Abstract
Freshwater ecosystems are strongly influenced by weather extremes such as heatwaves (HWs), which are predicted to increase in frequency and magnitude in the future. In addition to these climate extremes, the freshwater realm is impacted by the exposure to various classes of chemicals emitted by anthropogenic activities. Currently, there is limited knowledge on how the combined exposure to HWs and chemicals affects the structure and functioning of freshwater ecosystems. Here, we review the available literature describing the single and combined effects of HWs and chemicals on different levels of biological organization, to obtain a holistic view of their potential interactive effects. We only found a few studies (13 out of the 61 studies included in this review) that investigated the biological effects of HWs in combination with chemical pollution. The reported interactive effects of HWs and chemicals varied largely not only within the different trophic levels but also depending on the studied endpoints for populations or individuals. Hence, owing also to the little number of studies available, no consistent interactive effects could be highlighted at any level of biological organization. Moreover, we found an imbalance towards single species and population experiments, with only five studies using a multitrophic approach. This results in a knowledge gap for relevant community and ecosystem level endpoints, which prevents the exploration of important indirect effects that can compromise food web stability. Moreover, this knowledge gap impairs the validity of chemical risk assessments and our ability to protect ecosystems. Finally, we highlight the urgency of integrating extreme events into multiple stressors studies and provide specific recommendations to guide further experimental research in this regard.
Keywords: chemicals, combined effects, community, extreme events, freshwater, heatwave, micropollutants, multiple stressors
We reviewed the available literature describing the single and combined effects of heatwaves and chemicals on different levels of biological organization. We found that only few studies addressed these stressors in combination. Moreover, we highlight the imbalance towards single species and population experiments. This results in a knowledge gap for relevant community‐ and ecosystem‐level endpoints, which prevents the exploration of important indirect effects that can compromise food web stability. Finally, we highlight the urgency of integrating extreme events into multiple stressors studies and provide recommendations to guide further experimental research in this regard.
1. INTRODUCTION
Multiple anthropogenic disturbances drive the so‐called global change (Schlesinger, 2006), which is leading to various, and still unpredictable, changes in biodiversity, species interactions and ecosystem functioning (Cardinale et al., 2012). Among the several drivers of global change, Climate change is one of the key and most concerning ones (Parry et al., 2001). Climate change is composed of different processes and phenomena (IPCC, 2013), including extreme weather events such as heavy precipitations or heatwaves (HWs; Jentsch et al., 2007). HWs are characterized by a short‐term, rapid increase in temperature, which can impact all trophic levels, from microorganisms (Szymczak et al., 2020) to large predators (de Mira‐Mendes et al., 2019), across all ecosystem types (Stillman, 2019). HWs are of particular concern because their magnitude and frequency are predicted to increase in the future (Meehl & Tebaldi, 2004; Woolway et al., 2021). A growing amount of literature has described the effects of HWs on freshwater ecosystems (Bartosiewicz et al., 2016; Huber et al., 2010; Ledger & Milner, 2015; Maazouzi et al., 2008; Weisse et al., 2016; Woodward et al., 2016) and show that they can lead to high mortality rates (Mouthon & Daufresne, 2006; Strydom et al., 2020). This is particularly relevant for ectotherm animals (Brown et al., 2004; Cereja, 2020), which represent the vast majority (>95%) of species in aquatic ecosystems (Willmer et al., 2000). Temperature directly controls the metabolic rate of cells and their size (Gillooly et al., 2001). It also affects carbon allocation (García‐Carreras et al., 2018), population growth (Savage et al., 2004), carrying capacity (Fussmann et al., 2014) and ecosystem respiration (Yvon‐Durocher et al., 2010). Generally, ectotherm plants and animals show increased metabolism but reduced size at elevated temperature (Brown et al., 2004; Yvon‐Durocher et al., 2011; Zohary et al., 2021).
Extreme weather events are not the only disturbances affecting aquatic ecosystems. Among the large number of stressors affecting the freshwater realm (Birk et al., 2020), a serious, yet somewhat overlooked, dimension of global change is chemical pollution (Mazor et al., 2018; Rockström et al., 2009; Steffen et al., 2015). A review by Bernhardt et al. (2017) showed that chemicals are only partially considered as drivers of global change. Yet, the global annual application of pesticides grows constantly (Food and Agriculture Organization of the United Nations—FAOSTAT, 2020), and pharmaceuticals and personal care products are increasingly traced in freshwater ecosystems worldwide (Danner et al., 2019; Ebele et al., 2017). Micropollutants reaching water bodies act as selective stressors targeting different organisms based on their physicochemical properties and toxicological mode of action, leading to non‐random effects on communities (De Laender et al., 2016). Synthetic chemicals are known to reduce diversity (Bray et al., 2019) cause trophic interaction shifts (Schrama et al., 2017), affect ecosystem functioning (Spaak et al., 2017), and have been found to limit the overall ecological status of European rivers (Posthuma et al., 2020).
Heatwaves are predicted to increase more in their frequency, duration, and intensity particularly in spring and summer (Woolway et al., 2021). Spring and summer are the seasons when higher amounts of pesticides are generally applied (Phillips & Bode, 2004; Scheyer et al., 2007), whereas the emission of other micropollutants (e.g. pharmaceuticals and metals) is less time‐bound (Danner et al., 2019; Ebele et al., 2017). Surface water contamination by micropollutants has already been reported worldwide (Hughes et al., 2013; Sharma et al., 2019). Additionally, pesticide application (Kattwinkel et al., 2011) and pharmaceutical consumption is predicted to rise (Hughes et al., 2013). Shallow aquatic systems, such as shallow lakes, ponds, ditches, intermittent rivers and streams are expected to be the most impacted from the combined exposure of HWs and chemicals. In larger freshwater ecosystems, the concentration of chemicals may be lower due to dilution, whereas they are expected to experience less intense HWs due to their large thermal inertia (Woolway et al., 2021).
Multiple stressors research has received increasing attention in recent years (Orr et al., 2020), following the need for a more realistic and comprehensive assessment of the multiple drivers of global change across the different levels of biological organization. However, most research on climate change in a multiple stressor context focuses on warming under constant elevated temperature regimes (Arenas‐Sánchez et al., 2019; Piggott, Salis, et al., 2015; Piggott, Townsend, et al., 2015). The significance of increasing temperature on freshwater biota has been recognized long ago (Schindler, 1997), and the effects of elevated and constant temperatures have been largely studied in isolation (Döll et al., 2018) but occasionally also in combination with chemical stressors (Heugens et al., 2001; Holmstrup et al., 2010). Similarly, the relevance of extreme weather events as drivers of detrimental effects on biological systems was recognized more than a decade ago (Jentsch et al., 2007). Since then, scientists have made calls to drive the attention on extreme weather events, rather than on trends (Thompson et al., 2013; Woodward et al., 2016), and extreme temperature fluctuations have been shown to pose a greater risk to alter species performance than elevated mean temperature (Vasseur et al., 2014). Yet, we still lack a comprehensive understanding of the combined effects of extreme weather events and other stressors, such as chemical pollution, as well as of the mechanisms underpinning those effects in environmentally realistic species assemblages.
Understanding the combined effects of HWs and chemicals is pressing not only because they can interact resulting in effects larger then (synergism) or smaller than (antagonism) additive effects but also because their interactions are known to be dependent on the application order (Dinh et al., 2016). The toxicity of many micropollutants may increase for organisms previously exposed to warming, following the “climate change induced toxicant sensitivity” (CITS) concept (Hooper et al., 2013; Moe et al., 2013). Conversely, micropollutants can reduce the heat tolerance of organisms, according to the “toxicant induced climate change sensitivity” (TICS) concept (Hooper et al., 2013; Moe et al., 2013). Consequently, disentangling the processes causing these effects requires systematic testing of different exposure orders at different levels of organization.
The aim of this work was to critically evaluate the state of knowledge on the combined effects of HWs and chemicals with different physicochemical properties and toxicological modes of action, highlighting knowledge gaps. Here, we first develop predictions on how HWs and micropollutants may affect and interact on multiple endpoints spanning different organism groups and levels of organization (Figure 1). Then, we review laboratory, semi‐field and field studies assessing the effects of HWs alone and in combination with micropollutants on different trophic levels of freshwater ecosystems and used a food web approach to identify possible indirect effects that may be propagated across the different trophic levels. Finally, we provide recommendations for a better integration of HWs into multiple stressor's research and chemical risk assessment.
FIGURE 1.
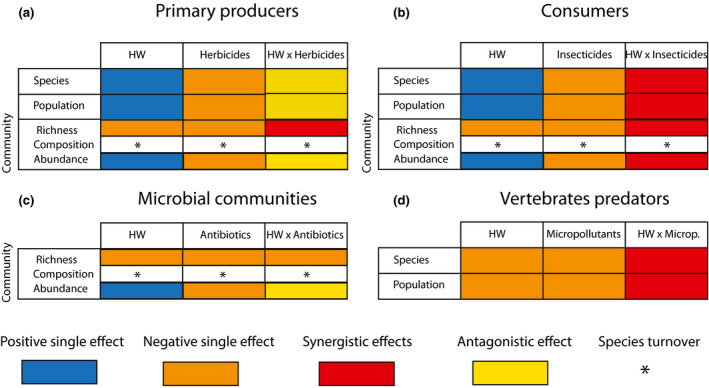
Predicted single and combined effects of heatwaves (HWs) and micropollutants on the different trophic levels (a, primary producers; b, consumers; c, microbial communities and d vertebrate predators) based on available literature (61 articles). Within each trophic level, the rows represent different types of endpoints. Columns show the effect of HWs and the chemical stressor that is projected to have the largest negative effects on the organism groups under consideration. Note that because micropollutants are expected to have mainly a negative direct effect on the different endpoints, we classified the possible synergism only as negative. The individual positive effects of HWs are related to the individual temperature‐stimulated increase in metabolism, which may result in increased population growth
2. MATERIALS AND METHODS
We performed a scoping review (Munn et al., 2018) and discussed the available information regarding the impact of HWs alone and in combination with micropollutants on different trophic levels. This allowed us to focus on the combined effects of HWs and the class of micropollutants that is expected to have the largest direct impact on the trophic level under investigation. This included, antibiotics for bacteria, herbicides for primary producers (phytoplankton, cyanobacteria and macrophytes), and insecticides for arthropods (micro‐ and macro‐crustacea and insects) and all of the above for vertebrate predators. We defined HW following The World Meteorological Association as “five or more consecutive days of prolonged heat in which the daily maximum temperature is higher than the average maximum temperature by 5°C (9°F) or more”. We also include a maximum based on Woolway et al. (2021), who projected maximum HWs of 27 days in the future (IPCC scenario RCP 2.6). These criteria were used to select studies, and we report HW’s characteristics in parentheses in the main text (i.e. duration, C: temperature of controls [reported only for laboratory studies], H: treatment temperature). Many of the studies we included aimed to investigate the effects of climate change but, because they used sudden increases of temperature larger than 5°C, they fit our criteria of a HW (rather than the more gradual temperature increases associated with Climate Change; IPCC, 2013). However, we excluded all experiments that included long (>1 week) acclimatization periods at high temperatures before chemical exposure and not testing the CITS or TICS phenomena, as they may have allowed adaptation and species recombination prior to the temperature increase. The complete report of the databases used for the literature search, the key words used for each trophic level as well as the complete list of the papers reviewed, are reported in Supporting Information (SI1, Table S1 and SI2, Table S1).
3. RESULTS
Our literature search resulted in 61 studies fitting our HW definition. Of these studies, 13 assessed the combined effects of HWs and chemicals. Only 21 studies explicitly tested the effects of HWs, whereas the others were conceived as conventional temperature raise studies, but their features suited our HW definition.
Studies assessing the combined effects of HW and chemical pollution included only one trophic level and were unequally spread between trophic levels: microbial communities (n = 0), primary producers (n = 3), primary consumers (n = 6) and predators (n = 4; Figure 2). No study assessed the combined effects of HWs and micropollutants on multitrophic systems.
FIGURE 2.
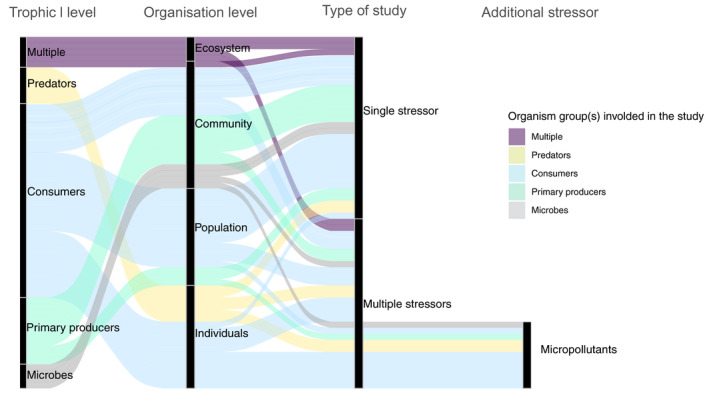
Alluvial plot showing the studies available dealing with heatwaves (HWs) only and HWs in combination with other stressors and the level of biological organization and trophic levels evaluated. The band width is proportional to the number of studies evaluated (total number of studies included = 61)
In the following subsections, we report the results of the literature review divided by trophic levels. Each subsection deals with one single trophic level. We first discuss the direct effects of HWs on each level and continue with the combined effects of HWs and micropollutants.
3.1. Microbes
3.1.1. Direct effects of HWs on microbial communities
Microbial communities comprise bacteria, fungi, protozoa, viruses and nematodes (Margulis et al., 1986). Aligning with the species‐sorting model (Leibold et al., 2004), microbial communities are shaped by the local environmental conditions (Logue & Lindström, 2010), such as temperature (Ziegler et al., 2019), salinity (Herlemann et al., 2011), resource availability (Pradeep Ram et al., 2020) or environmental degradation processes (Mykrä et al., 2017). Although some soil microbial communities are relatively tolerant (e.g. no significant changes in biomass or respiration) to HWs (de Oliveira et al., 2020; Pérez‐Guzmán et al., 2020), microbial communities in freshwater ecosystems have been found to have a higher sensitivity towards warmer temperature or HWs. This is due to faster conduction of temperature through water than terrestrial media (Singh & Devid, 2000; van Rooyen & Winterkorn, 1957). HWs may have an effect on the metabolism, biomass, composition and the stoichiometry of freshwater bacteria, as shown in experiments (10–30°C) where elevated temperature increased nutrient cycling on suspended bacteria from oligotrophic lakes (Phillips et al., 2017; Figure 3). The changes in stoichiometry are more pronounced when access to nutrients, particularly phosphorus, is lacking, thus resulting in larger effects of HWs in oligotrophic ecosystems compared with more eutrophic ones (Phillips et al., 2017). Further studies demonstrated that elevated temperatures in freshwater systems enhance both the growth rate and the leaf litter decomposition by aquatic fungi (Duarte et al., 2013), which promotes the organic nitrogen concentration in leaf litter (Kaushik & Hynes, 1971), and, in turn, stimulates microbial respiration rates (Stelzer et al., 2003; Figure 4). The biomass of freshwater microbial communities increases with temperature, which leads to an increased rate of leaf litter decomposition (Donnelly et al., 1990; Fernandes et al., 2012; Stelzer et al., 2003). This can enhance nutrient cycling, at least during short‐term periods.
FIGURE 3.
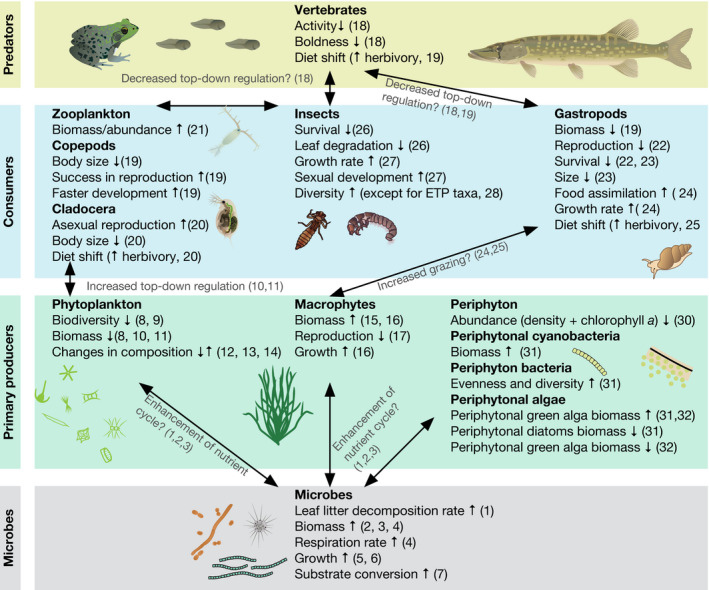
Graphical representation synthesizing the results found in the literature on the effects of heatwaves on aquatic food‐webs. Arrows connecting different organism groups indicate trophic and/or indirect effects. Upward arrows indicate an increase/raise and downward arrows indicate a decrease of the evaluated ecological parameter. Numbers in brackets refer to references: (1) Duarte et al. (2013), (2) Donnelly et al. (1990), (3) Fernandes et al. (2012), (4) Stelzer et al. (2003), (5) Zeng et al. (2014), (6) Zamarreňo et al. (2009), (7) Höfle (1979), (8) Remy et al. (2017), (9) Egger et al. (2012), (10) Velthuis et al. (2017), (11) O’Connor et al. (2009), (12) Maazouzi et al. (2008), (13) Bergkemper and Weisse (2017), (14) Weisse et al. (2016), (15) Bertani et al. (2016), (16) Hansson et al. (2020), (17) Li et al. (2017), (18) Mameri et al. (2020), (19) Carreira et al. (2016), (20) Nguyen et al. (2020), (21) Johnsen et al. (2020), (22) Cremona et al. (2020), (23) DeWhatley and Alexander (2018), (24) Leicht and Seppälä (2019), (25) Carreira et al. (2020), (26) Zhang et al. (2020), (27) Vander Vorste et al. (2017), (28) Prato et al. (2008), (29) Fornaroli et al. (2020), (30) Hao et al. (2020), (31) Piggott et al. (2015), (32) Bondar‐Kunze et al. (2021)
FIGURE 4.
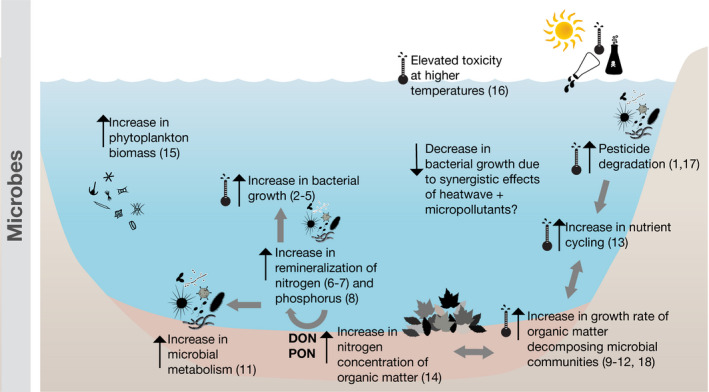
Conceptual overview of the potential combined effects of heatwaves and chemicals on microbial communities. Black upward or downward arrows indicate an increase or decrease of the respective processes. Grey arrows indicate direct and indirect effects on other processes. The thermometer symbol indicates an effect related to temperature only, whereas the symbols of the thermometer and of the chemical together indicate a combined effect of both. (1) Bighiu & Goedkoop (2021), (2) Zeng et al. (2014), (3) Zamarreňo et al. (2009), (4) Höfle (1979), (5) White et al. (1991), (6) Berthelot et al. (2019), (7) Hayes et al. (2019), (8) Klausmeier et al. (2004), (9) Duarte et al. (2013), (10) Donnelly et al. (1990), (11) Stelzer et al. (2003), (12) Fernandes et al. (2012), (13) Phillips et al. (2017), (14) Kaushik and Hynes (1971), (15) Pomeroy and Wiebe (1988), (16) Delnat et al. (2021), (17) Wickham et al. (2020), and (18) Arias Font et al. (2021)
Some microbial communities prosper with temperature increases, for example, functional groups such as ammonia‐oxidizing (Zeng et al., 2014) or biocalcifying (Zamarreňo et al., 2009) bacteria. Consequently, bacteria growing faster under HW conditions may outcompete slower‐growing bacteria, leading to changes in community structure (Fetzer et al., 2015). The assembly of microbial community changes due to changing environmental conditions (Langenheder & Lindström, 2019). This can alter biogeological fluxes and the availability of key elements for the ecosystem (Zhao et al., 2017; Figure 4). For instance, increased bacterial growth promoted by higher temperatures may lead to a phosphorus depletion for phytoplankton (Currie, 1990) and to food scarcity for herbivores. Conversely, a substantial increase in the bacterial abundance can, depending on the microbial community, facilitate the remineralization of nitrogen (Berthelot et al., 2019; Hayes et al., 2019) and phosphorus (Klausmeier et al., 2004), which precedes an increase in phytoplankton biomass that would, otherwise, function as a self‐limited nutrient sink (Pomeroy & Wiebe, 1988).
The ability of microbial communities to resist and recover from extreme climate events is still understudied but needed to help the characterization of ecosystem response to important ecological functions after extreme events (Bardgett & Caruso, 2020).
3.1.2. Combined effects of HWs and chemicals on microbial communities
Microbes living in freshwater habitats are exposed to micropollutants that form complex mixtures (Escher et al., 2020). Yet, it remains unknown how microbial communities might take up, degrade or metabolize micropollutants when simultaneously exposed to HWs and chronic chemical stress, as no studies were found investigating this (Figure 2).
Although lake microbial communities have been shown to be highly resilient to long‐term warming (i.e. composition was recovered within 1 week after the warming was interrupted; Shade et al., 2012), we do not know whether a simultaneous exposure to micropollutants may impair the recovery capacity. Similarly, it is unknown whether the shift in community composition resulting from HW (albeit short‐term) may increase the sensitivity towards chemicals.
Freshwater microbial organisms are capable of degrading pesticides and other synthetic chemicals (Mishra et al., 2020). Microbial degradation of micropollutants, thus, directly impact the exposure of other organism groups, which may benefit from an increased microbial degradation of toxic substances. Microbial communities repeatedly exposed to chemicals may enhance their biodegradative activity and reduce micropollutant persistence (Barra Caracciolo et al., 2015). However, this is only true when microbial populations able to degrade specific micropollutants are present in the environment (Barra Caracciolo et al., 2015; Coll et al., 2020). If such populations are removed by a disturbance (e.g. HWs), the biotransformation and degradation of chemicals may be impaired.
3.2. Primary producers
3.2.1. Direct effects of HWs on primary producers
Although metabolic theory (López‐Urrutia et al., 2006; O’Connor et al., 2011) and empirical data (O’Connor et al., 2009) indicate that respiration‐limited (heterotrophic) metabolism is more sensitive to changing temperature than photosynthesis‐limited (autotrophic) metabolism and production, negative effects of HWs have also been reported for primary producers (Eggers et al., 2012; Weisse et al., 2016). HWs have been shown to promote a shift in community composition, both in experimental systems and field studies (Bergkemper & Weisse, 2017; Blenckner et al., 2007; Maazouzi et al., 2008; Weisse et al., 2016). Generally, those compositional changes are linked to a reduction in phytoplankton diversity in microcosm experiments (Eggers et al., 2012; Remy et al., 2017; Weisse et al., 2016) as well as in field studies (Bergkemper & Weisse, 2017; Maazouzi et al., 2008; Figure 3). The reduction in species number is commonly driven by a decline of thermal sensitive species (Urrutia‐Cordero et al., 2017), which in turn allows more tolerant species to thrive and to increase their abundance (Eggers et al., 2012). Although a decline in richness is often reported as a consequence of a HW, some studies do not highlight a net decrease in species number, but rather a species turnover driven by the different species’ thermal sensitivities (Hansson et al., 2020; Li et al., 2017). Several studies show that compositional changes caused by HWs often promote cyanobacteria dominance, even in nutrient‐ and light‐deficient aquatic environments (Calderó‐Pascual et al., 2020). HWs can also promote cyanobacteria blooms by inducing a seasonal advancement and increasing their recruitment rates from sediments (Bergkemper & Weisse, 2017; Richardson et al., 2019; Urrutia‐Cordero et al., 2020). A common consequence of these blooms is the alteration of ecosystem functions, such as biomass production and chlorophyll‐a concentration, as well as toxicity through the release of cyanotoxins (Eggers et al., 2012; Remy et al., 2017).
Macrophytes have been shown to be sensitive to HWs, too. As for phytoplankton, their response to heat stress is species‐specific. Li et al. (2017) showed that Carya tomentosa exposed to extreme temperature fluctuations (+8°C, different durations) increased the mortality rate up to 60%. Oppositely, HWs tolerant species, such as Myriophyllum spicatum, exhibit opposite responses, by increasing their total abundance and biomass under HW (+8°C, different durations, Hansson et al., 2020). Nevertheless, when exposed to HWs, M. spicatum showed impaired sexual reproduction expressed as severe reduction in the number of flowers produced during the vegetative season, suggesting that the balance between vegetative and generative reproduction under HWs is shifted towards more asexual reproduction (Li et al., 2017).
The majority of the experimental set‐ups investigating the effects of HWs on primary producers have not included other trophic levels. The only experiment testing HW (+8°C, 7 days) effects on a freshwater food web (phytoplankton and zooplankton) reported reduced primary producer biomass as a result of an increased grazing activity from zooplankton (Velthuis et al., 2017). The same effects have been found by a similar study investigating a marine bi‐trophic community (+6°C, 8 days, O’Connor et al., 2009). Other studies have shown that HWs can influence the competition between algae and macrophytes. For instance, Bertani et al. (2016) found that after a natural HW, a shallow lake shifted from a phytoplankton‐dominating stage to a macrophyte‐dominating one. Such bottom‐up interactions triggered cascading effects at higher trophic levels, resulting in a decline in planktonic herbivores (Bertani et al., 2016).
Temperature can directly affect plant palatability. Zhang et al. (2019) showed that some aquatic macrophytes were less palatable to consumers (e.g. the aquatic gastropod Lymnaea stagnalis) when grown under constant elevated temperature. A reduction in plant palatability directly leads to decreased biomass, abundance and lower fitness of consumers (Zhang et al., 2019). Potentially, this could lead to a general decreased top‐down control also under HW stress, which may lead to unforeseen effects on ecosystem functioning.
3.2.2. Combined effects of HWs and micropollutants on primary producers
All the studies (n = 3) available on the combined effects of herbicides and HW on primary producers are single‐species laboratory tests (Delorenzo et al., 2013; Tasmin et al., 2014; Wilkinson et al., 2017). These show consistent trends for some endpoints and opposite trends for others. When the freshwater algae Pseudokirchneriella subcapitata was simultaneously exposed to high temperature and the herbicide diuron for six consecutive days, the acute toxicity of the herbicide decreased (6 days, C: 20°C, H: 30°C; Tasmin et al., 2014). The species’ growth was less reduced by diuron at high temperature compared to colder temperatures, and the photosynthetic activity was less impaired by the herbicide when undergoing the HW treatment (Figure 5; Tasmin et al., 2014). Delorenzo et al. (2013) studied the effects of increasing temperature and salinity on the toxicity of herbicides (irgarol, diuron, atrazine and ametryn, tested individually) to the phytoplankton species Dunaliella tertiolecta. The authors found that a HW exposure (5 days, C: 25°C, H: 35°C) generally decreased the negative effects of the herbicides on the chlorophyll‐a concentration, lipid content and starch content (Figure 5). However, opposite to Tasmin et al. (2014), under simultaneous exposure to HW and herbicides, growth rate and cell density were generally decreased compared with the treatment containing only herbicides (Figure 5; Delorenzo et al., 2013).
FIGURE 5.
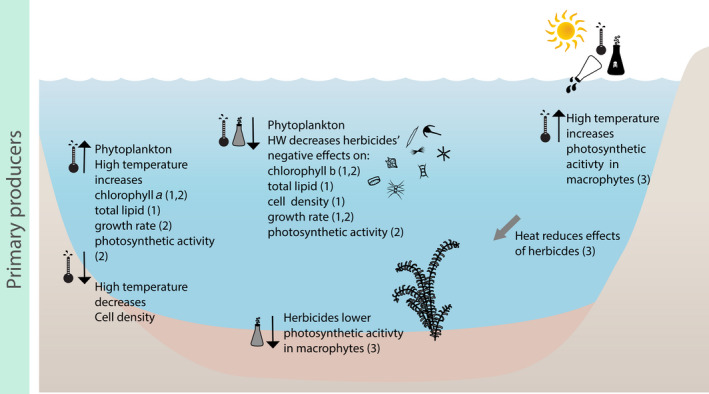
Conceptual overview of the single and combined effects of heatwaves and herbicide(s) on primary producers. Black upward or downward arrows indicate an increase or decrease of the respective processes. Grey arrows indicate direct and indirect effects on other processes. The thermometer symbol indicates an effect related to temperature only, whereas the symbols of the thermometer and of the chemical together indicate a combined effect. (1) Delorenzo et al. (2013), (2) Tasmin et al. (2014), and (3) Wilkinson et al. (2017)
The lower sensitivity of primary producers to herbicides, when simultaneously exposed to rapid temperature increase, is a frequently observed response across ecosystems (Gomes & Juneau, 2017). Wilkinson et al. (2017) observed that the decrease in photosynthetic activity in the seagrass Halophila ovalis exposed to diuron was reduced when exposed to extreme temperatures (3 days, C: 25°C, HW: 35°C).
The available studies highlight that herbicides and HWs have mostly an antagonistic (i.e. less than additive) effect on the photosynthetic efficiency and algal growth of primary producers (Delorenzo et al., 2013; Gomes & Juneau, 2017; Tasmin et al., 2014). The same trend has also been found by a recent review summarizing the combined effects of elevated temperature and herbicides on primary producers (Gomes & Juneau, 2017). Nevertheless, the sensitivity range of photosynthetic aquatic organisms to extreme temperature fluctuations and herbicides is wide, and further investigations are needed on the combined effects of HWs and herbicides on realistic species assemblages and on higher trophic levels. Potentially, the reduction in primary producers’ biomass caused by herbicides coupled with the shift to cyanobacteria dominance driven by HWs could reduce the availability of food source for consumers. Cyanobacteria are relatively tolerant to some classes of herbicides, particularly to the globally most applied phosphonate herbicide glyphosate (Annett et al., 2014; Forlani et al., 2008). The combined effects of HWs and herbicides could promote cyanobacteria blooms and reduce the portion of palatable phytoplankton and macrophytes for consumers. Such an effect could reduce consumers’ ability to carry out detoxification processes from cyanotoxins, leading to an overall biomass decline across the food web (Figure 3).
3.3. Primary consumers
3.3.1. Direct effects of HWs
Temperature plays a key role in ectotherms’ physiology, as their body temperature changes with the temperature of the environment. Thus, for ectotherms, changes in temperature result in alterations of the rate of all physiological and metabolic reactions (Brown et al., 2004). As a consequence, temperature drives the selection of specific individual traits as well as behavioural alterations. A recent mesocosm study on zooplankton community dynamics showed that under warming (sampling over 3 years, C: 11.7°C, HW: 19.7°C) smaller body size and asexual (parthenogenetic) reproduction dominated (Johnsen et al., 2020). Responses of tropical zooplankton to a HW (12 days, C: 26°C, HW: 34°C) revealed a faster development but a reduced body size, clutch size, hatching success and nauplii production for copepods (Nguyen et al., 2020). Similar warming‐driven selections were predicted under future climate models for shallow, eutrophic lakes in which copepod's biomass and abundance are projected to increase (Cremona et al., 2020). Under the same projections; however, cladoceras are expected to reduce their biomass, but not their abundance (Cremona et al., 2020).
The features of a HW, including duration and the speed of temperature increase over time, determines the magnitude of the adverse impact as investigated on the filtration capacity of Daphnia magna (Müller et al., 2018). Individuals showed an immediate negative response with rapid temperature changes (up to 20 days, T‐range: 11–29°C). More severe responses were noticed at higher temperatures and faster temperature changes, although quasi‐acclimatization with higher filtration capacities was reported (ca. 7 days, T‐range: 11–25°C; Müller et al., 2018).
Freshwater gastropods exposed to a HW showed thermal sensitivity through significantly higher mortality rates at high temperatures (10 days, C: 20°C, HW: 35 and 30°C; DeWhatley & Alexander, 2018). Similar observations were reported after a HW in 2003, when the snail populations of two European rivers strongly declined (Mouthon & Daufresne, 2008). HW effects were noticed also in the following years (2004, 2005), with the snail populations showing smaller cohorts (Mouthon & Daufresne, 2008).
Heatwaves effects can differ depending on the life‐history trait under investigation. For instance, hatching success of laid eggs not only increased with high temperature (7 days, C: 15°C, HW: 25°C), but also caused reduction in the size of eggs, egg survival rate and the number of hatched juveniles. However, the surviving offspring showed an increased developmental rate and growth (Leicht & Seppälä, 2019). The duration and speed of temperature increase of these extreme events appear to be crucial for the response direction of the organism's trait. Supporting this, two experimental HWs (1 and 7 weeks, C: 20°C, HW: 25°C) with identical magnitude but different duration have been found to increase snails’ food assimilation and growth rate, with only minor negative effects on reproduction (Carreira et al., 2020). Yet, responses on these endpoints were dependent on the HW duration, as the short HW caused a relatively stronger response (Carreira et al., 2020). Similar time‐dependent effects were reported for prolonged HWs (7, 9 or 11 days, C: 15°C, HW: 25°C) compared with short ones (1, 3, 5 days) with increased growth and reproduction only in the first week of observation and reduced levels of immune function only under prolonged HWs (Leicht et al., 2013).
To maintain higher metabolic rates at elevated temperatures, omnivorous ectotherms seem to change their diet by increasing herbivory instead of carnivory, which is also observed in other aquatic taxa, for example, zooplankton and fish. These temperature‐induced diet shifts may cascade through the aquatic food web, increasing top‐down pressures on primary producers (Zhang et al., 2020; Figure 3).
HW effects on benthic organisms and insects are poorly documented in freshwater research, and only a few publications studied their sensitivity towards such extreme events. Under laboratory conditions (15 days, C: 15°C, HW: 25°C), the amphipod Gammarus pulex showed behavioural alterations, consisting in vertical immigration to potential refuge areas (i.e. hyporheic zone; Vander Vorste et al., 2017). These tries of avoiding heat stress, came along with decreases in survival, leaf consumption and energy recourses. HWs may also favour the dominance of more resistant invasive species. In an experimental study, the allochthonous gammarid Dikerogammarus villosus was found to be less sensitive to HWs compared to the native G. pulex (Truhlar et al., 2014). This lower thermal sensitivity of the invasive species led to a rapid shift in macroinvertebrates’ community structure, which was linked to changes in nutrients dynamics (Maazouzi et al., 2011; Truhlar et al., 2014). Such changes in community structure after HWs have also been reported consistently in field studies (Daufresne et al., 2007), including the loss of the more sensitive Ephemeroptera, Plecoptera and Trichoptera taxa (Fornaroli et al., 2020).
Such individual‐ and community‐level changes seem to be linked to the temperature effects on the overall energy budget of the organisms, including energetic investments in homeostatic maintenance, growth, development and reproduction (Verberk et al., 2020). In accordance with these general findings, low energy reserves of the caddisfly larvae Stenopsyche marmorata were associated with high temperature (9 days, C: 10°C, HW: 25°C; Suzuki et al., 2018). Dinh et al. (2016) described that heat stress effects (6 days, C: 22°C, HW: 30°C) caused a reduction in the immune function (activity of phenoloxidase) and metabolic rate (activity of the electron transport system). Additionally, the authors pointed out the importance of delayed HW effects in shaping the overall stressor impact on the organism when other stressors are subsequently applied (TICS concept). Although Odonata are intermediary consumers/predators in aquatic food webs, we report here the effects of HWs on this organism group, as the following subsection only deals with vertebrate predators. Experimental warming studies on dragonfly larvae (Odonata) present strong thermal effects at environmentally relevant temperatures (ca. 35 days, C: ambient, HW: const. +5°C to ambient) with lower survival rates and premature emergence (Mccauley et al., 2015). Although no effects of increased temperature on the overall body size of adults were found, there was a significant interaction effect of temperature and sex with the trend for larger size with higher temperature being stronger in females than males.
3.3.2. Combined effects of HWs and chemicals on primary consumers
Interaction effect studies on HWs and chemical stressors towards aquatic primary and intermediate consumers are generally scarce (n = 6), and all of them were published within the last 10 years (Figure 6). In a microcosm experiment, negative synergistic effects were observed initially (4 and 14 days, C: 20°C, HW: 28°C) in several zooplankton taxa (Daphnia sp., Cyclopoida, and Copepoda nauplii) exposed to increased temperature and the insecticide lufenuron (Arenas‐Sánchez et al., 2019). Observed positive effects of increased temperature alone (16 days, C: 20°C, HW: 30°C) may be nullified through interactions with chemicals. This was noted for a cladoceran species (Moina micrura), which exposed to a HW only (16 days, C: 20°C, HW: 30°C) showed increased population size, but when exposed to a HW and the fungicide carbendazim simultaneously showed reduction in population size and net decrease of reproductive rate (Miracle et al., 2011). Another multiple stressor study with the freshwater gastropod Bellamya bengalensis reported detrimental effects on molecular endpoints caused by HWs in combination with the pesticide chlorpyrifos (60 days, C: 25°C, HW: 30°C, 35°C; Baag et al., 2021). In a multiple stressor experiment, Macaulay et al. (2021) exposed mayfly nymphs (Deleatidium spp.) to two subsequent HWs (both 6 days, C: 12°C, HW: 20°C). The first HW was combined with a non‐chemical stressor (e.g. starvation) and the second with the insecticide imidacloprid. The mayfly nymphs revealed a delayed negative synergistic interaction between a HW and imidacloprid (0.4 μg/L; Macaulay et al., 2021). In the same study, the second HW resulted in time‐dependent stressor interactions and time‐cumulative toxicity of imidacloprid affecting mayfly mobility. Lethal effects of imidacloprid were only observed when applied as single stressor, suggesting that previous exposure to the first HW might have increased the tolerance of the organisms to the combined effects of a HW and insecticide (Macaulay et al., 2021). Furthermore, there are reported synergistic HW–imidacloprid interactions in Deleatidium spp. and Coloburiscus humeralis on their moulting, immobility and mortality, suggesting temperature‐enhanced toxicity of the neonicotinoid insecticide (Macaulay et al., 2020). In another study with imidacloprid, Camp and Buchwalter (2016) showed that insecticide uptake in Isonychia bicolor as well as oxygen consumption rates increased significantly with increasing temperature (5 days, T‐range: 15, 18, 21, and 24°C). Additional uptake tests with several aquatic invertebrates (I. bicolor, Neocloeon triangulifer, Macaffertium modestum, Pteronarcys proteus, Acroneuria carolinensis, and Pleuroceridae sp.) indicated that all the species tested had significantly increased imidacloprid uptake with increasing temperatures (Camp & Buchwalter, 2016).
FIGURE 6.
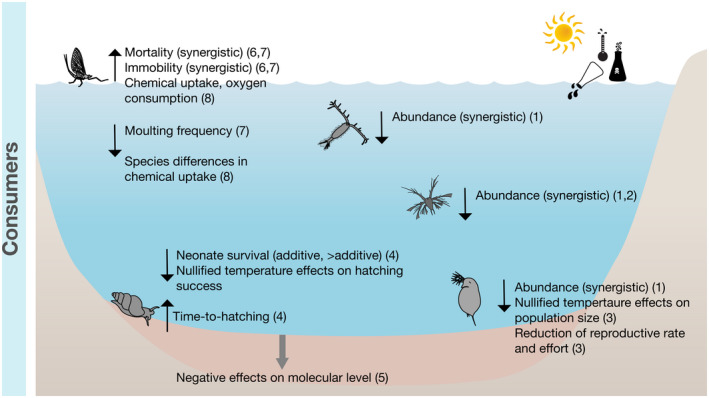
Conceptual overview of the combined effects of heatwaves and chemicals on primary consumers. Black upward or downward arrows indicate an increase or decrease of the respective ecological parameter. Grey arrows indicate direct and indirect effects on other processes. (1) Arenas‐Sánchez et al. (2019), (2) Van de Perre et al. (2018), (3) Miracle et al. (2011), (4) Kimberly and Salice (2013), (5) Baag et al. (2021), (6) Macaulay et al. (2021), (7) Macaulay et al. (2020), and (8) Camp and Buchwalter (2016)
3.4. Vertebrate predators
3.4.1. Direct effects of HWs on vertebrate predators
The focus in this section lays on larger vertebrate predators such as fish and amphibians, excluding macroinvertebrates and zooplankton. Increased water temperatures can affect the reproduction of fish by altering their spawning activity and impacting embryo development (Ashton et al., 2019; Warriner et al., 2020). Fish are known to respond to seasonal water temperatures by changes in movement behaviour, that is, the timing of migration or changes in microhabitat, seeking temperature refugia (Coutant, 2001). Consequently, HWs are likely to influence similar behavioural responses. In a mesocosm experiment, the swimming behaviour of the Iberian barbel (Luciobarbus bocagei), a Mediterranean freshwater fish, was impacted under HW conditions (6 days, C: 24.5°C, H: 29.7°C) resulting in decreased activity and boldness (Mameri et al., 2020). Next to direct consequences on individual performance, these temperature‐dependent behavioural changes also influence predator–prey interactions (Öhlund et al., 2014). Altered predator–prey interactions may have indirect effects on other trophic levels resulting from a decreased predation pressure on preys, that is, benthic invertebrates and other fish (Figure 3).
A similar indirect effect across trophic levels has been observed for tadpoles under HW conditions. Diet shifts of tadpoles occurred in HW treatments (various HW scenarios tested, see Carreira et al., 2016; Figure 3) with a general trend to increased herbivory in response to a higher temperature (Carreira et al., 2016). Consequently, the direct effect of the HW on the predator (i.e. its diet shift) has limited consequences on other predators but rather affects other trophic levels indirectly (i.e. increased consumption of macrophytes and decreased predation on insect larvae). Conversely, HWs can also have indirect effects on predators via a direct effect on their prey or lower trophic levels through bottom‐up mechanisms (Figure 3). Additionally, indirect effects within the same trophic level, for example, intensified interaction of predators through competition of food resources or microhabitats, may occur at least temporally.
3.4.2. Combined effects of HWs and micropollutants on vertebrate predators
The studies investigating the combined effects of HWs and micropollutants on predators (n = 7) include exposure experiments with metals, herbicides and antidepressants (Figure 7). The response categories of these studies comprise (i) effects only present in the combined exposure to HWs and micropollutants, (ii) effects dominated by the HW or (iii) effects in all treatments. It is worth noticing that this section reports mainly results of molecular endpoints. Extrapolation of effects in terms of fitness and reproduction are not easy because those are not typically measured.
FIGURE 7.
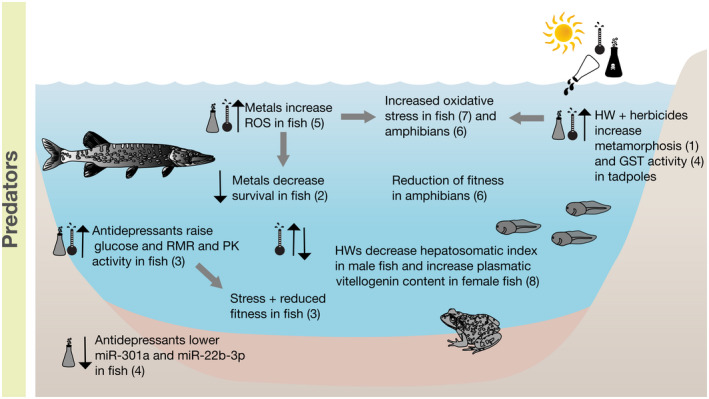
Conceptual overview of the single and combined effects of heatwaves and chemicals on predators. Black upward or downward arrows indicate an increase or decrease of the respective ecological parameter. Grey arrows indicate direct and indirect effects on other processes. The thermometer symbol indicates an effect related to temperature only, whereas the symbols of the thermometer and of the chemical together indicate a combined effect. (1) Freitas et al. (2016), (2) Park et al. (2020), (3) Mehdi et al. (2019), (4) Freitas et al. (2017), (5) Prophete et al. (2006), (6) Gripp et al. (2017), (7) Birnie‐Gauvin et al. (2017), and (8) Hani et al. (2019)
For the following examples, the sensitivity of the organisms was only evident when exposed to the combination of both stressors, whereas each single‐stressor application did not reveal significant effects. Tadpole metamorphosis was accelerated under HW conditions (7 days, C: 28°C, H: 34°C, diuron and its metabolite 3,4‐DCA) only when combined with an herbicide (Freitas et al., 2016). In another study exposing tadpoles to an herbicide under HW conditions (8 days, C: 28°C, H: 36°C, clomazone), the activity of the biotransformation enzyme glutathione‐S‐transferase (GST), increased with increasing dose. A GST rise indicates an increased oxidative stress, which may lead to a reduced fitness of amphibians (Gripp et al., 2017). Such an increase was not observed at the same herbicide concentrations at lower temperature (Freitas et al., 2017). The HW treatment alone indicated no significant difference in the activity of this phase II detoxification enzyme.
Similarly, the survival rate of zebrafish embryos after a HW treatment (7 days, C: 26°C, H: 34°C, cadmium) was not affected in the HW only treatment but showed a dose‐dependent reduction under joint cadmium exposure (Park et al., 2020). Furthermore, lower heart rates, increased cell death, upregulated genes for antioxidants and genes involved in apoptotic responses as well as DNA methylation were observed for the combined exposure. Another study with zebrafish revealed that glucose levels and routine metabolic rates, indicating stress and potentially reduced fitness, were increased in the combined exposure of a HW and an antidepressant (21 days, C: 27°C, H: 32°C, venlafaxine), again with no significant effects for the respective single stressor treatments (Mehdi et al., 2019).
The two following studies showed single stressor effects of the HW treatment and the combined exposure, but no effect on the micropollutant single stressor application. The pyruvate kinase activity in Danio rerio increased (21 days, C: 27°C, H: 32°C, venlafaxine), indicating an increase in metabolic costs for the organism (Mehdi et al., 2019). For the Japanese medaka, the production of intracellular reactive oxygen species (ROS) increased under HW condition (7 and 14 days, C: 25°C, H: 30°C, nickel chloride), and increased even more when combined with the exposure to a metal, which itself did not induce this effect (Prophete et al., 2006). An increase of ROS is associated to oxidative stress in fish, which can influence responses to environmental change and life history strategies (Birnie‐Gauvin et al., 2017).
The remaining two studies investigating the effect of HWs and micropollutants in predators did not show a combined effect mechanism of the tested stressors. No significant effect of combined exposures (15 days, C: 16°C, H: 21°C, cadmium) were detected and only the HW treatment decreased the hepatosomatic index in males and increased plasmatic vitellogenin content in female zebrafish (Hani et al., 2019). This result highlights the importance of considering different endpoints in multiple stressor research, as cadmium did show an interactive effect with HWs in zebrafish in the study mentioned earlier, investigating a different endpoint (Park et al., 2020). Conversely, the exposure to venlafaxine and a HW caused micro‐RNA to decrease in their single exposures in the same amount as for their combined exposure (21 days, C: 27°C, H: 32°C, venlafaxine), indicating no significant interaction effect in zebrafish (Ikert & Craig, 2020).
Although limited in their representation for the variety of top predators in freshwater ecosystems, these seven studies reveal a variety of combined effects of HWs and micropollutants on predators. Additionally, these studies highlight the importance of multiple stressor research, as only the combination of HWs and micropollutant revealed the adverse effects to predators. Chemical risk assessments based on exposure studies with standard temperature conditions are, thus, limited to detect the described effect occurring in combination with HWs.
Although the results of almost all mentioned studies indicate stronger effects of micropollutants on predators when simultaneously exposed to HWs, they also indicate that there are different response categories with different stressor interactions. To unravel the underlying mechanisms, more research is needed investigating how HWs and micropollutants interact in affecting predators’ responses.
4. CONCLUDING REMARKS AND RECOMMENDATIONS
This literature review has revealed that only 5 out of the 61 included studies investigated the effects of HWs using a multitrophic approach. Those studies only focus on the effects of HWs in isolation. HWs have been assessed mainly through single species and/or population experiments containing one trophic level. Although these approaches are essential to reveal physiological responses, they are limited in ecological realism, excluding the investigation of the effects of trophic and non‐trophic species interactions (Kéfi et al., 2015; Seibold et al., 2018). The lack of testing in complex species assemblages appears particularly problematic since ecological realism, and more community/ecosystem‐oriented studies have recently been recognized as essential for the advancement of multiple stressors research (Orr et al., 2020).
Generally, HWs are reported to cause compositional changes in all trophic levels analysed individually, although the mechanisms driving those changes may be different. Changes in community composition resulting from HW exposure could result in altered sensitivity towards chemical stressors. Yet, the scarcity of available studies does not allow to draw general conclusions on how HW‐driven compositional changes may affect community sensitivity towards different chemical classes. Furthermore, although a framework to mechanistically decompose the effects of multiple stressors on the different trophic levels has been recently propose (Bracewell et al., 2019; Van den Brink et al., 2019), at present, a mechanistic understanding of the processes underpinning the effects of HWs across organization levels in a multiple stressor context is missing. Mainly, this is linked to the insufficient availability of theoretical and empirical work trying to unravel the processes behind the observed effects. Nevertheless, this review has provided some insights on the mechanisms driving the combined effects of HWs and micropollutants. In the next sub‐sections we provide recommendations for further research.
4.1. Focus shift towards higher levels of biological organization
We suggest that the assessment of the effects of extreme events combined with other stressors should involve investigations at the community and ecosystem level, which are primary focus of water managers, risk assessors and policymakers interested in multiple stressor effects (Orr et al., 2020). Investigations at high levels of organization have the potential to unravel the combined effects of extreme events and other stressors on functional processes, species interactions and how impacts can cascade through the food web. This is particularly important since temperature effects mediated by species interactions may be larger than direct effects caused by extreme events (Higashi & Patten, 1989; Montoya et al., 2009). Ecosystem response projections from single‐species tests are challenging, as species performances differ depending on whether they are in monoculture or interacting in a community (Tabi et al., 2020). Moreover, it has been shown that food‐web length and the number of species at each trophic level can modify the overall effects of perturbations, including chemicals, on species assemblages (Zhao et al., 2019), highlighting the need to test multiple stressors on multitrophic and species rich communities (Seibold et al., 2018). An improved understanding of how high levels of organization processes respond under combined disturbances, based on empirical data, is needed, and represents the major knowledge‐gap identified in this review. In this context, micro‐ and mesocosm experiments represent one of the best ways to test the combined effects of micropollutants and extreme events at high levels of biological organization. Alongside with the experimental effort, the development of models able to predict combined effects of HWs and pollutants is needed to help ecosystems protection. Because testing all the possible stressors combination for all aquatic ecosystem types is unrealistic, we need modelling tools enabling us to project combined stressors’ effects on different organization levels.
4.2. Gradient testing
Experimental designs aiming to study effects of extreme events need to consider nonlinear responses. Non‐linear responses seem to be the norm in biological systems exposed to climatic variability, as a consequence of Jensen's inequality (Kreyling et al., 2018; Ruel & Ayres, 1999). Especially when effects of temperature are accounted for in freshwater systems, non‐linear responses have been reported across all levels of biological organization, from individuals (Bernhardt et al., 2018), to communities (Baranov et al., 2020) and ecosystem processes (Lv et al., 2020). Testing non‐linear responses requires the exploration of a wide gradient of the environmental driver(s) under investigation. Most experimental designs use only two levels of the tested drivers (i.e. applied/not applied; Kreyling et al., 2018), which can limit our ability to derive hypotheses on the mechanisms behind the observed effects. Adopting gradient designs, therefore, may allow researchers to perform multiple stressor experiments investigating more combinations of stressors and a larger gradient of those stressors. Furthermore, gradient approaches would consent to different realistic disturbance intensities (i.e. multiple temperature regimes or chemical concentrations) and HW durations to be tested. Experimental exploration along a large gradient of drivers’ intensities and durations may also help in determining stress levels that have long‐lasting legacies on communities’ biomass and composition (Jacquet & Altermatt, 2020). Moreover, more complex experimental designs allow studying different patterns of occurrence (i.e. reoccurring HWs, different timing of stressor application) and under different environmental scenarios. Finally, it has been pointed out that gradient designs allow more null models to be tested, which could improve our understanding of stressors’ interactions (Schäfer & Piggott, 2018).
4.3. Temporal dependency of stressor interactions and temporal dynamics of multiple stressors
The effects of multiple stressors (and their interactions) are temporal‐scale dependent (Garnier et al., 2017). That is, interactions between stressors can appear right after disturbance's application, as well as in the recovery phase. Yet, in multiple stressors studies, performing only a single sampling point after the stressors’ application is the rule (Beermann et al., 2018; Halstead et al., 2014; Piggott, Salis, et al., 2015; Piggott, Townsend, et al., 2015). Accordingly, we did not find any study assessing the combined effects of HWs and chemicals over time, which is required to describe potential adaptation and/or recovery. This is particularly relevant for the combination of HWs and micropollutant, as HWs, by definition, are temporary phenomena. Conversely, some micropollutants can persist in the aquatic environment for months or years (Arp et al., 2017), and many chemicals have a continuous discharge to the aquatic environment, maintaining exposure levels also for less persistent substances. Therefore, following the response of the study system(s) is important to detect interactions between stressors happening at different time points. Particularly, late stressors’ interactions may prevent the recovery of both compositional and functional endpoints. Furthermore, HWs are predicted to become more frequent (Meehl & Tebaldi, 2004) and may appear multiple times in a season (Woolway et al., 2021). The re‐occurrence of HWs in ecosystems stressed by persistent micropollutants (or receiving short‐lived micropollutants multiple times) makes it necessary to follow the system's response over time in order to identify late‐stage interactions and to take adequate protection measures.
Since perfect synchrony between stressors is rarely found in nature, designing experiments involving different order of stressor applications and measuring the organisms and ecosystem's responses over multiple time points is crucial (Jackson et al., 2021). Stressors’ sequence has consequences for the so‐called ecological memory, which is the ability of previous stressors to influence future responses (Hughes et al., 2019; Jackson et al., 2021). Among the literature we reviewed, only a single study explicitly investigated whether different orders of stressors application may result in different effects (Dinh et al., 2016). All the other studies reviewed here only tested simultaneous exposure to combined stressors. Testing different stressors’ sequence may be challenging as it requires large experimental designs. Yet, it appears to be a central research aspect, as the abovementioned CITS and TICS phenomena might produce unexpected effects across all levels of biological organization.
Some studies have explicitly assessed the effects of CITS and TICS, but this has been done prevalently at the individual and population level (Delnat et al., 2019; Verheyen & Stoks, 2019, 2020; Verheyen et al., 2019). Thus, consequences of these phenomena for higher levels of biological organization are still unexplored. Restricting the investigations of temporal dynamics of multiple stressors to the individual or population level will preclude the understanding of the role played by ecological memory in driving the responses at higher levels of biological organization.
Finally, the sampling of multiple time points can be translated in the measurement of different ecological metrics, such as resistance, recovery, resilience and invariability (Donohue et al., 2013, 2016; Hillebrand et al., 2018). Those ecological metrics have been shown to compose the different “dimensions” of ecological stability (Donohue et al., 2013; Pimm, 1984), which can be altered by different stressor combinations (Polazzo & Rico, 2021). Maintaining stable ecosystems is fundamental, as only stable systems can deliver functions and services consistently. Therefore, studying how HWs and micropollutants affect different stability indices, and how the order of stressors application plays in this context, may help to solve current managing conflicts, protect biodiversity in the long term, and guarantee the delivery of ecosystem functions and services.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supplementary Material
Supplementary Material
ACKNOWLEDGEMENTS
This work is part of the Innovative Training Network ECORISK2050 and was supported by the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska‐Curie grant agreement No 813124. A. Rico is supported by the Talented Researcher Support Programme—Plan GenT (CIDEGENT/2020/043) of the Generalitat Valenciana. M. Jackson is supported by the UK’s National Environment Research Council (NE/V001396/1).
Polazzo, F. , Roth, S. K. , Hermann, M. , Mangold‐Döring, A. , Rico, A. , Sobek, A. , Van den Brink, P. J. , & Jackson, M. C. (2022). Combined effects of heatwaves and micropollutants on freshwater ecosystems: Towards an integrated assessment of extreme events in multiple stressors research. Global Change Biology, 28, 1248–1267. 10.1111/gcb.15971
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no data sets were generated or analysed during the current study. However, all the articles used in this review are listed in [Link], [Link].
REFERENCES
- Annett, R. , Habibi, H. R. , & Hontela, A. (2014). Impact of glyphosate and glyphosate‐based herbicides on the freshwater environment. Journal of Applied Toxicology, 34, 458–479. 10.1002/jat.2997 [DOI] [PubMed] [Google Scholar]
- Arenas‐Sánchez, A. , López‐Heras, I. , Nozal, L. , Vighi, M. , & Rico, A. (2019). Effects of increased temperature, drought, and an insecticide on freshwater zooplankton communities. Environmental Toxicology and Chemistry, 38, 396–411. 10.1002/etc.4304 [DOI] [PubMed] [Google Scholar]
- Arias Font, R. , Khamis, K. , Milner, A. M. , Sambrook Smith, G. H. , & Ledger, M. E. (2021). Low flow and heatwaves alter ecosystem functioning in a stream mesocosm experiment. The Science of the Total Environment, 777, 146067. 10.1016/j.scitotenv.2021.146067 [DOI] [PubMed] [Google Scholar]
- Arp, H. P. H. , Brown, T. N. , Berger, U. , & Hale, S. E. (2017). Ranking REACH registered neutral, ionizable and ionic organic chemicals based on their aquatic persistency and mobility. Environmental Science: Processes & Impacts, 19, 939–955. 10.1039/C7EM00158D [DOI] [PubMed] [Google Scholar]
- Ashton, N. K. , Jensen, N. R. , Ross, T. J. , Young, S. P. , Hardy, R. S. , & Cain, K. D. (2019). Temperature and maternal age effects on burbot reproduction. North American Journal of Fisheries Management, 39(6), 1192–1206. 10.1002/nafm.10354 [DOI] [Google Scholar]
- Baag, S. , Mahapatra, S. , & Mandal, S. (2021). An Integrated and Multibiomarker approach to delineate oxidative stress status of Bellamya bengalensis under the interactions of elevated temperature and chlorpyrifos contamination. Chemosphere, 264. 10.1016/j.chemosphere.2020.128512 [DOI] [PubMed] [Google Scholar]
- Baranov, V. , Jourdan, J. , Pilotto, F. , Wagner, R. , & Haase, P. (2020). Complex and nonlinear climate‐driven changes in freshwater insect communities over 42 years. Conservation Biology, 34. 10.1111/cobi.13477 [DOI] [PubMed] [Google Scholar]
- Bardgett, R. D. , & Caruso, T. (2020). Soil microbial community responses to climate extremes: Resistance, resilience and transitions to alternative states. Philosophical Transactions of the Royal Society B: Biological Sciences, 375, 20190112. 10.1098/rstb.2019.0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barra Caracciolo, A. , Topp, E. , & Grenni, P. (2015). Pharmaceuticals in the environment: Biodegradation and effects on natural microbial communities. A review. Journal of Pharmaceutical and Biomedical Analysis, 106, 25–36. [DOI] [PubMed] [Google Scholar]
- Bartosiewicz, M. , Laurion, I. , Clayer, F. , & Maranger, R. (2016). Heat‐wave effects on oxygen, nutrients, and phytoplankton can alter global warming potential of gases emitted from a small shallow lake. Environmental Science and Technology, 50, 6267–6275. [DOI] [PubMed] [Google Scholar]
- Beermann, A. J. , Elbrecht, V. , Karnatz, S. , Ma, L. I. , Matthaei, C. D. , Piggott, J. J. , & Leese, F. (2018). Multiple‐stressor effects on stream macroinvertebrate communities: A mesocosm experiment manipulating salinity, fine sediment and flow velocity. Science of the Total Environment, 610–611, 961–971. 10.1016/j.scitotenv.2017.08.084 [DOI] [PubMed] [Google Scholar]
- Bergkemper, V. , & Weisse, T. (2017). Phytoplankton response to the summer 2015 heat wave—A case study from prealpine Lake Mondsee, Austria. Inland Waters, 7, 88–99. 10.1080/20442041.2017.1294352 [DOI] [Google Scholar]
- Bernhardt, E. S. , Rosi, E. J. , & Gessner, M. O. (2017). Synthetic chemicals as agents of global change. Frontiers in Ecology and the Environment, 15(2), 84–90. 10.1002/fee.1450 [DOI] [Google Scholar]
- Bernhardt, J. R. , Sunday, J. M. , Thompson, P. L. , & O’Connor, M. I. (2018). Nonlinear averaging of thermal experience predicts population growth rates in a thermally variable environment. Proceedings of the Royal Society B: Biological Sciences, 285(1886), 20181076. 10.1098/rspb.2018.1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani, I. , Primicerio, R. , & Rossetti, G. (2016). Extreme climatic event triggers a lake regime shift that propagates across multiple trophic levels. Ecosystems, 19(1), 16–31. 10.1007/s10021-015-9914-5 [DOI] [Google Scholar]
- Berthelot, H. , Duhamel, S. , L’Helguen, S. , Maguer, J.‐F. , Wang, S. , Cetinić, I. , & Cassar, N. (2019). NanoSIMS single cell analyses reveal the contrasting nitrogen sources for small phytoplankton. ISME Journal, 13, 651–662. 10.1038/s41396-018-0285-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bighiu, M. A. , & Goedkoop, W. (2021). Interactions with freshwater biofilms cause rapid removal of common herbicides through degradation‐evidence from microcosm studies. Environmental Science: Processes & Impacts, 23, 66–72. 10.1039/D0EM00394H [DOI] [PubMed] [Google Scholar]
- Birk, S. , Chapman, D. , Carvalho, L. , Spears, B. M. , Andersen, H. E. , Argillier, C. , Auer, S. , Baattrup‐Pedersen, A. , Banin, L. , Beklioğlu, M. , Bondar‐Kunze, E. , Borja, A. , Branco, P. , Bucak, T. , Buijse, A. D. , Cardoso, A. C. , Couture, R.‐M. , Cremona, F. , de Zwart, D. , … Hering, D. (2020). Impacts of multiple stressors on freshwater biota across spatial scales and ecosystems. Nature Ecology & Evolution, 4(8), 1060–1068. 10.1038/s41559-020-1216-4 [DOI] [PubMed] [Google Scholar]
- Birnie‐Gauvin, K. , Costantini, D. , Cooke, S. J. , & Willmore, W. G. (2017). A comparative and evolutionary approach to oxidative stress in fish: A review. Fish and Fisheries, 18(5), 928–942. 10.1111/faf.12215 [DOI] [Google Scholar]
- Blenckner, T. , Adrian, R. , Livingstone, D. M. , Jennings, E. , Weyhenmeyer, G. A. , George, D. G. , Jankowski, T. , Järvinen, M. , Aonghusa, C. N. , Nõges, T. , Straile, D. , & Teubner, K. (2007). Large‐scale climatic signatures in lakes across Europe: A meta‐analysis. Global Change Biology, 13, 1314–1326. 10.1111/j.1365-2486.2007.01364.x [DOI] [Google Scholar]
- Bondar‐Kunze, E. , Kasper, V. , & Hein, T. (2021). Responses of periphyton communities to abrupt changes in water temperature and velocity, and the relevance of morphology: A mesocosm approach. Science of the Total Environment. 10.1016/j.scitotenv.2021.145200 [DOI] [PubMed] [Google Scholar]
- Bracewell, S. , Verdonschot, R. C. M. , Schäfer, R. B. , Bush, A. , Lapen, D. R. , & Van den Brink, P. J. (2019). Qualifying the effects of single and multiple stressors on the food web structure of Dutch drainage ditches using a literature review and conceptual models. Science of the Total Environment, 684, 727–740. 10.1016/j.scitotenv.2019.03.497 [DOI] [PubMed] [Google Scholar]
- Bray, J. P. , Nichols, S. J. , Keely‐Smith, A. , Thompson, R. , Bhattacharyya, S. , Gupta, S. , Gupta, A. , Gao, J. , Wang, X. , Kaserzon, S. , Mueller, J. F. , Chou, A. , & Kefford, B. J. (2019). Stressor dominance and sensitivity‐dependent antagonism: Disentangling the freshwater effects of an insecticide among co‐occurring agricultural stressors. Journal of Applied Ecology, 56(8), 2020–2033. 10.1111/1365-2664.13430 [DOI] [Google Scholar]
- Brown, J. H. , Gillooly, J. F. , Allen, A. P. , Savage, V. M. , & West, G. B. (2004). Toward a metabolic theory of ecology. Ecology, 85, 1771–1789. 10.1890/03-9000 [DOI] [Google Scholar]
- Calderó‐Pascual, M. , de Eyto, E. , Jennings, E. , Dillane, M. , Andersen, M. R. , Kelly, S. , Wilson, H. L. , & McCarthy, V. (2020). Effects of consecutive extreme weather events on a temperate dystrophic lake: A detailed insight into physical, chemical and biological responses. Water, 12, 1411. 10.3390/w12051411 [DOI] [Google Scholar]
- Camp, A. A. , & Buchwalter, D. B. (2016). Can’t take the heat: Temperature‐enhanced toxicity in the mayfly Isonychia bicolor exposed to the neonicotinoid insecticide imidacloprid. Aquatic Toxicology. 10.1016/j.aquatox.2016.07.011 [DOI] [PubMed] [Google Scholar]
- Cardinale, B. J. , Duffy, J. E. , Gonzalez, A. , Hooper, D. U. , Perrings, C. , Venail, P. , Narwani, A. , Mace, G. M. , Tilman, D. , Wardle, D. A. , Kinzig, A. P. , Daily, G. C. , Loreau, M. , Grace, J. B. , Larigauderie, A. , Srivastava, D. S. , & Naeem, S. (2012). Biodiversity loss and its impact on humanity. Nature. 10.1038/nature11148 [DOI] [PubMed] [Google Scholar]
- Carreira, B. M. , Segurado, P. , Laurila, A. , & Rebelo, R. (2020). Heat waves trigger swift changes in the diet and life‐history of a freshwater snail. Hydrobiologia, 847. 10.1007/s10750-019-04155-3 [DOI] [Google Scholar]
- Carreira, B. M. , Segurado, P. , Orizaola, G. , Gonçalves, N. , Pinto, V. , Laurila, A. , & Rebelo, R. (2016). Warm vegetarians? Heat waves and diet shifts in tadpoles. Ecology, 97. 10.1002/ecy.1541 [DOI] [PubMed] [Google Scholar]
- Cereja, R. (2020). Critical thermal maxima in aquatic ectotherms. Ecological Indicators. 10.1016/j.ecolind.2020.106856 [DOI] [Google Scholar]
- Coll, C. , Bier, R. , Li, Z. , Langenheder, S. , Gorokhova, E. , & Sobek, A. (2020). Association between aquatic micropollutant dissipation and river sediment bacterial communities. Environmental Science & Technology, 54(22), 14380–14392. 10.1021/acs.est.0c04393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutant, C. C. (2001). Behavioral technologies for fish guidance. In Proceedings of the symposium behavioral technologies for fish guidance held at Charlotte, NC, USA, 30–31 August 1999 (Vol. 26). American Fisheries Society. [Google Scholar]
- Cremona, F. , Agasild, H. , Haberman, J. , Zingel, P. , Nõges, P. , Nõges, T. , & Laas, A. (2020). How warming and other stressors affect zooplankton abundance, biomass and community composition in shallow eutrophic lakes. Climatic Change, 159. 10.1007/s10584-020-02698-2 [DOI] [Google Scholar]
- Currie, D. J. (1990). Large‐scale variablity and interactions among phytoplankton, bacterioplankton, and phosphorus. Limnology and Oceanography, 35, 1437–1455. [Google Scholar]
- Danner, M. C. , Robertson, A. , Behrends, V. , & Reiss, J. (2019). Antibiotic pollution in surface fresh waters: Occurrence and effects. Science of the Total Environment. 10.1016/j.scitotenv.2019.01.406 [DOI] [PubMed] [Google Scholar]
- Daufresne, M. , Bady, P. , & Fruget, J. F. (2007). Impacts of global changes and extreme hydroclimatic events on macroinvertebrate community structures in the French Rhône River. Oecologia, 151. 10.1007/s00442-006-0655-1 [DOI] [PubMed] [Google Scholar]
- De Laender, F. , Rohr, J. R. , Ashauer, R. , Baird, D. J. , Berger, U. , Eisenhauer, N. , Grimm, V. , Hommen, U. , Maltby, L. , Meliàn, C. J. , Pomati, F. , Roessink, I. , Radchuk, V. , & Van den Brink, P. J. (2016). Reintroducing environmental change drivers in biodiversity‐ecosystem functioning research. Trends in Ecology & Evolution, 31, 905–915. 10.1016/j.tree.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mira‐Mendes, C. V. , Costa, R. N. , Dias, I. R. , Carilo Filho, L. M. , Mariano, R. , Le Pendu, Y. , & Solé, M. (2019). Effects of increasing temperature on predator‐prey interaction between beetle larvae and tadpoles. Studies on Neotropical Fauna and Environment, 54(3), 163–168. 10.1080/01650521.2019.1656519 [DOI] [Google Scholar]
- de Oliveira, A. B. , Cantarel, A. A. M. , Seiller, M. , Florio, A. , Bérard, A. , Hinsinger, P. , & Le Cadre, E. (2020). Short‐term plant legacy alters the resistance and resilience of soil microbial communities exposed to heat disturbance in a Mediterranean calcareous soil. Ecological Indicators, 108, 105740. 10.1016/j.ecolind.2019.105740 [DOI] [Google Scholar]
- Delnat, V. , Tran, T. T. , Verheyen, J. , Van Dinh, K. , Janssens, L. , & Stoks, R. (2019). Temperature variation magnifies chlorpyrifos toxicity differently between larval and adult mosquitoes. Science of the Total Environment. 10.1016/j.scitotenv.2019.07.030 [DOI] [PubMed] [Google Scholar]
- Delnat, V. , Verborgt, J. , Janssens, L. , & Stoks, R. (2021). Daily temperature variation lowers the lethal and sublethal impact of a pesticide pulse due to a higher degradation rate. Chemosphere, 263(600), 128114. 10.1016/j.chemosphere.2020.128114 [DOI] [PubMed] [Google Scholar]
- Delorenzo, M. E. , Danese, L. E. , & Baird, T. D. (2013). Influence of increasing temperature and salinity on herbicide toxicity in estuarine phytoplankton. Environmental Toxicology, 28(7), 359–371. 10.1002/tox.20726 [DOI] [PubMed] [Google Scholar]
- DeWhatley, M. C. , & Alexander, J. E. (2018). Impacts of elevated water temperatures on righting behavior and survival of two freshwater caenogastropod snails. Marine and Freshwater Behaviour and Physiology, 51. 10.1080/10236244.2018.1538699 [DOI] [Google Scholar]
- Dinh, K. V. , Janssens, L. , & Stoks, R. (2016). Exposure to a heat wave under food limitation makes an agricultural insecticide lethal: A mechanistic laboratory experiment. Global Change Biology, 22, 3361–3372. 10.1111/gcb.13415 [DOI] [PubMed] [Google Scholar]
- Döll, P. , Trautmann, T. , Gerten, D. , Schmied, H. M. , Ostberg, S. , Saaed, F. , & Schleussner, C. F. (2018). Risks for the global freshwater system at 1.5°C and 2°C global warming. Environmental Research Letters, 13, 044038. [Google Scholar]
- Donnelly, P. K. , Entry, J. A. , Crawford, D. L. , & Cromack, K. Jr (1990). Cellulose and lignin degradation in forest soils: Response to moisture, temperature, and acidity. Microbial Ecology, 20, 289–295. 10.1007/BF02543884 [DOI] [PubMed] [Google Scholar]
- Donohue, I. , Hillebrand, H. , Montoya, J. M. , Petchey, O. L. , Pimm, S. L. , Fowler, M. S. , Healy, K. , Jackson, A. L. , Lurgi, M. , McClean, D. , O'Connor, N. E. , O'Gorman, E. J. , & Yang, Q. (2016). Navigating the complexity of ecological stability. Ecology Letters, 19, 1172–1185. 10.1111/ele.12648 [DOI] [PubMed] [Google Scholar]
- Donohue, I. , Petchey, O. L. , Montoya, J. M. , Jackson, A. L. , McNally, L. , Viana, M. , Healy, K. , Lurgi, M. , O'Connor, N. E. , & Emmerson, M. C. (2013). On the dimensionality of ecological stability. Ecology Letters, 16, 421–429. 10.1111/ele.12086 [DOI] [PubMed] [Google Scholar]
- Duarte, S. , Fernandes, I. , Nogueira, M. J. , Cássio, F. , & Pascoal, C. (2013). Temperature alters interspecific relationships among aquatic fungi. Fungal Ecology, 6, 187–191. 10.1016/j.funeco.2013.02.001 [DOI] [Google Scholar]
- Ebele, A. J. , Abou‐Elwafa Abdallah, M. , & Harrad, S. (2017). Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerging Contaminants, 3(1), 1–16. 10.1016/j.emcon.2016.12.004 [DOI] [Google Scholar]
- Eggers, S. L. , Eriksson, B. K. , & Matthiessen, B. (2012). A heat wave and dispersal cause dominance shift and decrease biomass in experimental metacommunities. Oikos, 121, 721–733. 10.1111/j.1600-0706.2011.19714.x [DOI] [Google Scholar]
- Escher, B. I. , Stapleton, H. M. , & Schymanski, E. L. (2020). Tracking complex mixtures of chemicals in our changing environment. Science, 367, 388–392. 10.1126/science.aay6636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes, I. , Pascoal, C. , Guimarães, H. , Pinto, R. , Sousa, I. , & Cássio, F. (2012). Higher temperature reduces the effects of litter quality on decomposition by aquatic fungi. Freshwater Biology, 57, 2306–2317. 10.1111/fwb.12004 [DOI] [Google Scholar]
- Fetzer, I. , Johst, K. , Schawea, R. , Banitz, T. , Harms, H. , & Chatzinotas, A. (2015). The extent of functional redundancy changes as species’ roles shift in different environments. Proceedings of the National Academy of Sciences of the United States of America, 112(48), 14888–14893. 10.1073/pnas.1505587112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO)—FAOSTAT . (2020). 899 Database collection of the Food and Agriculture Organization of the United Nations. http://www.fao.org/faostat/en/#data [Google Scholar]
- Forlani, G. , Pavan, M. , Gramek, M. , Kafarski, P. , & Lipok, J. (2008). Biochemical bases for a widespread tolerance of cyanobacteria to the phosphonate herbicide glyphosate. Plant and Cell Physiology, 49. 10.1093/pcp/pcn021 [DOI] [PubMed] [Google Scholar]
- Fornaroli, R. , White, J. C. , Boggero, A. , & Laini, A. (2020). Spatial and temporal patterns of macroinvertebrate assemblages in the River Po Catchment (Northern Italy). Water, 12(9), 2452. 10.3390/w12092452 [DOI] [Google Scholar]
- Freitas, J. S. , Felício, A. A. , Teresa, F. B. , & Alves de Almeida, E. (2017). Combined effects of temperature and clomazone (Gamit®) on oxidative stress responses and B‐esterase activity of Physalaemus nattereri (Leiuperidae) and Rhinella schneideri (Bufonidae) tadpoles. Chemosphere, 185. 10.1016/j.chemosphere.2017.07.061 [DOI] [PubMed] [Google Scholar]
- Freitas, J. S. , Kupsco, A. , Diamante, G. , Felicio, A. A. , Almeida, E. A. , & Schlenk, D. (2016). Influence of temperature on the thyroidogenic effects of diuron and its metabolite 3,4‐DCA in tadpoles of the American bullfrog (lithobates catesbeianus). Environmental Science & Technology. 10.1021/acs.est.6b04076 [DOI] [PubMed] [Google Scholar]
- Fussmann, K. E. , Schwarzmüller, F. , Brose, U. , Jousset, A. , & Rall, B. C. (2014). Ecological stability in response to warming. Nature Climate Change, 4. 10.1038/nclimate2134 [DOI] [Google Scholar]
- García‐Carreras, B. , Sal, S. , Padfield, D. , Kontopoulos, D.‐G. , Bestion, E. , Schaum, C.‐E. , Yvon‐Durocher, G. , & Pawar, S. (2018). Role of carbon allocation efficiency in the temperature dependence of autotroph growth rates. Proceedings of the National Academy of Sciences of the United States of America, 115(31), E7361–E7368. 10.1073/pnas.1800222115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier, A. , Pennekamp, F. , Lemoine, M. , & Petchey, O. L. (2017). Temporal scale dependent interactions between multiple environmental disturbances in microcosm ecosystems. Global Change Biology, 23, 5237–5248. 10.1111/gcb.13786 [DOI] [PubMed] [Google Scholar]
- Gillooly, J. F. , Brown, J. H. , West, G. B. , Savage, V. M. , & Charnov, E. L. (2001). Effects of size and temperature on metabolic rate. Science, 293, 2248–2251. 10.1126/science.1061967 [DOI] [PubMed] [Google Scholar]
- Gomes, M. P. , & Juneau, P. (2017). Temperature and light modulation of herbicide toxicity on algal and cyanobacterial physiology. Frontiers in Environmental Science, 5, 1–17. 10.3389/fenvs.2017.00050 [DOI] [Google Scholar]
- Gripp, H. S. , Freitas, J. S. , Almeida, E. A. , Bisinoti, M. C. , & Moreira, A. B. (2017). Biochemical effects of fipronil and its metabolites on lipid peroxidation and enzymatic antioxidant defense in tadpoles (Eupemphix nattereri: Leiuperidae). Ecotoxicology and Environmental Safety, 136, 173–179. 10.1016/j.ecoenv.2016.10.027 [DOI] [PubMed] [Google Scholar]
- Halstead, N. T. , McMahon, T. A. , Johnson, S. A. , Raffel, T. R. , Romansic, J. M. , Crumrine, P. W. , & Rohr, J. R. (2014). Community ecology theory predicts the effects of agrochemical mixtures on aquatic biodiversity and ecosystem properties. Ecology Letters, 17, 932–941. 10.1111/ele.12295 [DOI] [PubMed] [Google Scholar]
- Hani, Y. M. I. , Turies, C. , Palluel, O. , Delahaut, L. , Bado‐Nilles, A. , Geffard, A. , Dedourge‐Geffard, O. , & Porcher, J.‐M. (2019). Effects of a chronic exposure to different water temperatures and/or to an environmental cadmium concentration on the reproduction of the threespine stickleback (Gasterosteus aculeatus). Ecotoxicology and Environmental Safety, 174, 48–57. 10.1016/j.ecoenv.2019.02.032 [DOI] [PubMed] [Google Scholar]
- Hansson, L.‐A. , Ekvall, M. K. , He, L. , Li, Z. , Svensson, M. , Urrutia‐Cordero, P. , & Zhang, H. (2020). Different climate scenarios alter dominance patterns among aquatic primary producers in temperate systems. Limnology and Oceanography, 65(10), 2328–2336. 10.1002/lno.11455 [DOI] [Google Scholar]
- Hao, B. , Wu, H. , Zhen, W. , Jo, H. , Cai, Y. , Jeppesen, E. , & Li, W. (2020). Warming effects on periphyton community and abundance in different seasons are influenced by nutrient state and plant type: A shallow lake mesocosm study. Frontiers in Plant Science, 11. 10.3389/fpls.2020.00404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, N. M. , Patoine, A. , Haig, H. A. , Simpson, G. L. , Swarbrick, V. J. , Wiik, E. , & Leavitt, P. R. (2019). Spatial and temporal variation in nitrogen fixation and its importance to phytoplankton in phosphorus‐rich lakes. Freshwater Biology, 64, 269–283. 10.1111/fwb.13214 [DOI] [Google Scholar]
- Herlemann, D. P. R. , Labrenz, M. , Jürgens, K. , Bertilsson, S. , Waniek, J. J. , & Andersson, A. F. (2011). Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME Journal, 5, 1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heugens, E. H. W. , Hendriks, A. J. , Dekker, T. , Van Straalen, N. M. , & Admiraal, W. (2001). A review of the effects of multiple stressors on aquatic organisms and analysis of uncertainty factors for use in risk assessment. Critical Reviews in Toxicology, 31(3), 247–284. 10.1080/20014091111695 [DOI] [PubMed] [Google Scholar]
- Higashi, M. , & Patten, B. C. (1989). Dominance of indirect causality in ecosystems. American Naturalist, 133. 10.1086/284919 [DOI] [Google Scholar]
- Hillebrand, H. , Langenheder, S. , Lebret, K. , Lindström, E. , Östman, Ö. , & Striebel, M. (2018). Decomposing multiple dimensions of stability in global change experiments. Ecology Letters, 21, 21–30. 10.1111/ele.12867 [DOI] [PubMed] [Google Scholar]
- Höfle, M. G. (1979). Effects of sudden temperature shifts on pure cultures of four strains of freshwater bacteria. Microbial Ecology, 5, 17–26. 10.1007/BF02010574 [DOI] [PubMed] [Google Scholar]
- Holmstrup, M. , Bindesbøl, A.‐M. , Oostingh, G. J. , Duschl, A. , Scheil, V. , Köhler, H.‐R. , Loureiro, S. , Soares, A. M. V. M. , Ferreira, A. L. G. , Kienle, C. , Gerhardt, A. , Laskowski, R. , Kramarz, P. E. , Bayley, M. , Svendsen, C. , & Spurgeon, D. J. (2010). Interactions between effects of environmental chemicals and natural stressors: A review. Science of the Total Environment, 408, 3746–3762. 10.1016/j.scitotenv.2009.10.067 [DOI] [PubMed] [Google Scholar]
- Hooper, M. J. , Ankley, G. T. , Cristol, D. A. , Maryoung, L. A. , Noyes, P. D. , & Pinkerton, K. E. (2013). Interactions between chemical and climate stressors: A role for mechanistic toxicology in assessing climate change risks. Environmental Toxicology and Chemistry, 32, 32–48. 10.1002/etc.2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, V. , Adrian, R. , & Gerten, D. (2010). A matter of timing: Heat wave impact on crustacean zooplankton. Freshwater Biology, 55, 1769–1779. 10.1111/j.1365-2427.2010.02411.x [DOI] [Google Scholar]
- Hughes, S. R. , Kay, P. , & Brown, L. E. (2013). Global synthesis and critical evaluation of pharmaceutical data sets collected from river systems. Environmental Science & Technology, 47(2), 661–677. 10.1021/es3030148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, T. P. , Kerry, J. T. , Connolly, S. R. , Baird, A. H. , Eakin, C. M. , Heron, S. F. , Hoey, A. S. , Hoogenboom, M. O. , Jacobson, M. , Liu, G. , Pratchett, M. S. , Skirving, W. , & Torda, G. (2019). Ecological memory modifies the cumulative impact of recurrent climate extremes. Nature Climate Change, 9, 40–43. 10.1038/s41558-018-0351-2 [DOI] [Google Scholar]
- Ikert, H. , & Craig, P. M. (2020). Chronic exposure to venlafaxine and increased water temperature reversibly alters microRNA in zebrafish gonads (Danio rerio). Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 33, 100634. 10.1016/j.cbd.2019.100634 [DOI] [PubMed] [Google Scholar]
- IPCC . (2013). Citation IPCC 2013. Clim. Chang. 2013—Phys. Sci. Basis, Contrib. Work. Gr. I to Fifth Assess. Rep. Intergov. Panel Clim. Chang. [Google Scholar]
- Jackson, M. C. , Pawar, S. , & Woodward, G. (2021). Ecology & evolution the temporal dynamics of multiple stressor effects: From individuals to ecosystems. Trends in Ecology & Evolution. 10.1016/j.tree.2021.01.005 [DOI] [PubMed] [Google Scholar]
- Jacquet, C. , & Altermatt, F. (2020). The ghost of disturbance past: Long‐term effects of pulse disturbances on community biomass and composition. Proceedings of the Royal Society B: Biological Sciences, 287(1930), 20200678. 10.1098/rspb.2020.0678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch, A. , Kreyling, J. , & Beierkuhnlein, C. (2007). A new generation of climate‐change experiments: Events, not trends. Frontiers in Ecology and the Environment 5, 365–374. [Google Scholar]
- Johnsen, M. A. , Stuparyk, B. R. , Cook, J. , & Vinebrooke, R. D. (2020). Resilience of pond communities to extreme thermal regime shifts: An alpine–montane reciprocal transplant experiment. Aquatic Sciences, 82(2). 10.1007/s00027-020-0709-1 [DOI] [Google Scholar]
- Kattwinkel, M. , Jan‐Valentin, K. , Foit, K. , & Liess, M. (2011). Climate change, agricultural insecticide exposure, and risk for freshwater communities. Ecological Applications, 21, 2068–2081. 10.1890/10-1993.1 [DOI] [PubMed] [Google Scholar]
- Kaushik, N. , & Hynes, H. (1971). The fate of the dead leaves that fall into streams. Archiv fur Hydrobiologie, 68, 465–515. [Google Scholar]
- Kéfi, S. , Berlow, E. L. , Wieters, E. A. , Joppa, L. N. , Wood, S. A. , Brose, U. , & Navarrete, S. A. (2015). Network structure beyond food webs: Mapping non‐trophic and trophic interactions on Chilean rocky shores. Ecology, 96, 291–303. 10.1890/13-1424.1 [DOI] [PubMed] [Google Scholar]
- Kimberly, D. A. , & Salice, C. J. (2013). Interactive effects of contaminants and climate‐related stressors: High temperature increases sensitivity to cadmium. Environmental Toxicology and Chemistry, 32(6), 1337–1343. 10.1002/etc.2198 [DOI] [PubMed] [Google Scholar]
- Klausmeier, C. A. , Litchman, E. , Daufresne, T. , & Levin, S. A. (2004). Optimal nitrogen‐to‐phosphorus stoichiometry of phytoplankton. Nature, 429, 171–174. 10.1038/nature02454 [DOI] [PubMed] [Google Scholar]
- Kreyling, J. , Schweiger, A. H. , Bahn, M. , Ineson, P. , Migliavacca, M. , Morel‐Journel, T. , Christiansen, J. R. , Schtickzelle, N. , & Larsen, K. S. (2018). To replicate, or not to replicat—That is the question: How to tackle nonlinear responses in ecological experiments. Ecology Letters, 21, 1629–1638. 10.1111/ele.13134 [DOI] [PubMed] [Google Scholar]
- Langenheder, S. , & Lindström, E. S. (2019). Factors influencing aquatic and terrestrial bacterial community assembly. Environmental Microbiology Reports, 11, 306–315. 10.1111/1758-2229.12731 [DOI] [PubMed] [Google Scholar]
- Ledger, M. E. , & Milner, A. M. (2015). Extreme events in running waters. Freshwater Biology, 60, 2455–2460. [Google Scholar]
- Leibold, M. A. , Holyoak, M. , Mouquet, N. , Amarasekare, P. , Chase, J. M. , Hoopes, M. F. , Holt, R. D. , Shurin, J. B. , Law, R. , Tilman, D. , Loreau, M. , & Gonzalez, A. (2004). The metacommunity concept: A framework for multi‐scale community ecology. Ecology Letters, 7, 601–613. 10.1111/j.1461-0248.2004.00608.x [DOI] [Google Scholar]
- Leicht, K. , Jokela, J. , & Seppälä, O. (2013). An experimental heat wave changes immune defense and life history traits in a freshwater snail. Ecology and Evolution, 3(15), 4861–4871. 10.1002/ece3.874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leicht, K. , & Seppälä, O. (2019). Direct and transgenerational effects of an experimental heatwave on early life stages in a freshwater snail. Freshwater Biology, 64. 10.1111/fwb.13401 [DOI] [Google Scholar]
- Li, Z. , He, L. , Zhang, H. , Urrutia‐Cordero, P. , Ekvall, M. K. , Hollander, J. , & Hansson, L.‐A. (2017). Climate warming and heat waves affect reproductive strategies and interactions between submerged macrophytes. Global Change Biology, 23, 108–116. 10.1111/gcb.13405 [DOI] [PubMed] [Google Scholar]
- Logue, J. B. , & Lindström, E. S. (2010). Species sorting affects bacterioplankton community composition as determined by 16S rDNA and 16S rRNA fingerprints. ISME Journal, 4, 729–738. 10.1038/ismej.2009.156 [DOI] [PubMed] [Google Scholar]
- López‐Urrutia, Á. , San Martin, E. , Harris, R. P. , & Irigoien, X. (2006). Scaling the metabolic balance of the oceans. Proceedings of the National Academy of Sciences of the United States of America, 103, 8739–8744. 10.1073/pnas.0601137103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv, W. , Zhang, L. , Niu, H. , Li, B. , Wang, Q. I. , Zhou, Y. , Wang, Y. , Jiang, L. , Liu, P. , Hong, H. , Jia, S. , Luo, C. , Tsechoe, D. , Zhou, H. , & Wang, S. (2020). Non‐linear temperature sensitivity of litter component decomposition under warming gradient with precipitation addition on the Tibetan plateau. Plant and Soil, 448, 335–351. 10.1007/s11104-020-04431-5 [DOI] [Google Scholar]
- Maazouzi, C. , Masson, G. , Izquierdo, M. S. , & Pihan, J. C. (2008). Midsummer heat wave effects on lacustrine plankton: Variation of assemblage structure and fatty acid composition. Journal of Thermal Biology, 33, 287–296. 10.1016/j.jtherbio.2008.03.002 [DOI] [Google Scholar]
- Maazouzi, C. , Piscart, C. , Legier, F. , & Hervant, F. (2011). Ecophysiological responses to temperature of the “killer shrimp” Dikerogammarus villosus: Is the invader really stronger than the native Gammarus pulex? Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 10.1016/j.cbpa.2011.03.019 [DOI] [PubMed] [Google Scholar]
- Macaulay, S. J. , Buchwalter, D. B. , & Matthaei, C. D. (2020). Water temperature interacts with the insecticide imidacloprid to alter acute lethal and sublethal toxicity to mayfly larvae. New Zealand Journal of Marine and Freshwater Research, 54(1), 115–130. 10.1080/00288330.2019.1614961 [DOI] [Google Scholar]
- Macaulay, S. J. , Hageman, K. J. , Piggott, J. J. , & Matthaei, C. D. (2021). Time‐cumulative effects of neonicotinoid exposure, heatwaves and food limitation on stream mayfly nymphs: A multiple‐stressor experiment. Science of the Total Environment. 10.1016/j.scitotenv.2020.141941 [DOI] [PubMed] [Google Scholar]
- Mameri, D. , Branco, P. , Ferreira, M. T. , & Santos, J. M. (2020). Heatwave effects on the swimming behaviour of a Mediterranean freshwater fish, the Iberian barbel Luciobarbus bocagei . Science of the Total Environment. 10.1016/j.scitotenv.2020.139152 [DOI] [PubMed] [Google Scholar]
- Margulis, L. , Chase, D. , & Guerrero, R. (1986). Microbial Communities: Invisible to the scrutiny of naturalists, most microbial communities have escaped description. BioScience, 36, 160–170. 10.2307/1310303 [DOI] [PubMed] [Google Scholar]
- Mazor, T. , Doropoulos, C. , Schwarzmueller, F. , Gladish, D. W. , Kumaran, N. , Merkel, K. , Di Marco, M. , & Gagic, V. (2018). Global mismatch of policy and research on drivers of biodiversity loss. Nature Ecology & Evolution, 2, 1071–1074. 10.1038/s41559-018-0563-x [DOI] [PubMed] [Google Scholar]
- Mccauley, S. J. , Hammond, J. I. , Frances, D. N. , & Mabry, K. E. (2015). Effects of experimental warming on survival, phenology, and morphology of an aquatic insect (Odonata). Ecological Entomology, 40(3), 211–220. 10.1111/een.12175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehl, G. A. , & Tebaldi, C. (2004). More intense, more frequent, and longer lasting heat waves in the 21st century. Science, 305, 994–997. 10.1126/science.1098704 [DOI] [PubMed] [Google Scholar]
- Mehdi, H. , Bragg, L. M. , Servos, M. R. , & Craig, P. M. (2019). Multiple stressors in the environment: The effects of exposure to an antidepressant (venlafaxine) and increased temperature on zebrafish metabolism. Frontiers in Physiology, 10. 10.3389/fphys.2019.01431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracle, M. R. , Nandini, S. , Sarma, S. S. S. , & Vicente, E. (2011). Endocrine disrupting effects, at different temperatures, on Moina micrura (Cladocera: Crustacea) induced by carbendazim, a fungicide. Hydrobiologia, 668. 10.1007/s10750-011-0638-z [DOI] [Google Scholar]
- Mishra, S. , Zhang, W. , Lin, Z. , Pang, S. , Huang, Y. , Bhatt, P. , & Chen, S. (2020). Carbofuran toxicity and its microbial degradation in contaminated environments. Chemosphere. 10.1016/j.chemosphere.2020.127419 [DOI] [PubMed] [Google Scholar]
- Moe, S. J. , De Schamphelaere, K. , Clements, W. H. , Sorensen, M. T. , Van den Brink, P. J. , & Liess, M. (2013). Combined and interactive effects of global climate change and toxicants on populations and communities. Environmental Toxicology and Chemistry, 32, 49–61. 10.1002/etc.2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya, J. M. , Woodward, G. , Emmerson, M. C. , & Solé, R. V. (2009). Press perturbations and indirect effects in real food webs. Ecology, 90. 10.1890/08-0657.1 [DOI] [PubMed] [Google Scholar]
- Mouthon, J. , & Daufresne, M. (2006). Effects of the 2003 heatwave and climatic warming on mollusc communities of the Saône: A large lowland river and of its two main tributaries (France). Global Change Biology, 12, 441–449. 10.1111/j.1365-2486.2006.01095.x [DOI] [Google Scholar]
- Mouthon, J. , & Daufresne, M. (2008). Population dynamics and life cycle of Pisidium amnicum (Müller) (Bivalvia: Sphaeriidae) and Valvata piscinalis (Müller) (Gastropoda: Prosobranchia) in the Saône river, a nine‐year study. Annales de Limnologie—International Journal of Limnology, 44(4), 241–251. 10.1051/limn:2008008 [DOI] [Google Scholar]
- Müller, M. F. , Colomer, J. , & Serra, T. (2018). Temperature‐driven response reversibility and short‐term quasi‐acclimation of Daphnia magna . PLoS One, 13. 10.1371/journal.pone.0209705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn, Z. , Peters, M. D. J. , Stern, C. , Tufanaru, C. , McArthur, A. , & Aromataris, E. (2018). Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Medical Research Methodology, 18. 10.1186/s12874-018-0611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykrä, H. , Tolkkinen, M. , & Heino, J. (2017). Environmental degradation results in contrasting changes in the assembly processes of stream bacterial and fungal communities. Oikos, 126, 1291–1298. 10.1111/oik.04133 [DOI] [Google Scholar]
- Nguyen, T. T. , Le, M. H. , Doan, N. X. , Pham, H. Q. , Vu, M. T. T. , & Dinh, K. V. (2020). Artificial light pollution increases the sensitivity of tropical zooplankton to extreme warming. Environmental Technology & Innovation. 10.1016/j.eti.2020.101179 [DOI] [Google Scholar]
- O’Connor, M. I. , Gilbert, B. , & Brown, C. J. (2011). Theoretical predictions for how temperature affects the dynamics of interacting herbivores and plants. American Naturalist, 178, 626–638. 10.1086/662171 [DOI] [PubMed] [Google Scholar]
- O’Connor, M. I. , Piehler, M. F. , Leech, D. M. , Anton, A. , & Bruno, J. F. (2009). Warming and resource availability shift food web structure and metabolism. PLoS Biology, 7. 10.1371/journal.pbio.1000178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhlund, G. , Hedström, P. , Norman, S. , Hein, C. L. , & Englund, G. (2014). Temperature dependence of predation depends on the relative performance of predators and prey. Proceedings of the Royal Society B: Biological Sciences. 10.1098/rspb.2014.2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr, J. A. , Vinebrooke, R. D. , Jackson, M. C. , Kroeker, K. J. , Kordas, R. L. , Mantyka‐Pringle, C. , Van den Brink, P. J. , De Laender, F. , Stoks, R. , Holmstrup, M. , Matthaei, C. D. , Monk, W. A. , Penk, M. R. , Leuzinger, S. , Schäfer, R. B. , & Piggott, J. J. (2020). Towards a unified study of multiple stressors: Divisions and common goals across research disciplines. Proceedings of the Royal Society B: Biological Sciences, 287(1926), 20200421. 10.1098/rspb.2020.0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, K. , Han, E. J. , Ahn, G. , & Kwak, I. S. (2020). Effects of combined stressors to cadmium and high temperature on antioxidant defense, apoptotic cell death, and DNA methylation in zebrafish (Danio rerio) embryos. Science of the Total Environment. 10.1016/j.scitotenv.2020.137130 [DOI] [PubMed] [Google Scholar]
- Parry, M. , Arnell, N. , McMichael, T. , Nicholls, R. , Martens, P. , Kovats, S. , Livermore, M. , Rosenzweig, C. , Iglesias, A. , & Fischer, G. (2001). Millions at risk: Defining critical climate change threats and targets. Global Environmental Change, 11(3), 181–183. 10.1016/S0959-3780(01)00011-5 [DOI] [Google Scholar]
- Pérez‐Guzmán, L. , Acosta‐Martínez, V. , Phillips, L. A. , & Mauget, S. A. (2020). Resilience of the microbial communities of semiarid agricultural soils during natural climatic variability events. Applied Soil Ecology, 149, 103487. 10.1016/j.apsoil.2019.103487 [DOI] [Google Scholar]
- Phillips, K. N. , Godwin, C. M. , & Cotner, J. B. (2017). The effects of nutrient imbalances and temperature on the biomass stoichiometry of freshwater bacteria. Frontiers in Microbiology, 8, 1–11. 10.3389/fmicb.2017.01692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, P. J. , & Bode, R. W. (2004). Pesticides in surface water runoff in south‐eastern New York State, USA: Seasonal and stormflow effects on concentrations. Pest Management Science. 10.1002/ps.879 [DOI] [PubMed] [Google Scholar]
- Piggott, J. J. , Salis, R. K. , Lear, G. , Townsend, C. R. , & Matthaei, C. D. (2015). Climate warming and agricultural stressors interact to determine stream periphyton community composition. Global Change Biology, 21, 206–222. 10.1111/gcb.12661 [DOI] [PubMed] [Google Scholar]
- Piggott, J. J. , Townsend, C. R. , & Matthaei, C. D. (2015). Climate warming and agricultural stressors interact to determine stream macroinvertebrate community dynamics. Global Change Biology, 21, 1887–1906. 10.1111/gcb.12861 [DOI] [PubMed] [Google Scholar]
- Pimm, S. L. (1984). The complexity and stability of ecosystems. Nature, 307, 321–326. 10.1038/307321a0 [DOI] [Google Scholar]
- Polazzo, F. , & Rico, A. (2021). Effects of multiple stressors on the dimensionality of ecological stability. Ecology Letters, 1–13. 10.1111/ele.13770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy, L. R. , & Wiebe, W. J. (1988). Energetics of microbial food webs. Hydrobiologia, 159, 7–18. 10.1007/BF00007363 [DOI] [Google Scholar]
- Posthuma, L. , Zijp, M. C. , De Zwart, D. , Van de Meent, D. , Globevnik, L. , Koprivsek, M. , Focks, A. , Van Gils, J. , & Birk, S. (2020). Chemical pollution imposes limitations to the ecological status of European surface waters. Scientific Reports, 10, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradeep Ram, A. S. , Keshri, J. , & Sime‐Ngando, T. (2020). Differential impact of top‐down and bottom‐up forces in structuring freshwater bacterial communities. FEMS Microbiology Ecology, 96, 1–13. [DOI] [PubMed] [Google Scholar]
- Prato, E. , Biandolino, F. , & Scardicchio, C. (2008). Implications for toxicity tests with amphipod Gammarus aequicauda: Effects of temperature and salinity on life cycle. Environmental Technology. 10.1080/09593330802379482 [DOI] [PubMed] [Google Scholar]
- Prophete, C. , Carlson, E. A. , Li, Y. , Duffy, J. , Steinetz, B. , Lasano, S. , & Zelikoff, J. T. (2006). Effects of elevated temperature and nickel pollution on the immune status of Japanese medaka. Fish & Shellfish Immunology, 21. 10.1016/j.fsi.2005.12.009 [DOI] [PubMed] [Google Scholar]
- Remy, M. , Hillebrand, H. , & Flöder, S. (2017). Stability of marine phytoplankton communities facing stress related to global change: Interactive effects of heat waves and turbidity. Journal of Experimental Marine Biology and Ecology, 497, 219–229. 10.1016/j.jembe.2017.10.002 [DOI] [Google Scholar]
- Richardson, J. , Feuchtmayr, H. , Miller, C. , Hunter, P. D. , Maberly, S. C. , & Carvalho, L. (2019). Response of cyanobacteria and phytoplankton abundance to warming, extreme rainfall events and nutrient enrichment. Global Change Biology, 25, 3365–3380. 10.1111/gcb.14701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockström, J. , Steffen, W. , Noone, K. , Persson, Å. , Chapin, F. S. , & Lambin, E. F. (2009). A safe operation space for humanity. Nature, 461, 472–475. [DOI] [PubMed] [Google Scholar]
- Ruel J. J., & Ayres M. P. (1999). Jensen’s inequality predicts effects of environmental variation. Trends in Ecology & Evolution, 14(9), 361–366. 10.1016/s0169-5347(99)01664-x [DOI] [PubMed] [Google Scholar]
- Savage, V. M. , Gillooly, J. F. , Brown, J. H. , West, G. B. , & Charnov, E. L. (2004). Effects of body size and temperature on population growth. American Naturalist, 163. 10.1086/381872 [DOI] [PubMed] [Google Scholar]
- Schäfer, R. B. , & Piggott, J. J. (2018). Advancing understanding and prediction in multiple stressor research through a mechanistic basis for null models. Global Change Biology, 24, 1817–1826. 10.1111/gcb.14073 [DOI] [PubMed] [Google Scholar]
- Scheyer, A. , Morville, S. , Mirabel, P. , & Millet, M. (2007). Variability of atmospheric pesticide concentrations between urban and rural areas during intensive pesticide application. Atmospheric Environment, 41(17), 3604–3618. 10.1016/j.atmosenv.2006.12.042 [DOI] [Google Scholar]
- Schindler, D. W. (1997). Widespread effects of climatic warming on freshwater ecosystems in North America. Hydrological Processes, 11(8), 1043–1067. [DOI] [Google Scholar]
- Schlesinger, W. H. (2006). Global change ecology. Trends in Ecology & Evolution, 21(6), 348–351. 10.1016/j.tree.2006.03.004 [DOI] [PubMed] [Google Scholar]
- Schrama, M. , Barmentlo, S. H. , Hunting, E. R. , van Logtestijn, R. S. P. , Vijver, M. G. , & van Bodegom, P. M. (2017). Pressure‐induced shifts in trophic linkages in a simplified aquatic food web. Frontiers in Environmental Science, 5, 1–10. 10.3389/fenvs.2017.00075 [DOI] [Google Scholar]
- Seibold, S. , Cadotte, M. W. , MacIvor, J. S. , Thorn, S. , & Müller, J. (2018). The necessity of multitrophic approaches in community ecology. Trends in Ecology & Evolution, 33, 754–764. 10.1016/j.tree.2018.07.001 [DOI] [PubMed] [Google Scholar]
- Shade, A. , Read, J. S. , Youngblut, N. D. , Fierer, N. , Knight, R. , Kratz, T. K. , Lottig, N. R. , Roden, E. E. , Stanley, E. H. , Stombaugh, J. , Whitaker, R. J. , Wu, C. H. , & McMahon, K. D. (2012). Lake microbial communities are resilient after a whole‐ecosystem disturbance. ISME Journal, 6. 10.1038/ismej.2012.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, A. , Kumar, V. , Shahzad, B. , Tanveer, M. , Sidhu, G. P. S. , Handa, N. , Kohli, S. K. , Yadav, P. , Bali, A. S. , Parihar, R. D. , Dar, O. I. , Singh, K. , Jasrotia, S. , Bakshi, P. , Ramakrishnan, M. , Kumar, S. , Bhardwaj, R. , & Thukral, A. K. (2019). Worldwide pesticide usage and its impacts on ecosystem. SN Applied Sciences, 1(11). 10.1007/s42452-019-1485-1 [DOI] [Google Scholar]
- Singh, D. N. , & Devid, K. (2000). Generalized relationships for estimating soil thermal resistivity. Experimental Thermal and Fluid Science, 22(3‐4), 133–143. 10.1016/S0894-1777(00)00020-0 [DOI] [Google Scholar]
- Spaak, J. W. , Baert, J. M. , Baird, D. J. , Eisenhauer, N. , Maltby, L. , Pomati, F. , Radchuk, V. , Rohr, J. R. , Van den Brink, P. J. , & De Laender, F. (2017). Shifts of community composition and population density substantially affect ecosystem function despite invariant richness. Ecology Letters, 20, 1315–1324. 10.1111/ele.12828 [DOI] [PubMed] [Google Scholar]
- Steffen, W. , Richardson, K. , Rockström, J. , Cornell, S. E. , Fetzer, I. , Bennett, E. M. , Biggs, R. , Carpenter, S. R. , de Vries, W. , de Wit, C. A. , Folke, C. , Gerten, D. , Heinke, J. , Mace, G. M. , Persson, L. M. , Ramanathan, V. , Reyers, B. , & Sörlin, S. (2015). Planetary boundaries: Guiding human development on a changing planet. Science, 347(6223), 10.1126/science.1259855 [DOI] [PubMed] [Google Scholar]
- Stelzer, R. S. , Heffernan, J. , & Likens, G. E. (2003). The influence of dissolved nutrients and particulate organic matter quality on microbial respiration and biomass in a forest stream. Freshwater Biology, 48, 1925–1937. 10.1046/j.1365-2427.2003.01141.x [DOI] [Google Scholar]
- Stillman, J. H. (2019). Heat waves, the new normal: Summertime temperature extremes will impact animals, ecosystems, and human communities. Physiology, 34, 86–100. 10.1152/physiol.00040.2018 [DOI] [PubMed] [Google Scholar]
- Strydom, S. , Murray, K. , Wilson, S. , Huntley, B. , Rule, M. , Heithaus, M. , Bessey, C. , Kendrick, G. A. , Burkholder, D. , Fraser, M. W. , & Zdunic, K. (2020). Too hot to handle: Unprecedented seagrass death driven by marine heatwave in a World Heritage Area. Global Change Biology, 26(6), 3525–3538. 10.1111/gcb.15065 [DOI] [PubMed] [Google Scholar]
- Suzuki, J. , Imamura, M. , Nakano, D. , Yamamoto, R. , & Fujita, M. (2018). Effects of water turbidity and different temperatures on oxidative stress in caddisfly (Stenopsyche marmorata) larvae. The Science of the Total Environment, 630, 1078–1085. 10.1016/j.scitotenv.2018.02.286 [DOI] [PubMed] [Google Scholar]
- Szymczak, A. , Ryo, M. , Roy, J. , & Rillig, M. C. (2020). Diversity of growth responses of soil saprobic fungi to recurring heat events. Frontiers in Microbiology, 11, 1–8. 10.3389/fmicb.2020.01326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabi, A. , Pennekamp, F. , Altermatt, F. , Alther, R. , Fronhofer, E. A. , Horgan, K. , Mächler, E. , Pontarp, M. , Petchey, O. L. , & Saavedra, S. (2020). Species multidimensional effects explain idiosyncratic responses of communities to environmental change. Nature Ecology & Evolution, 4, 1036–1043. 10.1038/s41559-020-1206-6 [DOI] [PubMed] [Google Scholar]
- Tasmin, R. , Shimasaki, Y. , Tsuyama, M. , Qiu, X. , Khalil, F. , Okino, N. , Yamada, N. , Fukuda, S. , Kang, I.‐J. , & Oshima, Y. (2014). Elevated water temperature reduces the acute toxicity of the widely used herbicide diuron to a green alga, Pseudokirchneriella subcapitata . Environmental Science and Pollution Research, 21, 1064–1070. 10.1007/s11356-013-1989-y [DOI] [PubMed] [Google Scholar]
- Thompson, R. M. , Beardall, J. , Beringer, J. , Grace, M. , & Sardina, P. (2013). Means and extremes: Building variability into community‐level climate change experiments. Ecology Letters, 16, 799–806. [DOI] [PubMed] [Google Scholar]
- Truhlar, A. M. , Dodd, J. A. , & Aldridge, D. C. (2014). Differential leaf‐litter processing by native (Gammarus pulex) and invasive (Dikerogammarus villosus) freshwater crustaceans under environmental extremes. Aquatic Conservation: Marine and Freshwater Ecosystems. 10.1002/aqc.2375 [DOI] [Google Scholar]
- Urrutia‐Cordero, P. , Ekvall, M. K. , Ratcovich, J. , Soares, M. , Wilken, S. , Zhang, H. , & Hansson, L.‐A. (2017). Phytoplankton diversity loss along a gradient of future warming and brownification in freshwater mesocosms. Freshwater Biology, 62, 1869–1878. 10.1111/fwb.13027 [DOI] [Google Scholar]
- Urrutia‐Cordero, P. , Zhang, H. , Chaguaceda, F. , Geng, H. , & Hansson, L. A. (2020). Climate warming and heat waves alter harmful cyanobacterial blooms along the benthic–pelagic interface. Ecology, 101, 1–12. 10.1002/ecy.3025 [DOI] [PubMed] [Google Scholar]
- Van den Brink, P. J. , Bracewell, S. A. , Bush, A. , Chariton, A. , Choung, C. B. , Compson, Z. G. , Dafforn, K. A. , Korbel, K. , Lapen, D. R. , Mayer‐Pinto, M. , Monk, W. A. , O'Brien, A. L. , Rideout, N. K. , Schäfer, R. B. , Sumon, K. A. , Verdonschot, R. C. M. , & Baird, D. J. (2019). Towards a general framework for the assessment of interactive effects of multiple stressors on aquatic ecosystems: Results from the Making Aquatic Ecosystems Great Again (MAEGA) workshop. Science of the Total Environment, 684, 722–726. 10.1016/j.scitotenv.2019.02.455 [DOI] [PubMed] [Google Scholar]
- Van de Perre, D. , Roessink, I. , Janssen, C. R. , Smolders, E. , De Laender, F. , Van den Brink, P. J. , & De Schamphelaere, K. (2018). The combined and interactive effects of zinc, temperature, and phosphorus on the structure and functioning of a freshwater community. Environmental Toxicology and Chemistry, 37(9), 2413–2427. 10.1002/etc.4201 [DOI] [PubMed] [Google Scholar]
- van Rooyen, M. , & Winterkorn, H. F. (1957). Theoretical and practical aspects of the thermal conductivity of soils and similar granular systems. Highway Research Board Bulletin 168—Fundam. Pract. Concepts Soil Freez. [Google Scholar]
- Vander Vorste, R. , Mermillod‐Blondin, F. , Hervant, F. , Mons, R. , & Datry, T. (2017). Gammarus pulex (Crustacea: Amphipoda) avoids increasing water temperature and intraspecific competition through vertical migration into the hyporheic zone: A mesocosm experiment. Aquatic Sciences. 10.1007/s00027-016-0478-z [DOI] [Google Scholar]
- Vasseur, D. A. , DeLong, J. P. , Gilbert, B. , Greig, H. S. , Harley, C. D. G. , McCann, K. S. , Savage, V. , Tunney, T. D. , & O'Connor, M. I. (2014). Increased temperature variation poses a greater risk to species than climate warming. Proceedings of the Royal Society B: Biological Sciences, 281(1779), 20132612. 10.1098/rspb.2013.2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velthuis, M. , de Senerpont Domis, L. N. , Frenken, T. , Stephan, S. , Kazanjian, G. , Aben, R. , Hilt, S. , Kosten, S. , van Donk, E. , & Van de Waal, D. B. (2017). Warming advances top‐down control and reduces producer biomass in a freshwater plankton community. Ecosphere, 8. 10.1002/ecs2.1651 [DOI] [Google Scholar]
- Verberk, W. C. , Buchwalter, D. B. , & Kefford, B. J. (2020). Energetics as a lens to understanding aquatic insect’s responses to changing temperature, dissolved oxygen and salinity regimes. Current Opinion in Insect Science. 10.1016/j.cois.2020.06.001 [DOI] [PubMed] [Google Scholar]
- Verheyen, J. , Delnat, V. , & Stoks, R. (2019). Increased daily temperature fluctuations overrule the ability of gradual thermal evolution to offset the increased pesticide toxicity under global warming. Environmental Science & Technology, 53(8), 4600–4608. 10.1021/acs.est.8b07166 [DOI] [PubMed] [Google Scholar]
- Verheyen, J. , & Stoks, R. (2019). Temperature variation makes an ectotherm more sensitive to global warming unless thermal evolution occurs. Journal of Animal Ecology, 88(4), 624–636. 10.1111/1365-2656.12946 [DOI] [PubMed] [Google Scholar]
- Verheyen, J. , & Stoks, R. (2020). Negative bioenergetic responses to pesticides in damselfly larvae are more likely when it is hotter and when temperatures fluctuate. Chemosphere, 243. 10.1016/j.chemosphere.2019.125369 [DOI] [PubMed] [Google Scholar]
- Warriner, T. R. , Semeniuk, C. A. D. , Pitcher, T. E. , & Love, O. P. (2020). Exposure to exogenous egg cortisol does not rescue juvenile Chinook salmon body size, condition, or survival from the effects of elevated water temperatures. Ecology and Evolution, 10(5), 2466–2477. 10.1002/ece3.6073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisse, T. , Gröschl, B. , & Bergkemper, V. (2016). Phytoplankton response to short‐term temperature and nutrient changes. Limnologica, 59, 78–89. 10.1016/j.limno.2016.05.002 [DOI] [Google Scholar]
- White, P. A. , Kalff, J. , Rasmussen, J. B. , & Gasol, H. M. (1991). The effect of temperature and algal biomass on bacterial production and specific growth rate in freshwater and marine habitats. Microbial Ecology, 21(1), 99–118. 10.1007/BF02539147 [DOI] [PubMed] [Google Scholar]
- Wickham, P. , Pandey, P. , Harter, T. , & Sandovol‐Solis, S. (2020). UV light and temperature induced fluridone degradation in water and sediment and potential transport into aquifer. Environmental Pollution, 265(Pt A), 114750. 10.1016/j.envpol.2020.114750 [DOI] [PubMed] [Google Scholar]
- Wilkinson, A. D. , Collier, C. J. , Flores, F. , Langlois, L. , Ralph, P. J. , & Negri, A. P. (2017). Combined effects of temperature and the herbicide diuron on Photosystem II activity of the tropical seagrass Halophila ovalis . Scientific Reports, 7, 1–11. 10.1038/srep45404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmer, P. , Stone, G. , & Johnston, I. (2000). Environmental physiology of animals. Blackwell Science. [Google Scholar]
- Woodward, G. , Bonada, N. , Brown, L. E. , Death, R. G. , Durance, I. , Gray, C. , Hladyz, S. , Ledger, M. E. , Milner, A. M. , Ormerod, S. J. , Thompson, R. M. , & Pawar, S. (2016). The effects of climatic fluctuations and extreme events on running water ecosystems. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1694), 20150274. 10.1098/rstb.2015.0274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolway, R. I. , Jennings, E. , Shatwell, T. , Golub, M. , Pierson, D. C. , & Maberly, S. C. (2021). Lake heatwaves under climate change. Nature, 589, 402–407. 10.1038/s41586-020-03119-1 [DOI] [PubMed] [Google Scholar]
- Yvon‐Durocher, G. , Jones, J. I. , Trimmer, M. , Woodward, G. , & Montoya, J. M. (2010). Warming alters the metabolic balance of ecosystems. Philosophical Transactions of the Royal Society B: Biological Sciences, 365(1549), 2117–2126. 10.1098/rstb.2010.0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvon‐Durocher, G. , Montoya, J. M. , Trimmer, M. , & Woodward, G. (2011). Warming alters the size spectrum and shifts the distribution of biomass in freshwater ecosystems. Global Change Biology, 17(4), 1681–1694. 10.1111/j.1365-2486.2010.02321.x [DOI] [Google Scholar]
- Zamarreňo, D. V. , May, E. , & Inkpen, R. (2009). Influence of environmental temperature on biocalcification by non‐sporing freshwater bacteria. Geomicrobiology Journal, 26. 10.1080/01490450902895962 [DOI] [Google Scholar]
- Zeng, J. , Zhao, D. , Yu, Z. , Huang, R. , & Wu, Q. L. (2014). Temperature responses of ammonia‐oxidizing prokaryotes in freshwater sediment microcosms. PLoS One, 9. 10.1371/journal.pone.0100653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P. , Grutters, B. M. C. , van Leeuwen, C. H. A. , Xu, J. , Petruzzella, A. , van den Berg, R. F. , & Bakker, E. S. (2019). Effects of rising temperature on the growth, stoichiometry, and palatability of aquatic plants. Frontiers in Plant Science, 9, 1–14. 10.3389/fpls.2018.01947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P. , van Leeuwen, C. H. A. , Bogers, D. , Poelman, M. , Xu, J. , & Bakker, E. S. (2020). Ectothermic omnivores increase herbivory in response to rising temperature. Oikos, 129. 10.1111/oik.07082 [DOI] [Google Scholar]
- Zhao, B. , Xing, P. , & Wu, Q. L. (2017). Microbes participated in macrophyte leaf litters decomposition in freshwater habitat. FEMS Microbiology Ecology, 93, 1–15. 10.1093/femsec/fix108 [DOI] [PubMed] [Google Scholar]
- Zhao, Q. , Van den Brink, P. J. , Carpentier, C. , Wang, Y. X. G. , Rodríguez‐Sánchez, P. , Xu, C. , Vollbrecht, S. , Gillissen, F. , Vollebregt, M. , Wang, S. , & De Laender, F. (2019). Horizontal and vertical diversity jointly shape food web stability against small and large perturbations. Ecology Letters, 22, 1152–1162. 10.1111/ele.13282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler, M. , Grupstra, C. G. B. , Barreto, M. M. , Eaton, M. , BaOmar, J. , Zubier, K. , Al‐Sofyani, A. , Turki, A. J. , Ormond, R. , & Voolstra, C. R. (2019). Coral bacterial community structure responds to environmental change in a host‐specific manner. Nature Communications, 10, 3092. 10.1038/s41467-019-10969-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohary, T. , Flaim, G. , & Sommer, U. (2021). Temperature and the size of freshwater phytoplankton. Hydrobiologia, 848(1), 143–155. 10.1007/s10750-020-04246-6 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Data Availability Statement
Data sharing not applicable to this article as no data sets were generated or analysed during the current study. However, all the articles used in this review are listed in [Link], [Link].


