95% CI) | 76.2 (73.6‐78.7) | 60.5 (55.6‐65.4) |
| 90% (95% CI) | 44.9 (41.9‐47.9) | 31.2 (26.5‐35.8) |
| Disease progression | ||
| Time to disease progression, months, median (95% CI) | 13.9 (12.8‐15.1) | 7.2 (6.2‐8.3) |
| Patients with disease progression c | 595 (50.8) | 302 (72.2) |
| PSA progression | 473 (40.4) | 241 (57.7) |
| Radiographic progression | 327 (27.9) | 190 (45.5) |
| Clinical progression | 163 (13.9) | 101 (24.2) |
Note: Data presented as n (%) unless otherwise stated. Data from FAS (n = 1727).
PSA progression was defined as a PSA rise of ≥25% and an absolute increase of ≥2 ng/mL above nadir.
Defined as best percentage change in PSA levels from baseline.
The percentage of patients with any type of disease progression calculated within the overall cohort; a patient can belong to more than one subcategory of disease progression.
3.3.2. Time to disease progression
Median time to disease progression (95% CI) was 13.9 months (12.8‐15.1) in Cohort 1 and 7.2 months (6.2‐8.3) in Cohort 2 (Table 2). Time to disease progression were also measured in Cohorts 3 and 4, but sample size limitations prevented conclusive analyses (Table S6).
3.3.3. Descriptive results of PRO summary scores
Baseline HRQoL was assessed using EQ‐5D‐5L (Figure 2A,B; Table S7) and FACT‐P scales (Figure 2C‐E; Table S8). EQ index, EQ‐VAS and FACT‐P scores increased slightly over time in both Cohorts 1 and 2, indicating a small improvement in HRQoL. Baseline pain scores, as assessed by BPI‐SF, were 2.01 in Cohort 1 and 2.22 in Cohort 2 (Figure 2F,G; Table S9). BPI‐SF scores remained comparable to baseline in both cohorts. Baseline HRQoL and pain was also assessed in Cohorts 3 and 4, but sample size limitations prevented conclusive analyses (Tables S7‐S9).
FIGURE 2.
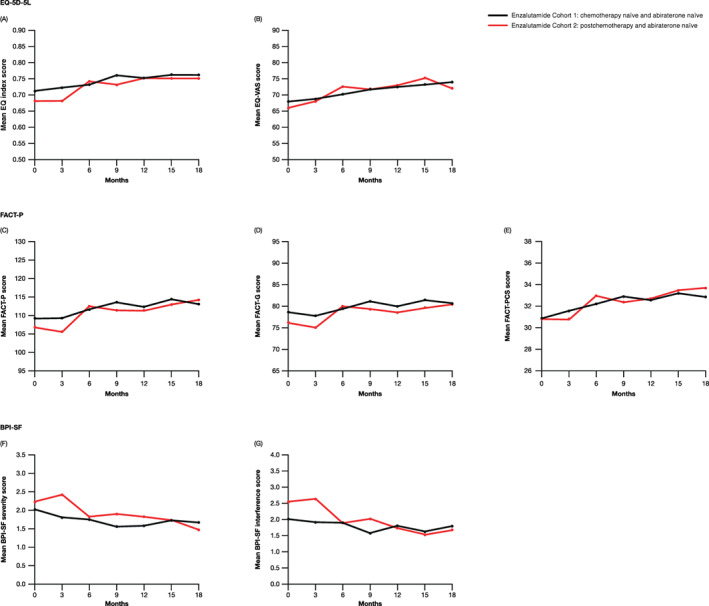
Mean EQ‐5D‐5L (A and B), FACT‐P (C‐E) and BPI‐SF (F and G) scores over time in Cohorts 1 and 2
3.3.4. MID analyses of PRO instruments
MID analyses on the PRO instruments were used to determine if patients improved, remained unchanged or worsened at visits up to month 9 (Table 3). When based on EQ index, the majority of patients in Cohorts 1 or 2 either reported improved (21%‐23%) or maintained (51%‐58%) HRQoL over time. Similarly, the majority of patients in Cohorts 1 and 2 improved (27%‐39%) or maintained (33%‐47%) their HRQoL over time based on EQ‐VAS scores.
TABLE 3.
Secondary endpoint: treatment response at selected study visits based on MIDs for the EQ‐5D‐5L, FACT‐P and BPI‐SF scales a
| Enzalutamide Cohort 1: chemotherapy naïve + abiraterone naïve (n = 1171) | Enzalutamide Cohort 2: postchemotherapy + abiraterone naïve (n = 418) | |||||
|---|---|---|---|---|---|---|
| Improve | No change | Worsen | Improve | No change | Worsen | |
| EQ index (MID, 0.12) b | ||||||
| 3 months | 184 (20.9) | 506 (57.5) | 190 (21.6) | 71 (20.7) | 192 (56.0) | 80 (23.3) |
| 6 months | 146 (21.8) | 357 (53.3) | 167 (24.9) | 56 (25.2) | 119 (53.6) | 47 (21.2) |
| 9 months | 117 (21.7) | 290 (53.7) | 133 (24.6) | 37 (23.0) | 83 (51.6) | 41 (25.5) |
| EQ‐VAS (MID, 7) c | ||||||
| 3 months | 238 (27.2) | 410 (46.9) | 227 (25.9) | 117 (34.3) | 116 (34.0) | 108 (31.7) |
| 6 months | 184 (27.6) | 292 (43.9) | 189 (28.3) | 87 (39.0) | 83 (37.2) | 53 (23.7) |
| 9 months | 160 (30.0) | 213 (40.1) | 158 (29.7) | 53 (32.9) | 65 (40.4) | 43 (26.7) |
| FACT‐P (MID, 6) d | ||||||
| 3 months | 267 (31.3) | 319 (37.4) | 266 (31.2) | 112 (33.1) | 91 (26.9) | 135 (39.9) |
| 6 months | 206 (31.5) | 207 (31.7) | 241 (36.9) | 73 (33.8) | 70 (32.4) | 73 (33.8) |
| 9 months | 178 (34.4) | 163 (31.5) | 176 (34.0) | 47 (29.9) | 48 (30.6) | 62 (39.5) |
| BPI‐SF severity (MID, 2) e | ||||||
| 3 months | 82 (10.0) | 640 (77.8) | 101 (12.3) | 53 (16.3) | 237 (72.2) | 36 (11.0) |
| 6 months | 71 (11.4) | 466 (74.6) | 88 (14.1) | 33 (15.2) | 160 (73.7) | 24 (11.1) |
| 9 months | 55 (10.9) | 378 (75.2) | 70 (13.9) | 19 (13.4) | 108 (76.1) | 15 (10.6) |
| BPI‐SF interference (MID, 1.25) e | ||||||
| 3 months | 166 (20.5) | 475 (58.6) | 169 (20.9) | 76 (23.9) | 171 (53.8) | 71 (22.3) |
| 6 months | 135 (22.3) | 364 (60.2) | 106 (17.5) | 50 (23.6) | 110 (51.9) | 52 (24.5) |
| 9 months | 102 (21.1) | 284 (58.7) | 98 (20.3) | 32 (22.9) | 78 (55.7) | 30 (21.4) |
Note: Data are presented as n (%) unless stated otherwise.
Due to patient dropout at months 12, 15 and 18, data are only shown from months 3‐9.
On EQ index, 0 = equivalent to dead and 1 = perfect health.
EQ‐VAS is measured on a scale from 0 to 100, with higher values indicating better QoL.
FACT‐P total score (range, 0‐156) is the combination of FACT‐G (range, 0‐108) and FACT‐PCS (range, 0‐48) subscale scores, with higher scores indicating better QoL.
BPI‐SF severity and interference are measured on a scale from 0 to 10, with higher scores indicating less pain severity or interference.
When assessing HRQoL based on FACT‐P, a composite of FACT‐G and FACT‐PCS subscales, patients in Cohorts 1 and 2 reported improvements (30%‐34%) or no change (27%‐37%) across visits. When the subscales were assessed individually, patients across both cohorts reported slightly fewer improvements on the FACT‐G and slightly more on the FACT‐PCS (Table S10).
A majority of patients in Cohorts 1 and 2 reported no change when assessing for pain severity (improved, 10%‐16%; no change, 72%‐78%) and for pain interference (improved, 21%‐24%; no change, 52%‐60%) based on the BPI‐SF scale. MID analyses for EQ‐5D‐5L, FACT‐P, BPI‐SF were also measured in Cohorts 3 and 4, but sample size limitations prevented conclusive analyses (Figure S3; Table S11).
3.4. Safety
The intended length of our study was 18 months, including at most one follow‐up visit posttreatment. The median treatment duration was 372 days in Cohort 1 and 253 days in Cohort 2 (Table 4). The treatment discontinuation rate was 58.9% in Cohort 1 and 74.4% in Cohort 2 (Table 1). Slightly more patients in Cohort 1 experienced dose interruptions (9.1%) than in Cohort 2 (5.7%), which may be due to a longer treatment duration (Table 4).
TABLE 4.
Overview of dosing status, TEAEs and deaths a per 100 patient‐years
| Enzalutamide Cohort 1: chemotherapy naïve + abiraterone naïve (n = 1175) | Enzalutamide Cohort 2: postchemotherapy + abiraterone naïve (n = 418) | |
|---|---|---|
| Treatment duration, median, days (IQR) | 372.0 (167‐533) | 253.0 (117‐509) |
| Dosing status per patient b | ||
| Dose modifications | 132 (11.2) | 29 (6.9) |
| Dose interruptions | 107 (9.1) | 24 (5.7) |
| n (%) | IR c | n (%) | IR c | |
|---|---|---|---|---|
| TEAEs | 599 (51.0) | 178.0 | 260 (62.2) | 292.9 |
| Treatment‐related TEAEs | 321 (27.3) | 52.6 | 113 (27.0) | 66.8 |
| Serious TEAEs | 236 (20.1) | 33.9 | 116 (27.8) | 53.4 |
| Serious treatment‐related TEAEs | 39 (3.3) | 4.1 | 17 (4.1) | 6.1 |
| TEAEs leading to treatment discontinuation | 247 (21.0) | 24.2 | 84 (20.1) | 25.9 |
| Treatment‐related TEAEs leading to treatment discontinuation | 122 (10.4) | 12.7 | 26 (6.2) | 8.6 |
| Deaths a | 91 (7.7) | 7.5 | 29 (6.9) | 8.1 |
| Most frequently reported TEAEs (in ≥5% of patients in any cohort) d | ||||
| Fatigue | 170 (14.5) | 15.7 | 60 (14.4) | 17.2 |
| Back pain | 79 (6.7) | 6.9 | 36 (8.6) | 10.6 |
| Asthenia | 77 (6.6) | 8.5 | 39 (9.3) | 12.2 |
| Decreased appetite | 68 (5.8) | 5.7 | 26 (6.2) | 7.5 |
| Malignant neoplasm progression | 61 (5.2) | 5.1 | 34 (8.1) | 9.5 |
| Hot flush | 60 (5.1) | 5.0 | 25 (6.0) | 7.0 |
| Nausea | 46 (3.9) | 4.2 | 30 (7.2) | 8.6 |
| Constipation | 43 (3.7) | 3.7 | 21 (5.0) | 6.1 |
| Bone pain | 42 (3.6) | 4.1 | 38 (9.1) | 11.7 |
| Arthralgia | 41 (3.5) | 4.0 | 26 (6.2) | 8.1 |
| General physical health deterioration | 30 (2.6) | 2.7 | 16 (3.8) | 4.7 |
| Anemia | 25 (2.1) | 3.0 | 25 (6.0) | 12.8 |
Note: Data presented as n (%) unless otherwise stated. Data from SAF (n = 1732).
Abbreviation: IQR, interquartile range; IR, incidence rate.
TEAEs and deaths were reported from the time of consent until 30 days following enzalutamide treatment discontinuation.
Patients can be counted in both dose changes and dose interruptions but will only count a maximum of once in each.
The IR, or the number of TEAEs per 100 patient‐years, is calculated as the number of TEAEs × 100, divided by the sum of treatment‐emergent period duration of all patients treated in the corresponding cohort in years.
TEAEs were sorted by frequency in Cohort 1, as this was the largest group.
Given the varied length of treatment duration across cohorts, TEAEs were normalized to 100 patient‐years to calculate an incidence rate (IR) [Table 4]. The occurrence of TEAEs was 51.0% (IR, 178.0) in Cohort 1 and 62.2% (IR, 292.9) in Cohort 2; the most frequently reported TEAE occurring in ≥5% of patients within either cohort was fatigue (Table 4). Hypertension was reported in low proportions in Cohorts 1 (2.1%) and 2 (2.4%).
The most frequently reported primary causes of treatment discontinuation were PSA progression and radiographic progression (Table 1); however, a proportion of patients also discontinued enzalutamide treatment due to TEAEs. Rates of treatment discontinuation due to TEAEs were comparable in Cohorts 1 (21.0%; IR, 24.2) and 2 (20.1%; IR, 25.9) [Table 4]. The most frequently reported TEAEs leading to permanent treatment discontinuation were fatigue (3.0%) for Cohort 1 and malignant neoplasm progression for Cohort 2 (4.3%). Safety was also assessed in Cohorts 3 and 4, but sample size limitations prevented conclusive analyses (Table S12).
4. DISCUSSION
To date, PREMISE is the largest European, observational study focusing on the clinical benefit and safety of enzalutamide in patients with mCRPC in the real‐world setting. TTF and secondary endpoints varied between cohorts. PSA progression and radiographic progression were the primary reasons for treatment failure in Cohorts 1 and 2, respectively. During the study, HRQoL scores slightly increased in abiraterone‐naïve cohorts, indicating a small improvement in health status, while BPI‐SF scores slightly decreased in all cohorts, indicating small reductions in pain severity and interference. Interpretation of results, in particular for the PRO data, from the cohorts that had received prior abiraterone was limited by their smaller sample size. The safety results observed in this real‐world setting were consistent with the known safety profile of enzalutamide from clinical studies.
The data from PREMISE confirm enzalutamide's clinical efficacy and tolerability in patients with mCRPC who underwent different prior treatments. Observed efficacy outcomes suggest chemotherapy‐ and abiraterone‐naïve patients received considerable clinical benefit from enzalutamide, while patients who previously received chemotherapy and abiraterone and who were further along in disease progression received minimal benefit. Other smaller observational and retrospective studies have shown that the clinical efficacy of enzalutamide is diminished when preceded by chemotherapy and abiraterone. 21 , 22 , 23 , 24 Preclinical in vitro data suggest that this diminished benefit of enzalutamide may be due to cross‐resistance following abiraterone treatment. 25 Taken together, the data highlight the rationale of using novel drugs such as enzalutamide at earlier stages in the disease continuum, as revealed by developments in other disease settings. 26 , 27 , 28
In our study, PSA progression was the most common type of disease progression, occurring in 41% to 63% of patients. We also observed that 28% to 46% of patients had radiographic progression; however, imaging was at the investigator's discretion, and asymptomatic or early radiographic progression may not have been detected. Our data, alongside similar findings from a PREVAIL post hoc analysis, 29 suggest radiographic progression may occur concurrently or in the absence of PSA progression. Further clinical application of these data would suggest that PSA monitoring should not be solely used to assess disease progression, especially in the latest stages of mCRPC disease. However, this should also be balanced by considerations around access to imaging in clinical practice vs the clinical trial setting and patients' overall benefit from treatment.
Patient baseline demographics were broadly comparable between the PREMISE cohorts and respective enzalutamide clinical trials; however, there are some differences such as age and time since prostate cancer diagnosis. 7 , 8 , 12 We also observed some differences in secondary endpoint outcomes between specific PREMISE cohorts and their respective trials. Chemotherapy‐ and abiraterone‐naïve patients in PREMISE had longer time to PSA progression compared to the equivalent population in PREVAIL (17.7 months in PREMISE vs 11.2 months in PREVAIL 7 ). These differences, however, could be due to longer PSA measurement intervals in PREMISE, which were based on investigator discretion rather than standardized PSA measurements in line with Prostate Cancer Clinical Trials Working Group (PCWG) 2 guidelines. 30
Calculated IRs combined with efficacy results suggest chemotherapy‐ and abiraterone‐naïve patients tolerated enzalutamide well. We observed, however, that a higher proportion of patients in Cohort 1 reported dose modifications and interruptions when compared to other cohorts. We hypothesize that this may be attributable to the longer observation period in Cohort 1 (median treatment duration, 372 days) when compared to other cohorts (132‐253 days), as rate of dose modification and interruptions were not time adjusted. Although hypertension was a frequently reported TEAE in PREVAIL (13%) 7 and AFFIRM (6.6%), 8 it was only reported in 0% to 2.9% of patients across all cohorts in PREMISE. TEAEs as the primary reason for treatment discontinuation were reported at a higher rate in PREMISE (12.7%‐22.7%) compared to respective trials (PREVAIL, 6%; AFFIRM, 8%; postabiraterone trial, 8%). 7 , 8 , 12 It is important to note that the highest proportions of TEAEs reported as the primary cause occurred in Cohort 4 (22.7%), a smaller (n = 96), but most heavily pretreated, cohort.
A key strength of our study is the selection of TTF as the primary endpoint and its relevance to the clinical setting. By accounting for various types of disease progression, death, potential toxicities and patient preference, TTF captures decision‐based nuances on enzalutamide dosing and cessation within individual clinical practices. Notably, we observed that PSA progression alone was documented as the primary reason for treatment failure in 16% to 24% of patients across all cohorts (Table 1). This result is contrary to PCWG2 guidelines, which recommend that radiographic or clinical progression is needed in addition to PSA progression in order to declare treatment failure. 30 Overall, this finding highlights the need to reinforce PCWG2 and 3 recommendations via continued education. Additional strengths include the range of participating countries involved (n = 16), the generalizability of the study data due to the nonrestrictive enrollment of eligible patients, and the inclusion of patient groups not typically captured in registrational trials, reflecting the outcomes in routine clinical practice.
Our study comes with some limitations. Due to the study's observational, nonrandomized design, data interpretation is limited due to lack of a comparative control group and formal statistical comparisons. The interpretation of efficacy and safety findings is limited by the small number of patients in Cohorts 3 and 4, which may impact the data's robustness. Due to follow‐up time limitations, many patients were censored, mainly in Cohort 1; thus, TTF estimations do not account for long‐term responders. Another limitation is potential selection bias, as recruitment at specific study sites may not be representative of the larger population and patients were not randomly assigned to enzalutamide treatment.
5. CONCLUSIONS
These data from a large cohort of unselected European patients confirmed that TTF with enzalutamide is prolonged in men with mCRPC receiving enzalutamide in the first‐line setting, with similar findings observed in secondary efficacy endpoints. Trends toward improved HRQoL and pain with enzalutamide were observed in abiraterone‐naïve patients regardless of prior chemotherapy. Safety results in this observational study were consistent with the known safety profile of enzalutamide in the clinical setting. These results support real‐world applicability and independent validation of data from enzalutamide clinical studies in men with mCRPC and extend these findings in examining outcomes in patient groups typically not represented in registrational studies.
CONFLICT OF INTEREST
Heather Payne has attended and received honoraria for advisory boards and lectures, has received travel funds and has served as a consultant for Astellas, AstraZeneca, Bayer, Boston Scientific, Ferring, Janssen, Novartis and Sanofi Aventis during the conduct of the study. Angus Robinson has served as a study investigator with Astellas during the conduct of this study. Bernard Rappe has served as a study investigator with Astellas during the conduct of this study. Serena Hilman has served as a study investigator with Astellas during the conduct of this study and has received financial support to attend educational events from Bayer and Janssen. Ugo De Giorgi has served as a consultant for Astellas, Bayer, BMS, Ipsen, Janssen, Novartis, Pfizer and Sanofi; has received research funding from AstraZeneca, Roche and Sanofi; and has received travel funds from BMS, Ipsen, Janssen, Pfizer and Roche during the conduct of the study. Steven Joniau has served as study investigator with Astellas during the conduct of our study; has received grants, honoraria and nonfinancial support from Astellas, Bayer, Ferring, Ipsen and Janssen; has received honoraria and nonfinancial support from Sanofi; and has received nonfinancial support from AstraZeneca, MDX Health, Pfizer and Roche during the conduct of the study. Roberto Bordonaro has served as a study investigator with Astellas during the conduct of this study. Stéphane Mallick has served as a study investigator with Astellas during the conduct of this study. Louis‐Marie Dourthe has served as a study investigator with Astellas during the conduct of this study and as an investigator for NSD, Janssen and Ipsen. Moisés Mira Flores has served as a study investigator with Astellas; has received grants and nonfinancial support from Astellas and Janssen; and has received nonfinancial support from Ipsen during the conduct of the study. Josep Gumà has served as a study investigator with Astellas; has received grants from Amgen, Ipsen, Pierre Fabre, Roche, Sanofi and Vifor; and has received personal fees from Sanofi. Benoit Baron was employed by Astellas during the conduct of the study. Aurea Duran and Alessandra Pranzo are employees of Astellas Pharma Europe Ltd. Alexis Serikoff is an employee of Astellas Pharma France. David Mott, Mike Herdman and Marco Pavesi have received grants from Astellas Pharma Europe Ltd. during the conduct of this study. David Mott and Mike Herdman are employed at the Office of Health Economics, a registered charity and an independent research organization that receives funding from a variety of private and public sector sources. Marco Pavesi is employed at the European Foundation for the Study of Chronic Liver Failure, which receives funding from a variety of private and public sector sources. Maria De Santis has served as a study investigator with Astellas; has received honoraria from Amgen, Astellas, AstraZeneca, Basilea, Bayer, BioClin, BMS, Calithera, Clovis, Eisai, Ferring, Incyte, Ipsen, Janssen, MSD, Merck, Novartis, Pfizer, Pierre Fabre Oncology, Roche, Sandoz, Sanofi, Seattle Genetics and Takeda; and has received nonfinancial support from the EAU Prostate Cancer Guidelines Panel, the ESMO Bladder Cancer Practice Guidelines Panel and the S3‐Leitlinie Harnblasenkarzinom panel during the conduct of the study.
ETHICS STATEMENT
PREMISE (NCT02495974) was conducted in compliance with European requirements for ensuring the rights of participants in noninterventional studies. Every participating study site received ethical approval from their respective ethics committees and all participating patients provided written informed consent prior to study initiation.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGMENTS
The authors are grateful to Dr Fernández Calvo Ovidio and Dr Borque Fernando Ȧngel for their impactful contributions to the study, and to Georgia Gourgioti, MSc, for her statistical assistance. Medical writing and editorial assistance were provided by Folabomi Oladosu, PhD, Jane Beck, MA, and Lauren Smith, BA (Hons), from Complete HealthVizion, funded by Astellas Pharma Inc and Pfizer Inc, the codevelopers of enzalutamide.
Payne H, Robinson A, Rappe B, et al. A European, prospective, observational study of enzalutamide in patients with metastatic castration‐resistant prostate cancer: PREMISE. Int. J. Cancer. 2022;150(5):837‐846. doi: 10.1002/ijc.33845
Funding information Pfizer; Astellas Pharma Inc
DATA AVAILABILITY STATEMENT
Researchers may request access to anonymized participant‐level data, trial‐level data and protocols from Astellas‐sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx. Further information is available from the corresponding author upon request.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356‐387. [DOI] [PubMed] [Google Scholar]
- 3. Mottet N, van den Bergh RCN, Briers E, et al. EAU—ESTRO—ESUR—SIOG Guidelines on Prostate Cancer ; 2018. http://uroweb.org/guideline/prostate-cancer/. Accessed November 2, 2020.
- 4. Tran C, Ouk S, Clegg NJ, et al. Development of a second‐generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Astellas Pharma US Inc , Medivation Inc. XTANDI US Prescribing Information ; 2019. https://www.astellas.us/docs/us/12A005-ENZ-WPI.pdf. Accessed March 19, 2020.
- 6. European Medicines Agency . Xtandi—Summary of opinion (post authorisation) ; 2021. https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-xtandi-ii-47-g_en.pdf. Accessed May 5, 2021.
- 7. Beer TM, Armstrong AJ, Sternberg CN, et al. Enzalutamide in men with chemotherapy‐naive metastatic prostate cancer (mCRPC): results of phase III PREVAIL study. J Clin Oncol. 2014;32(suppl):LBA1. [Google Scholar]
- 8. Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187‐1197. [DOI] [PubMed] [Google Scholar]
- 9. Loriot Y, Miller K, Sternberg CN, et al. Effect of enzalutamide on health‐related quality of life, pain, and skeletal‐related events in asymptomatic and minimally symptomatic, chemotherapy‐naive patients with metastatic castration‐resistant prostate cancer (PREVAIL): results from a randomised, phase 3 trial. Lancet Oncol. 2015;16:509‐521. [DOI] [PubMed] [Google Scholar]
- 10. Cella D, Ivanescu C, Holmstrom S, Bui CN, Spalding J, Fizazi K. Impact of enzalutamide on quality of life in men with metastatic castration‐resistant prostate cancer after chemotherapy: additional analyses from the AFFIRM randomized clinical trial. Ann Oncol. 2015;26:179‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fizazi K, Scher HI, Miller K, et al. Effect of enzalutamide on time to first skeletal‐related event, pain, and quality of life in men with castration‐resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol. 2014;15:1147‐1156. [DOI] [PubMed] [Google Scholar]
- 12. de Bono JS, Chowdhury S, Feyerabend S, et al. Antitumour activity and safety of enzalutamide in patients with metastatic castration‐resistant prostate cancer previously treated with abiraterone acetate plus prednisone for ≥24 weeks in Europe. Eur Urol. 2018;74:37‐45. [DOI] [PubMed] [Google Scholar]
- 13. Cheng HH, Gulati R, Azad A, et al. Activity of enzalutamide in men with metastatic castration‐resistant prostate cancer is affected by prior treatment with abiraterone and/or docetaxel. Prostate Cancer Prostatic Dis. 2015;18:122‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Delanoy N, Hardy‐Bessard A‐C, Efstathiou E, et al. Sequencing of taxanes and new androgen‐targeted therapies in metastatic castration‐resistant prostate cancer: results of the international multicentre retrospective CATS database. Eur Urol Oncol. 2018;1:467‐475. [DOI] [PubMed] [Google Scholar]
- 15. Caffo O, Wissing M, Bianchini D, et al. Survival outcomes from a cumulative analysis of worldwide observational studies on sequential use of new agents in metastatic castration‐resistant prostate cancer. Clin Genitourin Cancer. 2019;18:69‐76. [DOI] [PubMed] [Google Scholar]
- 16. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Qual Life Res. 2011;20:1727‐1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the Functional Assessment of Cancer Therapy—prostate instrument. Urology. 1997;50(6):920‐928. [DOI] [PubMed] [Google Scholar]
- 18. Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singapore. 1994;23:129‐138. [PubMed] [Google Scholar]
- 19. van Hout B, Janssen MF, Feng Y‐S, et al. Interim scoring for the EQ‐5D‐5L: mapping the EQ‐5D‐5L to EQ‐5D‐3L value sets. Value Health. 2012;15:708‐715. [DOI] [PubMed] [Google Scholar]
- 20. National Institute for Health and Care Excellence . Position Statement on Use of the EQ‐5D‐5L Value Set for England (updated October 2019); 2019. https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l. Accessed March 17, 2020.
- 21. Schmid SC, Geith A, Boker A, et al. Enzalutamide after docetaxel and abiraterone therapy in metastatic castration‐resistant prostate cancer. Adv Ther. 2014;31:234‐241. [DOI] [PubMed] [Google Scholar]
- 22. Badrising S, van der Noort V, van Oort IM, et al. Clinical activity and tolerability of enzalutamide (MDV3100) in patients with metastatic, castration‐resistant prostate cancer who progress after docetaxel and abiraterone treatment. Cancer. 2014;120:968‐975. [DOI] [PubMed] [Google Scholar]
- 23. Brasso K, Thomsen FB, Schrader AJ, et al. Enzalutamide antitumour activity against metastatic castration‐resistant prostate cancer previously treated with docetaxel and abiraterone: a multicentre analysis. Eur Urol. 2015;68:317‐324. [DOI] [PubMed] [Google Scholar]
- 24. Azad AA, Eigl BJ, Murray RN, Kollmannsberger C, Chi KN. Efficacy of enzalutamide following abiraterone acetate in chemotherapy‐naive metastatic castration‐resistant prostate cancer patients. Eur Urol. 2015;67:23‐29. [DOI] [PubMed] [Google Scholar]
- 25. Lombard AP, Liu L, Cucchiara V, et al. Intra versus inter cross‐resistance determines treatment sequence between taxane and AR‐targeting therapies in advanced prostate cancer. Mol Cancer Ther. 2018;17:2197‐2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone‐sensitive prostate cancer. J Clin Oncol. 2019;37:2974‐2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stenzl A, Dunshee C, De Giorgi U, et al. Effect of enzalutamide plus androgen deprivation therapy on health‐related quality of life in patients with metastatic hormone‐sensitive prostate cancer: an analysis of the ARCHES randomised, placebo‐controlled, phase 3 study. Eur Urol. 2020;78:603‐614. [DOI] [PubMed] [Google Scholar]
- 28. Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, castration‐resistant prostate cancer. N Engl J Med. 2020;382:2197‐2206. [DOI] [PubMed] [Google Scholar]
- 29. Bryce AH, Alumkal JJ, Armstrong A, et al. Radiographic progression with nonrising PSA in metastatic castration‐resistant prostate cancer: post hoc analysis of PREVAIL. Prostate Cancer Prostatic Dis. 2017;20:221‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials working group. J Clin Oncol. 2008;26:1148‐1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
Researchers may request access to anonymized participant‐level data, trial‐level data and protocols from Astellas‐sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx. Further information is available from the corresponding author upon request.


