Abstract
Background and Aim(s)
Liver steatosis, as the main feature of metabolic associated fatty liver disease (MAFLD), was associated with the progression of liver fibrosis and metabolic syndrome, which needed to be estimated accurately. In this study, we explored the significance of appendicular skeletal muscle index (ASMI) in evaluating liver steatosis of MAFLD patients.
Methods
Eight hundred and ninety-nine cases with MAFLD from 2017 to 2018 National Health and Nutrition Examination Surveys (NHANES) database were included. All the analyzed data were obtained from NHANES database. The association between ASMI and liver steatosis were evaluated using R and EmpowerStats.
Results
MAFLD individuals were randomly divided into a training (n = 450) and validation cohort (n = 449). In univariate analysis, HbA1c, arms fat, arms lean mass, legs lean mass, trunk lean mass, total fat, total lean mass and ASMI were significantly associated with liver steatosis (p < 0.05). Multivariate analysis showed that HbA1c (OR: 1.6732; 95% CI: 1.2753–2.1929, p = 0.0002) and ASMI (OR: 1.6723; 95% CI: 1.1760–2.5204, p = 0.0052) were independently associated with severe liver steatosis. ASMI accurately evaluated severe liver steatosis with an AUROC of 0.73 and 0.81 in training and validation cohort, respectively. Compared with ASMI only, ASMI combined with HbA1c improved the AUROC to 0.85 and 0.88. Furthermore, the AUROC of our model was superior to FLI in the evaluation of liver steatosis.
Conclusion
ASMI combined with HbA1c has good evaluation value for liver steatosis in MAFLD patients, which might be beneficial for the management of MAFLD clinically.
Keywords: appendicular skeletal muscle index (ASMI), HbA1c, liver, steatosis, metabolic associated fatty liver disease (MAFLD)
Introduction
Non-alcoholic fatty liver disease (NAFLD) was the most common liver disease globally, affecting about a quarter of the population. It imposed a significant health and economic burden on all societies (1). Most studies confirmed that the prevalence of NAFLD was often accompanied by the occurrence of a variety of metabolic disorders, which might aggravate liver injury of NAFLD (2, 3). Due to the heterogeneity of patients with NAFLD, a panel of experts put forward that the metabolic (dysfunction) associated fatty liver disease (MAFLD) might be a more appropriate overarching term (4). The most significant advantages of diagnostic criteria in MAFLD was the definition of liver steatosis and the presence of metabolic abnormalities (5). It had been reported that significant steatosis was associated with fibrosis progression in patients with NAFLD, and the degree of liver steatosis was associated with metabolic syndrome and cardiovascular risk (3). Therefore, it was essential to estimate liver steatosis accurately in patients with NAFLD/MAFLD. At present, liver biopsy was still the gold standard for measuring liver fat content, however, it was an invasive operation, making it unreasonable to evaluate liver steatosis routinely. Therefore, it was necessary for us to find a simple and effective non-invasive method to accurately evaluate liver steatosis in MAFLD patients.
Appendicular skeletal muscle index (ASMI) was a parameter that reflects the weight of appendicular muscles per square meter of height (kg/m2) (6). Usually, ASMI was often used to evaluate skeletal muscle in patients with chronic liver disease (e.g., cirrhosis and end-stage liver disease), so as to reflect the body condition of patients indirectly (6, 7). ASMI also was associated with the progression of chronic metabolic diseases. For instance, the skeletal muscle mass index was negatively correlated with liver steatosis in males with type 2 diabetes (8). Furthermore, researchers found that the destruction of the relationship between skeletal muscle and liver accelerated the progression of NAFLD (9, 10). The underlying mechanism was that excessive energy produced by skeletal muscle during exercise might be stored in the liver in the form of lipids, which would increase the accumulation of liver fat (11). However, the correlation between ASMI and the degree of liver steatosis was unclear. In this study, we will estimate the value of ASMI in evaluating liver steatosis in patients with MAFLD through a population-based data from National Health and Nutrition Examination Surveys (NHANES).
Materials and Methods
Study Design and Participants
Datasets from 2017 to 2018 NHANES required for the cross-sectional study were downloaded from the NHANES web site.1 The NHANES database was a nationally representative survey of the United States conducted annually by CDC’s National Center for Health Statistics (CDC/NCHS), often used in NAFLD (MAFLD) research (12). The study was approved by the NCHS research ethics review board. Informed consents were obtained from all participants in this study. The study protocol also conformed to the ethical guidelines of the Declaration of Helsinki revised in 2013.
In total, 9,254 individuals were initially identified from the database 2017–2018 NHANES. Individuals who were younger than 18 years (n = 3,398), without Fibroscan data (n = 737) or with ineligible Fibroscan data (n = 374), CAP < 248 (n = 2,006) and without dual-energy X-ray absorptiometry (DXA) data and biochemistry data (n = 1,851) were excluded. As a consequence, 889 individuals were included in the final analysis and divided into discovery cohort (n = 450) and validation cohort (n = 449) randomly (Figure 1).
FIGURE 1.
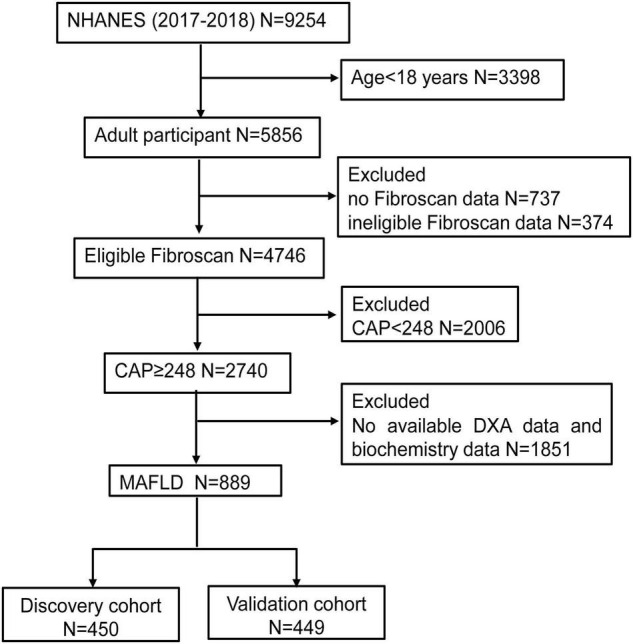
The flow chart of participants selection. NHANES, National Health and Nutrition Examination Surveys; CAP, Controlled attenuation parameter; DXA, dual-energy X-ray absorptiometry; MAFLD, Metabolic associated fatty liver disease.
Diagnostic Criteria of Metabolic Associated Fatty Liver Disease
Metabolic associated fatty liver disease (MAFLD) was defined by the evidence of liver steatosis in adults (CAP ≥ 248 detected by VCTE in this study) (13, 14) and one of the following three criteria, namely overweight/obesity (body mass index, BMI ≥ 25 kg/m2), T2DM or evidence of metabolic dysregulation. Furthermore, among lean/normal weight individuals (BMI < 25 kg/m2) with liver steatosis who did not have T2DM, the presence of metabolic dysregulation was defined as the presence of two or more of the following metabolic risk abnormalities: (1) waist circumference ≥ 102 cm in men or 88 cm in women, (2) blood pressure (BP) ≥ 130/85 mmHg, (3) serum triglycerides (TG) ≥ 1.70 mmol/L, (4) high-density lipoprotein cholesterol (HDL-C) < 1.0 mmol/L for men or < 1.3 mmol/L for women, (5) prediabetes [i.e., fasting plasma glucose (FPG) 5.6–6.9 mmol/L, or 2-h post-load glucose levels (2 h PBG) 7.8–11.0 mmol/L or HbA1c 5.7–6.4%], (6) HOMA-IR score ≥ 2.5, and (7) plasma C-reactive protein (CRP) level > 2 mg/L (5).
Vibration-Controlled Transient Elastography
Vibration-controlled transient elastography (VCTE) was performed using the Fibroscan model 502 V2 Touch (Echosens, Paris, France) equipped with both a medium (M) or extra-large (XL) probes. Examinations were considered reliable only if at least 10 liver stiffness measurements (LSM) were obtained after a fasting time of at least 3 h, with an interquartile range/median <30%. In this study, the severity of liver steatosis in MAFLD patients was defined by CAP value detected by VCTE [Mild/Moderate steatosis (S1-S2: 248–279 dB/m) and Severe steatosis (S3 ≥ 280 dB/m)] (13, 15). Mild/Moderate steatosis defined as non-Severe steatosis.
Body Composition Measurement
Body composition such as lean mass, fat mass, and bone mineral density in MAFLD patients was assessed by DXA scanning. Scans were acquired on Hologic QDR-4500A fan-beam densitometers (Hologic, Inc., Bedford, Massachusetts) using software version Apex 3.2. Further details of the DXA examination were documented on the NHANES website (see text footnote 1). Skeletal muscle indices were calculated by dividing lean mass of the respective body compartments with height squared: appendicular skeletal muscle index (ASMI) = (arms lean mass + legs lean mass)/height2, arm muscle index (AI) = arms lean mass/height2, leg muscle index (LI) = legs lean mass/height2.
Variables
ASMI, as the independent variable, was measured by DXA scanning during the mobile examination center (MEC) visit and calculated by researchers, and CAP, as dependent variables were determined by VCTE measurement. For covariables, continuous variables included age (years), anthropometric measures, FPG (mmol/L), total cholesterol (TC) (mmol/L), TG (mmol/L), HDL-C (mmol/L), total bilirubin (TBIL, μmol/L), alanine aminotransferase (ALT, U/L), aspartate aminotransferase (AST, U/L), γ-glutamyl transpeptidase (GGT, U/L), albumin (ALB, g/L), alkaline phosphatase (ALP, U/L), uric acid (UA, μmol/L), HbA1c (%); categorical variables included gender, race, metabolic diseases, and average alcoholic drinks.
Statistical Analysis
We constructed univariate and multivariate analysis to explore the association between ASMI and severity of steatosis in MAFLD patients. Furthermore, by logistic regression analysis, we established calculated the AUROC of different models for severe liver steatosis, the dependent variable of the study was dichotomous variable (non-Severe steatosis or Severe steatosis). All statistical analyses were conducted by using R 4.0.22 and EmpowerStats.3 Sample weights were used to calculate all estimates according to the analytical guideline provided by NCHS. A p-value < 0.05 was considered as statistically significant.
Results
Baseline and Dual-Energy X-ray Absorptiometry Characteristics of Study Population
A total of 899 individuals with MAFLD were extracted from 2017 to 2018 NHANES database according to the screening criteria in this study. Subsequently, the cohort was randomly divided into a training cohort (n = 450) and validation cohort (n = 449) (Figure 1). The mean age in the training cohort was 41.2 years and 255 individuals (56.7%) were male, while the mean age in the validation cohort was 41.3 years and 261 individuals (58.1%) were male. No matter in training cohort or validation cohort, MAFLD patients showed high BMI (>35 kg/m2), waist circumference (>104 cm) and hip circumference (>109 cm). In our research population, MAFLD patients often have a variety of metabolic diseases (Hypertension in training/validation cohort: 13.3%/15.8%; Diabetes in training/validation cohort: 9.1%/10.3%) and a history of heavy drinking (Table 1). Except for PLT (p = 0.007), LSM data (p = 0.048) and the prevalence of thyroid problem (p = 0.026), no statistically differences were observed in anthropometrics data, blood test parameters, CAP data, prevalence of metabolic disorders (p > 0.05). The details of the baseline characteristics of MAFLD patients in two cohorts were presented in Table 1. We also collected DXA data, including lean mass, fat mass and Bone mineral content (BMC) in total body, arms, legs and trunk, and DXA index, including AI, LI, and ASMI. We observed that there were no differences in the DXA characteristics between the training and validation cohorts (p > 0.05) (Table 2).
TABLE 1.
Baseline characteristics of MAFLD patients in the 2017–2018 NHANES.
| Variables | Training Cohort (n = 450) | Validation Cohort (n = 449) | P-value |
| Age (year) | 41.2 ± 11.4 | 41.3 ± 12.2 | 0.964 |
| Gender, n (%) | 0.683 | ||
| Male | 255 (56.7) | 261 (58.1) | |
| Female | 195 (43.3) | 188 (41.9) | |
| Anthropometrics | |||
| Weight (kg) | 90.8 ± 18.0 | 89.4 ± 18.2 | 0.240 |
| BMI (kg/m2) | 31.9 ± 5.4 | 31.5 ± 6.4 | 0.249 |
| Waist circumference (cm) | 105.7 ± 12.6 | 104.5 ± 13.5 | 0.155 |
| Hip circumference (cm) | 110.0 ± 10.9 | 109.6 ± 12.3 | 0.527 |
| Race, n (%) | 0.626 | ||
| Mexican American | 68 (15.2) | 76 (16.9) | |
| Other Hispanic | 37 (8.3) | 44 (9.7) | |
| Non-Hispanic | 320 (71.2) | 302 (67.2) | |
| Other races | 24 (5.3) | 28 (6.2) | |
| Blood test | |||
| FPG (mmol/L) | 5.6 ± 1.8 | 5.6 ± 2.0 | 0.974 |
| HbA1c (%) | 5.7 ± 1.0 | 5.7 ± 1.0 | 0.244 |
| ALT (U/L) | 28.9 ± 18.8 | 27.6 ± 18.6 | 0.323 |
| AST (U/L) | 23.5 ± 13.5 | 22.9 ± 11.4 | 0.472 |
| ALP (U/L) | 78.3 ± 23.8 | 78.2 ± 19.3 | 0.915 |
| GGT (U/L) | 35.0 ± 41.0 | 34.8 ± 30.8 | 0.965 |
| ALB (g/L) | 41.1 ± 3.2 | 41.5 ± 3.0 | 0.078 |
| TBIL (μmol/L) | 7.4 ± 4.9 | 7.6 ± 3.9 | 0.456 |
| TG (mmol/L) | 2.0 ± 1.5 | 2.0 ± 1.550 | 0.694 |
| TC (mmol/L) | 5.1 ± 1.0 | 5.0 ± 1.0 | 0.487 |
| HDL-C (mmol/L) | 1.3 ± 0.3 | 1.2 ± 0.4 | 0.397 |
| Creatinine (μmol/L) | 77.7 ± 49.0 | 75.2 ± 17.6 | 0.302 |
| UA (μmol/L) | 331.3 ± 77.2 | 340.7 ± 82.9 | 0.080 |
| PLT (109/L) | 257.0 ± 59.6 | 246.0 ± 62.5 | 0.007 |
| HGB (g/dL) | 14.5 ± 1.5 | 14.5 ± 1.4 | 0.673 |
| VCTE | |||
| CAP (dB/m), n (%) | 0.772 | ||
| S1 (248–267) | 76 (17.2) | 76 (16.9) | |
| S2 (268–279) | 68 (15.1) | 61 (13.5) | |
| S3 (≥280) | 305 (67.7) | 313 (69.6) | |
| IQRc | 0.225 | ||
| 35.4 ± 18.7 | 33.9 ± 17.7 | ||
| LSM (kPa), n (%) | 0.048 | ||
| F0 (<6.3) | 78 (17.2) | 76 (16.9) | |
| F1 (6.3–8.2) | 68 (15.1) | 61 (13.5) | |
| F2 (8.3–10.4) | 20 (4.6) | 15 (3.3) | |
| F3 (10.5–12.4) | 3 (0.7) | 8 (1.7) | |
| F4 (≥12.5) | 6 (1.3) | 11 (2.2) | |
| IQRe | 0.659 | ||
| 0.8 ± 0.6 | 0.8 ± 0.7 | ||
| Ratio: stiffness IQRe/median E | 0.758 | ||
| 13.8 ± 6.1 | 13.6 ± 6.0 | ||
| Hypertension | 60 (13.3) | 71 (15.8) | 0.299 |
| Diabetes | 41 (9.1) | 46 (10.3) | 0.644 |
| Gout | 12 (2.7) | 14 (3.1) | 0.299 |
| CHD | 5 (1.0) | 3 (0.7) | 0.287 |
| Thyroid problem | 38 (8.4) | 21 (4.6) | 0.026 |
| Average alcoholic drinks, n (%) | |||
| <4 drinks/day | 244 (54.3) | 283 (63.0) | |
| 4–8 drinks/day | 60 (13.3) | 29 (6.4) | |
| >8 drinks/day | 8 (1.7) | 13 (2.9) | |
| NA | 138 (30.7) | 124 (27.6) | |
Continuous variables are shown as mean ± standard deviation (SD). Categorical values are shown as n (%). MAFLD, Metabolic-associated fatty liver disease; BMI: body mass index; FPG, fasting plasma glucose; HbA1c, ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, γ-glutamyl transpeptidase; ALB, albumin; TBIL, total bilirubin; TG, triglycerides; TC, Total cholesterol; HDL-C, high-density lipoprotein cholesterol; UA, uric acid; PLT, platelet; HGB, hemoglobin; VCTE, Vibration Controlled Transient Elastography; CAP, controlled attenuation parameter; IQRc, CAP interquartile range; LSM, liver stiffness measurements; IQRe, Stiffness E interquartile range; CHD, Coronary Heart Disease. NA, Not available.
TABLE 2.
DXA characteristics of MAFLD patients in the 2017–2018 NHANES.
| Variables | Training cohort (n = 450) | Validation Cohort (n = 449) | P-value |
| Absolute value | |||
| Total lean mass (kg) | 56.7 ± 12.5 | 55.8 ± 11.6 | 0.264 |
| Total fat mass (kg) | 32.0 ± 95.4 | 31.4 ± 10.7 | 0.323 |
| Total BMC (g) | 2443.6 ± 454.7 | 2436.7 ± 409.3 | 0.813 |
| Arms lean mass (kg) | 6.9 ± 2.1 | 6.7 ± 2.0 | 0.127 |
| Arms fat (kg) | 4.0 ± 1.5 | 3.8 ± 1.5 | 0.043 |
| Arms BMC (g) | 390.3 ± 97.6 | 384.0 ± 85.4 | 0.304 |
| Legs lean mass (kg) | 18.0 ± 4.3 | 17.9 ± 4.1 | 0.589 |
| Legs fat (kg) | 10.5 ± 3.8 | 10.5 ± 4.2 | 0.966 |
| Legs BMC (g) | 908.1 ± 204.0 | 915.7 ± 184.4 | 0.559 |
| Trunk lean mass (kg) | 28.6 ± 6.2 | 28.0 ± 5.6 | 0.159 |
| Trunk fat (kg) | 15.6 ± 5.3 | 15.8 ± 5.6 | 0.657 |
| Trunk BMC (g) | 632.1 ± 130.2 | 629.6 ± 121.4 | 0.773 |
| Index | |||
| Arms muscle index (AI) (kg/m2) | 2.39 ± 0.56 | 2.32 ± 0.57 | 0.061 |
| Legs muscle index (LI) (kg/m2) | 6.28 ± 1.08 | 6.23 ± 1.18 | 0.570 |
| ASMI (kg/m2) | 8.66 ± 1.56 | 8.55 ± 1.67 | 0.291 |
MAFLD, Metabolic-associated fatty liver disease; BMC, Bone mineral content; AI, Arms muscle index; LI, Legs muscle index; ASMI, Appendicular skeletal muscle index.
Model Development
Univariate and multivariate logistic regression analysis were used to analyze the related factors for liver steatosis in MAFLD patients. According to the univariate analysis, HbA1c, arms fat, arms lean mass, legs lean mass, trunk lean mass, total fat, total lean mass, and ASMI were significantly associated with liver steatosis (p < 0.05). These significant variables were further conducted to the multivariate analysis. Multivariate analysis showed that HbA1c [odds ratio (OR): 1.6732; 95% CI: 1.2753–2.1929, p = 0.0002] and ASMI (OR, 1.6723; 95% CI: 1.1760–2.5204, p = 0.0052) were independently associated with severe liver steatosis in MAFLD patients (Table 3).
TABLE 3.
Univariate and multivariate analysis of variables associated with liver steatosis.
| Univariate analysis |
Multivariate analysis |
|||
| Variables | OR (95%CI) | P-value | OR (95%CI) | P-value |
| Age (year) | 0.9980 (0.9494, 1.0490) | 0.9363 | ||
| Gender, n (%) | 0.4040 (0.1079, 1.5127) | 0.1785 | ||
| Waist circumference (cm) | 1.0702 (1.0296, 1.1125) | 0.0006 | ||
| Hip circumference (cm) | 1.0366 (0.9940, 1.0812) | 0.0934 | ||
| BMI (kg/m2) | 1.1265 (1.0400, 1.2201) | 0.0035 | ||
| ALT (U/L) | 1.0266 (1.0096, 1.0439) | 0.0020 | ||
| AST (U/L) | 1.0310 (1.0101, 1.0523) | 0.0035 | ||
| GGT (U/L) | 1.0023 (0.9939, 1.0108) | 0.5912 | ||
| ALB (g/L) | 0.9981 (0.8351, 1.1930) | 0.9835 | ||
| ALP (U/L) | 1.0050 (0.9821, 1.0285) | 0.6710 | ||
| CRE (μmol/L) | 0.9974 (0.9730, 1.0224) | 0.8369 | ||
| HDL-C (mmol/L) | 0.0981 (0.0091, 1.0523) | 0.0551 | ||
| HbA1c (%) | 1.5812 (1.2275, 2.0370) | 0.0004 | 1.6723 (1.2753, 2.1929) | 0.0002 |
| Arms fat (kg) | 1.0003 (1.0000, 1.0006) | 0.0425 | ||
| Arms BMC (g) | 1.0031 (0.9974, 1.0089) | 0.2850 | ||
| Arms lean mass (kg) | 1.0003 (1.0000, 1.0006) | 0.0336 | ||
| Legs fat (kg) | 1.0001 (1.0000, 1.0002) | 0.2255 | ||
| Legs BMC (g) | 1.0006 (0.9977, 1.0034) | 0.6991 | ||
| Legs lean mass (kg) | 1.0001 (1.0000, 1.0003) | 0.0486 | ||
| Trunk fat (kg) | 1.0000 (0.9999, 1.0001) | 0.8382 | ||
| Trunk BMC (g) | 1.0027 (0.9982, 1.0073) | 0.2366 | ||
| Trunk lean mass (kg) | 1.0002 (1.0001, 1.0003) | 0.0020 | ||
| Total fat (kg) | 1.0000 (1.0000 1.0001) | 0.0389 | ||
| Total BMC (g) | 1.0027 (0.9982, 1.0073) | 0.2366 | ||
| Total lean mass (kg) | 1.0001 (1.0000, 1.0001) | 0.0085 | ||
| ASMI (kg/m2) | 1.6308 (1.1329, 2.3475) | 0.0085 | 1.7216 (1.1760 2.5204) | 0.0052 |
BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyl transpeptidase; ALB, albumin; ALP, alkaline phosphatase; HDL-C, high-density lipoprotein cholesterol; CRE, creatinine; BMC, Bone mineral content; ASMI, Appendicular skeletal muscle index.
Performance of Arms Muscle Index, Legs Muscle Index, Appendicular Skeletal Muscle Index, and Appendicular Skeletal Muscle Index Combined With HbA1c for Evaluating Severe Liver Steatosis in Metabolic Associated Fatty Liver Disease Patients
AI accurately evaluated severe liver steatosis in MAFLD patients with an AUROC of 0.72 in training cohort and 0.83 in validation cohort, sensitivities of 92 and 78% and specificities of 50 and 82% were obtained in training cohort and validation cohort, respectively (Table 4 and Figure 2A). The LI evaluated the severity liver steatosis in MAFLD patients with an AUROC of 0.72 in training cohort and 0.76 in validation cohort, sensitivities of 92 and 78% and specificities of 51 and 70% were obtained in training cohort and validation cohort, respectively (Table 4 and Figure 2B). The ASMI demonstrated an AUROC of 0.73 in training cohort and 0.81 in validation cohort for evaluating severe liver steatosis in MAFLD patients. The combination of ASMI and HbA1c improved performance of ASMI only, increasing the AUROC to 0.85 and 0.88, respectively. In training cohort, sensitivity was 92% and specificity was 52% for ASMI alone, turned to 75 and 86% by the addition of HbA1c, respectively. Similarly, in validation cohort, sensitivity was 89% and specificity was 72% for ASMI alone, turned to 100 and 68% by the addition of HbA1c, respectively (Table 4 and Figures 2C–E). Furthermore, using linear regression, the combination of ASMI and HbA1c also could evaluate liver steatosis conveniently and effectively [Liver steatosis (CAP value) = 200.95812 + 6.88228 × ASMI + 7.75809 × HbA1c]. We also calculated the diagnostic value of the fatty liver index (FLI, a non-invasive approach to discriminate individuals with NAFLD) for liver steatosis severity in this study. In training cohort, the FLI had an AUROC of 0.82 for identifying severe liver steatosis in MAFLD patients. Sensitivity and specificity were 83 and 76%, respectively. While in validation cohort, the AUROC was 0.84, the sensitivity was 89% and the specificity was 74% (Table 4 and Figure 2F). The full performance characteristics of AI, LI, ASMI, ASMI combined with HbA1c, as well as FLI were provided in Table 4.
TABLE 4.
Association of ASMI with severity of steatosis in MAFLD patients.
| Models | Cohort | AUROC | Cut-off value | Sensitivity | Specificity |
| Arm muscle index (AI) | Training | 0.72 | 0.42 | 0.92 | 0.50 |
| Validation | 0.83 | 0.60 | 0.78 | 0.82 | |
| Leg muscle index (LI) | Training | 0.72 | 0.43 | 0.92 | 0.51 |
| Validation | 0.76 | 0.48 | 0.78 | 0.70 | |
| ASMI | Training | 0.73 | 0.61 | 0.92 | 0.52 |
| Validation | 0.81 | 0.48 | 0.89 | 0.72 | |
| HbA1c | Training | 0.78 | 0.46 | 0.58 | 0.90 |
| Validation | 0.78 | 0.61 | 0.67 | 0.79 | |
| ASMI + HbA1c | Training | 0.85 | 0.61 | 0.75 | 0.86 |
| Validation | 0.88 | 0.68 | 1.00 | 0.68 | |
| Fatty liver index (FLI) | Training | 0.82 | 0.59 | 0.83 | 0.76 |
| Validation | 0.84 | 0.63 | 0.89 | 0.74 |
AI, Arms muscle index; LI, Legs muscle index; ASMI, Appendicular skeletal muscle index; FLI, Fatty liver index.
FIGURE 2.
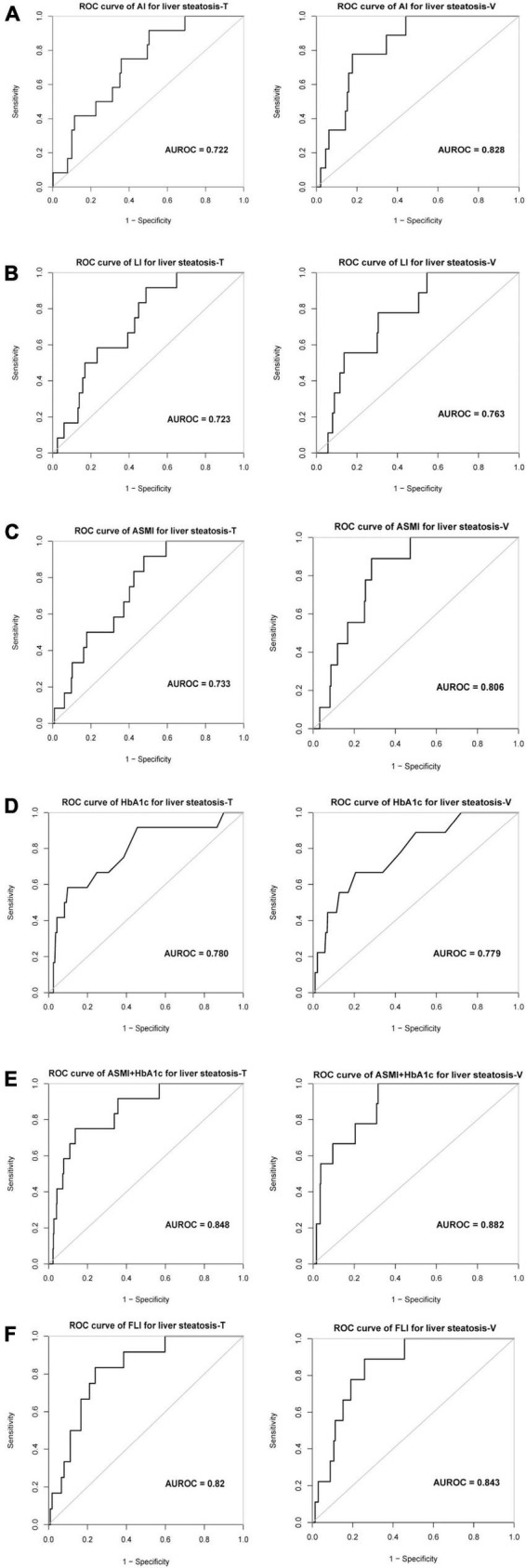
The evaluation value of different indexes on severe liver steatosis of MAFLD patients. ROC curve and AUROC value of (A) AI, (B) LI, (C) ASMI, (D) HbA1c, (E) ASMI + HbA1c, and (F) FLI for liver steatosis in training and validation cohort. AI, Arms muscle index; LI, Legs muscle index; ASMI, Appendicular skeletal muscle index; FLI, Fatty liver index; AUROC, the area under the receiver operating characteristic.
Discussion
This was the first and large population-based cross-sectional study that evaluated severe liver steatosis by skeletal muscle index and metabolic disorders in MAFLD patients. In this study, we found that ASMI and HbA1c were associated with liver steatosis in MAFLD patients independently. Notably, ASMI combined with HbA1c achieved a good predictive value for evaluating liver steatosis in MAFLD patients.
Steatosis was a crucial pathological manifestation in the liver of patients with MAFLD (16). It was well-known that advanced steatosis was associated with progression of fibrosis in NAFLD patients (17). Besides, the degree of liver steatosis was related to the occurrence of metabolic syndrome and cardiovascular risk (18). Therefore, an accurate estimation of the liver steatosis was necessary for patients with MAFLD. Previous studies had shown that CAP value detected by VCTE was significantly correlated with the severity of steatosis evaluated by liver biopsy (19), and demonstrated the accuracy of CAP for diagnosis of NAFLD (20–22). Furthermore, Yu et al. demonstrated that CAP detected by VCTE were highly accurate for assessing liver steatosis in patients of MAFLD and T2DM (23). Therefore, CAP value could accurately reflect liver steatosis in MAFLD patients.
In our study, we found that ASMI combined with HbA1c had good diagnostic performance for liver steatosis detected by VCTE in patients with MAFLD. Skeletal muscle had been considered as a vital organ for whole body metabolism, since it was a primary site for glucose uptake and storage, and it was also a reservoir of amino acids stored as protein (24). Previous studies showed that the crosstalk between muscle and liver might play an important role in the pathogenesis of NASH. For instance, reduced appendicular skeletal muscle mass as well as increased visceral fat mass might adversely affect the risk of NAFLD development and progression (25, 26). Many other researches also assumed that low ASM mass or ASMI was associated with more severe liver steatosis and/or hepatocellular ballooning in NAFLD patients (27–29). However, there were no published paper explored the relationship between ASMI and liver steatosis in patients with MAFLD. Intriguingly, our study found ASMI was positively related to liver steatosis in MAFLD patients, which was different from the results in NAFLD patients. Firstly, skeletal muscle was an important organ for storing and releasing lipids in response to fatty acid oversupply, excessive fatty acids and energy storage may lead to the increase the mass of skeletal muscle. In this situation, fatty acids released from skeletal muscle might aggravate insulin resistance and liver steatosis in MAFLD patients (30, 31). Secondly, liver steatosis and ASMI both were affected by BMI (a parameter calculated by height and weight) (32, 33), in our study, most individuals with large ASMI also showed higher height and weight, and the corresponding liver steatosis was more serious. Thirdly, MAFLD was associated with a variety of metabolic abnormalities, which might affect the relationship between ASMI and steatosis. For instance, HbA1c was a metabolic index which was positively associated with liver steatosis in our study. One recent study showed that higher mean HbA1c was associated with higher grade of steatosis and ballooned hepatocytes (34). Indeed, compared with ASMI only, ASMI combined with HbA1c increased the AUROC from 0.73 to 0.85 for assessing severe liver steatosis in MAFLD patients. One previous study reported that FLI was one of the most accurate algorithms for the non-invasive diagnosis of NAFLD in both lean and overweight/obese population (35, 36), while the AUROC of our model was superior to FLI in MAFLD patients. All variables in the non-invasive clinical model established in this study were objective results and easy to obtain, which could be a better evaluation method for liver steatosis in patients with MAFLD.
There were some limitations in this study. Firstly, liver steatosis of MAFLD patients was detected by Fibroscan, not by liver biopsy, because it was impracticable to perform invasive tests such as biopsies in a large population-based study. Secondly, ASMI was a parameter calculated based on the appendicular skeletal muscle mass obtained from dual-energy X-ray absorptiometry (DXA), some medical institutions might not have the objective conditions for this operation. Thirdly, ASMI only assessed muscle weight, muscle length and muscle fiber type were not considered. Finally, there was still a lack of another group of MAFLD patients for external validation. We will perform the validation analysis deeply in the future.
Conclusion
We constructed a clinical diagnostic method for non-invasive evaluation the degree of steatosis in MAFLD patients, with fewer parameters (ASMI and HbA1c) and high accuracy. The clinical method might be beneficial to reduce the financial cost of liver biopsy and convenient for clinicians to identify the degree of steatosis in patients with MAFLD clinically.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics Statement
The studies involving human participants were reviewed and approved by the NCHS Research Ethics Review Board. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
RJ and XW: study concept and design. XL, JY, and BL: acquisition of data. RJ, XW, LW, FL, and HR: analysis and interpretation of data. RJ, XW, FL, and HR: drafting of the manuscript. FL and HR: critical revision of the manuscript for important intellectual content. All authors have made a significant contribution to this study and have approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
- MAFLD
metabolic associated fatty liver disease
- ASMI
appendicular skeletal muscle index
- NHANES
National Health and Nutrition Examination Surveys
- NAFLD
Non-alcoholic fatty liver disease
- MRI-PDFF
magnetic resonance imaging-derived proton density fat fraction
- VCTE
Vibration-controlled transient elastography
- NCHS
National Center for Health Statistics
- DXA
dual-energy X-ray absorptiometry
- BMI
body mass index
- BP
blood pressure
- TG
triglycerides
- HDL-C
high-density lipoprotein cholesterol
- FPG
fasting plasma glucose
- CRP
C-reactive protein
- AI
arm muscle index
- LI
leg muscle index
- TC
cholesterol
- GGT
γ -glutamyl transpeptidase
- ALB
albumin
- ALP
alkaline phosphatase
- UA
uric acid
- HbA1c
hemoglobin A1C
- BMC
Bone mineral content
- OR
odds ratio
- FLI
fatty liver index.
Footnotes
Funding
This study was supported by the National Natural Science Foundation of China (NSFC) (81870407 LW and 81870406 HR), Peking University Medicine Fund of Fostering Young Scholars’ Scientific & Technological Innovation, and the Fundamental Research Funds for the Central Universities (BMU2021PYB010 XW), Chinese foundation for hepatitis prevention and control—TianQing liver disease research fund subject (TQGB20210139 XW), and Qi-Min Project (HR).
References
- 1.Eslam M, Sanyal AJ, George J, International Consensus P. Mafld: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. (2020) 158:1999–2014.e1. 10.1053/j.gastro.2019.11.312 [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of Nafld and Nash: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2018) 15:11–20. 10.1038/nrgastro.2017.109 [DOI] [PubMed] [Google Scholar]
- 3.Lee H, Lee YH, Kim SU, Kim HC. Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: a nationwide cohort study. Clin Gastroenterol Hepatol. (2021) 19:2138–47.e10. 10.1016/j.cgh.2020.12.022 [DOI] [PubMed] [Google Scholar]
- 4.Eslam M, Ahmed A, Despres JP, Jha V, Halford JCG, Wei Chieh JT, et al. Incorporating fatty liver disease in multidisciplinary care and novel clinical trial designs for patients with metabolic diseases. Lancet Gastroenterol Hepatol. (2021) 6:743–53. 10.1016/S2468-1253(21)00132-1 [DOI] [PubMed] [Google Scholar]
- 5.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. (2020) 73:202–9. 10.1016/j.jhep.2020.03.039 [DOI] [PubMed] [Google Scholar]
- 6.Lindqvist C, Brismar TB, Majeed A, Wahlin S. Assessment of muscle mass depletion in chronic liver disease: dual-energy X-Ray absorptiometry compared with computed tomography. Nutrition. (2019) 61:93–8. 10.1016/j.nut.2018.10.031 [DOI] [PubMed] [Google Scholar]
- 7.Carey EJ, Lai JC, Wang CW, Dasarathy S, Lobach I, Montano-Loza AJ, et al. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl. (2017) 23:625–33. 10.1002/lt.24750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto Y, Osaka T, Fukuda T, Tanaka M, Yamazaki M, Fukui M. The relationship between hepatic steatosis and skeletal muscle mass index in men with Type 2 diabetes. Endocr J. (2016) 63:877–84. 10.1507/endocrj.EJ16-0124 [DOI] [PubMed] [Google Scholar]
- 9.Polyzos SA, Margioris AN. Sarcopenic obesity. Hormones (Athens). (2018) 17:321–31. 10.1007/s42000-018-0049-x [DOI] [PubMed] [Google Scholar]
- 10.Altajar S, Baffy G. Skeletal muscle dysfunction in the development and progression of nonalcoholic fatty liver disease. J Clin Transl Hepatol. (2020) 8:414–23. 10.14218/JCTH.2020.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Q, Bengmark S, Qu S. The role of hepatic fat accumulation in pathogenesis of non-alcoholic fatty liver disease (Nafld). Lipids Health Dis. (2010) 9:42. 10.1186/1476-511X-9-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National health and nutrition examination survey: sample design, 2011-2014. Vital Health Stat 2. (2014) 162:1–33. [PubMed] [Google Scholar]
- 13.Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Ledinghen V, et al. Individual patient data meta-analysis of controlled attenuation parameter (Cap) technology for assessing steatosis. J Hepatol. (2017) 66:1022–30. 10.1016/j.jhep.2016.12.022 [DOI] [PubMed] [Google Scholar]
- 14.Cassinotto C, Boursier J, de Ledinghen V, Lebigot J, Lapuyade B, Cales P, et al. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, fibroscan, and arfi with liver biopsy. Hepatology. (2016) 63:1817–27. 10.1002/hep.28394 [DOI] [PubMed] [Google Scholar]
- 15.Weng Z, Ou W, Huang J, Singh M, Wang M, Zhu Y, et al. Circadian misalignment rather than sleep duration is associated with Mafld: a population-based propensity score-matched study. Nat Sci Sleep. (2021) 13:103–11. 10.2147/NSS.S290465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, et al. Comparison of Mafld and Nafld diagnostic criteria in real world. Liver Int. (2020) 40:2082–9. 10.1111/liv.14548 [DOI] [PubMed] [Google Scholar]
- 17.Ajmera V, Park CC, Caussy C, Singh S, Hernandez C, Bettencourt R, et al. Magnetic resonance imaging proton density fat fraction associates with progression of fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. (2018) 155:307–10.e2. 10.1053/j.gastro.2018.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyersohn NM, Mayrhofer T, Corey KE, Bittner DO, Staziaki PV, Szilveszter B, et al. Association of hepatic steatosis with major adverse cardiovascular events, independent of coronary artery disease. Clin Gastroenterol Hepatol. (2021) 19:1480–8.e14. 10.1016/j.cgh.2020.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahn JM, Paik YH, Min SY, Cho JY, Sohn W, Sinn DH, et al. Relationship between controlled attenuation parameter and hepatic steatosis as assessed by ultrasound in alcoholic or nonalcoholic fatty liver disease. Gut Liver. (2016) 10:295–302. 10.5009/gnl15155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abeysekera KWM, Fernandes GS, Hammerton G, Portal AJ, Gordon FH, Heron J, et al. Prevalence of steatosis and fibrosis in young adults in the UK: a population-based study. Lancet Gastroenterol Hepatol. (2020) 5:295–305. 10.1016/S2468-1253(19)30419-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Ledinghen V, Vergniol J, Capdepont M, Chermak F, Hiriart JB, Cassinotto C, et al. Controlled Attenuation Parameter (Cap) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. (2014) 60:1026–31. 10.1016/j.jhep.2013.12.018 [DOI] [PubMed] [Google Scholar]
- 22.Shimizu M, Suzuki K, Kato K, Jojima T, Iijima T, Murohisa T, et al. Evaluation of the effects of dapagliflozin, a Sodium-Glucose Co-Transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with Type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab. (2019) 21:285–92. 10.1111/dom.13520 [DOI] [PubMed] [Google Scholar]
- 23.Yu H, Liu H, Zhang J, Jia G, Yang L, Zhang Q, et al. Accuracy of fibrotouch in assessing liver steatosis and fibrosis in patients with metabolic-associated fatty liver disease combined with Type 2 diabetes mellitus. Ann Palliat Med. (2021) 10:9702–14. 10.21037/apm-21-2339 [DOI] [PubMed] [Google Scholar]
- 24.Argiles JM, Campos N, Lopez-Pedrosa JM, Rueda R, Rodriguez-Manas L. Skeletal muscle regulates metabolism via interorgan crosstalk: roles in health and disease. J Am Med Dir Assoc. (2016) 17:789–96. 10.1016/j.jamda.2016.04.019 [DOI] [PubMed] [Google Scholar]
- 25.van den Hoek AM, de Jong J, Worms N, van Nieuwkoop A, Voskuilen M, Menke AL, et al. Diet and exercise reduce pre-existing nash and fibrosis and have additional beneficial effects on the vasculature, adipose tissue and skeletal muscle via organ-crosstalk. Metabolism. (2021) 124:154873. 10.1016/j.metabol.2021.154873 [DOI] [PubMed] [Google Scholar]
- 26.Li G, Rios RS, Wang XX, Yu Y, Zheng KI, Huang OY, et al. Sex influences the association between appendicular skeletal muscle mass to visceral fat area ratio and non-alcoholic steatohepatitis in patients with biopsy-proven non-alcoholic fatty liver disease. Br J Nutr. (2021):1–8. [Online ahead of print], 10.1017/S0007114521002415 [DOI] [PubMed] [Google Scholar]
- 27.Lee YH, Jung KS, Kim SU, Yoon HJ, Yun YJ, Lee BW, et al. Sarcopaenia is associated with Nafld independently of obesity and insulin resistance: nationwide surveys (Knhanes 2008-2011). J Hepatol. (2015) 63:486–93. 10.1016/j.jhep.2015.02.051 [DOI] [PubMed] [Google Scholar]
- 28.Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. (2017) 66:123–31. 10.1016/j.jhep.2016.08.019 [DOI] [PubMed] [Google Scholar]
- 29.Hsing JC, Nguyen MH, Yang B, Min Y, Han SS, Pung E, et al. Associations between body fat, muscle mass, and nonalcoholic fatty liver disease: a population-based study. Hepatol Commun. (2019) 3:1061–72. 10.1002/hep4.1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szendroedi J, Yoshimura T, Phielix E, Koliaki C, Marcucci M, Zhang D, et al. Role of diacylglycerol activation of pkctheta in lipid-induced muscle insulin resistance in humans. Proc Natl Acad Sci U S A. (2014) 111:9597–602. 10.1073/pnas.1409229111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Therkelsen KE, Pedley A, Speliotes EK, Massaro JM, Murabito J, Hoffmann U, et al. Intramuscular fat and associations with metabolic risk factors in the framingham heart study. Arterioscler Thromb Vasc Biol. (2013) 33:863–70. 10.1161/ATVBAHA.112.301009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng TC. Role of sarcopenia in nonalcoholic fatty liver disease: definition is crucially important. Hepatology. (2018) 68:788–9. 10.1002/hep.29910 [DOI] [PubMed] [Google Scholar]
- 33.Peng TC, Wu LW, Chen WL, Liaw FY, Chang YW, Kao TW. Nonalcoholic fatty liver disease and sarcopenia in a western population (NHANES III): the importance of sarcopenia definition. Clin Nutr. (2019) 38:422–8. 10.1016/j.clnu.2017.11.021 [DOI] [PubMed] [Google Scholar]
- 34.Alexopoulos AS, Crowley MJ, Wang Y, Moylan CA, Guy CD, Henao R, et al. Glycemic control predicts severity of hepatocyte ballooning and hepatic fibrosis in nonalcoholic fatty liver disease. Hepatology. (2021) 74:1220–33. 10.1002/hep.31806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li C, Guo P, Zhang R, Zhang M, Li Y, Huang M, et al. Both Whr and Fli as better algorithms for both lean and overweight/obese Nafld in a Chinese population. J Clin Gastroenterol. (2019) 53:e253–60. 10.1097/MCG.0000000000001089 [DOI] [PubMed] [Google Scholar]
- 36.Koehler EM, Schouten JN, Hansen BE, Hofman A, Stricker BH, Janssen HL. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin Gastroenterol Hepatol. (2013) 11:1201–4. 10.1016/j.cgh.2012.12.031 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.


