Abstract
Of the more than 6,000 members of the most speciose avian clade, Passeriformes (perching birds), only the five species of dippers (Cinclidae, Cinclus) use their wings to swim underwater. Among nonpasserine wing‐propelled divers (alcids, diving petrels, penguins, and plotopterids), convergent evolution of morphological characteristics related to this highly derived method of locomotion have been well‐documented, suggesting that the demands of this behavior exert strong selective pressure. However, despite their unique anatomical attributes, dippers have been the focus of comparatively few studies and potential convergence between dippers and nonpasseriform wing‐propelled divers has not been previously examined. In this study, a suite of characteristics that are shared among many wing‐propelled diving birds were identified and the distribution of those characteristics across representatives of all clades of extant and extinct wing‐propelled divers were evaluated to assess convergence. Putatively convergent characteristics were drawn from a relatively wide range of sources including osteology, myology, endocranial anatomy, integument, and ethology. Comparisons reveal that whereas nonpasseriform wing‐propelled divers do in fact share some anatomical characteristics putatively associated with the biomechanics of underwater “flight”, dippers have evolved this highly derived method of locomotion without converging on the majority of concomitant changes observed in other taxa. Changes in the flight musculature and feathers, reduction of the keratin bounded external nares and an increase in subcutaneous fat are shared with other wing‐propelled diving birds, but endocranial anatomy shows no significant shifts and osteological modifications are limited. Muscular and integumentary novelties may precede skeletal and neuroendocranial morphology in the acquisition of this novel locomotory mode, with implications for understanding potential biases in the fossil record of other such transitions. Thus, dippers represent an example of a highly derived and complex behavioral convergence that is not fully associated with the anatomical changes observed in other wing‐propelled divers, perhaps owing to the relative recency of their divergence from nondiving passeriforms.
Keywords: Cinclus, endocast, ethology, integument, myology, osteology, Pan‐Alcidae, Pan‐Sphenisciformes, Passeriformes, Pelecanoididae, Plotopteridae
1. INTRODUCTION
Cinclidae are unique among passerines in that they have the ability to actively propel themselves underwater with their wings. There are five extant species of dippers, which range through North and South America, Africa, Asia, and Europe—an exceptionally broad geographic distribution for a clade with so few species (del Hoyo et al., 2005; Figure 1). All five extant species of dipper are relatively similar in size, body shape (semi‐fusiform), behavior, and song, with the primary interspecific differences being limited to plumage pattern (del Hoyo et al., 2005; Figure 2). Dependent on fast‐flowing, clear, relatively pristine riverine environments, dippers primarily prey upon invertebrates but are known to sometimes consume small fish, their eggs, and fry (del Hoyo et al., 2005).
FIGURE 1.
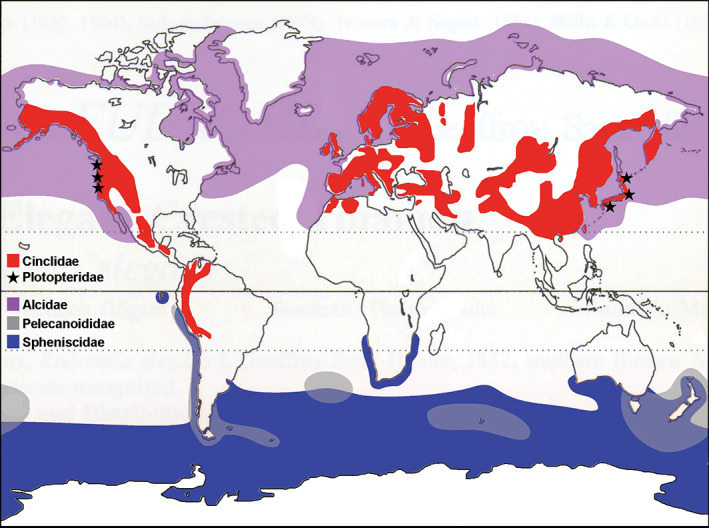
Map depicting the geographic ranges of wing‐propelled diving clades. Ranges for extant species correspond to those summarized in del Hoyo et al. (1992, 1996, 2005). The geographic range of Pan‐Alcidae extended further to the south in the Western Atlantic Ocean as recently as the Pleistocene (Smith & Clarke, 2015). Fossil localities for Plotopteridae are those summarized by Mayr et al. (2015)
FIGURE 2.

An American Dipper Cinclus mexicanus (photo courtesy of D. Field). Note the robust and strongly curved unguals, short tail, and narial slit
The monophyly of extant Cinclidae is supported by molecular and morphological evidence, and all five extant species are united in a single genus, Cinclus (del Hoyo et al., 2005; Voelker, 2002). A phylogeny based on mtDNA inferred that the Eurasian species Cinclus cinclus (which also ranges into northern Africa) and Cinclus pallasii form a clade that is in turn sister to a clade uniting the North American Cinclus mexicanus and the closely related South American Cinclus leucocephalus and Cinclus schultzi (Voelker, 2002). However, statistical support for this branching pattern was weak, and alternative hypotheses that the North American or South American lineages could instead be the sister to the remaining species could not be rejected.
Dippers are oscine passeriforms, consistently placed in the clade Musicapoidea in the results of molecular based phylogenetic analyses and most recently recovered as sister taxon to a clade uniting all other families of Musicapoidea except for Elachuridae (Barker et al., 2002; Oliveros et al., 2019). Divergence time analyses estimate the dipper lineage split from other Musicapoidea around 20 million years ago (Oliveros et al., 2019), and that the basal divergence in crown dippers occurred ~4 million years ago (Voelker, 2002). This leaves a relatively wide interval for the origin of wing‐propelled diving in dippers, as the evolution of this trait could have occurred at any point between the divergence of stem dippers and the origin of crown Cinclidae.
The sparse and problematic fossil record of Cinclidae offers little insight into resolving this issue. The fossil record of dippers is limited to a few Pleistocene records, all referred to the extant species C. cinclus (Michailidis et al., 2018; Sanchez‐Marco, 1999; Tyrberg, 1998). Three putative species of dipper were described from the Miocene of Hungary, Cinclus gaspariki, Cinclus minor, and Cinclus major (Kessler, 2013; Kessler & Hír, 2012). However, those taxa were named based on highly fragmentary material (carpometacarpus, carpometacarpus fragment, distal ulna) and no apomorphies of Cinclidae were cited to support their referral to Cinclidae (Kessler, 2013; Kessler & Hír, 2012). Cinclus has not been included in a phylogenetic analysis of osteological characters and our evaluation of extant Cinclus carpometacarpi and ulnae did not identify any unambiguous apomorphies that would allow for referral of isolated exemplars of those elements to Cinclidae (or differentiation from near outgroup taxa such as Catharus). Therefore, we consider those Miocene specimens to be too incomplete to support referral to Cinclidae. Thus, it remains unknown precisely when in their evolutionary history dippers developed their derived method of underwater locomotion and what morphological and behavioral steps may have been involved in this evolutionary transition. Still, it is clear based on the wide geographic distribution of this species‐depauperate clade that dippers evolved to successfully exploit a niche (freshwater wing‐propelled diver) that is wholly unoccupied by not only the other 6,000 species of passeriforms, but by any other avian clade.
Convergent evolution in all of its many described forms (e.g., morphological, genetic, behavioral, cognitive) has long been a topic of interest to evolutionary biologists because it provides potential insights regarding homoplastically repeated patterns of selection (reviewed by McGhee, 2011; Lamichhaney et al., 2019). Not surprisingly, anatomical modifications associated with wing‐propelled diving have been well‐documented and evolutionary convergence of musculoskeletal characteristics, especially in the forelimbs, have been previously noted (Clarke et al., 2007; Ksepka & Clarke, 2010; Marples, 1952; Olson, 1980; Olson & Hasegawa, 1979; Raikow et al., 1988; Simpson, 1946; Smith, 2010; Smith & Clarke, 2011, 2015; Storer, 1960). Wing‐propelled diving (hereafter abbreviated as WPD) is a relatively rare form of locomotion among birds, known in only ~50 of the >10,000 extant species of birds (Smith & Clarke, 2015), all of which belong to four clades: Cinclidae (dippers); Pan‐Alcidae (auks, puffins, and allies; n = 23 spp.); Pan‐Sphenisciformes (penguins; n = 17–20 spp.); and Pelecanoididae (diving petrels; all five spp. classified in the genus Pelecanoides; Figure 3). Interestingly, there are more species of WPD birds known from the fossil record than there are extant species, including more than 50 extinct penguins, approximately 35 extinct pan‐alcids, two named extinct species of diving petrel, and perhaps as many as 10 species of the entirely extinct plotopterids (Mayr, 2009; Mayr et al., 2015; Ksepka & Ando, 2011; Ksepka & Clarke, 2010; Olson, 1985a, 1985b; Smith, 2011a; Worthy et al., 2007).
FIGURE 3.
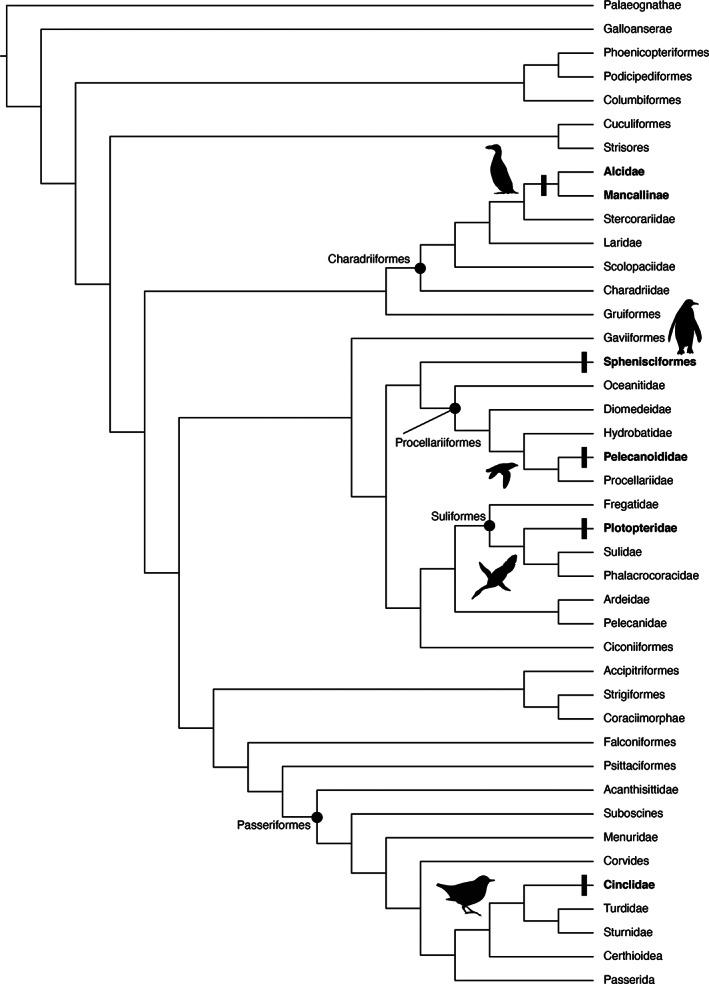
Systematic position of wing‐propelled diving birds (bolded) and their near outgroups in Aves. The tree is simplified from Ksepka et al. (2020) with the position of Cinclidae modified based on Oliveros et al. (2019) and the position of Plotopteridae based on Mayr et al. (2021)
While the majority of diversity among penguins and pan‐alcids are represented by extinct species (Ksepka & Clarke, 2010; Smith & Clarke, 2015), and the plotopterids have been extinct since the middle Miocene (Smith, 2010), extant auks and penguins have been the subject of intense study and inform the interpretation of fossils. WPD birds have exapted the flight stroke for underwater propulsion. However, underwater flight entails different biomechanical requirements or constraints than aerial flying (Habib, 2010; Habib & Ruff, 2008), as water is more than 800 times denser than air. In contrast to the plethora of studies devoted to auks, penguins, and plotopterids, dippers have been the focus of comparatively little attention. Historically, dippers were once considered to merely cling to rocks along the bottom of streambeds. However, more recent studies have documented that at least three species actively propel themselves with their wings underwater (del Hoyo et al., 2005; contra Mayr et al., 2020; Video 1).
Here, we summarize anatomical and ecological data on all WPD clades and assess the levels of convergent characteristics shared among these taxa. We provide detailed comparisons between dippers and the most closely related non‐WPD passeriforms, thrushes and starlings, in order to test the hypothesis that dippers have anatomically converged with other WPD birds. Newly presented osteological details of Cinclidae may serve to assist in the identification of additional dipper fossils that could inform lingering questions about the timing and pattern of the evolution of this intriguing clade of songbirds. These comparisons not only provide new insights about the evolution of dippers, but also broaden our understanding of the evolution of WPD across Aves.
2. MATERIALS AND METHODS
WPD birds are defined herein as species that feed primarily using winged underwater propulsion and that do not frequently employ their hindlimbs to produce thrust while diving. Five clades meet this definition: Pan‐Sphenisciformes, Pan‐Alcidae, Plotopteridae (inferred from the shortened tarsometatarsus and compressed humeri characteristic of this extinct clade), Pelecanoididae, and Cinclidae. Reports of WPD in the two South American dipper species have yet to be fully substantiated despite anecdotal data, and all species of dippers also infrequently employ feeding strategies other than WPD, including wading, probing, leaf turning, and rare aerial flycatching (del Hoyo et al., 2005). While there are a very limited number of additional examples of WPD among other clades of birds (e.g., some species of shearwaters; del Hoyo et al., 1992), only the five clades listed above are composed entirely of species that habitually use this locomotor behavior. Additional species of diving birds employ both the wings and hind limbs during underwater swimming to different degrees (e.g., shearwaters, loons, cormorants, and gannets; del Hoyo et al., 1992; Storer, 1960; Wang & Clarke, 2014). Some species of non‐alcid charadriiforms have also been documented to propel themselves with their wings underwater as a means of escape, particularly during early stages of ontogeny (e.g., Tringa tetanus, see discussion in Smith & Clarke, 2015, and references cited therein). Finally, although the ethology of kingfishers (Alcedinidae) does not approach locomotive behavior resembling true WPD, it is worth noting that many members of this clade do use their wings to assist in their ascent from the water after plunge‐diving to capture prey (del Hoyo et al., 2011), a potential step in the evolution of WPD that has possibly been overlooked. These facultative aqueous wing‐propelling taxa are not treated as WPDs and are not the focus of comparisons with Cinclidae herein.
Osteological comparisons—Because of their putatively convergent nature, investigations into the osteological similarities of WPD birds have a relatively long history (Howard, 1953, 1969; Ksepka & Clarke, 2010; Marples, 1952; Olson, 1981, 1985a, 1985b; Olson & Hasegawa, 1979; Raikow et al., 1988; Smith, 2011a, 2011b; Smith & Clarke, 2012, 2015; Storer, 1960; Wang & Clarke, 2014). A review of pertinent literature identified 16 osteological characteristics that, because of their prevalence among well‐studied WPD birds such as penguins and pan‐alcids, have been previously suggested to be convergent skeletal modifications associated with WPD. An additional seven characteristics drawn from neuroanatomical, ecological, and integumentary observations were also identified for a total of 23 potentially convergent WPD‐associated characteristics. A description of each characteristic and its known distribution is provided below. It should be noted that some characteristics, which are known to be more broadly associated with diving birds and that are not exclusive to WPD (e.g., increased hemoglobin and myoglobin levels; reduction or lack of a hallux), were not included in the broad comparisons between WPD clades. Differences between Cinclus and other nondiving passeriforms were also investigated and are reported herein.
Skeletonized specimens representing four of the five species of extant dipper and three species of cathartid thrush representing the near outgroup to dippers (Klicka et al., 2005; Oliveros et al., 2019) were measured and directly evaluated for discrete osteological characters (see online Appendix 1). Skeletal specimens of the Rufous‐throated Dipper Cinclus schulzii were not available to us. Discrete morphological data for nonpasseriforms were primarily drawn from the following phylogenetic and endocranial studies: Pan‐Alcidae (Smith, 2014; Smith & Clarke, 2012); Pan‐Sphenisciformes (Ksepka et al., 2012; Ksepka & Clarke, 2010); Pelecanoididae (Ksepka & Clarke, 2010; Smith, 2010); Plotopteridae (Kawabe et al., 2013; Smith, 2010). Osteological and neuroanatomical terminology follow that summarized by Baumel and Witmer (1993) and osteological measurements were taken according to the methods outlined by von Den Driesch (1976). Avian body mass data are those of Dunning (2008).
The following osteological specimens were evaluated: American Dipper C. mexicanus (FMNH 288098, 288103, 336653, UMMZ 99329, 99330, 155407, 155862, 159400, USNM 320181, 343488, 345548, 611141, 641431, 641437); White‐throated (aka European) Dipper C. cinclus (USNM 136001, 499067, 637547, 637549, 637551); White‐capped Dipper C. leucocephalus (FMNH 433712); Brown (aka Asian) Dipper C. pallasii USNM 16688, 289927, 292206, 292946, 319050, 319594, 321550); Veery Catharus fuscescens (FMNH 317416, 331893, 331889, 331897, 331902, 349168, 470350, 475717, 475718, 475719); Swainson's Thrush Catharus ustulatus (FMNH 495801, 495811, 495812, 495813, 495815, 495816, 495817, 495818, 495819, 495859, CU 1819); Hermit Thrush Catharus guttatus (FMNH 492720, 494069, 494080, 494081, 494082, 494092, 494093, 494094, 494095, 494096). Three additional specimens were used to further explore forelimb element cross‐sections. These data were accessed on www.MorphoSource.org and were scanned using computed tomography (CT). The Field Museum of Natural History provided access to the C. mexicanus specimen (FMNH 288367), the collection of which was funded by oVert TCN. The files for specimen fmnh:birds:288367 were downloaded from www.MorphoSource.org, provided by Duke University, can be found at ark:/87602/m4/M73645 and was scanned at the University of Chicago PaleoCT scanning facility on a GE phoenix v|tome|x s scanner. The specimen was scanned at 100 kV, 300 μA, at 200 ms, resulting in isotropic voxels of 75 μm. Yale Peabody museum provided access to the data for Pelecanoides urinatrix (YPM 120642), the collection of which was funded by oVert TCN. The files for P. urinatrix (ypm:vz:ypm orn:120642) were downloaded from www.MorphoSource.org and can be found at ark:/87602/m4/M100459. It was scanned at the Research Museums Center at the University of Michigan Museum of Zoology on a Nikon XT H225ST CT scanner. It was scanned at 85 kV, 200 μA at 250 ms with a resolution of 76 μm. This specimen's cranial pneumaticity was also examined. Catharus minimus (UF‐O 52130) was scanned at the University of Florida Nanoscale Research Facility on a GE phoenix v|tome|x m240 scanner at 75 kV, 200 μA at 200 ms with a resolution of 58 μm. The data were downloaded from MorphoSource.org and can be accessed at ark:/87602/m4/M40419. The CT data for all three of these digital specimens were analyzed and graphic representations produced using VG Studio Max 3.4 (Volume Graphics; https://www.volumegraphics.com). In addition, dried skulls of the American Dipper C. mexicanus (USNM 630605), Hermit Thrush C. guttatus (USNM 634096), and Samoan Starling Aplonis atrifusca (Sturnidae; USNM 498061) were scanned at the Ohio University MicroCT Scanning facility (OUμCT) on a General Electric eXplore Locus in vivo μCT scanner. These were scanned at 80 kV, 450 μA, 400 ms, with 1,200 views, yielding isotropic voxels of 44 μm. Those CT data were digitally highlighted and modeled in 3D using the segmentation tools in Avizo (Thermo Fisher Scientific; http://www.thermofisher.com). The data were segmented without filtration in Avizo using both automated (Magic Wand) and manual (Paintbrush) techniques; given that the specimens were all well‐ossified adult dried skulls (i.e., with no soft tissue), segmentation was straightforward and did not require any advanced processing. All CT‐derived images and reconstructions are available via MorphoSource (https://www.morphosource.org/projects/000377999).
Data on sex and body mass as well as 14 osteological dimensions (e.g., length of elements and width of individual osteological features) were collected for 57 individuals representing three species of Catharus and four species of Cinclus. A phylogenetic principal components analysis (Revell, 2009) was conducted and the resulting phylomorphospace was plotted for Cinclus and Catharus species with the loadings for the pPCA on the axes as an exploratory analysis. The relationship between this suite of osteological measurements of Cinclus and Catharus specimens were evaluated using a hierarchical linear model with phylogenetic correlations to compare how each of the above osteological measurements scaled with body mass for each genus while correcting for sex, allowing us to estimate divergence in scaling between the species.
To further investigate the relationship between the relative size of skeletal elements of Catharus and Cinclus, the correlation between the lengths of major limb bones (e.g., humerus, ulna, femur ratios) in each of those genera were calculated using phylogenetic correlations averaged across 100 different phylogenetic trees derived from Jetz et al. (2012) and employing the R packages phytools (Revell, 2012) and corrplot (Wei & Simko, 2017). Scaling relationships between major limb bones and body mass in Catharus and Cinclus were estimated by conducting a hierarchical phylogenetic regression using sex and size (geometric mean of all osteological measurements) to predict bone lengths, with the phylogeny, species, genus, and individuals as grouping factors to correct for autocorrelations. This analysis used a stan‐language model implemented in brms (Bürkner, 2017, 2018, 2019; Stan Development Team, 2019) running four chains for 20,000 generations, with the first 10,000 discarded as burn‐in. The bone measurements were used as separate response variables with their variance modeled as a function of average mass, sex, species, with the variance further pooled by genus and phylogeny (the only two “random” or grouping effects).
All four chains achieved convergence as evidenced by Rhat values of 1.00. Each measurement (e.g., keel length, deltopectoral crest length, pygostyle length) was separately predicted from the size, sex, genus, and phylogenetic variance–covariance matrix. All fits were made simultaneously and estimates for both the slope and intercept were corrected for phylogenetic signal.
To reduce the chance of spurious results, all parameters were shrunk towards a common value via partial pooling (Gelman et al., 2012; Gelman & Loken, 2013), and so strong evidence was needed to find that any two of the same type of parameters (e.g., slopes) differed. Slopes between body size and osteological measurements were initially modeled as following a normal distribution centered on 0.5 with a standard deviation of 1, while intercepts were modeled as normally distributed with a center of zero and standard deviation of 1. The differences between genera were especially pooled, with an expected difference of zero and standard deviation of 0.1. This makes the test for differences conservative, as it takes strong evidence to overcome the shared tight prior and suggest that two genera differ in a given measurement. For every post‐burn‐in generation, we compared the estimated Cinclus and Catharus effect on each measurement. Raw measurement data and analysis scripts have been made available online (see Supporting Information Materials and https://www.morphosource.org/projects/000377999). The following equations were used to analyze discrete data:
Endocast reconstruction—The endocranial neuroanatomy of birds is highly variable and has been demonstrated to reflect not only phylogeny, but ecology and aspects of cognition (Georgi & Sipla, 2008; Iwaniuk & Hurd, 2005; Ksepka et al., 2020; Smith & Clarke, 2012; Witmer et al., 2008). The internal cavities of the braincase of American Dipper C. mexicanus (USNM 630605), Hermit Thrush C. guttatus (USNM 634096), and Samoan Starling A. atrifusca (Sturnidae; USNM 498061) were digitally highlighted and modeled in 3D using the segmentation tools in Avizo (Thermo Fisher Scientific; http://www.thermofisher.com), producing endocasts of the brain and endosseous labyrinths (Balanoff et al., 2016; Witmer et al., 2008). Brain endocasts in birds are widely regarded as faithful proxies for the structure and volume of the brain itself (Balanoff et al., 2016; Early et al., 2020; Iwaniuk & Nelson, 2002). Raw endocast data and digital reconstructions are available upon request.
Myology, internal anatomy, and integument—Soft‐tissue anatomy is important to consider in understanding the full extent of potential swimming and diving adaptations and for making comparisons between dippers and closely related but non‐WPD passeriforms. A soft‐tissue dissection of a formalin‐preserved American Dipper was performed along with dissections of two species of Turdidae (Swainson's Thrush and Veery; all specimens FMNH uncatalogued). Characteristics of integument (e.g., feather density, relative length of primaries and rectrices, narial coverings), the relative size of external nares as well as differences in levels of subcutaneous fatty tissues and relative size of integumentary glands were assessed. Special consideration was also given to the primary flight musculature (m. pectoralis and m. supracoracoideus) as both of these muscles provide thrust during WPD (Kozlova, 1961; Watanabe et al., 2020). The following formalin‐preserved and skinned specimens were also evaluated: C. mexicanus (USNM 530549, 196710); C. pallasii (USNM 233716, 233715); C. cinclus (USNM 162889, 126770); C. leucocephalus (USNM 387740, 387744); C. schulzii (USNM 264511).
Early shifts in integument preceding musculoskeletal shifts have been reported in the penguin stem lineage and some shifts in wing feathering even precede loss of flight in waterbirds (Clarke et al., 2010; Wang & Clarke, 2015). Integumentary traits in alcids, penguins, and diving petrels were evaluated from the following sources (Clarke et al., 2010; Kulp et al., 2018; McKitrick, 1991; Schreiweis, 1982; Smith, 2014; Wang & Clarke, 2015; Watanabe et al., 2020). From an osteological perspective, plotopterids closely approach the highly derived features of penguins (e.g., decreased maneuverability of forelimb; Raikow et al., 1988). However, because plotopterids are known only from skeletal fossil remains, myological, and integumentary characteristics (e.g., lack of feather tracts, narial covering) are unknown (Kawabe et al., 2013; Mayr et al., 2015; Olson & Hasegawa, 1979).
Ethology—Behavioral characteristics were primarily sourced from the review of the clade by del Hoyo et al. (2005). However, to contextualize our anatomical findings, data on dive depth, dive duration, reproductive strategies, and song complexity were also confirmed by observing American Dippers and Brown Dippers in the wild (NAS personal obs.), and additional observations were made from videos of dippers filmed underwater in the wild (Video 1).
Institutional Abbreviations—AMNH, American Museum of Natural History, New York City, New York, USA; BM, Bruce Museum, Greenwich, Connecticut, USA; CU, Clemson University's Campbell Museum of Natural History, Clemson, South Carolina, USA; FMNH, Field Museum of Natural History, Chicago, Illinois, USA; NCSM, North Carolina Museum of Natural Sciences, Raleigh, North Carolina, USA; PSM, Puget Sound Museum, Tacoma, Washington, USA; SAM, South African Museum, Cape Town, South Africa; UF‐O University of Florida Ornithology Department, Gainesville, Florida, USA; UMMZ, University of Michigan Museum of Zoology, Ann Arbor, Michigan, USA; USNM, Smithsonian Institution, National Museum of Natural History, Division of Birds, Washington District of Columbia, USA; UWBM, University of Washington Burke Museum, Seattle, Washington, USA; YPM, Yale Peabody Museum of Natural History, New Haven, Connecticut, USA.
3. TRAITS SHARED BY WPD TAXA
Although there are some exceptions (details on distribution below), this suite of characteristics is largely absent in non‐WPD outgroups, suggesting that these characteristics are convergently shared by phylogenetically disparate clades of WPD birds. The presence or absence of the full suite of 23 characteristics was evaluated in the five clades of WPD birds relative to a representative of their nearest non‐WPD sister taxa: Cinclidae (Cinclus) relative to Catharus and Turdus (thrushes and starlings) (Klicka et al., 2005); Pan‐Alcidae relative to Stercorariidae (skuas) (Smith & Clarke, 2015), Pan‐Sphenisciformes relative to Procellariiformes (excluding Pelecanoididae which are well nested within that clade; Hackett et al., 2008), Pelecanoides relative to Puffinus (Hackett et al., 2008), and Plotopteridae relative to Suloidea (specific comparisons made with Morus bassanus and Sula sula; (Mayr et al., 2015).
3.1. Skull and endocranium
1. External narial opening, reduced in size and covered by narial flaps and/or feathering. Reduced or covered external nares prevent aspiration of water while diving in many species of birds. The external nares of Cinclidae are covered by a flap of cornified rhamphotheca and are at least partially feathered in some species (e.g., C. mexicanus; see Figure 4). The rhamphothecal covering of the bony narial opening (i.e., the external nares) is covered with short dense feathers in most alcids, whereas it is instead reduced to a narrow slit in puffins and auklets (Smith, 2011a, 2014), In most extant penguin species, however, the rhamphotheca extends to largely or completely cover the external nares shortly after hatching and a functional open nares remains only in Spheniscus and Eudyptula, with a very small opening present in Pygoscelis adeliae (Zusi, 1975). The nares of diving petrels are tube‐shaped, but are reduced in size and face dorsally, rather than anteriorly as in other Procellariiformes (del Hoyo et al., 1992). Available fossil skulls of plotopterids do not preserve the rhamphotheca, preventing the assessment of narial covering (Kawabe et al., 2013).
FIGURE 4.
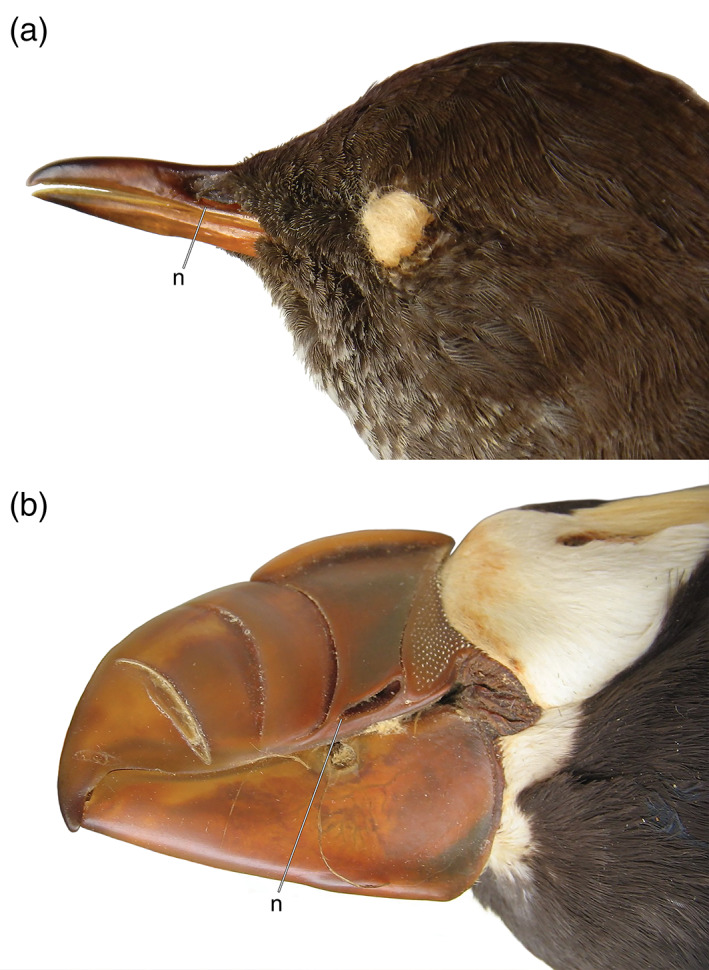
Beaks of wing‐propelled divers in lateral view showing the slit‐like external nares (n): (a) Cinclus mexicanus (Cinclidae, American Dipper; USNM 530549) and (b) Fratercula cirrhata (Alcidae, Tufted Puffin; USNM 589729)
2. Cerebellum, absence of fissures and folds. The dorsal and posterior aspects of the cerebellum of most birds are characterized by visible fissures or folds (Iwaniuk et al., 2006). In many species of diving birds including pan‐alcids (Smith & Clarke, 2012), penguins, loons (Ksepka et al., 2012), and plotopterids (Kawabe et al., 2013), these folds are obscured in digital endocast reconstructions by a thickened layer of meningeal tissue. In some groups such as diving petrels, they are reduced but minimally retained (Kawabe et al., 2013). In comparison to the sampled outgroup taxon, C. guttatus, the cerebellar folds of C. mexicanus are somewhat reduced, but still distinctly visible (Figure 5). The function of a thickened layer of meningeal tissue, obscuring the cerebellar folds, is not known but has been suggested to be related to withstanding pressures exerted on the brain while diving at depth (Smith & Clarke, 2012).
FIGURE 5.
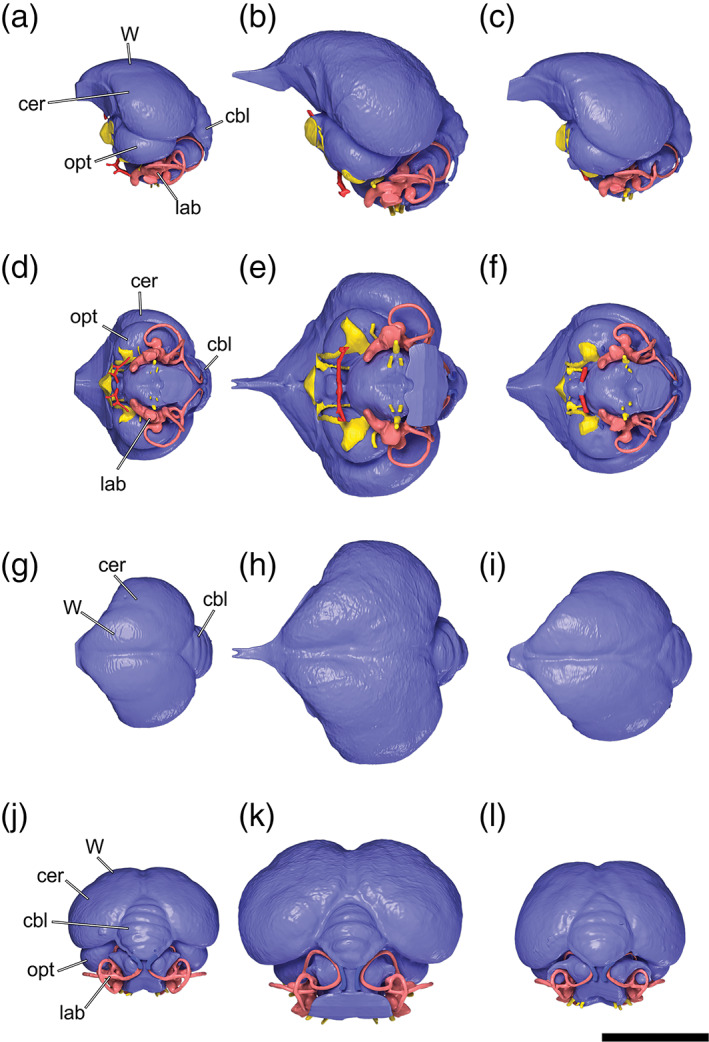
Brain endocasts of a, d, g, j, Catharus guttatus (Turdidae, Hermit Thrush, USNM 634096); b, e, h, k, Aplonis atrifusca (Sturnidae, Samoan Starling, USNM 498061); and c, f, i, l, Cinclus mexicanus (Cinclidae, American Dipper, USNM 630605) in left lateral view (a–c), ventral view (d–f), dorsal view (g–i), and caudal views (j–l), all based on surface renders of μCT scan data. Anatomical abbreviations: Cbl, cerebellum; cer, cerebral hemisphere; lab, endosseous labyrinth; opt, optic lobe; W, wulst. Scale bar = 1 cm
3. Occipital sinus, indistinct. In most extant birds the occipital sinus is a linear feature visible along the dorsal surface of the cerebellum (Butler & Hodos, 2005). In pan‐alcids, diving petrels and penguins the occipital sinus is obscured by a thickened layer of meningeal tissue (Kawabe et al., 2013; Ksepka et al., 2012; Smith & Clarke, 2012, fig. 16). In contrast to other WPDs, the occipital sinus of plotopterids is distinctly visible on the posterodorsal cerebellum (Kawabe et al., 2013). The occipital sinus is not visible on the endocasts of C. mexicanus or its near outgroup taxon, C. guttatus, suggesting that the lack of this feature in dippers is not related to diving habitus (Figure 5). As with the obscured cerebellar folds (see above), the function of a thickened layer of meningeal tissue, obscuring the occipital sinus, is not precisely known but has been suggested to be related to withstanding pressures exerted on the brain while diving at depth (Smith & Clarke, 2012).
4. Olfactory bulb, reduced relative size. In comparison with non‐WPD outgroups, the relative size of the olfactory bulb in pan‐alcids, penguins and diving petrels is reduced (Kawabe et al., 2013; Ksepka et al., 2012; Smith & Clarke, 2012, fig. 16). The olfactory bulb does not appear to be reduced in dippers (Figure 5) or plotopterids (Kawabe et al., 2013). Penguins and pan‐alcids are putatively dependent on visual signals to locate prey and navigate to their breeding locations. In contrast, some petrels use smell to navigate to their nesting localities (Grubb, 1979). The olfactory bulb is not reduced in dippers compared to outgroups (Figure 5), but it should be noted that the olfactory bulb is already very small in passerines. Dippers forage and navigate based on visual cues, so olfaction is probably of limited importance during dives (del Hoyo et al., 2005).
5. Cranial pneumaticity, reduced. There is an extensive literature documenting the reduction of skeletal pneumaticity in diving birds (Bellairs & Jenkin, 1960; Gutzwiller et al., 2013; Ksepka et al., 2012; O'Connor, 2004; Witmer, 1990). The pneumatic spaces in the cranium of dippers are not noticeably reduced in comparison with Catharus and Turdus (see Figure 6).
FIGURE 6.
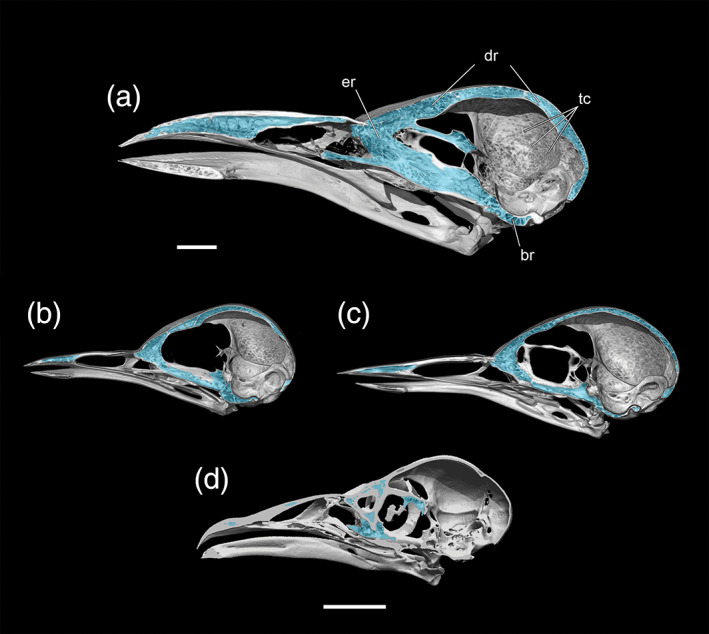
Medial views of the right side of sagittally sectioned skulls (based on volume renders of μCT scan data) of a, Aplonis atrifusca (Sturnidae, Samoan Starling, USNM 498061); b, Catharus guttatus (Turdidae, Hermit Thrush, USNM 634096); and c, Cinclus mexicanus (Cinclidae, American Dipper, USNM 630605); d, Pelecanoides urinatrix (YPM 120642) showing the minor differences in the extent of craniofacial pneumaticity. Anatomical abbreviations: Br, basicranial recesses; dr, dorsal recesses; er, ethmoid recesses; tc, trabecular cells. Scale bars = 1 cm
3.2. Sternum
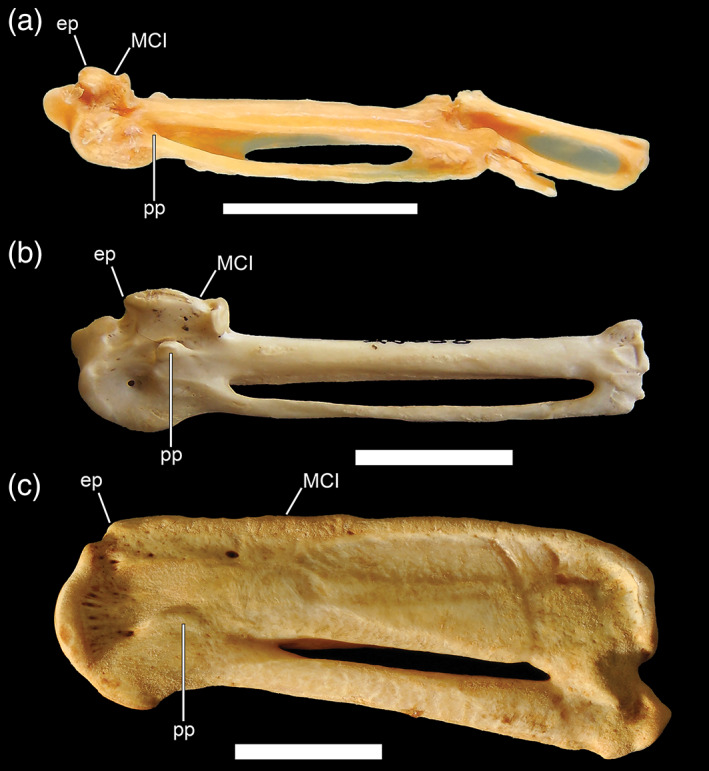
6. Sternum, elongate. The sternum of many diving birds is elongate (i.e., high length to width ratio) in comparison with nondiving taxa (Storer, 1960). This is true of pan‐alcids (Smith, 2011a), penguins (Ksepka & Clarke, 2010), and plotopterids (Smith, 2010). However, the sterna of diving petrels are not elongate in comparison with their near outgroups and those of dippers are only slightly elongate (Figure 7).
FIGURE 7.
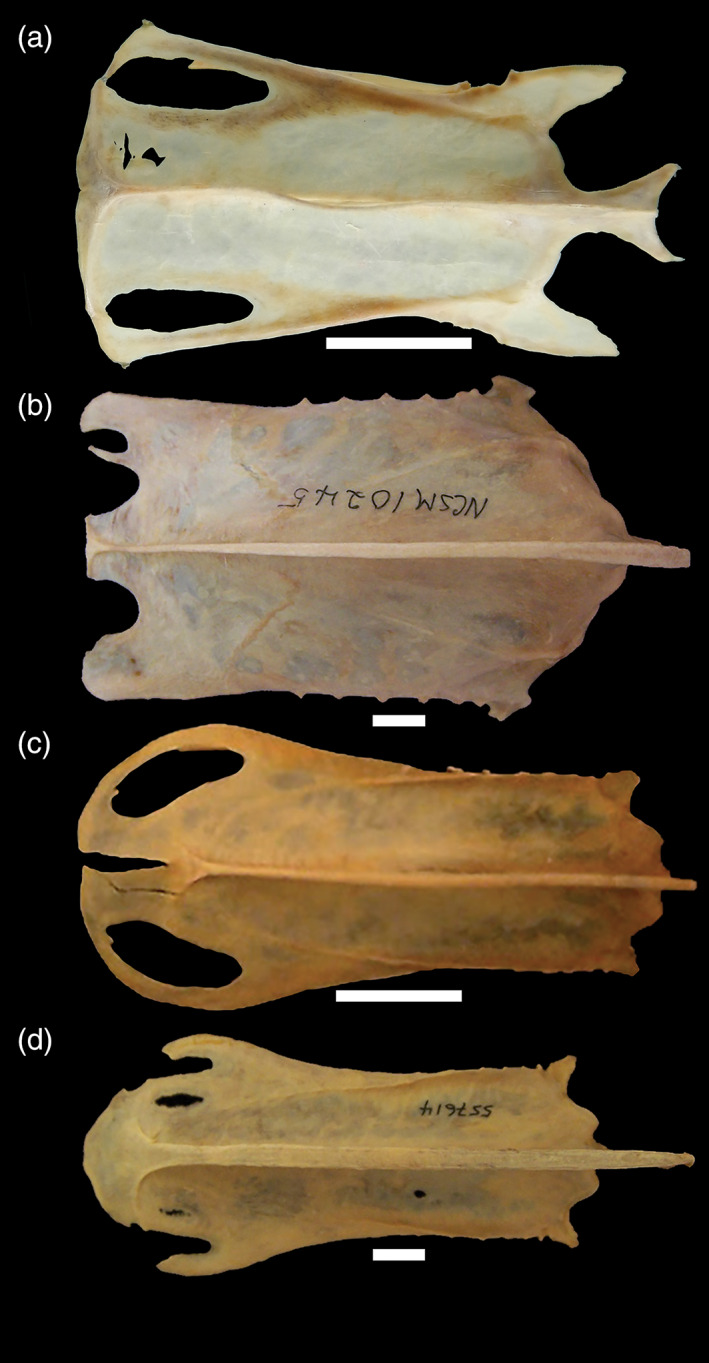
Sterna of wing‐propelled divers (and a nonwing‐propelled diving gull for comparison) in ventral view: a, Cinclus pallasii (Cinclidae, Brown Dipper; USNM 319050); b, Larus marinus (Laridae, Black‐backed Gull; NCSM 10245); c, Aethia pusilla (Alcidae, Least Auklet; NCSM 17734); d, Cerorhinca monocerata (Alcidae, Rhinoceros Puffin; USNM 557614). All scale bars = 1 cm
3.3. Forelimb
7. Wing elements (humerus, radius, ulna), shafts dorsoventrally compressed. The wing elements of pan‐alcids, penguins, and plotopterids are distinctly compressed (or flattened) in comparison with their nondiving outgroup taxa (Ksepka & Clarke, 2010, fig. 1; Olson & Hasegawa, 1979; Smith, 2011b). The humerus of Pelecanoides is compressed, though the remaining wing elements show little modification. Some other genera of Procellariiformes such as Oceanites and Puffinus also possess a less distinctly flattened humerus. The shafts of the humerus, radius, and ulna of dippers are not dorsoventrally compressed (Figure 8).
FIGURE 8.
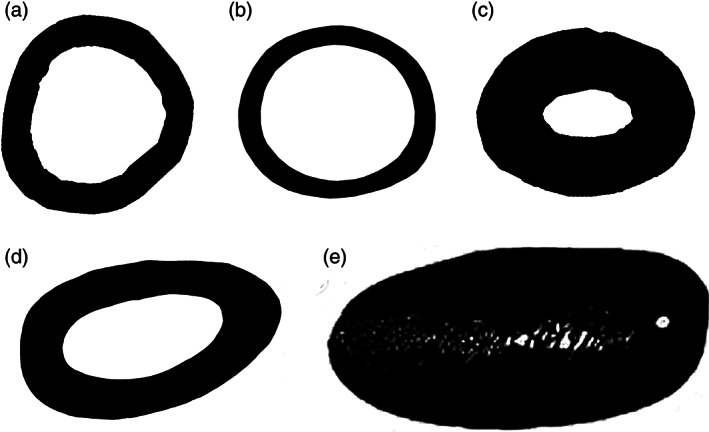
Humeral cross‐sections showing the relative cortical bone thickness and dorsoventral compression of the mid‐shaft region: a, Cinclus mexicanus (Cinclidae, American Dipper; FMNH 288367); b, Catharus minimus (Turdidae, Gray‐cheeked Thrush; FLMNH 52130); c, Pelecanoides urinatrix (Pelecanoididae, Common Diving Petrel; YPM 120642); d, Alca torda (Alcidae, Razorbill; NCSM 22058); e, Aptenodytes forsteri (Spheniscidae, Emperor Penguin; AMNH 3728). Images not to scale for comparison
8. Humerus, m. supracoracoideus scar distally elongate. The insertion of m. supracoracoideus on the proximal humerus of most charadriiforms (e.g., Larus marinus) forms a rounded scar, while in pan‐alcids this scar is distally elongate (crista m. supracoracoidei; Baumel & Witmer, 1993). The latter is true in penguins (Ksepka & Clarke, 2010), diving petrels, and plotopterids (Smith, 2010). The supracoracoideus scar of dippers is actually relatively shorter (relative to humerus length) than that of Catharus (Figure 9).
FIGURE 9.
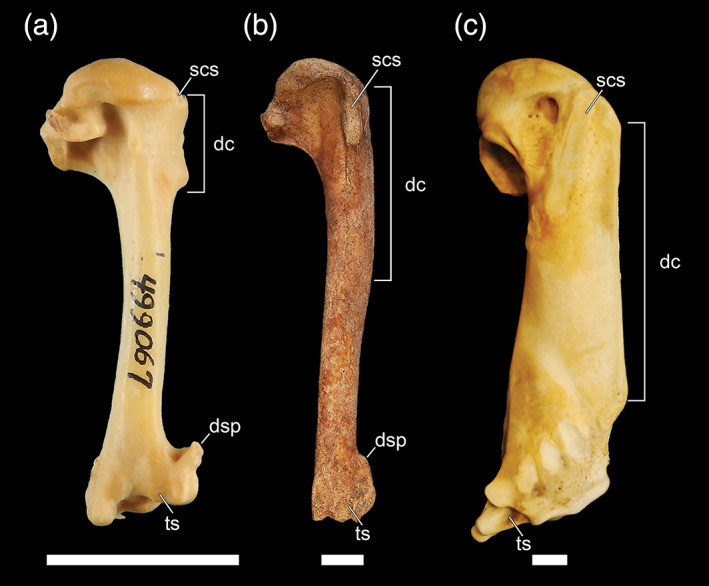
Humeri of wing‐propelled divers in ventral view: a, Cinclus cinclus (Cinclidae, White‐throated Dipper; USNM 499067); b, Pinguinus impennis (Alcidae, Great Auk; USNM 623465); c, Aptenodytes forsteri (Spheniscidae, Emperor Penguin; AMNH 8110). Anatomical abbreviations: dc, deltopectoral crest; dsp, dorsal supracondylar process; scs, supracoracoideus scar; ts, tricipital sulcus. Scale bars = 1 cm
9. Humerus, deltopectoral crest distally elongate. The deltopectoral crest extends distally along the anterodorsal margin of the humeral shaft to a point roughly one‐third to one‐half of the distance towards the distal end of the shaft in most species of alcids (e.g., Pinguinus; Figure 9), and well past the halfway point in many species of penguins (Ksepka & Clarke, 2010) and plotopterids (Smith, 2010). In dippers and diving petrels, the deltopectoral crest is not elongate relative to that of their near outgroups.
10. Humerus, dorsal supracondylar process reduced (relative to nondiving immediate outgroup taxa). The attachment point for m. extensor carpi forms a prominent dorsally directed process in many charadriiforms (e.g., L. marinus), while in all alcids this attachment forms a medial extension along shaft of the humerus, but does not project as far dorsally. The dorsal supracondylar process of diving petrels is also reduced in relation to those observed in most other Procellariiformes, though a reduced process is also observed in storm petrels. In plotopterids and extant penguins, the dorsal supracondylar process is essentially absent (a reduced, compact tubercle is present in some stem penguins). The dorsal supracondylar process of dippers does not significantly vary in its dorsal projection or relative size as compared to Catharus and Turdus (Figure 9).
11. Ulna:humerus ratio, reduced relative length. Reduction (and sometimes fusion) of distal forelimb elements is a recurring trend in WPD birds; most evident in the flipper‐like forelimbs of penguins (Ksepka & Clarke, 2010), and to a lesser degree in flightless auks (Smith, 2011b). Much of the length reduction typically occurs in the radius and ulna (Wang & Clarke, 2014). The shortening of these elements provides needed mechanical advantage for WPD birds coping with the challenges of locomoting in a medium as dense as water (Habib, 2010; Habib & Ruff, 2008). The ulna and radius of both diving petrels and plotopterids are also considerably shorter than their humeri as compared to near outgroups (Smith, 2010). While the absolute length of the ulnae of dippers are longer than their humeri (see osteological measurement data provided in Supporting Information), the relative length of the ulna as compared to the humerus in Cinclus is reduced relative to Catharus (Figure 10).
FIGURE 10.
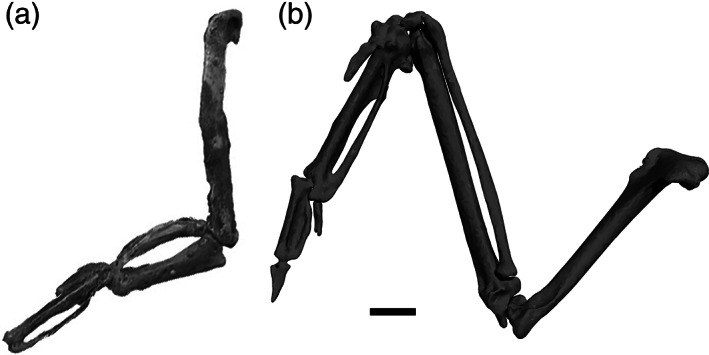
Wing elements of a, Mancalla sp. (Alcidae, Lucas Auk; composite LACM 154560; modified from Smith (2011b, fig. 16); not to scale; oblique dorsal view) and b, Cinclus mexicanus (Cinclidae, American Dipper; rendered from CT scan of FMNH 288367; oblique ventral view). Note that in Mancalla, the ulna is shorter than both the humerus and the carpometacarpus, a condition otherwise known only in hummingbirds among all extant Aves (Smith, 2011b). In contrast, the ulnae of dippers are longer than both the humerus and carpometacarpus but are also relatively shorter than the ulnae of thrushes. Scale bar = 3.5 mm
12. Humerus, development of trochlear ridges/grooves for the scapulotriceps and humerotriceps tendons. Deep paired sulci for the scapulotriceps and humerotriceps tendons are well developed in penguins, plotopterids, and alcids (Ksepka & Clarke, 2010; Smith, 2010, 2014) and are weakly developed in diving petrels. These grooves remain indistinct in dippers (Figure 9).
13. Metacarpal I, distally elongate. Metacarpal I and the associated structure on its proximal end (i.e., the extensor process; see below) provide an attachment point(s) for m. extensor carpi radialis (Baumel & Witmer, 1993), a muscle involved in flexion of the distal wing. In contrast with the rather abrupt proximal termination of metacarpal I in most charadriiforms, the first metacarpal of most alcids (e.g., Alca torda; Figure 11) extends distally (fused) about one third of the length of metacarpal II, while in Mancalla species (e.g., Mancalla cedrosensis) and crown penguins metacarpal I is even more elongate, terminating approximately at the midpoint of metacarpal II. In crown penguins, metacarpal I further lacks a facet for a free alular digit (retained in some stem taxa). Metacarpal I is also distally elongate in comparison to near outgroup taxa in plotopterids (Smith, 2010). Dippers also possess a slightly elongate first metacarpal when compared to Catharus; however, the degree to which dippers have elongated this element is significantly less than that of other WPD birds.
FIGURE 11.
Carpometacarpi of a, Cinclus pallasii (Cinclidae, Brown Dipper; USNM 292206; also including digit II, phalanx 1, and digit III, phalanx 1); b, Alca torda (Alcidae, Razorbill; NCSM 20058), and c, Spheniscus demersus (Spheniscidae, African Penguin; SAM, uncatalogued) in ventral view. Anatomical abbreviations: ep, extensor process; MCI, first metacarpal. Scale bars = 1 cm
14. Carpometacarpus, reduction of extensor process. In addition to the distal elongation of metacarpal I (Figure 11), the extensor process (located on the proximal end of metacarpal I) is reduced or effectively absent in all WPDs except Cinclus (Ksepka & Clarke, 2010; Smith, 2010, 2014). This process serves as the attachment site for m. extensor metacarpi radialis, which serves to automate the extension of the distal part of the wing. The extensor process is unmodified in Cinclus relative to its outgroup.
15. Ulnare, enlarged. The ulnare (one of the free carpals) is greatly enlarged in penguins and plotopterids (Ksepka & Clarke, 2010; Smith, 2010), a feature putatively associated with the stiffened, flipper‐like wing. The ulnare of the flightless auk Pinguinus impennis is somewhat enlarged (although not to the degree seen in penguins), whereas the ulnare in extant volant alcids is not significantly larger than in other charadriiforms. The ulnare of the extinct flightless mancalline auks remains unknown (Smith, 2011a). The ulnare of diving petrels and dippers are not significantly larger than those of their respective near outgroups examined for this study (Figure 12).
FIGURE 12.
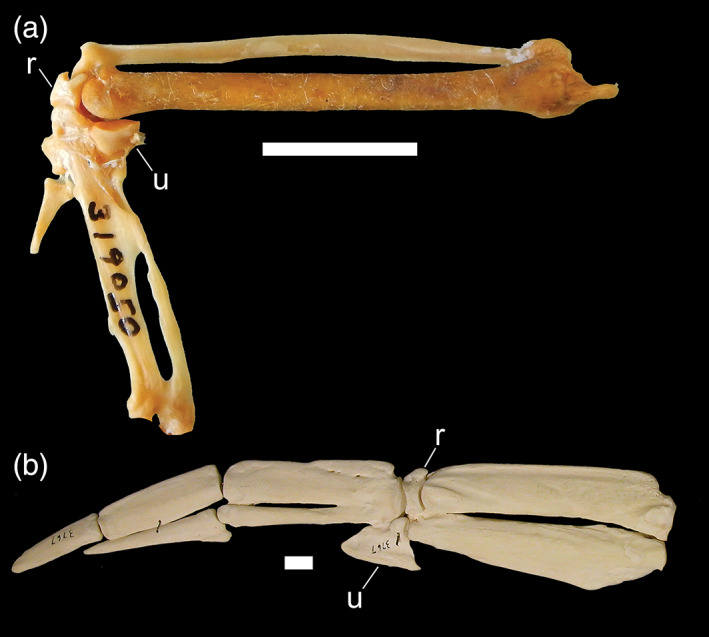
Distal wing elements of a, Cinclus pallasii (Cinclidae, Brown Dipper; USNM 319050); and b, Aptenodytes forsteri (Spheniscidae, Emperor Penguin; AMNH 3767) depicting the range of relative size of free carpals among wing‐propelled divers (dorsal view). Anatomical abbreviations: r, radiale; u, ulnare. Scale bars = 1 cm
16. Forelimb element cortices, relatively thick. The forelimb skeletal elements (particularly the humerus) have relatively thick cortices (i.e., pachyostosis or osteosclerosis) in pan‐alcids (Smith & Clarke, 2012), penguins (Ksepka et al., 2015), plotopterids (Olson & Hasegawa, 1979), and diving petrels (Figure 8). Pneumatic spaces are reduced in general, and the cortices of the hind limb elements (e.g., femur) are thickened in some WPD taxa as well, contributing to the overall greater density of the skeleton in those WPDs. While this trend is superficially similar to that seen in flightless terrestrial species of birds (e.g., moa) that are not constrained by the biophysics of flight, greater bone density serves as a functional adaptation to decrease buoyancy in WPD birds (Smith & Clarke, 2012). The cortices of dippers are not substantially thicker than are those of thrushes (Figure 8).
17. Scapula, broad distal blade. The distal portion of the scapula (i.e., the scapular blade) of penguins and plotopterids is expanded into a paddle‐like shape (early stem penguins retain a less expanded scapular blade). This feature is absent in pan‐alcids and diving petrels. Although the distal scapular blade of dippers does not approach the extreme width of penguins and plotopterids, it is nonetheless, significantly broader than that of Catharus (Figure 13).
FIGURE 13.
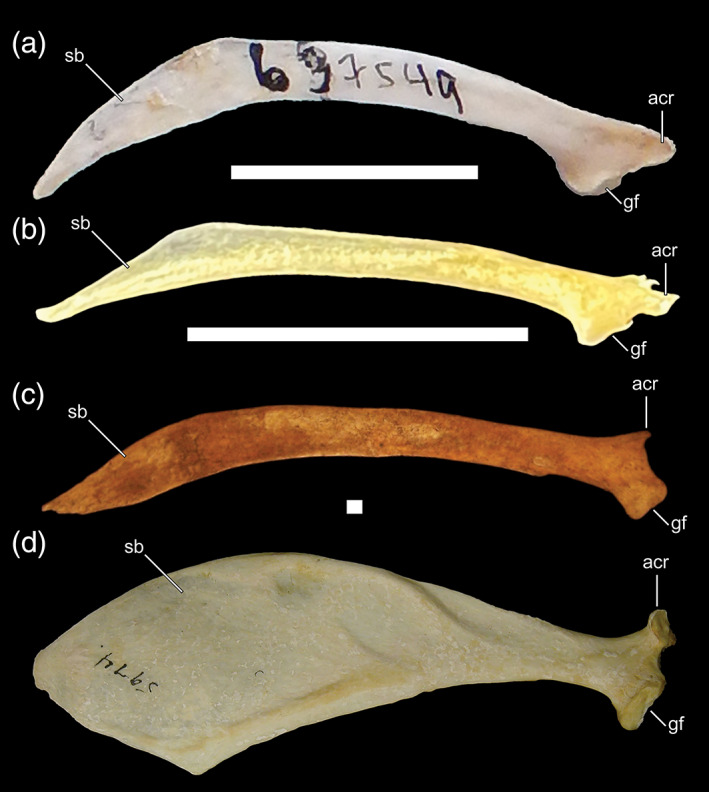
Lateral view of scapulae. a, Cinclus cinclus (Cinclidae, White‐throated Dipper; USNM 637549); b, Catharus guttatus (Turdidae, Hermit Thrush; BM; uncatalogued); c, Pinguinus impennis (Alcidae, Great Auk; USNM 623465; modified from Smith (2011a, fig. A2.22); d, Eudyptes chrysolophus (Spheniscidae, Macaroni Penguin; AMNH 5974; modified from Ksepka and Clarke (2010, fig. 24; not to scale). Anatomical abbreviations: acr, acomion; gf, glenoid facet; sb, scapular blade. Scale bars for Cinclus and Pinguinus = 25 mm. Scale bar for Catharus = 1 cm
3.4. Hindlimb
18. Tarsometatarsus, short. Compared to closely related outgroup taxa, the tarsometatarsus is relatively short compared to close relatives in all WPD birds. Although this may reflect the fact that these WPD taxa rely primarily on their wings for propulsion in the water, it should be noted that some penguin species walk great distances to reach their breeding colonies (e.g., Emperor Penguin Aptenodytes forsteri). Thus, the reduction of the tarsometatarsus may also serve some function such as reducing drag during WPD or shifting center of gravity to accommodate the larger pectoral musculature when on land.
3.5. Vertebrae and ribs
19. Uncinate processes, not fused to thoracic ribs. The uncinate processes of the thoracic ribs are frequently fused to the posteriorly adjacent ribs in many birds, providing rigidity to the rib cage and as well as respiratory functions (Codd, 2010). The uncinate processes remain unfused in penguins, diving petrels (but not other procellariiforms), alcids (Smith, 2014), as well as in the foot‐propelled diving loon (Gavia; see Ksepka & Clarke, 2010, character 129). The condition in plotopterids is unknown. In contrast, the uncinate processes are fused to the thoracic ribs in most outgroups, with limited fusion noted in Larus, Stercorarius, Oceanites, and Puffinus. The uncinate processes of dippers are not fused to the thoracic ribs, in contrast with Catharus, in which they are fully fused.
20. Pygostyle, long and straight. When compared with closely related nondiving taxa, the pygostyle of WPD pan‐alcids, penguins, plotopterids, and diving petrels is relatively long and straight (Chu, 1998; Felice & O'Connor, 2014; Smith, 2011b, 2013, 2014). Pygostyle morphology in dippers is not noticeably different from that of cathartid thrushes or Turdus (Figure 14).
FIGURE 14.
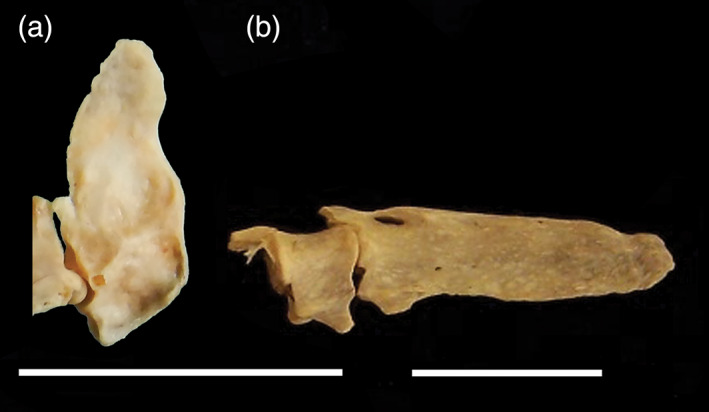
Pygostyle and last free caudal vertebra in left lateral view: a, Cinclus cinclus (Cinclidae, White‐throated Dipper; USNM 637551); b, Alca torda (Alcidae, Razorbill; NCSM 20058). Scale bars = 1 cm
3.6. Other characteristics
21. Apteria, absent. The feathers of most birds grow in distinct tracks along the body (pterylae), with bare patches of skin between (apteria). Apteria are lacking in penguins and dippers, but present in thrushes, diving petrels and pan‐alcids (del Hoyo et al., 2005, 1992; Konyukhov, 1996; Ksepka & Clarke, 2010). The condition in plotopterids is unknown.
22. M. supracoracoideus, relatively large. In penguins, m. supracoracoideus accounts for a much larger percentage of the total muscle mass than in Procellariiformes (Schreiweis, 1982). The same is true of pan‐alcids (Hudson et al., 1969), and Pelecanoides (McKitrick, 1991) compared to other Charadriiformes and Procellariiformes, respectively. Likewise, the supracoracoideus muscle in dippers is relatively larger than in Catharus (Figure 15). The state in plotopterids is unknown.
FIGURE 15.
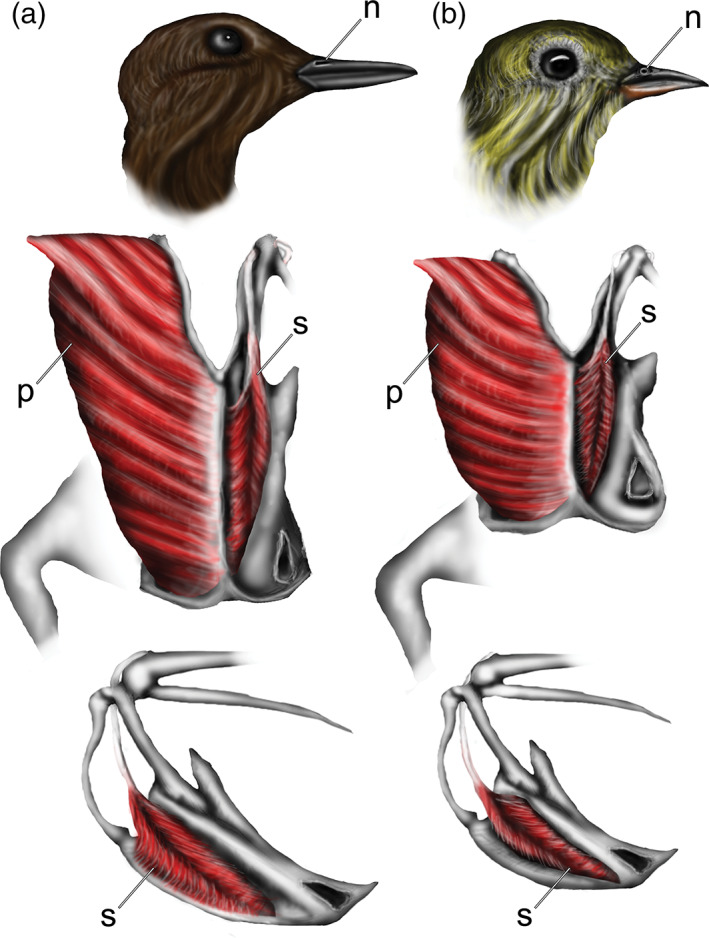
Cranial integument, narial morphology, and myological comparison: a, Cinclus mexicanus (Cinclidae, American Dipper); b, Catharus ustulatus (Turdidae, Swainson's Thrush). Note that in Cinclus the nares (n) are elongate slits; the sternum (s) is relatively elongate as compared to Catharus, and m. pectoralis major (p; on right of sternal keel in middle row) and m. supracoracoideus (s; on left of sternal keel in middle row) are relatively larger. Top row in right lateral view; middle row in ventral view; bottom row in left lateral view
23. Range of body mass (i.e., body mass value of largest species minus value of smallest species). Living penguins (Spheniscidae) display the largest range of body mass (~37,000 g) among all extant birds (Dunning, 2008; Smith, 2012; Smith, 2015). Moreover, when including stem penguins, the range of body mass approaches 80,000 g, a similar value to that estimated for Plotopteridae (Dyke et al., 2011; Jadwiszczak, 2001; Smith, 2015). Pan‐Alcidae have the largest range of body mass among extant Charadriiformes (~900 g; Smith, 2015). Thus, there is a consistent trend of relatively increased range of body mass (in comparison to other birds) in three phylogenetically distinct lineages of WPD birds. In contrast, the range of body mass in dippers and diving petrels is not significantly greater than that of their nondiving outgroup taxa (Dunning, 2008) and the range of body mass in the largest radiation of modern birds (i.e., the ~6,500 species of extant Passeriformes) is only ~1,100 g. Ongoing work suggests that body mass of WPDs has evolved under fewer or different constraints than those of other birds (Smith, 2015). Interestingly, it is likely that volant WPD taxa may be strongly constrained with respect to body mass by the competing demands of aerial and aqueous flight, whereas flightless WPD birds are free to increase their body size by as much as two orders of magnitude. A theoretical lmit of ~1 kg has been proposed for volant WPD taxa (Elliott et al., 2013; Stonehouse, 1967; Storer, 1960). However, the 2.1 kg body mass estimate for the volant Pliocene auk Alca stewarti contradicts that hypothesis (discussed by Smith, 2015).
4. RESULTS
Osteology—There are some distinct osteological differences between dippers and thrushes that are putatively associated with WPD. The phylogenetic PCA suggests that the primary differences between dippers and thrushes manifest in the tradeoff between flight muscle attachments (relative size of the m. supracoracoideus scar and the distal extent of the deltopectoral crest; Figure 16). Counterintuitively, these two muscle attachment points are smaller relative to overall body size in dippers than in thrushes, opposite to the trend observed in other WPD birds and in apparent conflict with the larger muscle bodies observed in dippers as compared to thrushes (see myological results below).
FIGURE 16.
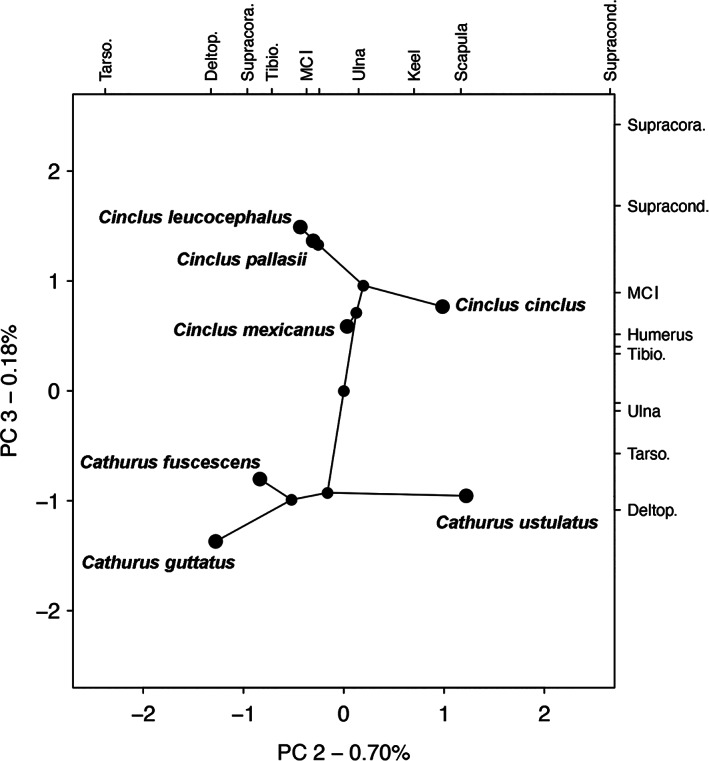
Phylomorphospace showing the principal component axes 2 and 3 for Catharus and Cinclus. The two axes are those that characterize the major differences in length of elements and features between the two groups (the first axis is largely size). The loadings for each trait are shown on the top (for PC2) and at right (for PC3). The relationship between the supracondylar process and deltopectoral crest characterize PC3 which shows the greatest difference between the dippers and thrushes. Abbreviations: Deltop., deltopectoral crest; MCI, metacarpal one; Supracond., dorsal supracondylar process length; Supracora., supracoracoideus scar length; Tarso, tarsometatarsus; Tibio, tibiotarsus
Interelement correlations show that Cinclus exhibits tight isometric scaling between elements (strong, positive correlations between bones), while Catharus thrushes show a more typical pattern of variable allometries seen in the relative lengths of different elements (Figure 17). Consistent with previous findings that proposed that ulnar shortening is highly selected for in WPD birds (Wang & Clarke, 2014), we found reduced ulnar length relative to humeral length in Cinclus relative to Catharus.
FIGURE 17.
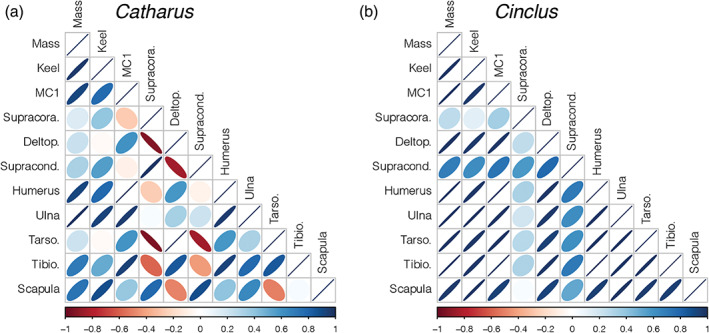
Correlogram showing the pairwise correlation between skeletal elements of Catharus and Cinclus. A perfect correlation (r = 1) is represented as a line, while no correlation (r = 0) is represented as a perfect circle. Ovals indicate a correlation between 0 and 1, with a more circular shape indicating lower correlation. The sign of the correlation is indicated by both the orientation of the oval and by its color (red color and pointing towards the left upper corner indicates a negative correlation, and blue indicates a positive correlation). These phylogenetically corrected correlations were determined between elements within a genus according to the phylogenies from Jetz et al. (2012). a, Correlations in the genus Catharus showing some negative relationships between certain wing and leg traits (red). b, Correlations in the genus Cinclus showing that in dippers all elements scale positively with one another, although leg and wing traits have weaker correlations
Hierarchical regressions all converged, with all chains exhibiting Rhat values <1.01 and effective sample sizes all over 6,000 (detailed results provided in Supporting Information). Bayesian R 2 values for the morphological measurements varied from 0.08 for the supracoracoideus to 0.82 for the distal scapular breadth (dorsal supracondylar process extension, 0.13; deltopectoral crest length, 0.20; tarsometatarsus length, 0.39; tibiotarsal ratio, 0.66; humeroulnar ratio, 0.78; first metacarpal, 0.69; keel length, 0.56; pygostyle length, 0.65; Figure 18). Uncertainty was high due to the small number of species compared between the two genera, but they differed significantly (p < .05) in the distal scapular breadth, with Cinclus having a proportionally wider scapula, even when accounting for sex and phylogeny. For the other measures, despite high uncertainty, there were consistent differences in the estimated values across the posteriors (Figure 18). In addition to the significantly and substantially broader distal scapulae and shorted ulnae, when compared to thrushes, dippers were found across the posterior estimates to have a longer sternal keel and sternum, and a more elongate first metacarpal. All of those characters are consistent with convergence on the phenotype of other WPD birds. Although the means of the posterior distributions of the extension of the dorsal supracondylar process of the humerus and the length of the pygostyle in Cinclus were not significantly different from those of Catharus, the full range of values recovered suggests some degree of anatomical modification (Figure 18). Also, compared with Catharus the uncinate processes of Cinclus are not fused to adjacent thoracic ribs, potentially providing increased ability to accommodate flexion in the torso or perhaps respiratory specialization related to diving (Codd, 2010). In summary, approximately half of the 10 statistical comparisons of osteological features indicated that the anatomy of Cinclus varied from that of Catharus in ways that would be predicted by the shared morphology of other WPD birds and additional morphologies (e.g., unfused uncinate processes) are also suggestive of only moderate osteological convergence.
FIGURE 18.
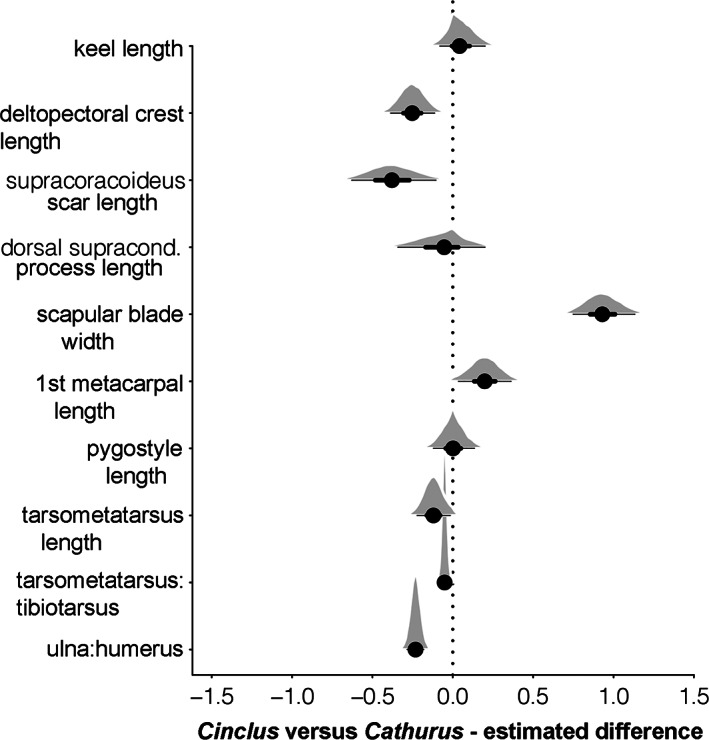
Phylogenetic hierarchical regressions showing the best estimate of each genus‐specific regression of body mass against each osteological measurement. Standard Bayesian 50% confidence intervals are shown. Positive values equate to larger relative size. The ulna:humerus ratio and tarsometatarsus:tibiotarsus ratio show the greatest allometric difference between genera, although the divergence is not large enough to be statistically clear at the p < .05 level. Bayesian R 2 values for the regressions are 0.91 ± 0.01 for keel length, 0.46 ± 0.08 for the deltopectoral crest, 0.40 ± 0.08 for supracondylar length, 0.84 ± 0.03 for pygostyle length, 0.82 ± 0.03 for ulna:humerus ratio, and 0.83 ± 0.03 for tarsometatarsus:Tibiotarsus ratio
Endocranial anatomy and pneumatization—There is an extensive literature documenting the reduction of skeletal pneumaticity, including that of cranial features, in diving birds (Bellairs & Jenkin, 1960; Gutzwiller et al., 2013; O'Connor, 2004; Witmer, 1990). In this context it is reasonable to hypothesize that dippers would show reduced pneumaticity to reduce buoyancy. Despite differing lifestyles, however, both Cinclus and Catharus, which are similar in size, share surprisingly similar amounts of pneumatic invasion of the skull (Figure 6). The starling (Aplonis) was considerably more pneumatic than either the dipper or thrush, although the greater extent of pneumaticity may be partly attributed to the larger size of the starling in our sample, in that pneumaticity often has a size‐related (allometric) component (Smith, 2012; Verheyen, 1953; Winkler, 1979). As shown in sagittal section (Figure 6), the extent of the dorsal pneumatic cavities in the skull roof is very similar in the dipper and the thrush, and, if anything, the pneumaticity is slightly more extensive in Cinclus, continuing more caudally to cover nearly the entire cerebellar region, whereas the roof of the cerebellum is apneumatic in Catharus. The ethmoid recesses are again comparable in pneumatic extent in the Cinclus and Catharus, but the latter shows slightly more extensive basicranial pneumaticity. Nevertheless, subtle differences aside, there is certainly no evidence of the major reduction in craniofacial pneumaticity that one might expect as an adaptation given the semi‐aquatic lifestyle of dippers. Furthermore, we did not identify decreased pneumaticity (i.e., increased density) in postcranial elements such as the humerus or coracoid of dippers, areas that display decreases in pneumaticity in many WPDs (e.g., penguins and auks; Ksepka & Clarke, 2010; Smith & Clarke, 2014).
The endocasts of the brain and inner ear of Cinclus are extremely similar in morphology to those of the thrush and starling in our sample, suggesting that differences in locomotor and feeding ecology are not strongly tracked by the surficial structure of the brain and endosseous labyrinth (Figures 5 and 19). Compared to many nonpasserines, the endocrania of all three species are posteriorly shifted within the skull and markedly rotated dorsally with respect to the lateral semicircular canals (Figure 20), owing to the relatively large size of the orbits. The major brain regions (e.g., cerebral hemispheres, optic lobes, cerebellum) are similarly sized in all sampled exemplars (Figure 5). This is especially true of the dipper and thrush which are similar in overall relative size. The role of allometric scaling is unassessed in this small sample but no doubt plays a role in the subtle differences between the larger starling and smaller dipper and thrush. Olfactory bulbs are small in all three species, and the cerebral hemispheres of the telencephalon (forebrain) are by far the largest regions of the brain. Likewise, the size and position of the Wulst, a paired projection from the surface of the telencephalon, and corresponding to the underlying hyperpallium, is rostrally situated and volumetrically conserved across all three species we examined. The optic lobes, corresponding to the underlying optic tectum, are modest in size and seem to be rotated rostrally about the center of the cerebrum. The trigeminal system appears similarly sized in all three species, suggesting no gross differences in somatosensory capabilities in dippers relative to the other species. Likewise, the cerebellum is grossly similar in overall size and conformation of the folia among the species, again suggesting no obvious differences in sensorimotor coordination. The floccular recesses (i.e., housing the cerebellar auricles) do appear to be moderately larger in Cinclus; however, the uncertain polarity of this character in our sample leaves it unclear as to whether or not this is a potential adaptation for enhanced gaze stabilization (Witmer et al., 2003). Interestingly, the flocculus in all three species takes an almost recurrent path to wrap around the common crus of the inner ear to project into the extremely conserved position (Witmer et al., 2008) within the rostral semicircular canal.
FIGURE 19.
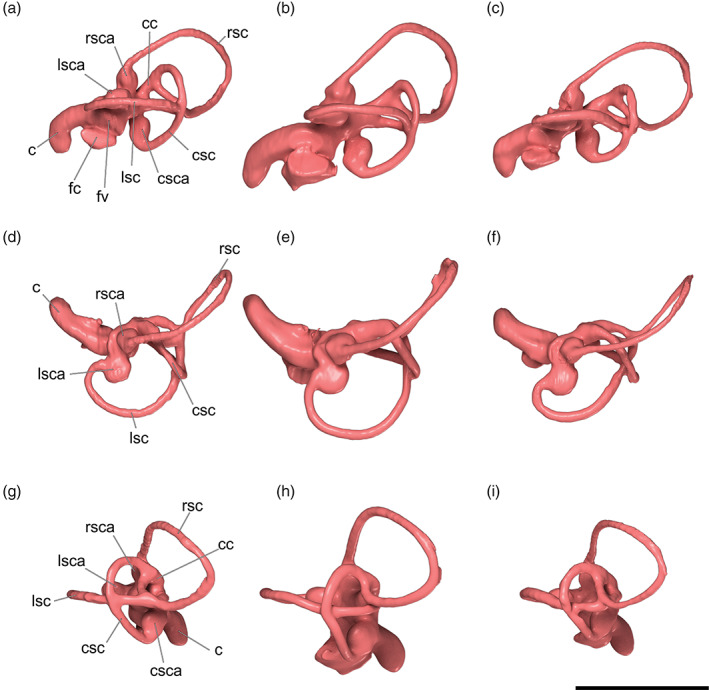
Left endosseous labyrinths of the inner ear of a, d, g, Catharus guttatus (Turdidae, Hermit Thrush, USNM 634096); b, e, h, Aplonis atrifusca (Sturnidae, Samoan Starling, USNM 498061); and c, f, i, Cinclus mexicanus (Cinclidae, American Dipper, USNM 630605) in left lateral view (a–c), dorsal view (d–f), and caudal view (g–i), all based on surface renders of μCT scan data. Anatomical abbreviations: c, cochlear duct; cc, crus communis; csc, caudal semicircular canal; csca, ampulla of the caudal semicircular canal; fc, fenestra cochleae; fv, fenestra vestibuli; lsc, lateral semicircular canal; lsca, ampulla of the lateral semicircular canal; rsc, rostral semicircular canal; rsca, ampulla of the rostral semicircular canal. Scale bar = 0.5 cm
FIGURE 20.
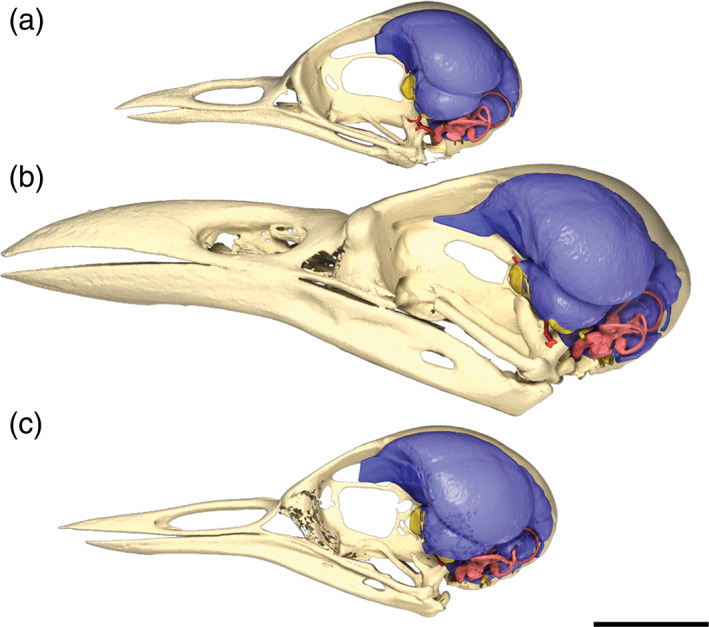
Skulls of a, Catharus guttatus (Turdidae, Hermit Thrush, USNM 634096); b, Aplonis atrifusca (Sturnidae, Samoan Starling, USNM 498061); and c, Cinclus mexicanus (Cinclidae, American Dipper, USNM 630605) in left lateral view (based on surface renders of μCT scan data) with the brain endocast and endosseous labyrinth superimposed in position. The lateral semicircular canals are oriented horizontally showing the natural alert posture of the head (Witmer et al., 2008) and the rotation of the brain to accommodate the large eyeball within the orbit. Scale bar = 1 cm
Much like the brain endocasts, the endosseous labyrinths of the inner ear in sampled passerines are, as expected, extremely different from those of non‐passerine WPD birds (Figure 19). All three passerines in our sample show extreme rotation of the labyrinth to an almost horizontal conformation overall. Rather than itself being a functional adaptation, it is possible that the driving force for this rotation of the labyrinth pertains to spatial constraints imposed by enlargement of the optic lobes and cerebrum (common among songbirds), along with the dorsal rotation of the brain noted above (see Figure 20). Overall, the brain, inner ear, and skull pneumaticity of dippers is quite similar to those of thrushes.
4.1. Ethology
Myology—While the brain and skull were found to be remarkably similar, dissections revealed some notable differences between Cinclus and Catharus that are most parsimoniously attributed to their differing ecologies. M. pectoralis major is the largest avian muscle in all volant birds (relatively diminutive in flightless species; e.g., Ostrich) and is primarily responsible for the downstroke of powered flight (aerial or subaqueous). In Cinclus, this muscle is craniocaudally elongate and relatively more massive compared to that of Catharus (Figure 15). Likewise, the keel of the sternum extends relatively further caudally (accounting for overall length differences between these taxa), caudal to the lower extremity in Cinclus. By contrast, in Catharus the sternal keel is shorter, merging with the sternal plate cranial to the lower extremity of the sternum. As noted by Crisp (1865), the larger muscle mass in Cinclus is a likely adaptation for subaqueous swimming, as it provides greater downstroke power needed to overcome powerful unidirectional river currents.
The m. supracoracoideus is used in the upstroke of powered flight and lies deep to m. pectoralis major. M. supracoracoideus originates on the ventral surface of the sternum at the base of the keel and continues as a long tendon that passes through the triosseal canal, inserting primarily onto the dorsal tubercle of the proximal humerus (George & Burger, 1966). As in m. pectoralis major, the muscle body of m. supracoracoideus in Cinclus is also more craniocaudally elongate and dorsoventrally extensive relative to that seen in Catharus. This is not unexpected as, in contrast to aerial flight, a powered upstroke is employed during WPD to provide thrust throughout the wingbeat (Watanuki et al., 2006). Thus, it is unsurprising that m. supracoracoideus is enlarged in WPD birds in comparison with non‐WPD sister taxa (e.g., Stercorariidae vs. Pan‐Alcidae, Hudson et al., 1969; Sphenisciformes vs. Procellariiformes, Schreiweis, 1982). The muscle fibers of m. supracoracoideus in Cinclus extend to the ventral margin of the sternal keel, covering the majority of the lateral surface of the keel as well as a large portion of the sternal plate (Figure 15). In contrast, the ventral third of the keel of Catharus is not covered by m. supracoracoideus.
In general, the musculature of the hindlimb and tail region of Cinclus is more massive relative to that of Catharus, a feature also noted by Crisp (1865). It remains uncertain whether this robust musculature is correlated to diving behavior. Dippers do not appear to extensively use their legs or tail while diving (see discussion of tail use below), though the more strongly developed legs at least may be related to underwater rock‐gripping. In addition, the eyes of Cinclus are also adapted for an amphibious lifestyle, with a greater development of an irideal sphincter muscle, which controls eye accommodation under increased pressure (Goodge, 1960). As in other diving birds, dippers are known to have higher concentrations of myoglobin and hemoglobin in their tissues than do nondiving birds (muscle and blood, respectively; Kooyman & Ponganis, 1998; Milsom et al., 1973), and it is conceivable that increased muscle mass combined with slow basal metabolic rate could help lower the metabolic costs of maintaining relatively increased muscle mass.
Integument and internal anatomy—Integumentary structures were also investigated to identify adaptations potentially related to WPD. The keratinous beak of Cinclus possesses a narrow, slit‐like narial opening, as opposed to the more oval‐shaped external nares of Catharus and most other passerines (Figure 4). The bony narial opening in the skull of Cinclus and Catharus are similar in relative size and shape, suggesting that the change in shape of the narial opening in the rhamphotheca of Cinclus may be related to diving. Supporting this interpretation, the same condition is found in some Pan‐Alcidae (e.g., Fratercula), and some penguins. In penguins, a large narial opening is present in the skull. In most extant penguin species, however, the rhamphotheca extends to largely or completely cover the external nares shortly after hatching (Zusi, 1975). Furthermore, dippers, as well as some penguins possess a valve‐like flap that covers the narial slit while diving, whereas in extant Alcidae, the nares are covered with short dense feathers in all excepting the puffins (del Hoyo et al., 2005, 1992, 1996). The external nares of diving petrels are reduced in size and face dorsally, rather than anteriorly as in other Procellariiformes (del Hoyo et al., 1992). Plotopterids possess long, slit‐like bony narial openings in their skull (Smith, 2010), suggesting that the rhamphothecal covering would be similar; however, the keratinous beak of plotopterids is unknown. Thus, narial modifications appear to be a frequent, yet independently acquired feature among WPD birds, including Cinclus.
Our examinations of formalin‐preserved specimens and skins also confirmed that the feathering of adult Cinclus is markedly different than that of Catharus. The microstructure of the down feathers of Cinclus is distinct, and the density of these feathers is nearly double that of Catharus (Davenport et al., 2009). The rachises of the contour feathers are also relatively thicker and longer, and the number of contour feathers is increased as compared to Catharus (Davenport et al., 2009; del Hoyo et al., 2005). Most notably, increased feather number in Cinclus is linked to highly reduced, nearly absent apteria meaning that more feathers cover the body without increased density per unit area (Davenport et al., 2009). Apteria are also absent in extant penguins (del Hoyo et al., 2005). Whether or not the flattened rachis and reduction or loss of the central vacuole that has been documented in the highly derived feathers of penguins and proposed to be related to hydrodynamic demands (Kulp et al., 2018) has not, to our knowledge, been studied in auks, diving petrels, or dippers.
Dippers possess rather short, rounded wings, superficially not unlike those of volant auks (Figure 21). Wing shape along with dorsal and ventral covert feather length in Cinclus is largely consistent with those of other passerines (Wang & Clarke, 2015). However, although Cinclus wing shape was recovered well within passerine morphospace, it is noteworthy that both dorsal and ventral covert feather length is slightly greater than in Turdus and Sturnus (Wang & Clarke, 2015). Thus, while Cinclus coverts do not approach the length seen in other WPD taxa, their vector of change is in the same direction (Wang & Clarke, 2015, fig. 2). In addition, as in some other diving birds (e.g., grebes, some ducks, and some alcids), dippers simultaneously molt all of their primaries, becoming flightless for a period of up to 2 weeks after their young are fledged (del Hoyo et al., 2005; Ginn & Melville, 1983). Simultaneous molt of primary feathers has been proposed as a preadaptation to the permanent loss of flight (Terrill, 2020), and while there is no evidence that Cinclidae is on an evolutionary path towards flightlessness, correlations between the evolution of WPD and molt strategy have yet to be studied in detail.
FIGURE 21.
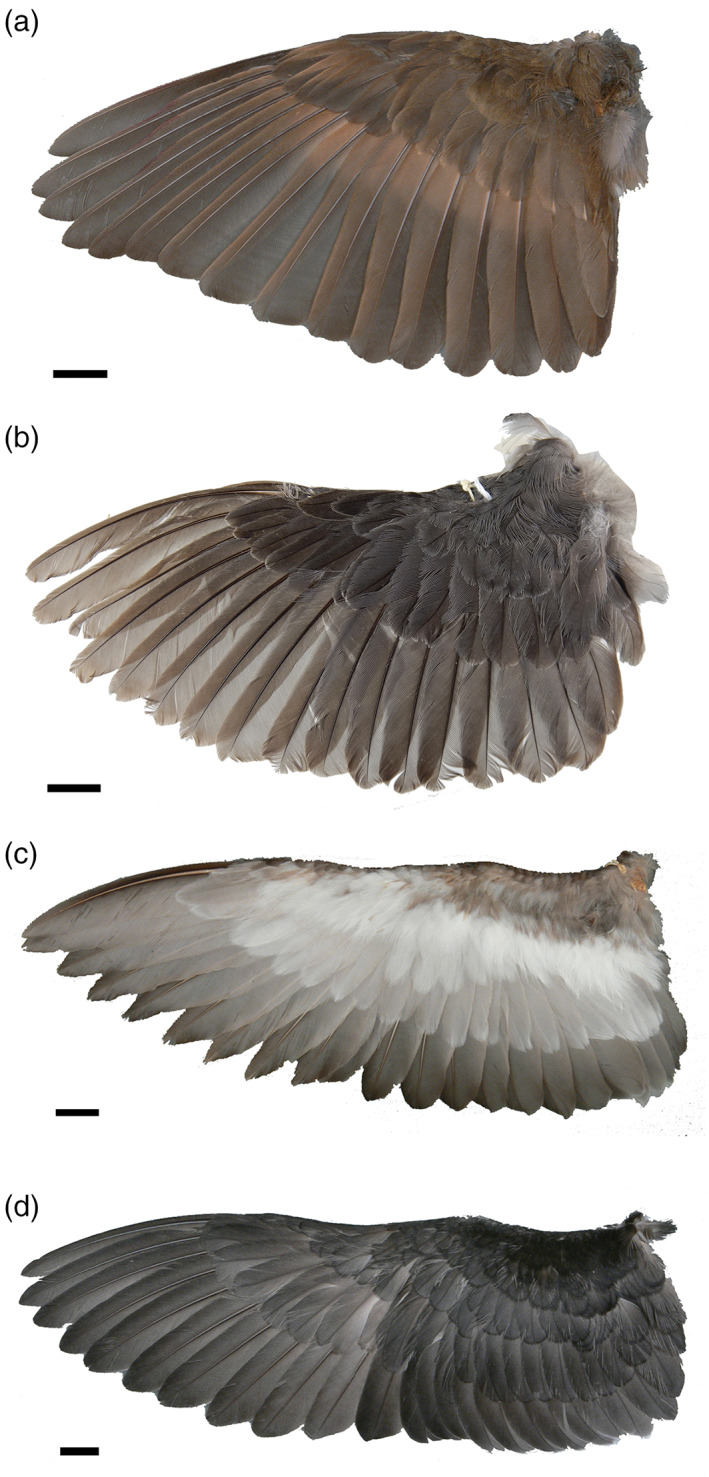
Comparison of wing shape of selected WPD birds in dorsal view: a, Catharus guttatus (Turdidae, Hermit Thrush; PSM 11204); b, Cinclus mexicanus (Cinclidae, American Dipper; USNM 640895); c, Aethia pusilla (Alcidae, Least Auklet; UWBM 48412); d, Pelecanoides urinatrix (Pelecanoididae, Common Diving Petrel; PSM 17233). Scale bars = 1 cm
Finally, with respect to feathering, it is worth mentioning that the rectrices of dippers are noticeably shorter than those of thrushes and starlings, and the stubby tail of dippers is a diagnostic field characteristic used to recognize the silhouette of these unique birds (Figure 2). Likewise, the tails of other WPD birds including penguins, auks, and diving petrels are shortened in comparison with their non‐WPD sister taxa. The short tail of WPD birds has been proposed as an adaptation for diving (Storer, 1960)—as employing a longer tail as an underwater rudder would conceivably increase hydrodynamic drag. A recent study found that a population of C. cinclus had elongated their tails over a 20‐year period (1985–2005) and postulated this morphological trend was a result of decreased stream flow owing to global warming (Moreno‐Rueda & Rivas, 2007), perhaps providing evidence that tail feather length in dippers is functionally correlated with some aspect of diving. Interestingly, the rectrices of the American dipper we dissected were relatively longer than those of the thrushes we evaluated and the tail feathers of the individual shown in the Video 1 are also noticeably longer than what is considered typical for that species and for dippers in general. It should be noted that the stream in which the dipper in the video is foraging is relatively slow moving, not unlike the conditions described for the study area of Moreno‐Rueda and Rivas (2007). Whether or not the rectrix length of dippers is ecophenotypically plastic and what hydrodynamic function that may serve warrants further study.
In Cinclus, the skin was generally thicker than that of Catharus (subjective assessment not a histological comparison). The dissected Cinclus specimen also possessed a thick layer of fatty tissue directly beneath the skin, whereas Catharus by contrast had almost no subcutaneous fat. These integumentary modifications potentially provide increased insulation from the typically frigid stream waters in which dippers forage.
Our dissections of formalin‐preserved specimens also confirmed that the uropygial gland at the base of the tail which secretes fluids used for waterproofing the plumage, is considerably larger in Cinclus than in Catharus. A survey of 1,433 species spanning Aves found that the uropygial gland of C. mexicanus was larger than any other sampled passerine and was only superseded in relative weight by three seabirds (Sterna albifrons, Phalaropus fulicarius, and Oceanodroma melania; Johnston, 1988). It is striking, however, that the relative size of the uropygial gland of dippers exceeds that of all other WPD birds (penguins, 9 spp. sampled; diving petrels, 3 spp. sampled; alcids, 11 spp. sampled; Johnston, 1988). Whether or not the microstructural characteristics of dipper feathers, or those of WPD birds in general, are modified to accommodate increased application of uropygial gland secretions remains unknown. Only relative sizes of the glands but not raw data were provided by Johnston (1988), thus future studies taking into account allometric scaling may better disentangle the relative influence of body size, plumage differences, phylogeny, and ecology on uropygial gland size.
Cinclus possesses wide, ridged pads of skin beneath the toes that were not observed in Catharus. The increased size of these toe pads is consistent with Cinclus' characteristic underwater rock‐gripping and branch‐grabbing behaviors. Unlike other WPD birds and like thrushes, dippers do not have webbing between their toes (i.e., palmate foot type). Like extant alcids, dippers use their feet to propel themselves along the surface of the water but do not rely on their legs and feet for propulsion once their bodies are completely submerged underwater (Video 1). It is plausible, however, that increased development of the leg musculature, as well as large toe pads and large, strongly curved unguals (Figures 2 and 22), may serve as an adaption to resisting the force of currents while gripping rocks or branches underwater.
FIGURE 22.

Underside of preserved specimen of Cinclus leucocephalus (Cinclidae, White‐capped Dipper; USNM 387744) showing the thickened foot pads, large and strongly curved unguals, and short rectrices characteristic of dippers. Scale bar = 1 cm
5. DISCUSSION
The results of our comparisons between dippers, their nondiving sister taxa, and other passeriforms reveals that Cinclus lacks the majority of characteristics that are widely shared by other WPD birds, possessing only 11 of the 23 putatively convergent WPD traits evaluated (findings summarized in Table 1). However, two of those characteristics (olfactory bulb size and visible occipital sinus) are also present in Catharus and thus cannot be attributed to WPD behavior in dippers. At the other end of the spectrum, penguins possess all 23 WPD characteristics, auks possess 20, plotopterids possessed 16–21 (range owing to missing data), and diving petrels possess 14. There are multiple potential explanations for fewer WPD traits in dippers. Dippers are the only freshwater WPD birds, and thus their lesser degree of convergence versus pelagic taxa may be in part due to distinctly different habitat‐linked locomotory ecology. The way in which dippers dive from the surface (rather than plunging from above as some petrels do) is a hypothetical step in one of several models of the evolution of WPD that was previously proposed in Pan‐Alcidae and based on the behaviors observed in other non‐WPD charadriiforms (Smith & Clarke, 2015). As Cinclidae consists of only five species, the aquatic feeding behaviors of other closely related passerines will require study to determine if analogs for putative evolutionary steps in the evolution of WPD in dippers are reflected in the behaviors and anatomy of other taxa. Likewise, it cannot be ignored that dippers and the four pelagic lineages are characterized by vastly different ancestral states and timings of clade origin. Comparison of estimated lineage age with the quantity of derived characteristics reveals a general trend of older lineages possessing a higher proportion of derived characteristics (Figure 23). This is however, a very coarse comparison and should not be interpreted as evidence that all lineages of WPD are evolving in similar stepwise fashion towards an evolutionary endpoint. However, given the relatively young age of the Cinclidae lineage, there is of course, potential for dippers to accrue more‐derived morphologies over time. We suggest that subsequent studies will likely draw stronger conclusions by treating WPD behavior along a spectrum rather than as a binary character.
TABLE 1.
Summary of characters associated with WPD
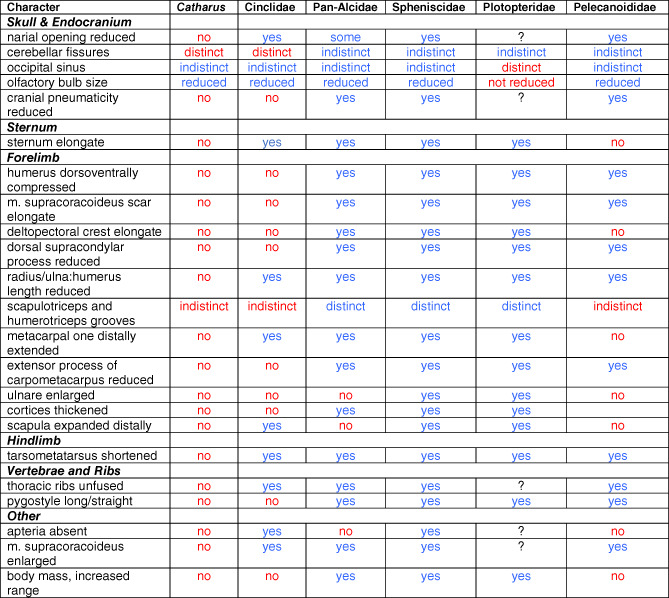
|
Note: Characteristics consistent with WPD convergence appear in blue and those characteristics that are inconsistent with convergence among WPD taxa appear in red. The state of five of the 23 selected characteristics are unknown (missing data denoted by “?”) in Plotopteridae because those data are not preserved in currently described fossils.
FIGURE 23.
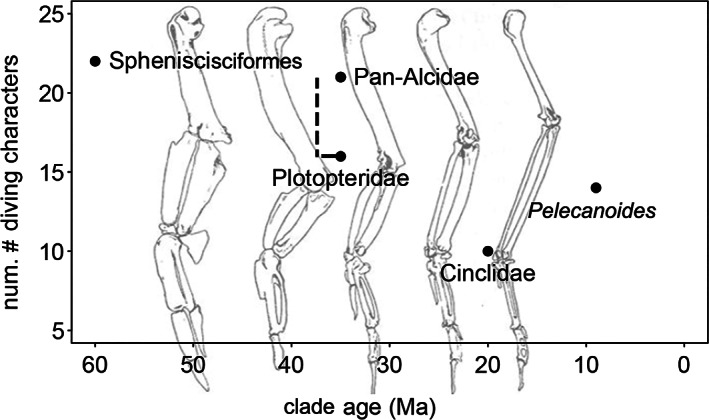
Comparison of clade age and quantity of convergent characters associated with wing‐propelled diving. The range depicted for Plotopteridae includes the uncertainty that exists because of the current state of anatomical knowledge based on the clade's incomplete fossil records. Background image of wing elements (from left to right; Spheniscus, Mancalla, Pinguinus, Alca, Larus) modified from Storer (1960)
The statistical comparison of osteological measurements of dippers and thrushes produced a mixture of expected and quite unexpected results. Analyses confirmed the broader distal scapulae and greater length of the sternum in dippers that was evident during dissections but revealed that many of the features that may be expected to be present in a bird with such derived behavior were, in fact, absent. The pygostyle of dippers is not relatively longer than that of thrushes. Unexpectedly, the length of the deltopectoral crest and m. supracoracoideus scars of the proximal humerus appear to be shorter (relative to overall size) in dippers than in thrushes (Figure 18). Although we did not attempt to precisely characterize relative tendon volume between dippers and thrushes, the tendons that insert along the deltopectoral crest and m. supracoracoideus scars of the humerus were not noticeably larger or different in character. The visibly larger flight muscles of dippers (as compared to thrushes; Figure 15) observed in dissection confirmed the findings of Crisp (1865). That the scars that serve as attachment points for these relatively large muscles would be smaller in dippers than in non‐WPD thrushes is perplexing and warrants further biomechanical study. A shift towards a tendinous rather than fleshy attachment or a subtle change in lever arm cannot be excluded. While these changes in flight muscle insertion may be apomorphic in dippers, that changes in muscular insertions occur after an increase in the relative mass of the flight muscles merits further investigation as pattern general to the evolution of WPD. Notable associated myological and osteological differences have been documented between flightless auks and penguins (Smith & Clarke, 2015; Watanabe et al., 2020), but myological comparisons of volant WPD birds remain to be made.
As in many diving birds, dippers do have increased levels of hemoglobin in their blood (del Hoyo et al., 2005) and in contrast with other passerines of similar body mass, dippers have substantially lower basal metabolic rates (Murrish, 1970). Despite their relatively short‐duration and shallow dives, dippers do possess a suite of characteristics that facilitate this extremely derived behavior—a behavior not even approximated by the other 6,000 or more species of passerines. However, osteological similarities may be linked to the large body size and deeper dive depths not observed in dippers. Although there are some notable exceptions, dive depth and duration are loosely correlated with increasing body mass in both penguins and alcids (Watanuki & Burger, 1999; though see discussion in Smith, 2011a). The considerable depths frequented by pelagic taxa are not available to dippers in freshwater streams and dive depths among dippers are typically less than 2 m, with average dive duration lasting less than 30 s (del Hoyo et al., 2005). Body mass of the five species of dippers ranges from ~40 to 90 g (del Hoyo et al., 2005). Therefore, although dive depths of extinct and extant alcids and penguins as well as putatively those of plotopterids (e.g., Copepteryx spp., ~7 kg; Dyke et al., 2011; see discussion in Smith, 2015), far exceed those of dippers, dive durations of dippers are consistent with that of the smaller species of alcids (Smith, 2011a; Smith & Clarke, 2015). For example, the average dive duration of the Dovekie (Alle alle; ~160 g) is ~40 s (Watanuki & Burger, 1999; also see data summarized in table 8.9 of Smith, 2011a) and typical dive depths of the smallest alcid, the Least Auklet (Aethia pusilla; ~85 g), are only 10–15 m (Obst et al., 1995), which markedly contrasts with the much deeper and longer dives of larger alcids (Croll & McLaren, 1993).
Compared with thrushes, dippers show marked differences in integument. They have reduced keratinous narial openings, thicker skin and subcutaneous fat deposits. They further show a thicker layer of down feathering and contour feathers of greater length, rigidity, and overall relative quantity (in that they lack apteria; Davenport et al., 2009). Dorsal and ventral covert feathers are also more elongate (Wang & Clarke, 2015). Although not ubiquitous, all of these modifications are shared with other WPD clades. Integumentary shifts, particularly in the shape of body contour feathers, occur early in penguin evolution as Eocene penguins already exhibited short, undifferentiated primary feathers and broad contour feather shafts (Clarke et al., 2010). Other shifts in feathering in dippers merit further study. Loss or reduction of the central vacuole and flattening of the rachis barb and barbules may confer the observed change in rigidity (Kulp et al., 2018). Differences in subcutaneous fat levels are interesting as they suggest the acquisition of an aquatic habitus is associated with novel selective pressures related to heat loss. However, lacking detailed life history observations from the individuals dissected, it is as yet unclear how much of the difference in fat thickness relates to differences between these taxa versus seasonal individual variation. Interestingly, with respect to heat regulation, and in stark contrast to the countercurrent heat exchange documented in penguins that functions to prevent frostbite in the wings and feet (Frost et al., 1975; Thomas & Fordyce, 2008; Trawa, 1970), dippers are actually susceptible to overheating and purportedly dissipate heat through their legs and feet (Murrish, 1970).
The brains of dippers and thrushes did not display any marked differences (Figure 5), and explanations for the fine scale neuroanatomical differences observed in dippers (or lack thereof) warrants further investigation with a larger sample of Musicapoidea. The increased levels of meningeal tissue that tend to obscure the cerebellar folds and occipital sinus during endocast reconstruction of other WPD has been proposed as a possible adaptation for coping with the extreme pressures associated with long, deep dives (Smith & Clarke, 2012). However, plotopterids lack a thickened meningeal layer (Kawabe et al., 2013) and the diving‐related hypothesis has yet to be assessed in a broad phylogenetic context. The lack of thickened meningeal tissue surrounding the brain of dippers might be considered to be consistent with that hypothesis. However, considering the details of the brain, inner ear, and skull pneumaticity, the overriding impression is how unremarkable all of these attributes are in dippers compared to other oscines given their divergent ecology. It is absolutely true that there could be differences in the underlying neural wiring that would be undetected in our analyses of endocasts, but certainly nothing can be discerned grossly, suggesting subtle neural changes imparting the marked behavioral shifts. Although there are slight differences in the endocranial anatomy and relative brain volume of volant alcids and stem‐penguins relative to their non‐WPD sister taxa, the most profound anatomical changes in those groups occur in flightless pan‐alcids and more‐derived extant penguin lineages (Ksepka et al., 2020; Proffitt et al., 2016; Smith & Clarke, 2012). The relative recency of the divergence of dippers from non‐WPD passerines coupled with their retention of aerial flight may be partly responsible for the lack of neuroanatomical specialization in the clade.
Dippers are oscine songbirds and not surprisingly, the vocalizations of dippers have been described as musical and wren‐like (del Hoyo et al., 2005; NAS, field observations). This stands in contrast with penguins, alcids, and diving petrels, all of which have what can arguably be characterized as rather harsh, simple, nonmelodic vocalizations. However, the alarm calls of dippers are loud and quite shrill so as to overcome the roar of fast‐moving streams (del Hoyo et al., 2005). Clearly, phylogeny plays a larger role in constraining some traits than does the influence of WPD. Likewise, the stream and forest environment that dippers inhabit is drastically different from the pelagic environment of non‐passerine WPD. Finally, colonial nesting, which is common (but not ubiquitous) in other WPD and many other sea birds, is not known in dippers, exerts quite different communication challenges for reproducing pairs, and suggests that vocalizations are not closely associated with the constraints imposed by WPD.
6. CONCLUSION
Dippers represent an example of a highly derived and complex behavioral convergence that is not fully associated with the anatomical changes observed in other WPDs, perhaps owing to the relative recency of their divergence from nondiving passeriforms. Penguins, plotopterids and petrels are all part of the water bird clade (Aequornithes sensu Hackett et al., 2008; Jarvis et al., 2014) and thus, share some degree of phylogenetic constraints and range of phenotypic plasticity. Although the placement of Charadriiformes is less well‐supported (sometimes united with “waterbirds” sensu Prum et al., 2015), the fossil record of charadriiforms clearly shows that they did not begin to heavily exploit the marine realm until the Miocene (Smith & Clarke, 2015). Given the terrestrial origins of Charadriiformes and the geologically recent transition to aqueous ecologies of some members of the clade, charadriiforms may provide a better analog for the evolution of dippers from terrestrial passerine ancestors. Perhaps these newly presented data for Cinclidae will facilitate the identification of additional dipper fossils that could further inform lingering questions about the timing and pattern of the evolution of this behaviorally and anatomically intriguing clade of songbirds.
Just as volant WPD birds should not be viewed as taxa on an evolutionary trajectory towards flightlessness (contra Storer, 1960; see discussion by Smith, 2015), dippers should not necessarily be considered less derived than other volant WPD birds. Although dippers do lack many of the anatomical features of nonpasseriform WPD, behaviorally they have evolved the capability to propel themselves with their wings underwater in search of prey, an evolutionary leap that has not been achieved by any other genus of passeriforms. Dippers provide another example of the variety of paths that evolution takes to allow different lineages to inhabit similar niches. In summary, perhaps the decreased degree of convergence observed in dippers is not unexpected given their ecological differences from other WPD. For example, dippers do not dive as deeply as alcids and penguins and do not plunge dive as diving petrels do, so lack of increased pneumaticity is not wholly unexpected if the increased pressure experienced by birds at depth or upon impact with the water is not experienced by dippers. Moreover, penguins acquired much of their derived anatomical features quite early in their evolutionary history and have consistently filled the niche of flightless, pelagic WPD bird for more than 60 million years, whereas pan‐alcids have successfully integrated both aerial and underwater flight for at least 30 million years, even if at least two lineages (Pinguinus and Mancallinae) did evolve to be flightless (Figure 23; Smith, 2011b; Smith & Clarke, 2011). However, WPDs are not embarked on an evolutionary trajectory towards flightlessness (Smith, 2015) and only time will tell if dippers continue to converge on the phenotype that is to varying degrees, shared by other WPD. Older and more complete fossils of dippers are required to answer lingering questions about the timing and pattern of acquisition of traits linked to the evolution of WPD in Cinclidae. This study clearly shows that there is a range of anatomical modifications associated with WPD and active selection for features associated with this derived method of locomotion. Fundamentally, WPD is a rare method of locomotion that is accompanied by similar anatomical changes across several lineages, and that remains an informative system for investigations of convergence.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
N. Adam Smith: Conceptualization (lead); data curation (lead); formal analysis (equal); visualization (lead); writing – original draft (lead); writing – review and editing (lead). Krista L Koeller: Conceptualization (supporting); data curation (supporting); formal analysis (supporting); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Julia A. Clarke: Conceptualization (supporting); data curation (supporting); formal analysis (supporting); funding acquisition (supporting); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting). Dan Ksepka: Conceptualization (supporting); data curation (supporting); formal analysis (supporting); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting). Jonathan S. Mitchell: Conceptualization (supporting); data curation (supporting); formal analysis (equal); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting). Ali Nabavizadeh: Conceptualization (supporting); data curation (supporting); formal analysis (supporting); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting). Ryan C. Ridgely: Conceptualization (supporting); data curation (supporting); formal analysis (supporting); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting). Lawrence M. Witmer: Conceptualization (supporting); data curation (supporting); formal analysis (supporting); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting).
VIDEO LEGEND FOR LINKED VIDEOS
VIDEO 1: An American Dipper (Cinclus mexicanus) using its wings to propel itself underwater in search of prey in a stream in Alaska, USA. Video captured by Bob Armstrong and used with permission.
Supporting information
Appendix S1: Supporting Table
ACKNOWLEDGMENTS
N. Adam Smith was supported by the JC Meeker Postdoctoral Fellowship at the Field Museum and by the Benson Endowment at Clemson University's Campbell Geology Museum. Daniel T. Ksepka was supported by United States National Science Foundation (NSF) grant DEB 1556615. Early work on this project was also supported by NSF DEB 0949897 to Julia A. Clarke. Lawrence M. Witmer and Ryan C. Ridgley were supported by NSF IBN‐0343744, IOB‐0517257, IOS‐1050154, and IOS‐1456503. For access to specimens the authors thank B. Marks at FMNH, H. James and C. Milensky at USNM, and J. Hinshaw at UMMZ. The authors also thank Bob Armstrong for permission to share his video of Cinclus mexicanus, and Amanada Jackson, the Assistant Director for Assistive Technology Services at the University of Florida Disability Resource Center, who provided accessibility advice.
Smith, N. A. , Koeller, K. L. , Clarke, J. A. , Ksepka, D. T. , Mitchell, J. S. , Nabavizadeh, A. , Ridgley, R. C. , & Witmer, L. M. (2022). Convergent evolution in dippers (Aves, Cinclidae): The only wing‐propelled diving songbirds. The Anatomical Record, 305(7), 1563–1591. 10.1002/ar.24820
This article includes AR WOW Video, which can be viewed at Video 1: https://players.brightcove.net/656326989001/default_default/index.html?videoId=6279772503001.
Funding information United States National Science Foundation (NSF), Grant/Award Numbers: IOS‐1456503, IOS‐1050154, IOB‐0517257, IBN‐0343744, DEB 0949897, DEB 1556615
DATA AVAILABILITY STATEMENT
All supporting data (CT images, reconstructions, measurements, and R scripts) have been made freely available via MorphoSource (https://www.morphosource.org/projects/000377999) and as online journal Supporting Information Materials.
REFERENCES
- Balanoff, A. M. , Bever, G. S. , Colbert, M. W. , Clarke, J. A. , Field, D. J. , Gignac, P. M. , Ksepka, D. T. , Ridgely, R. , Smith, N. A. , Torres, C. , Walsh, S. , & Witmer, L. (2016). Best practices for digitally constructing endocranial casts: Examples from birds and their dinosaurian relatives. Journal of Anatomy, 229, 173–190. 10.1111/joa.12378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker, F. K. , Barrowclough, G. F. , & Groth, J. G. (2002). A phylogenetic hypothesis for passerine birds: Taxonomic and biogeographic implications of an analysis of nuclear DNA sequence data. Proceedings of the Royal Society of London. Series B: Biological Sciences, 269, 295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumel, J. J. , & Witmer, L. (1993). Osteologica. In Baumel J. J., King A. S., Breazile J. E., Evans H. E., & Vande Berge J. C. (Eds.), Handbook of avian anatomy: Nomina Anatomica Avium (Vol. 23, pp. 45–132). Nuttall Ornithological Club. [Google Scholar]
- Bellairs, A. A. , & Jenkin, C. R. (1960). The skeleton of birds. In Marshall J. A. (Ed.), Biology and comparative physiology of birds (pp. 241–300). Academic Press. [Google Scholar]
- Bürkner, P. C. (2017). brms: An R package for Bayesian multilevel models using Stan. Journal of Statistical Software, 80, 1–28. [Google Scholar]
- Bürkner, P. C. (2018). Advanced Bayesian multilevel modeling with the R package brms. The R Journal, 10(1), 395–411. [Google Scholar]
- Bürkner, P. C. (2019). thurstonianIRT: Thurstonian IRT models in R. Journal of Open Source Software, 4, 1662. 10.21105/joss.01662 [DOI] [Google Scholar]
- Butler, A. B. , & Hodos, W. (2005). Comparative vertebrate Neuroanatomy: Evolution and adaptation. Wiley. [Google Scholar]
- Chu, P. C. (1998). A phylogeny of the gulls (Aves: Larinae) inferred from osteological and integumentary characters. Cladistics, 14, 1–43. [DOI] [PubMed] [Google Scholar]
- Clarke, J. A. , Ksepka, D. T. , Salas‐Gismondi, R. , Altimirano, A. J. , Shawkey, M. D. , & D'Alba, L. (2010). Fossil evidence for evolution of the shape and color of penguin feathers. Science, 330, 954–957. [DOI] [PubMed] [Google Scholar]
- Clarke, J. A. , Ksepka, D. T. , Stucchi, M. , Urbina, M. , Giannini, N. , Bertelli, S. , Narváez, Y. , & Boyd, C. A. (2007). Paleogene equatorial penguins challenge the proposed relationship between biogeography, diversity, and Cenozoic climate change. Proceedings of the National Academy of Sciences of the United States of America, 104, 11545–11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd, J. R. (2010). Uncinate processes in birds: Morphology, physiology and function. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 156, 303–308. [DOI] [PubMed] [Google Scholar]
- Crisp, E. (1865). On the anatomy and habits of the Water‐ousel (Cinclus aquaticus). In Proceedings of the Zoological Society of London (Vol. 33, pp. 49–52). London: Taylor and Francis. [Google Scholar]
- Croll, D. A. , & McLaren, E. (1993). Diving metabolism and thermoregulation in Common and Thick‐billed Murres. Journal of Comparative Physiology B, 163, 160–166. [DOI] [PubMed] [Google Scholar]
- Davenport, J. , O'Halloran, J. , Hannah, F. , McLaughlin, O. , & Smiddy, P. (2009). Comparison of plumages of White‐throated Dipper Cinclus cinclus and Blackbird Turdus merula . Waterbirds, 32, 169–178. [Google Scholar]
- del Hoyo, J. , Elliott, A. , & Christie, D. (2005). Handbook of the birds of the world. Vol. 10: Cuckoo‐shrikes to Thrushes. Lynx Edicions. [Google Scholar]
- del Hoyo, J. , Elliott, A. , & Christie, D. (2011). Handbook of the birds of the world. Vol 11: Old World Flycathchers to Old World Warblers. Lynx Edicons. [Google Scholar]
- del Hoyo, J. , Elliott, A. , & Sargatal, J. (1992). Handbook of the birds of the world. v1, Ostrich to Ducks. Lynx Edicions. [Google Scholar]
- del Hoyo, J. , Elliott, A. , & Sargatal, J. (1996). Handbook of the birds of the world. Vol. 3: Hoatzin to Auks. Lynx Edicions. [Google Scholar]
- Dunning, J. B. J. (2008). CRC handbook of avian body masses (2nd ed.). CRC Press. [Google Scholar]
- Dyke, G. J. , Wang, X. , & Habib, M. B. (2011). Fossil plotopterid seabirds from the Eo‐Oligocene of the Olympic peninsula (Washington state, USA): Descriptions and functional morphology. PLoS One, 6, e25672. 10.1371/journal.pone.0025672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early, C. M. , Iwaniuk, A. N. , Ridgely, R. C. , & Witmer, L. M. (2020). Endocast structures are reliable proxies for the sizes of corresponding regions of the brain in extant birds. Journal of Anatomy, 237, 1162–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott, K. H. , Ricklefs, R. E. , Gaston, A. J. , Hatch, S. A. , Speakman, J. R. , & Davoren, G. K. (2013). High flight costs, but low dive costs, in auks support the biomechanical hypothesis for flightlessness in penguins. Proceedings of the National Academy of Sciences of the United States of America, 110, 9380–9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felice, R. N. , & O'Connor, P. M. (2014). Ecology and caudal skeletal morphology in birds: The convergent evolution of pygostyle shape in underwater foraging taxa. PLoS One, 9, e89737. 10.1371/journal.pone.0089737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost, P. G. H. , Siegfried, W. R. , & Greenwood, P. J. (1975). Arteriovenous heat‐exchange systems in Jackass Penguin Spheniscus demersus . Journal of Zoology, 175, 231–241. [Google Scholar]
- Gelman, A. , Hill, J. , & Yajima, M. (2012). Why we (usually) don't have to worry about multiple comparisons. Journal of Research on Educational Effectiveness, 5, 189–211. [Google Scholar]
- Gelman, A , & Loken, E . (2013). The garden of forking paths: Why multiple comparisons can be a problem, even when there is no “fishing expedition” or “p‐hacking” and the research hypothesis was posited ahead of time. Columbia University, Department of Statistics. https://forrt.org/curated_resources/the-garden-of-forking-paths-why-multiple/
- George, J. C. , & Burger, A. J. (1966). Avian myology. Academic Press. [Google Scholar]
- Georgi, J. A. , & Sipla, J. S. (2008). Comparative and functional anatomy of balance in aquatic reptiles and birds. In Thewissen J. G. M. & Nummela S. (Eds.), Sensory evolution on the threshold (pp. 233–256). University of California Press. [Google Scholar]
- Ginn, H. B. , & Melville, D. S. (1983). Moult in birds. The British Trust for Ornithology. [Google Scholar]
- Goodge, W. R. (1960). Adaptations for amphibious vision in the dipper (Cinclus mexicanus). Journal of Morphology, 107, 79–91. [DOI] [PubMed] [Google Scholar]
- Grubb, T. C. (1979). Olfactory guidance of Leach's Storm Petrel to the breeding Island. The Wilson Bulletin, 91, 141–143. [Google Scholar]
- Gutzwiller, S. C. , Su, A. , & O'Connor, P. M. (2013). Postcranial pneumaticity and bone structure in two clades of neognath birds. The Anatomical Record, 296, 867–876. [DOI] [PubMed] [Google Scholar]
- Habib, M. B. (2010). The structural mechanics and evolution of aquaflying birds. Biological Journal of the Linnean Society, 99, 687–698. [Google Scholar]
- Habib, M. B. , & Ruff, C. B. (2008). The effects of locomotion on the structural characteristics of avian limb bones. Zoological Journal of the Linnean Society, 153, 601–624. [Google Scholar]
- Hackett, S. J. , Kimball, R. T. , Reddy, S. , Bowie, R. C. K. , Braun, E. L. , Braun, M. J. , Chojnowski, J. L. , Cox, W. A. , Han, K. L. , & Harshman, J. (2008). A phylogenomic study of birds reveals their evolutionary history. Science, 320, 1763–1768. [DOI] [PubMed] [Google Scholar]
- Howard, H. (1969). A new avian fossil from Kern County, California. Condor, 71, 68–69. [Google Scholar]
- Howard, H. (1953). An early bird. Los Angeles County Museum Quarterly, 10, 12–13. [Google Scholar]
- Hudson, G. E. , Hoff, K. M. , Berge, J. V. , & Trivette, E. C. (1969). A numerical study of the wing and leg muscles of Lari and Alcae. Ibis, 111, 459–524. [Google Scholar]
- Iwaniuk, A. N. , Hurd, P. L. , & Wylie, D. R. W. (2006). Comparative morphology of the avian cerebellum: I. degree of foliation. Brain, Behavior and Evolution, 68, 45–62. [DOI] [PubMed] [Google Scholar]
- Iwaniuk, A. N. , & Hurd, P. L. (2005). The evolution of cerebrotypes in birds. Brain, Behavior and Evolution, 65, 215–230. [DOI] [PubMed] [Google Scholar]
- Iwaniuk, A. N. , & Nelson, J. E. (2002). Can endocranial volume be used as an estimate of brain size in birds? Canadian Journal of Zoology, 80, 16–23. [Google Scholar]
- Jadwiszczak, P. (2001). Body size of Eocene Antarctic penguins. Polish Polar Research, 22(2), 147–158. [Google Scholar]
- Jarvis, E. D. , Mirarab, S. , Aberer, A. J. , Li, B. , Houde, P. , Li, C. , Ho, S. Y. W. , Faircloth, B. C. , Nabholz, B. , & Howard, J. T. (2014). Whole‐genome analyses resolve early branches in the tree of life of modern birds. Science, 346, 1320–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetz, W. , Thomas, G. H. , Joy, J. B. , Haartmann, K. , & Mooers, A. O. (2012). The global diversity of birds in space and time. Nature, 491, 444–448. [DOI] [PubMed] [Google Scholar]
- Johnston, D. W. (1988). Morphological atlas of the avian uropygial gland. British Museum. [Google Scholar]
- Kawabe, S. , Ando, T. , & Endo, H. (2013). Enigmatic affinity in the brain morphology between plotopterids and penguins, with a comprehensive comparison among water birds. Zoological Journal of the Linnaean Society, 170, 467–493. [Google Scholar]
- Kessler, E. (2013). Neogene songbirds (Aves, Passeriformes) from Hungary. Hantkeniana, 8, 37–149. [Google Scholar]
- Kessler, E. , & Hír, E. (2012). Eszak‐Magyaroszag madarvilaga a miocenben II. Resz [the avifauna of North Hungary during the Miocene]. Foldtani Kozlony, 142, 149–168. [Google Scholar]
- Klicka, J. , Voelker, G. , & Spellman, G. M. (2005). A molecular phylogenetic analysis of the “true thrushes” (Aves: Turdinae). Molecular Phylogenetics and Evolution, 34, 486–500. [DOI] [PubMed] [Google Scholar]
- Konyukhov, N. B. (1996). Morphoecological and ethological differences in the family Alcidae (Aves). Biology Bulletin, 23, 369–374. [Google Scholar]
- Kooyman, G. L. , & Ponganis, P. J. (1998). The physiological basis of diving to depth: Birds and mammals. Annual Review of Physiology, 60, 19–32. [DOI] [PubMed] [Google Scholar]
- Kozlova, E. V. (1961). Fauna of USSR. BIRDS. Charadriiformes, suborder Alcae. Publications, Agencies and Staff of the U.S. Department of Commerce. [Google Scholar]
- Ksepka, D. T. , & Ando, T. (2011). Penguins past, present, and future: Trends in the evolution of the Sphenisciformes. In Dyke G. & Kaiser G. (Eds.), Living dinosaurs (pp. 155–186). John Wiley & Sons. [Google Scholar]
- Ksepka, D. T. , Balanoff, A. M. , Smith, N. A. , Bever, G. S. , Bhullar, B.‐A. S. , Bourdon, E. , Braun, E. L. , Burleigh, J. G. , Clarke, J. A. , & Colbert, M. W. (2020). Tempo and pattern of avian brain size evolution. Current Biology, 30, 2026–2036. [DOI] [PubMed] [Google Scholar]
- Ksepka, D. T. , Balanoff, A. M. , Walsh, S. , Revan, A. , & Ho, A. (2012). Evolution of the brain and sensory organs in Sphenisciformes: New data from the stem penguin Paraptenodytes antarcticus . Zoological Journal of the Linnean Society, 166, 202–219. [Google Scholar]
- Ksepka, D. T. , & Clarke, J. A. (2010). The basal penguin (Aves: Sphenisciformes) Perudyptes devriesi and a phylogenetic evaluation of the penguin fossil record. Bulletin of the American Museum of Natural History, 337, 1–77. [Google Scholar]
- Ksepka, D. T. , Werning, S. , Sclafani, M. , & Boles, Z. (2015). Bone histology in extant and fossil penguins (Aves: Sphenisciformes). Journal of Anatomy, 227, 611–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulp, F. B. , D'Alba, L. , Shawkey, M. D. , & Clarke, J. A. (2018). Keratin nanofiber distribution and feather microstructure in penguins. The Auk: Ornithological Advances, 135, 777–787. [Google Scholar]
- Lamichhaney, S. , Card, D. S. , Grayson, P. , Tonini, J. F. R. , Bravo, G. A. , Näpflin, K. , Termignoni‐Garcia, F. , Torres, C. , Burbrink, F. , Clarke, J. A. , Sackton, T. B. , & Edwards, S. V. (2019). Integrating natural history collections and comparative genomics to study the genetic architecture of convergent evolution. Philophical Transactions of the Royal Society of London. Series B, Biological Sciences, 374(1777), 20180248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marples, B. J. (1952). Tertiary penguins of New Zealand. New Zealand Geological Survey Paleontological Bulletin, 20, 1–66. [Google Scholar]
- Mayr, G. (2009). Paleogene fossil birds. Springer‐Verlag. [Google Scholar]
- Mayr, G. , de Pietri, V. L. , Love, L. , Mannering, A. A. , Bevitt, J. J. , & Scofield, R. P. (2020). First complete wing of a stem group sphenisciform from the Paleocene of New Zealand sheds light on the evolution of the penguin flipper. Diversity, 12, 46. [Google Scholar]
- Mayr, G. , Goedert, J. L. , & Vogel, O. (2015). Oligocene plotopterid skulls from western North America and their bearing on the phylogenetic affinities of these penguin‐like seabirds. Journal of Vertebrate Paleontology, 35, e943764. [Google Scholar]
- Mayr, G. , Goedert, J. L. , De Pietri, V. L. , & Scofield, R. P. (2021). Comparative osteology of the penguin‐like mid‐Cenozoic Plotopteridae and the earliest true fossil penguins, with comments on the origins of wing‐propelled diving. Journal of Zoological Systematics and Evolutionary Research, 59, 264–276. [Google Scholar]
- McGhee, G. R. (2011). Convergent evolution: Limited forms most beautiful. MIT Press. [Google Scholar]
- McKitrick, M. C. (1991). Forelimb myology of loons (Gaviiformes), with comments on the relationship of loons and tubenoses (Procellariiformes). Zoological Journal of the Linnean Society, 102, 115–152. [Google Scholar]
- Michailidis, D. , Konidaris, G. E. , Athanassiou, A. , Panagopoulou, E. , & Harvati, K. (2018). The ornithological remains from Marathousa 1 (middle Pleistocene; Megalopolis Basin, Greece). Quaternary International, 497, 85–94. [Google Scholar]
- Milsom, W. K. , Johansen, K. , & Millard, R. W. (1973). Blood respiratory properties in some Antarctic birds. The Condor, 75, 472–474. [Google Scholar]
- Moreno‐Rueda, G. , & Rivas, J. M. (2007). Recent changes in allometric relationships among morphological traits in the dipper (Cinclus cinclus). Journal of Ornithology, 148, 489–494. [Google Scholar]
- Murrish, D. E. (1970). Responses to temperature in the dipper, Cinclus mexicanus . Comparative Biochemistry and Physiology, 34, 859–869. [Google Scholar]
- O'Connor, P. M. (2004). Pulmonary pneumaticity in the postcranial skeleton of extant Aves: A case study examining Anseriformes. Journal of Morphology, 261, 141–161. [DOI] [PubMed] [Google Scholar]
- Obst, B. S. , Russell, R. W. , Hunt, G. L. , Eppley, Z. A. J. , & Harrison, N. M. (1995). Foraging and energetics of Least Auklets (Aethia pusilla) breeding on three Bering Sea islands. Physiological Zoology, 68, 647–672. [Google Scholar]
- Oliveros, C. H. , Field, D. J. , Ksepka, D. T. , Barker, F. K. , Aleixo, A. , Andersen, M. J. , Alström, P. , Benz, B. W. , Braun, E. L. , Braun, M. J. , Bravo, G. A. , Brumfield, R. T. , Chesser, R. T. , Claramunt, S. , Cracraft, J. , Cuervo, A. M. , Derryberry, E. M. , Glenn, T. C. , Harvey, M. G. , … Faircloth, B. C. (2019). Earth history and the passerine superradiation. Proceedings of the National Academy of Sciences of the United States of America, 116, 7916–7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, S. L. (1980). A new genus of penguin‐like pelecaniform bird from the Oligocene of Washington (Pelecaniformes: Plotopteridae). Papers in Avian Paleontology Honoring Hildegarde Howard . 51–57. Natural History Museum of Los Angeles County Contributions in Science. [Google Scholar]
- Olson, S. L. (1981). A third species of (Mancalla) from the late Pliocene San Diego formation of California (Aves: Alcidae). Journal of Vertebrate Paleontology, 1, 97–99. [Google Scholar]
- Olson, S. L. (1985a). Early Pliocene Procellariiformes (Aves) from Langebaanweg, South‐Western Cape Province, South Africa. Annals of the South African Museum, 95, 123–145. [Google Scholar]
- Olson, S. L. (1985b). The fossil record of Birds. Avian Biology, 8, 79–238. [Google Scholar]
- Olson, S. L. , & Hasegawa, Y. (1979). Fossil counterparts of giant penguins from the North Pacific. Science, 206, 688–689. [DOI] [PubMed] [Google Scholar]
- Proffitt, J. V. , Clarke, J. A. , & Scofield, R. P. (2016). Novel insights into early neuroanatomical evolution in penguins from the oldest described penguin brain endocast. Journal of Anatomy, 229, 228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prum, R. O. , Berv, J. S. , Dornburg, A. , Field, D. J. , Townsend, J. P. , Lemmon, E. M. , & Lemmon, A. R. (2015). A comprehensive phylogeny of birds (Aves) using targeted next‐generation DNA sequencing. Nature, 526, 569–573. [DOI] [PubMed] [Google Scholar]
- Raikow, R. J. , Bicanovsky, L. , & Bledsoe, A. H. (1988). Forelimb joint mobility and the evolution of wing‐propelled diving in birds. The Auk, 105, 446–451. [Google Scholar]
- Revell, L. J. (2009). Size‐correction and principal components for interspecific comparative studies. Evolution, 63, 3258–3268. [DOI] [PubMed] [Google Scholar]
- Revell, L. J. (2012). Phytools: An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3, 217–223. [Google Scholar]
- Sánchez‐Marco, A. (1999). Implications of the avian fauna for paleoecology in the Early Pleistocene of the Iberian Peninsula. Journal of Human Evolution, 37, 375–388. [DOI] [PubMed] [Google Scholar]
- Schreiweis, D. O. (1982). A comparative study of the appendicular musculature of penguins (Aves, Sphenisciformes). Smithsonian Contributions to Zoology, 341, 1–46. [Google Scholar]
- Simpson, G. G. (1946). Fossil penguins. Bulletin of the American Museum of Natural History, 87, 1–99. [Google Scholar]
- Smith, N. A. (2011a). Systematics and evolution of extinct and extant Pan‐Alcidae (Aves, Charadriiformes): Combined phylogenetic analyses, divergence estimation, and paleoclimatic interactions. University of Texas at Austin. [Google Scholar]
- Smith, N. A. (2011b). Taxonomic revision and phylogenetic analysis of the flightless Mancallinae (Aves, Pan‐Alcidae). ZooKeys, 91, 1–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, N. A. (2013). A new species of auk (Charadriiformes, pan‐Alcidae) from the Miocene of Mexico. The Condor, 115, 77–83. [Google Scholar]
- Smith, N. A. (2014). The fossil record and phylogeny of the auklets (Pan‐Alcidae, Aethiini). Journal of Systematic Palaeontology, 12, 217–236. [Google Scholar]
- Smith, N. A. (2015). Evolution of body mass in the Pan‐Alcidae (Aves, Charadriiformes): The effects of combining neontological and paleontological data. Paleobiology, 42, 8–26. [Google Scholar]
- Smith, N. A. , & Clarke, J. A. (2011). An Alphataxonomic revision of extinct and extant razorbills (Aves, Alcidae): A combined morphometric and phylogenetic approach. Ornithological Monographs, 72, 1–61. [Google Scholar]
- Smith, N. A. , & Clarke, J. A. (2012). Endocranial anatomy of the Charadriiformes: Sensory system variation and the evolution of wing‐propelled diving. PLoS One, 7, e49584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, N. A. , & Clarke, J. A. (2014). Osteological histology of the Pan‐Alcidae (Aves, Charadriiformes): Correlates of wing‐propelled diving and flightlessness. The Anatomical Record, 297, 188–199. [DOI] [PubMed] [Google Scholar]
- Smith, N. A. , & Clarke, J. A. (2015). Systematics and evolution of the Pan‐Alcidae (Aves, Charadriiformes). Journal of Avian Biology, 46, 125–140. [Google Scholar]
- Smith, N. D. (2010). Phylogenetic analysis of Pelecaniformes (Aves) based on osteological data: Implications for waterbird phylogeny and fossil calibration studies. PLoS One, 5, e13354. 10.1371/journal.pone.0013354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, N. D. (2012). Body mass and foraging ecology predict evolutionary patterns of skeletal pneumaticity in the diverse “waterbird” clade. Evolution: International journal of organic. Evolution, 66, 1059–1078. [DOI] [PubMed] [Google Scholar]
- Stan Development Team . (2019). RStan: The R interface to Stan. R package version 2.18.2.
- Stonehouse, B. (1967). The general biology and thermal balances of penguins. Advances in Ecological Research, 4, 131–196. [Google Scholar]
- Storer, R. W. (1960). Evolution in the diving birds. Proceedings of the XII International Ornithological Congress, 2, 694–707. [Google Scholar]
- Terrill, R. S. (2020). Simultaneous wing molt as a catalyst for the evolution of flightlessness in Birds. The American Naturalist, 196, 775–784. 10.5061/dryad.1rn8pk0r3 [DOI] [PubMed] [Google Scholar]
- Thomas, D. B. , & Fordyce, R. E. (2008). The heterothermic loophole exploited by penguins. Australian Journal of Zoology, 55, 317–321. [Google Scholar]
- Trawa, G. (1970). Note preliminaire sur la vascularisation des membres des Spheniscides de terre Adelie. L'Oiseau Rev Francais d'Ornith, 40, 142–156. [Google Scholar]
- Tyrberg, T. (1998). Pleistocene birds of the Palearctic: A catalogue. Nuttall Ornithological Club. [Google Scholar]
- Verheyen, R. (1953). Contribution a l'étude de la structure pneumatique du crâne chez les oiseaux. Institut Royal des Sciences Naturelles de Belgique, Bulletin, 29, 1–24. [Google Scholar]
- Voelker, G. (2002). Molecular phylogenetics and the historical biogeography of dippers (Cinclus). Ibis, 144, 577–584. [Google Scholar]
- Von Den Driesch, A. (1976). A guide to the measurement of animal bones from archaeological sites. Peabody Museum Bulletin, 1, 103–129. [Google Scholar]
- Wang, X. , & Clarke, J. A. (2014). Phylogeny and forelimb disparity in waterbirds. Evolution, 68, 2847–2860. [DOI] [PubMed] [Google Scholar]
- Wang, X. , & Clarke, J. A. (2015). The evolution of avian wing shape and previously unrecognized trends in covert feathering. Proceedings of the Royal Society B: Biological Sciences, 282, 20151935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, J. , Field, D. J. , & Matsuoka, H. (2020). Wing musculature reconstruction in extinct flightless auks ((Pinguinus) and (Mancalla)) reveals incomplete convergence with penguins (Spheniscidae) due to differing ancestral states. Integrative Organismal Biology, 3(1), obaa040. 10.1093/iob/iobaa040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanuki, Y. , & Burger, A. E. (1999). Body mass and dive duration in alcids and penguins. Canadian Journal of Zoology, 77, 1838–1842. [Google Scholar]
- Watanuki, Y. , Wanless, W. , Harris, M. , Lovvorn, J. R. , Miyazaki, M. , Tanaka, H. , & Sato, K. (2006). Swim speeds and stroke patterns in wing‐propelled divers: A comparison among alcids and a penguin. Journal of Experimental Biology, 209, 1217–1230. [DOI] [PubMed] [Google Scholar]
- Wei, T. , & Simko, V. (2017). R package “corrplot”: Visualization of a correlation matrix (Version 0.84). https://github.com/taiyun/corrplot
- Winkler, R. (1979). Zur Pneumatisation des Schädeldachs der Vögel. Ornithologische Beobachter, 76, 449–119. [Google Scholar]
- Witmer, L. M. (1990). The craniofacial air sac system of Mesozoic birds (Aves). Zoological Journal of the Linnean Society, 100, 327–378. [Google Scholar]
- Witmer, L. M. , Chatterjee, S. , Franzosa, J. , & Rowe, T. (2003). Neuroanatomy of flying reptiles and implications for flight, posture and behaviour. Nature, 425, 950–953. [DOI] [PubMed] [Google Scholar]
- Witmer, L. M. , Ridgely, R. C. , Dufeau, D. L. , & Semones, M. C. (2008). Using CT to peer into the past: 3D visulaization of the brain and ears regions of birds, crocodiles, and nonavian dinosaurs. In Endo H. & Frey R. (Eds.), Anatomical imagimng: Towards a new morphology (pp. 67–88). Springer‐Verlag. [Google Scholar]
- Worthy, T. H. , Tennyson, A. J. D. , Jones, C. , McNamara, J. A. , & Douglas, B. J. (2007). Miocene waterfowl and other birds from Central Otago, New Zealand. Journal of Systematic Palaeontology, 5, 1–39. [Google Scholar]
- Zusi, R. L. (1975). An interpretation of skull structure in penguins. In Stonehouse B. (Ed.), The biology of penguins (pp. 59–84). University Park Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Table
Data Availability Statement
All supporting data (CT images, reconstructions, measurements, and R scripts) have been made freely available via MorphoSource (https://www.morphosource.org/projects/000377999) and as online journal Supporting Information Materials.


