Abstract
Permafrost thaw is a major potential feedback source to climate change as it can drive the increased release of greenhouse gases carbon dioxide (CO2) and methane (CH4). This carbon release from the decomposition of thawing soil organic material can be mitigated by increased net primary productivity (NPP) caused by warming, increasing atmospheric CO2, and plant community transition. However, the net effect on C storage also depends on how these plant community changes alter plant litter quantity, quality, and decomposition rates. Predicting decomposition rates based on litter quality remains challenging, but a promising new way forward is to incorporate measures of the energetic favorability to soil microbes of plant biomass decomposition. We asked how the variation in one such measure, the nominal oxidation state of carbon (NOSC), interacts with changing quantities of plant material inputs to influence the net C balance of a thawing permafrost peatland. We found: (1) Plant productivity (NPP) increased post‐thaw, but instead of contributing to increased standing biomass, it increased plant biomass turnover via increased litter inputs to soil; (2) Plant litter thermodynamic favorability (NOSC) and decomposition rate both increased post‐thaw, despite limited changes in bulk C:N ratios; (3) these increases caused the higher NPP to cycle more rapidly through both plants and soil, contributing to higher CO2 and CH4 fluxes from decomposition. Thus, the increased C‐storage expected from higher productivity was limited and the high global warming potential of CH4 contributed a net positive warming effect. Although post‐thaw peatlands are currently C sinks due to high NPP offsetting high CO2 release, this status is very sensitive to the plant community's litter input rate and quality. Integration of novel bioavailability metrics based on litter chemistry, including NOSC, into studies of ecosystem dynamics, is needed to improve the understanding of controls on arctic C stocks under continued ecosystem transition.
Keywords: C storage, decomposition, litter chemistry, NOSC, peat, permafrost thaw, plant community change, Stordalen Mire
Thawing permafrost in peat‐dominated ecosystems is a major potential feedback source to climate change as it can drive the increased release of greenhouse gases carbon dioxide (CO2) and methane (CH4). Although post‐thaw peatlands are currently C sinks because high plant productivity offsets high CO2 release from decomposition, this status is very sensitive to the plant community's litter input rate and quality. Integration of novel bioavailability metrics based on litter chemistry into studies of ecosystem dynamics, as introduced here, is needed to improve the understanding of controls on arctic C stocks under continued ecosystem transition.
1. INTRODUCTION
As ecosystems warm due to climate change, carbon (C) cycling processes are expected to alter, leading to changes in the amount of C stored in plants and soils with potential feedbacks to atmospheric C and the rate of warming. As temperatures warm and atmospheric CO2 increases, plant communities may become more productive leading to increased CO2 uptake; but warming temperatures may also increase CO2 release by speeding soil decomposition (Euskirchen et al., 2009; Herbert et al., 1999). The net effect of these opposing processes on global climate will depend on both environmental conditions and on how changes in plant physiology and community composition alter the quantity and quality of organic material supplied to the soil through litter (Saleska et al., 2002). This is especially true in Arctic ecosystems which are undergoing major transitions as permafrost thaws, making feedbacks to global climate change particularly difficult to predict. In addition to releasing historically unavailable frozen C pools for decomposition, increased temperatures and nutrient release from thawing permafrost may drive a surge of CO2 uptake as arctic plants increase photosynthetic rates and/or are replaced by more productive species which may increase the quantity of litter inputs to the soil (Bäckstrand et al., 2010; Euskirchen et al., 2009; Mekonnen et al., 2021; Varner et al., 2021). In some places, this rise in primary productivity can equal or even exceed the C released from the decomposition of previously frozen soil C over the growing season (Hicks Pries et al., 2013, 2015; Schuur et al., 2009). Conversely, shifts in plant community composition may also increase the rate at which C is returned to the atmosphere through decomposition if they lead to major changes in the quality of litter deposition (Hartley et al., 2012; Wild et al., 2014, 2016). Understanding of how differences in litter quality interact with changes in environmental and microbial factors to determine C storage versus release remains poorly constrained. While some of these changes may be short‐lived (e.g., nutrient release from recently thawed permafrost), others can be expected to persist over the long term (e.g., compositional shifts to more productive plant species; Mekonnen et al., 2019). Therefore, long‐term prediction and modeling of the implications of arctic warming for net C balance require that we improve the understanding of the mechanisms by which changes in plant community composition can simultaneously impact C uptake through differences in primary productivity, and C release from decomposition through differences in litter quantity and quality.
Permafrost thaw‐altered C uptake rates may result from changes in plant growth both above‐ and belowground. Warmer seasons can contribute to earlier snowmelt and later ground freeze that can lead to a long plant growing season (Richardson et al., 2013; Wolkovich et al., 2012). Thus, warmer temperatures have been found to lead to increased aboveground plant biomass in the Antarctic tundra (Day et al., 2008) as well as Arctic and Subarctic uplands (Camill et al., 2001; Hartley et al., 2012). Below ground, the volume of soil accessible to plant roots increases as the active layer deepens due to permafrost thaw (Iversen et al., 2015), which can result in increases to belowground biomass with thaw as roots grow without the limitations imposed by permafrost (Blume‐Werry et al., 2019). Collectively, these changes can result in increased above‐ and belowground biomass and thereby an increase in ecosystem C stock (Camill et al., 2001; Hartley et al., 2012).
Thaw‐induced changes in growth patterns may also result in differences in nutrient content and decomposability of plant litter. Since aboveground and belowground plant tissues have different nutrient compositions, shifts in ratios of aboveground to belowground biomass and litter production may result in different nutrient input rates (Zechmeister‐Boltenstern et al., 2015). Additionally, permafrost thaw can increase plant nutrient content through increased availability. Thaw releases biologically important amounts of plant‐available nitrogen both directly and through the decomposition of permafrost organic matter (Keuper et al., 2012). Increased N availability can be augmented by lengthening of the growing season which allows substantial growth and nutrient uptake by plants to continue belowground late into the season, even after peak aboveground growth (Blume‐Werry et al., 2015, 2019; Keuper et al., 2017), potentially impacting long‐term vegetation dynamics and carbon cycling (Riley et al., 2021). Higher levels of nutrients in biomass (living tissue) and litter in post‐thaw plant types can be expected to increase the decomposability of this material, thus increasing the rate of C and nutrient cycling after the thaw. However, nutrient content alone cannot predict the decomposability of plant litter, particularly where microbial decomposition is limited by factors such as oxygen and electron acceptor availability – as it is in wetlands. A more detailed chemical analysis of the decomposability of organic material is possible using high‐resolution mass spectrometry such as FT‐ICR MS which has been used previously to show differences in the composition of soil organic matter with permafrost thaw (Fudyma, Toyoda, et al., 2021; Hodgkins et al., 2014). Spectrometric techniques have great potential for understanding the decomposability of plant litter because they allow investigators to identify differences in bioavailability of material based on factors such as chemical complexity and the energetic favorability of decomposition for particular compounds (Fudyma, Toyoda, et al., 2021). For example, the nominal oxidation state of carbon (NOSC) of each compound in a sample can be used to indicate the potential thermodynamic energy yield on oxidation of the C of each molecule (Keiluweit et al., 2016; Wilson & Tfaily, 2018). The diversity of compounds present in a sample can be used as an indication of the diversity of microbial metabolic pathways that may be able to degrade it (AminiTabrizi et al., 2020). Thus, detailed chemistry measurements provide potential ways to predict rates of decomposition of litter that may vary with hydrology and oxygen availability.
Peat‐dominated permafrost systems are of particular concern when it comes to understanding the dynamics underlying changes in C storage because they hold large quantities of C (Hodgkins et al., 2014; Houghton, 2007), and because permafrost thaw can lead to peat inundation and wetland creation driving the substantial release of CH4 to the atmosphere (Bäckstrand et al., 2010; Hodgkins et al., 2014). The much higher radiative forcing caused by CH4 gas means that permafrost thaw can result in net positive climate forcing even when net C storage is increasing (Bäckstrand et al., 2010).
In peat‐dominated permafrost systems, permafrost‐underlain areas (palsas) are often relatively dry and characterized by arctic heath‐type communities dominated by dwarf shrubs, mosses, and small sedges (Malmer et al., 2005). Thaw leads to soil subsidence, which can result in the creation of wet areas, particularly sphagnum‐dominated bogs and/or sedge‐dominated fens (Malmer et al., 2005). These changes in plant community composition substantially alter the decomposition environment as well as the quantity and quality of litter inputs. Plant functional types (PFTs) found in wet fen areas formed after permafrost thaw (mostly graminoids) are generally more nutrient‐rich than functional types typical of permafrost‐underlain areas (dwarf shrubs, forbs, mosses, and lichen) or those in more ombrotrophic transitional areas (sphagnum moss; Fudyma et al., 2019; Malmer et al., 2005; Wang & Moore, 2014). These differences in nutrient content are expected to alter decomposition rates, and indeed, in northern climates, leaf litter decomposability has been found to be lowest in sphagnum mosses, higher in evergreen shrubs, graminoids, and deciduous shrubs, and highest in forbs (Dorrepaal et al., 2005). However, post‐permafrost thaw, litter can also decompose in an oxygen‐limited environment where the bioavailability of organic compounds may depend more strongly on electron acceptor availability than on nutrient content. Little is currently known about the role that litter chemical diversity and NOSC have on its susceptibility to decomposition.
Here, we assessed the impact of plant litter quality and quantity on C input to thawing permafrost soils in the Stordalen Mire ecosystem. This system contains soils at various stages of thaw that have been studied using biogeochemical, microbiological, ecosystem science, and modeling approaches for decades (Bäckstrand et al., 2010; Chang et al., 2019; Hodgkins et al., 2014; McCalley et al., 2014; Woodcroft et al., 2018). In spite of these efforts, the roles of changes in plant productivity and litter quantity and quality in determining net‐C balance remain under‐explored. It is essential to understand the mechanisms controlling C cycling in order to predict how further ecosystem changes may alter C storage. Such mechanistic understanding of climate‐critical ecosystems will allow more accurate modeling of how these ecosystems will feed back into the larger climate system with continued warming. Therefore, we tested the hypotheses that plant community changes post‐permafrost thaw results in (1) increased plant productivity (NPP) and litter inputs to the soil, and (2) increased quality (based on biochemical availability) and decomposability of plant litter inputs, leading to (3) decreased residence time of plant C limiting the net C storage effect of the increased productivity.
2. METHODS
2.1. Site description
Stordalen Mire is a well‐studied system for thawing permafrost located 10 km east of Abisko, Sweden at 68°21′20″N, 19°02′84″E, and is 363 m above sea level (with ecologically relevant microtopography across the Mire spanning several meters’ elevation). The site is managed by the Abisko Scientific Research Station and the Integrated Carbon Observation System. Within the Mire, there are three main habitats spanning a permafrost thaw gradient: permafrost underlain (palsa), initial/partial permafrost thaw (bog), and complete thaw (fen). Palsas consist of raised, permafrost underlain areas characterized by low shrubs (e.g., Betula nana, Empetrum nigrum, Andromeda polifolia, Vaccinium spp.), forbs (e.g., Rubus chamaemorus), graminoids (e.g., Eriophorum vaginatum), lichens, and drier mosses (including Sphagnum fuscum). Bogs are wetter, though still perched above the regional water table, low‐lying areas often still underlain by permafrost lenses characterized by more hydric species of Sphagnum (e.g., Sphagnum balticum) and small sedges (e.g., Eriophorum vaginatum, Carex rotundata). Fens are formed after complete permafrost thaw and collapse. They are characterized by standing or flowing water, and hydric Sphagnum and sedge species (e.g., Eriophorum angustifolium, Carex rotundata, Carex rostrata). The 15 main plant species found there belong to six plant function types: forbs (Comarum palustre, Rubus chamaemorus), evergreen shrubs (Andromeda polifolia, Empetrum nigrum, Vaccinium vitis‐idaea), deciduous shrubs (Betula nana, Vaccinium microcarpum, Vaccinium uliginosum), mosses (mainly Sphagnum sp.), graminoids (Carex canescens, C. rotundata, C. rostrata, Eriophorum angustifolium, Eriophorum vaginatum), and horsetails (Equisetum sp.). As of 2010, 49% of the area within Stordalen was made up of intact palsa, 37% was made up of partially thawed bog, 12% was made up of fully thawed fen (Bäckstrand et al., 2010). From 1970 to 2014, palsa and bog areas each shrank by 11% while fen areas expanded by 100% (Varner et al., 2021).
2.2. Plant standing biomass
Standing aboveground and belowground biomass for each habitat type was sampled in 2015, between July 13–24 which corresponded to the middle of the growing season (Olefeldt et al., 2012). Habitat types were determined by plant community composition and water‐level based on classifications by Malmer et al. (2005). Palsa sites were determined by the presence of woody shrubs, primarily E. nigrum and A. polifolia. Bog sites were primarily identified by Sphagnum spp. and E. vaginatum growth and vicinity to still intact palsa regions. Fen sites were identified by the amount of sedges such as E. angustifolium and water level. Two‐quarter meter quadrats (0.25 cm × 0.25 cm) were placed at each of five replicate locations at each of the three habitat types along the thaw gradient. Biomass was sampled by cutting out a block of peat around the edges of the quarter meter quadrat. Palsa plots were sampled to a depth of 15 cm and the entire peat block was removed from the hole. Plants were then separated from the peat block with roots and stems intact in order to allow for correct identification. For bog and fen sites, all living Sphagnum stems were removed first. Sedges were then extracted individually, including as much of the root system as could be removed by hand (up to 60 cm depth). All plants were identified based on aboveground structures, then belowground portions were separated and all samples were dried at 30°C to constant mass. Dried samples were weighed for total biomass calculations and then subsampled for C and nutrient analysis. These measurements were used for total standing biomass and nutrient composition at the peak of the growing season.
2.3. Leaf tissue and annual litter deposition
Leaf tissue and litter deposition were measured in the spring, summer, and fall of 2017 as follows. In early May 2017 (prior to full thaw), two quarter meter quadrats (0.25 cm × 0.25 cm) were marked at five different sites for each habitat type. To sample the nutrient status of litter remaining after the winter, standing litter was collected from each of the five individuals of each sedge species, and 20 fallen leaves were collected from the ground for each species of forb/shrub (except Empetrum nigrum and Andromeda polifolia because of the difficulty of finding the small evergreen leaves which disappear rapidly into the surrounding vegetation). The total number of individuals of each sedge species per quadrat was counted in order to calculate the total standing litter from the previous year.
To measure the total peak season leaf tissue, in late July, corresponding to peak growing season, quadrats were revisited and percent cover of litter and live leaf material were measured. Additionally, the total number of individuals (or branch tips for shrubs) of each species in each quadrat was measured. All living leaf material was collected and the number of leaves per branch or stem was counted from eight branches (for shrubs except Empetrum nigrum) or eight individuals (for forbs/sedges) from plants outside of the quadrats at each site. For Empetrum nigrum, eight branches were cut just below the last green leaves, the length of each of these branch tips was recorded, and the leaves were collected.
To measure annual litter inputs, in autumn, the sites were monitored so that each plant species was sampled as its leaves were senesced. This resulted in the following sampling dates: September 15 for Rubus chamaemorus, Betula nana, Vaccinium uliginosum, October 8 for Andromeda polifolia, October 20 for Eriophorum vaginatum, Vaccinium vitis‐idaea, Empetrum nigrum, Eriophorum angustifolium, Carex rotundata, Carex rostrata. Litter was collected from a subset of individuals or branches of each species from each plot such that the total mass of leaves per species per plot could be calculated using the summer measurements of the number of individuals or branches of each species per plot. This provided an estimate of the annual litter input from each plant species measured in mass per area based on the two quarter meter quadrats used for each site (with n = 5 sites per each of three habitat types). All plant material samples were dried at 60°C for 48 h or until they reached constant mass, then weighed and subsampled for C and nutrient analysis.
2.4. Sphagnum growth
Sphagnum growth was measured using bottle brushes placed in each of the five bog plots as soon as they were thawed enough in the spring of 2017. For each plot, eight bottle brushes were monitored monthly through September and replaced if they were overgrown. At each monitoring date, the median distance between the top of the wire and the top of the Sphagnum spp. was recorded. In October, eight brushes were removed and a 5 cm × 5 cm area of Sphagnum spp. was sampled. The lengths of the capitula of a representative sample were recorded, the stalks were separated from the capitula, and the stalks were cut to 3 cm in length. Stem and capitulum samples remained separate as they were dried at 60°C until they reached constant mass then weighed and subsampled for C and nutrient analysis.
2.5. Carbon and nutrient analyses
Dried plant tissues were individually ground into a homogeneous powder using a Wiley Mini‐Mill (3383‐L10, Thomas Wiley) then re‐dried in a large‐capacity drying oven (5EM, Cat # 51221093, "Precision" Jouan, Inc.) at 45°C for 48 h, ensuring material was fully dry without the risk of losing volatiles. The relative content of total carbon, nitrogen, and sulfur (CNS) was measured in triplicate by a VarioEL Cube Elemental Analyzer (ElementarAnalysensysteme GmbH) equipped with an autosampler for automated analysis of multiple samples. This analysis oxidizes the sample through flash combustion, proven best for finding the concentration of carbon, hydrogen, nitrogen, and sulfur (Krotz & Giazzi, 2011). The remaining material (200 mg, dry weight) was tested for other significant plant nutrients (P, K, Fe, Mg, Mn, Ca, Al, Zn). Before analysis, these samples were suspended in a strong acid solution (tracegrade metal HNO3 and ultrapure H2O2), and heated in a digestion block (DigiPREP MS, 50ml 48 Pos, SCP Science) followed by a microwave (Ethos 320TC, Milestone). In some cases (<20% of samples), 200 mg of sample was not available so acid dilutions were adjusted to the weight available (150, 100, or 50 mg). The nutrient content of samples was measured using inductively coupled plasma — optical emission spectrometry (Optima 8300DV, PerkinElmer) equipped with an autosampler. ICP‐OES was re‐calibrated using standards and blanks every 10 samples. ICP‐OES uses plasma to identify elements by their emission spectra and is known for its low detection limits, providing accurate detection of micro‐nutrients in small plant samples (Chaves et al., 2010).
2.6. Plant bioavailability analysis
We used Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FTICR‐MS) to assess differences in organic matter quality and decomposability of plant tissues (Wilson & Tfaily, 2018). Briefly, 0.5 g each of leaf, stem, and root tissues from two replicates of each of 13 representative plant samples from each habitat were collected from the vicinity of the main sampling sites and immediately flash frozen until analysis using water extraction and direct injection onto a 12 T Bruker ESI‐FTICR‐MS spectrometer operation in negative mode (see Wilson et al., 2021 for detailed methodology). For each sample, 96 individual scans were averaged and then internally calibrated using organic matter homologous series separated by 14 Da (i.e. CH2 groups). The mass measurement accuracy was <1 ppm for singly charged ions across a broad m/z range (i.e., 200 < m/z < 1200). Chemical formulas assignments were made using an in‐house built software program following the Compound Identification Algorithm, described by Kujawinski and Behn (2006) and modified by Minor et al. (2012) and based on the following “Golden Rules” criteria: S/N > 7, and mass measurement error <1 ppm, taking into consideration the presence of C, H, O, N, S, and P and excluding other elements. All observed ions in the spectra were singly charged based on the identification of 1.0034 Da spacing found between isotopic forms of the same molecule (e.g., between 12Cn and 12Cn‐1–13C1). Two technical replicates were collected for most samples and, when available, peaks present in either (or both) spectra were combined and the signal intensities were averaged for downstream analysis. In total, the dataset included 119,164 peaks of which 85,617 (72%) were assigned a molecular formula. The molecular formulae were then used to calculate the nominal oxidation state of carbon (NOSC = 4 − (4C + H − 3N – 2O + 5P − 2S)/C) of each compound (Keiluweit et al., 2016). This calculation provides insight into the thermodynamic energy yield on oxidation of the C and is another approach for measuring organic matter quality (Wilson & Tfaily, 2018).
2.7. Chemical transformation and network analysis
To identify potential decomposition pathways for each plant tissue and identify the close association between related metabolites within each plant tissue, the mass differences between FTICR–MS peaks within each sample were compared with the precise mass differences for commonly observed biochemical transformations adopted from (Breitling et al., 2006). These include a wide range of reactions that use organic compounds containing C, H, O, and/or N, S, and P as terminal electron acceptors. For example, a mass difference of 2.0156 Da represents a hydrogenation/dehydrogenation reaction, whereas a mass difference of 71.0371 Da indicates a reaction in which one alanine (C3H5NO) was lost. This approach is possible because of the ultra‐high mass accuracy of FTICR–MS. These possible transformations were used to create networks of connectivity based on potential decomposition pathways for each plant tissue sample using Cytoscape 3.8.1 and the MetaNetter 2 plug‐in (Shannon et al., 2003) as previously described in (AminiTabrizi et al., 2020; Fudyma, Chu, et al., 2021; Fudyma, Toyoda, et al., 2021). Network analysis was performed to calculate the network heterogeneity and the clustering coefficient of each sample. For plant tissues, network heterogeneity is defined as the coefficient of variation of the number of connections between nodes (Dong & Horvath, 2007). As such, it reflects the tendency of a network to contain hub nodes where a hub is a node with a number of connections that greatly exceeds the average number of connections for a node in that network. Thus, higher network heterogeneity signifies networks with large numbers of hub nodes that have many more connections than the majority of (non‐hub) nodes. In the case of plant metabolites, this signifies a system with a greater diversity of potential chemical transformation pathways (i.e., the same compound could be transformed through a multitude of reaction pathways). The clustering coefficient of a node is the number of triangles (3‐loops) that pass through this node, relative to the maximum number of 3‐loops that could pass through the node. High values indicate that nodes in a network have (on average) a large number of interconnections. Thus, high values indicate that metabolites in a network are structurally related. So, in the context of metabolomics, the clustering coefficient of a network signifies how closely related the plant metabolites are in a specific network, and network heterogeneity signifies the diversity of degradation pathways possible in that network.
2.8. Decomposition rates
To test the relationship between bioavailability indices and decomposition rates, litter from a characteristic species from each habitat (Table 1, E. vaginatum for palsa, Sphagnum spp. for bog, and E. angustifolium for fen) was added to incubations of peat in bottles with aerobic headspace from the same habitat in the following way:
TABLE 1.
Species found in each habitat grouped by plant functional type
| Palsa | Bog | Fen | |
|---|---|---|---|
| Deciduous shrub | Betula nana | Betula nana | |
| Vaccinium uliginosum | |||
| Evergreen shrub | Andromeda polifolia | ||
| Empetrum nigrum | Andromeda polifolia | ||
| Vaccinium microcarpum | |||
| Vaccinium vitis‐idaea | Vaccinium microcarpum | ||
| Forb | Comarum palustre | ||
| Rubus chamaemorus | Rubus chamaemorus | Rubus chamaemorus | |
| Graminoid | Carex canescens | ||
| Carex rostrata | Carex rostrata | ||
| Carex rotundata | Carex rotundata | ||
| Eriophorum angustifolium | Eriophorum angustifolium | ||
| Eriophorum vaginatum | Eriophorum vaginatum | Eriophorum vaginatum | |
| Moss | Polytrichum strictum | Polytrichum strictum | |
|
Sphagnum fuscum feather mosses |
|||
| Sphagnum spp. | Sphagnum spp. | ||
| Lichen | lichen | ||
| Horsetails | Equisetum spp. | ||
| Mean richness | 8.4 | 5.4 | 2.8 |
| Mean Shannon diversity | 1.54 | 0.95 | 0.86 |
| Mean Pilou's evenness | 0.73 | 0.57 | 0.85 |
Bold species names indicate habitats where that species is common (i.e., found in at least 3 plots). Richness, Shannon Diversity, and Pilou's evenness are mean values across five replicates for each site. Bold values are significantly different from both other sites at p < .05; italic values are different from each other but not the third site at p < .05; based on ANOVA with Tukey HSD.
Plants representative of each phase of permafrost thaw (Palsa: Eriophorum vaginatum, Bog: Sphagnum spp., Fen: Eriophorum angustifolium) were isotopically labeled by growing them in a high‐13CO2 atmosphere chamber (with a paired natural‐abundance control). Entire plants (including root systems and soil matrix) were collected intact for labeling from the field early in the arctic growing season (around June 20, 2015). Sedges were trimmed to stimulate the growth of fresh leaves and Sphagnum height was marked against stakes inserted in the pots to monitor stem elongation. The subsequent 13CO2 labeling period consisted of 8 weeks of growth from June 25 through August 11: The peak summer growth period for this site (Bäckstrand et al., 2010; Johansson & Linder, 1975). We regulated chamber air over the entire growing period to ensure high atmospheric 13C enrichment. After 8‐weeks, all new aboveground plant growth (identified as newly produced leaves for sedges and stem elongation beyond the initial marker for Sphagnum) was harvested and tested for isotopic enrichment using mass spectrometry at the University of Stockholm Stable Isotope Laboratory. The final 13C content of plant material prior to addition to incubations was 52.4 atom percent (at‐%) for E. vaginatum and E. angustifolium, and 22.9 at‐% for Sphagnum.
The 13C labeled litter was placed in soil incubations in order to track its decomposition. Incubations contained peat material collected from the same palsa, bog, and fen areas as the plants. Peat material was collected from the top 5 cm at each site, homogenized with minimal aeration, and refrigerated overnight. Each incubation was maintained with an aerobic headspace and contained an equal volume (38 ml) of peat at field saturation from one of the 3 habitat types. Incubations were either un‐amended (no‐litter control) or amended with the dried ground (to ensure homogeneity) aboveground litter (either unlabeled or labeled) from the corresponding habitat type at a ratio of 0.2 g litter per g dry‐peat equivalent (based on bulk density measures of wet peat samples). Each treatment (no‐litter, unlabeled litter, labeled litter) was replicated nine times for each habitat type resulting in a total of 81 incubations. Incubations were maintained at 10°C (which is within the normal range for surface soil temperatures in the summer) except during gas sampling. We sampled headspace air in each incubation jar for total concentration and isotopic composition of CO2 and CH4 on days 1, 3, 5, and every 3 days thereafter for 40 days after beginning the incubations, flushing with 400 ppm CO2 tank air (δ13C = −34‰) after each measurement.
Gas production from the litter was separated from that from soil using isotope partitioning based on the following:
where CO2tot is the total CO2 produced from labeled litter addition incubations, f tot is the 13CO2/total CO2 produced from labeled litter addition incubations, f unl is the 13CO2/total CO2 from unlabeled litter addition incubations, f litter is the 13C/total C of labeled litter, α litter is the 13CO2 produced from labeled litter incubation/f inc, is the isotopic ratio of natural abundance litter and soil incubation based on: Csoil which is the total C content of soil added to incubations, f soil is the 13C/total C of soil added to incubations, Clitter is the total C content of unlabeled litter added to incubations (note this is the same for labeled litter), f unlitter is the 13C/total C of unlabeled litter added to incubations.
This approach accounted for any differences in the fractionation of decomposition processes between soil alone versus soil plus litter addition incubations. This calculation was repeated for CH4 fluxes and combined with the CO2 fluxes to calculate total C loss. The rate of total C released from litter over the first 5 days of incubation was used to calculate decomposition rate and residence time for each litter type.
2.9. Flux measurements
Annual net ecosystem exchanges (NEE) of CO2 and CH4 from 2010 to 2014 were measured using transparent plexiglas autochambers (3 in each habitat for a total of 9) obtained from the Isogenie Database (Bolduc et al., 2020, IsoGenieDB: https://isogenie‐db.asc.ohio‐state.edu/). The combined radiative impact of CO2 and CH4 fluxes was calculated using a CH4 100 year global warming potential (GWP) of 28 (Boucher et al., 2009; IPCC, 2014). Thus, the sum of annual CO2 flux and 28× annual CH4 flux (both in gC m−2year−1) gave the total flux in terms of CO2 equivalents. Net ecosystem CO2 exchange was partitioned into photosynthetic and respiratory components during a July 2012 measurement campaign which quantified ecosystem respiration as the CO2 flux measured in the dark from shrouded autochambers. Dark flux measurements were paired with full light measurements and GPP was estimated as ecosystem respiration (CO2 flux to the atmosphere in the dark) minus NEE (CO2 flux to the atmosphere in full light, with uptake fluxes defined negative). All nine autochambers were distributed over the 0900–0300 time period. To get 24‐h estimates of potential GPP (assuming full light) and ecosystem respiration, missing hours were filled using linear interpolation between existing data points, then values were summed across all hours of a day.
2.10. Calculations and statistical analysis
Total photosynthetic tissue and annual litter production rates were calculated by first measuring the dry mass per leaf then multiplying by the number of leaves per branch, branches per plant, and plants per plot. Total C and nutrient content were calculated by multiplying the total mass by the measured percent C and nutrient content. For Sphagnum, only the capitulum was considered part of photosynthetic tissue since there is evidence that 80%–100% of net C gain takes place here (Laing et al., 2014; Wallén et al., 1988). Sphagnum litter was considered to consist of stem tissue only in a quantity equal to the amount of stem elongation measured over the course of the growing season.
C:nutrient data were not normally distributed, and had different distributions in 2015 versus 2017 collections. The significance of differences in C:nutrient ratios among habitat types and plant functional types (PFTs) were tested using ordinal logistic tests. For nutrient stoichiometry data, the Bonferroni correction was applied to the significance level to account for repeated testing of different nutrient data in the same samples. Tests were performed at the PFTs and habitat type levels, but only at the species level for Eriophorum vaginatum because most species were not abundant enough in more than one thaw stage for reliable statistical analysis. Tests were conducted using JMP Pro 14 (SAS corporation).
Site and PFT mean values for standing biomass and total C, and nutrient stocks were calculated in R studio running R version 3.6.0 (R Core Team, 2021; RStudio Team, 2021). Site means were normally distributed and the significance of differences among sites was tested using analysis of variance (ANOVA) and Tukey post hoc tests.
NOSC scores for each sample were calculated as the mean of the scores across all compounds present in a sample. Site‐level scores were calculated as biomass weighted means of the scores for each plant species and tissue type found in a habitat. Site‐level scores for network heterogeneity, clustering coefficients, and nutrient ratios were calculated in the same way. Mean values across sites were calculated using ANOVA with Tukey post hoc tests. These calculations were performed in R studio running R version 4.0.2 (R Core Team, 2021; RStudio Team, 2021).
3. RESULTS
3.1. Total carbon and nutrient stocks and flows across thaw gradient habitats
The diversity of plants contributing to aboveground and belowground nutrient stocks decreased with thaw, with mosses dominating the bog and graminoids dominating the fen (Figure 1, Table 1). Palsa habitats had the most diversity and evenness in PFT contributions to biomass, with four of five PFTs represented at each site sampled (Figure 1, Table 1). The total aboveground biomass and photosynthetic tissue were not significantly different across the stages of the thaw gradient, but total belowground biomass was significantly greater in the palsa than in the later thaw stages (ANOVA with Tukey HSD p < .05). Aboveground biomass in particular was highly variable between replicate sites of the same type due to high heterogeneity in plant species composition and growth density and the relatively small plot size.
FIGURE 1.
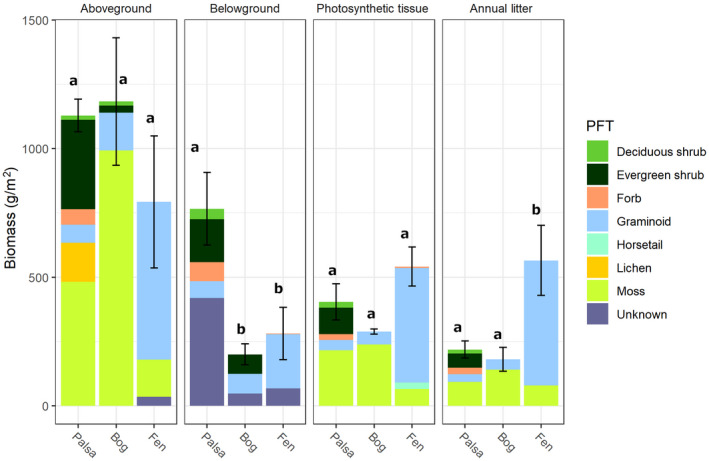
Plant community composition. Standing biomass (aboveground, belowground, photosynthetic tissue) or annual litter input at each state of permafrost thaw (palsa, bog, fen), by plant functional type. Letters indicate significant differences between different thaw stages in total pool size or flux (ANOVA, Tukey HSD p < .05). Error bars show the standard error of mean total values
In contrast to the standing biomass stocks, total litter production was significantly greater in the fen than in the palsa or bog (ANOVA with Tukey HSD p < .05; Figure 1). This pattern resulted in post‐thaw fen habitats having significantly greater total annual nutrient inputs from litter than did palsa or bog for C, N, and S, but not P or K (Figure 2, Table S1).
FIGURE 2.
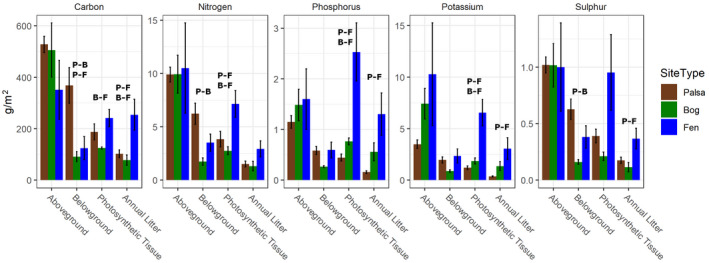
Total nutrient content in plant tissues. Total carbon (C), nitrogen (N), sulfur (S), phosphorus (P), and potassium (K) in standing aboveground biomass, belowground biomass, photosynthetic tissue, and annual litter inputs at each thaw stage (palsa, bog, fen). Letters indicate significant differences between different identified thaw stages (P, palsa; B, bog; F, fen) in total pool size or flux (ANOVA, Tukey HSD p < .05). Error bars are the standard error of the mean
Both peak season primary productivity and respiration increased with the transition from palsa or bog to fen, but the magnitude of increase was much greater for primary productivity (Figure 3a). There was very little difference in primary productivity between palsa and bog and a slight decrease in ecosystem respiration in the bog. These shifts resulted in total CO2 uptake and net carbon balance (NCB) increasing progressively with thaw, but total CH4 release also increased, resulting in a net increase in climate radiative forcing (Figure 3b, Table S1).
FIGURE 3.
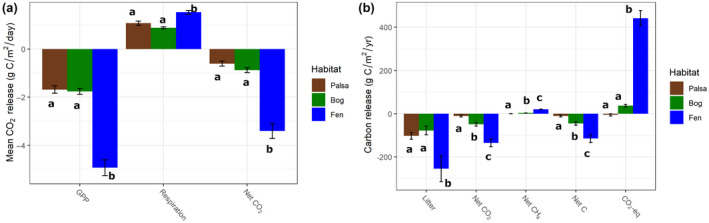
Net ecosystem fluxes. (a) Gross primary productivity, ecosystem respiration, and net CO2 flux across the thaw gradient (with positive values indicating net flux to the atmosphere) estimated as a 24‐h average from July measurements (peak growing season) under light and dark conditions in autochambers. (b) Flows of C to the atmosphere via litter, net CO2 exchange, net CH4 exchange, net C balance (from both CO2 and CH4), and in CO2‐equivalents (assuming a CH4 Global Warming Potential of 28)
3.2. Plant stoichiometry
Aboveground plant nutrient ratios were primarily driven by leaf nutrient content because most species at this site are mainly comprised of leaf tissue (i.e., lack woody stems), and even the shrubs are extremely small and therefore make up a relatively small proportion of biomass (Figures 1 and 4). Leaf nutrient content was generally lowest in mosses, graminoids, and evergreen shrubs, while forbs and deciduous shrubs generally had the highest nutrient content (p < .0125, Figure S1, Table S2). This pattern was consistent within each habitat such that the most nutrient‐rich plants were always forbs or deciduous shrubs where present (Figure S4, Tables S2–S5). Each PFT generally showed consistent leaf nutrient ratios in each habitat except for graminoid leaf phosphorus (Figure S1c) and potassium (Figure S1d) content which were lower in bog and fen than in palsa. In a number of cases, there were apparent differences within plant functional types between habitats that were not statistically significant (e.g., moss C:K in the palsa). This result may have been influenced by low sample numbers for species that were less common in a particular habitat.
FIGURE 4.
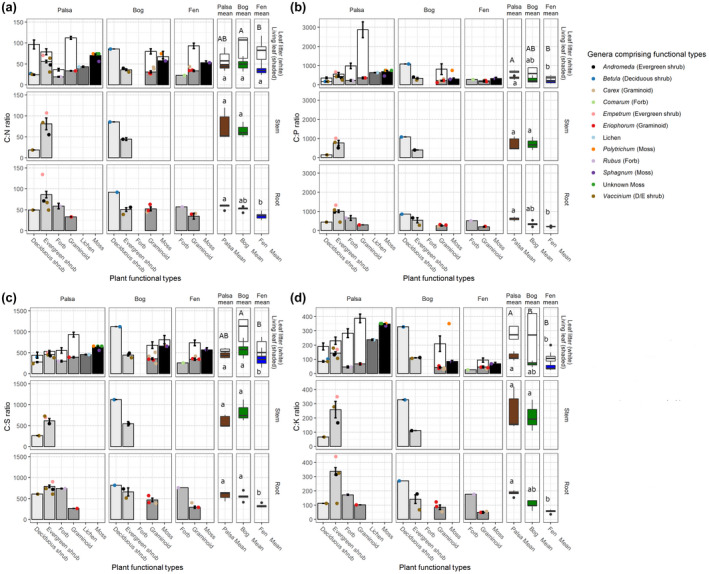
Plant nutrient ratios by functional type. Nutrient content of plant functional types (PFT), by tissue type (rows) and permafrost thaw stage (palsa, bog, fen) (columns) for (a) carbon:nitrogen (C:N), (b) carbon:phosphorus (C:P), c) carbon:sulfur (C:S), and (d) carbon:potassium (C:K). Shaded bars show nutrient concentrations in living tissues, while white bars show nutrient concentrations in senesced leaf tissues. Colored dots represent genus‐level means for the species within each PFT. Colored box plots represent biomass‐weighted mean nutrient concentrations for each stage of permafrost thaw. Statistical differences are based on the Dunn test or Wilcox test (where limited to two comparisons) controlled for false discovery rate with Benjamini‐Hochberg method using Q = 0.05
Although most plant species and functional types at Stordalen Mire were abundant in only one particular thaw stage, some were abundant enough in multiple stages to assess intraspecific variation between thaw stages and inter‐species variation within a PFT. For example, graminoids existed at all three stages in high abundance and Eriophorum vaginatum and Eriophorum angustifolium were each common in two of these (Figure 1, Table 1). We found no significant difference between C:nutrient ratios of E. angustifolium in the bog versus the fen (Figure S7). However, E. vaginatum was significantly more P and K rich in the bog than the palsa (Figure S8). Comparing these species to each other in the bog (the only thaw stage where they are both found), we found significantly higher P content in E. angustifolium than in E. vaginatum but no significant differences in other nutrient ratios (Figure S9).
For all PFTs, leaf tissue nutrient content sampled at peak growing season differed significantly from litter sampled at the end of the growing season and from a litter from the previous year sampled after overwintering (Figure S10). Litter from the prior year also showed some significant differences from the end of season litter, often a lower nutrient content than the end of season litter (Figure S10, Table S5). End of season litter C:nutrient ratios did not differ significantly between PFTs when assessed regardless of habitat, despite largely varied physical characteristics, though graminoids appeared to have the lowest nutrient concentrations (Figure S2, Table S3). When assessed within each habitat, graminoid litter was significantly lower in S and P than shrub litter and appeared lower in all nutrients than all other plants (Figure 4, Figure S3).
Root nutrient content did not differ significantly by habitat within PFTs. However, within each habitat, PFTs differed significantly from each other primarily in N and S content, with graminoids generally having the highest nutrient content, consistent with the aboveground differences (Figure 4, Figures S3 and S6, Table S4).
3.3. Plant decomposability indices
The potential decomposability of litter was measured using NOSC and chemical reaction networks. Networks showed variation in structure between plant species and tissue types with clustering coefficients ranging from 0.0935 (Eriophorum angustifolium leaf) to 0.1560 (Sphagnum plant) and network heterogeneity ranging from 0.477 (Empetrum nigrum stem) to 0.754 (Eriophorum angustifolium root; Figure 5). Clustering coefficients are based on the average number of interconnections between nodes and high values indicate that metabolites in a network are structurally related. Aggregation at the habitat level showed higher clustering of compounds (indicating structurally related metabolites) in the bog than other habitats, primarily driven by the high clustering of Sphagnum spp. compounds (Figure 5). Plant tissues from fen areas had the highest heterogeneity of networks (indicating many pathways to production or degradation of each compound), likely driven especially by the high heterogeneity of pathways (edges) connecting compounds found in samples of Eriophorum angustifolium roots (Figure 5). Such high heterogeneity of a network indicates many potential degradation pathways and implies that the tissue is likely to be more quickly and easily decomposed by a broad variety of microorganisms (AminiTabrizi et al., 2020). Habitat transition from palsa to bog, but not palsa to fen, resulted in a significant decrease in the average bioavailability of plant materials as measured by NOSC (Figure 5, Figure S11). This pattern was likely driven by the very low NOSC of Sphagnum spp., which is the major component of bog vegetation (Figures 1 and 5). NOSC and clustering coefficients appeared to be better predictors of leaf decomposition rate across three time‐points of decomposition (Figure 6).
FIGURE 5.
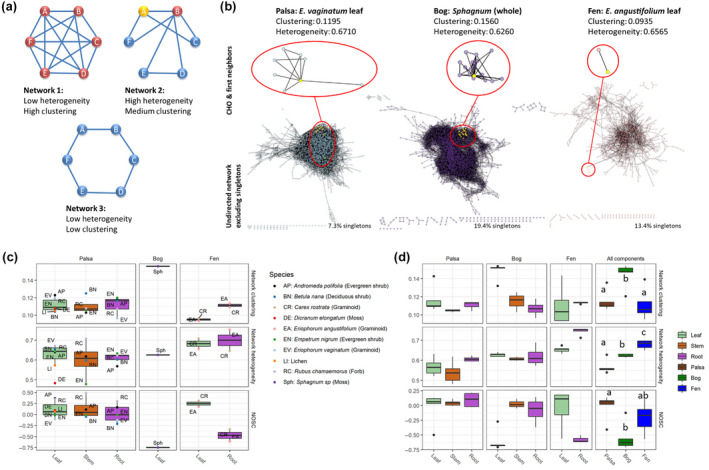
Plant chemistry network characteristics and NOSC. (a) Examples of networks with different clustering coefficients and heterogeneity, with nodes colored by the number of connecting edges (red = 5, yellow = 3, blue = 2). Network 1: all nodes are equally connected resulting in low heterogeneity and as connected as possible resulting in a high clustering coefficient. Network 2: Node B is a highly connected hub node while others have very few connections resulting in a high variance in connectivity across nodes (Dong & Horvath, 2007) which creates high heterogeneity and an intermediate clustering coefficient (since the average number of connections between nodes is neither maximal nor minimal). Network 3: All nodes are equally connected resulting in low heterogeneity, and the average number of connections between nodes is very low resulting in a low clustering coefficient. (b) Visualization of networks for litter used in incubations with peat from palsa (E. vaginatum leaf), bog (Sphagnum), and fen (E. angustifolium leaf). Zoomed‐in areas show sub‐networks for a single compound (CHO) and its first neighbors to illustrate differences in clustering and heterogeneity. (c) Differences in bioavailability of plant litter based on FT‐ICR MS analyses showing average nominal oxidation state of carbon (NOSC), network heterogeneity, and network clustering coefficient for each tissue type of each plant species grouped by permafrost thaw stage and tissue type. Both network heterogeneity and clustering coefficient have a possible range from 0 to 1, while NOSC may range from −4 to +4. (d) biomass‐weighted average NOSC, network heterogeneity, and clustering coefficient for each tissue type as well as total plant biomass (all components) at each thaw stage. Mass‐weighted indices were calculated based on biomass contribution per field‐sampling plot for each species. Variation within a thawing stage is driven by species composition differences between plots. Different letters indicate significant differences based on ANOVA and Tukey HSD tests with p < .05
FIGURE 6.
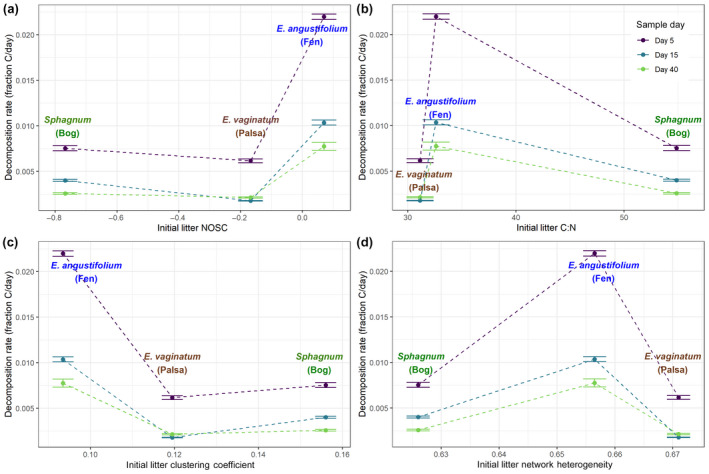
Impacts of plant litter chemistry on decomposition rate. Carbon decomposition rate (sum of CO2 and CH4) at days 5, 15, and 40 for plant litter characteristic of each stage of permafrost thaw. Litter was added to peat from the corresponding thaw stage and incubated with aerobic headspace. Decomposition rates are plotted versus (a) average nominal oxidation state of carbon of the litter, (b) clustering coefficient of compounds found in litter, (c) network heterogeneity of compounds found in litter, and (d) bulk carbon:nitrogen ratio of the litter. Error bars show standard error across replicate incubations for each time point: 9 for day 5, 6 for day 15, 3 for day 40. All values are significantly different at p < .05 based on ANOVA with Tukey HSD except for Fen:Day 40‐Bog:Day 5, Palsa:Day 40‐Palsa:Day 15, Bog:Day 40‐Palsa:Day 15, Bog:Day 40‐Bog:Day 15, Bog:Day 40‐Palsa:Day 40
4. DISCUSSION
We tested the hypotheses that permafrost thaw‐driven ecosystem succession in peat dominated systems would result in: (1) increased plant productivity and litter input to the soil, and (2) increased quality (based on biochemical availability) and decomposability of plant litter inputs, leading to (3) decreased residence time of decomposing plant C limiting the net C storage effect of the increased productivity. We found that, across the permafrost thaw gradient, C in aboveground standing biomass remained the same (albeit with large spatial heterogeneity) but declined in belowground biomass (Figures 1 and 2), indicating the importance of improving measurements of the relationship between aboveground and belowground plant C pools to benchmark models. Net carbon uptake (both CO2 and NCB) increased across the thaw gradient, but so did C inputs from litter (Figure 3), indicating decreasing residence time of C in plant communities. Summer CO2 respiration rates also increased, but to a much smaller degree than the increase in productivity (Figure 3). Meanwhile, CH4 production increased substantially, resulting in an increase in climate forcing despite the increase in C storage (Figure 3). Despite the limited change in habitat‐level C:nutrient ratios (Figure 4), total nutrient input rates increased (Figure 2), and plant compound bioavailability (based on NOSC) in leaf litter increased (Figure 5). The nominal oxidation state of carbon (NOSC), a measure of the energetic availability of a compound for microbial decomposition, and litter chemical complexity were promising predictors of the decomposition rate of litter (Figure 6). Taken together, we found that permafrost thaw led to an overall increase in the rate of C and nutrient cycling with higher productivity that produced more easily degradable litter driving higher decomposition rates (Figure 7). The results of this study highlight the importance of taking into account changes in plant chemistry when modeling decomposition rates across a plant community transition. Climate change projections indicate that this region is likely to become substantially warmer and wetter (Bintanja & Andry, 2017), which is promoting the conversion of both palsa and bog habitat to fen (Johansson et al., 2006; Malmer et al., 2005; Varner et al., 2021). The maintenance of these fen areas as C sinks will depend on the high NPP continuing to offset rising decomposition rates with increasing temperatures. Understanding the relationship between litter chemistry and decomposition will be essential to predicting the feedbacks between these areas and the global climate.
FIGURE 7.
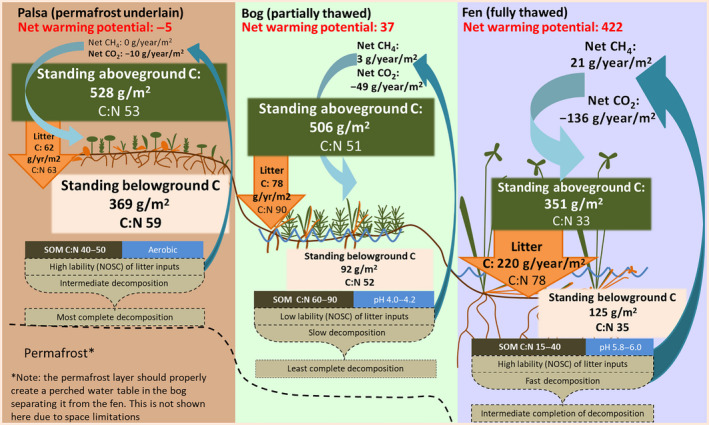
Overview of changing C stocks, flows, and key ecosystem properties across permafrost thaw gradient. While standing stocks of C in plant biomass decrease across the permafrost thaw gradient, the rate of C cycling increases. This is driven by increased uptake (NPP) and faster transfer of NPP to SOM via litter with thaw (as seen in fully thawed fen versus palsa or bog areas). High bioavailability of litter in the fully thawed fen results in rapid decomposition with a large contribution to CO2 and especially CH4 production but the high volume of litter input results in incomplete decomposition. In pre‐thaw (palsa) areas, high bioavailability but smaller volume of litter and aerobic conditions result in near‐complete decomposition of inputs but a smaller contribution to C emissions than in post‐thaw areas. In intermediate thaw (bog) areas, the intermediate volume of low bioavailability litter and anaerobic conditions result in slower and incomplete decomposition with intermediate C emissions. Overall, this leads to positive feedback from permafrost thaw to global warming
4.1. Plant C uptake and input to soil
Although NPP was higher in fens than palsas, aboveground standing biomass (C stock) was not, and belowground biomass was even lower (Figures 1 and 3). These results were surprising given that in arctic systems, climate warming is expected to result in active layer deepening (Iversen et al., 2015) and longer growing seasons (Richardson et al., 2013; Wolkovich et al., 2012). Factors, that in turn, are generally predicted to increase plant root growth (Blume‐Werry et al., 2019; Keuper et al., 2020) and standing biomass, as is occurring in upland areas near our site (Day et al., 2008; Hartley et al., 2012). While it is possible that our methodology underestimated deep sedge roots, our results were within the range of root measurements for sedges in the Fine Root Ecology Database (Figure S12; Iversen et al., 2021), suggesting the observation of decreased belowground biomass with thaw may not be an artifact. Our aboveground biomass findings may differ from previous research because we assessed the full plant community, with its changes in composition, whereas many studies reporting increased plant biomass and productivity are based on changes within the same species or plant functional type. Thus, our study shows the importance of including plant community transition, in addition to within‐community growth patterns, when quantifying changes in NPP, biomass, and soil C resulting from climate change. It will be important for models of ecosystem response to include such shifts in plant community composition in order to accurately predict warming‐induced changes to C cycling (Bouskill et al., 2020).
It should also be noted that the lack of significant differences in aboveground biomass and photosynthetic tissue was partly due to high heterogeneity in plant growth patterns at our site, particularly in the density and composition of aboveground biomass. We suggest that this heterogeneity is driven by differences in environmental conditions within habitat types. For instance, variation in water table depth may have led to large differences in the abundance and species of Sphagnum spp. and sedges found at bog and fen sites. Similarly, soil moisture differences in palsa sites may have driven variability in lichen versus woody plant cover. Because of the substantial differences in NPP and bioavailability between these different PFTs, further characterization of the drivers of this plant community variation would aid in accurate scaling of C cycling predictions.
Despite the heterogeneity, our system did show a shift towards increasing dominance of sedges post‐thaw which resulted in increased total nutrient contents in photosynthetic tissues and litter inputs (Figures 1, 2 and 4). This shift also resulted in an increase in the proportion of aboveground biomass and nutrients that entered the peat as litter on an annual basis. Although there was no increase in biomass or photosynthetic tissue, we found that net primary productivity during peak growing season in post‐thaw fen habitats was more than twice that in palsa or bog (Figure 3). This result coincided with a similarly large increase in aboveground litter inputs in the fen (Figure 1). Thus, we observed a faster rate of C movement through the plant community in fen areas. Such increases in C inputs through litter can lead to increased C storage if decomposition rates are slow, but they may also lead to increased C release through decomposition and thus an overall faster rate of C cycling. Previous research has shown that the fen is a larger C sink than the palsa or bog (Bäckstrand et al., 2010; Varner et al., 2021), but also that it produces more CH4. Our results also showed that ecosystem respiration at peak growing season was higher in the fen (Figure 3). The increased litter inputs likely fuel increasing decomposition rates, especially if they also correspond to increases in litter quality and bioavailability across the thaw gradient.
4.2. Plant nutrient content and decomposability
We expected that the changes in plant functional type with thaw would lead to higher nutrient concentrations in plant tissues. Prior studies have shown that fen species have higher N and P concentrations throughout the growing season compared to bog and palsa species (Wang & Moore, 2014), and that evergreen woody plants generally have lower nutrient concentrations in aboveground biomass than deciduous or herbaceous plants (Aerts, 1996). The differences between plant functional types at our site were consistent with this previous work (Figure 4, Figures S1 and S4). Graminoids had the most variable leaf nutrient concentrations, which were likely due to the interspecific differences between Eriophorum species and intraspecific differences in E. vaginatum between sites (Figure 4, Figures S7–S9). Because of the variation between functional types within a site, there were no significant differences in biomass‐weighted site‐level nutrient content between palsa and post‐thaw bog areas. However, there was a consistent, if slight, increase in leaf nutrient content in post‐thaw fen areas, particularly in K (Figure 4). This trend was more pronounced in root tissues where nutrient concentrations increased more strongly with the thaw, likely due to the shift toward graminoids which consistently showed the highest root nutrient concentrations (Figure 4, Figures S3 and S6). Differences in nutrient content between litter types were less than the differences in nutrient content between leaf types, with only forbs showing higher concentrations of N and P than other functional types (Figure 4, Figures S2 and S5), and only P and K content increasing significantly at the habitat level with thaw (Figure 4). These findings are generally consistent with the prediction of increased nutrient availability post‐permafrost thaw due to both direct releases from permafrost and from the decomposition of organic matter (Keuper et al., 2012). They are also consistent with findings of higher P and K in plant tissues in fens than bogs which are likely also related to nutrient availability (Wojtuń, 1994).
While plant material with high nutrient concentrations is generally expected to decompose more rapidly, nutrient content alone is not diagnostic for bioavailability. A more mechanistically linked prediction of bioavailability can be obtained through detailed chemical analysis using FTICR‐MS. This approach allows us to calculate the nominal oxidation state of carbon (NOSC) for each compound in a sample, which indicates the potential thermodynamic energy yield on oxidation of the C of each molecule (Keiluweit et al., 2016; Wilson & Tfaily, 2018). Higher NOSC values are thus indicative of higher energy yield through decomposition, which should be predictive of decomposition rate. Based on NOSC values, we found that the bioavailability of plant leaf material was substantially higher in palsa and fen areas than in bog, but root material in the fen was significantly less bioavailable than the other two habitats (Figure 5). The low leaf bioavailability in the bog was driven by the prevalence of Sphagnum spp. which is high in phenolic compounds that are very difficult to degrade (Fudyma et al., 2019; Wilson et al., 2021). In contrast, the sedge species found in the fen have a higher concentration of bioavailable amino sugars in their leaves (Wilson et al., 2021) which are likely to result in rapid decomposition. The fact that the contrasting low bioavailability of sedge roots decreased the overall bioavailability of biomass in the fen suggests that root litter may be important for C storage in these areas.
Another factor that can influence decomposition rates is the diversity of compounds available for microbial decomposition (AminiTabrizi et al., 2020). We used mass differences between the compounds in each sample to identify how they were related to each other in terms of the chemical transformations that could occur during decomposition. By creating networks of interactions, we were able to use network heterogeneity as an indication of the diversity of different types of transformations that could occur and clustering coefficients to indicate the similarity of these different transformations. We found high clustering of compound networks for Sphagnum spp. indicating that metabolites in the bog tended to be highly structurally related as compared to in the palsa or fen (Figure 5). In line with this result, high heterogeneity of transformations in sedge roots drove particularly high network heterogeneity in the fen, indicating that a more diverse set of reactions is taking place in these areas (Figure 5). These results indicate that plant material in the palsa and fen is more bioavailable (based on NOSC), more structurally heterogeneous (based on clustering coefficient), and represents a more diverse set of potential decomposition pathways (based on network heterogeneity) than the bog.
4.3. C residence time
The greater bioavailability of plant material predicted by aspects of its chemical composition was confirmed by our incubation experiment. We found that NOSC and network clustering coefficient were good predictors of initial decomposition rate (Figure 6). In contrast, the C:N ratio, which has more traditionally been used as a predictor of decomposability, did not show a consistent relationship with decomposition rate. These relationships were maintained throughout the first 40 days of decomposition which accounted for 16% (in the bog), 11% (in the palsa), and 46% (in the fen) of the C in the plant litter. This high decomposition rate of fen litter — more than three times that of litter from the palsa or bog — could result in dramatically different residence times. This idea is further supported by the simultaneous high summer NPP, litter deposition rate, and respiration rate which should lead to shorter C residence times in fen areas (Figure 3).
While these differences in residence time are only in reference to litter from a single‐species, the strong dominance of the selected species in the bog and fen means they are likely strong indicators of overall process rates in these areas. Data from the field shows that the degree of overlap between compounds found in plant material (including all major plant species) and in peat is least in the palsa (25%) as compared to the bog (47%) and fen (43%) and that palsa peat has the lowest NOSC values (Wilson et al., 2021). The low degree of overlap and low NOSC in the palsa indicate that the majority of compounds in the plant material are degraded and, therefore, no longer present in the peat. The greater degradation in the palsa is not surprising given that bog and fen areas are water‐logged and the resultant O2 limitation can lead to a build‐up of lignins and other less bioavailable compounds (Freeman et al., 2001; Hodgkins et al., 2014). The greater degradation in the palsa contrasts with the slower decomposition rates measured in the litter incubations but may be explained by the much more diverse and woody plant community which may not have been as well represented by a single species as bog and fen areas. Additionally, palsa and bog areas receive less than half the litter inputs of the fen on an annual basis. The high litter input rates in the fen may result in a greater overlap of litter and peat compounds than the other habitats simply because they exceed even a fast rate of decomposition. Indeed, the lack of a very high degree of overlap between plant and peat compounds in the fen despite very high litter input rates suggests that rapid decomposition must be occurring despite the water‐logged conditions (Figure 7). These results are consistent with previous research which found faster initial decomposition of litter in post‐thaw sites but that this fast decay rate reached an asymptote with more mass remaining than at pre‐thaw sites (Malhotra et al., 2018). A potential explanation is that the combination of high litter input rates and anaerobic conditions allow microbial communities to primarily decompose only the most easily degradable compounds, leaving less easily decomposed components of litter to accumulate.
The rapid initial decomposition rates in post‐thaw fen sites may be related to the high microbial diversity at these sites (Hough et al., 2020; Mondav et al., 2017) which can perform a more diverse set of reactions (Woodcroft et al., 2018) leading to the high diversity of transformations taking place in this habitat despite low diversity of litter inputs. Thus, the high bioavailability of plant litter inputs appears to drive faster decomposition rates by allowing for more functionally diverse microbial communities. If this mechanism is broadly generalizable it suggests that the chemical bioavailability of plant litter can be used to predict microbial diversity, decomposition rates, and C storage in peatlands undergoing plant community succession due to climate change. Although most global‐scale land models do not explicitly represent microbial activity or diversity (e.g., CLM5, Lawrence et al., 2019; ELM, Zhu et al., 2019), some site to regional‐scale models do (e.g., ecosys; Chang et al., 2019, 2020; Xu et al., 2015). For these more microbially sophisticated models, the observed relationships we found here would be useful to inform or benchmark ecosystem models aimed at predicting the impacts of global change.
5. CONCLUSIONS
The rapid, multifaceted changes taking place in arctic ecosystems make it difficult to accurately predict their interactions with the global climate. However, increasing mechanistic understanding of the controls on productivity and decomposition rates has the potential to increase the accuracy of land models. We showed how an emerging approach to studying litter bioavailability based on chemical complexity and energetics can improve understanding of the mechanisms underlying changing C release from thawing permafrost systems. The use of NOSC shows promise as a measure of potential decomposition rates of litter. However, further validation remains to determine if it can be used predictively across a broad range of litter types. If so, NOSC could be an integral parameter to include in models of C cycling, especially those aiming to predict the consequences of climate change. NOSC may also prove to be a useful addition to the conceptualization of the slow‐to‐fast plant economics spectrum. This framework predicts that plants in resource‐poor areas will be slower growing with lower productivity, thicker cuticles, lower specific leaf area, longer leaf life‐spans, and will produce lower quality litter (Reich, 2014; Wright et al., 2004). Meanwhile, plants with greater resource availability are expected to be faster growing with higher productivity, less waxy cuticles, higher specific leaf area, shorter leaf life spans and produce lower quality litter (Reich, 2014; Wright et al., 2004). In our thawing permafrost peatland study system, we saw that climate change drove a shift from lower resource availability palsas and bogs dominated by plants on the slower end of the plant economics spectrum toward higher resource availability fens dominated by plants on the faster end of the plant economics spectrum. We showed that this trait economics spectrum is propagated through to impacts on soil C, where litter at the fast end of the spectrum can fuel faster decomposition for a given set of environmental constraints. This fast‐slow spectrum both above and belowground was not strongly related to nutrient ratios, but was related to the energetic favorability of litter chemistry for decomposition (i.e. NOSC). This result from a system that is becoming wetter with warming is in line with the findings from alpine meadows which are becoming drier and seeing the reverse shift: transition from fast to slow plant traits results in slower decomposition (Harte et al., 2015). In both studies, a key finding is that litter chemical composition depended on the changing vegetation composition, which affects soil organic matter decomposition and thereby the net ecosystem carbon fluxes. Models that represent vegetation dynamics should consider these dynamics to accurately predict future ecosystem carbon cycling.
6. APPENDIX
6.1. The IsoGenie Coordinators
Steve Frolking (Department of Earth Sciences and Institute for the Study of Earth, University of New Hampshire, Oceans and Space, Durham, NH 03824, USA); Suzanne B. Hodgkins (Microbiology Department, The Ohio State University, OH 43210, USA; ORC iD: 0000‐0002‐0489‐9207); Carmody K. McCalley (Thomas H. Gosnell School of Life Sciences, Rochester Institute of Technology, Rochester, NY, 14623); William T. Cooper (Department of Chemistry & Biochemistry, Florida State University, Tallahassee, FL 32306, USA); Jeffrey P. Chanton (Earth Ocean and Atmospheric Sciences, Florida State University, Tallahassee, FL 32306, USA; ORC iD: 0000‐0002‐3303‐9708); Matthew B. Sullivan (Department of Environmental Science, University of Arizona, Tucson, AZ 85721, USA; The Ohio State University, Microbiology Department, OH 43210, USA; Center of Microbiome Science, The Ohio State University, OH 43210, USA; The Byrd Polar and Climate Research Center, The Ohio State University, OH 43210, USA; ORC iD: 0000‐0001‐8398‐8234); Gene W. Tyson (Centre for Microbiome Research, School of Biomedical Science, Translational Research Institute, Queensland University of Technology, Woolloongabba, QLD, Australia; ORC iD: 0000‐0001‐8559‐9427); Eoin L. Brodie (Climate and Ecosystem Sciences Division, Lawrence Berkeley Laboratory, Berkeley, CA 94720, USA; ORC iD: 0000‐0002‐8453‐8435); Ben B. Woodcroft (Centre for Microbiome Research, Queensland University of Technology, School of Biomedical Science, Translational Research Institute, Woolloongabba, QLD, Australia; ORC iD: 0000‐0003‐0670‐7480); Sky Dominguez (Ecology & Evolutionary Biology Department, University of Arizona, Tucson, AZ 85721, USA).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Study design: Moira Hough, S. Rose Vining, Emily Pickering Pedersen, Ellen Dorrepaal, Virginia Rich, Scott R. Saleska. Fieldwork: Moira Hough, S. Rose Vining, Emily Pickering Pedersen, Ryan Lawrence. Lab work: Moira Hough, S. Rose Vining, Samantha McCabe, Rachel M. Wilson, Malak M. Tfaily. Data analysis: Moira Hough, S. Rose Vining, Samantha McCabe, Gil Bohrer, Rachel M. Wilson, Ryan Lawrence. Funding acquisition & site development: Moira Hough, Virginia I. Rich, Scott R. Saleska, S. Rose Vining, Patrick M. Crill, Ruth K. Varner Steven J. Blazewicz, Malak M. Tfaily, William J. Riley, S. Rose Vining, The IsoGenie Coordinators. Writing & intellectual development: Moira Hough, Samantha McCabe, Kuang‐Yu Chang, William J. Riley, Scott R. Saleska, Virginia I. Rich, Malak M. Tfaily, Ellen Dorrepaal, Gil Bohrer.
Supporting information
Fig S1‐12
Table S1‐6
ACKNOWLEDGMENTS
This study was supported by the United States Department of Energy Office of Science Biological and Environmental Research Grants DE‐SC0021349 and DE‐AC02‐05CH11231, the Genomic Science Program of the United States Department of Energy Office of Biological and Environmental Research grants DE‐SC0010580 and DE‐SC0016440, and the National Science Foundation Biology Integration Institutes Program Award # 2022070. A National Science Foundation Graduate Research Fellowship supported MH. The Northern Ecosystems Research for Undergraduates program (NERU; National Science Foundation REU site EAR‐1063037) supported RL. A fellowship through the Howard Hughes Medical Institute (HHMI) grant number 5200642 supported SRV.
Hough, M. , McCabe, S. , Vining, S. R. , Pickering Pedersen, E. , Wilson, R. M. , Lawrence, R. , Chang, K.‐Y. , Bohrer, G. , The IsoGenie Coordinators , Riley, W. J. , Crill, P. M. , Varner, R. K. , Blazewicz, S. J. , Dorrepaal, E. , Tfaily, M. M. , Saleska, S. R. , & Rich, V. I. (2022). Coupling plant litter quantity to a novel metric for litter quality explains C storage changes in a thawing permafrost peatland. Global Change Biology, 28, 950–968. 10.1111/gcb.15970
Malak M. Tfaily, Scott R. Saleska, Virginia Rich should be considered joint senior authors.
†The IsoGenie Coordinators names are listed in the Appendix section.
Contributor Information
Moira Hough, houghm@email.arizona.edu.
Malak M. Tfaily, Email: tfaily@arizona.edu.
Scott R. Saleska, Email: saleska@arizona.edu.
Virginia I. Rich, Email: virginia.isabel.rich@gmail.com.
The IsoGenie Coordinators:
Steve Frolking, Suzanne B. Hodgkins, Carmody K. McCalley, William T. Cooper, Jeffrey P. Chanton, Matthew B. Sullivan, Gene W. Tyson, Eoin L. Brodie, Ben J. Woodcroft, and Sky Dominguez
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in the Isogenie database at https://isogenie‐db.asc.ohio‐state.edu/.
REFERENCES
- Aerts, R. (1996). nutrient resorption from senescing leaves of perennials: Are there general patterns? The Journal of Ecology, 84(4), 597–608. 10.2307/2261481 [DOI] [Google Scholar]
- AminiTabrizi, R. , Wilson, R. M. , Fudyma, J. D. , Hodgkins, S. B. , Heyman, H. M. , Rich, V. I. , Saleska, S. R. , Chanton, J. P. , & Tfaily, M. M. (2020). Controls on soil organic matter degradation and subsequent greenhouse gas emissions across a permafrost thaw gradient in Northern Sweden. Frontiers in Earth. 10.3389/feart.2020.557961 [DOI] [Google Scholar]
- Bäckstrand, K. , Crill, P. M. , Jackowicz‐Korczyñski, M. , Mastepanov, M. , Christensen, T. R. , & Bastviken, D. (2010). Annual carbon gas budget for a subarctic peatland, Northern Sweden. Biogeosciences, 7(1), 95–108. 10.5194/bg-7-95-2010 [DOI] [Google Scholar]
- Bintanja, R. , & Andry, O. (2017). Towards a rain‐dominated Arctic. Nature Climate Change, 7(4), 263–267. 10.1038/nclimate3240 [DOI] [Google Scholar]
- Blume‐Werry, G. , Milbau, A. , Teuber, L. M. , Johansson, M. , & Dorrepaal, E. (2019). Dwelling in the deep—strongly increased root growth and rooting depth enhance plant interactions with thawing permafrost soil. The New Phytologist, 223(3), 1328–1339. 10.1111/nph.15903 [DOI] [PubMed] [Google Scholar]
- Blume‐werry, G. , Wilson, S. D. , Kreyling, J. , & Milbau, A. (2015). The hidden season: Growing season is 50 % longer below than above ground along an arctic elevation gradient. The New Phytologist. [DOI] [PubMed] [Google Scholar]
- Bolduc, B. , Hodgkins, S. B. , Varner, R. K. , Crill, P. M. , McCalley, C. K. , Chanton, J. P. , Tyson, G. W. , Riley, W. J. , Palace, M. , Duhaime, M. B. , Hough, M. A. ; IsoGenie Project Coordinators, IsoGenie Project Team, A2A Project Team ; Saleska, S. R. , Sullivan, M. B. , & Rich, V. I. (2020). The IsoGenie database: An interdisciplinary data management solution for ecosystems biology and environmental research. PeerJ, 8, e9467. 10.7717/peerj.9467 [DOI] [Google Scholar]
- Boucher, O. , Friedlingstein, P. , Collins, B. , & Shine, K. P. (2009). The indirect global warming potential and global temperature change potential due to methane oxidation. Environmental Research Letters, 4(4), 044007. 10.1088/1748-9326/4/4/044007 [DOI] [Google Scholar]
- Bouskill, N. J. , Riley, W. J. , Zhu, Q. , Mekonnen, Z. A. , & Grant, R. F. (2020). Alaskan carbon‐climate feedbacks will be weaker than inferred from short‐term experiments. Nature Communications, 11(1), 5798. 10.1038/s41467-020-19574-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitling, R. , Ritchie, S. , Goodenowe, D. , Stewart, M. L. , & Barrett, M. P. (2006). Ab initio prediction of metabolic networks using Fourier transform mass spectrometry data. Metabolomics, 2(3), 155–164. 10.1007/s11306-006-0029-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camill, P. , Lynch, J. A. , Clark, J. S. , Brad Adams, J. , & Jordan, B. (2001). Changes in biomass, aboveground net primary production, and peat accumulation following permafrost thaw in the boreal peatlands of Manitoba, Canada. Ecosystems, 4(5), 461–478. 10.1007/s10021-001-0022-3 [DOI] [Google Scholar]
- Chang, K. , Riley, W. J. , Brodie, E. L. , McCalley, C. K. , Crill, P. M. , & Grant, R. F. (2019). Methane production pathway regulated proximally by substrate availability and distally by temperature in a high‐latitude mire complex. Journal of Geophysical Research. Biogeosciences, 124(10), 3057–3074. 10.1029/2019JG005355 [DOI] [Google Scholar]
- Chang, K.‐Y. , Riley, W. J. , Crill, P. M. , Grant, R. F. , & Saleska, S. R. (2020). Hysteretic temperature sensitivity of wetland CH4 fluxes explained by substrate availability and microbial activity. Biogeosciences, 17(22), 5849–5860. [Google Scholar]
- Chaves, E. S. , dos Santos, E. J. , Araujo, R. G. O. , Oliveira, J. V. , Frescura, V. L. A. , & Curtius, A. J. (2010). Metals and phosphorus determination in vegetable seeds used in the production of biodiesel by ICP OES and ICP‐MS. Microchemical Journal, 96(1), 71–76. 10.1016/j.microc.2010.01.021 [DOI] [Google Scholar]
- Day, T. A. , Ruhland, C. T. , & Xiong, F. S. (2008). Warming increases aboveground plant biomass and C stocks in vascular‐plant‐dominated Antarctic tundra. Global Change Biology, 14(8), 1827–1843. 10.1111/j.1365-2486.2008.01623.x [DOI] [Google Scholar]
- Dong, J. , & Horvath, S. (2007). Understanding network concepts in modules. BMC Systems Biology, 1, 24. 10.1186/1752-0509-1-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrepaal, E. , Cornelissen, J. H. C. C. , Aerts, R. , Wallén, B. , & van Logtestijn, R. S. P. P. (2005). Are growth forms consistent predictors of leaf litter quality and decomposability across peatlands along a latitudinal gradient? The Journal of Ecology, 93(4), 817–828. 10.1111/j.1365-2745.2005.01024.x [DOI] [Google Scholar]
- Euskirchen, E. S. , Mcguire, A. D. , Chapin, F. S. , Yi, S. , & Thompson, C. C. (2009). Changes in vegetation in northern Alaska under scenarios of climate change, 2003–2100: Implications for climate feedbacks. Ecological Applications, 19(4), 1022–1043. [DOI] [PubMed] [Google Scholar]
- Freeman, C. , Ostle, N. , & Kang, H. (2001). An enzymic “latch” on a global carbon store. Nature, 409(6817), 149. 10.1038/35051650 [DOI] [PubMed] [Google Scholar]
- Fudyma, J. D. , Chu, R. K. , Graf Grachet, N. , Stegen, J. C. , & Tfaily, M. M. (2021). Coupled biotic‐abiotic processes control biogeochemical cycling of dissolved organic matter in the Columbia river hyporheic zone. Frontiers in Water, 2. 10.3389/frwa.2020.574692 [DOI] [Google Scholar]
- Fudyma, J. D. , Lyon, J. , AminiTabrizi, R. , Gieschen, H. , Chu, R. K. , Hoyt, D. W. , Kyle, J. E. , Toyoda, J. , Tolic, N. , Heyman, H. M. , Hess, N. J. , Metz, T. O. , & Tfaily, M. M. (2019). Untargeted metabolomic profiling of Sphagnum fallax reveals novel antimicrobial metabolites. Plant Direct, 3(11), e00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudyma, J. D. , Toyoda, J. G. , Chu, R. K. , Weitz, K. K. , Heyman, H. M. , Eder, E. , Hoyt, D. W. , Gieschen, H. , Graf Grachet, N. , Wilson, R. M. , & Tfaily, M. M. (2021). Sequential abiotic‐biotic processes drive organic carbon transformation in peat bogs. Journal of Geophysical Research. Biogeosciences, 126(2). 10.1029/2020jg006079 [DOI] [Google Scholar]
- Harte, J. , Saleska, S. R. , & Levy, C. (2015). Convergent ecosystem responses to 23‐year ambient and manipulated warming link advancing snowmelt and shrub encroachment to transient and long‐term climate‐soil carbon feedback. Global Change Biology, 21(6), 2349–2356. 10.1111/gcb.12831 [DOI] [PubMed] [Google Scholar]
- Hartley, I. P. , Garnett, M. , Sommerkorn, M. , Hopkins, D. W. , Fletcher, B. J. , Sloan, V. L. , Phoenix, G. K. , & Wookey, P. A. (2012). A potential loss of carbon associated with greater plant growth in the European Arctic. Nature Climate Change, 2(12), 875–879. 10.1038/nclimate1575 [DOI] [Google Scholar]
- Herbert, D. A. , Rastetter, E. B. , Shaver, G. R. , & Ågren, G. I. (1999). Effects of plant growth characteristics on biogeochemistry and community composition in a changing climate. Ecosystems, 2(4), 367–382. 10.1007/s100219900086 [DOI] [Google Scholar]
- Hicks Pries, C. E. , Schuur, E. A. G. , & Crummer, K. G. (2013). Thawing permafrost increases old soil and autotrophic respiration in tundra: Partitioning ecosystem respiration using 13C and 14C. Global Change Biology, 19(2), 649–661. [DOI] [PubMed] [Google Scholar]
- Hicks Pries, C. E. , Van Logtestijn, R. S. P. , Schuur, E. A. G. , Natali, S. M. , Cornelissen, J. H. C. , Aerts, R. , & Dorrepaal, E. (2015). Decadal warming causes a consistent and persistent shift from heterotrophic to autotrophic respiration in contrasting permafrost ecosystems. Global Change Biology, 21(12), 4508–4519. 10.1111/gcb.13032 [DOI] [PubMed] [Google Scholar]
- Hodgkins, S. B. , Tfaily, M. M. , McCalley, C. K. , Logan, T. A. , Crill, P. M. , Saleska, S. R. , Rich, V. I. , & Chanton, J. P. (2014). Changes in peat chemistry associated with permafrost thaw increase greenhouse gas production. Proceedings of the National Academy of Sciences of the United States of America, 111(16), 5819–5824. 10.1073/pnas.1314641111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough, M. , McClure, A. , Bolduc, B. , Dorrepaal, E. , Saleska, S. , Klepac‐Ceraj, V. , & Rich, V. (2020). Biotic and environmental drivers of plant microbiomes across a permafrost thaw gradient. Frontiers in Microbiology, 11, 796. 10.3389/fmicb.2020.00796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton, R. A. (2007). Balancing the global carbon budget. Annual Review of Earth and Planetary Sciences, 35(1), 313–347. 10.1146/annurev.earth.35.031306.140057 [DOI] [Google Scholar]
- IPCC . (2014). Climate change 2014: Synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change. In Core Writing Team , Pachauri R. K., & Meyer L. A. (Eds.). IPCC, 151 pp. [Google Scholar]
- Iversen, C. M. , McCormack, M. L. , Baer, J. K. , Powell, A. S. , Chen, W. , Collins, C. , Fan, Y. , Fanin, N. , Freschet, G. T. , Guo, D. , Hogan, J. A. , Kou, L. , Laughlin, D. C. , Lavely, E. , Liese, R. , Lin, D. , Meier, I. C. , Montagnoli, A. , Roumet, C. , … Zadworny, M. (2021). Fine‐root ecology database (FRED): A global collection of root trait data with coincident site, vegetation, edaphic, and climatic data, version 3. Oak Ridge National Laboratory, TES SFA, U.S. Department of Energy. 10.25581/ornlsfa.014/1459186 [DOI] [Google Scholar]
- Iversen, C. M. , Sloan, V. L. , Sullivan, P. F. , Euskirchen, E. S. , McGuire, A. D. , Norby, R. J. , Walker, A. P. , Warren, J. M. , & Wullschleger, S. D. (2015). The unseen iceberg: Plant roots in arctic tundra. The New Phytologist, 205(1), 34–58. 10.1111/nph.13003 [DOI] [PubMed] [Google Scholar]
- Johansson, L. G. , & Linder, S. (1975). The seasonal pattern of photosynthesis of some vascular plants on a subarctic mire. In Wielgolaski F. E. (Ed.), Fennoscandian tundra ecosystems. Ecological studies (analysis and synthesis) (Vol. 16). Springer. 10.1007/978-3-642-80937-8_21 [DOI] [Google Scholar]
- Johansson, T. , Malmer, N. , Crill, P. M. , Friborg, T. , Åkerman, J. H. , Mastepanov, M. , & Christensen, T. R. (2006). Decadal vegetation changes in a northern peatland, greenhouse gas fluxes and net radiative forcing. Global Change Biology, 12(12), 2352–2369. 10.1111/j.1365-2486.2006.01267.x [DOI] [Google Scholar]
- Keiluweit, M. , Nico, P. S. , Kleber, M. , & Fendorf, S. (2016). Are oxygen limitations under recognized regulators of organic carbon turnover in upland soils? Biogeochemistry, 127(2–3), 157–171. 10.1007/s10533-015-0180-6 [DOI] [Google Scholar]
- Keuper, F. , Bodegom, P. M. , Dorrepaal, E. , Weedon, J. T. , Hal, J. , Logtestijn, R. S. P. , & Aerts, R. (2012). A frozen feast: Thawing permafrost increases plant‐available nitrogen in subarctic peatlands. Global Change Biology, 18(6), 1998–2007. 10.1111/j.1365-2486.2012.02663.x [DOI] [Google Scholar]
- Keuper, F. , Dorrepaal, E. , van Bodegom, P. M. , van Logtestijn, R. , Venhuizen, G. , van Hal, J. , & Aerts, R. (2017). Experimentally increased nutrient availability at the permafrost thaw front selectively enhances biomass production of deep‐rooting subarctic peatland species. Global Change Biology, 23(10), 4257–4266. 10.1111/gcb.13804 [DOI] [PubMed] [Google Scholar]
- Keuper, F. , Wild, B. , Kummu, M. , Beer, C. , Blume‐Werry, G. , Fontaine, S. , Gavazov, K. , Gentsch, N. , Guggenberger, G. , Hugelius, G. , Jalava, M. , Koven, C. , Krab, E. J. , Kuhry, P. , Monteux, S. , Richter, A. , Shahzad, T. , Weedon, J. T. , & Dorrepaal, E. (2020). Carbon loss from northern circumpolar permafrost soils amplified by rhizosphere priming. Nature Geoscience, 13(8), 560–565. 10.1038/s41561-020-0607-0 [DOI] [Google Scholar]
- Krotz, L. , & Giazzi, G. (2011). Achieving rapid, accurate, and reliable nitrogen determination in soils using dynamic flash combustion. Thermo Fisher Scientific. https://en.cnki.com.cn/Article_en/CJFDTotal‐SBKY201106011.htm [Google Scholar]
- Kujawinski, E. B. , & Behn, M. D. (2006). Automated analysis of electrospray ionization Fourier transform ion cyclotron resonance mass spectra of natural organic matter. Analytical Chemistry, 78(13), 4363–4373. 10.1021/ac0600306. PMID: 16808443 [DOI] [PubMed] [Google Scholar]
- Laing, C. G. , Granath, G. , Belyea, L. R. , Allton, K. E. , & Rydin, H. (2014). Tradeoffs and scaling of functional traits in Sphagnum as drivers of carbon cycling in peatlands. Oikos, 123, 817–828. 10.1111/oik.01061 [DOI] [Google Scholar]
- Lawrence, D. M. , Fisher, R. A. , Koven, C. D. , Oleson, K. W. , Swenson, S. C. , Bonan, G. , Collier, N. , Ghimire, B. , Kampenhout, L. , Kennedy, D. , Kluzek, E. , Lawrence, P. J. , Li, F. , Li, H. , Lombardozzi, D. , Riley, W. J. , Sacks, W. J. , Shi, M. , Vertenstein, M. , … Zeng, X. (2019). The community land model version 5: Description of new features, benchmarking, and impact of forcing uncertainty. Journal of Advances in Modeling Earth Systems, 11(12), 4245–4287. [Google Scholar]
- Malhotra, A. , Moore, T. R. , Limpens, J. , & Roulet, N. T. (2018). Post‐thaw variability in litter decomposition best explained by microtopography at an ice‐rich permafrost peatland. Arctic, Antarctic, and Alpine Research, 50(1), e1415622. 10.1080/15230430.2017.1415622 [DOI] [Google Scholar]
- Malmer, N. , Johansson, T. , Olsrud, M. , & Christensen, T. R. (2005). Vegetation, climatic changes and net carbon sequestration in a North‐Scandinavian subarctic mire over 30 years. Global Change Biology, 11(11), 1895–1909. 10.1111/j.1365-2486.2005.01042.x [DOI] [Google Scholar]
- McCalley, C. K. , Woodcroft, B. J. , Hodgkins, S. B. , Wehr, R. A. , Kim, E.‐H. , Mondav, R. , Crill, P. M. , Chanton, J. P. , Rich, V. I. , Tyson, G. W. , & Saleska, S. R. (2014). Methane dynamics regulated by microbial community response to permafrost thaw. Nature, 514(7523), 478–481. [DOI] [PubMed] [Google Scholar]
- Mekonnen, Z. A. , Riley, W. J. , Berner, L. T. , Bouskill, N. J. , Torn, M. S. , Iwahana, G. , Breen, A. L. , Myers‐Smith, I. H. , Criado, M. G. , Liu, Y. , Euskirchen, E. S. , Goetz, S. J. , Mack, M. C. , & Grant, R. F. (2021). Arctic tundra shrubification: A review of mechanisms and impacts on ecosystem carbon balance. Environmental Research Letters, 16(5), 053001. [Google Scholar]
- Mekonnen, Z. A. , Riley, W. J. , Randerson, J. T. , Grant, R. F. , & Rogers, B. M. (2019). Expansion of high‐latitude deciduous forests driven by interactions between climate warming and fire. Nature Plants, 5(9), 952–958. 10.1038/s41477-019-0495-8 [DOI] [PubMed] [Google Scholar]
- Minor, E. C. , Steinbring, C. J. , Longnecker, K. , & Kujawinski, E. B. (2012). Characterization of dissolved organic matter in Lake Superior and its watershed using ultrahigh resolution mass spectrometry. Organic Geochemistry, 1(43), 1–11. 10.1016/j.orggeochem.2011.11.007 [DOI] [Google Scholar]
- Mondav, R. , McCalley, C. K. , Hodgkins, S. B. , Frolking, S. , Saleska, S. R. , Rich, V. I. , Chanton, J. P. , & Crill, P. M. (2017). Microbial network, phylogenetic diversity and community membership in the active layer across a permafrost thaw gradient. Environmental Microbiology, 19(8), 3201–3218. 10.1111/1462-2920.13809 [DOI] [PubMed] [Google Scholar]
- Olefeldt, D. , Roulet, N. T. , Bergeron, O. , Crill, P. , Bäckstrand, K. , & Christensen, T. R. (2012). Net carbon accumulation of a high‐latitude permafrost palsa mire similar to permafrost‐free peatlands. Geophysical Research Letters, 39(3), 10.1029/2011GL050355 [DOI] [Google Scholar]
- R Core Team . (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R‐project.org/ [Google Scholar]
- RStudio Team . (2021). RStudio: Integrated development environment for R. RStudio, PBC. http://www.rstudio.com/ [Google Scholar]
- Reich, P. B. (2014). The world‐wide “fast‐slow” plant economics spectrum: A traits manifesto. The Journal of Ecology, 102(2), 275–301. 10.1111/1365-2745.12211 [DOI] [Google Scholar]
- Richardson, A. D. , Keenan, T. F. , Migliavacca, M. , Ryu, Y. , Sonnentag, O. , & Toomey, M. (2013). Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agricultural and Forest Meteorology, 169, 156–173. 10.1016/j.agrformet.2012.09.012 [DOI] [Google Scholar]
- Riley, W. J. , Mekonnen, Z. A. , Tang, J. Y. , Zhu, Q. , Bouskill, N. J. , & Grant, R. F. (2021). Non‐growing season plant nutrient uptake controls Arctic tundra vegetation composition under future climate. Environmental Research Letters, 16(7). 10.1088/1748-9326/ac0e63 [DOI] [Google Scholar]
- Saleska, S. R. , Shaw, M. R. , Fischer, M. L. , Dunne, J. A. , Still, C. J. , Holman, M. L. , & Harte, J. (2002). Plant community composition mediates both large transient decline and predicted long‐term recovery of soil carbon under climate warming. Global Biogeochemical Cycles, 16(4), 3‐1–3‐18. 10.1029/2001GB001573 [DOI] [Google Scholar]
- Schuur, E. A. G. , Vogel, J. G. , Crummer, K. G. , Lee, H. , Sickman, J. O. , & Osterkamp, T. E. (2009). The effect of permafrost thaw on old carbon release and net carbon exchange from tundra. Nature, 459(7246), 556–559. [DOI] [PubMed] [Google Scholar]
- Shannon, P. , Markiel, A. , Ozier, O. , Baliga, N. S. , Wang, J. T. , Ramage, D. , Amin, N. , Schwikowski, B. , & Ideker, T. (2003). Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Research, 13(11), 2498–2504. 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner, R. K. , Crill, P. M. , Frolking, S. , McCalley, C. K. , Burke, S. A. , Elizabeth, C. J. P. H. , Saleska, S. , & Palace, M. W. (2021). Permafrost thaw driven changes in hydrology and vegetation cover increase trace gas emissions and climate forcing in Stordalen Mire from 1970–2014. Philosophical Transactions of the Royal Society of London. Series A: Mathematical and Physical Sciences. 10.1098/rsta.2021.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallén, B. , Falkengren‐Grerup, U. , & Malmer, N. (1988). Biomass, productivity and relative rate of photosynthesis of Sphagnum at different water levels on a South Swedish peat bog. Ecography, 11, 70–76. 10.1111/j.1600-0587.1988.tb00782.x [DOI] [Google Scholar]
- Wang, M. , & Moore, T. R. (2014). Carbon, nitrogen, phosphorus, and potassium stoichiometry in an ombrotrophic peatland reflects plant functional type. Ecosystems, 673–684. 10.1007/s10021-014-9752-x [DOI] [Google Scholar]
- Wild, B. , Gentsch, N. , Čapek, P. , Diáková, K. , Alves, R. J. E. , Bárta, J. , Gittel, A. , Hugelius, G. , Knoltsch, A. , Kuhry, P. , Lashchinskiy, N. , Mikutta, R. , Palmtag, J. , Schleper, C. , Schnecker, J. , Shibistova, O. , Takriti, M. , Torsvik, V. L. , Urich, T. , … Richter, A. (2016). Plant‐derived compounds stimulate the decomposition of organic matter in arctic permafrost soils. Scientific Reports, 6(February), 25607. 10.1038/srep25607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild, B. , Schnecker, J. , Alves, R. J. E. , Barsukov, P. , Bárta, J. , Čapek, P. , Gentsch, N. , Gittel, A. , Guggenberger, G. , Lashchinskiy, N. , Mikutta, R. , Rusalimova, O. , Šantrůčková, H. , Shibistova, O. , Urich, T. , Watzka, M. , Zrazhevskaya, G. , & Richter, A. (2014). Input of easily available organic C and N stimulates microbial decomposition of soil organic matter in arctic permafrost soil. Soil Biology & Biochemistry, 75, 143–151. 10.1016/j.soilbio.2014.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, R. M. , Hough, M. A. , Verbeke, B. A. , Hodgkins, S. B. , Coordinators, T. I. G. , Chanton, J. P. , Saleska, S. R. , Rich, V. I. , & Tfaily, M. M. (2021). Plant organic matter inputs exert a strong control on soil organic matter decomposition in a thawing permafrost peatland. Science of the Total Environment, in review. 10.1101/2021.10.20.465126 [DOI] [PubMed] [Google Scholar]
- Wilson, R. M. , & Tfaily, M. M. (2018). Advanced molecular techniques provide new rigorous tools for characterizing organic matter quality in complex systems. Journal of Geophysical Research. Biogeosciences, 123(6), 1790–1795. 10.1029/2018JG004525 [DOI] [Google Scholar]
- Wojtuń, B. , & Wojtun, B. (1994). Element contents of sphagnum mosses of peat bogs of lower Silesia (Poland). The Bryologist, 97(3), 284–295. 10.2307/3243461 [DOI] [Google Scholar]
- Wolkovich, E. M. , Cook, B. I. , Allen, J. M. , Crimmins, T. M. , Betancourt, J. L. , Travers, S. E. , Pau, S. , Regetz, J. , Davies, T. J. , Kraft, N. J. B. , Ault, T. R. , Bolmgren, K. , Mazer, S. J. , McCabe, G. J. , McGill, B. J. , Parmesan, C. , Salamin, N. , Schwartz, M. D. , & Cleland, E. E. (2012). Warming experiments underpredict plant phenological responses to climate change. Nature, 485(7399), 494–497. [DOI] [PubMed] [Google Scholar]
- Woodcroft, B. J. , Singleton, C. M. , Boyd, J. A. , Evans, P. N. , Hoelzle, R. D. , Lamberton, T. O. , McCalley, C. K. , Hodgkins, S. B. , Wilson, R. M. , Chanton, J. P. , Crill, P. M. , Saleska, S. R. , Rich, V. I. , & Tyson, G. W. (2018). Genome‐centric metagenomic insights into microbial carbon processing across a permafrost thaw gradient. Nature. [DOI] [PubMed] [Google Scholar]
- Wright, I. J. , Reich, P. B. , Westoby, M. , Ackerly, D. D. , Baruch, Z. , Bongers, F. , Cavender‐Bares, J. , Chapin, T. , Cornelissen, J. H. C. , Diemer, M. , Flexas, J. , Garnier, E. , Groom, P. K. , Gulias, J. , Hikosaka, K. , Lamont, B. B. , Lee, T. , Lee, W. , Lusk, C. , … Villar, R. (2004). The worldwide leaf economics spectrum. Nature, 428(6985), 821–827. [DOI] [PubMed] [Google Scholar]
- Xu, X. , Elias, D. A. , Graham, D. E. , Phelps, T. J. , Carroll, S. L. , Wullschleger, S. D. , & Thornton, P. E. (2015). A microbial functional group‐based module for simulating methane production and consumption: Application to an incubated permafrost soil. Journal of Geophysical Research. Biogeosciences, 120(7), 1315–1333. 10.1002/2015JG002935 [DOI] [Google Scholar]
- Zechmeister‐Boltenstern, S. , Keiblinger, K. M. , Mooshammer, M. , Penuelas, J. , Richter, A. , Sardans, J. , & Wanek, W. (2015). The application of ecological stoichiometry to plant‐microbial‐soil organic matter transformation. Ecological Monographs, 85(2), 133–155. [Google Scholar]
- Zhu, Q. , Riley, W. J. , Tang, J. , Collier, N. , Hoffman, F. M. , Yang, X. , & Bisht, G. (2019). Representing nitrogen, phosphorus, and carbon interactions in the E3SM land model: Development and global benchmarking. Journal of Advances in Modeling Earth Systems, 11(7), 2238–2258. 10.1029/2018MS001571 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐12
Table S1‐6
Data Availability Statement
The data that support the findings of this study are openly available in the Isogenie database at https://isogenie‐db.asc.ohio‐state.edu/.


