Abstract
Aims
To test whether the Stop‐tabac smartphone application (app) increased smoking cessation rates.
Design
A two‐arm, parallel‐group, individually randomized, double‐blind, controlled trial.
Setting and Participants
A total of 5293 daily smokers (Stop‐tabac = 2639, control = 2654) enrolled on app stores and on the internet in 2019–20, who lived in France or Switzerland.
Intervention and comparator
The Stop‐tabac application includes immediate feedback during episodes of craving and withdrawal; individually tailored counseling messages with notifications sent during 6 months; a discussion forum; fact sheets; modules on nicotine replacement therapy and e‐cigarettes; and calculators of cigarettes not smoked, money saved and days of life gained since quitting. The control application included five brief pages and calculators as above.
Measurements
Primary outcome: self‐reported smoking cessation after 6 months (no puff of tobacco in the past 4 weeks), with non‐responders counted as smokers. Secondary outcome: self‐reported use of nicotine medications.
Findings
Participants were aged 36 years on average; 66% were women who smoked 15 cigarettes/day, and 64% screened positive for depression. Stop‐tabac participants used the app over a longer period than control participants (23 versus 11 days, P < 0.001). Smoking cessation rates after 6 months were 9.9% in the Stop‐tabac group versus 10.3% in the control group (odds ratio = 0.96, 95% confidence interval = 0.80–1.45, P = 0.63). Rates of use of nicotine medications after entry in the study were 38 versus 30% after 6 months (χ2 = 8.3, P = 0.004) in the Stop‐tabac and control groups. After 6 months, 26% of participants in the Stop‐tabac group and 8% in the control group said that the app helped them ‘a lot’ or ‘enormously’ to quit smoking (χ2 = 113, P < 0.001).
Conclusions
In smokers enrolled on the app stores and the internet, allocation to the Stop‐tabac smoking cessation app did not increase smoking cessation rates, but increased rates of use of nicotine medications.
Keywords: Mobile phone, nicotine replacement therapy, randomized controlled trial, smartphone, smoking, smoking cessation, tobacco dependence
INTRODUCTION
Background
Mobile health (mHealth) tools can extend smoking cessation counseling beyond specialized clinics, doctors’ offices and quitlines, and reach smokers who never receive support from these sources. Text messaging on mobile phones is one of the most affordable effective interventions to assist tobacco cessation [1]. A Cochrane meta‐analysis found that text messaging was effective for smoking cessation, but that smartphone applications (apps) had no effect [2]. However, there were only five eligible trials of smartphone apps in this review, and the conclusion was of low certainty due to the inconsistency and imprecision of the trials [2]. Thus, good‐quality trials in large samples are needed [2, 3].
Literature reviews concluded that the average quality of smartphone apps for smoking cessation was poor, that few apps adhered to guidelines for treating tobacco dependence or provided individually tailored feedback and that most apps used simplistic tools only [4, 5, 6, 7]. One review concluded that only 4% of the 50 most downloaded smoking cessation apps had any scientific support, and that only half of the scientifically vetted apps were still available to consumers at the time of the review [6]. There is a multi‐billion‐dollar mHealth industry [4, 8], but very few apps are based on science and very few have been evaluated via randomized controlled trials.
Too few smokers use nicotine replacement therapy (NRT) during quit attempts, either because of misconceptions about the nature of addiction and about the benefits and risks of NRT, or because of NRT's cost [9]. When they use NRT, many smokers use it without behavioral support and therefore do not obtain the full benefit this treatment offers, in particular because of poor compliance [10]. Therefore, there is a need to produce mass‐level behavioral support for people who either are prescribed NRT but do not receive behavioral support, or purchase NRT without a prescription.
The app tested in this study
The Stop‐tabac smartphone app, available in French, German and Italian (and formerly in English), is a stand‐alone intervention intended at motivating and helping smokers to quit smoking. It has been available at no charge since 2012 for iOS (Apple) and Android, and will still be available in the future. The app was developed by the authors and by experts in addictions; this was the fourth version of the app. This app is based on behavior change and addiction theories [11, 12, 13], on guidelines and literature reviews on tobacco dependence treatments [1, 14], on the applicants’ research and experience with smokers and on suggestions made by users. A review independent from the authors ranked Stop‐tabac among the best five smoking cessation apps world‐wide [7]. The app was used by 24 000 people every month before we started this study, but new downloads were deactivated 1 month before we started enrolling study participants in order to enroll naive users only.
Objectives
The primary objective of this study was to test whether the French version of the Stop‐tabac application improved smoking cessation rates in daily smokers and helped them maintain abstinence over 6 months. Secondary objectives were to assess whether the outcome was influenced by the personal characteristics of participants (dependence level, sex and age, smoking history and depression) and to assess whether the app had any effect on motivation to quit, quit attempts, cigarettes smoked per day and use of NRT, e‐cigarettes and heated tobacco [13].
METHODS
Study design and participants
A two‐arm, parallel‐group, superiority, individually randomized, double‐blind, controlled trial with follow‐up after 1 week, 1 month and 6 months. Participants were 5293 daily cigarette smokers who lived in Switzerland or France. Recruitment was through self‐identification and self‐selection; participants either found the app directly on the Apple App Store or Google Play Store, or via advertisements on the internet (including on Stop‐tabac.ch, Stop‐alcool.ch and Stop‐cannabis.ch). The study protocol was published [15].
Outcomes
Primary outcome
Self‐reported smoking cessation after 6 months (no puff of tobacco in the past 4 weeks), which is the criterion recommended by the American Food and Drug Administration (FDA) [16].
Secondary outcomes
Point prevalence of smoking abstinence at 6 months (no puff of tobacco in the previous 7 days)
The Russell Standard: continuous 6‐month (at 6 months) or 1‐month (at 1 month) smoking abstinence allowing for smoking five or fewer cigarettes after the target quit date, but no cigarette in the past 7 days [17].
Abstinence of tobacco use after 1 week and after 1 month (no use in the previous 7 days), use of nicotine medications (we did not ask whether medications were obtained with a medical prescription), use of e‐cigarettes and heated tobacco after entry in the study, use of any smoking cessation ‘app’ for mobile devices after entry in the study (including Stop‐tabac) and perceived usefulness of the study app.
In smokers at follow‐up: motivation to quit, quit attempts since the target quit date and cigarettes per day.
Quit date in quitters at follow‐up.
Data collected automatically by the app during 6 months.
Dates of the first and last times the app was opened, number of different days when the app was opened, number of different pages (screens) seen on the app (two views of the same screen were counted as two occurrences); these data were collected in all 5293 participants (no missing data).
Inclusion criteria
Daily cigarette smoker.
Has been a daily smoker for at least 1 year.
Aged ≥ 18 years.
Sets a target quit date within 1 month of enrollment and commits to quit on this date.
Provides informed consent on‐line.
Commits to answer all follow‐up questionnaires.
Commits to use the app.
Owns a smartphone (Android or iOS) and has regular access to e‐mail.
Provides a postal address, a telephone number and a valid e‐mail address.
Lives in Switzerland or in France.
Exclusion criterion
Self‐report of prior use of the Stop‐tabac app.
Enrollment, informed consent
Once on the app stores, participants downloaded the app. At this point, the app only displayed a screen that instructed them to visit the study website (with link), where they read the consent form and answered the baseline questionnaire. Participants received the intervention at no charge and were not paid.
Randomization
Randomization took place after we received participants’ answers to the questionnaire and after we verified eligibility. Eligibility was assessed automatically by computer algorithms. We performed an automated check of e‐mail addresses, names, age and sex to avoid double registrations. Eligible participants received an e‐mail message with a link to a webpage displaying a personal code to access their allocated version of the app. Randomization was used only in those who clicked on this link, which ensured that we had a valid e‐mail address for each participant. Randomization was performed by a computer using a list of random numbers with a 1:1 ratio, with a script written by our team in Perl language. Participants inserted their personal code manually in the app on their smartphone, and at this point they accessed either the Stop‐tabac or the control app. This procedure ensured that people registered only once, that all of them downloaded the app and opened it at least once and that they had access to their intended intervention only. Participants who did not enter their personal code in the app were excluded after randomization, as specified in the protocol [15] (Figure 1).
FIGURE 1.
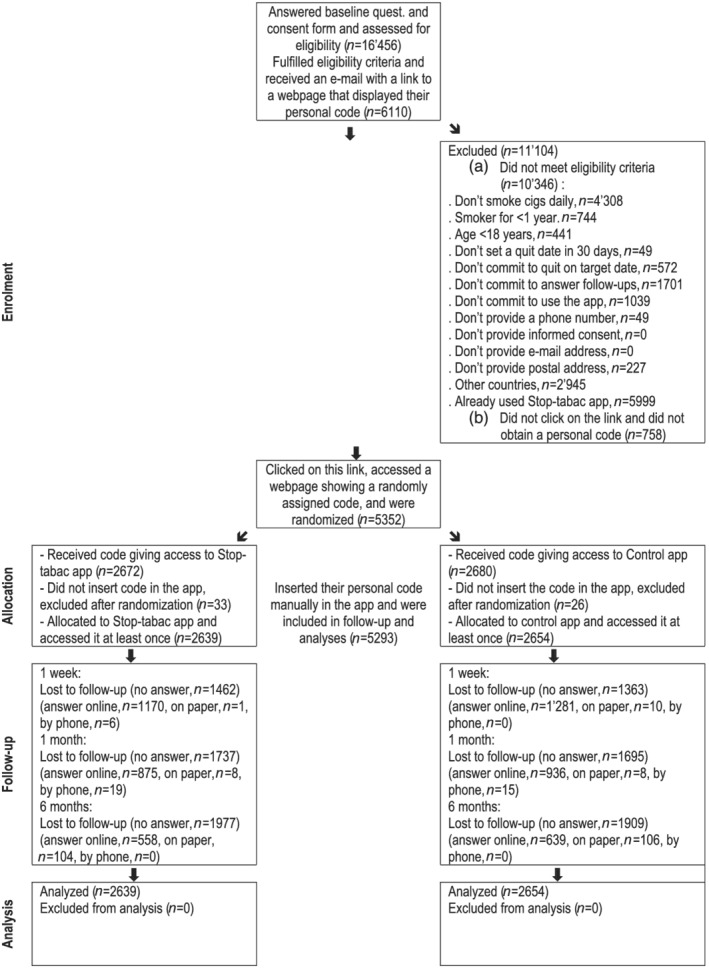
Flow‐chart of study participants
Blinding
Participants were blinded to their assignment group and to the existence of a control group. We waited until the end of data collection to inform them of the existence of the control version and to give them access to the full version. The collection of on‐line questionnaires was fully automatic. Assistants who sent text messages to non‐respondents and collected questionnaires by postal mail and over the telephone were blinded to the group assignment of participants. The data analyst was also blinded, and the data were unblinded only after the main results were written up. After randomization, we only contacted participants in both study groups for the follow‐up surveys.
Intervention
The Stop‐tabac app includes:
Brief information pages on tobacco dependence, withdrawal symptoms, treatments, relapse situations, mood management, risks of smoking and benefits of quitting and heated tobacco products.
Calculators: number of cigarettes not smoked, money saved and days of life gained since quitting.
Ecological momentary intervention when participants experienced challenging situations: a ‘Difficulty’ button gave access to relevant information in three categories: relapse, craving level (rated with a slider graded 1–5 followed by a personalized message) and withdrawal symptoms.
A quiz (42 questions) which allows the transmission of knowledge in a playful manner. Users could either answer all quiz questions at once or a few questions at a time.
Telephone numbers of the quitlines in France or Switzerland, dialed automatically.
The ‘Coach’, an automatic system that produces individually tailored feedback messages and sends reminders (push notifications within the app during 6 months, sent weekly during the first month then every 2 weeks), based on each user's target quit date, tobacco dependence level, perceived advantages and drawbacks of smoking and motivation to quit. The app does not send text messages on mobile phones.
A discussion forum (in written format) moderated by a psychologist helped by volunteer moderators [18]. Users were not prompted to engage with the discussion forum.
A module aimed at increasing use of, and compliance with, NRT that includes fact sheets (NRT utilization, benefits and side‐effects), a series of brief individually tailored messages sent during 3 months after the quit date based on users’ responses to questions on current NRT use, knowledge about NRT, intention to use NRT and perceived effect on withdrawal symptoms.
A module on electronic cigarettes that includes a series of brief individually tailored feedback messages based on users’ responses to questions on current vaping, intention to use e‐cigarettes, opinions about the effects of vaping on smoking cessation and nicotine withdrawal symptoms, perceived side‐effects and perceived addictiveness of e‐cigarettes. The e‐cigarette module did not encourage people to use e‐cigarettes as smoking cessation aids, but presented studies showing that e‐cigarettes increase the odds of quitting smoking.
The Stop‐tabac app belongs to the University of Geneva. It was developed under the responsibility of the authors, who have no financial interest in this app and no conflict of interest with the pharmaceutical, tobacco or e‐cigarette industries. The development and maintenance of the was supported by the Swiss Tobacco Prevention Fund (at the Swiss Ministry of Health), and the app never received support from the pharmaceutical, tobacco or e‐cigarette industries. During the study, registration in this study was mandatory to access the app.
The conditions of enrollment in, and of utilization of, the app in the trial were similar to conditions outside the trial. In particular, users found the app directly on the app stores and on our websites, the app was used ad libitum, we did not give users any instructions regarding frequency of use and we did not use any prompts or reminders to trigger utilization, apart from the notifications normally sent within the app itself. There is no human involvement in this intervention; apart for the moderation of the discussion forum, this moderation is aimed at preventing trolling and at resolving conflicts between users. The moderators do not provide smoking cessation counselling.
Control group
The control app was also named Stop‐tabac; it used the same graphic design as the complete app but included a few features only: five brief information pages (100–300 words each, on the addictiveness of nicotine, reasons to quit, deciding to quit, finding other pleasurable things to do and money savings) and calculators as above. We designed the control app in this way to reduce the likelihood that control participants realized that they received a control app. We do not think that this content was sufficient to produce an impact, but enough to prevent the potential problem that the control app was so unsatisfactory that control participants accessed other support more frequently than intervention participants.
Baseline data
The baseline questionnaire, in French, was administered through a mobile‐friendly website, accessible via smartphone or computer. Baseline variables included smoking behavior (see Inclusion criteria), tobacco dependence (cigarettes/day and minutes to first cigarette), current use of NRT, e‐cigarettes and heated tobacco, age and sex, a two‐item screening test for depression [19], telephone number and postal and e‐mail addresses (Table 1). Baseline data were collected between 10 April 2019 and 25 March 2020, i.e. mainly before the first COVID‐19 lockdown that took place on 16–17 March 2020 in Switzerland and France, respectively.
TABLE 1.
Baseline characteristics of daily smokers enrolled on application (app) stores and on the internet in 2019–20 and randomly assigned to using either the Stop‐tabac app or a control app
| Stop‐tabac app | Control app | |
|---|---|---|
| n = 2639 | n = 2654 | |
| Age, years, mean ± SD | 35 ± 11 | 36 ± 11 |
| Women, % | 67 | 64 |
| Country of residence, % France (the rest = Switzerland) | 82 | 82 |
| Two‐item screening test for depression, % positive | 64 | 63 |
| Duration of daily smoking, years, mean ± SD (median) | 17 ± 11 (16) | 17 ± 11 (15) |
| Cigarettes smoked per day, mean ± SD (median) | 16 ± 7 (15) | 16 ± 8 (15) |
| Minutes to first cigarette of the day, mean ± SD (median) | 45 ± 95 (15) | 52 ± 96 (20) |
| Currently smoke cigars, pipe, waterpipe or cannabis, % | 27 | 27 |
| Currently use electronic cigarettes, % | 18 | 18 |
| Currently use heated tobacco (IQOS, glo, Ploom, etc.), % | 2 | 3 |
| Currently use nicotine medications, % | 16 | 18 |
| Days until their target quit date, mean ± SD (median) | 7 ± 10 (1) | 6 ± 10 (1) |
SD = standard deviation.
Follow‐up
Participants received an invitation by e‐mail to answer the on‐line follow‐up questionnaires 1 week, 1 month and 6 months after their target quit date. Reminders were sent to non‐responders via e‐mail, and this data collection system was automatic. Assistants sent non‐responders three reminders via text messages (or WhatsApp), non‐responders received the questionnaire by postal mail, and then non‐responders were contacted by telephone [20]. Follow‐up took place from April 2019 to April 2020 (7 days), from May 2019 to August 2020 (1 month) and from October 2019 to November 2020 (6 months).
Sample size
We expected quit rates of 12.5% in the Stop‐tabac group and 10% in the control group after 6 months [odds ratio (OR) = 1.28] [2, 21, 22]; 5200 participants were needed to detect this effect with a power of 80% and a confidence interval (CI) of 95%.
Statistics
For the primary outcome and all other outcomes in Table 2 we used the baseline number of participants as the denominator; participants absent at follow‐up were counted as smokers, under the assumption that data were missing not at random. This assumption is not necessarily verified, but it is common practice in smoking cessation studies; e.g. the Cochrane Tobacco Group's guidelines recommend regarding dropouts as smokers [2]. The main analysis was a comparison of the proportions of abstinent smokers (as defined above) after 6 months. We also report results in responders only with no imputation for missing data (Table 3), under the assumption that data were missing completely at random. We conducted subgroup analyses to test whether the outcome was associated with participants’ characteristics and with the utilization of the app (from data automatically collected by the app). We used χ2 tests and ORs with 95% CIs to compare proportions, and Mann–Whitney U‐tests to compare medians. Each follow‐up point was analysed independently of the others.
TABLE 2.
Smoking cessation end‐points, with non‐responders counted as smokers
| Stop‐tabac app, | Control app, | Odds ratio (95% CI) | P‐value | |
|---|---|---|---|---|
| N = 2639 | N = 2654 | |||
| n (%) | n (%) | |||
| After 1 week, self‐report of no smoking in the previous 7 days | 495 (18.8) | 498 (18.8) | 0.99 (0.86–1.13) | 0.99 |
| After 1 month, self–report of no smoking in the previous: | ||||
| 7 days | 419 (15.9) | 428 (16.1) | 0.98 (0.85–1.14) | 0.81 |
| 4 weeks | 363 (13.8) | 350 (13.2) | 1.05 (0.90–1.23) | 0.55 |
| After 1 month, Russell Standard: ≤ 5 cigarettes in past 4 weeks plus no puff of tobacco in past 7 days | 385 (14.6) | 395 (14.9) | 0.98 (0.84–1.14) | 0.76 |
| After 6 months, self–report of no smoking in the previous: | ||||
| 7 days | 298 (11.3) | 318 (12.0) | 0.94 (0.79–1.11) | 0.43 |
| 4 weeks (primary outcome) | 262 (9.9) | 274 (10.3) | 0.96 (0.80–1.45) | 0.63 |
| After 6 months, Russell Standard: ≤ 5 cigarettes in past 6 months plus no puff of tobacco in past 7 days | 244 (9.2) | 250 (9.4) | 0.98 (0.81–1.18) | 0.82 |
CI = confidence interval.
TABLE 3.
Other end–points in responders at follow–up, with no imputation for missing data
| Stop‐tabac, | Control, | Stop‐tabac, | Control, | Stop‐tabac, | Control, | |
|---|---|---|---|---|---|---|
| n = 1177, | n = 1291, | n = 902, | n = 959 | n = 662, | n = 745 | |
| 7 days | 1 month | 6 months | ||||
| No puff of tobacco in the past 7 days, % | 42 | 39 | 47 | 45 | 45 | 43 |
| No puff of tobacco in past 4 weeks, % | – | – | 40 | 37 | 40 | 37 |
| Did not smoke any tobacco after their target quit date, % | 42 | 39 | 39 | 37 | 31 | 30 |
| Smoked 1–5 cigarettes after their target quit date, % | 23 | 26 | 18 | 18 | 16 | 12 |
| Used nicotine medications after entry in the study, % | 35 | 30** | 34 | 29** | 38 | 30** |
| Used nicotine medications after entry in the study, among those who were not already using them at baseline, % | 22 | 16*** | 23 | 17** | 29 | 20*** |
| Used heated tobacco after entry in the study, % | 2 | 3 | 4 | 5 | 11 | 12 |
| Used e‐cigarettes with nicotine after entry in the study, % | 24 | 23 | 28 | 24 | 32 | 28 |
| Used e‐cigarettes with nicotine after entry in the study, among those who were not already using them at baseline, % | 8 | 8 | 12 | 9 | 16 | 15 |
| Used a smartphone app for smoking cessation after entry in the study (including Stop‐tabac), % | 59 | 45*** | 54 | 40*** | 57 | 45*** |
| The study app helped them quit smoking ‘a lot + enormously’, % | 22 | 9*** | 24 | 10*** | 26 | 8*** |
| In former smokers: interval between target quit date and actual quit date, days, mean | 1 | 3 | 7 | 8 | 50 | 65 |
|
In smokers at follow‐up: Cigarettes per day, mean |
8 |
7 |
9 |
8 |
10 |
11 |
| Seriously tried to quit smoking after entry in the study, % | 69 | 69 | 64 | 67 | 72 | 78 * |
| Has firmly decided to quit smoking, % | 59 | 59 | 47 | 49 | 41 | 36 |
P ≤ 0.05.
P ≤ 0.01.
P ≤ 0.001.
Participants could withdraw from the study at any point, without having to justify their decision. The app was not modified during the trial. The study protocol was implemented as published [15]. The study was conducted and results are presented in conformity with the Consolidated Standards of Reporting Trials (CONSORT) guidelines [22]. There was no data‐monitoring committee. The data were accessible to the two co‐investigators. We used IBM SPSS statistics version 26.
ETHICS APPROVAL
The study was submitted to the cantonal ethics committee in Geneva (Req‐2018‐00356) who answered that, according to Swiss law, the app being no medical device, the study did not legally need approval from this commission. Therefore, the commission did not formally review our proposal, but answered that: ‘everything indicates that this study will take place in compliance with the general ethical principles applicable to any research involving people’ [e‐mail dated 16 May 2018; address: Commission Cantonale d'Ethique de la Recherche (CCER), rue Adrien‐Lachenal 8, 1207 Geneva, Switzerland]. No consent was needed for publication.
AVAILABILITY OF DATA AND MATERIALS
A data management plan was submitted as required by the Swiss National Science Foundation. After publication of the main results, the anonymized data will be made publicly available. The shared data file, with anonymized participant‐level data, will be fully documented, which means that the variable labels and value labels will include the full wording of questions and of response options. An accompanying Readme.txt file will include a full description of data collection procedures and analyses. The registration and consent form and the baseline and follow‐up questionnaires are available at: https://archive.org/details/@stopdependance_ch.
RESULTS
Enrollment
The baseline questionnaire was answered by 16 456 people, 6110 (37%) of whom were eligible and received an e‐mail with a link to a webpage displaying their personal code; 5352 clicked on this link and obtained their randomly assigned personal code and 5293 inserted this code into the app and were therefore included into the trial (Figure 1). Most participants were women (66%), average age 36 years (range = 19–79 years); 64% screened positive on the two‐item test for depression, participants smoked 15 cigarettes/day (median) and smoked their first cigarette of the day 15–20 minutes after waking, 17% were using nicotine medications and 18% were using e‐cigarettes at baseline (Table 1). Participants lived in all the regions (‘Departments’) of France and all the Cantons of French‐speaking Switzerland.
Participation in follow‐up surveys
Participation rates at follow‐up in the Stop‐tabac and control groups were 45 and 49% after 7 days (χ2 = 8.7, P = 0.003), 34 and 36% after 1 month (χ2 = 2.2, P = 0.14) and 25 and 28% after 6 months (χ2 = 6.0, P = 0.01). No privacy breaches were reported by participants.
Impact of the app on smoking cessation
At 6 months, the 4‐week smoking abstinence rates (primary outcome) were similar in both study groups (Stop‐tabac = 9.9%, control = 10.3%, OR = 0.96, 95% CI = 0.80–1.45). Other smoking cessation end‐points showed no between‐group differences (Table 2). In an analysis limited to survey respondents at follow‐up with no imputation for missing data, there were no between‐group differences in quit rates at follow‐up (Table 3).
Impact on smoking behavior
In smokers at follow‐up, there were no between‐group differences for cigarettes/day, motivation to quit and quit attempts (Table 3). In ex‐smokers, intervals between the target quit date set at baseline and the actual quit date reported at follow‐up were similar in both study groups (Table 3).
Smoking cessation in subgroups
Depressed participants were less likely to quit smoking than non‐depressed participants, but the app had no effect on smoking cessation whether or not participants were depressed at baseline. The app had no effect on smoking cessation, independently from sex, age, tobacco dependence levels or years of daily smoking (data not shown). Among participants who had stopped smoking after 1 week or 1 month, relapse rates at subsequent follow‐ups were similar in both study groups (data not shown).
Impact on use of NRT, e‐cigarettes and heated tobacco
At each follow‐up, the proportions of participants who reported having used nicotine medications after entry in the study were 5–8 percentage points higher in the Stop‐tabac group than in the control group (each P ≤ 0.01, Table 3). The effect of the app on NRT use did not differ across subgroups (data not shown). The proportions of participants who reported having used e‐cigarettes or heated tobacco products after entry into the study were similar in both groups at all follow‐ups (Table 3).
Utilization of smartphone apps for smoking cessation
At each follow‐up, 13–14% more participants in the Stop‐tabac group than in the control group said that they used a smartphone app for smoking cessation after their target quit date (each P ≤ 0.001, Table 3). This question covered any app, including Stop‐tabac. From a free‐text field, the apps most used were Stop‐tabac (65% of answers), Kwit (8%), Tabac‐info‐service (7%) and Smokefree Buddy (3%).
Perceived helpfulness of the app
At each follow‐up, participants in the Stop‐tabac group were two to three times more likely than participants in the control group to report that the study app helped them ‘a lot’ or ‘enormously’ to quit smoking (each P ≤ 0.001, Table 3). In the Stop‐tabac group after 1 month, women were more likely than men to report that this app helped them to quit smoking ‘a lot’ or ‘enormously’ (30 versus 21%, χ2 = 19, P = 0.002). There was no association between perceived helpfulness and the other characteristics of participants (data not shown).
Utilization data collected automatically by the app
Participants in the Stop‐tabac group used the app during a longer period than participants in the control group (median interval between first and last use: 23 versus 11 days; 5th and 95th centiles: 0–306 days (Stop‐tabac) and 1–267 days (control, P < 0.001, U = 2 996 499). Stop‐tabac users opened the app over more different days than control participants (median 5 versus 3 days; 5th and 95th centiles: 1–66 days (Stop‐tabac) and 1–39 days (control, P < 0.001, U = 2 859 400) and viewed more screens (median 26 versus 14 screens; 5th and 95th centiles: 1–331 screens (Stop‐tabac) and 1–144 screens (control, P < 0.001, U = 2 552 347). Fewer people in the Stop‐tabac group (16%) than in the control group (21%) used the app for only 1 day, and more used it during the whole 6 months (15 versus 11%, P < 0.001).
Utilization in subgroups
In the whole sample, the duration of utilization of the app was longer in men than in women (interval between first and last use 18 versus 15 days, U = 2 969 079, P = 0.001), in older versus younger participants (+1.4 days of use per year of age, t = 11.2, P < 0.001), in people who lived in Switzerland versus France (36 versus 14 days, U = 1 636 340, P < 0.001), in those who were not depressed versus those depressed (24 versus 13 days, U = 2 817 796, P < 0.001), in those who were using NRT at baseline versus those who were not (21 versus 15 days, U = 1 819 629, P < 0.001) and in those who set a target quit date soon after baseline versus those who delayed their quit date (–0.5 days of use per additional day of delay in the target quit date, t = 4.1, P < 0.001).
Smoking cessation and frequency of use
Data collected automatically by the app indicated that at 6 months, former smokers had viewed more screens/pages than those who failed to quit (81 versus 31 screens, U = 144 499, P < 0.001), had opened the app on more different days (on 22 versus 7 different days, U = 136 719, P < 0.001) and had used it longer (median interval between first and last use 155 versus 41 days, U = 147 290, P < 0.001).
DISCUSSION
The Stop‐tabac smartphone app did not increase smoking cessation rates or improve smoking behavior in those who failed to quit. These results are consistent with a Cochrane meta‐analysis which found no effect of smartphone apps on smoking cessation (five studies, 3820 participants, low‐certainty evidence) [2].
Nevertheless, the Stop‐tabac app increased the proportion of NRT users. Uptake of and compliance with NRT is usually low in people who obtain these products without a medical prescription [23]. This result suggests that the app's module on NRT warrants further investigation and development.
There are several reasons why this app had no effect on smoking behavior. First, the app was perhaps unsatisfactory. However, it was iteratively improved during 7 years and was rated among the best five smoking cessation apps globally [7]. Therefore, our results can probably not be attributed to shortcomings in the quality of the app. The app could, however, be improved by sending text messages by smartphone, as text messages have proved effective [2], or e‐mail messages. Secondly, participants probably did not use the app frequently enough. Thirdly, even the best possible stand‐alone app may not be sufficient to treat tobacco addiction. Finally, the quit rate in our control group was quite high (19% after 1 month) and it may be difficult to push quit rates much higher, even with the best possible self‐help intervention.
User engagement and retention in the Stop‐tabac app were good in comparison with other studies of mHealth apps [24]. However, this was not sufficient to produce an impact upon smoking behavior, in particular because our participants were addicted and depressed. Improving the intensity of duration of utilization of mHealth apps in general is a priority [24].
The control app's content was minimal; only 8% of participants said the control app helped them to quit smoking, and participants did not use it very intensively. We do not believe this was sufficient to produce an effect on smoking cessation. The relatively high quit rate in the control group is probably better explained by the self‐selection of enrollees in this trial (they were highly motivated to quit).
Contrasting with these results, Stop‐tabac users were more likely than participants in the control group to report that the app helped them to quit smoking, and former smokers used the app more frequently than participants who failed to quit, suggesting either that better adhesion to the intervention produced greater benefits or that those who were more motivated were more likely to both quit smoking and use the app. As previously reported, engagement‐related issues may reduce mHealth efficacy [25, 26].
The app might prevent relapse in people who had recently quit smoking, but this effect was not captured by this study because we enrolled current smokers only.
Implications for research, development and practice
Further developments of the app could include prompts to increase the duration and intensity of app use, actions to increase users’ involvement in the discussion forum, the implementation of several improvements suggested by users and the addition of text and e‐mail messages. Smoking cessation apps should perhaps be integrated into programs that also offer in‐person or group counseling, medications, vaping products, financial incentives or telephone support. These apps should perhaps aim at improving utilization of these interventions, rather than at increasing quit rates as stand‐alone interventions. Future research could investigate which components within apps are most effective; e.g. decision aids [26].
Strengths
We included a sample large enough to detect the hypothesized effect, the follow‐up was long enough to fulfill inclusion criteria in Cochrane Reviews and the study included an adequate control group. Any daily smoker who visited the app stores could participate, the age range was large and participants lived everywhere in France and French‐speaking Switzerland, which increases the generalizability of our findings, and illustrates how apps can help difficult‐to‐reach smokers. Finally, the enrollment process in the study was similar to the natural process of downloading the app outside the study (i.e. both inside and outside the study people found the app either directly on the app stores or on websites), and the app used in the study is the same as the app available on the app stores, which ensures that our results are generalizable to all users of this app. The Stop‐tabac app will be transferred in 2022 to the Swiss Tobacco Prevention Fund, which ensures that it will have access to financial resources and will still be available in the future.
Limitations
Participation rates at follow‐up were low, but similarly low response rates are common in on‐line studies [27] and attrition rates were not very different between study groups. Participants consisted of volunteers and may not be representative of all smokers. Our sample included large proportions of women, depressed people, users of e‐cigarettes (18% in our sample versus 7% of smokers in the Swiss population in 2017 [28]) and NRT users (17% in our sample versus 3% of smokers in the Swiss population in 2016 [29]), which may limit generalizability.
Two‐thirds of participants screened positive for depression, much greater than the prevalence of depression in smokers in the general population (e.g. 32% in the United States and Australia) [30]. Depression had no impact upon between‐group differences on the primary outcome, but the high rates of depression may have reduced the intervention's efficacy by reducing engagement with the app.
The difference in attrition between Swiss and French participants cannot be explained by the cultural specificity of the app, and is more probably related to the fact that the researchers were identified as Swiss.
Participants in both study groups could easily download other smoking cessation apps from the app stores, which may have contaminated the control group.
Almost all data for the first three surveys (95% at baseline and after 7 days, 87% at 1 month) were collected before the first COVID‐19 lockdown; thus, the lockdown had at most a marginal impact on enrollment and on the 7‐day and 1‐month surveys. However, only 28% of 6‐month data was collected before the lockdown; thus, the lockdown may have had some impact on the 6‐month follow‐up, as research suggests that smokers were more likely to try to quit smoking but also to increase their cigarette consumption during this lockdown [31, 32].
CONCLUSIONS
The Stop‐tabac smartphone app had no impact upon smoking cessation but increased the proportion of users of nicotine medications. It was more engaging and was perceived as more helpful than a control app.
DECLARATION OF INTERESTS
None.
AUTHOR CONTRIBUTIONS
Jean‐Francois Etter: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; supervision; validation. Yasser Khazaal: Conceptualization; funding acquisition; investigation; methodology; resources.
CLINICAL TRIAL REGISTRATION
ISRCTN Registry: ISRCTN11318024, 17 May 2018. http://www.isrctn.com/ISRCTN11318024.
ACKNOWLEDGEMENTS
This study was supported by the Swiss National Science Foundation, grant 32 003‐179 369, CHF 194942 (EUR 182200, USD 200700). J.F.E.’s salary is paid by the University of Geneva; Y.K.’s salary is paid by the Lausanne University Hospitals. Vincent Baujard developed the software for data collection and managed and maintained the on‐line data collection system. Evelyne Laszlo supervised the development of both versions of the application (Stop‐tabac and control) and moderated the discussion forum ‘The Tribe’. Open Access Funding provided by Universite de Geneve.
Etter J‐F, Khazaal Y. The Stop‐tabac smartphone application for smoking cessation: a randomized controlled trial. Addiction. 2022;117:1406–1415. 10.1111/add.15738
Funding information Swiss Tobacco Prevention Fund 32003‐179369. Open Access Funding provided by Universite de Geneve.
REFERENCES
- 1. West R, Raw M, McNeill A, Stead L, Aveyard P, Bitton J, et al. Health‐care interventions to promote and assist tobacco cessation: a review of efficacy, effectiveness and affordability for use in national guideline development. Addiction. 2015;110:1388–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y, Dobson R. Mobile phone text messaging and app‐based interventions for smoking cessation. Cochrane Database Syst Rev. 2019;(10). 10.1002/14651858.CD006611.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Regmi K, Kassim N, Ahmad N, Tuah NA. Effectiveness of mobile apps for smoking cessation: a review. Tob Prev Cessat. 2017;3(12):1–11. 10.18332/tpc/70088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abroms LC, Lee Westmaas J, Bontemps‐Jones J, Ramani R, Mellerson J. A content analysis of popular smartphone apps for smoking cessation. Am J Prev Med. 2013;45:732–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoeppner BB, Hoeppner SS, Seaboyer L. How smart are smartphone apps for smoking cessation? A content analysis. Nicotine Tob Res. 2016;18:1025–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haskins BL, Lesperance D, Gibbons P, Boudreaux ED. A systematic review of smartphone applications for smoking cessation. Transl Behav Med. 2017;7:292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel R, Sulzberger L, Li G, Mair J, Morley H, Shing MN, et al. Smartphone apps for weight loss and smoking cessation: quality ranking of 120 apps. NZ Med J. 2015;128:73–6. [PubMed] [Google Scholar]
- 8. Bakker D, Kazantzis N, Rickwood D, Rickard N. Mental health smartphone apps: review and evidence‐based recommendations for future developments. J Med Internet Res Ment Health. 2016;3:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cokkinides VE, Ward E, Jemal A, Thun MJ. Under‐use of smoking‐cessation treatments: results from the National Health Interview Survey, 2000. Am J Prev Med. 2005;28:119–22. [DOI] [PubMed] [Google Scholar]
- 10. Hartmann‐Boyce J, Hong B, Livingstone‐Banks J, Wheat H, Fanshawe TR. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database Syst Rev. 2019;6:CD009670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. West R. Theory of Addiction. Oxford, UK: Blackwell; 2006. [Google Scholar]
- 12. Bandura A. Human agency in social cognitive theory. Am Psychol. 1989;44(9):1175–84. [DOI] [PubMed] [Google Scholar]
- 13. Godin G, Kok G. The theory of planned behavior: a review of its applications to health‐related behaviors. Am J Health Promot. 1996;11:87–98. [DOI] [PubMed] [Google Scholar]
- 14. Anderson JE, Jorenby DE, Scott WJ, Fiore MC. Treating tobacco use and dependence: an evidence‐based clinical practice guideline for tobacco cessation. Chest. 2002;121:932–41. [DOI] [PubMed] [Google Scholar]
- 15. Etter JF, Khazaal Y. The Stop‐tabac smartphone application for smoking cessation: study protocol for a randomized controlled trial in the general population. Trials. 2020;21:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. US Food and Drug Administration (FDA) . Transcript of the Joint Meeting of the Nonprescription Drugs Advisory Committee and the Drug Abuse Advisory Committee of the Food and Drug Administration. Rockville, MD: FDA; 1995. [Google Scholar]
- 17. West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100:299–303. [DOI] [PubMed] [Google Scholar]
- 18. Burri M, Baujard V, Etter JF. A qualitative analysis of an internet discussion forum for recent ex‐smokers. Nicotine Tob Res. 2006;8:S13–9. [DOI] [PubMed] [Google Scholar]
- 19. Whooley MA, Avins AL, Miranda J, Browner WS. Case‐finding instruments for depression. Two questions are as good as many. J Gen Intern Med. 1997;12:439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dillman DA. Mail and internet surveys: the tailored design method. New York, NY: John Wiley & Sons; 2000. [Google Scholar]
- 21. Shahab L, McEwen A. Online support for smoking cessation: a systematic review of the literature. Addiction. 2009;104:1792–804. [DOI] [PubMed] [Google Scholar]
- 22. Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet. 2001;357:1191–4. [PubMed] [Google Scholar]
- 23. Bansal MA, Cummings KM, Hyland A, Giovino GA. Stop‐smoking medications: who uses them, who misuses them, and who is misinformed about them? Nicotine Tob Res. 2004;6:S303–10. [DOI] [PubMed] [Google Scholar]
- 24. Owen JE, Jaworski BK, Kuhn E, Makin‐Byrd KN, Ramsey KM, Hoffman JE. mHealth in the wild: using novel data to examine the reach, use, and impact of PTSD coach. J Med Internet Res Ment Health. 2015;2:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fleming TM, de Beurs D, Khazaal Y, Gaggioli A, Riva G, Botella C, et al. Maximizing the impact of e‐therapy and serious gaming: time for a paradigm shift. Front Psychol. 2016;7:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Torous J, Nicholas J, Larsen ME, Firth J, Christensen H. Clinical review of user engagement with mental health smartphone apps: evidence, theory and improvements. Evid Based Ment Health. 2018;21:116–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cook C, Heath F, Thompson RL. A meta‐analysis of response rates in web‐ or internet‐based surveys. Educ Psychol Meas. 2000;60:821–36. [Google Scholar]
- 28. Swiss Federal Office of Statistics (OFS) . Enquête suisse sur la santé 2017—Consommation de tabac en Suisse. Neuchâtel, Switzerland: OFS; 2020. [Google Scholar]
- 29. Gmel G, Kuendig H, Notari L, Gmel C. Monitorage suisse des addictions: consommation d'alcool. In: tabac et drogues illégales en Suisse en 2016. Lausanne, Switzerland: Addiction Suisse; 2017. p. 68. [Google Scholar]
- 30. Lawrence D, Mitrou F, Zubrick SR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health. 2009;9:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bar‐Zeev Y, Shauly M, Lee H, Neumark Y. Changes in smoking behaviour and home‐smoking rules during the initial COVID‐19 lockdown period in Israel. Int J Environ Res Public Health. 2021;18:1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jackson SE, Garnett C, Shahab L, Oldham M, Brown J. Association of the COVID‐19 lockdown with smoking, drinking and attempts to quit in England: an analysis of 2019–20 data. Addiction. 2021;116:1233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data management plan was submitted as required by the Swiss National Science Foundation. After publication of the main results, the anonymized data will be made publicly available. The shared data file, with anonymized participant‐level data, will be fully documented, which means that the variable labels and value labels will include the full wording of questions and of response options. An accompanying Readme.txt file will include a full description of data collection procedures and analyses. The registration and consent form and the baseline and follow‐up questionnaires are available at: https://archive.org/details/@stopdependance_ch.


