Abstract
The incidence of human infection with Campylobacter jejuni is increasing in most developed countries and the reason for this is largely unknown. Although poultry meat is considered to be a major source, it is evident that other reservoirs exist, possibly common to humans and poultry. Environmental sources are believed to be important reservoirs of Campylobacter infection in broiler chicken flocks. We investigated the potential importance of wildlife as a source of infection in commercial poultry flocks and in humans by comparing the serotype distributions, fla types, and macrorestriction profiles (MRPs) of C. jejuni isolates from different sources. The serotype distribution in wildlife was significantly different from the known distributions in broilers and humans. Considerable sero- and genotype diversity was found within the wildlife collection, although two major groups of isolates within serotype O:12 and the O:4 complex were found. Common clonal lines among wildlife, chicken, and/or human isolates were identified within serotype O:2 and the O:4 complex. However, MRPs of O:12 and O:38 strains isolated from wildlife and other sources indicated that some clonal lines propagated in a wide selection of animal species but were not detected in humans or broilers in this study. The applied typing methods successfully identified different clonal groups within a strain collection showing large genomic diversity. However, the relatively low number of wildlife strains with an inferred clonal relationship to human and chicken strains suggests that the importance of wildlife as a reservoir of infection is limited.
Campylobacter jejuni is the major cause of acute bacterial gastroenteritis in Denmark and in many other developed countries (9, 28). Most cases of campylobacteriosis occur sporadically, with the principal route of infection believed to be food (5). However, routes of transmission are rarely established and a wide range of zoonotic and environmental risk factors have been identified. In Denmark, approximately 50% of Danish poultry flocks are infected with C. jejuni (8, 35). Environmental sources are believed to be important reservoirs for Campylobacter infections in broiler chicken flocks. Several epidemiological investigations of human Campylobacter infections from different countries have been reported, and frequently identified risk factors are consumption of undercooked chicken meat, pets in the household, contaminated drinking water, and foreign travel (4, 13; J. Neimann, personal communication). However, the picture of the relative importance of known and unknown sources remains unclear. The incidence of human Campylobacter infections reaches a peak in July-August, at the same time or even before the broiler infections reach a peak (M. Madsen, A. Wedderkopp, J. Engberg, and T. Hald, Abstr. COST ACTION 97 Workshop, abstr. 1, 1999), indicating the possible existence of a common infection reservoir(s) that can affect humans and broiler chickens simultaneously. July and August cover the warmest summer period in Denmark, i.e., the high season for barbecues, open-air dinners, and for garden vegetables and garden and forest berries that may be consumed without being thoroughly cleaned.
The importance of wild birds and mammals as reservoirs and potential sources of Campylobacter infections in poultry production and as direct sources of human infections has not been investigated in detail. However, several studies have shown the occurrence of Campylobacter spp. in wild animals in Scandinavian countries (7, 11, 27). In the present study we have investigated the Penner serotype distribution in wildlife and the presence of common clonal lines of C. jejuni in wildlife, broiler flocks, and humans by using PCR-restriction fragment length polymorphism (PCR-RFLP) analysis of the flaA gene (fla typing) and macrorestriction profiling using pulsed-field gel electrophoresis (MRP-PFGE).
MATERIALS AND METHODS
Isolates.
All C. jejuni isolates used in the present study originated between 1996 and 1998. Isolation was done after primary inoculation onto modified charcoal cefoperazone desoxycholate agar plates (Oxoid CM739, SR 155) and incubation at 42°C under microaerobic conditions for 24 to 48 h. The isolates were identified to the species level on the basis of standardized conventional methods: morphology, mobility, catalase, oxidase, indoxyl acetate hydrolysis, hippurate hydrolysis, and susceptibility to nalidixic acid and cephalothin (19, 20).
Origin of isolates.
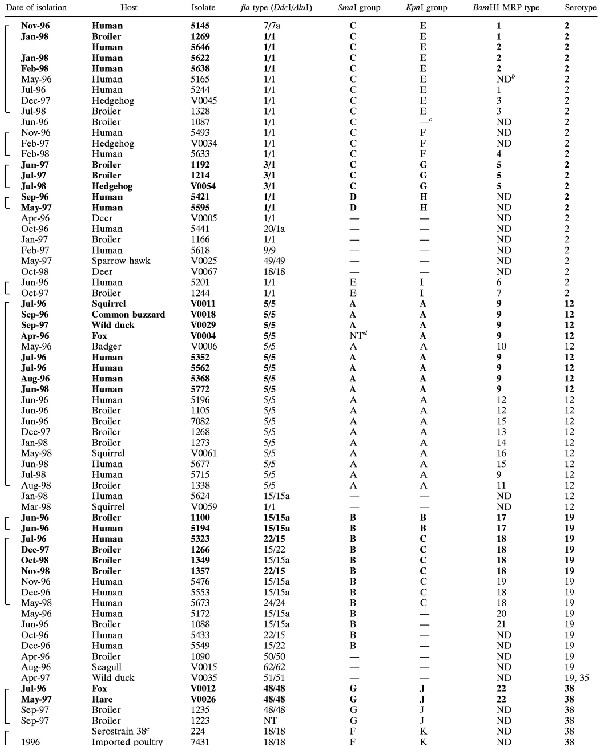
Details of the strains examined are given in Table 1. Wildlife isolates (n = 47) were obtained from feces or from the intestinal tract of wild mammals (n = 32) and birds (n = 15) that were found dead or dying in the wild and submitted to the Danish Veterinary Laboratory for bacteriological analysis. Isolates V0022 and V0023 originated from two hedgehogs that had been kept in the same house prior to death and were submitted to the laboratory on the same day. Isolates V0013 and V0020 originated from two squirrels that were found dead in a garden and were submitted to the laboratory on the same day.
TABLE 1.
Serotypes and genotypes of 120 C. jejuni isolates from wildlife, humans, and broiler flocksa
 |
|---|
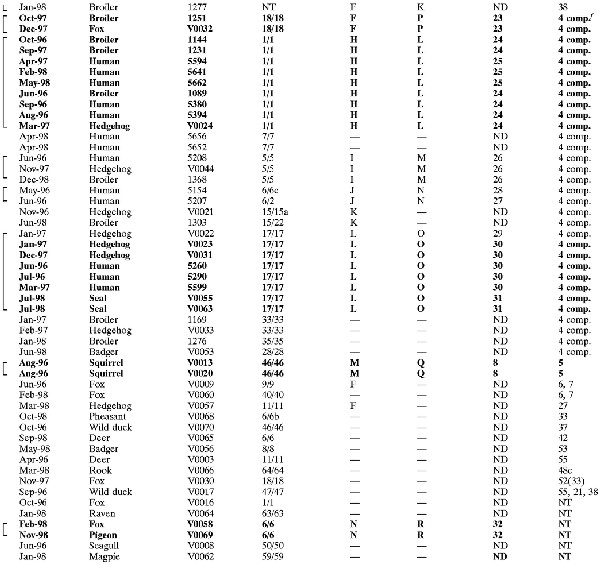 |
Boldface for all fields indicates that isolates are indistinguishable by all typing methods. Use of boldface for some fields indicates that minor differences in one typing result were seen. Brackets indicate that isolates belong to SGs, where SmaI and KpnI MRPs each could be derived from at least one other MRP within that group by one to three band differences.
ND, not done.
—, unique genotype, not belonging to an SG.
NT, nontypeable.
Type strain, serotype O:38.
4 comp., serotype O:4 complex.
Broiler chicken isolates (n = 29) originated from collections of fecal isolates sampled at the time of slaughter from healthy broilers as part of an ongoing surveillance program for campylobacters in broilers in Denmark carried out at the Danish Veterinary Laboratory (7, 8, 17, 35). Human isolates (n = 44) originated from a collection of Campylobacter isolates from stools of clinical cases of diarrhea that were submitted to the Statens Serum Institut for bacteriological examination. Isolates 5595 and 5618 were isolated from patients that had been abroad prior to onset of illness. C. jejuni isolates from broiler flocks and human cases were selected at random to represent the most common serotypes found among the wildlife isolates (O:4, O:12, and O:2). In addition, O:19 was chosen for comparison because of its association with Guillain-Barré syndrome, and O:38 was chosen because preliminary typing results indicated that the wildlife isolates of this serotype constituted a clonal group. The selection was done based on a database of serotyped isolates. It was assured that the three years were all represented in each serotype, if possible, but geography or prior knowledge of genotypes was not taken into account during the selection. None of the serotyped isolates from human patients obtained from 1996 to 1998 was of serotype O:38. Instead, one O:38 isolate from food and one Penner reference strain of O:38 were included for comparison.
Typing methods. (i) Serotyping.
Serotyping was performed according to the Penner serotyping scheme as previously described (17) but with the use of the full set of 66 antisera of the system (47 C. jejuni antisera and 19 C. coli antisera). The antisera used were prepared in house.
(ii) PCR-RFLP profiles.
PCR-RFLP profiles of the flaA gene were produced as previously described using restriction endonucleases DdeI and AluI (25). Computer-assisted identification using GelCompar (Applied Maths, Kortrijk, Belgium) was used for identification of RFLP profiles in a database based on approximately 600 C. jejuni isolates, and profiles were assigned to previously defined profile types (16, 23–26). In cases when a match could not be found, a new profile type was defined.
(iii) PFGE analyses.
PFGE analyses were performed as described by On et al. (21) using the restriction endonucleases SmaI, KpnI, and BamHI (Gibco-BRL, Glasgow, Scotland) with the modification that 1% agarose gels (Pulsed Field Certified Agarose; Bio-Rad, Hercules, Calif.) were used for all gels, and the following ramping parameters were used for BamHI digests: 2 to 5 s, 11 h; 6 to 12 s, 6 h; 15 to 20 s, 5 h.
MRPs were compared visually and assigned to arbitrarily defined MRP types. Repeated gel runs were done to confirm profile identity or similarity. MRPs with each restriction endonuclease were assigned to MRP groups such that all MRPs within a group could be derived from at least one other profile within that group by one to three band differences (33).
Statistical analysis.
The chi-square test was used to determine the differences in serotype distributions between different reservoirs.
RESULTS
Serotypes.
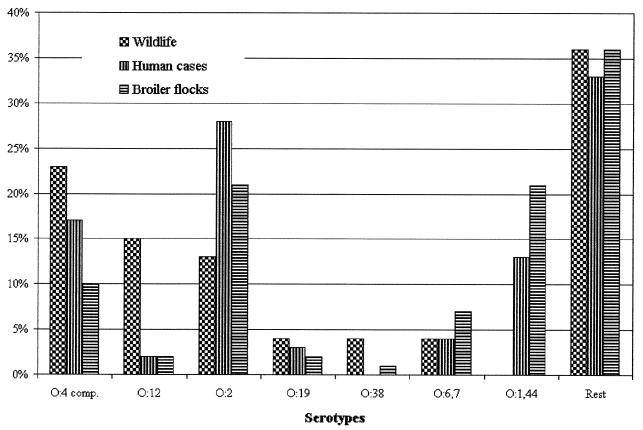
The serotype distribution of isolates from wildlife is presented in Fig. 1. For comparison, the serotype distributions for C. jejuni isolates from humans (n = 661) and broiler chickens (n = 244) in Denmark in the years 1996 to 1998 are also included (3, 7; E. M. Nielsen, unpublished data). The dominant serotypes in wildlife were O:4 complex (11 of 47), O:12 (7 of 47), and O:2 (6 of 47). Serotype O:1,44 was not found in wildlife. There were significantly more O:12 isolates in wildlife than in humans and broilers (P < 0.001 using the chi-square test), and the difference in the occurrence of O:1,44 isolates was also significant (P < 0.001). A number of serotypes were sporadically found in wildlife. Among those serotypes, O:6,7 represented 4 to 7% of the isolates found in humans and broilers, and O:5 and O:37 in humans and O:5 and O:27 in broilers represented 2 to 3% of the C. jejuni isolates in these sources during 1996 to 1998. The remaining serotypes (O:33, O:42, O:53, O:55, O:48, and O:52) were very rare among human and broiler isolates (less than 1% each). The observed serotype distribution in wildlife isolates differed significantly from the known serotype distribution in broiler isolates (P < 0.001) and human isolates (P < 0.001).
FIG. 1.
Serotype distribution of isolates from wildlife, humans, and broilers. Data for humans and broilers are based on 661 and 244 serotyped isolates, respectively, as part of the national surveillance scheme from 1996 to 1998 (16).
Flagellin PCR-RFLP typing and MRP.
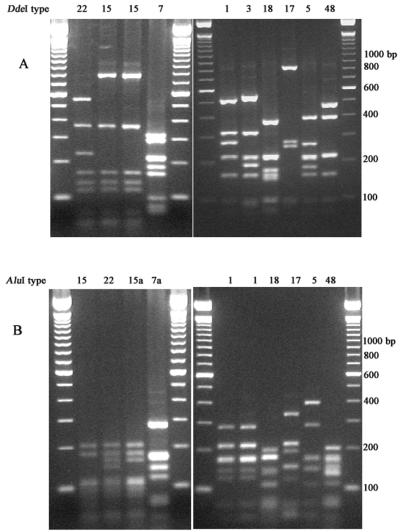
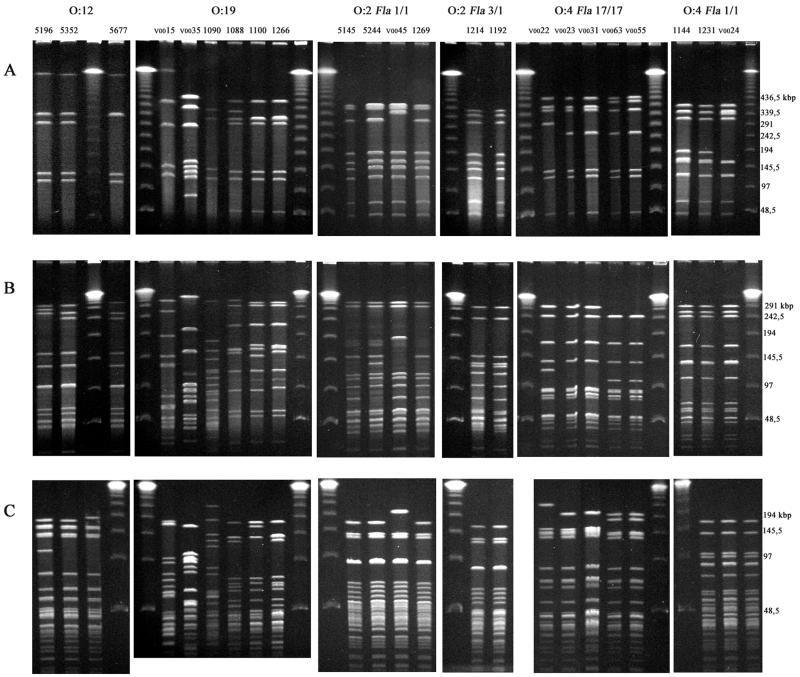
Representative fla profiles are shown in Fig. 2. General features of fla profiles were as previously described (25). A total of 32 different fla types (DdeI profile type and AluI profile type) were identified among the 120 isolates under study (Table 1). Two isolates, both belonging to serotype O:38, did not yield a PCR product by the protocol used and were therefore considered nontypeable by fla typing. fla types 1/1, 11/11, 15/15a, 15/22, 18/18, 46/46, and 5/5 were found in two or more different serotypes. Representative MRPs of isolates from different sources obtained with the three restriction enzymes are shown in Fig. 3. General features of MRPs were as previously described (21, 25). A total of 82 different KpnI MRPs and 59 different SmaI MRPs were identified. Some isolates with identical SmaI profiles could be distinguished by KpnI, and the reverse was also seen. MRPs with each restriction endonuclease were divided into groups so that every MRP within a group could be derived from at least one other MRP within that group by one to three band differences. Several similarity groups (SGs) were found where isolates belonged to the same SmaI group and the same KpnI group (Table 1). This similarity was in every case accompanied by comparable similarity in the BamHI profile. Selected MRPs of SGs within serotypes O:12, O:2, and O:4 complex are shown in Fig. 3.
FIG. 2.
fla profiles using restriction endonucleases DdeI (A) and AluI (B) of isolates 5323, 1266, 1349, 5145, 1231, V0054, 1251, V0022, 1105, and 1235. Molecular weight marker, 100-bp ladder.
FIG. 3.
MRPs using restriction endonucleases SmaI (A), KpnI (B), and BamHI (C) of isolates representing serotypes O:12; O:19; O:2, and O:4. The numbers above the SmaI photographs refer to isolate numbers listed in Table 1. Molecular weight marker, λ ladder.
Comparison of serotyping and molecular methods.
Ninety-five combinations of sero- and genotypes were seen among the 120 examined isolates. Seventeen identical groups (IGs) (isolates that could not be distinguished by any typing method) could be identified, with 5 five of them covering more than one reservoir (Table 1; representative MRPs are shown in Fig. 3). Within serotypes O:2 and O:4 complex, IGs were found that contained wildlife isolates together with human and/or broiler isolates, implicating five wildlife isolates altogether.
SGs (17 in total) covered 81 isolates, including the aforementioned IGs (68% of the total isolate collection) (Table 1). Nineteen wildlife isolates were included in SGs that also implicated human and/or broiler isolates, of which 11 (all isolated from mammals) belonged to those serotypes that are dominant in humans and broilers (O:2 and the O:4 complex). Plotting of the presumed origin of isolates and visual analysis revealed no spatial clustering that could be related to IGs or SGs (data not shown). Likewise, groups were widespread over time (Table 1).
DISCUSSION
The difference in serotype distribution of wildlife isolates and human or broiler isolates was of statistical significance, despite the limited number of wildlife isolates. This suggests that the importance of wildlife as a campylobacter source for humans or broilers is limited. On the contrary, human and broiler isolates show larger serotype overlap.
To investigate this issue in depth, we compared the genotypes of wildlife isolates with those of human and broiler isolates representing serotypes that are epidemiologically important in regard to humans and broilers (O:2, O:4 complex, and O:19) or that constitute clonal groups in the wildlife collection (O:12 and O:38). In spite of the large type diversity among isolates in this study, a significant percentage of the isolates (36%) were part of IGs that could not be distinguished by any of the applied typing methods (Table 1). In particular, the use of three different restriction endonucleases that cover multiple sites throughout the bacterial genome has been used to define clonal identity where all strain patterns match (21) and may indicate epidemiological relationship. Some of the clonal lines identified in this study were widespread over time as well as in geography, which is congruent with the premise that some clones are genetically stable (14) and thus establishes the validity of using high-resolution genotyping for investigating complex epidemiologies.
The SGs, formed on the basis of similarity in three sets of MRPs, covered two-thirds of isolates. This way of grouping isolates takes into account the inadequacy of inferring strain relationships using MRPs with a limited number of fragments (<10) like the SmaI profiles (4, 21), and it is consistent with generally accepted criteria for the evaluation of strain relatedness by PFGE typing (31, 33). We presume that similarity in all three sets of MRPs and identical serotype and fla type is concordant with strains being clonally related, as suggested previously (16, 21, 31), and that such strains may share common characteristics, for instance, with regard to survival or colonization of different hosts.
In general, we found that SGs in most cases overlap with fla types, supporting the argument that fla types can be conserved within clonal lines (30). However, this was not the case among the O:19 isolates under study, where fla profiles within the SG showed minor variation (Fig. 2, fla profiles 22, 15, and 15a), possibly consistent with hypervariable regions in the fla genes of these isolates (22).
The identification of clonally related isolates from wild animals, broiler flocks, and/or humans suggests that exchanges of C. jejuni strains between reservoirs do take place. It is, however, not possible from the epidemiological data presented here to establish the general direction of a given infection link. A bidirectional flow of C. jejuni isolates between reservoirs, as well as a ubiquitous occurrence of the identified clones, could explain this finding.
The majority of wildlife isolates (36 of 47) do not seem to share any relationship with those human and broiler isolates from the two important serotypes, O:2 and O:4 complex, that were included in this study. In particular, the identification of the O:12 group, which occurs with an increased frequency in wildlife compared to the other sources, is unexplained. Interestingly, in a New Zealand study O:12 strains were the most common serotype among surface water samples, and the SmaI MRPs of O:12 isolates were similar to the fla type 5/5 group (10). Farm management and behavior of wild animals, as well as the inherent fastidiousness of campylobacters and the differences in the virulence properties and colonization potential among C. jejuni strains, may be important factors in this context.
It is common practice in Denmark that used litter from broiler houses is stored at a farm until it is used as fertilizer, thereby enabling a potential transfer of Campylobacter spp. from broiler flocks to animals that inhabit the area close to farmlands and potential further spread to other wild animals. A recent Danish study suggested that stored litter acts as a continuous source of campylobacter for the broiler flocks raised on farms (26).
Other transmission routes between humans and the external environment could be via hedgehogs that have been kept (hospitalized) in private homes or fed in the garden, which was the case with a significant proportion of the hedgehogs included in this study. Indeed, two clones belonging to the O:4 complex have been isolated from humans and hedgehogs. A Norwegian study found that hedgehog-human contact could explain an outbreak of Salmonella enterica serovar Enteritidis (32).
Previous Scandinavian studies have shown that C. jejuni is widespread in nature, but the carriage rates in wild birds (7, 12) and in mammals (7) have been found to be less than 30 and 20%, respectively. By contrast, in commercial poultry flocks 50 to 100% of animals in a flock have been found to be infected (1, 34), and in a dog breeding colony 40 to 86% of animals have been found to be infected (15). Recent studies have shown that domestic animals (pigs, cattle) housed in production facilities with a high individual-to-area ratio may carry Campylobacter spp. for extended periods (18, 36). However, wild animals in general are more dispersed in the landscape, meaning that, for instance, coprophagy occurs infrequently and the animals generally eat more varied feed. Coprophagic behavior in penguins was used to explain the finding of C. jejuni isolates bearing a close resemblance to human strains, suggesting their recent introduction to sub-Antarctica by human visitors or migrating birds (2). Feed composition and coprophagy may influence the composition of the intestinal flora (29) and may also have importance for colonization by Campylobacter spp.
Additional investigations of the C. jejuni prevalence and type distribution in wildlife would further elucidate the importance of the infection pressure from wildlife to broilers and man. In addition, typing studies involving a larger number of isolates from different wild animals known to have close contact to poultry farms as well as isolates from broilers are needed to establish the direction and the significance of the C. jejuni infection route.
ACKNOWLEDGMENTS
This study was partly supported by grants from the Danish Broiler Meat Association and from the Danish Ministry of Food, Agriculture and Fisheries.
We thank Lis Nielsen and Connie Jenning Sørensen for excellent technical assistance with the genotyping profiles.
REFERENCES
- 1.Achen M, Morishita T Y, Ley E C. Shedding and colonization of Campylobacter jejuni in broilers from day-of-hatch to slaughter age. Avian Dis. 1998;42:732–737. [PubMed] [Google Scholar]
- 2.Broman T, Bergström S, On S L W, Palmgren H, McCafferty D J, Sellin M, Olsen B. Isolation and characterization of Campylobacter jejuni subsp. jejuni from macaroni penguins (Eudyptes chrysolophus) in the subantarctic region. Appl Environ Microbiol. 2000;66:449–452. doi: 10.1128/aem.66.1.449-452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brondsted T, Hald T, Jorgensen B B, editors. Annual report on zoonoses in Denmark 1999. Copenhagen, Denmark: Danish Zoonosis Centre; 2000. [Google Scholar]
- 4.Eberhart-Phillips J, Walker N, Garrett N, Bell D, Sinclair D, Rainger W, Bates M. Campylobacteriosis in New Zealand: results of a case-control study. J Epidemiol Community Health. 1997;51:686–691. doi: 10.1136/jech.51.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman C R, Neimann J, Wegener H C, Tauxe R V. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations. In: Nachamkin I, Blaser M J, editors. Campylobacter. 2nd ed. Washington, D.C.: ASM Press; 2000. pp. 121–138. [Google Scholar]
- 6.Gibson J, Lorenz E, Owen R J. Lineages within Campylobacter jejuni defined by numerical analysis of pulsed-field gel electrophoretic DNA profiles. J Med Microbiol. 1997;46:157–163. doi: 10.1099/00222615-46-2-157. [DOI] [PubMed] [Google Scholar]
- 7.Hald T, Wegener H C, Larsen S K, Flensburg J, editors. Annual report of zoonoses in Denmark 1996. Copenhagen, Denmark: Danish Zoonosis Centre; 1997. [Google Scholar]
- 8.Hald T, Wegener H C, Larsen S K, Flensburg J, editors. Annual Report on Zoonoses in Denmark 1997. Copenhagen, Denmark: Danish Zoonosis Centre; 1998. [Google Scholar]
- 9.Hald T, Wegener H C, Jorgensen B B, editors. Annual report on zoonoses in Denmark 1998. Copenhagen, Denmark: Danish Zoonosis Centre; 1999. [Google Scholar]
- 10.Hudson J A, Nicol C, Wright J, Whyte R, Hasell S K. Seasonal variation of Campylobacter types from human cases, veterinary cases, raw chicken, milk and water. J Appl Microbiol. 1999;87:115–124. doi: 10.1046/j.1365-2672.1999.00806.x. [DOI] [PubMed] [Google Scholar]
- 11.Kakoyiannis C K, Winter P J, Marshall R B. The relationship between intestinal Campylobacter species isolated from animals and humans as determined by BRENDA. Epidemiol Infect. 1998;100:379–387. doi: 10.1017/s0950268800067133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapperud G, Rosef O. Avian wildlife reservoir of Campylobacter fetus subsp. jejuni, Yersinia spp., and Salmonella spp. in Norway. Appl Environ Microbiol. 1983;45:375–380. doi: 10.1128/aem.45.2.375-380.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapperud G, Skjerve E, Bean N H, Ostroff S M, Lassen J. Risk factors for sporadic Campylobacter infections: results of a case-control study in southeastern Norway. J Clin Microbiol. 1992;30:3117–3121. doi: 10.1128/jcm.30.12.3117-3121.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manning G, Duim B, Wassenaar T M, Wagenaar J, Ridley A, Newell D G. Evidence for a genetically stable clone of Campylobacter jejuni. Appl Environ Microbiol. 2001;67:1185–1189. doi: 10.1128/AEM.67.3.1185-1189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newton C M, Newell D G, Wood M, Baskerville M. Campylobacter infection in a closed dog breeding colony. Vet Rec. 1988;123:152–154. doi: 10.1136/vr.123.6.152. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen E M, Engberg J, Fussing V, Petersen L, Brogren C-H, On S L W. Evaluation of phenotypic and genotypic methods for subtyping of Campylobacter jejuni isolates from humans, poultry, and cattle. J Clin Microbiol. 2000;38:3800–3810. doi: 10.1128/jcm.38.10.3800-3810.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen E M, Engberg J, Madsen M. Distribution of serotypes of Campylobacter jejuni and C. coli from Danish patients, poultry, cattle and swine. FEMS Immunol Med Microbiol. 1997;19:47–56. doi: 10.1111/j.1574-695X.1997.tb01071.x. [DOI] [PubMed] [Google Scholar]
- 18.On S L W, Atabay H I, Corry J E L. Clonality of Campylobacter sputorum bv. Paraureolyticus determined by macrorestriction profiling and biotyping, and evidence for long-term persistent infection in cattle. Epidemiol Infect. 1999;122:175–182. doi: 10.1017/s0950268898001824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.On S L W, Holmes B. Effect of inoculum size on the phenotypic characterization of Campylobacter species. J Clin Microbiol. 1991;29:923–926. doi: 10.1128/jcm.29.5.923-926.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.On S L W, Holmes B. Assessment of enzyme detection tests useful in identification of campylobacteria. J Clin Microbiol. 1992;30:746–749. doi: 10.1128/jcm.30.3.746-749.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.On S L W, Nielsen E M, Engberg J, Madsen M. Validity of SmaI-defined genotypes of Campylobacter jejuni examined by SalI, KpnI, and BamHI polymorphisms: evidence of identical clones infecting humans, poultry, and cattle. Epidemiol Infect. 1998;120:231–237. doi: 10.1017/s0950268898008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parkhill J, Wren B W, Mungall K, Ketley J M, Churcher C, Basham D, Chillingworth T, Davies R M, Feltwell T, Holroyd S, Jagels K, Karlyshev A V, Moule S, Pallen M J, Penn C W, Quail M A, Rajandream M-A, Rutherford K M, Vliet A H M, Whitehead S, Barrell B G. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 23.Petersen, L., and D. G. Newell. The ability of Fla-typing schemes to discriminate between strains of Campylobacter jejuni. J. Appl. Microbiol., in press. [DOI] [PubMed]
- 24.Petersen, L., E. M. Nielsen, and S. L. W. On. Serotype and genotype diversity and hatchery transmission of Campylobacter jejuni in commercial broiler flocks. Vet. Microbiol., in press. [DOI] [PubMed]
- 25.Petersen L, On S L W. Efficacy of flagellin gene typing for epidemiological studies of Campylobacter jejuni in poultry estimated by comparison with macrorestriction profiling. Lett Appl Microbiol. 2000;31:14–19. doi: 10.1046/j.1472-765x.2000.00757.x. [DOI] [PubMed] [Google Scholar]
- 26.Petersen L, Wedderkopp A. Evidence that certain clones of Campylobacter jejuni persist during successive broiler flock rotations. Appl Environ Microbiol. 2001;67:2739–2745. doi: 10.1128/AEM.67.6.2739-2745.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosef O, Kapperud G, Lauwers S, Gondrosen B. Serotyping of Campylobacter jejuni, Campylobacter coli, and Campylobacter laridis from domestic and wild animals. Appl Environ Microbiol. 1985;49:1507–1510. doi: 10.1128/aem.49.6.1507-1510.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skirrow M B. Diseases due to Campylobacter, Helicobacter and related bacteria. J Comp Pathol. 1994;111:113–149. doi: 10.1016/s0021-9975(05)80046-5. [DOI] [PubMed] [Google Scholar]
- 29.Smith H W. Observations on the flora of the alimentary tract of animals and factors affecting its composition. J Pathol Bacteriol. 1965;89:95–122. [PubMed] [Google Scholar]
- 30.Stanley J, Linton D, Sutherland K, Jones C, Owen R J. High-resolution genotyping of Campylobacter coli identifies clones of epidemiologic and evolutionary significance. J Infect Dis. 1995;172:1130–1134. doi: 10.1093/infdis/172.4.1130. [DOI] [PubMed] [Google Scholar]
- 31.Struelens M J. Consensus guidelines for appropriate use and evaluation of microbial epidemiologic typing systems. Clin Microbiol Infect. 1996;2:2–11. doi: 10.1111/j.1469-0691.1996.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 32.Søbstad Ø, Blinkenberg J, Bergesen E, Digranes A, Tveit I, Heir E, Kapperud G, Stavnes T-L, Hasseltvedt V, Iversen B G. Transmission of salmonellosis through hedgehogs in Norway. Eurosurveillance Wkly. 2000;38:1–2. [Google Scholar]
- 33.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas L M, Long K A, Good R T, Panaccio M, Widders P R. Genotypic diversity among Campylobacter jejuni isolates in a commercial broiler flock. Appl Environ Microbiol. 1997;63:1874–1877. doi: 10.1128/aem.63.5.1874-1877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wedderkopp A, Rattenborg E, Madsen M. National surveillance of Campylobacter in broilers at slaughter in Denmark in 1998. Avian Dis. 2000;44:993–999. [PubMed] [Google Scholar]
- 36.Weijtens M J B M, van der Plas J, Bijker P G H, Urlings H A P, Koster D, van Logtestijn J G, Huis in't Veld J H J. The transmission of Campylobacter in piggeries; an epidemiological study. J Appl Microbiol. 1997;83:693–698. doi: 10.1046/j.1365-2672.1997.00301.x. [DOI] [PubMed] [Google Scholar]