Abstract
Psychedelic drugs are gaining attention from the scientific community as potential new compounds for the treatment of psychiatric diseases such as mood and substance use disorders. The 5‐HT2A receptor has been identified as the main molecular target, and early studies pointed to an effect on the expression of neuroplasticity genes. Analysing RNA‐seq data from the prefrontal cortex of rats chronically treated with lysergic acid diethylamide (LSD), we describe the psychedelic‐induced rewiring of gene co‐expression networks, which become less centralised but more complex, with an overall increase in signalling entropy typical of highly plastic systems. Intriguingly, signalling entropy mirrors, at the molecular level, the increased brain entropy reported through neuroimaging studies in human, suggesting the underlying mechanisms of higher‐order phenomena. Moreover, from the analysis of network topology, we identify potential transcriptional regulators and propose the involvement of different cell types in psychedelics’ activity.
Psychedelics are emerging drugs for the therapy of psychiatric conditions, but their molecular mechanisms of action have not been yet fully elucidated. Analysing RNA‐seq data of rats chronically treated with lysergic acid diethylamide (LSD), we show that psychedelics induce the reorganization of gene co‐expression networks in rats’ prefrontal cortex (PFC), with a global increase in signalling entropy and network de‐centralization. Based on gene networks’ structure, we identify novel potential molecular regulators of their transcriptional effects (e.g. Tcf4) and suggest increased cell variability and plasticity as being involved in their mechanisms of action.
Abbreviations
- Arnt
aryl hydrocarbon receptor nuclear translocator
- BDNF
brain derived neurotrophic factor
- CTRL
control
- DOI
1‐(2,5‐dimethoxy‐4‐iodophenyl)‐2‐aminopropane
- FDR
false discovery rate
- Egr1
early growth response protein 1
- GO
gene ontology
- LSD
lysergic acid diethylamide
- ME
module eigengene
- MEG
magnetoencephalography
- fMRI
functional magnetic resonance imaging
- PCA
principal component analysis
- PFC
prefrontal cortex
- PTSD
post‐traumatic stress disorder
- RPM
reads per million
- Tcf4
transcription factor 4
- Tet1
ten‐eleven translocation methylcytosine dioxygenase 1
- TF
transcription factor
- WGCNA
weighted gene co‐expression network analysis
1. INTRODUCTION
Psychedelics, such as lysergic acid diethylamide (LSD), psilocybin and the substituted amphetamine 1‐(2,5‐dimethoxy‐4‐iodophenyl)‐2‐aminopropane (DOI), are psychoactive compounds that induce profound acute subjective effects in humans, affecting perception, behaviour, and mood through activation of 5‐HT2A receptors (Nichols, 2004). The alterations in perception and thought processes can be interpreted as transient psychotic‐like states (Nichols, 2016) that resemble symptoms of mental disorders such as schizophrenia (Schmid et al., 2015; Vollenweider et al., 1998). Hence, they have been used to mimic schizophrenia in animal models (Marona‐Lewicka et al., 2011; Martin et al., 2014), similarly to the previously employed NMDA receptor antagonists ketamine and PCP (phencyclidine) (Winship et al., 2019). However, psychedelics have also been recently shown to produce long‐lasting beneficial effects in patients with mood and substance use disorders: several pilot clinical trials have shown that single acute administrations of psilocybin or LSD produce symptoms’ reduction for at least six months (Carhart‐Harris et al., 2016, 2017, 2018; Davis et al., 2020; Griffiths et al., 2016; Santos et al., 2016), contrary to most classical therapeutics for mood disorders (e.g. serotonin reuptake inhibitors), which require weeks to exert their effects. Moreover, single administrations of psilocybin produce long‐lasting changes in personality traits (e.g. higher openness) that extend also to healthy subjects (Erritzoe et al., 2018; MacLean et al., 2011). Importantly, these treatments have been proven safe, without subsequent persisting psychosis, drug abuse or any significant impairment in cognitive or physical functioning (Studerus et al., 2011). Nevertheless, in rare instances perceptual alterations can reoccur as “benign flashbacks” (Martinotti et al., 2018), also known as hallucinogen persisting perception disorder.
The molecular target responsible for psychedelics’ behavioural and psychological effects in humans has been identified as the 5‐HT2A receptor (Madsen et al., 2019; Preller et al., 2018; Vollenweider & Preller, 2020). Nevertheless, molecular mechanisms underlying acute changes in brain activity and lasting psychological effects have been less investigated. Neuroplasticity‐related genes and immediate‐early genes have been reported to be over‐expressed upon psychedelics’ administration in rodent models, especially in areas with dense 5‐HT2A receptor expression like the prefrontal cortex (PFC) (Benekareddy et al., 2013; González‐Maeso et al., 2003, 2007; Jefsen et al., 2020; Nichols et al., 2003). These results have been confirmed and expanded through the use of high‐throughput technologies, profiling the whole transcriptome, such as microarrays (Nichols & Sanders‐Bush, 2002, 2004) and, more recently, RNA sequencing (Donovan et al., 2021; Martin et al., 2014; de la Fuente Revenga et al., 2021).
Given their relevance for basic research and the study of schizophrenia, on the one hand, and as promising therapeutic agents for psychiatric disorders on the other, much research effort has been devoted to investigating their mechanisms of action. In particular, most research interest has been focussed on neuroimaging (e.g. magnetoencephalography—MEG—or functional magnetic resonance imaging—fMRI) of subjects during psychedelics’ acute effects, leading to the observation of increased variability of spontaneous brain activity and changes in brain networks’ connectivity (Preller et al., 2018, 2019, 2020). Using these measurements, the entropy (variability or information content) of brain activity has been quantified and shown to increase upon psychedelics (Carhart‐Harris et al., 2014). Interestingly, the acute increase of brain entropy under LSD correlates with changes in personality traits after two weeks (Lebedev et al., 2016). This theory has gained additional support since its first formulation through new measurements (Carhart‐Harris, 2018; Herzog et al., 2020; Lebedev et al., 2016; Schartner et al., 2017).
More generally, entropy is a measure of the number of possible states of a system and can manifest at different levels in signals of various kinds, not only from the brain's electrical activity but also from gene expression. In cell biology, it intuitively quantifies cell plasticity and robustness to perturbations. Indeed, stem cells have been shown to have high transcriptional entropy that decreases across differentiation (Gulati et al., 2020; Teschendorff & Enver, 2017), and tumours, in particular those resistant to drug treatments, display higher entropy than normal tissue (Conforte et al., 2019; Nijman, 2020; Savino et al., 2020). Both systems are highly plastic, able to adapt to the environment and diversify in response to stimuli. However, diversity can also increase with the loss of stable organising principles, correlating with dysfunctions such as ageing (Hernando‐Herraez et al., 2019).
Here, analysing the RNA‐seq data of rats chronically treated with LSD (Martin et al., 2014), we dig deeper into the impact of psychedelics on gene expression. We investigate how gene co‐expression networks reorganise upon LSD and identify Tcf4 (Transcription factor 4) as a potential player in network regulation. Moreover, we find differences in network topology for microenvironmental or neuronal networks, suggesting that both cell compartments might be involved in the long‐term effects of repeated LSD administration. Finally, we observe that the increase in brain entropy after a single dose of the drug is paralleled by increased transcriptional entropy, which declines after a few days in the absence of a new intake. Repeated drug administration results in a sustained increase in transcriptional entropy, lasting at least a few weeks after discontinuation of the drug. Moreover, we identify epigenetic factors that might be responsible for these lasting effects, and the potential involvement of alternative splicing patterns and transposable elements’ activation in the overall activity of psychedelics.
2. MATERIALS AND METHODS
2.1. Data collection and pre‐processing
2.1.1. RNA‐seq data of rats chronically treated with LSD
Drug treatment and tissue collection for the chronic LSD cohort were described previously (Martin et al., 2014). Briefly, male Sprague‐Dawley rats (8–10 weeks of age; 180–200 g) were divided into two groups: saline control, and LSD treated. LSD‐treated animals were injected (i.p.) with 0.16 mg/kg of LSD tartrate every other day for 90 days. Saline‐treated animals were injected with sterile saline every other day for 90 days on the same day as LSD‐treated animals. 28 days after the final treatment, rats were decapitated without anaesthesia, brains rapidly removed, quickly frozen on dry ice, and stored at −80°C until dissection. The mPFC from one hemisphere was dissected from frozen brain and processed for mRNA, protein, and gDNA using the Norgen All‐In‐One kit. The purified RNA was then sent for library prep and sequencing by the Genome Sciences Resource core at Vanderbilt University as described in Martin et al., 2014. Raw sequence data were reanalysed in this study.
Reads were trimmed and quality checked with TrimGalore (https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/), aligned to the Rattus norvegicus genome (rn6) with Hisat2 (Kim et al., 2019), and counts obtained with HTSeq‐count (Anders et al., 2015). The resulting processed data were RPM (reads per million) normalised and only genes with at least 5 reads in at least 10 samples were retained for further analyses.
Data have been deposited to the Gene Expression Omnibus (GSE179379).
2.1.2. Microarray data of rats after 90 min of LSD treatment
Experimental setting and data collection are described in Nichols & Sanders‐Bush, 2004. Raw data were normalised with the rma function from the Affy package (Gautier et al., 2004). Probes were mapped to gene symbols with the GPL85 annotation from Gene Expression Omnibus.
Data have been deposited to the Gene Expression Omnibus (GSE179378). RNA‐seq and microarray data are part of the same SuperSeries (GSE179380).
2.1.3. RNA‐seq of mice's cortical neurons upon treatment with DOI
Counts were downloaded from Gene Expression Omnibus (GSE161626). Technical replicates were averaged and then normalised and log transformed similarly to rats’ PFC data.
Splicing junctions were quantified with SGSeq (Goldstein et al., 2016). Transposable elements were quantified with TEtranscripts (Jin et al., 2015), after aligning the reads with Hisat2 allowing reporting up to 1000 mapping sites per read.
2.2. Subsampling
Subsampling was performed by randomly selecting 10 million reads from the original fastq files, and repeating the pre‐processing, as described above.
2.3. Differential expression and functional enrichment
Differential gene expression was performed with DESeq2 (Love et al., 2014) on count data. Gene Ontology enrichment was calculated with the enrichGO function from the clusterProfiler package (Yu et al., 2012), using “Biological Process” or “Molecular Function” GO categories and default parameters. For modules’ enrichment, all genes belonging to a module different from the “grey” (unconnected) module were used as background.
Transcription Factors (TFs) were defined based on the Gene Ontology category GO0003700, downloaded from Biomart (https://www.ensembl.org/biomart/).
Targets of each TF were obtained from the ChEA database, and downloaded from https://maayanlab.cloud/Enrichr/#stats (ChEA_2016).
An exploratory enrichment analysis of differentially spliced genes has been performed with Enrichr (https://maayanlab.cloud/Enrichr/). The Wikipathway gene lists has been downloaded from https://maayanlab.cloud/Enrichr/#stats (Wikipathway_2021_Human) and used for testing the enrichment of significantly differentially spliced genes.
The GSEA analysis of genes with the highest entropy change was performed with the fgsea (Korotkevich et al., 2021) and msigdbr packages (https://CRAN.R‐project.org/package=msigdbr), using the category “C5” (comprising GO categories).
2.4. Co‐expression networks
The co‐expression network was obtained with the blockwiseModules function from the WGCNA package (Langfelder & Horvath, 2008), setting the parameters networkType and TOMtype to “signed”, and beta = 12.
The module eigengene (ME) was calculated with the function moduleEigengenes, and weighted degree within each module (kWithin) was calculated with the function intramodularConnectivity.fromExpr, setting networkType=”signed” and power = 12.
Differential activity of modules was tested comparing ME between treatment groups with the Wilcoxon test, and then correcting for multiple testing and obtaining false discovery rates for each module.
Connectivity represents genes’ weighted degree, with weights based on the adjacency matrix, either across the whole network or considering only a module's genes. The connectivity in a specific condition was obtained by calculating the adjacency using only samples belonging to that group.
Module eigengene in different datasets was obtained as the projection of samples on the first principal component of a PCA (principal component analysis, function prcomp) performed on rats’ PFC data using only the genes of the chosen module.
2.5. Signalling entropy
Signalling entropy was calculated with the SCENT R package (Teschendorff & Enver, 2017), either using the provided PPI network or the co‐expression network built in this work. Input data (RNA‐seq) were log transformed with an offset of 1.1.
2.6. Between‐sample entropy
Between‐sample entropy was obtained as 1‐correlation, with the correlation obtained from pairwise Pearson's correlation between samples using gene expression profiles or splicing junction usage profiles.
2.7. Gene mapping
ID mapping and orthologs identification was performed with the biomaRt R package (Durinck et al., 2009). In case of multiple IDs mapping to the same symbol, the ID with the highest average expression across all dataset's values was chosen.
2.8. Statistical analyses
All statistical analyses were performed with R 4.0.4 (R Core Team, 2018).
Packages used for plotting are R base graphics, ggplot2 (Valero‐Mora, 2010) and ggsignif (https://CRAN.R‐project.org/package=ggsignif), and pheatmap (https://CRAN.R‐project.org/package=pheatmap).
Groups’ means were compared with the Wilcoxon rank sum test, while correlations’ statistical significance was calculated with the cor.test function. Only tests passing the threshold FDR <0.05 (false discovery rate) are shown. Non‐parametric tests were employed given the limited sample size, hence no assessment of data normality was needed. No tests for outliers were performed and all collected data were used.
Given that data had already been collected, no specific analysis to predetermine the sample size was performed. Nevertheless, the primary data here employed comprised 10 samples per group, highly exceeding previous similar studies (Nichols & Sanders‐Bush, 2004).
No randomisation or blinding procedures were applied in this study.
We confirm that institutional ethical approval was not required for this study.
The study was not pre‐registered.
A preprint of this work was posted on Biorxiv (doi: https://doi.org/10.1101/2021.06.23.449556).
3. RESULTS
3.1. Chronic LSD exposure has a long‐term effect on the epigenetic machinery
We made use of the previously described system of rats chronically treated with LSD, which show persistent altered locomotor activity and social interactions long after drug discontinuation (Marona‐Lewicka et al., 2011) and have hence been proposed as a rat model system for the study of schizophrenia. RNA‐seq data on the mPFC (medial prefrontal cortex) of these rats four weeks after cessation of the drug showed persisting transcriptional effects and enrichment for differential regulation of schizophrenia‐related genes (Martin et al., 2014).
We further pursued the investigation of these high depth RNA‐seq data to study the rearrangements of gene co‐expression networks in response to prolonged, repeated LSD administration.
First, from the analysis of differentially expressed genes (Table S1), we observed increased expression of genes related to neuroplasticity and neurotransmission, confirming previous reports of bdnf (brain derived neurotrophic factor) up‐regulation and of increased dendritogenesis upon psychedelics treatment (Ly et al., 2018). Indeed, “dendrite development” is amongst the top significantly enriched Gene Ontology (GO) categories for up‐regulated genes (Table S2). Also, we found significant enrichment for circadian rhythm genes, possibly underlying the alteration of sleep cycles (Barbanoj et al., 2008). Interestingly, “covalent chromatin modification” and “histone modification” showed strong enrichment for up‐regulated genes, among which we found Tet1 (Ten‐eleven translocation methylcytosine dioxygenase 1), involved in the erasure of DNA methylation (Wu & Zhang, 2017). This indicates that repeated psychedelics’ administration affects the epigenetic machinery, thus suggesting a mechanism for their long‐term effects. Down‐regulated genes were found to be primarily involved in oxidative phosphorylation (Table S3).
We then built gene co‐expression networks to investigate potential regulatory relationships between genes and their changes upon treatment. We applied the widely used WGCNA algorithm (Weighted Gene Co‐expression Network Analysis) (Zhang & Horvath, 2005) and identified eighteen clusters of genes (modules, Table S4), six of which show differential activity between LSD and Ctrl (control) conditions (Figure 1), as quantified through the module eigengene (ME), an ideal meta‐gene representing the whole module's expression. Modules higher in LSD‐treated rats are enriched for regulation of chromatin organisation, vesicle‐mediated transport in synapse, and cell‐cell adhesion (Table S5), indicating that our observations on differentially expressed GO categories are robust and independent of the analysis method. Although modules down‐regulated upon treatment do not show significant enrichment for GO biological processes, they include genes with molecular functions such as GTP‐binding, hydrolase activity, and structural constituents of ribosomes (Table S6). However, it is important to note that these are not the only enriched categories for each module (Tables S5 and S6), and labels assigned based on the most significantly enriched GO categories should not be interpreted strictly to exclude other categories.
FIGURE 1.
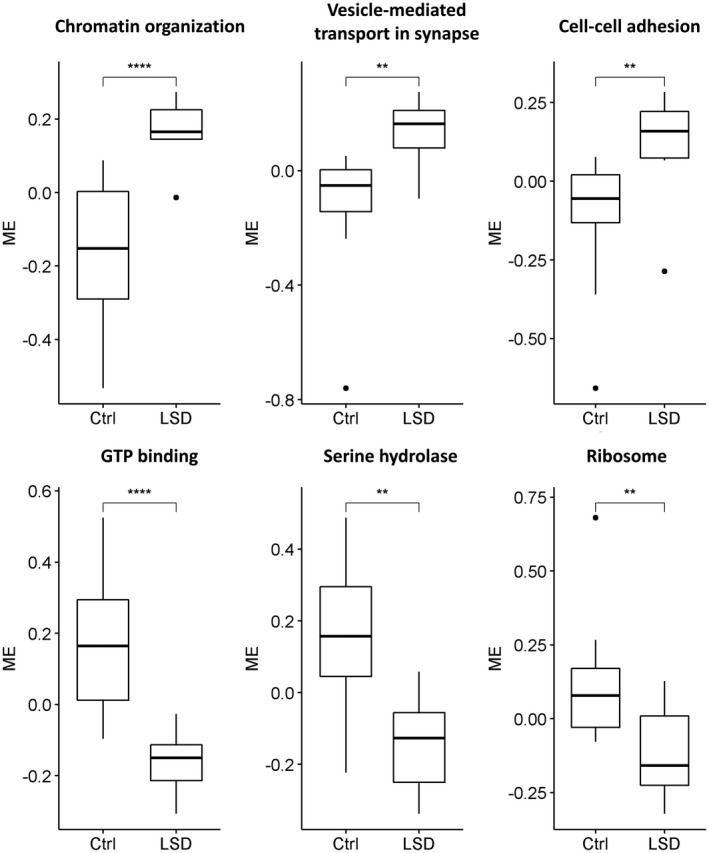
LSD‐regulated co‐expression modules. Three modules, enriched for the GO categories “chromatin organisation”, “vesicle‐mediated transport in synapse” and “cell‐cell adhesion”, respectively, are more active in LSD‐treated rats than in controls, while the three modules enriched for the molecular functions “GTP‐binding”, “serine hydrolase” and “ribosome” are less active in the LSD condition. Activity is quantified through the module eigengene (ME), the projection of samples on the first principal component obtained using the genes of the module. N = 10, number of rats per treatment group. Significance is obtained with the Wilcoxon rank sum test. *0.05, **0.01, ***0.001, ****0.0001
Using the network's topological features, we looked for potential regulators of the LSD modules. Indeed, genes centrally located within the network and displaying many connections (hubs) have been reported to be crucial for the system's maintenance (Jeong et al., 2001; Pržulj et al., 2004). Hence, we selected genes amongst the first 10 central transcription factors (TFs) in each module and tested whether there is independent evidence of their regulatory activity on the same module's genes using the ChEA database (Lachmann et al., 2010). With these criteria, we identified Tcf4 as a potential regulator of the “Vesicle transport” module (false discovery rate for each of the three available gene sets <3*10−4), and Arnt (Aryl hydrocarbon receptor nuclear translocator) as a potential regulator of the “Cell‐cell adhesion” module (false discovery rate = 0.0009), but no other genes passed these stringent filters.
3.2. Transcriptional entropy increases with LSD treatment
We investigated the signalling variability of gene networks by measuring transcriptional entropy. As mentioned in the introduction, transcriptional entropy has been paradoxically associated with both plasticity and ageing. Nevertheless, different measures have been used between the two contexts: in the first, entropy is quantified from the number of signalling paths in a protein‐protein interaction network or from the number of expressed genes in each sample (Gulati et al., 2020; Teschendorff & Enver, 2017), whereas in the second, differences between samples have been used as a metric to define entropy (Hernando‐Herraez et al., 2019). To distinguish these scenarios, we named these two kinds of entropies as “signalling” and “between‐sample” entropy, schematically described in Figure 2.
FIGURE 2.
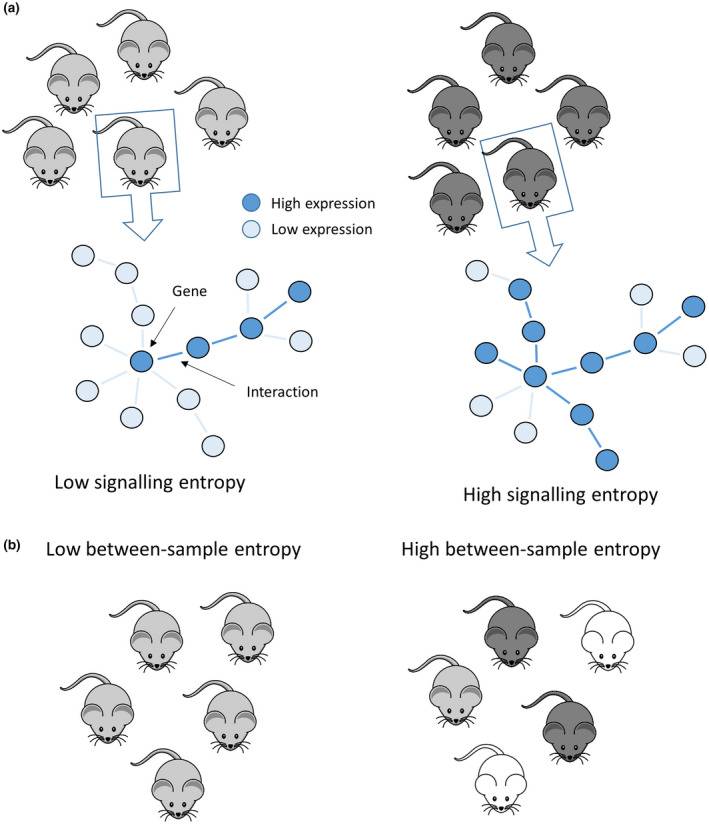
Schematic representation of signalling and between‐sample entropies. (a) Within each subject, a gene‐interaction network is obtained and the number of possible paths from each node is calculated based on gene expression levels. A few paths correspond to low entropy, while many paths correspond to high entropy. Hence, for each subject, a gene‐level entropy measure is obtained and then summarised as the overall subject specific signalling entropy. (b) Between‐sample entropy is defined as the variability between different subjects in a group. Hence, homogenous groups have low entropy, while diversified groups have high entropy
The first type of entropy is particularly interesting in this context. Networks with high signalling entropy have many active (expressed) nodes, which are available for interactions with their first neighbours in the network. In a multi‐cellular sample such as the whole mPFC, the genes that contribute to network complexity could derive from any of the sampled cells. Hence, many expressed genes might indicate: (1) a high cell‐type diversification (each cell with its type‐specific genes); (2) the existence of many functional states of the same cell type (with distinct gene expression); (3) a homogenous population of cells, with individual cells expressing many genes. For each of these possibilities, nodes with many connections are not expected to interact simultaneously with all their neighbours but to interact with one or a few in a specific timeframe or spatial location, taking part in different pathways in different instances. Hence, from the molecular point of view, a highly entropic network can be thought of as a network with many different potential states, which can be found simultaneously across distinct cell types or in different times/spatial locations of the same cell.
On the other side, between‐sample entropy considers the variability across subjects instead of quantifying gene interactions variability. This means that treatments leading to unpredictable effects on different subjects increase between‐sample entropy.
To ensure the robustness of our observations, we measured signalling entropy based on both protein‐protein interaction (PPI) networks, as in the originally published method (Teschendorff & Enver, 2017), or on our co‐expression network. Moreover, as additional measures of within‐sample variability or information content, we quantified the number of splicing junctions used in each sample and the proportion of reads mapped to transposable elements. With all metrics, we found a significant increase in the transcriptional complexity of the PFC of rats chronically treated with LSD with respect to saline controls, even after a withdrawal period of four weeks (Figure 3a–d). Of note, none of these measures is influenced by sequencing depth since differences are retained also by randomly sampling the data to have the same number of reads in each sample (Figure S1). On the contrary, we observed a decrease in between‐sample entropy, revealed by the lower samples’ divergence in the LSD condition at the gene expression and splicing usage levels (Figure 3e–f). Taken together, these observations suggest that LSD treatment induces increased plasticity‐like and reduced ageing‐like entropy.
FIGURE 3.
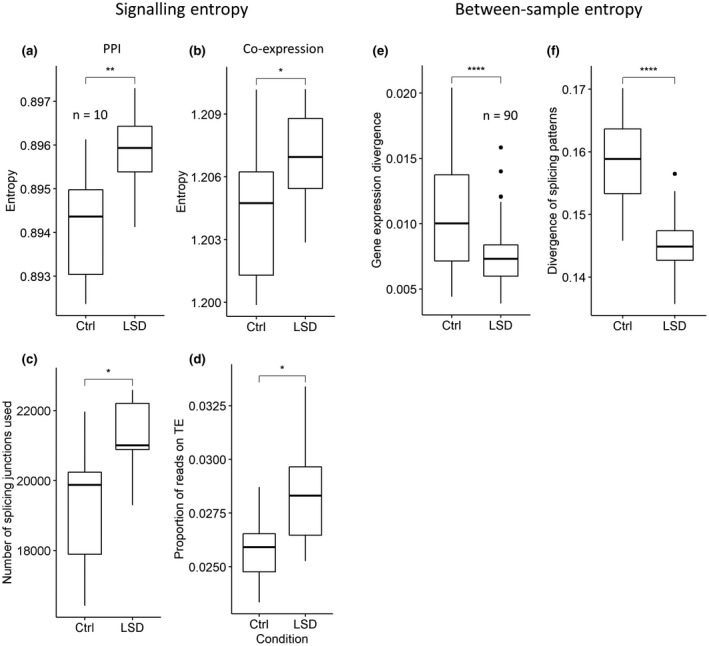
Signalling entropy increases in the LSD condition, while between‐sample entropy decreases. (a) Signalling entropy based on the PPI network; (b) signalling entropy based on the co‐expression network; (c) signalling diversification quantified as the number of different splicing junction used in each sample or (d) from the proportion of reads in each library mapping to transposable elements. (a–d n = 10, number of rats per treatment group). Between‐sample entropy measured from the correlation between samples using (e) gene expression or (f) splicing junction usage. (e–f n = 90, number of pairwise correlations between individual rats) Significance is obtained with the Wilcoxon rank sum test. *0.05, **0.01, ***0.001, ****0.0001
3.3. Co‐expression networks reorganise toward a less centralised topology
We next studied which nodes most strongly contribute to the overall signalling entropy increase, ranking the genes based on their average entropy change between the LSD and saline conditions. Performing a GSEA analysis on the ranked gene list, RNA processing and splicing, chromosome organisation, DNA repair, cell cycle, extracellular matrix, and chromosome organisation resulted as significantly enriched amongst the genes with the highest increase in entropy. Of note, increased entropy in genes regulating RNA processing and splicing could explain the larger set of splicing junctions detected in the LSD treatment group. Interestingly, the genes with altered splicing patterns are enriched for genes regulated by the splicing factor Nova.
In line with an increase in entropy, the overall connectivity of the co‐expression network decreases (Figure 4a), as previously shown for cancer networks (Schramm et al., 2010). Interestingly, not all modules rearrange their connections in the same way: despite most modules decreasing their co‐expression in the LSD‐treated group, indicating a de‐centralisation of the network, three display increased intramodular connectivity, indicating a tighter regulation of corresponding functions (Figure 4b). For the same three modules, the nodes that increase co‐expression connections tend to be the most central, while for the remaining modules we observe the opposite trend (Figure 4c, d). Accordingly, in most modules entropy increases more strongly for peripheral nodes, and only a few modules make an exception (Figure 4d). Of these, the “mesenchyme development” module shows coherent correlation sign between centrality and change in connectivity/entropy. This indicates that for most modules the nodes increasing signalling connections, reflecting on both entropy and intramodular weighted degree, are the most peripheral in the module, while hubs tend to lose connections. Intriguingly, the modules showing strong opposite trends, and therefore increasing the compactness and centralisation of the network, are enriched for mesenchyme development, regulation of immune system and extracellular matrix, categories that could reflect microenvironmental reorganisation, and suggesting that different cell types might be affected by prolonged LSD treatment in different ways.
FIGURE 4.
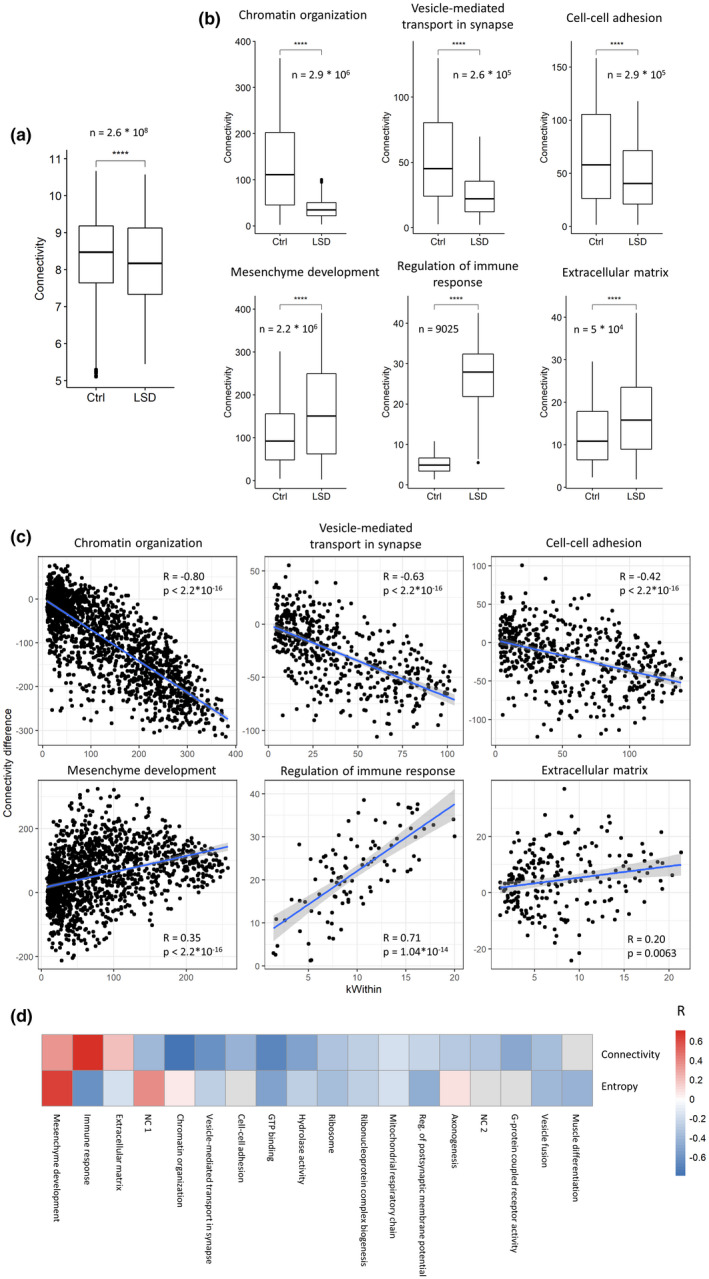
Different modules differently reorganise their structure upon LSD. (a) The overall network connectivity decreases in the LSD condition (n = 2.6 * 108, number of genes’ pairwise correlations per treatment group); (b) connectivity changes in the LSD‐induced modules and in three additional modules showing opposite trend (n = number of genes’ pairwise correlations per module per treatment group); (c) Gene‐wise relationship between change in connectivity and network centrality, measured as the intramodular connectivity (kWIthin). Together with each scatter plot, Pearson's correlations and p‐values are reported. (d) Heatmap summarising the correlation between node's centrality and connectivity change (first row) or entropy change (second row) for each module. Red indicates positive and blue indicates negative correlation. Non‐significant correlations are shown in grey
3.4. Persisting effects of repeated treatment resemble the transient effects of a single treatment
To explore the temporal dynamics of the transcriptional changes that we observe in chronically treated rats, we took advantage of two additional transcriptomic datasets, analysing PFCs from rat brains harvested 90 min after an acute administration of LSD (Nichols & Sanders‐Bush, 2004), or neurons isolated from the PFC of mice after 24 h, 48 h or 7 days of DOI administration (de la Fuente Revenga et al., 2021).
We envisioned at least two possible temporal dynamics: in the first model, psychedelics induce the well‐established transcriptional changes in neuroplasticity genes without immediately affecting the organisation of co‐expression modules nor transcriptional complexity, exerting this effect only after repeated treatments; in a second model, the network changes that we detect are induced acutely, and potentially show different post‐treatment dynamics in the case of single or multiple treatments.
Therefore, we tested whether our findings could be replicated in the two aforementioned single‐dose datasets. Co‐expression modules increasing in expression with chronic LSD treatment are strongly over‐expressed also after a single treatment with DOI and return to the baseline at 7 days (Figure S2). Additionally, the potential transcriptional regulator of the “vesicle transport” module, Tcf4, shows the same trend upon DOI treatment (Figure S3a). Modules down‐regulated upon chronic LSD treatment do not show a clear pattern, with only a slight trend toward down‐regulation at 24h and 48h of the GTP‐binding module (Figure S2). This suggests that, while the first three groups of genes are induced by acute treatment and require multiple administrations for sustained expression, the last three modules are repressed only upon chronic treatment. No significant differences could be detected in the microarray of LSD’s acute effects, possibly related to the small sample size (N = 3), to differences in the technologies, or to the smaller number of genes analysed in the array with respect to genome‐wide RNA‐seq (Figures S3b, S4). Nevertheless, we cannot exclude the lack of differential expression being because of differences in the biological system.
Similarly, we tested the change in signalling entropy: entropy is significantly increased by DOI at 24 h after treatment, returning to baseline at 7 days (Figure 5a), while in the LSD dataset we observed a trend towards increased signalling entropy in the treated group (Figure 5b), which nevertheless did not reach significance, likely because of the small sample size. Unfortunately, the small number of samples in the two additional datasets did not allow to have a reliable measure of between‐sample entropy.
FIGURE 5.
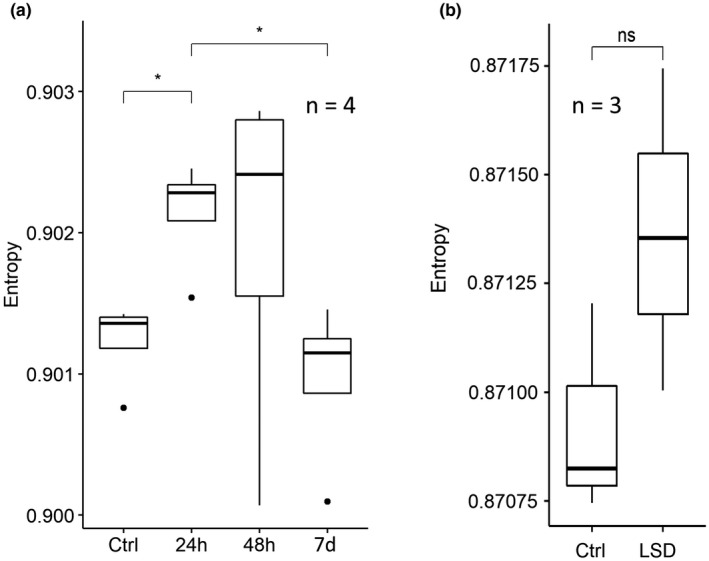
Signalling entropy transiently increase upon a single psychedelic's administration. (a) Signalling entropy of mice's PFC neurons at different time points after a single DOI’s administration (n = 4, number of mice per group); (b) Signalling entropy in rat's PFC 90 min after LSD treatment (n = 3, number of samples per group, each comprising two pooled rats’ PFCs). Significance is obtained with the Wilcoxon rank sum test. *0.05, ns = not significant
Therefore, these additional datasets do not support the first model: signalling entropy increases acutely even after a single treatment.
4. DISCUSSION
Psychedelic drugs are gaining attention from the scientific community as potential new compounds for the treatment of psychiatric diseases such as depression and PTSD (post‐traumatic stress disorder), but also as highways to explore the neurobiology of human consciousness. Also, the psychedelics’ induced alterations in perception and thought processes mimic symptoms of mental disorders such as schizophrenia, and hence they have been employed in animal models to study this disease.
Neuroimaging studies have shown their effects on brain connectivity networks in humans, where they induce the reorganisation of interacting areas leading to diminished activity of the default mode network and to increased variability of the electromagnetic signal, quantified as an increase in the entropy.
The main molecular target has been identified as the serotonin 5‐HT2A receptor. Early studies pointed to an effect on neuroplasticity‐related genes, but an extensive investigation of psychedelics’ induced gene expression changes is still lacking. Nevertheless, gene expression patterns and interactions strongly characterise the functioning of biological systems, implying that behavioural changes are often mediated by alterations in gene expression. The transcriptome, studied through microarray or RNA sequencing, is the most accessible layer of gene expression, and RNA‐seq is increasingly employed to explore the molecular mechanisms implicated in physiological, pathological, and drug‐induced processes.
Analysing RNA‐seq data from prefrontal cortices of rats chronically treated LSD three months after drug discontinuation (e.g. no drug or recent drug on‐board), we observe long‐lasting changes in gene expression, particularly in neuroplasticity and neurotransmission genes, in line with previous reports, but also in circadian rhythm and, importantly, in epigenetic modifiers. Indeed, Tet1, involved in the erasure of DNA methylation (Wu & Zhang, 2017), increases its expression upon LSD, and the “histone modification” GO category shows strong enrichment for up‐regulated genes. This indicates that repeated psychedelics’ administration affects the epigenetic machinery, thus proposing a mechanism for their long‐term effects. Interestingly, Tet1 is recruited by Egr1 (Early growth response protein (1) (Sun et al., 2019), one of the classic immediate‐early genes which expression is induced by psychedelics (González‐Maeso et al., 2003). Of note, it has been recently shown that a single dose of DOI is able to alter the PFC epigenetic status in mice up to 7 days after administration (de la Fuente Revenga et al., 2021), suggesting that a single exposure is sufficient to induce epigenetic reorganisation.
Applying gene co‐expression network analysis, we confirm these observations identifying modules of tightly connected LSD‐induced genes enriched for chromatin modification, vesicle‐mediated transport in synapse and cell‐cell adhesion. On the other hand, down‐regulated modules are enriched for GTP‐binding, hydrolase activity and ribosome. From the topology of the co‐expression network, we propose Tcf4 as a potential regulator of the up‐regulated “Vesicle transport” module. Notably, Tcf4 regulates neuritogenesis and neuronal migration, and its loss decreases spine density in the cortex and in the hippocampus (Crux et al., 2018).
Importantly, we describe an overall increase in transcriptional/signalling entropy, potentially reflecting an overall increase in available transcriptional states. Intriguingly, transcriptional entropy mirrors, at the molecular level, the increased brain entropy reported through neuroimaging studies in human.
High transcriptional entropy is typical of stem cells, and decreases with differentiation, paralleling cells’ developmental potential. Nevertheless, transcriptional entropy has also been associated with aging, reflecting the disruption of a well‐organised and functional system.
Using multiple metrics, we show that LSD‐induced transcriptional entropy is more reflective of a plastic stem‐like state than an aged state, suggesting the induction of a potentiality‐expansion process with organised and reproducible features.
Indeed, we employ the previously defined metric of “signalling entropy” (Teschendorff & Enver, 2017), based on the number of signalling paths in a protein‐protein interaction or co‐expression network, which we show to increase in the LSD‐treated group. When obtained from a heterogenous population of cells, this measure can be interpreted as a high between‐cell variability, because of the co‐existence of different cell types or functional states (Nijman, 2020), but also as an increased individual‐cell potentiality.
Previous studies have reported increased brain entropy quantified through various measures, each with its assumptions and interpretations, which can be broadly classified into two groups: the variability of the activation signal in a specific brain area, and the variability of between‐areas connectivity. Nevertheless, one element is shared in both groups: the variability of the signal is measured across time. Indeed, this is a fundamental difference with respect to transcriptional entropy, captured in a fixed timeframe. Moreover, despite all of them describing the brain at some level, the objects defining various entropy measures are intrinsically different. Hence, the conclusions that can be driven from the analysis of each metric do not necessarily describe the same phenomenon.
However, we can speculate how different metrics might be related from the biological point of view. Indeed, there have been numerous attempts to link brain connectivity networks and gene networks and define which genes are responsible for specific connectivity patterns (Diez & Sepulcre, 2018; Fornito et al., 2019), despite no similar studies have been performed to formally compare different measures of brain entropy.
Nevertheless, it is possible to envision models of brain functioning where signalling entropy might explain both local and connectivity variability. For example, the signalling entropy indicates the presence of cells with highly heterogeneous functional states, thus likely responding differently to stimuli and hence potentially resulting in variable firing rates. Similarly, this functional variability might lead to higher variability in the connection with other brain regions.
It is important to stress that this interpretative framework is likely simplistic and merely speculative. Still, the parallels that we observe between previously defined brain entropies and signalling entropy indicate that investigating their mechanistic relationships would improve our understanding of brain functioning at different levels and facilitate the interpretation of these measures.
In addition, we imply other players in transcriptional diversification since LSD‐treated rats display re‐activation of transposable elements and increased alternative splicing sites’ usage. In particular, the most reliably alternatively spliced genes are enriched for targets of the Nova splicing factor, known to control the splicing of synaptic proteins (Ule et al., 2005).
Transposable elements (TEs) are a class of repeated DNA sequences with the ability to mobilise and change locations in the genome (Ahmadi et al., 2020). Despite being mostly inactive in somatic cells, efficient transposition was detected in neural progenitor cells and mature neurons (MacIa et al., 2017). They have been reported to be both beneficial and pathological to the organism (Biémont, 2010), and associated with both neurodegenerative and psychiatric diseases and plasticity. For example, in first episode schizophrenia, hypomethylation of HERV‐K locus was reported (Forner et al., 2019), and L1 insertions were found significantly elevated in post‐mortem dorsolateral prefrontal cortex of patients with schizophrenia (Doyle et al., 2017). Nevertheless, they have been proposed to have a fundamental role in promoting evolution and also increasing cells’ variability through the generation of somatic mosaicism (Paquola et al., 2017), creating a greater potential for the adaptation of genetic networks.
Disentangling individual modules’ topological reorganisation, we distinguish two opposite processes: (1) most modules decrease their connectivity paralleling the increased entropy, and redistribute connections towards module's periphery, hence reducing its centralisation (Figure 6a); (2) a few modules increase their overall connectivity and centralisation. Interestingly, the modules increasing connectivity are enriched for GO categories indicative of the involvement of microenvironment: mesenchyme development, regulation of immune response and extracellular matrix. This suggests a potential involvement of non‐neuronal cells in long‐term LSD effects. In particular, mesenchymal stem cells (MSCs) are stem cells found in many adult tissues, including the brain (Appaix, 2014), and can differentiate into neurons (Pavlova et al., 2012; Zeng et al., 2015; Zhao et al., 2015) and glial cells (George et al., 2019) to replace damaged tissues (Dimarino et al., 2013), thus promoting neuroprotection, regeneration and repair (Qu et al., 2008; Thomi et al., 2019). They have immunomodulatory properties, mitigating the inflammation related to stroke or neurological diseases (Feng et al., 2020; Liu et al., 2019; Salari et al., 2020). Interestingly, both mesenchyme‐ and immune‐related modules display similar topological restructuring.
FIGURE 6.
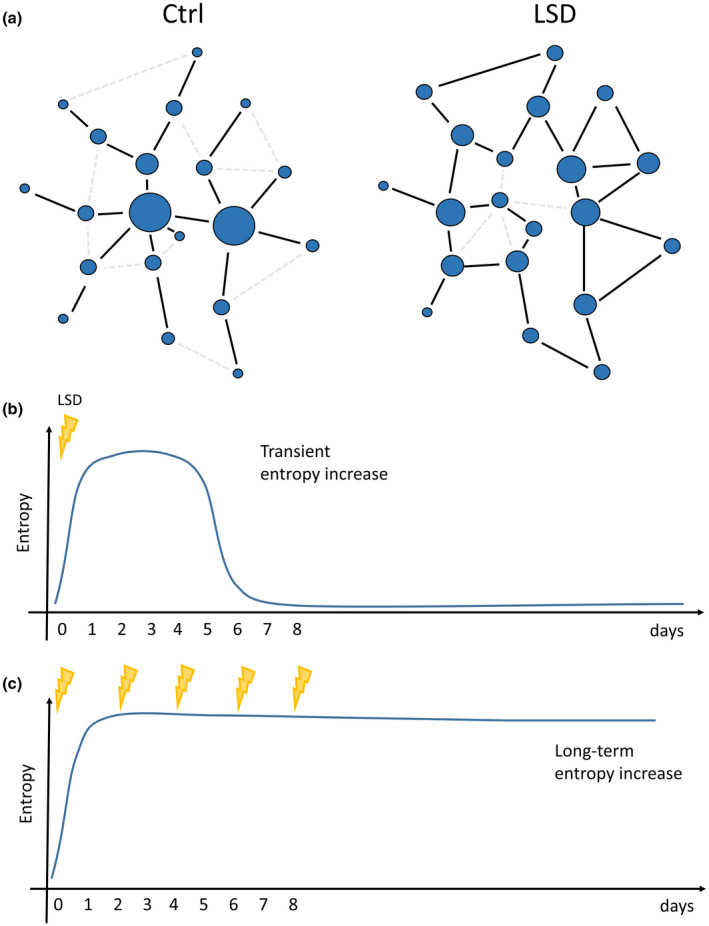
Schematic representations of models of LSD‐induced changes in network organisation. (a) Most gene co‐expression modules reorganise toward a less centralised structure, with central hubs losing connections and peripheral nodes acquiring connections. Overall, the number of available paths across the network increases, resulting in increased signalling entropy. In the proposed model, a single dose of psychedelic drug induces a transient increase in signalling entropy that returns to baseline after 7 days (b), while repeated administrations induce a sustained entropy increase even after discontinuation of the drug (c)
In line with our observations suggesting non‐neuronal populations’ involvement, in particular of the immune system, psychedelics have been shown to exert anti‐inflammatory effects, which led to their proposal as treatments for neurodegenerative diseases such as Alzheimer disease (Vann Jones and O’Kelly 2020; Family et al., 2020; Flanagan & Nichols, 2018).
Finally, we explored the temporal dynamics of co‐expression modules and signalling entropy by comparing our results with other available datasets. We acknowledge that none of these conditions is ideal for our aim since the LSD dataset has been generated with a different platform (Affymetrix U34A), possibly generating technical batch effects, and comprises only three pooled independent replicates of two animals each, limiting the statistical power, while the second utilises a different psychedelic drug, DOI, with non‐perfectly overlapping effects with LSD (González‐Maeso et al., 2003). Another limitation is that the LSD set used rats, while the DOI set mice. Nevertheless, both LSD‐induced modules and signalling entropy display similar trends in PFC neurons upon DOI treatment, increasing at 24 h and returning to background after 7 days. Hence, we propose a model of the transcriptional response to psychedelics where a single dose triggers a transient reorganisation of the gene networks that are sustained at long‐term only after several administrations (Figure 6b).
We hypothesise that the short‐term reorganisation and entropy increase induced by LSD allows for the formation of new synaptic connections and hence novel neuronal networks that can be maintained after the treatment, resulting in the long‐lasting beneficial effects that psychedelics have proven to exert in clinical settings. Frequent and repeated administrations, however, may result in a prolonged increase in the entropy and decentralisation of co‐expression networks that could be reflective of the psychotic‐like state observed in the chronically treated rats. Additional time‐course analyses and different treatment schemes will be needed to test this model.
Signalling entropy increase could be interpreted as increased cell diversity within the same subject (Nijman, 2020), but could also reflect higher individual cells’ potential (Teschendorff & Enver, 2017). With the available data, it is impossible distinguishing between these two mechanisms, for which single cell RNA‐seq experiments would be necessary (Papalexi & Satija, 2018). Indeed, future studies investigating the biological interpretation of signalling entropy and the definition of metrics less prone to alternative readings will be extremely insightful. The indirect activation by LSD of several neuronal cell types has previously been shown (Martin & Nichols, 2016), but how each cell population responds to this class of compounds and how cell heterogeneity is affected remains largely unknown.
Additionally, comparing experiments with different administration schedules showed differences in the persistence of transcriptional rewiring, and additional time‐course experiments with longer time frames will be needed to elucidate the dynamics of gene expression response.
In conclusion, analysing transcriptomic data of LSD‐treated rats, we identify increased signalling entropy paralleling the psychedelics‐induced increase in brain entropy observed at higher levels, identify epigenetic alterations that could explain long‐term effects, and imply alternative splicing, transposable elements’ activity, and the involvement of additional cell components such as the mesenchyme in the mechanism of action of psychedelics.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
Aurora Savino conceived the work, performed the analysis, and wrote the manuscript. Charles Nichols provided the data and wrote the manuscript.
Supporting information
Supplementary Material
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
ACKNOWLEDGEMENTS
This research was funded by the Italian Ministry of University and Research (MIUR), which funded Aurora Savino’s PhD scholarship, and also supported by the NIH grants MH083689 from NIMH (_100000025) and DA02189 from NIDA (_100000026) to Charles D. Nichols.
All experiments were conducted in compliance with the ARRIVE guidelines. Open Access Funding provided by Universita degli Studi di Torino within the CRUI‐CARE Agreement.
Savino, A. , & Nichols, C. D. (2022). Lysergic acid diethylamide induces increased signalling entropy in rats’ prefrontal cortex. Journal of Neurochemistry, 162, 9–23. 10.1111/jnc.15534
This article is part of the special issue “ Psychedelics and Neurochemistry ”
[Correction added on 11th May 2022, after first online publication: CARE funding statement has been added.]
Contributor Information
Aurora Savino, Email: aurora.savino@unito.it.
Charles D. Nichols, Email: aurora.savino@unito.it, Email: cnich1@lsuhsc.edu.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in the Gene Expression Omnibus database at https://www.ncbi.nlm.nih.gov/geo/ (GSE179380).
REFERENCES
- Ahmadi, A. , Toma, I. , De, V.‐T. , Eftekhariyan, G. M. R , & Sadeghi, I. (2020). Transposable elements in brain health and disease. Ageing Research Reviews, 64. 10.1016/j.arr.2020.101153 [DOI] [PubMed] [Google Scholar]
- Anders, S. , Pyl, P. T. , & Huber, W. (2015). HTSeq–a Python framework to work with high‐throughput sequencing data. Bioinformatics, 31, 166–169. 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appaix, F. (2014). Brain mesenchymal stem cells: The other stem cells of the brain? World Journal of Stem Cells, 6, 134. 10.4252/wjsc.v6.i2.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbanoj, M. J. , Riba, J. , Clos, S. , Giménez, S. , Grasa, E. , & Romero, S. (2008). Daytime Ayahuasca administration modulates REM and slow‐wave sleep in healthy volunteers. Psychopharmacology (Berl), 196, 315–326. 10.1007/s00213-007-0963-0 [DOI] [PubMed] [Google Scholar]
- Benekareddy, M. , Nair, A. R. , Dias, B. G. , Suri, D. , Autry, A. E. , Monteggia, L. M. , & Vaidya, V. A. (2013). Induction of the plasticity‐Associated immediate early gene Arc by stress and hallucinogens: Role of brain‐derived neurotrophic factor. International Journal of Neuropsychopharmacology, 16, 405–415. 10.1017/S1461145712000168 [DOI] [PubMed] [Google Scholar]
- Biémont, C. (2010). A brief history of the status of transposable elements: From junk DNA to major players in evolution. Genetics, 186, 1085–1093. 10.1534/genetics.110.124180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart‐Harris, R. L. (2018). The entropic brain—revisited. Neuropharmacology, 142, 167–178. 10.1016/j.neuropharm.2018.03.010 [DOI] [PubMed] [Google Scholar]
- Carhart‐Harris, R. L. , Bolstridge, M. , Day, C. M. J. , Rucker, J. , Watts, R. , Erritzoe, D. E. , Kaelen, M. , Giribaldi, B. , Bloomfield, M. , Pilling, S. , Rickard, J. A. , Forbes, B. , Feilding, A. , Taylor, D. , Curran, H. V. , & Nutt, D. J. (2018). Psilocybin with psychological support for treatment‐resistant depression: six‐month follow‐up. Psychopharmacology (Berl), 235, 399–408. 10.1007/s00213-017-4771-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart‐Harris, R. L. , Bolstridge, M. , Rucker, J. , Day, C. M. J. , Erritzoe, D. , Kaelen, M. , Bloomfield, M. , Rickard, J. A. , Forbes, B. , Feilding, A. , Taylor, D. , Pilling, S. , Curran, V. H. , & Nutt, D. J. (2016). Psilocybin with psychological support for treatment‐resistant depression: an open‐label feasibility study. The Lancet Psychiatry, 3, 619–627. 10.1016/S2215-0366(16)30065-7 [DOI] [PubMed] [Google Scholar]
- Carhart‐Harris, R. L. , Leech, R. , Hellyer, P. J. , Shanahan, M. , Feilding, A. , Tagliazucchi, E. , Chialvo, D. R. , & Nutt, D. (2014). The entropic brain: A theory of conscious states informed by neuroimaging research with psychedelic drugs. Frontiers in Human Neuroscience, 8, 1–22. 10.3389/fnhum.2014.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart‐Harris, R. L. , Roseman, L. , Bolstridge, M. , Demetriou, L. , Pannekoek, J. N. , Wall, M. B. , Tanner, M. , Kaelen, M. , McGonigle, J. , Murphy, K. , Leech, R. , Curran, H. V. , & Nutt, D. J. (2017). Psilocybin for treatment‐resistant depression: FMRI‐measured brain mechanisms. Scientific Reports, 7, 1–11. 10.1038/s41598-017-13282-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforte, A. J. , Tuszynski, J. A. , Silva, F. A. B. , & da Carels, N. (2019). Signaling complexity measured by shannon entropy and its application in personalized medicine. Frontiers in Genetics, 10, 1–14. 10.3389/fgene.2019.00930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crux, S. , Herms, J. , & Dorostkar, M. M. (2018). Tcf4 regulates dendritic spine density and morphology in the adult brain. PLoS One, 13, 2–10. 10.1371/journal.pone.0199359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, A. K. , Barrett, F. S. , May, D. G. , Cosimano, M. P. , Sepeda, N. D. , Johnson, M. W. , Finan, P. H. , & Griffiths, R. R. (2020). Effects of psilocybin‐assisted therapy on major depressive disorder: A randomized clinical trial. JAMA Psychiatry, 78(5), 1–9. 10.1001/jamapsychiatry.2020.3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente Revenga, M. , Zhu, B. , Guevara, C. A. , Naler, L. B. , Saunders, J. M. , Zhou, Z. , Toneatti, R. , Sierra, S. , Wolstenholme, J. T. , Beardsley, P. M. , Huntley, G. W. , Lu, C. , & González‐Maeso, J. (2021). Prolonged epigenomic and synaptic plasticity alterations following single exposure to a psychedelic in mice. Cell Reports, 37(3), 109836. 10.1016/j.celrep.2021.109836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez, I. , & Sepulcre, J. (2018). Neurogenetic profiles delineate large‐scale connectivity dynamics of the human brain. Nature Communications, 9, 1–10. 10.1038/s41467-018-06346-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimarino, A. M. , Caplan, A. I. , & Bonfield, T. L. (2013). Mesenchymal stem cells in tissue repair. Frontiers in Immunology, 4, 201. 10.3389/fimmu.2013.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan, L. L. , Johansen, J. V. , Ros, N. F. , Jaberi, E. , Linnet, K. , Johansen, S. S. , Ozenne, B. , Issazadeh‐Navikas, S. , Hansen, H. D. , & Knudsen, G. M. (2021). Effects of a single dose of psilocybin on behaviour, brain 5‐HT2A receptor occupancy and gene expression in the pig. European Neuropsychopharmacology, 42, 1–11. 10.1016/j.euroneuro.2020.11.013 [DOI] [PubMed] [Google Scholar]
- Doyle, G. A. , Crist, R. C. , Karatas, E. T. , Hammond, M. J. , Ewing, A. D. , Ferraro, T. N. , Hahn, C. G. , & Berrettini, W. H. (2017). Analysis of LINE‐1 elements in DNA from postmortem brains of individuals with schizophrenia. Neuropsychopharmacology, 42, 2602–2611. 10.1038/npp.2017.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durinck, S. , Spellman, P. T. , Birney, E. , & Huber, W. (2009). Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nature Protocols, 4, 1184–1191. 10.1038/nprot.2009.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erritzoe, D. , Roseman, L. , Nour, M. M. , MacLean, K. , Kaelen, M. , Nutt, D. J. , & Carhart‐Harris, R. L. (2018). Effects of psilocybin therapy on personality structure. Acta Psychiatrica Scand., 138, 368–378. 10.1111/acps.12904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Family, N. , Maillet, E. L. , Williams, L. T. J. , Krediet, E. , Carhart‐Harris, R. L. , Williams, T. M. , Nichols, C. D. , Goble, D. J. , & Raz, S. (2020). Safety, tolerability, pharmacokinetics, and pharmacodynamics of low dose lysergic acid diethylamide (LSD) in healthy older volunteers. Psychopharmacology (Berl), 237, 841–853. 10.1007/s00213-019-05417-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Y. , Guo, M. , Zhao, H. , Han, S. , Dong, Q. , & Cui, M. (2020) Mesenchymal‐stem‐cell–derived extracellular vesicles mitigate trained immunity in the brain. Frontiers in Bioengineering and Biotechnology, 8. 10.3389/fbioe.2020.599058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan, T. W. , & Nichols, C. D. (2018). Psychedelics as anti‐inflammatory agents. International Review of Psychiatry, 30, 363–375. 10.1080/09540261.2018.1481827 [DOI] [PubMed] [Google Scholar]
- Forner, L. , Andreguetto, T. N. , Lopes, A. M. , Buosi, P. , Borghi, F. A. , Facincani, I. S. , Fernandes‐Ferreira, R. , Oliveira‐Brancati, C. I. F. , de Almeida, E. A. , Felício, A. A. , Martins, D. P. , Filho, G. M. A. , & Souza, D. R. S. (2019). Genetic and biochemical biomarkers related to oxidative stress in patients with schizophrenia. Genetics and Molecular Research, 18, 1–8. 10.4238/gmr18259 [DOI] [Google Scholar]
- Fornito, A. , Arnatkevičiūtė, A. , & Fulcher, B. D. (2019). Bridging the gap between connectome and transcriptome. Trends in Cognitive Sciences, 23, 34–50. 10.1016/j.tics.2018.10.005 [DOI] [PubMed] [Google Scholar]
- Gautier, L. , Cope, L. , Bolstad, B. M. , & Irizarry, R. A. (2004). affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics, 20, 307–315. 10.1093/bioinformatics/btg405 [DOI] [PubMed] [Google Scholar]
- George, S. , Hamblin, M. R. , & Abrahamse, H. (2019). Differentiation of mesenchymal stem cells to neuroglia: In the context of cell signalling. Stem Cell Reviews and Reports, 15, 814–826. 10.1007/s12015-019-09917-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, L. D. , Cao, Y. , Pau, G. , Lawrence, M. , Wu, T. D. , Seshagiri, S. , & Gentleman, R. (2016). Prediction and quantification of splice events from RNA‐Seq data. PLoS One, 11, e0156132. 10.1371/journal.pone.0156132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González‐Maeso, J. , Weisstaub, N. V. , Zhou, M. , Chan, P. , Ivic, L. , Ang, R. , Lira, A. , Bradley‐Moore, M. , Ge, Y. , Zhou, Q. , Sealfon, S. C. , & Gingrich, J. A. (2007). Hallucinogens recruit specific cortical 5‐HT2A receptor‐mediated signaling pathways to affect behavior. Neuron, 53, 439–452. 10.1016/j.neuron.2007.01.008 [DOI] [PubMed] [Google Scholar]
- González‐Maeso, J. , Yuen, T. , Ebersole, B. J. , Wurmbach, E. , Lira, A. , Zhou, M. , Weisstaub, N. , Hen, R. , Gingrich, J. A. , & Sealfon, S. C. (2003). Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5‐hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. Journal of Neuroscience, 23, 8836–8843. 10.1523/JNEUROSCI.23-26-08836.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths, R. R. , Johnson, M. W. , Carducci, M. A. , Umbricht, A. , Richards, W. A. , Richards, B. D. , Cosimano, M. P. , & Klinedinst, M. A. (2016). Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life‐threatening cancer: A randomized double‐blind trial. Journal of Psychopharmacology, 30, 1181–1197. 10.1177/0269881116675513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati, G. S. , Sikandar, S. S. , Wesche, D. J. , Manjunath, A. , Bharadwaj, A. , Berger, M. J. , Ilagan, F. , Kuo, A. H. , Hsieh, R. W. , Cai, S. , Zabala, M. , Scheeren, F. A. , Lobo, N. A. , Qian, D. , Yu, F. B. , Dirbas, F. M. , Clarke, M. F. , & Newman, A. M. (2020). Single‐cell transcriptional diversity is a hallmark of developmental potential. Science, 367(6476), 405–411. 10.1126/science.aax0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernando‐Herraez, I. , Evano, B. , Stubbs, T. , Commere, P. H. , Jan, B. M. , Clark, S. , Andrews, S. , Tajbakhsh, S. , & Reik, W. (2019). Ageing affects DNA methylation drift and transcriptional cell‐to‐cell variability in mouse muscle stem cells. Nature Communications, 10, 1–11. 10.1038/s41467-019-12293-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog, R. , Mediano, P. A. M. , Rosas, F. E. , Carhart‐Harris, R. , Perl, Y. S. , Tagliazucchi, E. , & Cofre, R. (2020). A mechanistic model of the neural entropy increase elicited by psychedelic drugs. Scientific Reports, 10, 1–12. 10.1038/s41598-020-74060-6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jefsen, O. H. , Elfving, B. , Wegener, G. , & Müller, H. K. (2020). Transcriptional regulation in the rat prefrontal cortex and hippocampus after a single administration of psilocybin. Journal of Psychopharmacology, 35(4), 483–493. 10.1177/0269881120959614 [DOI] [PubMed] [Google Scholar]
- Jeong, H. , Mason, S. P. , Barabási, A.‐L. , & Oltvai, Z. N. (2001). Lethality and centrality in protein networks. Nature, 411, 41. 10.1038/35075138 [DOI] [PubMed] [Google Scholar]
- Jin, Y. , Tam, O. H. , Paniagua, E. , & Hammell, M. (2015). TEtranscripts: a package for including transposable elements in differential expression analysis of RNA‐seq datasets. Bioinformatics, 31, 3593–3599. 10.1093/bioinformatics/btv422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D. , Paggi, J. M. , Park, C. , Bennett, C. , & Salzberg, S. L. (2019). Graph‐based genome alignment and genotyping with HISAT2 and HISAT‐genotype. Nature Biotechnology, 37, 907–915. 10.1038/s41587-019-0201-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkevich, G. , Sukhov, V. , Budin, N. , Shpak, B. , Artyomov, M. N. , & Sergushichev, A. (2021). Fast gene set enrichment analysis. bioRxiv, 60012. 10.1101/060012 [DOI] [Google Scholar]
- Lachmann, A. , Xu, H. , Krishnan, J. , Berger, S. I. , Mazloom, A. R. , & Ma’ayan, A. (2010). ChEA: Transcription factor regulation inferred from integrating genome‐wide ChIP‐X experiments. Bioinformatics, 26, 2438–2444. 10.1093/bioinformatics/btq466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfelder, P. , & Horvath, S. (2008). WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics, 9, 559. 10.1186/1471-2105-9-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedev, A. V. , Kaelen, M. , Lövdén, M. , Nilsson, J. , Feilding, A. , Nutt, D. J. , & Carhart‐Harris, R. L. (2016). LSD‐induced entropic brain activity predicts subsequent personality change. Human Brain Mapping, 37, 3203–3213. 10.1002/hbm.23234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Zeng, R. , Wang, Y. , Huang, W. , Hu, B. , Zhu, G. , Zhang, R. , Li, F. , Han, J. , & Li, Y. (2019). Mesenchymal stem cells enhance microglia M2 polarization and attenuate neuroinflammation through TSG‐6. Brain Research, 1724, 146422. 10.1016/j.brainres.2019.146422 [DOI] [PubMed] [Google Scholar]
- Love, M. I. , Huber, W. , & Anders, S. (2014). Moderated estimation of fold change and dispersion for RNA‐seq data with DESeq2. Genome Biology, 15, 550. 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly, C. , Greb, A. C. , Cameron, L. P. , Wong, J. M. , Barragan, E. V. , Wilson, P. C. , Burbach, K. F. , Soltanzadeh Zarandi, S. , Sood, A. , Paddy, M. R. , Duim, W. C. , Dennis, M. Y. , McAllister, A. K. , Ori‐McKenney, K. M. , Gray, J. A. , & Olson, D. E. (2018). Psychedelics promote structural and functional neural plasticity. Cell Reports, 23, 3170–3182. 10.1016/j.celrep.2018.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia, A. , Widmann, T. J. , Heras, S. R. , Ayllon, V. , Sanchez, L. , Benkaddour‐Boumzaouad, M. , Muñoz‐Lopez, M. , Rubio, A. , Amador‐Cubero, S. , Blanco‐Jimenez, E. , Garcia‐Castro, J. , Menendez, P. , Ng, P. , Muotri, A. R. , Goodier, J. L. , & Garcia‐Perez, J. L. (2017). Engineered LINE‐1 retrotransposition in nondividing human neurons. Genome Research, 27, 335–348. 10.1101/gr.206805.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean, K. A. , Johnson, M. W. , & Griffiths, R. R. (2011). Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. Journal of Psychopharmacology, 25(11), 1453–1461. 10.1177/0269881111420188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen, M. K. , Fisher, P. M. , Burmester, D. , Dyssegaard, A. , Stenbæk, D. S. , Kristiansen, S. , Johansen, S. S. , Lehel, S. , Linnet, K. , Svarer, C. , Erritzoe, D. , Ozenne, B. , & Knudsen, G. M. (2019). Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology January, 44(7), 1328–1334. 10.1038/s41386-019-0324-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marona‐Lewicka, D. , Nichols, C. D. , & Nichols, D. E. (2011). An animal model of schizophrenia based on chronic LSD administration: Old idea, new results. Neuropharmacology, 61, 503–512. 10.1016/j.neuropharm.2011.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, D. A. , Marona‐Lewicka, D. , Nichols, D. E. , & Nichols, C. D. (2014). Chronic LSD alters gene expression profiles in the mPFC relevant to schizophrenia. Neuropharmacology, 83, 1–8. 10.1016/j.neuropharm.2014.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, D. A. , & Nichols, C. D. (2016). Psychedelics recruit multiple cellular types and produce complex transcriptional responses within the brain. EBioMedicine, 11, 262–277. 10.1016/j.ebiom.2016.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinotti, G. , Santacroce, R. , Pettorruso, M. , Montemitro, C. , Spano, M. C. , Lorusso, M. , Giannantonio, M. , & di Lerner, A. G. (2018). Hallucinogen persisting perception disorder: Etiology, clinical features, and therapeutic perspectives. Brain Sciences, 8(3), 47. 10.3390/brainsci8030047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, C. D. , Garcia, E. E. , & Sanders‐Bush, E. (2003). Dynamic changes in prefrontal cortex gene expression following lysergic acid diethylamide administration. Molecular Brain Research, 111, 182–188. 10.1016/S0169-328X(03)00029-9 [DOI] [PubMed] [Google Scholar]
- Nichols, C. D. , & Sanders‐Bush, E. (2002). A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology, 26, 634–642. 10.1016/S0893-133X(01)00405-5 [DOI] [PubMed] [Google Scholar]
- Nichols, C. D. , & Sanders‐Bush, E. (2004). Molecular genetic responses to lysergic acid diethylamide include transcriptional activation of MAP kinase phosphatase‐1, C/EBP‐β and ILAD‐1, a novel gene with homology to arrestins. Journal of Neurochemistry, 90, 576–584. 10.1111/j.1471-4159.2004.02515.x [DOI] [PubMed] [Google Scholar]
- Nichols, D. E. (2004). Hallucinogens. Pharmacology & Therapeutics, 101, 131–181. 10.1016/j.pharmthera.2003.11.002 [DOI] [PubMed] [Google Scholar]
- Nichols, D. E. (2016). Psychedelics. Pharmacological Reviews, 68(2), 264–355. 10.1124/pr.115.011478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijman, S. M. B. (2020). Perturbation‐driven entropy as a source of cancer cell heterogeneity. Trends in Cancer, 6, 454–461. 10.1016/j.trecan.2020.02.016 [DOI] [PubMed] [Google Scholar]
- Papalexi, E. , & Satija, R. (2018). Single‐cell RNA sequencing to explore immune cell heterogeneity. Nature Reviews Immunology, 18, 35–45. 10.1038/nri.2017.76 [DOI] [PubMed] [Google Scholar]
- Paquola, A. C. M. , Erwin, J. A. , & Gage, F. H. (2017). Insights into the role of somatic mosaicism in the brain. Current Opinion in Systems Biology, 1, 90–94. 10.1016/j.coisb.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova, G. , Lopatina, T. , Kalinina, N. , Rybalkina, E. , Parfyonova, Y. , Tkachuk, V. , & Revishchin, A. (2012). In vitro neuronal induction of adipose‐derived stem cells and their fate after transplantation into injured mouse brain. Current Medicinal Chemistry, 19, 5170–5177. 10.2174/092986712803530557 [DOI] [PubMed] [Google Scholar]
- Preller, K. H. , Burt, J. B. , Ji, J. L. , Schleifer, C. H. , Adkinson, B. D. , Stämpfli, P. , Seifritz, E. , Repovs, G. , Krystal, J. H. , Murray, J. D. , Vollenweider, F. X. , & Anticevic, A. (2018). Changes in global and thalamic brain connectivity in LSD‐induced altered states of consciousness are attributable to the 5‐HT2A receptor. Elife, 7, 1–31. 10.7554/eLife.35082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preller, K. H. , Duerler, P. , Burt, J. B. , Ji, J. L. , Adkinson, B. , Stämpfli, P. , Seifritz, E. , Repovš, G. , Krystal, J. H. , Murray, J. D. , Anticevic, A. , & Vollenweider, F. X. (2020). Psilocybin induces time‐dependent changes in global functional connectivity. Biological Psychiatry, 88(2), 197–207. 10.1016/j.biopsych.2019.12.027 [DOI] [PubMed] [Google Scholar]
- Preller, K. H. , Razi, A. , Zeidman, P. , Stämpfli, P. , Friston, K. J. , & Vollenweider, F. X. (2019). Effective connectivity changes in LSD‐induced altered states of consciousness in humans. Proceedings of the National Academy of Sciences of the United States of America, 116, 2743–2748. 10.1073/pnas.1815129116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pržulj, N. , Wigle, D. A. , & Jurisica, I. (2004). Functional topology in a network of protein interactions. Bioinformatics, 20, 340–348. 10.1093/bioinformatics/btg415 [DOI] [PubMed] [Google Scholar]
- Qu, C. , Mahmood, A. , Lu, D. , Goussev, A. , Xiong, Y. , & Chopp, M. (2008). Treatment of traumatic brain injury in mice with marrow stromal cells. Brain Research, 1208, 234–239. 10.1016/j.brainres.2008.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Salari, V. , Mengoni, F. , Gallo, F. , Del, B. G. , & Fabene, P. F. (2020). The anti‐inflammatory properties of mesenchymal stem cells in epilepsy: Possible treatments and future perspectives. International Journal of Molecular Sciences, 21, 1–15. 10.3390/ijms21249683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos, R. G. , Osório, F. L. , Crippa, J. A. S. , Riba, J. , Zuardi, A. W. , Hallak, J. E. C. , dos Santos, R. G. , Osório, F. L. , Crippa, J. A. S. , Riba, J. , Zuardi, A. W. , & Hallak, J. E. C. (2016). Antidepressive, anxiolytic, and antiaddictive effects of ayahuasca, psilocybin and lysergic acid diethylamide (LSD): A systematic review of clinical trials published in the last 25 years. Therapeutic Advances in Psychopharmacology, 6(3), 193–213. 10.1177/2045125316638008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino, A. , Provero, P. , & Poli, V. (2020). Differential co‐expression analyses allow the identification of critical signalling pathways altered during tumour transformation and progression. International Journal of Molecular Sciences, 21, 1–23. 10.3390/ijms21249461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schartner, M. M. , Carhart‐Harris, R. L. , Barrett, A. B. , Seth, A. K. , & Muthukumaraswamy, S. D. (2017). Increased spontaneous MEG signal diversity for psychoactive doses of ketamine, LSD and psilocybin. Scientific Reports, 7, 1–12. 10.1038/srep46421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid, Y. , Enzler, F. , Gasser, P. , Grouzmann, E. , Preller, K. H. , Vollenweider, F. X. , Brenneisen, R. , Müller, F. , Borgwardt, S. , & Liechti, M. E. (2015). Acute effects of lysergic acid diethylamide in healthy subjects. Biological Psychiatry, 78, 544–553. 10.1016/j.biopsych.2014.11.015 [DOI] [PubMed] [Google Scholar]
- Schramm, G. , Kannabiran, N. , & König, R. (2010). Regulation patterns in signaling networks of cancer. BMC Systems Biology, 4(1). 10.1186/1752-0509-4-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studerus, E. , Kometer, M. , Hasler, F. , & Vollenweider, F. X. (2011). Acute, subacute and long‐term subjective effects of psilocybin in healthy humans: A pooled analysis of experimental studies. Journal of Psychopharmacology, 25, 1434–1452. 10.1177/0269881110382466 [DOI] [PubMed] [Google Scholar]
- Sun, Z. , Xu, X. , He, J. , Murray, A. , Sun, M. , Wei, X. , Wang, X. , McCoig, E. , Xie, E. , Jiang, X. , Li, L. , Zhu, J. , Chen, J. , Morozov, A. , Pickrell, A. M. , Theus, M. H. , & Xie, H. (2019). EGR1 recruits TET1 to shape the brain methylome during development and upon neuronal activity. Nature Communications, 10, 1–12. 10.1038/s41467-019-11905-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschendorff, A. E. , & Enver, T. (2017). Single‐cell entropy for accurate estimation of differentiation potency from a cell’s transcriptome. Nature Communications, 8, 1–15. 10.1038/ncomms15599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomi, G. , Surbek, D. , Haesler, V. , Joerger‐Messerli, M. , & Schoeberlein, A. (2019). Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia‐mediated neuroinflammation in perinatal brain injury. Stem Cell Research & Therapy, 10, 1–16. 10.1186/s13287-019-1207-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ule, J. , Ule, A. , Spencer, J. , Williams, A. , Hu, J. S. , Cline, M. , Wang, H. , Clark, T. , Fraser, C. , Ruggiu, M. , Zeeberg, B. R. , Kane, D. , Weinstein, J. N. , Blume, J. , & Darnell, R. B. (2005). Nova regulates brain‐specific splicing to shape the synapse. Nature Genetics, 37, 844–852. 10.1038/ng1610 [DOI] [PubMed] [Google Scholar]
- Valero‐Mora, P. M. (2010). ggplot2: Elegant Graphics for Data Analysis. Journal of Statistical Software, 35. 10.18637/jss.v035.b01 [DOI] [Google Scholar]
- Vann, J. S. A. , & O’Kelly, A. (2020). Psychedelics as a treatment for Alzheimer’s disease dementia. Frontiers in Synaptic Neuroscience, 12, 10–15. 10.3389/fnsyn.2020.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider, F. X. , & Preller, K. H. (2020). Psychedelic drugs: Neurobiology and potential for treatment of psychiatric disorders. Nature Reviews Neuroscience, 21, 611–624. 10.1038/s41583-020-0367-2 [DOI] [PubMed] [Google Scholar]
- Vollenweider, F. X. , Vollenweider‐Scherpenhuyzen, M. F. , Bäbler, A. , Vogel, H. , & Hell, D. (1998). Psilocybin induces schizophrenia‐like psychosis in humans via a serotonin‐2 agonist action. NeuroReport, 9, 3897–3902. 10.1097/00001756-199812010-00024 [DOI] [PubMed] [Google Scholar]
- Winship, I. R. , Dursun, S. M. , Baker, G. B. , Balista, P. A. , Kandratavicius, L. , Maia‐de‐Oliveira, J. P. , Hallak, J. , & Howland, J. G. (2019). An overview of animal models related to schizophrenia. Canadian Journal of Psychiatry, 64, 5–17. 10.1177/0706743718773728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X. , & Zhang, Y. (2017). TET‐mediated active DNA demethylation: Mechanism, function and beyond. Nature Reviews Genetics, 18, 517–534. 10.1038/nrg.2017.33 [DOI] [PubMed] [Google Scholar]
- Yu, G. , Wang, L.‐G. , Han, Y. , & He, Q.‐Y. (2012). clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS: A Journal of Integrative Biology, 16, 284–287. 10.1089/omi.2011.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, X. , Qiu, X.‐C. , Ma, Y.‐H. , Duan, J.‐J. , Chen, Y.‐F. , Gu, H.‐Y. , Wang, J.‐M. , Ling, E.‐A. , Wu, J.‐L. , Wu, W. , & Zeng, Y.‐S. (2015). Integration of donor mesenchymal stem cell‐derived neuron‐like cells into host neural network after rat spinal cord transection. Biomaterials, 53, 184–201. 10.1016/j.biomaterials.2015.02.073 [DOI] [PubMed] [Google Scholar]
- Zhang, B. , & Horvath, S. (2005). A general framework for weighted gene co‐expression network analysis. Statistical Applications in Genetics and Molecular Biology, 4(1). 10.2202/1544-6115.1128 [DOI] [PubMed] [Google Scholar]
- Zhao, E. , Jia, Y. , Wang, D. , Wen, G. , Guan, W. , Jing, L. , & Deng, Y. (2015). Effect of p65 gene inhibited by siRNA on differention of rat marrow mesenchymal stem cells into neurons. Zhongguo Ying Yong Sheng Li Xue Za Zhi, 31, 254–258. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Data Availability Statement
The data that support the findings of this study are openly available in the Gene Expression Omnibus database at https://www.ncbi.nlm.nih.gov/geo/ (GSE179380).


