Short abstract
See related editorial by Lundebjerg et al.
INTRODUCTION
Progress in drug development is giving rise to optimism that a disease‐modifying therapy (DMT) for Alzheimer's disease (AD) may become available. 1 However, healthcare systems seem to lack the capacity to provide patients with timely access to treatment. 2
In Italy, patients' evaluation in memory clinics, so‐called “Centres for Dementia and Cognitive Decline” (CDCD), is standardized with the use of neuropsychological tests and neuroimaging. 3 , 4 In Italy, there are more than 500 CDCDs, which are coordinated by either neurologists or geriatricians, leading to different patients' characteristics.
Very few studies have investigated the epidemiology of eligibility for a DMT in AD. 5 Recently, Canevelli et al. claimed that a marginal proportion (<1%) of patients with cognitive disorders attending “real‐world” geriatric services would benefit from DMT such as aducanumab. 6 These numbers differ from the RAND Corporation simulation, which estimated that the proportion of potential eligible patients was around one third of an MCI population. 2
The recent U.S. FDA's approval of aducanumab 7 has elicited a heated debate on clinical effectiveness and generalizability of findings arguing for caution before planning reorganization of care resources. 8 In November 2020, the FDA Drugs Advisory Committee voted that aducanumab's trial data did not provide sufficient efficacy evidence. Afterward, FDA approved the drug arguing that amyloid reduction constituted a suitable surrogate for clinical benefit. 9 Remarkably, the initial labeling covering anyone with AD was updated to patients with MCI or mild dementia due to AD. 10
Phase 3 studies on treatments with anti‐amyloid monoclonal antibodies require rigid eligibility criteria to provide homogeneous sample populations and minimize safety/tolerability concerns. Notwithstanding, it is a matter of controversy whether these stringent criteria adequately apply to real‐world populations.
METHODS
To characterize the proportion of patients potentially eligible to aducanumab in the “real world,” the research criteria were applied to patients referred to the neurological CDCD unit of a University Hospital in Brescia (Italy) across a 5‐year interval (2015–2019). Patients underwent a standardized assessment, including cognitive evaluation, neuroimaging, and either amyloid positron emission tomography (PET) or cerebrospinal fluid (CSF). The local ethics committee authorized data collection as part of a clinical registry. Written informed consent was obtained from patients or proxies.
RESULTS
A total of 1143 patients at their first visit were consecutively recruited (mean ± SD age = 74.0 ± 8.6; education = 7.4 ± 2.7; Mini‐Mental State Examination [MMSE] = 22.6 ± 4.2; months to diagnosis = 8.0 ± 5.1). Six hundred and twenty‐two patients were excluded because of not meeting the prespecified criteria (e.g., diagnosis of non‐AD dementia, MMSE score < 24, and/or evident functional impairment), whereas 98 because of the absence of objective cognitive impairment. Thus, the target population included 423 (37.0%) patients with mild congitive impairment (MCI). As shown in Figure 1, 121 of 423 (28.6%) carried significant laboratory abnormalities or clinically relevant comorbidities; 57 (19%) patients showed significant cerebrovascular abnormalities on magnetic resonance imaging (MRI), leaving a target population of 245 (60%) patients. Of these, 178 underwent either amyloid PET or CSF while 37 refused further evaluation and 30 were lost to follow‐up. Finally, 138 patients (mean age = 68.2 ± 6.5; education = 8.0 ± 3.2; MMSE = 26.1 ± 0.9) showed positive amyloid status. The proportion of patients eligible to aducanumab was 12.0% of the total sample of patients referred to the CDCD, that is, 32.6% of the target MCI population (Figure 1).
FIGURE 1.
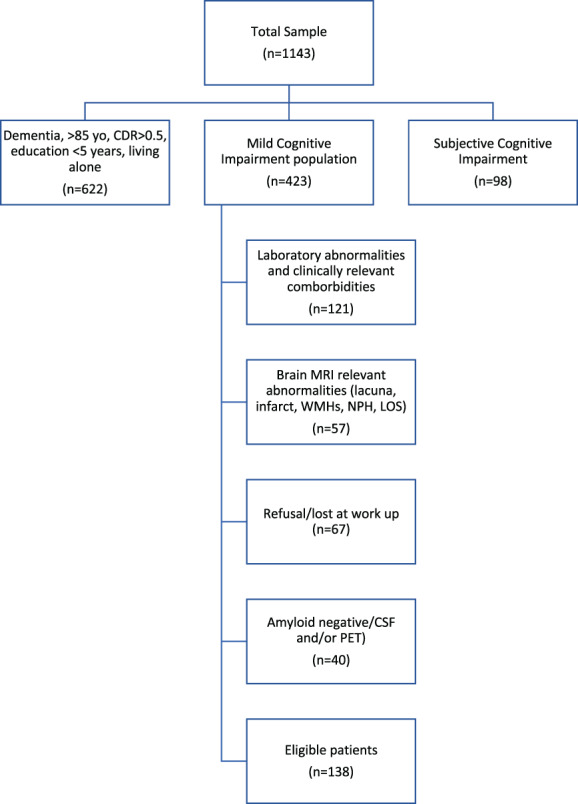
Application of the eligibility criteria to “real‐world” patients attending a Center for Dementia and Cognitive Decline. CDR, clinical dementia rating; CSF, cerebrospinal fluid; NPH, normal pressure hydrocephalus; LOS, space‐occupying lesion; MRI, magnetic resonance imaging; PET, positron emission tomography; WMHs, white matter hyperintensities; yo, years old
DISCUSSION
Although these findings should be cautiously interpreted as they were derived from a single CDCD experience, they are in agreement with RAND simulation 2 and argue for “real‐world” eligibility being dependent on the clinical setting. Indeed, geriatric CDCDs are generally devoted to an older and frail population while neurologic settings are more committed to advanced diagnostics. 11 Our results showed that the proportion of eligible patients with cognitive disorders attending “real‐world” CDCDs exceeds 30% of MCI patients. This observation should encourage a deep reflection in order to plan a healthcare resources' reorganization, even if DMT would be limited to early AD. In fact, referring to a survey by the local Brescia Health Protection Agency, 12 in an area of 1,206,895 inhabitants, the incidence of AD was 2.5/1000, making the annually estimated number of eligible patients of 915, that is, ~45,000 nationally. Accordingly, with the prospected elevated price tag per year, not accounting for ancillary expenses (i.e., amyloid PET, CSF analysis, and sequential MRIs), the drug's potential impact on annual care expenditures might represent a great challenge for healthcare systems. This increasingly debated issue argues for the urge of formal cost‐effectiveness analysis either in countries (i.e., United States) where most eligible patients would be on Medicare or in countries (i.e., Italy) characterized by national health insurance programs with universal coverage.
Still, many barriers exist along the diagnostic journey of AD and addressing the capacity constraints may turn out to be very challenging. Nevertheless, the advancement of diagnostics and therapeutics should encourage considering an extensive planning at national and local levels.
CONFLICT OF INTEREST
Alessandro Padovani has received honoraria for presentations at scientific meetings and/or research funding from Nutricia, Roche, GE Health, Eli‐Lilly, Biogen, and Pfizer. All other authors have no real or potential conflict of interest related to this work.
AUTHOR CONTRIBUTIONS
Alessandro Padovani: study conception, interpretation of data, and drafting of the manuscript. Alberto Benussi: study conception, design, and writing of the manuscript. Salvatore Caratozzolo: data collection and interpretation of data. Andrea Pilotto: interpretation of data and drafting the manuscript. Luca Rozzini: data collection. Gioacchino Tedeschi: interpretation of data and drafting of the manuscript.
SPONSOR'S ROLE
The sponsors had no role in design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
ACKNOWLEDGMENTS
Open Access Funding provided by Universita degli Studi di Brescia within the CRUI‐CARE Agreement.
Funding information This work was supported by the Airalzh‐AGYR2020 grant issued to Alberto Benussi.
See related editorial by Lundebjerg et al.
REFERENCES
- 1. The Lancet Neurology . Alzheimer's disease: a time for cautious optimism. Lancet Neurol. 2015;14(8):779. doi: 10.1016/S1474-4422(15)00154-4 [DOI] [PubMed] [Google Scholar]
- 2. Hlavka JP, Mattke S, Liu JL. Assessing the preparedness of the health care system infrastructure in six European countries for an Alzheimer's treatment. Rand Health Q. 2019;8(3):2. doi: 10.7249/rr2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Di Fiandra T, Canevelli M, Di Pucchio A, Vanacore N. The Italian Dementia National Plan. Ann Ist Super Sanita. 2015;51(4):261‐264. doi: 10.4415/ANN_15_04_02 [DOI] [PubMed] [Google Scholar]
- 4. Boccardi M, Nicolosi V, Festari C, et al. Italian consensus recommendations for a biomarker‐based aetiological diagnosis in mild cognitive impairment patients. Eur J Neurol. 2020;27(3):475‐483. doi: 10.1111/ene.14117 [DOI] [PubMed] [Google Scholar]
- 5. Epelbaum S, Paquet C, Hugon J, et al. How many patients are eligible for disease‐modifying treatment in Alzheimer's disease? A French national observational study over 5 years. BMJ Open. 2019;9(6):1‐11. doi: 10.1136/bmjopen-2019-029663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Canevelli M, Rossi PD, Astrone P, Consorti E, Vanacore N, Cesari M. “Real world” eligibility for aducanumab. J Am Geriatr Soc. 2021;XX:XX. doi: 10.1111/jgs.17390 [DOI] [PubMed] [Google Scholar]
- 7. Cavazzoni P. FDA's decision to approve new treatment for Alzheimer's disease. https://www.fda.gov/drugs/news‐events‐human‐drugs/fdas‐decision‐approve‐new‐treatment‐alzheimers‐disease. Accessed September 26, 2021.
- 8. Cummings J, Aisen P, Lemere C, Atri A, Sabbagh M, Salloway S. Aducanumab produced a clinically meaningful benefit in association with amyloid lowering. Alzheimers Res Ther. 2021;13(1):10‐12. doi: 10.1186/s13195-021-00838-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rabinovici GD. Controversy and progress in Alzheimer's disease – FDA approval of aducanumab. N Engl J Med. 2021;385(9):771‐774. doi: 10.1056/NEJMp2111320 [DOI] [PubMed] [Google Scholar]
- 10. Alexander GC, Knopman DS, Emerson SS, et al. Revisiting FDA approval of aducanumab. N Engl J Med. 2021;385(9):769‐771. doi: 10.1056/NEJMp2110468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Canevelli M, Di Pucchio A, Marzolini F, et al. A national survey of Centers for Cognitive Disorders and Dementias in Italy. J Alzheimers Dis. 2021;24:1‐9. doi: 10.3233/JAD-210634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. UO Epidemiologia . Monitoraggio demenze ‐ aggiornamento al 2018. 2019. https://www.ats-brescia.it/documents/3432658/8475219/dati_demenze_2018REV.pdf/c4b4fbf1-0905-3af2-102a-f4dc8132303d. Accessed September 26, 2021.


