Abstract
Background
Up to 15% of people aged 60 and over are anemic, and the prevalence of anemia increases with age. In older men and women, anemia is associated with increases in the risk of death and all‐cause hospitalization, poor functional capacity, quality of life, and depression.
Methods and Results
We reviewed the literature describing anemia in aging populations, focusing on the specific diagnostic criteria of anemia and potential causes in older men and women. Even after extensive etiologic workup that involves careful medical history, physical examination, laboratory measurements, and additional studies such as bone marrow biopsy, anemia of aging is unexplained in up to 40% of older patients with anemia. As a result, treatment options remain limited.
Conclusions
The prevalence of unexplained anemia of aging (UAA; also called unexplained anemia of the elderly, UAE), its deleterious impacts on health, physical function, and quality of life, and the lack of effective treatment or therapy guidelines represent a compelling unmet clinical need. In this review and consensus document, we discuss the scope of the problem, possible causes of UAA, diagnostic criteria, and potential treatment options. Because even mild anemia is strongly linked to poor clinical outcomes, it should receive clinical attention rather than simply being considered a normal part of aging.
Keywords: erythropoietin, hemoglobin, physical function
Key points
The prevalence of unexplained anemia of aging (UAA) increases with age.
UAA is diagnosed by exclusion of known causes of anemia.
UAA is associated with decreased quality of life and increased mortality.
Why does this paper matter?
Because of detrimental effects on health and lack of therapeutic guidelines, UAA represents an unmet clinical need.
UNEXPLAINED ANEMIA IS PREVALENT IN AGING POPULATIONS
Anemia, defined by the World Health Organization criteria as circulating hemoglobin (Hb) <12 g/dl in women and <13 g/dl in men, is common among individuals over the age of 60. 1 , 2 , 3 The prevalence of anemia increases with age and is particularly common among the oldest and most frail: in a retrospective study of more than 19,000 hospital patients, the incidence of anemia rose from 15% at the ages of 64–69 to 37% in those over aged 90 (Figure 1). Many anemia cases have no clear underlying cause, a population that we will refer to as unexplained anemia of aging (UAA).
FIGURE 1.
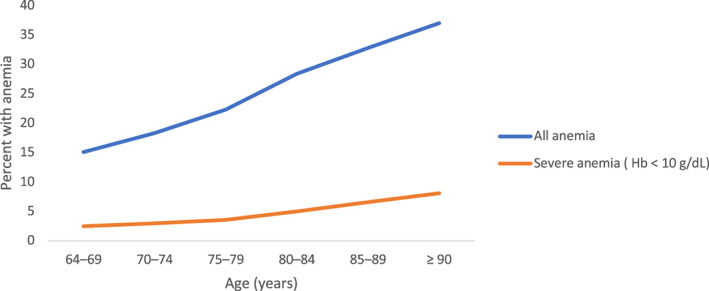
Prevalence of anemia increases with age. Prevalence of anemia (WHO criteria: Hb < 12 g/dl in women and <13 g/dl in men) and severe anemia (Hb < 10 g/dl) in a cohort of 19,758 inpatients and outpatients aged ≥64 years with complete blood counts treated at Innsbruck Medical University Hospital between October 1, 2004 and September 29, 2005. Source: Data from Reference 8
In one of the first population‐based studies to describe UAA, Guralnik et al. examined the third National Health and Nutrition Examination Survey (NHANES) of 4199 community‐dwelling men and women over the age of 65 years. 4 One third of cases of anemia could be attributed to nutritional causes, and another third were associated with inflammation or chronic kidney disease (CKD). This left one third of cases without a clear etiology. A comparable proportion of mild anemia (defined as a Hb concentration of 10.0–11.9 g/dl in women and 10.0–12.9 g/dl in men) is unexplained: in an Italian cohort of 8744 individuals between aged 65–84, mild anemia had no identifiable cause in 26.4% of cases. 5
In a wide range of population‐based studies of anemia, 25%–44% of cases could be classified as UAA (Table 1). This holds true whether the study population is community‐based, hospital inpatient, or long‐term care residents. Importantly, the prevalence of UAA remains high even when the potential cause of anemia is rigorously examined. In study populations consisting of hematology clinic outpatients and patients referred to anemia referral clinics, in which cohorts were subjected to comprehensive examinations, the frequency of UAA is 35%–44%. For example, Artz et al. 6 examined a community‐dwelling, predominantly African‐American (69%) group of patients over the age of 65 who had been referred to an anemia clinic and even after intensive evaluations of causes, UAA was the most common category in both white and African‐American patients. Michalak et al. 7 retrospectively examined medical records of 981 patients over the age of 60 and found that anemia was unexplained in 48 of 169 patients, with a UAA prevalence of 4.9%. The prevalence of UAA increased from 2.9% in those between 60 and 69 to 12.3% in patients ≥80 years of age.
TABLE 1.
Prevalence of diagnostic causes of anemia in older patients, including UAA
| Source | N | Age | %CKD | %IDA | %ACI/ACD | %B12/folate deficiency | %UAA |
|---|---|---|---|---|---|---|---|
| NHANES III 4 | 2096 | ≥65 | 8.2 | 16.6 | 19.7 |
B12: 5.9 B6: 6.4 Both: 2.0 |
33.6 |
| Health and Anemia 5 | 8744 | ≥65 | 15.0 | 16.0 | 17.4 | 10.1 | 26.4 |
| Community dwelling outpatients 64 | 190 | ≥65 | 4.0 | 12.0 | 6.0 | NA | 35 |
| InChianti 65 | 582 | ≥65 | 10.4 | 17.6 | 24.5 | 10.5 | 37.2 |
|
Leiden (≥ 85y) 66 |
490 | ≥85 years | 7.0 | 32.4 | 20.1 | 8.7 | 25.4 |
| Polish Clinic patients 7 | 981 | ≥60 | NA | NA | NA | NA | 28.4 |
| Innsbruck Medical University cohort 8 | 4117 | ≥64 | 11.3 | 14.4 | 62.1 |
B12: 2.0 B6: 6.7 |
‐ |
| Community dwelling 6 | 174 | ≥65 | 9.8 | 25 | 9.8 | 1 | 44 |
Abbreviations: ACD, anemia of chronic disease; ACI, anemia of chronic inflammation; CKD, chronic kidney disease; IDA, iron deficiency anemia; UAA, unexplained anemia of aging.
DIAGNOSTIC CRITERIA AND ETIOLOGY FOR UAA
The etiology of anemia in older patients is complex and falls into four often overlapping categories: (1) deficiencies of nutrients, including iron, vitamin B12, and folic acid; (2) anemia of inflammation or chronic disease, congestive heart failure (CHF), malignancy, auto‐immune disease or infections; (3) chronic kidney disease (CKD); (4) hematologic malignancies 6 ; and (5) UAA, which is diagnosed mainly by exclusion. Although there is not yet a consensus regarding its etiology, UAA is a recognized disease among older patients with anemia who do not meet the standard criteria for anemia subclassification. 8 , 9 , 10
The causes of UAA are likely multifactorial, with variable contributions from renal disease, endocrine deficiency (blunted erythropoietin response), chronic inflammation, androgen deficiency, nascent myelodysplasia, and other underlying conditions. Ferrucci et al. 11 reported a significant correlation between low circulating testosterone and anemia in community‐dwelling men and women over the age of 65 from the InChianti study, a relationship that was independent of circulating erythropoietin levels. 12 Tettamanti et al. 5 suggested a number of potential causes for low Hb in people over 65 years, including undiagnosed myelodysplastic syndrome, hypogonadism (testosterone <275 ng/ml), impaired bone marrow response to EPO (decreased responsiveness), low‐grade chronic inflammation (elevated IL‐6, TNF‐α, or hepcidin), vitamin D deficiency [25(OH) < 20 ng/ml], and unrecognized iron deficiency. Aging is also associated with dysfunction of hematopoiesis, evidenced by an age‐related decrease in the number of hematopoietic stem cells in the bone marrow and the circulation 13 . 14 Many men and women with anemia have elevated inflammatory markers in the absence of a diagnosed acute or chronic disease, 11 and should be considered and treated similarly to patients with UAA.
Although not unexplained, perioperative bleeding represents a significant cause of anemia for many older patients undergoing hip fracture repair. 15 Preoperative anemia in older patients is also common (up to 20% of patients) with limited treatment options. For example, intravenous iron is relatively save but not effective in raising hemoglobin levels in this patient group. 16 The drop in hemoglobin resulting from a hip fracture prior to surgery is substantial, 17 and the drop is exacerbated during the surgery. In the early postoperative period after hip fracture repair surgery, uncorrected anemia (Hb <100 g/L) was an independent risk factor for inability to walk on the third postoperative day, independent of the type of surgery or prefracture function.
Guralnik et al. 4 , 18 used the criteria established for the NHANES study to identify potential causes of anemia (left column, Table 2). In cases of anemia (Hb < 13 g/dl), UAA could be ruled out if a patient meets any these criteria. The right column of Table 2 contains our proposed expansion of the exclusion criteria used by Guralnik et al.
TABLE 2.
Diagnostic exclusion criteria for unexplained anemia of aging
| Potential cause | Guralnik et al. criteria | Expanded criteria |
|---|---|---|
| Diagnosis of anemia |
World Health Organization criteria: Men <13 g/dl Women <12 g/dl |
men and postmenopausal women <13 g/dl |
| Iron Status |
Ferritin <12 ng/ml Transferrin saturation < 15% |
Ferritin <40 ng/ml 67 Transferrin saturation < 15% |
| B12 | < 200 pg/ml | < 200 pg/ml |
| Folate |
red blood cell folate <102.6 ng/ml Home exam: serum folate <2.6 ng/ml |
Red blood cell folate <102.6 ng/ml Alternative: serum folate <2.6 ng/ml |
| Chronic kidney disease | Creatinine clearance <30 ml/m | Creatinine clearance <30 ml/m |
| Chronic inflammation | Serum iron <60 μg/dl | Diagnosed inflammatory disease and serum iron <60 μg/dl 10 |
| Thyrotropin/TSH | <0.1 mU/ml or >10 mU/ml (except in cases of corrective thyroid therapy without erythroid response) | |
| Other | No history of evidence of hematologic malignancy or myelodysplastic syndrome |
ANEMIA IS ASSOCIATED WITH MORBIDITY AND MORTALITY
Anemia is not only more common in older people but is also more likely to be associated with harm. A recent study 19 in a large cohort of 138,670 subjects demonstrated that in individuals older than 60 years, anemia was associated with elevated mortality and poorer quality of life (QOL), whereas in individuals aged 60 or younger, anemia had no effect on mortality and a very limited impact on health‐related quality of life. In that study, lower QOL resulted from lower physical function subscales. After adjusting for age, sex, type II diabetes, and comorbidity in a population of 17,030 community‐dwelling men and women over 66 years of age, anemia was associated with increased risks of all‐cause hospitalization (HR = 2.16, confidence interval [CI] 1.88–2.48) and death (HR: 4.29, CI 3.55–5.12). 20 Consistent with this, in men and women over the age of 65, anemia is associated with a higher risk of various poor health outcomes, including poor functional capacity, poor QOL, 21 depression, 22 sarcopenia, and poor muscle quality. 23 Similarly, data from the EPESE cohort 24 of older Americans demonstrated that after controlling for age, sex, cognitive status, blood creatinine, and comorbid conditions, individuals with anemia had more disabilities, and poorer functional status than people without anemia. Decline in performance with lower hemoglobin was also observed in people with hemoglobin levels above the WHO clinical threshold for anemia. Of course, factors other than age can exacerbate the risk of anemia; prevalence in older black men and women is substantially greater than that of the general population, 25 with black women 80–85 years old having a prevalence that is 6.4 times greater than the population average.
For older individuals with comorbid disease, the deleterious effects associated with anemia are similarly clear. For example, among patients >75 years old with stable angina, anemia is associated with elevated rates of death (34% increase for every 1 g/dl decrease in Hb, p < 0.01), increased cardiac death (28% increase for every 1 g/dl decrease in Hb, p < 0.01), and increased major adverse clinical events (23% increase for Hb <13.3 g/dl). 2
THE NEGATIVE EFFECTS OF ANEMIA ON PHYSICAL FUNCTION IN OLDER PEOPLE ARE UNDER‐ASCERTAINED AND UNDER‐REPORTED
Progressive, age‐associated decreases in functional capacity, strength, and physical activity are well‐described consequences of aging with multiple etiologies. Reduced hemoglobin is associated with low aerobic capacity, strength, and endurance in all adults, including older men and women. 26 Slower walking speed, an important biomarker of aging and prognostic of mortality and other adverse outcomes, is strongly associated with maximal aerobic capacity in older people. 27
Functional capacity, in turn, is strongly associated with health‐related outcomes in older men and women. In particular, the short physical performance battery (SPPB), a combined functional measurement of usual walking speed, time to stand up and sit down from a chair five times, and standing balance, is a powerful predictor of disability, institutionalization, and death. 28 , 29 Usual walking speed, one of the components of the SPPB, is strongly and independently associated with health‐related outcomes and mortality risk. 30 This simple assessment is so strongly linked to health‐related outcomes in geriatric patients that it has been termed the “sixth vital sign.” 31 The SPPB (or usual walking speed) can be easily measured in a limited amount of space with no requirement for specialized equipment. In addition, the Functional Assessment of Chronic Illness Therapy (FACIT) is a brief, standardized set of 13 questions 32 that can identify functional differences between patients with or without anemia.
Although these measurements are strongly linked to outcomes, they are not commonly assessed in geriatric patients. Perhaps because these functional tests are not a routine component of a geriatric assessment by most health care providers, decreases in function are not often recognized and, as a result, lower Hb levels in geriatric patients may be considered inconsequential, with little effect on functional status or quality of life. We strongly recommend the routine use of a standardized assessment of functional capacity and fatigue during routine office visits so that such deficits may be quickly identified, and appropriate therapies may be implemented. However, current treatment options for UAA are extremely limited. By definition, the etiology of UAA is not understood and traditional treatment options such as iron, folate, B12, or improved nutrition often have little or no effect on resolving the anemia. The use of erythropoietin stimulating agents may be effective, but are generally used only for severe anemia, and even then only rarely.
PHARMACOECONOMICS: ANEMIA LEADS TO HIGH HEALTHCARE COSTS IN OLDER PEOPLE
Use of healthcare resources and overall medical costs are significantly higher among patients with anemia. Anemia accounts for 2.8 million physician office visits and 890,000 emergency department visits per year in the United States. 33 One‐quarter of patients admitted to the hospital for anemia are readmitted to the emergency department within 30 days, 34 with the likelihood of unplanned readmission increasing with the severity of anemia at first discharge. 35 Following admission, anemia is associated with elevated in‐hospital mortality and longer stays. 36 , 37
These trends are exacerbated in the older people, in whom anemia is associated with up to fourfold greater risk of overall mortality and twofold greater risk of hospitalization. 20 , 38 Hospital stays are longer among older patients with anemia 37 , 39 : average length of stay increases 4–5 days in patients with moderate anemia and 7–10 days in the most severe cases. 40 As a consequence of the combined increases in hospitalization, readmission, length of stay, morbidity, and mortality, patients with anemia experience medical costs more than twice as high as those of patients without anemia of comparable age, sex, comorbidities, and insurance status. 41 This holds true for patients overall, as well as for populations with specific comorbidities: greater costs have been reported for patients with anemia and chronic kidney disease, 42 chronic obstructive pulmonary disease 43 or colorectal disease requiring surgery. 44 Anemia is also strongly associated with frailty in older men and women 45 : an increase in one point of hemoglobin concentration is associated with a 14% risk reduction of being frail (OR = 0.86, 95%IC = 0.79–0.94).
Anemia drives up healthcare costs in the older people by increasing the risk of falls. Individuals with anemia over the age of 65 years are twice as likely to experience recurrent falling 46 and are 3‐fold more likely to have a history of falls when controlling for age, gender, arthritis, and residence type. 40 Anemia is associated with a 66% increase in the incidence of injurious falls among people 65 years and older 47 (10% of all injurious falls in this age group). Risk rises as Hb level decreases, but importantly, even individuals with the mildest anemia (Hb 12–12.9 g/dl) are significantly more likely to experience falls. 40 , 47
Fall injuries are the fifth largest category of personal health care spending in the United States. People over 65 sustain more than 3.2 million injurious falls per year 48 and the ensuing care is responsible for up to $48–49 billion in costs. 49 , 50 A retrospective analysis of claims for injurious falls from more than 30 health plans (mean patient age, 76 years) performed in 2005 found that anemia increased costs by $1855 per patient per month, and $2811 for falls causing hip fracture. 51 Between 1996 and 2013, spending on falls increased 25%–50%, depending on the category of care, 49 and expenditures are predicted to continue rising. 50 Among older nursing home residents with a hip fracture, anemia is associated with an increased mortality risk (HR = 1.6, 95% CI 1.1–2.5). 52 Anemia is 4‐fold more prevalent among nursing home residents in the US than in community‐dwelling older people (56% overall; 64% for males and 53% for females) and was associated with a 2‐fold increase in risk of falling. 53
CLINICAL CONSEQUENCES OF MILD ANEMIA
These studies demonstrate that anemia is associated with elevated morbidity and mortality in older men and women. It is important to note that these risks are also present for patients on the mild end of the anemia spectrum. Awareness of these clinical consequences is particularly important because of the high prevalence of mild anemia among patients with anemia. The vast majority of UAA cases (91.7%) are classified as mild (Hb ≥ 10 g/dl), 7 with most patients presenting with hypoproliferative anemia with normocytic indices. 5 Moreover, the proportion of patients with anemia that can be classified as mild (Hb ≥ 10 g/dl) increases with age (Figure 1).
Relative to age‐matched nonanemic people, patients with mild anemia experience declines in mobility and physical performance. 4 Moreover, mild anemia is prospectively associated with clinically relevant outcomes such as risk of hospitalization and all‐cause mortality 38 (Table 3). Mortality increases steadily as Hb declines: in women over 65, Hb of 11 g/dl is associated with significantly greater mortality than the low‐normal WHO cutoff of 12 g/dl; conversely, women with Hb of 14 g/dl experienced a 24% reduction in mortality relative to patients below the cutoff. 54 The significant differences in mortality risk among people near the threshold for anemia diagnosis strongly implies that the overall health consequences of anemia cannot be attributed to individuals with severely low Hb.
TABLE 3.
Mild anemia increases hospitalization and mortality
| Percent hospitalized | Percent deceased | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Years of follow‐up | 0.5 | 1.0 | 1.5 | 2.0 | 0.5 | 1.0 | 1.5 | 2.0 | |
| Women | Nonanemic | 5 | 11 | 16 | 21 | 0.5 | 2 | 2.5 | 5 |
| Mildly anemic | 13 | 20 | 26 | 29 | 3 | 7.5 | 10 | 13 | |
| Men | Nonanemic | 10 | 18 | 24 | 30 | 1 | 2 | 4 | 10 |
| Mildly anemic | 17 | 28 | 35 | 42 | 5 | 9 | 14 | 17 | |
Note: Percent of patients experiencing one or more hospitalizations or deceased, stratified by sex and mild anemia status, over a 2‐year follow‐up in the Health and Anemia population‐based study (2003–2007). Mild anemia was defined as Hb concentration between 10.0 and 11.9 g/dl in women and between 10.0 and 12.9 g/dl in men. Source: From Reference 38.
Among both male and female participants in the Cardiovascular Health Study, baseline Hb status was strongly associated with health‐related outcomes, and a decrease in Hb over 3 years was associated with decline in cognitive function. 3 The authors concluded that reduced blood Hb was present in many older patients who were at elevated risk for adverse outcomes but who would not have been identified as anemic according to the WHO criteria.
Together, these associations with poor clinical outcomes indicate that mild anemia should receive clinical attention rather than simply being considered a normal part of aging. 4
DEFINING ANEMIA THRESHOLDS IN MEN AND WOMEN
The WHO definition of anemia uses different Hb thresholds for men and women, but these differences are based largely on population averages for all adults, rather than on specific clinical outcomes related to Hb values <12 g/dl for women and <13 g/dl for men. According to the WHO criteria, the prevalence of anemia increases with advancing age to a greater extent in men than in women 4 as a result of different sex‐related thresholds for diagnosis. However, there is little metabolic or physiological rationale for a different threshold between postmenopausal women and older men. Importantly, women with a Hb concentration between 12.0 and 13.0 g/dl (anemic in men but not in women, according to the WHO definition) experienced a significantly lower QOL than women with Hb > 13 g/dl. Mild anemia, estimated to affect 11.1% in the population over the age of 65, 5 is defined by WHO criteria as Hb levels of 11.0–11.9 in women and 11.0–12.9 g/dl in men. The differences in these ranges (0.9 g/dl in women vs 1.9 g/dl in men) result in different therapeutic guidelines, potentially leading to undertreatment of older women with anemia. Recently, Simonsick et al. 55 examined fatigue and anemia in a cohort of older men and women from the Baltimore Longitudinal Study on Aging. They reported that even subclinical anemia, defined as Hb of 12–12.9 g/dl for women and 13–13.9 g/dl for men, was associated with fatigability. They also demonstrated that fatigability was predictive of subsequent clinical anemia. The observation that reduced QOL is observed in older women with Hb values up to 13 g/dl implies that the threshold for anemia in postmenopausal women should be similar to that in men. Such a change in diagnostic criteria would allow mild anemia in older people to be defined in the same range, and to be treated according to the same guidelines, in both sexes.
THERAPIES FOR UAA
At the present time, there is no consensus optimal therapy for UAA, 56 in large part because a single targetable deficit has not been characterized. Currently, erythropoietin‐stimulating agents (ESA) such as epoetin alfa and darbepoetin alfa are used to treat anemia in patients with chronic kidney disease (CKD), patients receiving dialysis, or in patients with nonmyeloid malignancies where anemia is due to effect of concomitant myelosuppressive chemotherapy. 57 A retrospective analysis 58 of patients with anemia over aged 60 revealed that those with UAA had inappropriately low EPO levels after adjusting for Hb, eGFR, and comorbidities. Gowanlock et al. 59 performed a retrospective analysis of the use of epoetin alfa to treat UAA in 570 patients with anemia over aged 60. They observed a larger increase in Hb in UAA (47%) and CKD (54%) relative to other etiologies (22%) and found that a baseline EPO level < 200 IU/L independently predicted treatment response. Agnihotri et al. 60 conducted a randomized controlled trial to examine the effects of epoetin alfa versus placebo in older (≥65 years; mean age 76.1 ± 7.2 years) women with anemia (Hb ≤ 11.5 g/dl). Patients randomized to epoetin alfa had improved fatigue and QOL relative to the placebo group, and achieved Hb was directly related to improvements in fatigue and QOL. These data imply that patients over the age of 65 years are quite responsive to ESAs.
Idiopathic cytopenia of unknown significance with anemia has been proposed to be synonymous with UAA, 10 and Maggio et al. 61 suggested that UAA is a result of the cumulative effects of age‐associated changes in androgen production, low insulin like growth factor 1, and low thyroid hormone levels. At present, however, there is no consensus on the definitive etiology for UAA. Consequently, the diagnosis of UAA is one of exclusion, according to the criteria enumerated in Table 2. The criteria in this table have been somewhat expanded using data published after the paper by Guralnik et al. Moreover, despite strong evidence that even mild anemia is associated with fatigue and reduced quality of life, there is no consensus for treatment options.
Guralnik et al. 18 suggested that patients with UAA may have age‐associated deficiencies in the ability to sense hypoxia or erythropoietin (EPO), and consequently require higher rates of erythropoietin production to maintain normal erythrocyte production, a finding supported by data from the Baltimore Longitudinal Study of Aging. 62 Ferrucci et al. 11 separately hypothesized that in many anemic older people, elevated inflammation may prevent EPO from being upregulated sufficiently to meet the need for red cell production.
Several potential treatment options for UAA have been suggested 56 or are in development, including hepcidin antagonists, hypoxia‐inducible factor (HIF)‐prolyl hydroxylase inhibitors (PHIs), the transforming growth factor beta superfamily ligands, and androgens. The HIF‐PHIs are of particular importance. The HIF pathway is a critical component of the cellular response to lower oxygen, and accordingly plays a major role in the regulation of iron metabolism and erythropoiesis. HIF prolyl hydroxylases (PHDs) post‐translationally modify HIF, targeting it for degradation by the proteasome; HIF‐PHIs prevent this degradation and increase HIF activity under normoxia. 63 Unlike ESAs, which are delivered via injection, this class of drugs is orally delivered small molecules that stimulate production of EPO at physiologic levels. Because a HIF‐PHI has been approved for a chronic indication (CKD) in multiple nations, it is reasonable to consider this class of drugs as candidate treatments for UAA.
CONCLUSIONS
Anemia is prevalent in men and women over the age of 65 years and becomes progressively more common with age. Anemia is associated with reductions in functional capacity and quality of life, as well an increased risk of death from all causes. Roughly one third of anemia in older patients is unexplained (UAA). Although the WHO diagnostic thresholds for anemia are most frequently used, evidence supports the use of a single criterion of Hb < 13 g/dl in both older men and postmenopausal women. Even mild anemia (Hb 11.0–12.9) is associated with poor clinical outcomes, lower QOL, and elevated mortality. Although UAA is currently diagnosed by exclusion of identifiable causes for anemia, the diagnostic criteria for UAA are clearly defined, and the UAA patient population experiences significant functional decline, morbidity, and mortality. In part due to the lack of consensus on the etiology of UAA, treatment options are extremely limited at present. Novel therapies are currently in development for the treatment of this highly prevalent condition. Finally, we strongly recommend routine assessment of functional capacity and quality of life in all older patients by healthcare providers, particularly those suffering from anemia, using standardized tools that will help to identify functional deficits and changes in functional capacity in older patients with anemia.
CONFLICT OF INTEREST
No other conflicts are reported.
AUTHOR CONTRIBUTIONS
William Evans is a consultant for BioAge Labs, Inc. All authors contributed equally to the writing and editing of this manuscript.
SPONSOR'S ROLE
BioAge Labs provided honoraria to some of the co‐authors to participate in a virtual meeting to discuss the topic of unexplained anemia of aging. The review was written and approved by the authors. BioAge Labs was provided a copy of the manuscript prior to submission for review.
ACKNOWLEDGMENTS
This manuscript resulted from a meeting on the topic of Anemia and Aging funded with hon oraria provided by BioAge Labs, Inc.
Guralnik J, Ershler W, Artz A, et al. Unexplained anemia of aging: Etiology, health consequences, and diagnostic criteria. J Am Geriatr Soc. 2022;70(3):891‐899. doi: 10.1111/jgs.17565
Funding information BioAge Labs, Inc
REFERENCES
- 1. Ania BJ, Suman VJ, Fairbanks VF, Rademacher DM, Melton LJ 3rd. Incidence of anemia in older people: an epidemiologic study in a well defined population. J Am Geriatr Soc. 1997;45(7):825‐831. [DOI] [PubMed] [Google Scholar]
- 2. Artz AS, Fergusson D, Drinka PJ, et al. Prevalence of anemia in skilled‐nursing home residents. Arch Gerontol Geriatr. 2004;39(3):201‐206. [DOI] [PubMed] [Google Scholar]
- 3. Zakai NA, French B, Arnold AM, et al. Hemoglobin decline, function, and mortality in the elderly: the cardiovascular health study. Am J Hematol. 2013;88(1):5‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263‐2268. [DOI] [PubMed] [Google Scholar]
- 5. Tettamanti M, Lucca U, Gandini F, et al. Prevalence, incidence and types of mild anemia in the elderly: the "health and anemia" population‐based study. Haematologica. 2010;95(11):1849‐1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Artz AS, Thirman MJ. Unexplained anemia predominates despite an intensive evaluation in a racially diverse cohort of older adults from a referral anemia clinic. J Gerontol A Biol Sci Med Sci. 2011;66(8):925‐932. [DOI] [PubMed] [Google Scholar]
- 7. Michalak SS, Rupa‐Matysek J, Hus I, Gil L. Unexplained anemia in the elderly—a real life analysis of 981 patients. Arch Med Sci. 2020;16(4):834‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bach V, Schruckmayer G, Sam I, Kemmler G, Stauder R. Prevalence and possible causes of anemia in the elderly: a cross‐sectional analysis of a large European university hospital cohort. Clin Interv Aging. 2014;9:1187‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Halawi R, Moukhadder H, Taher A. Anemia in the elderly: a consequence of aging? Expert Rev Hematol. 2017;10(4):327‐335. [DOI] [PubMed] [Google Scholar]
- 10. Stauder R, Valent P, Theurl I. Anemia at older age: etiologies, clinical implications, and management. Blood. 2018;131(5):505‐514. [DOI] [PubMed] [Google Scholar]
- 11. Ferrucci L, Guralnik JM, Woodman RC, et al. Proinflammatory state and circulating erythropoietin in persons with and without anemia. Am J Med. 2005;118(11):1288. [DOI] [PubMed] [Google Scholar]
- 12. Maggio M, Snyder PJ, Ceda GP, et al. Is the haematopoietic effect of testosterone mediated by erythropoietin? The results of a clinical trial in older men. Andrology. 2013;1(1):24‐28. [DOI] [PubMed] [Google Scholar]
- 13. Groarke EM, Young NS. Aging and hematopoiesis. Clin Geriatr Med. 2019;35(3):285‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Haan G, Lazare SS. Aging of hematopoietic stem cells. Blood. 2018;131(5):479‐487. [DOI] [PubMed] [Google Scholar]
- 15. Foss NB, Kristensen MT, Kehlet H. Anaemia impedes functional mobility after hip fracture surgery. Age Ageing. 2008;37(2):173‐178. [DOI] [PubMed] [Google Scholar]
- 16. Smith A, Moon T, Pak T, Park B, Urman RD. Preoperative anemia treatment with intravenous iron in patients undergoing major orthopedic surgery: a systematic review. Geriatr Orthop Surg Rehabil. 2020;11:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith GH, Tsang J, Molyneux SG, White TO. The hidden blood loss after hip fracture. Injury. 2011;42(2):133‐135. [DOI] [PubMed] [Google Scholar]
- 18. Guralnik JM, Ershler WB, Schrier SL, Picozzi VJ. Anemia in the elderly: a public health crisis in hematology. Hematology Am Soc Hematol Educ Program. 2005;2005(1):528‐532. [DOI] [PubMed] [Google Scholar]
- 19. Wouters H, van der Klauw MM, de Witte T, et al. Association of anemia with health‐related quality of life and survival: a large population‐based cohort study. Haematologica. 2019;104(3):468‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Culleton BF, Manns BJ, Zhang J, Tonelli M, Klarenbach S, Hemmelgarn BR. Impact of anemia on hospitalization and mortality in older adults. Blood. 2006;107(10):3841‐3846. [DOI] [PubMed] [Google Scholar]
- 21. Lucca U, Tettamanti M, Mosconi P, et al. Association of mild anemia with cognitive, functional, mood and quality of life outcomes in the elderly: the "health and anemia" study. PLoS One. 2008;3(4):e1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Onder G, Penninx BW, Cesari M, et al. Anemia is associated with depression in older adults: results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2005;60(9):1168‐1172. [DOI] [PubMed] [Google Scholar]
- 23. Cesari M, Penninx BW, Lauretani F, et al. Hemoglobin levels and skeletal muscle: results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59(3):249‐254. [DOI] [PubMed] [Google Scholar]
- 24. Penninx BW, Guralnik JM, Onder G, Ferrucci L, Wallace RB, Pahor M. Anemia and decline in physical performance among older persons. Am J Med. 2003;115(2):104‐110. [DOI] [PubMed] [Google Scholar]
- 25. Le CH. The prevalence of anemia and moderate‐severe anemia in the US population (NHANES 2003‐2012). PLoS One. 2016;11(11):e0166635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Celsing F, Svedenhag J, Pihlstedt P, Ekblom B. Effects of anaemia and stepwise‐induced polycythaemia on maximal aerobic power in individuals with high and low haemoglobin concentrations. Acta Physiol Scand. 1987;129(1):47‐54. [DOI] [PubMed] [Google Scholar]
- 27. Fiser WM, Hays NP, Rogers SC, et al. Energetics of walking in elderly people: factors related to gait speed. J Gerontol A Biol Sci Med Sci. 2010;65(12):1332‐1337. [DOI] [PubMed] [Google Scholar]
- 28. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower‐extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guralnik JM, Simonsick EM, Ferrucci L, Wallace RB. Short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85‐M94. [DOI] [PubMed] [Google Scholar]
- 30. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. J Aging Phys Act. 2015;23(2):314‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94(2):528‐538. [DOI] [PubMed] [Google Scholar]
- 33. CDC . Anemia or Iron Deficiency 2016.
- 34. Brunner‐La Rocca HP, Peden CJ, Soong J, Holman PA, Bogdanovskaya M, Barclay L. Reasons for readmission after hospital discharge in patients with chronic diseases‐information from an international dataset. PLoS One. 2020;15(6):e0233457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lin RJ, Evans AT, Chused AE, Unterbrink ME. Anemia in general medical inpatients prolongs length of stay and increases 30‐day unplanned readmission rate. South Med J. 2013;106(5):316‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Krishnasivam D, Trentino KM, Burrows S, et al. Anemia in hospitalized patients: an overlooked risk in medical care. Transfusion. 2018;58(11):2522‐2528. [DOI] [PubMed] [Google Scholar]
- 37. Zaninetti C, Klersy C, Scavariello C, Bastia R, Balduini CL, Invernizzi R. Prevalence of anemia in hospitalized internal medicine patients: correlations with comorbidities and length of hospital stay. Eur J Intern Med. 2018;51:11‐17. [DOI] [PubMed] [Google Scholar]
- 38. Riva E, Tettamanti M, Mosconi P, et al. Association of mild anemia with hospitalization and mortality in the elderly: the health and anemia population‐based study. Haematologica. 2009;94(1):22‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Migone De Amicis M, Poggiali E, Motta I, et al. Anemia in elderly hospitalized patients: prevalence and clinical impact. Intern Emerg Med. 2015;10(5):581‐586. [DOI] [PubMed] [Google Scholar]
- 40. Dharmarajan TS, Norkus EP. Mild anemia and the risk of falls in older adults from nursing homes and the community. J Am Med Dir Assoc. 2004;5(6):395‐400. [DOI] [PubMed] [Google Scholar]
- 41. Nissenson AR, Wade S, Goodnough T, Knight K, Dubois RW. Economic burden of anemia in an insured population. J Manag Care Pharm. 2005;11(7):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. St Peter WL, Guo H, Kabadi S, et al. Prevalence, treatment patterns, and healthcare resource utilization in Medicare and commercially insured non‐dialysis‐dependent chronic kidney disease patients with and without anemia in the United States. BMC Nephrol. 2018;19(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Halpern MT, Zilberberg MD, Schmier JK, Lau EC, Shorr AF. Anemia, costs and mortality in chronic obstructive pulmonary disease. Cost Eff Resour Alloc. 2006;4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Feng S, Greenberg J, Moloo H, Thavorn K, McIsaac DI. Hospital cost associated with anemia in elective colorectal surgery: a historical cohort study. Can J Anaesth. 2019;66(8):877‐885. [DOI] [PubMed] [Google Scholar]
- 45. Steinmeyer Z, Delpierre C, Soriano G, et al. Hemoglobin concentration; a pathway to frailty. BMC Geriatr. 2020;20(1):202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Penninx BW, Pluijm SM, Lips P, et al. Late‐life anemia is associated with increased risk of recurrent falls. J Am Geriatr Soc. 2005;53(12):2106‐2111. [DOI] [PubMed] [Google Scholar]
- 47. Duh MS, Mody SH, Lefebvre P, Woodman RC, Buteau S, Piech CT. Anaemia and the risk of injurious falls in a community‐dwelling elderly population. Drugs Aging. 2008;25(4):325‐334. [DOI] [PubMed] [Google Scholar]
- 48. Burns ER, Stevens JA, Lee R. The direct costs of fatal and non‐fatal falls among older adults ‐ United States. J Safety Res. 2016;58:99‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dieleman JL, Baral R, Birger M, et al. US spending on personal health care and public health, 1996–2013. JAMA. 2016;316(24):2627‐2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Florence CS, Bergen G, Atherly A, Burns E, Stevens J, Drake C. Medical costs of fatal and nonfatal falls in older adults. J Am Geriatr Soc. 2018;66(4):693‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lefebvre PM, Samir H, Duh MS, Woodman RC, Buteau S, Tak Piech C. Impact of anemia on the medical cost of injurious falls in the elderly. Blood. 2005;106: Abstract 2238. [Google Scholar]
- 52. Berry SD, Samelson EJ, Bordes M, Broe K, Kiel DP. Survival of aged nursing home residents with hip fracture. J Gerontol A Biol Sci Med Sci. 2009;64(7):771‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pandya N, Bookhart B, Mody SH, Funk Orsini PA, Reardon G. Study of anemia in long‐term care (SALT): prevalence of anemia and its relationship with the risk of falls in nursing home residents. Curr Med Res Opin. 2008;24(8):2139‐2149. [DOI] [PubMed] [Google Scholar]
- 54. Chaves PH, Xue QL, Guralnik JM, Ferrucci L, Volpato S, Fried LP. What constitutes normal hemoglobin concentration in community‐dwelling disabled older women? J Am Geriatr Soc. 2004;52(11):1811‐1816. [DOI] [PubMed] [Google Scholar]
- 55. Simonsick EM, Patel KV, Schrack JA, Ferrucci L. Fatigability as a predictor of subclinical and clinical anemia in well‐functioning older adults. J Am Geriatr Soc. 2020;68(10):2297‐2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Busti F, Marchi G, Lira Zidanes A, Castagna A, Girelli D. Treatment options for anemia in the elderly. Transfus Apher Sci. 2019;58(4):416‐421. [DOI] [PubMed] [Google Scholar]
- 57. Bohlius J, Bohlke K, Castelli R, et al. Management of cancer‐associated anemia with erythropoiesis‐stimulating agents: ASCO/ASH clinical practice guideline update. J Clin Oncol. 2019;37(15):1336‐1351. [DOI] [PubMed] [Google Scholar]
- 58. Gowanlock Z, Sriram S, Martin A, Xenocostas A, Lazo‐Langner A. Erythropoietin levels in elderly patients with anemia of unknown etiology. PLoS One. 2016;11(6):e0157279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gowanlock Z, Sriram S, Martin A, Xenocostas A, Lazo‐Langner A. Erythropoiesis‐stimulating agents in elderly patients with anemia: response and cardiovascular outcomes. Blood Adv. 2017;1(19):1538‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Agnihotri P, Telfer M, Butt Z, et al. Chronic anemia and fatigue in elderly patients: results of a randomized, double‐blind, placebo‐controlled, crossover exploratory study with epoetin alfa. J Am Geriatr Soc. 2007;55(10):1557‐1565. [DOI] [PubMed] [Google Scholar]
- 61. Maggio M, De Vita F, Fisichella A, et al. The role of the multiple hormonal dysregulation in the onset of "anemia of aging": focus on testosterone, IGF‐1, and thyroid hormones. Int J Endocrinol. 2015;2015:1‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ershler WB, Sheng S, McKelvey J, et al. Serum erythropoietin and aging: a longitudinal analysis. J Am Geriatr Soc. 2005;53(8):1360‐1365. [DOI] [PubMed] [Google Scholar]
- 63. Haase VH. Hypoxia‐inducible factor‐prolyl hydroxylase inhibitors in the treatment of anemia of chronic kidney disease. Kidney Int Suppl (2011). 2021;11(1):8‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Price EA, Mehra R, Holmes TH, Schrier SL. Anemia in older persons: etiology and evaluation. Blood Cells Mol Dis. 2011;46(2):159‐165. [DOI] [PubMed] [Google Scholar]
- 65. Chaves PH, Semba RD, Leng SX, et al. Impact of anemia and cardiovascular disease on frailty status of community‐dwelling older women: the women's health and aging studies I and II. J Gerontol A Biol Sci Med Sci. 2005;60(6):729‐735. [DOI] [PubMed] [Google Scholar]
- 66. den Elzen WP, de Craen AJ, Wiegerinck ET, Westendorp RG, Swinkels DW, Gussekloo J. Plasma hepcidin levels and anemia in old age. The Leiden 85‐plus study. Haematologica. 2013;98(3):448‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Roy CN, Snyder PJ, Stephens‐Shields AJ, et al. Association of testosterone levels with anemia in older men: a controlled clinical trial. JAMA Intern Med. 2017;177(4):480‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]


