Summary
Background and objectives
Molluscum contagiosum (MC) is a common viral infection. Hypersensitivity reactions reminiscent of Gianotti‐Crosti syndrome, termed Gianotti‐Crosti syndrome‐like reaction (GCLR), have been reported in a subset of patients. We report a series of patients with GCLR, better delineating its clinical presentation and course.
Patients and methods
Retrospective chart review of all children presenting with GCLR at our Pediatric Skin Center between 2015 and 2020.
Results
26 children (14 boys) with a median age of 6.5 (3–11.3) years were included. GCLR involved the extensor surfaces of the extremities in all patients. More widespread eruptions also affected the trunk and face in 7 (27 %) and 6 (23 %) children respectively. Involvement of the skin overlying the Achilles tendons was a new finding in 4 (15 %) children. Itch was the predominant symptom in 20 (77 %) patients. The rash responded to topical and/or systemic corticosteroids and resolved within four weeks. GCLR was followed by clearance of MC in all patients within 9 (4–24) weeks.
Conclusions
GCLR is a characteristic acute, wide‐spread, pruritic papular eruption, which often leads to emergency consultations and anxiety in affected patients. GCLR responds well to corticosteroid treatment, has a benign course, and heralds the healing of MC.
Keywords: molluscum contagiosum, Gianotti‐Crosti syndrome‐like reaction
Introduction
Molluscum contagiosum (MC) is a common skin infection, mostly affecting young individuals aged 5–10 years [1]. While self‐limited, lesions may be numerous and lead to molluscum dermatitis associated with significant pruritus, especially in children with atopic dermatitis. Furthermore, MC may become inflamed and form cutaneous abscesses [2]. Rarely, a hypersensitivity reaction reminiscent of papular acrodermatitis of childhood (Gianotti‐Crosti syndrome, GCS) has been described [2, 3, 4]. The term Gianotti‐Crosti syndrome‐like reaction (GCLR) was proposed for such cases presenting with acute itchy papules and plaques involving the extensor surfaces of the extremities [2].
Despite a reported frequency of 4.9 % in the study by Berger et al., only few anecdotal cases of GCLR have since been reported [2, 5]. Therefore, it is not surprising that familiarity and experience with this reaction is limited among general practitioners, pediatricians, and dermatologists alike. Here, we report a series of patients with GCLR and describe its clinical presentation and course in detail to increase awareness of this specific rash.
Patients and methods
All consecutive patients under the age of 18 years treated for GCLR at our Pediatric Skin Center during a six‐year period (2015–2020) were included in this study. Referral was either by pediatricians or office‐based dermatologists, including our teledermatology service, or by self‐referral to our pediatric emergency department. A retrospective chart review was performed. Gender, age, duration and treatment of MC prior to GCLR, as well as clinical characteristics, response to treatment, and course of GCLR were recorded. Morphology and distribution of GCLR were assessed based on physician's notes and clinical photographs where available. Missing data on treatment response and outcome were collected by telephone calls.
Clinical diagnostic criteria for GCLR included an eruptive, papular rash favoring the extensor surfaces of the extremities and appearing concurrently with an active MC infection. A rash consistent with any other known skin disorder precluded a diagnosis of GCLR. The severity of GCLR was assessed using the Physician's Global Assessment (PGA), which comprises a simple 5‐point scoring system ranging from 0 (clear) to 4 (severe) points [6].
Clinical data were entered into a computerized database (Excel 2013®, Microsoft Corp., Redmond, USA). For the descriptive analysis, median and range were used as appropriate for continuous variables and number and percentage for categorical variables.
Approval from the Zürich Cantonal Ethics Committee (Kantonale Ethikkommission Zürich) was obtained for this study as required by local standards. All parents or caregivers gave written consent to the project.
Results
Patient characteristics
Twenty‐six patients (14 male, 54 %) with a median age of 6.5 years (range 3.0–11.3) were included. Characteristics are summarized in Table 1. Seven children (27 %) presented directly to our pediatric emergency department. The median duration of MC at the onset of GCLR was eight months (range 1–36). Two thirds of children had received specific treatment for their MC infection prior to the GCLR.
Table 1.
Patient characteristics. Data calculated in proportion to all treated patients*/physician‐referred patients**
| n = 26 | % | |
|---|---|---|
| Gender | ||
|
– male – female |
14 12 |
54 46 |
| Age at inclusion (y; median, range) | 6.5 (3.0–11.3) | |
| Atopy | ||
|
– yes – no – unknown |
11 13 2 |
42 50 8 |
| Duration of MC at onset of GCLR (months; median, range) | 8 (1–36) | |
|
Previous therapy for MC – KOH – cantharidin – curettage – other |
18 9 3 4 5 |
69 50* 17* 22* 28* |
| Morphology of MC at initiation of GCLR | ||
|
– inflammatory – non‐inflammatory – unknown |
21 4 1 |
81 15 4 |
| Referral to Pediatric Dermatology by | ||
|
– office‐based physician (pediatrician, dermatologist, GP) – of which by teledermatology – emergency department |
19 8 7 |
73 42** 27 |
Clinical characteristics of GCLR
The diagnosis of GCLR was made for all children based on clinical presentation. Skin biopsies were not performed. Molluscum contagiosum was found in all patients. In 21 (81 %) of them the MC were inflamed at the onset of GCLR, characterized by local erythema, scaling and crusting (Figure 1). The GCLR was characterized by the sudden onset of symmetrical erythematous papules (100 %), plaques (46 %), papulovesicles (15 %), urticarial papules/plaques (8 %), and/or target‐like papules/plaques (8 %) (Table 2, Figures 1, 2). Different morphologies in the same individual were a common finding. All skin lesions were persistent for at least several days. Intense pruritus was a prominent feature in 20 (77 %) children. One child reported pain associated with the GCLR. The eruption was considered mild to moderate in 19 children (73 %) based on the PGA.
Figure 1.
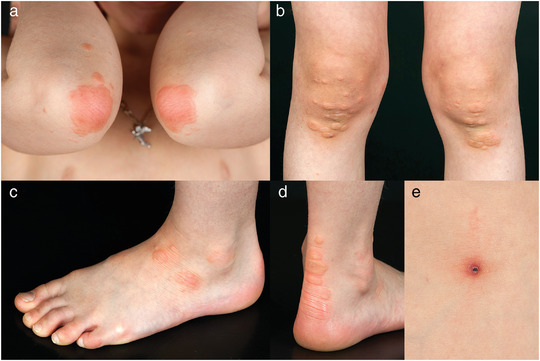
Typical presentation of Gianotti‐Crosti syndrome‐like reaction due to molluscum contagiosum infection with pruritic papules and larger plaques overlying elbows (a), knees (b), ankles (c), and Achilles tendons (d). Concomitant inflammatory change in molluscum contagiosum (e).
Table 2.
Clinical characteristics of Gianotti‐Crosti syndrome‐like reaction
| n = 26 | % | |
|---|---|---|
| Morphology of GCLR | ||
|
– papules – plaques – papulovesicles – urticarial papules/plaques – target‐like papules/plaques |
26 12 4 2 2 |
100 46 15 8 8 |
| Affected body areas | ||
|
– extremities – upper extremities – accentuation over extensor surfaces/elbows – dorsal hands – palms – lower extremities – accentuation over extensor surfaces/knees – Achilles tendon – dorsal feet – soles – face – trunk – buttocks |
26 25 22 7 1 23 18 4 6 1 6 7 5 |
100 96 85 27 4 88 69 15 23 4 23 27 19 |
| Severity of GCLR (PGA) | ||
|
– 4 – 3 – 2 – 1 |
1 10 9 6 |
4 38 35 23 |
| Symptoms associated with GCLR | ||
|
– pruritus – pain – asymptomatic |
20 1 5 |
77 4 19 |
| Treatment for GCLR performed | ||
|
– yes – no |
20 6 |
77 23 |
| Total duration of GCLR (days; median, range)* | 23.5 (10–150) | |
| Resolution of MC after GCLR | ||
|
– yes – unknown |
21 5 |
81 19 |
| Time until resolution of MC after onset of GCLR (weeks; median, range) | 9 (4–24) |
Information available for 18 patients (69 %).
Figure 2.
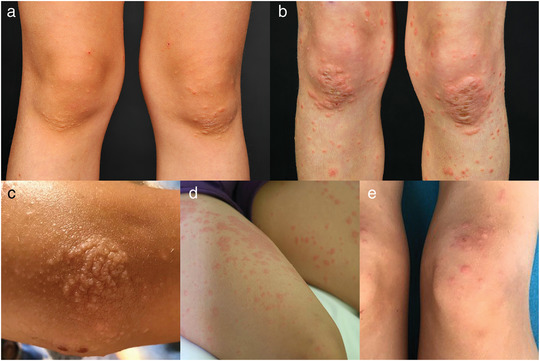
Spectrum of morphologies observed in Gianotti‐Crosti syndrome‐like reaction due to molluscum contagiosum. Papules (a), plaques (b), papulovesicles (c), urticarial papules (d), target‐like papules and plaques (e).
The GCLR involved the extensor surfaces of the extremities in all children. In 22 children (85 %), the upper and lower extremities were simultaneously affected. An accentuation over large joints (elbows, knees, ankles) was a typical feature. A peculiar finding was the involvement of the skin overlying the Achilles tendon, occurring in 15 % of subjects (Figure 1). The face and trunk were involved in approximately a quarter of the children. Disseminated skin lesions occurred in five individuals (19 %).
Treatment of GCLR was initiated in 20 children (77 %). Treatment details are presented in Table 3. Potent topical corticosteroids were by far the most common therapy, prescribed in 95 % of treated children. Five children (25 %) with more extensive involvement and severe pruritus received systemic corticosteroids (prednisolone 1 mg/kg per day tapered over a period of 7 days). The majority of patients reported a quick and significant improvement of pruritus and rash after initiation of treatment.
Table 3.
Treatment characteristics of Gianotti‐Crosti syndrome‐like reaction
| n = 20 | % | |
|---|---|---|
| Treatment prescribed | ||
|
– potent topical corticosteroids – systemic corticosteroids – emollients |
19 5 12 |
95 25 60 |
| Duration of corticosteroid treatment (days; median, range) | ||
|
– topical corticosteroids – systemic corticosteroids |
14 (5–35) 7 (6–10) |
|
| Treatment response of pruritus | ||
|
– significant – moderate – slight – unknown/n/a |
13 2 1 4 |
65 10 5 20 |
| Treatment response of rash | ||
|
– significant – moderate – slight – unknown |
14 2 1 3 |
70 10 5 15 |
We identified a median total disease duration of GCLR of 23.5 (10–150) days. In one patient, the GCLR manifestations had a waxing and waning course over 150 days, with quick resolution after consultation and treatment with topical corticosteroids. Once the GCLR resolved no recurrences were observed.
The MC resolved in all children within a median of 9 (4–24) weeks after the onset of the GCLR.
Discussion
Gianotti‐Crosti syndrome‐like reaction was first described as “the bump that rashes” by Berger et al. in 2012 [2]. Since then, only two small series with a total of four cases have appeared in the Spanish literature in 2019 [5]. The scarcity of available data may explain why many physicians are unfamiliar with this condition. We here describe a relatively large series of 26 cases, encountered in a single center within a period of six years. This reflects our impression that GCLR is a rather frequent pediatric dermatosis in clinical practice, but often remains unrecognized. Although we were unable to calculate the exact percentage of children with GCLR compared to all children with MC infection treated at our institution, the reported frequency of 4–5 % seems plausible based on our experience [2].
We found an even gender distribution in our cohort unlike the male preponderance suggested by previous reports [2]. In line with the recognized increased susceptibility of atopic children to develop MC, we found a high prevalence of atopy in 42 % of children affected by GCLR [1, 2, 7]. The link between atopy and the risk of developing GCLR remains unclear to date; however, atopy also appears to be a risk factor for classic GCS [2, 8].
GCLR in our cohort developed at a median age of 6.5 years, consistent with the observations of Berger and Estébanez et al., who reported median ages of 5.5 and six years, respectively, for patients with GCLR [2, 5]. This observation is also in good agreement with the median age of six years for children with MC infection [1].
GCLR involved the upper and/or lower extremities in all children, always presenting with a predominant involvement of the extensor surfaces. As clues to the diagnosis of GCLR we identified accentuated red plaques over large joints such as elbows, knees, and ankles, as well as the involvement of the skin overlying the Achilles tendons. Furthermore, we found severe itch as a dominant symptom in 80 % of our patients. A more diverse clinical presentation including papulovesicles, urticarial papules, and even target‐like lesions was present in a minority of patients (Figure 2).
An additional helpful indicator of GCLR is the presence of newly inflamed MC lesions, which was observed in 80 % of our patients. This phenomenon likely represents the mounting of a specific immune response to MC lesions which consecutively leads to GCLR in a subset of patients [9].
The onset of the GCLR is often rapid with the onset of a severely pruritic rash, leading to considerable anxiety in patients and caregivers and frequently resulting in emergency department visits.
In our cohort, 69 % of children had received treatment for their MC infection, most commonly with a local application of KOH, curettage, or cantharidin. We did not observe a clear relationship between treatment of MC and the occurrence of GCLR. However, in one subject the GCLR started one day after treatment with cantharidin. Berger et al. reported onset of GCLR within one month after institution of MC treatment in 38 % of patients, suggesting that MC therapy may serve as a trigger for GCLR [2].
In line with the few reports available, GCLR resolved in all patients within three to four weeks, followed by clearance of the MC infection five to six weeks thereafter. Our data thus supports the view of GCLR as a sign of imminent healing of MC infection.
Although the pathogenesis of GCLR has not been conclusively determined, it likely represents a delayed‐type hypersensitivity reaction to MC antigens [2, 9, 10]. This is supported by its occurrence in the late phase of MC infection and the clearance of MC during its course. We did not perform skin biopsies in our cohort; however, previous reports show a perivascular infiltrate composed of lymphocytes, histiocytes and a few eosinophils in the superficial and mid‐dermis [2].
Classic GCS due to Epstein‐Barr virus, Hepatitis B virus, other viral pathogens or vaccines is characterized by the appearance of monomorphic, small, asymptomatic papules involving the cheeks and extensor surfaces of the extremities [11, 12]. In contrast, GCLR due to MC tends to present with larger, pruritic papules and plaques. Involvement of the cheeks, as a hallmark of classic GCS, only occurs in a minority of patients [2]. GCLR tends to have a shorter course than classic GCS with a better response to anti‐inflammatory treatment. The differences between GCLR and classic GCS are summarized in Table 4.
Table 4.
Comparison of the clinical features of Gianotti‐Crosti syndrome‐like reaction (GCLR) vs. classical Gianotti‐Crosti syndrome (GCS)
| GCLR | Classic GCS | |
|---|---|---|
| Trigger | Molluscum contagiosum | Epstein‐Barr virus, Hepatitis B virus, vaccinations, many other (mostly viral) pathogens |
| Morphology |
Polymorphic: papules/plaques > papulovesicles, urticarial or target‐like lesions |
Monomorphic: papules |
| Distribution |
Relatively localized, predominantly over extensor surfaces of large joints: elbows, knees, Achilles tendon |
More generalized: extensor surfaces of the extremities, buttocks, face |
| Pruritus | Severe | Slight/absent |
| Duration | 2–6 weeks | 6–10 weeks |
| Response to topical corticosteroid treatment | Good | Minimal |
This study is limited by its retrospective design. In addition, data sets were incomplete for some patients.
In conclusion, our report highlights the characteristic clinical features of GCLR, raising awareness of this likely underdiagnosed skin disorder. Previously unknown clinical findings are reported. GCLR should be considered in all children presenting with an acute pruritic symmetrical rash, predominantly involving the extensor surfaces of the extremities, with concomitant occurrence of MC lesions. GCLR has a benign course, responds well to potent anti‐inflammatory treatment and is a harbinger of MC clearance.
Conflict of interest
None.
Acknowledgement
The patients in this manuscript have given written informed consent to publication of their case details.
Open Access Funding provided by Universitat Zurich.
References
- 1. Osio A, Deslandes E, Saada V et al. Clinical characteristics of molluscum contagiosum in children in a private dermatology practice in the greater Paris area, France: A prospective study in 661 patients. Dermatology 2011; 222(4): 314–20. [DOI] [PubMed] [Google Scholar]
- 2. Berger EM, Orlow SJ, Patel RR, Schaffer JV. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: The bump that rashes. Arch Dermatol 2012; 148(11): 1257–64. [DOI] [PubMed] [Google Scholar]
- 3. Rocamora V, Romani J, Puig L, de Moragas J. Id reaction to molluscum contagiosum. Pediatr Dermatol 1996; 13(4): 349–50. [DOI] [PubMed] [Google Scholar]
- 4. Carrascosa J, Just M, Ribera M, Ferrándiz C. Papular acrodermatitis of childhood related to poxvirus and parvovirus B19 infection. Cutis 1998; 61(5): 265–7. [PubMed] [Google Scholar]
- 5. Estébanez A, Silva E, Guillen S et al. [Gianotti‐crosti syndrome‐like reaction secondary to molluscum contagiosum]. An Pediatr (Engl Ed) 2020; 93(1): 49–50. [DOI] [PubMed] [Google Scholar]
- 6. Pascoe VL, Enamandram M, Corey KC et al. Using the physician global assessment in a clinical setting to measure and track patient outcomes. JAMA Dermatology 2015; 151(4): 375–81. [DOI] [PubMed] [Google Scholar]
- 7. Kim JP, Chao LX, Simpson EL, Silverberg JI. Persistence of atopic dermatitis (AD): A systematic review and meta‐analysis. J Am Acad Dermatol 2016; 75(4): 681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chuh A, Zawar V, Lee A, Sciallis G. Is Gianotti‐Crosti syndrome associated with atopy? A case‐control study and a postulation on the intrinsic host factors in Gianotti‐Crosti syndrome. Pediatr Dermatol 2016; 33(5): 488–92. [DOI] [PubMed] [Google Scholar]
- 9. Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics 2012; 129(4): e1072–5. [DOI] [PubMed] [Google Scholar]
- 10. Leung AKC, Sergi CM, Lam JM, Leong KF. Gianotti‐Crosti syndrome (papular acrodermatitis of childhood) in the era of a viral recrudescence and vaccine opposition. World J Pediatr 2019; 15(6): 521–7. [DOI] [PubMed] [Google Scholar]
- 11. Brandt O, Abeck D, Gianotti R, Burgdorf W. Gianotti‐Crosti syndrome. J Am Acad Dermatol 2006; 54(1): 136–45. [DOI] [PubMed] [Google Scholar]
- 12. Chun A, Zawar V, Swallls GF et al. Pityriasis rosea, Gianotti‐Crosti syndrome, asymmetric periflexural exanthem, papular‐purpuric gloves and socks syndrome, eruptive pseudoangiomatosis, and eruptive hypomelanosis: Do their epidemiological data substantiate infectious etiologies? Infect Dis Rep 2016; 8(1): 12–28. [DOI] [PMC free article] [PubMed] [Google Scholar]


