ABSTRACT
Atypical femoral fractures (AFFs) occurring during the course of osteoporosis treatment usually lead to discontinuation of anti‐resorptive (AR) drugs. However, the risk of fracture after an AFF is unknown. We conducted a follow‐up study of patients with AFF matched 1:3 for age and gender with patients with a peripheral major osteoporotic fracture (pMOF), in the setting of a fracture liaison service, to investigate the incidence of subsequent low‐trauma fractures. Fifty‐five patients with AFF (95% women, age [mean ± standard deviation] 75 ± 10 years, 89% exposed to AR drugs), followed for 6.2 ± 3.7 years, were compared to 165 matched controls with a pMOF (hip 85%) followed for 4.3 ± 2.6 years. During the follow‐up, 38% of patients in the AFF group and 16% in the pMOF group received AR therapies. Continuation of AR drugs after an AFF was associated with contralateral AFF in 27% of subjects. The risks of new low‐trauma, major osteoporotic and imminent (within 2 years) fractures, were similar between the two groups: incidence rate ratio (95% confidence interval [CI]) of subsequent fracture following AFF relative to pMOF, 1.30 (95% CI, 0.82–2.04), 1.28 (95% CI, 0.74–2.15), and 1.11 (95% CI, 0.54–2.15), respectively. Moreover, the risk of sustaining multiple fractures per participant was significantly increased among patients with AFF compared to pMOF (hazard ratio 1.48 [95% CI, 1.00–2.19]; p = 0.049). When taking mortality into account, the risk of subsequent fractures tended to be higher in the AFF group (sub‐hazard ratio 1.42 [95% CI, 0.95–2.12]). In conclusion, patients who sustained an AFF are at high risk of subsequent fragility fractures, at least equal or even greater to the risk observed after a pMOF. However, continuation of AR drugs increases the risk of contralateral AFF. Therefore, optimal modalities for secondary fracture prevention after AFF require further evaluation. © 2021 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Keywords: ANTIRESORPTIVES, THERAPEUTICS, FRACTURE RISK ASSESSMENT, OSTEOPOROSIS, DISEASES AND DISORDERS OF/RELATED TO BONE
Introduction
Atypical femoral fractures (AFFs) are rare insufficiency fractures of the subtrochanteric or diaphyseal region of the femur, which occur in a number of metabolic bone disorders but are mainly recognized as a long‐term complication in subjects with long‐term anti‐resorptive (AR) therapies.( 1 , 2 , 3 ) AFFs have been associated with the duration of treatment with AR therapies; ie, incidence increasing from three to more than 50 cases per 100,000 person‐years between 1 and 10 years of exposure.( 4 , 5 ) However, the benefit of AR drugs on fracture prevention in patients with established osteoporosis or at high risk of fractures largely exceeds the risk of AFF, with about 1200 fractures prevented for one AFF associated with 3 years of bisphosphonate therapy.( 6 , 7 , 8 ) Even in the context of bone fragility, clinical management of patients who sustained an AFF generally includes cessation of AR treatments, which decreases the risk of AFF by 70% after 1 year, despite the long‐term remnant effect of bisphosphonates in bones.( 1 , 9 ) Low‐quality evidence is available concerning medical management of patients with AFF, whereby the possibility of a second AFF is balanced with the need to prevent future fragility fractures in high‐risk patients. The current attitude is case‐specific, but a consensus‐based strategy includes surgical fixation depending on the pattern of the fracture (intramedullary nailing in majority of cases), discontinuation of AR treatments, adequate calcium and vitamin D supplementation, and consideration of teriparatide for patients with delayed healing.( 6 , 10 )
Poor postoperative outcomes (required revision surgery, implant failure, and delayed radiological union) have been reported after AFF,( 11 , 12 , 13 ) whereas no excess risk of mortality was established in AFF patients when compared to the general population.( 14 ) It is noteworthy that data are lacking on the incidence of subsequent fragility fractures after an AFF. In particular, it remains unknown whether AFF should be considered as a predictor of imminent fracture risk, similarly to a recent osteoporotic fracture.( 15 , 16 )
In this follow‐up study, we compared the incidence of new fragility fractures following an AFF to the one following an osteoporotic fracture.
Patients and Methods
Study design and population
We conducted an observational follow‐up study at Geneva University Hospitals, Switzerland between August 2000 and March 2020. Cases of AFF were identified based on radiographic assessment of all incident femoral fractures admitted to the orthopedic ward and enrolled in our fracture liaison service (FLS) in addition to patients referred to our bone diseases clinic.( 17 , 18 ) All patients aged 18 years and older with an AFF corresponding to the 2013 American Society of Bone and Mineral Research Task Force criteria( 10 ) were considered, including also rare cases of atypical periprosthetic femoral fractures, as reported.( 19 , 20 , 21 , 22 ) Exclusion criteria were as follows: osteogenesis imperfecta,( 23 ) no follow‐up or follow‐up limited to less than 12 months, and absence of available matched‐control. AFF were classified as complete or incomplete, subtrochanteric or diaphyseal, and X‐rays were screened to identify contralateral incomplete AFF at baseline. Each case of AFF was matched for age (±2 years) and gender with three control patients, randomly identified within our FLS or within the Geneva Retirees Cohort (GERICO; http://www.isrctn.com/ISRCTN11865958), a community‐dwelling cohort of elderly healthy subjects, whose characteristics were previously described.( 24 ) Control patients had sustained a low‐trauma peripheral major osteoporotic fracture (pMOF), mostly hip fractures, and some proximal humerus or forearm fractures for the few younger controls (hip fracture being very rare at this age, matching with hip fracture was not possible in these cases). The study was approved by the Ethics committee, University of Geneva, Geneva, Switzerland.
Data collection
Baseline characteristics of patients were recorded, including age, gender, body mass index (BMI), prior low‐trauma fractures, as well as characteristics of AR treatment (type of drugs, duration before baseline, and continuation during follow‐up). All comorbidities at the time of the index fracture were collected to calculate the Charlson's comorbidity index adjusted for age. Areal bone mineral density (aBMD) of the lumbar spine, femoral neck, and total hip was measured by dual‐energy X‐ray absorptiometry (DXA) at the closest time to AFF or pMOF, for cases and controls, respectively. Osteoporotic status was defined as a T‐score ≤ −2.5 SD at least at one site among lumbar spine, femoral neck, and total hip.( 25 )
Data about subsequent vertebral and peripheral low‐trauma fractures, as well as new atypical femoral fractures and mortality, were obtained retrospectively from the electronic medical records, until March 2020. Outcomes of patients in the GERICO cohort were recorded prospectively during the follow‐up visits planned at 3‐year intervals. In both groups, the use of any anti‐osteoporosis drugs was also recorded, using all electronic medical reports available over the follow‐up. All incident fractures were confirmed by X‐rays or medical reports. Low‐trauma fractures were defined as occurring without any fall/impact or from a fall from a standing height or less. Traumatic and pathological fractures were not recorded. New moderate or severe morphometric vertebral fractures according to Genant semiquantitative classification were also included when vertebral fracture assessment or any spine films were available.( 26 ) We defined vertebral, hip, forearm, and proximal humerus fractures as incident major osteoporotic fractures (MOF), and the first 2 years following the index fracture as the period to assess imminent fracture risk.( 16 ) New contralateral AFFs were also recorded and analyzed specifically, and not included as subsequent low‐trauma fractures.
Statistical analysis
Baseline characteristics were compared between groups with chi‐square test for categorical variables and a Mann‐Whitney test for continuous variables. For these variables, mean imputation was used if less than 5% of data were not available. Time to first subsequent fracture was calculated as the time between the index AFF or pMOF, and the subsequent fracture. Second AFFs were analyzed separately. Multiple fractures that occurred on the same day for an individual subject were treated as a single event. The Andersen‐Gill model was used to estimate time to multiple subsequent fractures occurring at several time points. For those who did not sustain a subsequent fracture, follow‐up time was calculated as time to either death or the last follow‐up record. Person‐time incidence rates (per 1000 patient‐years) were used to represent the incidence rate of subsequent fragility fractures and compare incidence rate ratio between groups. As a sensitivity analysis, Fine and Gray regression for survival analyses were secondly fitted to account for competing mortality risk and estimate sub‐hazard ratio (SHR) with 95% confidence interval (CI) on the association between the index fracture type and subsequent fracture occurrence.( 27 ) Additional univariate Cox proportional hazard models were applied to investigate the predictors of fractures among baseline characteristics, in each group. A p value of <0.05 was considered statistically significant. Analyses were conducted with STATA version 14.0 (StataCorp LP, College Station, TX, USA).
Results
Among 82 AFFs identified between August 2000 and July 2018, 55 were included in our study. The main reason for exclusion was loss of follow‐up or follow‐up less than one year (Fig. 1). The AFF group included 51 (93%) complete AFFs, all surgically treated, and four (7%) incomplete AFFs. Among these incomplete AFFs, two of them were treated with prophylactic nailing and one received teriparatide. Twelve (22%) AFFs occurred at the subtrochanteric region of the femur and 43 (78%) at the femoral diaphysis. Two of them were sub‐prosthetic AFFs (both exposed to AR and discontinued after AFF, one treated with curved plate, the other with hip prosthesis). Patients were mostly women (94%), and mean age was 75 years (range, 56–90 years) (Table 1). Forty‐nine patients (89%) were treated or had been treated with AR drugs at the time of AFF (43 bisphosphonates, one denosumab, five bisphosphonate followed by denosumab), whereas six patients (11%) had no current or previous exposure to these drugs. Mean duration of AR treatment in treated patients was 7.5 ± 4.6 years (range, 3 months to 20 years). AR therapies were given mainly for osteoporosis and prevention of fragility fractures, except in one patient with metastatic breast cancer. Delayed fracture healing or non‐union was observed in 11 patients (20%) after surgical management, including five who continued AR therapies after the AFF.
Fig. 1.
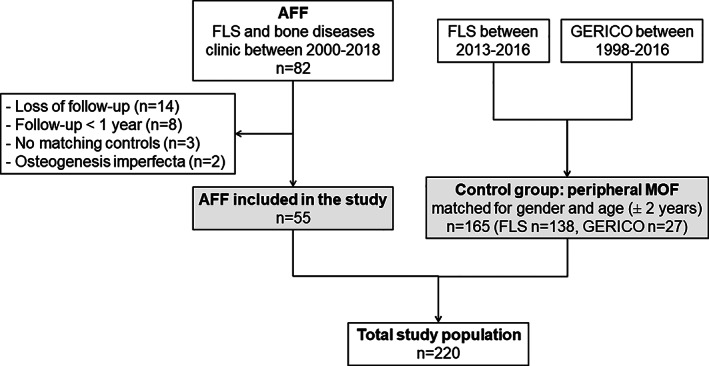
Study flowchart. AFF = atypical femoral fracture; FLS = Fracture Liaison Service; GERICO = Geneva Retirees Cohort.
Table 1.
Baseline Characteristics and Follow‐Up of Patients With AFF and With pMOFs
| Characteristic | AFF (n = 55) | pMOF (n = 165) | p |
|---|---|---|---|
| Gender (women), (%) | 95 | 95 | 1.000 |
| Age (years), mean ± SD | 75 ± 10 | 75 ± 10 | 0.896 |
| Weight (kg), mean ± SD | 66 ± 13 | 65 ± 15 | 0.951 |
| Height (cm), mean ± SD | 158 ± 8 | 161 ± 9 | 0.005 |
| Body mass index (kg/m2), mean ± SD | 26.3 ± 5.1 | 25.0 ± 5.4 | 0.063 |
| Charlson Comorbidity Index, mean ± SD | 3.9 ± 1.6 | 4.6 ± 2.1 | 0.013 |
| Prior clinical fracture (excluding index AFF or pMOF) (%) | 67 | 33 | <0.001 |
| Lumbar spine T‐score, mean ± SD a | −1.4 ± 1.7 | −1.3 ± 1.6 | 0.799 |
| Total hip T‐score, mean ± SD a | −1.3 ± 1.3 | −1.7 ± 1.0 | 0.216 |
| Femoral neck T‐score, mean ± SD a | −1.9 ± 0.9 | −2.0 ± 1.0 | 0.626 |
| Osteoporotic status on DXA a | 0.980 | ||
| Normal BMD, (%) | 14 | 13 | |
| Osteopenia, (%) | 40 | 40 | |
| Osteoporosis, (%) | 46 | 47 | |
| Prior AR therapy, (%) b | 89 | 12 | <0.001 |
| AR therapy during follow‐up, (%) c | 38 | 16 | <0.001 |
| Follow‐up duration (years), mean ± SD | 6.2 ± 3.7 | 4.3 ± 2.6 | <0.001 |
Bold values are significant at p <0.05.
AFF = atypical femoral fracture; AR = anti‐resorptive; BMD = bone mineral density; DXA = dual energy X‐ray absorptiometry; pMOF = peripheral major osteoporotic fracture; SD = standard deviation.
Osteoporosis defined as at least one T‐score ≤ −2.5 SD and osteopenia as at least one T‐score between −1 and −2.5 SD with none ≤ − 2.5 SD at the lumbar spine, total hip, or femoral neck. Data available for a subgroup of patients (spine n = 148, total hip n = 131, femoral neck n = 136).
Before index fracture.
Continued or started after index fracture or during the follow‐up.
The index fracture in the matched control group (pMOF group) were hip (n = 140, 85%), forearm (n = 16, 10%), and proximal humerus (n = 9, 5%) fractures. The baseline characteristics and follow‐up of patients in the AFF group and control group are reported in Table 1. As compared with the control group, patients in the AFF group had more prevalent fractures (67% versus 33%; p < 0.001, excluding the index one) and were more often on AR drugs before (89% versus 12%; p < 0.001) and after (38% versus 16%; p < 0.001) the index fracture. Furthermore, the AFF group had lower Charlson comorbidity index adjusted for age (p = 0.013), and longer follow‐up duration (p < 0.001). Weight and body mass index were comparable between groups, but height was slightly lower in the AFF group. Osteoporosis on DXA was detected in 46% and 47% of patients in the AFF and pMOF groups, respectively.
In the AFF group, a contralateral AFF was observed in 19 patients (35%). At time of first AFF, contralateral AFF was observed in 14 patients (25%) (13 contralateral incomplete AFFs and one complete AFF). Five patients (9%) developed a contralateral AFF later during the follow‐up (two incomplete AFFs, time ranges 1.5 to 4.7 years after index AFF). The characteristics of patients with a concomitant contralateral AFF were not different from those without contralateral AFF (Supplemental Table S1). Among 15 incomplete contralateral AFFs, eight of them were treated with prophylactic nailing and one received teriparatide. Patients who developed a contralateral subsequent AFF over the follow‐up had lower BMI (p = 0.026). Continuation of AR drugs after an AFF was associated with subsequent contralateral AFF in 27% of subjects (versus 4% in those who discontinued AR drugs, p = 0.031).
A total number of 66 incident fractures occurred in 31 patients in the AFF group (56%), and 74 fractures in 61 patients of the pMOF group (37%) over the course of follow‐up. Fracture sites are detailed in Supplemental Tables S2 and S3, and their distribution was different between the groups, the most frequent ones being vertebrae, pelvis, and humerus in the AFF group, and hip, vertebrae, and humerus in the pMOF group. The incidence of new low‐trauma fractures was 142 (95% CI, 100–202) per 1000 patient‐years in the AFF group, and 109 (95% CI, 85–140) per 1000 patient‐years in the pMOF group. The incidence rate ratios (IRRs) and 95% CIs of subsequent fracture following AFF relative to pMOF were 1.30 (95% CI, 0.82–2.04), p = 0.117 for all low‐trauma fractures, and 1.28 (95% CI, 0.74–2.15), p = 0.171 for incident MOF (Table 2). In Cox‐regression analyses, hazard ratio for all low‐trauma fractures following AFF relative to pMOF was HR 1.22 (95% CI, 0.79–1.90), p = 0.369. In multivariate Cox‐regression including height, Charlson comorbidity score, fracture prior to the index one, and use of AR drugs after the index fracture, as potential confounders, adjusted HR for subsequent fracture was 1.15 (95% CI, 0.71–1.89), p = 0.568. We did not include in this model the use of AR drugs before the index fracture because of its high collinearity with the exposure (AFF).
Table 2.
Incidence of Fracture and Mortality During the Follow‐Up
| Parameter | All incident low‐trauma fractures | Incident MOF | Mortality | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients n (%) | PY | Incidence per 1000 PY (95% CI) | IRR (95% CI) | p | Patients n (%) | PY | Incidence per 1000 PY (95% CI) | IRR (95% CI) | p | Patients n (%) | PY | Incidence per 1000 PY (95% CI) | IRR (95% CI) | p | |
| Total follow‐up | |||||||||||||||
| AFF (n = 55) | 31 (56) | 218 | 142 (100–202) | 1.30 (0.82–2.04) | 0.117 | 23 (42) | 220 | 104 (69–157) | 1.28 (0.74–2.15) | 0.171 | 5 (9) | 340 | 15 (6–35) | 0.29 (0.09, 0.75) | 0.002 |
| pMOF (n = 165) | 61 (37) | 560 | 109 (85–140) | 46 (28) | 562 | 82 (61–109) | 36 (22) | 713 | 50 (36–70) | ||||||
| Imminent risk* | |||||||||||||||
| AFF (n = 55) | 13 (24) | 91 | 143 (83–246) | 1.11 (0.54–2.15) | 0.364 | 9 (16) | 105 | 86 (45–165) | 0.86 (0.36–1.86) | 0.359 | 0 (0) | 105 | 0 | NA | 0.038 |
| pMOF (n = 165) | 36 (22) | 280 | 128 (93–178) | 30 (18) | 301 | 100 (70–143) | 9 (5) | 318 | 28 (15–54) | ||||||
Bold values are significant at p < 0.05.. AFF = atypical femoral fracture; CI = confidence interval; IRR = incidence rate ratio; MOF = major osteoporotic fracture; pMOF = peripheral major osteoporotic fracture; PY = patient‐years. * follow‐up censored at maximum 2 years
The incidences of new low trauma fractures were 109 (95% CI 60, 197) per 1000 patient‐years in the AFF group without osteoporosis and 186 (95% CI 108, 320) per 1000 patient‐years in the AFF group with osteoporosis (IRR 1.70 [95% CI, 0.70–4.19], p = 0.100).
When restricting the follow‐up period to 2 years after the index fracture, the imminent fracture risk was also similar between the two groups (IRR 1.11 [95% CI, 0.54–2.15], p = 0.364 for all incident low‐trauma fractures; IRR 0.86 [95% CI, 0.36–1.86], p = 0.359 for incident pMOF). Similar results were obtained in subgroups analyses considering only AFF matched with controls with hip fractures (Supplemental Table S4) or considering only AFF with prior exposure to AR drugs (Supplemental Table S5).
Multiple fracture events occurred in 12 (22%) patients in the AFF group and nine (5%) in the pMOF group. Using an Andersen‐Gill model allowing for analysis of multiple fractures per participant, the risk of new low‐trauma fractures was significantly increased in patients with AFF compared to those with pMOF (HR 1.48 [95% CI, 1.00–2.19], p = 0.049). In univariate analyses in the AFF group, using the same model considering the possibility of multiple fractures events per participant, we identified that the only and strongest predictor of new fractures was osteoporosis on DXA at the time of the index AFF (all subsequent fractures, HR 1.99 [95% CI, 1.12–3.56], p = 0.020; subsequent MOF, HR 2.52 [95% CI, 1.15–5.52], p = 0.021). Gender, prior fracture to the index one, use of AR therapies before the index fracture, and discontinuation of AR drugs during the follow‐up, were not associated with subsequent fractures.
Mortality was significantly lower in the AFF group (15 per 1000 patient‐years) compared to the pMOF group (50 per 1000 patient‐years), with an IRR of 0.29 (95% CI, 0.09–0.75), p = 0.002 (Table 2). In models taking into account competing mortality risk in fracture risk estimates, the risk of new low‐trauma fractures tended to be higher in the AFF group compared to the pMOF group (sub‐hazard ratio 1.42 [95% CI, 0.95–2.12], p = 0.087 for all incident low‐trauma fractures; 1.40 [95% CI, 0.88–2.23], p = 0.152 for incident pMOF) (Fig. 2).
Fig. 2.
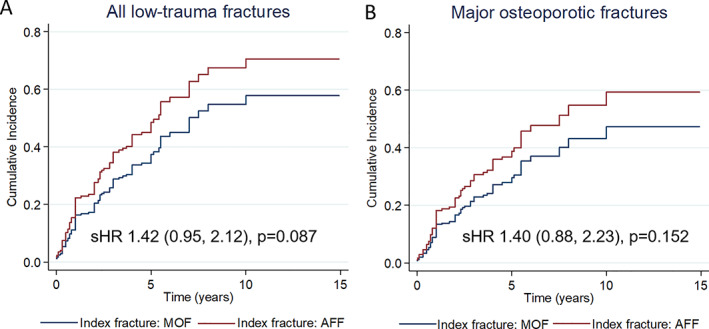
Estimated cumulative risk of new low‐trauma fractures* (A) and pMOF (B) taking into account mortality risk, after an index AFF or pMOF. * Contralateral AFF were not recorded as new low‐trauma fractures. AFF = atypical femoral fracture; pMOF = major osteoporotic fracture; sHR = sub‐hazard ratio (95% confidence interval).
Discussion
To our knowledge, this is the first study that provides longitudinal data regarding the risk of subsequent low‐trauma fractures in subjects who have experienced an AFF. Patients in the AFF group had more frequently sustained a prior clinical fracture and subsequently used AR drugs, which is not surprising because long‐term AR drugs use is a major risk factor for AFF.( 10 ) Despite a higher proportion of prior and subsequent treatment with anti‐osteoporotic drugs in the AFF group, the incidence of new fragility fractures following an AFF was at least as high as following a pMOF, itself a well‐recognized risk factor of subsequent fracture. Both groups were at very high risk of fractures, with similar proportion of patients with osteoporosis on DXA at start of the follow‐up. The incidence of secondary fractures was particularly high in the two study groups (142 and 109 fractures per 1000 patient‐years, respectively). In comparison, the incidence of fracture in the general Swiss population is 20 to 30 fractures per 1000 patients‐years between the age of 70 and 79 years.( 28 ) The incidence of subsequent fractures after a low‐trauma fracture reported in the Dubbo Osteoporosis Epidemiology Study in Australia was 63 subsequent fractures per 1000 patient‐years in women between the age of 70 and 79 years.( 15 ) In our study, the risk of imminent fracture was also high (24%–22%) and not different between the two groups (IRR 1.11 [95% CI, 0.54–2.15]). These results were obtained with a follow‐up censored at 2 years for all patients, excluding a potential bias induced by the difference in follow‐up duration between groups. Furthermore, the only and strongest predictive factor of subsequent fracture in AFF patients was the osteoporotic status on DXA. Taken together, these data indicate a particularly high incidence of fractures in patients who sustained an AFF, particularly in osteoporotic ones, supporting the concept of persistent bone fragility in these patients despite previous long‐term AR therapies. Fracture sites description reveals that two‐thirds of new fractures were major osteoporotic fractures (vertebral fractures, hip, humeral, or forearm fractures).
Mortality was more important in the pMOF group compared to the AFF group (22% versus 9%; p = 0.036), which is consistent with higher Charlson comorbidity index at baseline and the increased mortality risk observed after hip fractures, which account for 85% of pMOF in our study.( 29 , 30 , 31 ) Furthermore, previous studies did not report excess mortality associated with AFF when compared to non‐AFF and general population.( 14 , 32 ) In a sensitivity analysis comparing the risk of new fracture between the AFF and pMOF groups, and integrating competing mortality, a nonsignificant trend of 40% increase in fracture risk was observed in the AFF group compared to the pMOF group (Fig. 2). In addition, the risk of multiple fractures was significantly higher in the AFF group compared to the pMOF group. Taken together, these data support the need to consider prevention of subsequent fractures in patients with AFF.
Thirty‐five percent of patients with AFF developed a contralateral AFF, the majority concomitantly, as previously reported (22% to 63% according to studies).( 4 , 10 , 33 ) Subsequent contralateral AFFs were associated with the continuation of AR drugs after the index AFF. This is consistent with the risk of AFF increasing with longer duration of bisphosphonate use and greater adherence.( 4 , 8 , 34 ) A recent study showed that the radiographic progression of incomplete AFF contralateral to a complete AFF was associated with a higher frequency of postoperative bisphosphonates use (61% in progression group versus 25% in non‐progression group).( 35 ) In a retrospective cohort of 126 patients with AFF, the risk of contralateral AFF was 54% in patients who continued bisphosphonates for more than 3 years after the first AFF compared to 19% if bisphosphonates were stopped in the first few months following the event.( 36 , 37 ) These data suggest the relevance of AR drugs discontinuation after AFF. Furthermore, in patients receiving bisphosphonates, their discontinuation is associated with a rapid and significant decrease in the risk of AFF.( 8 , 9 )
The issue raised by these data is how to prevent subsequent fractures after AFF. These patients are at very high risk of major osteoporotic fracture, but continuation of AR therapies after the index AFF increases the risk of contralateral subsequent AFF, without being associated with a lower incidence of subsequent fracture in this observational study. Considering these data, discontinuation of AR drugs, as proposed currently in order to prevent contralateral AFF, appears to be an insufficient strategy, despite the promotion of classical general measures such as optimizing nutritional intakes, adequate calcium, and vitamin D intake or supplements, and prevention of falls.( 6 , 10 , 38 ) In this context, prophylactic intramedullary nailing should also be considered in patients with intractable pain, non‐union or active incomplete fractures.( 39 ) After healing of bilateral, surgically managed AFFs, AR drugs might be continued in patients at high risk of subsequent fragility fractures.( 38 ) In addition, anabolic agents, such as teriparatide, abaloparatide, or romosozumab, might be of potential interest, particularly in osteoporotic patients. This is reinforced by the particular high incidence of new vertebral fractures observed in patients who sustained an AFF (42%), suggesting teriparatide and other anabolics as potential treatments of choice in these patients. Whether these drugs might also improve AFF healing in this context is less established in observational studies.( 40 , 41 , 42 , 43 ) The results of the first randomized placebo‐controlled trial testing teriparatide for the healing of incomplete AFF (TAFF trial), were recently reported. In this 2‐year study, teriparatide failed to demonstrate benefits on clinical and radiographic healing outcomes in incomplete AFFs.( 44 ) In addition, data are lacking on the effect of teriparatide on BMD, bone turnover markers, and fracture prevention in the specific population of patients with AFF, and usually with long prior exposure to AR drugs. It also remains unknown how to maintain the benefit of teriparatide and manage bone fragility after 18 to 24 months of teriparatide, a period after which bisphosphonates or denosumab are proposed in the general osteoporotic population. Concerning romosozumab, which also has AR properties, its association with the risk of AFF is questionable and there is currently no data on its use after an AFF.( 38 )
We recognize this study has some limitations. AFF being a rare complication, the sample size is limited, even with nearly 20 years of recruitment in our center with approximately 450 osteoporotic hip fractures per year.( 45 ) Therefore, the risk equivalency or risk increase of fractures in the AFF group compared to the pMOF group, which are discussed in this work, are based on analyses with notorious power limitations. The identification of our cases of AFF was mainly through our FLS, and therefore most of them were complete AFF. Also, as any retrospective study and medical record‐based data collection, there was no systematic follow‐up and possibility of missing data or underestimation of the proportion of patients receiving AR drugs in the control group. Despite matching for age and gender, some factors such as follow‐up duration, Charlson's comorbidity index, and bisphosphonate exposure were not well balanced between AFF and pMOF groups and could have impact our results to some extent. These data illustrate on one side the high exposure to AR therapies in AFF patents and on the other side the treatment gap of osteoporosis after an MOF (16% receiving AR therapies).( 46 ) Furthermore, 27 (16%) of the 165 pMOF patients were from a community‐dwelling database, whereas all AFF patients were identified in an hospital setting (Fig. 1). Nevertheless, our study is the first follow‐up study that addresses fracture risk after AFF. Our finding of a particularly high risk of fracture following an AFF needs to be replicated in prospective studies to definitely confirm that AFF requires secondary fracture prevention.
In conclusion, this study shows that patients who sustained an AFF are at very high risk of subsequent fractures, at least equal or even greater if considering multiple fractures risk, to the risk of fracture observed after a pMOF. In parallel, the risk of a subsequent AFF was increased in patients with an index AFF who did not discontinue AR therapies. Hence there is an urgent need to design randomized controlled trials (RCTs) to evaluate the risks and benefits of osteoporosis therapies in patients with AFF.
Disclosures
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. RR reported personal fees from Abiogen, Danone, Echolight, Mithra, ObsEva, Pfizer consumer health, and Theramex, outside the submitted work; SF reported grants and other from AMGEN, other from UCB, other from Radius, grants and other from Agnovos, outside the submitted work; EB reported personal fees from AMGEN, Nestlé, outside the submitted work. All other authors did not report any disclosures.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/jbmr.4461.
Supporting information
Appendix S1. Supporting Information
Acknowledgments
This study was supported by grants from the Geneva University Hospitals and Faculty of Medicine Clinical Research Center, the HUG Private Foundation, and the Fondation pour la Recherche sur l'Ostéoporose et les Maladies Osseuses de Genève. None of the funders had any influence on the study design, implementation, and analysis, and on interpretation of the data. Open Access Funding provided by Universite de Geneve.
[Correction added on 13 April 2022, after first online publication: ‘Open Access Funding provided by Universite de Geneve’ funding statement has been added.]
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Schilcher J, Koeppen V, Aspenberg P, Michaëlsson K. Risk of atypical femoral fracture during and after bisphosphonate use. N Engl J Med. 2014;371(10):974‐976. [DOI] [PubMed] [Google Scholar]
- 2. Schneider JP, Hinshaw WB, Su C, Solow P. Atypical femur fractures: 81 individual personal histories. J Clin Endocrinol Metab. 2012;97(12):4324‐4328. [DOI] [PubMed] [Google Scholar]
- 3. Lo JC, Grimsrud CD, Ott SM, Chandra M, Hui RL, Ettinger B. Atypical femur fracture incidence in women increases with duration of bisphosphonate exposure. Osteoporos Int. 2019;30(12):2515‐2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27(12):2544‐2550. [DOI] [PubMed] [Google Scholar]
- 5. Meier RP, Perneger TV, Stern R, Rizzoli R, Peter RE. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Arch Intern Med. 2012;172(12):930‐936. [DOI] [PubMed] [Google Scholar]
- 6. Black DM, Abrahamsen B, Bouxsein ML, Einhorn T, Napoli N. Atypical femur fractures: review of epidemiology, relationship to bisphosphonates, prevention, and clinical management. Endocr Rev. 2019;40(2):333‐368. [DOI] [PubMed] [Google Scholar]
- 7. Khosla S, Bilezikian JP, Dempster DW, et al. Benefits and risks of bisphosphonate therapy for osteoporosis. J Clin Endocrinol Metab. 2012;97(7):2272‐2282. [DOI] [PubMed] [Google Scholar]
- 8. Black DM, Geiger EJ, Eastell R, et al. Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N Engl J Med. 2020;383(8):743‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schilcher J, Koeppen V, Aspenberg P, Michaëlsson K. Risk of atypical femoral fracture during and after bisphosphonate use. Acta Orthop. 2015;86(1):100‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29(1):1‐23. [DOI] [PubMed] [Google Scholar]
- 11. Teo BJ, Koh JS, Goh SK, Png MA, Chua DT, Howe TS. Post‐operative outcomes of atypical femoral subtrochanteric fracture in patients on bisphosphonate therapy. Bone Joint J. 2014;96‐B(5):658‐664. [DOI] [PubMed] [Google Scholar]
- 12. Egol KA, Park JH, Rosenberg ZS, Peck V, Tejwani NC. Healing delayed but generally reliable after bisphosphonate‐associated complete femur fractures treated with IM nails. Clin Orthop Relat Res. 2014;472(9):2728‐2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schultz DS, Gass HM, Barfield WR, Demos HA, Hartsock LA. Surgical complications associated with atypical femur fractures attributed to bisphosphonate use. J Surg Orthop Adv. 2018;27(1):14‐20. [PubMed] [Google Scholar]
- 14. Kharazmi M, Hallberg P, Schilcher J, Aspenberg P, Michaëlsson K. Mortality after atypical femoral fractures: a cohort study. J Bone Miner Res. 2016;31(3):491‐497. [DOI] [PubMed] [Google Scholar]
- 15. Center JR, Bliuc D, Nguyen TV, Eisman JA. Risk of subsequent fracture after low‐trauma fracture in men and women. JAMA. 2007;297(4):387‐394. [DOI] [PubMed] [Google Scholar]
- 16. Roux C, Briot K. Imminent fracture risk. Osteoporos Int. 2017;28(6):1765‐1769. [DOI] [PubMed] [Google Scholar]
- 17. Ing‐Lorenzini K, Desmeules J, Plachta O, Suva D, Dayer P, Peter R. Low‐energy femoral fractures associated with the long‐term use of bisphosphonates: a case series from a Swiss university hospital. Drug Saf. 2009;32(9):775‐785. [DOI] [PubMed] [Google Scholar]
- 18. Chevalley T, Hoffmeyer P, Bonjour JP, Rizzoli R. An osteoporosis clinical pathway for the medical management of patients with low‐trauma fracture. Osteoporos Int. 2002;13(6):450‐455. [DOI] [PubMed] [Google Scholar]
- 19. Leclerc JT, Michou L, Vaillancourt F, Pelet S, Simonyan D, Belzile EL. Prevalence and characteristics of atypical periprosthetic femoral fractures. J Bone Miner Res. 2019;34(1):83‐92. [DOI] [PubMed] [Google Scholar]
- 20. Lee YK, Park CH, Kim KC, Hong SH, Ha YC, Koo KH. Frequency and associated factor of atypical periprosthetic femoral fracture after hip arthroplasty. Injury. 2018;49(12):2264‐2268. [DOI] [PubMed] [Google Scholar]
- 21. MacKenzie SA, Ng RT, Snowden G, Powell‐Bowns MFR, Duckworth AD, Scott CEH. Periprosthetic atypical femoral fractures exist and are associated with duration of bisphosphonate therapy. Bone Joint J. 2019;101‐B(10):1285‐1291. [DOI] [PubMed] [Google Scholar]
- 22. Robinson Jde D, Leighton RK, Trask K, Bogdan Y, Tornetta P 3rd. Periprosthetic atypical femoral fractures in patients on long‐term bisphosphonates: a multicenter retrospective review. J Orthop Trauma. 2016;30(4):170‐176. [DOI] [PubMed] [Google Scholar]
- 23. Meier RP, Ing Lorenzini K, Uebelhart B, Stern R, Peter RE, Rizzoli R. Atypical femoral fracture following bisphosphonate treatment in a woman with osteogenesis imperfecta—a case report. Acta Orthop. 2012;83(5):548‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Biver E, Durosier‐Izart C, Chevalley T, van Rietbergen B, Rizzoli R, Ferrari S. Evaluation of radius microstructure and areal bone mineral density improves fracture prediction in postmenopausal women. J Bone Miner Res. 2018;33(2):328‐337. [DOI] [PubMed] [Google Scholar]
- 25. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1‐129. [PubMed] [Google Scholar]
- 26. Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8(9):1137‐1148. [DOI] [PubMed] [Google Scholar]
- 27. Leslie WD, Lix LM, Wu X. Competing mortality and fracture risk assessment. Osteoporos Int. 2013;24(2):681‐688. [DOI] [PubMed] [Google Scholar]
- 28. Svedbom A, Ivergård M, Hernlund E, Rizzoli R, Kanis JA. Epidemiology and economic burden of osteoporosis in Switzerland. Arch Osteoporos. 2014;9:187. [DOI] [PubMed] [Google Scholar]
- 29. Katsoulis M, Benetou V, Karapetyan T, et al. Excess mortality after hip fracture in elderly persons from Europe and the USA: the CHANCES project. J Intern Med. 2017;281(3):300‐310. [DOI] [PubMed] [Google Scholar]
- 30. von Friesendorff M, McGuigan FE, Wizert A, et al. Hip fracture, mortality risk, and cause of death over two decades. Osteoporos Int. 2016;27(10):2945‐2953. [DOI] [PubMed] [Google Scholar]
- 31. Trombetti A, Herrmann F, Hoffmeyer P, Schurch MA, Bonjour JP, Rizzoli R. Survival and potential years of life lost after hip fracture in men and age‐matched women. Osteoporos Int. 2002;13(9):731‐737. [DOI] [PubMed] [Google Scholar]
- 32. Gani L, Anthony N, Dacay L, Tan P, Chong LR, King TF. Incidence of atypical femoral fracture and its mortality in a Single Center in Singapore. JBMR Plus. 2021;5(8):e10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Probyn L, Cheung AM, Lang C, et al. Bilateral atypical femoral fractures: how much symmetry is there on imaging? Skeletal Radiol. 2015;44(11):1579‐1584. [DOI] [PubMed] [Google Scholar]
- 34. Wang Z, Ward MM, Chan L, Bhattacharyya T. Adherence to oral bisphosphonates and the risk of subtrochanteric and femoral shaft fractures among female Medicare beneficiaries. Osteoporos Int. 2014;25(8):2109‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee KJ, Min BW, Bae KC, Cho CH, Lee SW, Kim BS. Progression of asymptomatic contralateral femur in patients with complete atypical femoral fracture, according to initial radiographic findings. J Bone Joint Surg Am. 2021;103(2):123‐130. [DOI] [PubMed] [Google Scholar]
- 36. Dell RM, Greene D, Tran D. Stopping bisphosphonate treatment decreases the risk of having a second atypical femur fracture. Paper presented at: American Academy of Orthopaedic Surgeons (AAOS) Annual Meeting; 2012. Feb 7‐11; San Francisco, CA, USA. [Google Scholar]
- 37. Dell R, Greene D. A proposal for an atypical femur fracture treatment and prevention clinical practice guideline. Osteoporos Int. 2018;29(6):1277‐1283. [DOI] [PubMed] [Google Scholar]
- 38. van de Laarschot DM, McKenna MJ, Abrahamsen B, et al. Medical management of patients after atypical femur fractures: a systematic review and recommendations from the European Calcified Tissue Society. J Clin Endocrinol Metab. 2020;105(5):1682‐1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koh A, Guerado E, Giannoudis PV. Atypical femoral fractures related to bisphosphonate treatment: issues and controversies related to their surgical management. Bone Joint J. 2017;99‐B(3):295‐302. [DOI] [PubMed] [Google Scholar]
- 40. Im GI, Lee SH. Effect of teriparatide on healing of atypical femoral fractures: a systemic review. J Bone Metab. 2015;22(4):183‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miyakoshi N, Aizawa T, Sasaki S, et al. Healing of bisphosphonate‐associated atypical femoral fractures in patients with osteoporosis: a comparison between treatment with and without teriparatide. J Bone Miner Metab. 2015;33(5):553‐559. [DOI] [PubMed] [Google Scholar]
- 42. Shin WC, Moon NH, Jang JH, Seo HU, Suh KT. A retrospective bicenter comparative study of surgical outcomes of atypical femoral fracture: potential effect of teriparatide on fracture healing and callus formation. Bone. 2019;128:115033. [DOI] [PubMed] [Google Scholar]
- 43. Zhang HY, Weng HL, Li M, Zhang J. Different surgical outcomes in a patient with bilateral atypical femoral fracture related to bisphosphonate use with or without teriparatide treatment. Osteoporos Int. 2019;30(11):2349‐2354. [DOI] [PubMed] [Google Scholar]
- 44. Tile L, Bleakney R, Tomlinson G, et al. Teriparatide for the healing of incomplete atypical femur fractures: the TAFF trial. J Bone Miner Res. 2020;35(Suppl 1):23. https://www.asbmr.org/meetings/annualmeeting/AbstractDetail?aid=5ce3b97c‐2f08‐4fa3‐9b05‐5728cafc195f. [Google Scholar]
- 45. Chevalley T, Guilley E, Herrmann FR, Hoffmeyer P, Rapin CH, Rizzoli R. Incidence of hip fracture over a 10‐year period (1991‐2000): reversal of a secular trend. Bone. 2007;40(5):1284‐1289. [DOI] [PubMed] [Google Scholar]
- 46. Kanis JA, Norton N, Harvey NC, et al. SCOPE 2021: a new scorecard for osteoporosis in Europe. Arch Osteoporos. 2021;16(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


