Abstract
Aim
To analyse the efficacy of non‐surgical therapy (NST) in terms of pocket closure (PC) and changes in percentage and number of pockets.
Materials and Methods
Three databases (PubMed, EMBASE, and Scopus) were searched up to January 2020. Prospective studies with a minimum follow‐up of 12 months and presenting data in terms of PC or number or percentage of pocket depths (PDs) before and after NST on systemically healthy patients were included. Random‐effect meta‐analyses were performed.
Results
After screening 4610 titles and abstracts, 27 studies were included. Of these, 63.9% of PC was reported by one study. The percentage of PDs ≤3 mm changed from 39.06% to 64.11% with a weighted mean difference (WMD) of 26.14% (p < .001). This accounted for a relative increase of healthy sites of 64.13%. The mean percentage of PD ≥5 mm was 28.23% and 11.71% before and after treatment, respectively, with a WMD of 15.50% (p < .001). The WMD in the number of PDs ≥5 mm before and after treatment was 24.42 (p = .036). The mean number of residual PPD ≥5 after NST was 14.13.
Conclusions
NST is able to eradicate the majority of the pockets. However, residual pockets after NST may remain and should be considered cautiously for further treatment planning.
Keywords: non‐surgical periodontal treatment, pocket closure, residual pockets, scaling and root planing
Clinical Relevance.
Scientific rationale for study: This systematic review aimed to analyse the efficacy of NST in terms of pocket closure and changes in the percentage and number of pockets.
Principal findings: NST is effective in eradicating between one‐half and two‐thirds of existing pocket depths (PDs). Residual PDs may often remain and may require further attention.
Practical implications: Efficacy of NST should be evaluated in terms of residual PDs.
1. INTRODUCTION
The primary aims of periodontal treatment are to prevent tooth loss and arrest the progression of periodontitis. Since these outcomes usually require long‐term studies and multiple periodontal examinations with time, in clinical trials and daily clinical practice surrogate outcomes such as clinical attachment level (CAL), pocket depth (PD), or bleeding on probing (BoP) are often used to predict the risk of tooth loss and disease progression (Tomasi & Wennström, 2017). In particular, residual PD ≥5 mm, especially when associated with persisting BoP, was claimed as a site‐specific, positive predictive factor for further clinical attachment loss during supportive periodontal therapy (SPT) (Claffey et al., 1990; Claffey & Egelberg, 1995; Matuliene et al., 2008; Chapple et al., 2018).
Non‐surgical periodontal therapy (NST) has been suggested as the ideal initial treatment for patients suffering from periodontitis (Lindhe et al., 1982). NST consists of subgingival debridement, subgingival scaling, and root planing. Theoretically, these are well‐differentiated procedures. However, they are performed clinically at the same time, being named “scaling and root planning” (SRP), which is considered the cornerstone of cause‐related therapy (Laleman et al., 2017; Graziani et al., 2018). A consistent amount of evidence has indicated that SRP is effective in controlling inflammation, reducing PD, and improving CAL (Van der Weijden & Timmerman, 2002; Sanz‐Sanchez et al., 2012; Smiley et al., 2015). However, SRP is technically demanding, and complete calculus removal is difficult to achieve (Rabbani et al., 1981; Buchanan & Robertson, 1987; Brayer et al., 1989; Rateitschak‐Pluss et al., 1992; Zafar et al., 2021). NST is less effective especially at mobile teeth and/or in deep sites and at posterior teeth (particularly in molars with furcation involvements) (D'Aiuto et al., 2005; Rateitschak‐Pluss et al., 1992; Serino et al., 2001; Tomasi et al., 2007; Jiao et al., 2017). It is therefore common that residual PDs remain after NST (Sanz‐Sanchez et al., 2020).
Residual PDs have been traditionally considered as signs of incomplete periodontal treatment, as they have been associated with an increased risk of disease progression at the patient and site level during SPT (Claffey et al., 1990; Claffey & Egelberg, 1995; Matuliene et al., 2008). Residual PDs must be recorded at the end of NST and through SPT in order to tailor the treatment plan on specific patient‐centered needs. Further treatment may include periodontal surgeries, aiming at PD reduction, or personalized SPT, as shorter recall intervals have been associated with increased periodontal stability along time even in the presence of PD ≥5 mm (Ramseier et al., 2019). However, the efficacy of NST has usually been reported in terms of mean values of PD reduction, residual PD, and CAL gain, which are not indicators of treatment success or periodontal stability. Conversely, pocket closure (PC), intended as residual PD ≤4 mm or PD ≤3 mm at site level or the number and percentage of residual pockets after NST at patient level, has seldom been reported. Thus, the aim of this systematic review was to analyse the efficacy of NST in terms of PC and changes in percentage and number of pockets.
2. MATERIALS AND METHODS
2.1. Protocol development and focused question
The protocol of this systematic review was made apriori, agreed upon by all authors and registered in the PROSPERO International Prospective Register of Systematic Reviews hosted by the Centre for Reviews and Dissemination, University of York, National Institute for Health Research (United Kingdom; CRD42020149759). This systematic review was reported according to the PRISMA (Preferred Reporting Items for Systematic Review and Meta‐Analyses) statement (Moher et al., 2009) and answered the following focused question: “What is the efficacy of non‐surgical periodontal treatment in terms of PC of the patients affected by periodontitis?”
2.2. Screening methods
2.2.1. Eligibility criteria
In order to select pertinent publications, the research question was designed according to the following PICOST question:
Population: Otherwise healthy patients affected by periodontitis
Intervention: NST
Comparison: No need for comparison
- Outcomes:
- Percentage or number of closed pockets (PD ≤3 mm or PD ≤4 mm) out of the total number of pockets at baseline (PD > 3 mm or PD > 4 mm)
- Changes in the percentage of pockets at different thresholds before and after NST
- Mean number of residual pockets after NST at different thresholds
- Changes in gingival or bleeding indices
- Changes in plaque indices
- Percentage of cases with the need of re‐treatment
- Complications and patient‐related outcomes (PROMs) study design: Prospective study design
Type of study: prospective studies
Timing: ≥1‐year follow‐up after treatment.
Eligible studies were included if they met the following criteria:
Abstract present
English language
Prospective study design
Related to periodontal NST
Otherwise healthy patients affected by periodontitis
Patient‐level data about the number or percentage of pockets (%PD) at baseline and at 1‐year follow‐up after the treatment available.
Studies were excluded if they met the following exclusion criteria:
Adjunct(s) used in all the groups included in the study
Follow‐up period shorter than 1 year after NST
Less than 10 patients.
Two independent reviewers (Filippo Citterio and Moontaek Chang) initially screened the articles based on the titles and abstracts. In order to avoid the exclusion of possibly relevant papers, the articles selected by one of the two reviewers were considered for full text reading. Inter‐rater agreement between reviewers was assessed by mean of Cohen's kappa. Then, the reviewers proceeded to full text reading and excluded the articles that did not fulfil the inclusion criteria. Disagreement regarding inclusion or exclusion of the retrieved papers was resolved by discussion and if necessary by a third examiner (Gian Marco Piccoli). Again, inter‐rater agreement was assessed with the kappa coefficient.
2.3. Types of intervention
All types of NST were considered irrespective of the type of instrumentation or the modality of treatment.
2.4. Information source and search
Electronic search
Electronic search was performed on MEDLINE via PUBMED, on EMBASE via Ovid, and on SCOPUS with the strings reported in Figure S1 using a combination of MeSH terms and free text words (Limits: Humans; English; up to January 2020).
Manual search
All reference lists of the selected studies and previously published systematic reviews were checked for cross‐references. The following journals were hand‐searched: Journal of Clinical Periodontology, Journal of Periodontology, The International Journal of Periodontics and Restorative Dentistry and Journal of Periodontal Research.
2.5. Data extraction
Two examiners (Filippo Citterio and Giacomo Gualini) read the selected papers and collected relevant data by filling a specific table in duplicate. Any disagreement was discussed with a third examiner (Moontaek Chang).
If a study was comparing more than two arms that fulfilled the inclusion criteria, the data from the groups of interest were extracted.
2.6. Risk‐of‐bias assessment
Quality analysis of included randomized clinical trial (RCT) was performed according to the Cochrane Collaboration risk‐of‐bias tool for randomized trials (Rob2) (Sterne et al., 2019) and the CONSORT statement (Moher et al., 2001) (Table S1). Risk of bias in non‐controlled before and after longitudinal studies and non‐randomized controlled trials was assessed using modifications of ROBINS‐I tool as proposed by Cochrane Review (Tables S2 and S3).
2.7. Meta‐analysis
Random‐effect meta‐analyses were performed to pool data according to the different outcomes reported at 12 months. Weighted means (WM), weighted mean differences (WMD), 95% confidence intervals (CI), and prediction intervals were provided to compare the percentage and number of PDs, and full mouth bleeding score (FMBS) and full mouth plaque score (FMPS) before and after NST. Heterogeneity was expressed by I 2 (Higgins & Thompson, 2002). OpenMeta [Analyst] intercooled software was used to perform all analyses. Statistical significance was defined as a p < .05.
3. RESULTS
3.1. Search results
The electronic and hand searches provided 5090 papers. After removal of duplicates, 4610 papers were retrieved. Title and abstract screening led to the rejection of 4437 papers. Agreement was deemed good (kappa = 0.714; SE of kappa = 0.024; CI 95% 0.667–0.761; number of observed agreements: 4482 or 97.22% of the observations). One‐hundred and seventy‐three papers were selected for full text reading. For data analysis, 27 papers including 29 independent groups that underwent NST were finally selected after full text reading. Agreement was deemed very good (kappa = 0.885; SE of kappa = 0.046; CI 95% 0.795–0.975; number of observed agreements: 169 or 96.57% of the observations). Quantitative assessment by meta‐analysis was performed on a total of 22 study groups from 20 independent studies. Seven studies were excluded from quantitative assessment since they presented exclusively data at the site level (Badersten et al., 1984; Becker et al., 1988; Sato et al., 1993; Timmerman et al., 1996; Ramberg et al., 2001; Serino et al., 2001; Yen et al., 2008; Wong et al., 2012) (Figure 1).
FIGURE 1.
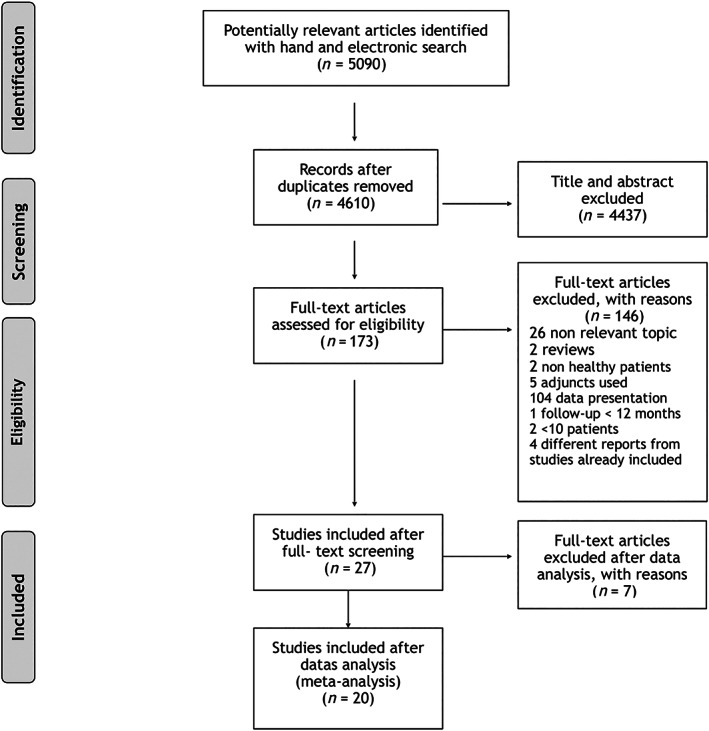
Flow‐chart of selection and reasons for exclusion
Meta‐analysis was performed according to specific outcome reported in the studies pooling papers with same outcomes such as number or percentage of pockets at baseline and follow‐up for different PD categories.
3.2. Study characteristics
Studies characteristics are reported in Table S4. Studies varied significantly in study design, NST protocol, use of mouthwashes, inclusion criteria for patients, and handling of possible modifying factors such as systemic diseases and habits. The reported PD categories varied among studies. According to %PDs, 10 studies reported PD ≤3 mm and PD = 4–6 mm (Badersten et al., 1984; Lindhe & Nyman, 1985; Becker et al., 1988; Sato et al., 1993; Ramberg et al., 2001; Rosling et al., 2001; Serino et al., 2001; Yen et al., 2008; Shiloah et al., 2014; Giannelli et al., 2015), 2 studies PD ≤4 mm (Timmerman et al., 1996; Sampaio et al., 2011), 1 study PD ≥4.5 mm (Sanz‐Sanchez et al., 2015), 2 studies PD ≥4 mm (Westfeld et al., 1998; Rosa et al., 2011), 6 studies PD ≥5 mm (Wan et al., 2009; Feres et al., 2012; Mestnik et al., 2012; Goncalves et al., 2015; Tekce et al., 2015; Morales et al., 2016), 3 studies PD ≥6 mm (Wong et al., 2012; Tekce et al., 2015; Morales et al., 2016), and 11 studies PD ≥7 mm (Badersten et al., 1984; Lindhe & Nyman, 1985; Becker et al., 1988; Ramberg et al., 2001; Rosling et al., 2001; Serino et al., 2001; Shiloah et al., 2014; Giannelli et al., 2015; Tekce et al., 2015; Morales et al., 2016; Giannelli et al., 2018). Other studies reported sparsely for other PD categories such as PD = 5–6 mm or PD = 4–5 mm (see Table S5). Five studies reported the number of pockets for PD ≥4 mm, PD ≥4.5 mm, PD ≥5 mm, PD ≥6 mm, and PD ≥7 mm (Sanz‐Sanchez et al., 2015; Baelum & Lopez, 2016; Morales et al., 2016; Borges et al., 2017; Cosgarea et al., 2017).
3.3. Risk of bias
Risk of bias around RCT ranged from “Some concerns” to “High risk”. No study was deemed to have “Low risk” of bias. Results from the Rob.2 tool are reported in Figure 2a. Non‐randomized controlled studies and uncontrolled before and after studies were deemed all at moderate risk due to missing information regarding pre‐intervention trends and patterns that may have acted as confounders for the outcome of interest (Figure 2b,c). However, though it was impossible to consider those studies at “Low risk”, the likelihood that the presence of some true confounders before the start of the therapy may have significantly hampered the results is limited.
FIGURE 2.
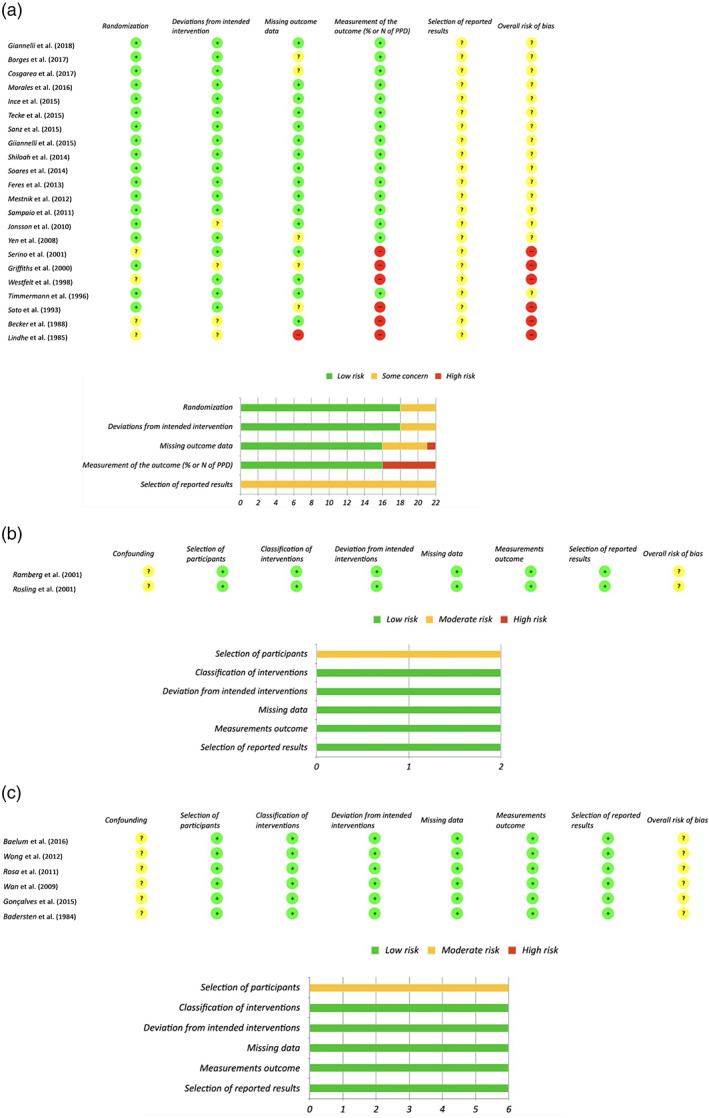
(a) Risk of bias in randomized controlled clinical trials. (b) Risk of bias in uncontrolled trials. (c) Risk of bias in before and after studies [Colour figure can be viewed at wileyonlinelibrary.com]
3.4. Outcomes
3.4.1. Percentage or number of closed pockets (PD ≤3 mm or PD ≤ 4 mm) out of the total number of pockets at baseline (PD > 3 mm or PD > 4 mm)
No study reported the primary outcome of PC. Data for PC were provided by the authors of one paper (Cosgarea et al., 2017). In that cohort, 26 patients with a baseline mean number of PD ≥4 mm of 61.48 ± 22.13 had PC on a mean of 39.00 ± 17.30 sites at 1‐year follow‐up. This accounted for 63.9% of PC.
3.4.2. Changes in the percentage of pockets before and after NST (PD ≤ 3 mm, PD ≤ 4 mm, PD ≥ 4 mm, PD ≥5 mm)
WM percentage of PDs before and after NST, 95% confidence intervals and prediction intervals, WMDs between the two evaluations, and relative changes after NST, intended as proportions of pocket reduction or increase after NST, are collected in Table 1.
TABLE 1.
Changes in the percentages of pockets before and after non‐surgical therapy and relative changes after NST, intended as proportions of pocket reduction or increase after NST
| Pocket depth category | Included studies (groups) | Before NST | One year after NST | Changes in the percentage of pockets | |||||
|---|---|---|---|---|---|---|---|---|---|
| WM (%) | 95% CI | WM (%) | 95% CI | WMD (%) | 95% CI | 95% prediction interval | |||
| Relative increase (%) a | |||||||||
| PD ≤3 mm | 4 | 39.06 | 17.61/60.52 | 64.11 | 37.81/90.41 | — 26.14* | −39.87/−12.40 | −90.72/38.43 | 64.13 |
| PD ≤4 mm | 1 | 44.20 | 31.02/57.38 | 87.47 | 76.68/100.26 | — | — | — | 73.19 |
| Relative reduction (%) a | |||||||||
| PD 4–6 mm | 5 | 41.04 | 19.05/63.03 | 33.63 | 16.87/50.40 | 12.03* | 4.40/19.66 | −22.99/47.06 | 18.05 |
| PD ≥4 mm | 2 (3) | 52.74 | 48.42/57.96 | 18.15 | 9.00/27.24 | 34.01 | 28.63/39.39 | — | 65.59 |
| PD ≥5 mm | 6 (7) | 28.23 | 6.52/50.54 | 11.71 | 7.88/15.54 | 15.50* | 7.86/23.14 | −11.76/42.76 | 58.52 |
| PD ≥6 mm | 2 | 24.60 | −20.11/69.31 | 7.33 | −5.81/20.46 | 17.17* | 14.41/48.74 | — | 70.20 |
| PD ≥7 mm | 7 | 13.65 | 8.05/19.25 | 3.40 | 1.66/5.14 | 10.27* | 5.22/15.31 | −5.32/29.86 | 75.09 |
Abbreviations: NST, non‐surgical therapy; WM, weighted mean; WMD, weighted mean difference.
p < .001.
Percentage calculated as (100 − “% of PD” value; e.g., 100 − 52.74 = 47.26 in “PD < 4 mm”).
Percentage of PD ≤3 mm
Meta‐analysis of four studies revealed a statistically significant increase of %PD ≤3 mm, i.e., healthy sites after NST, which accounted for a 26.14% change (95% CI: 39.87%–12.40%; p < .001) (Lindhe & Nyman, 1985; Rosling et al., 2001; Shiloah et al., 2014; Giannelli et al., 2015). Percentage of PD ≤3 mm before and 1 year after NST changed from 39.06% (95% CI: 17.61%–60.52%) to 64.11% (95% CI: 37.81%–90.41%) with a relative increase of healthy sites of 64.13% (Figure 3a).
FIGURE 3.
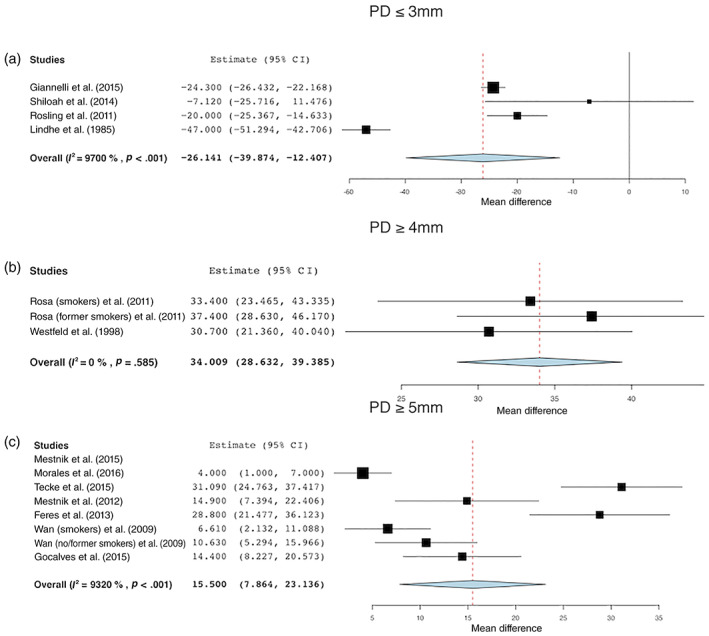
Changes in the percentage of sites with (a) PD ≤3 mm, (b) PD ≥4 mm, and (c) PD ≥5 mm before and after treatment [Colour figure can be viewed at wileyonlinelibrary.com]
Percentage of PD ≤4 mm
One study (Sampaio et al., 2011) reported data on this category. The reported percentage of PD ≤4 mm was 44.2% (±13.18%) and 87.47% (±12.79%), respectively, before and after treatment, that is, an increase of about 77.4%.
Percentage of PD ≥4 mm
Three groups in two studies (Westfeld et al., 1998; Rosa et al., 2011) were analysed for %PD ≥4 mm, but no statistically significant differences before and after NST were found (WMD: 34.01%; 95% CI: 28.63%–39.39%; p = .585). The percentage of PD ≥4 mm before treatment was 52.74% (95% CI: 48.42%–57.96%) and after treatment 18.15% (95% CI: 9.00%–27.24%) (Figure 3b).
Percentage of PD ≥5 mm
Meta‐analysis of data from six studies including seven different patient groups revealed that a reduction of %PD ≥5 mm amounted to 15.50% after NST (95% CI: 7.86%–23.14%; p < .001) (Wan et al., 2009; Feres et al., 2012; Mestnik et al., 2012; Goncalves et al., 2015; Tekce et al., 2015; Morales et al., 2016). The mean %PD ≥5 mm was 28.23% (95% CI: 6.52%–50.54%) and 11.71% (95% CI: 7.88%–15.54%) before and after treatment, respectively (Figure 3c).
Percentage of PD 4–6 mm
For %PD 4–6 mm, data were pooled from four studies (Lindhe & Nyman, 1985; Rosling et al., 2001; Shiloah et al., 2014; Giannelli et al., 2015). NST produced a statistically significant reduction of 12.03% of PD 4–6 mm (95% CI: 4.40%–19.66%, p < .001) 1 year after treatment. The mean %PD 4–6 mm was 41.04% (95% CI: 19.05%–63.03%) and 33.63% (95% CI: 16.87%–50.40%) before and after treatment, respectively (Figure 4a).
FIGURE 4.
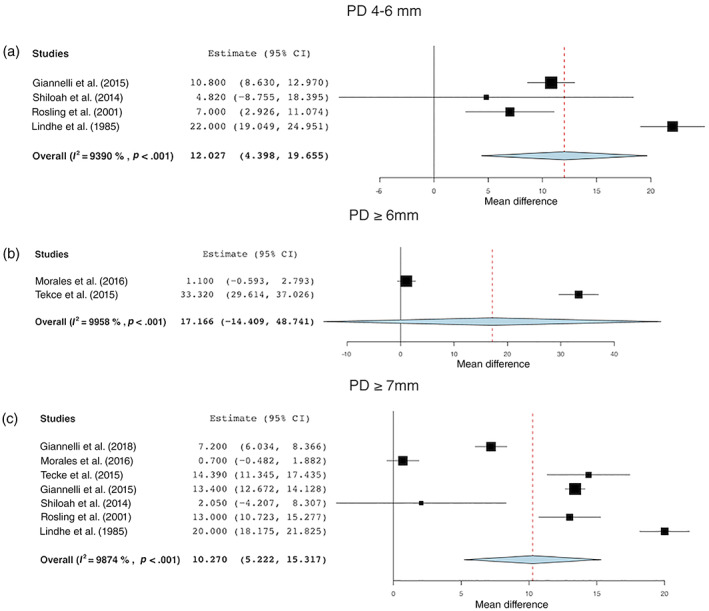
Changes in the percentage of sites with (a) PD 4–6 mm, (b) PD ≥6 mm, and (c) PD ≥7 mm before and after treatment [Colour figure can be viewed at wileyonlinelibrary.com]
Percentage of PD ≥4.5 mm
One study reported %PD ≥4.5 mm before and after treatment, finding a significant reduction of −6.24% ± 8.5% (Sanz‐Sanchez et al., 2015).
Percentage of PD ≥6 mm
A statistically significant reduction of 17.17% (95% CI: –14.41% to 48.74%; p < .001) was found for %PD ≥6 mm data pooled from two studies (Morales et al., 2016; Tekce et al., 2015). The mean %PD≥6 mm was 24.60% (95% CI: −20.11% to 69.31%) and 7.33% (95% CI: −5.81% to 20.46%) before and after treatment, respectively (Figure 4b).
Percentage of PD ≥7 mm
Meta‐analysis of seven studies showed that the WMD %PD ≥7 mm before and after NST was 8.98% (95% CI: 5.22%–15.31%; p < .001) (Lindhe & Nyman, 1985; Rosling et al., 2001; Shiloah et al., 2014; Giannelli et al., 2015; Tekce et al., 2015; Morales et al., 2016; Giannelli et al., 2018). The mean %PD≥7 mm was 13.65% (95% CI: 8.05%–19.25%) and 3.40% (95% CI: 1.66%–5.14%) before and after treatment, respectively (Figure 4c).
3.4.3. Mean number of residual pockets at the end of therapy
The number of pockets before and after NST was an outcome of interest in five studies (Sanz‐Sanche et al., 2015; Baelum & Lopez, 2016;Morales et al., 2016; Borges et al., 2017; Cosgarea et al., 2017). The study by Morales et al. (2016) however did not report the standard deviation but reported only the mean number of pockets (Figure 5).
FIGURE 5.
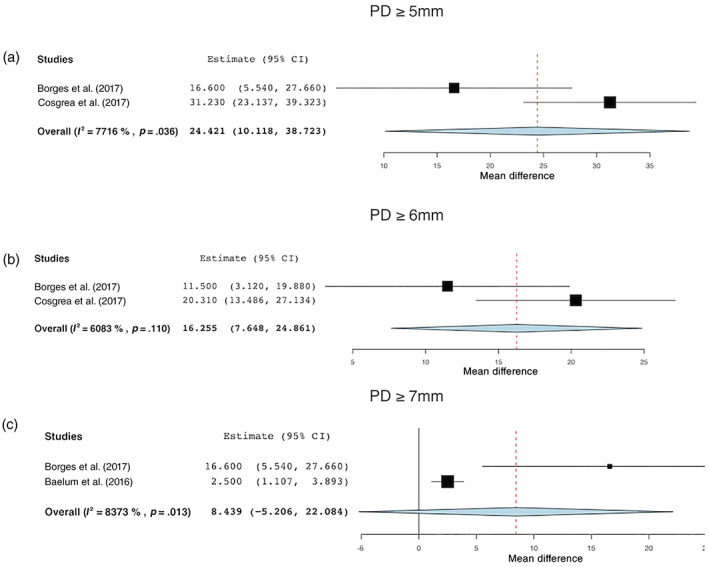
Changes in the number of sites with (a) PD ≥5 mm, (b) PD ≥6 mm, and (c) PD ≥7 mm before and after treatment [Colour figure can be viewed at wileyonlinelibrary.com]
PD ≥4 mm
The study by Baelum and Lopez (2016) reported a significant reduction (from 38.0 ± 32.2 to 9.3 ± 10.5) 12 months after therapy.
PD ≥4.5 mm
The study by Sanz‐Sanchez et al. (2015) reported a significant reduction of the number of PD ≥4.5 of −0.49 ± 0.45 with a baseline number of pockets of 5.72 ± 0.5.
PD ≥5 mm
For PD ≥5 mm, the WMD in the number of pockets before and after treatment was 24.42 (95% CI: 10.12–38.72; p = .036). The mean number of pockets with PD ≥5 mm before and after treatment was, respectively, 39.01 (95% CI: 33.04%–44.97%) and 14.13 (95% CI: 5.89–22.37) (Figure 5a).
PD ≥6 mm
For PD ≥6 mm, the WMD before and after treatment did not reach statistical significance (WMD = 16.255; 95% CI: 7.648–24.861; p = .110). The WM number of pockets was 24.60 (95% CI: −20.107 to 69.307) and 7.33 (95% CI: –5.80 to 20.46) before and after NST, respectively (Figure 5b).
PD ≥7 mm
For PD ≥7, the WMD in the number of pockets before and after treatment was 8.44 (95% CI: –5.206 to 22.084; p = .013). The mean number of pockets with PD≥7 mm was 7.28 (95% CI: −0.94 to 15.50) and 2.91% (95% CI: −1.59 to 7.39) before and after treatment, respectively (Figure 5c).
3.4.4. Changes in gingival or bleeding indices
FMBS
FMBS before and after treatment was reported in 16 patient groups in 15 independent studies. The WM FMBS before treatment was 57.37% (95% CI: 47.21%–67.54%). After treatment, it was reduced to 21.86% (95% CI: 16.23%–27.50%). The WMD was 35.61% (95% CI: 24.83%–46.39%).
3.4.5. Changes in plaque indices
FMPS
FMPS after treatment was reported in 15 groups out of 13 independent studies. The WM FMPS before NST was 62.41% (95% CI: 48.36%–76.47%) and after NST 28.75% (95% CI: 25.15%–32.36%). The WMD was 29.55% (95% CI: 20.75%–38.36%).
3.4.6. Percentage of cases with the need of re‐treatment
No study reported the percentage of cases in need of re‐treatment or sites that experienced further periodontal breakdown.
3.4.7. Harms, adverse effects, and PROMs
Harms and adverse effects related to the technique were not reported in any of the studies. The most common complications reported during follow‐up were the incidence of attachment loss at healthy sites and hypersensitivity.
PROMs were reported only in two studies (Wong et al., 2012; Giannelli et al., 2018), which reported that patients prefer to have NST with laser adjunction and that NST increased their quality of life, respectively.
4. DISCUSSION
The present systematic review addressed the efficacy of NST in terms of PC 1 year after treatment. Data from one study found 63.9% of PC. However, the available evidence about the efficacy of NST in terms of PC is limited and generally unreported. Four studies reported the %PD ≤3 mm before and after NST, and one study reported the %PD ≤4 mm.
The thresholds PD ≤3 mm and PD ≥4 mm or PD ≤4 mm and PD ≥5 mm allow drawing indirect conclusions on PC assuming PD ≤3 mm and PD ≤4 mm as healthy sites, respectively.
Considering PC at PD ≤3 mm, the data by Cosgarea et al. (2017) provided evidence of PC in 39.00 ± 17.30 out of the 61.48 ± 22.13 PDs ≥4 mm at baseline. The studies reporting %PD ≤3 mm demonstrated that NST is able to nearly double the percentage of healthy sites in patients affected from periodontitis from 39% to 64% 1 year after NST. The meta‐analysis we have performed for the percentage of PD≥4 mm did not find a statistically significant reduction (WMD: 34.01%; p = .585), even though a trend towards a reduction was still evident with a change from 52.74% (95% CI: 48.42%–57.96%) at baseline and 18.15% (95% CI: 9.00%–27.24%) after NST. However, the study by Baelum and Lopez (2016) showed a significant reduction in the number of PD ≥4 mm with approximately 73% less pockets after treatment.
Even though NST may be considered ideal for the initial treatment of periodontitis, we observe that a remarkable percentage of PDs remains after NST and that the majority of patients will need to address residual PDs either by means of surgical procedures or tailoring the recall intervals of SPT according to specific patient‐centred needs. However, the actual evaluation on the efficacy of NST directly relates to at least two parameters: (1) the baseline characteristics of the patients, and (2) the threshold selected for periodontal health in terms of PD.
The baseline conditions of the patients in the included studies for this systematic review may have influenced the outcomes of NST across studies, as suggested also by the large prediction intervals. It has been demonstrated that baseline PD, tooth type, mobility, and smoking affect the likelihood of PC (D'Aiuto et al., 2005; Tomasi et al., 2007; Jiao et al., 2017). Unfortunately, these factors have not been reported homogeneously in the selected studies, and the diagnostic criteria for the patients varied consistently. The new classification of periodontal diseases and conditions should help selecting patients, providing more homogeneous samples across studies (Tonetti et al., 2018).
Whether PD ≤3 mm may be considered as an ideal threshold to distinguish periodontally healthy sites from diseased ones is still debatable. Sites with PD ≤3 mm were traditionally considered healthy since they did not benefit from periodontal treatment in the literature and showed even CAL loss after SRP (Lindhe et al., 1982; Kaldahl et al., 1996; Becker et al., 2001). However, PD = 4 mm has been associated only with a limited increase in the odds ratio of tooth loss both at the tooth and site level and not with a risk of disease progression at the patient level (Matuliene et al., 2008). Hence, it may still be considered compatible with periodontal health, especially in patients with a reduced periodontium (Chapple et al., 2018). If the treatment goal of periodontal therapy is to maintain periodontal stability during SPT, the number of pockets with PD ≥5 or 6 mm may be considered a better predictor of periodontal breakdown and should be targeted with additional periodontal treatment or shorter recall intervals during SPT (Ramseier et al., 2019). The data we were able to retrieve from the meta‐analysis showed that NST was able to reduce the number and the percentage of PD ≥5 mm from 39.01 to 14.13 and from 28.23% to 11.71%, respectively. This finding clearly supports the potentialities of NST. If the threshold for disease is PD ≥5 mm, NST is able to eradicate approximately two‐thirds of the pockets, resulting in 90% of the sites being ≤4 mm. These results are in agreement with those reported in a recent systematic review in which PC was observed in 74% of the sites (95% CI: 64%–85%) (Suvan et al., 2019) and with a previous report on the probability of PC after NST, in which 62.4% of the pockets were closed after 3 months (Tomasi et al., 2007).
The fact that the mean number of PDs ≥5 mm after NST is 14.13 suggests that a remarkable proportion of patients are at risk of further disease progression and thus in need of additional treatment. Having ≥9 sites with PD ≥5 mm has in fact been associated with disease progression at the patient level, and PD ≥5 mm has been significantly associated with increased odds ratio of tooth loss at the tooth and site level (Matuliene et al., 2008; Salvi et al., 2014). Therefore, PDs ≥5 mm after NST may be considered an unsatisfactory treatment outcome, making NST alone unable to achieve treatment goals.
Changing the threshold to PD ≥6 mm NST did not show a significant effect in reducing the number of diseased sites. This is again in agreement with a systematic review which compared the efficacy of access flap to NST. It was found that the WM PD reduction for NST at deep sites was approximately 2 mm, which is generally not enough to close pockets with a baseline PD ≥6 mm (Sanz‐Sanchez et al., 2020). A possible explanation for the lack of effect at sites with PD ≥6 mm may be found in the ineffectiveness of SRP in deeper pockets (D'Aiuto et al., 2005; Tomasi et al., 2007) and in part by the baseline conditions of the patients in these studies. In particular, it seems that the patients enrolled in the study by Tekce et al. (2015) were affected by the most severe and generalized forms of periodontitis at baseline compared to the ones in studies pooled for percentage of PD categories. Importantly, PD ≥6 mm has been identified in both retrospective and prospective studies as a risk indicator of disease progression and tooth loss (Claffey & Egelberg, 1995; Matuliene et al., 2008), and residual PD ≥6 mm after NST should be definitely considered as an indicator of incomplete periodontal treatment. This further enlightens the fact that NST, which has been shown to leave a mean number of 7.33 sites with PD ≥6 mm, may be ineffective in cases of severe periodontitis to achieve periodontal stability along the time.
The minimum follow‐up period for inclusion in this review was 1 year. This threshold has been selected because improvement of clinical conditions in PD ≥6 mm has been observed to occur during a period, which approximately last for 9–12 months after NST (Badersten et al., 1984; Cobb, 2002).
One of the major shortcomings of this review is that we were not able to retrieve much data about PC. No study reported directly for PC, intended as the percentage of the number of sites with a baseline PD > 3 mm which resulted in PD ≤3 mm or PD ≤4 mm 1 year after NST. The majority of data in the included studies for this review reported about percentages of different PD thresholds before and after treatment. This has two main limitations. First, data about the percentage of PDs have not been directly associated with increased risk of tooth loss or disease progression. Second, the estimation of PC was indirectly calculated since no direct information was available on the actual diseased sites that were eradicated after treatment. This, in particular, prevented us from performing proper analysis on the efficacy of NST on the PC, and we were only able to estimate the efficacy of NST based on different thresholds. Finally, heterogeneity was high among studies included in meta‐analyses.
In conclusion, the present systematic review supports the role of NST as an efficient periodontal treatment strategy. PC may occur in approximately two‐thirds of the pockets, but more data are required. NST is able to reduce the percentage or number of pockets between one‐half and two‐thirds of the sites. Hence, a consistent proportion of patients may be in need of additional treatment including surgical procedures or shortened recall intervals during SPT. Reporting of PC as outcome is important and should be included in future studies.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
Conception or design of the work: Filippo Citterio, Giacomo Gualini, Moontaek Chang, Mario Aimetti; Data collection: Filippo Citterio, Giacomo Gualini, Moontaek Chang, Gian Marco Piccoli; Data analysis and interpretation: Filippo Citterio, Giacomo Gualini, Moontaek Chang, Mario Aimetti, Federica Romano; Drafting the article: Filippo Citterio, Giacomo Gualini, Giacomo Baima, Moontaek Chang; Critical revision of the article: Marta Giraudi, Giulia Maria Mariani, Valeria Manavella, Mario Aimetti. All authors gave final approval of the version to be published.
Supporting information
TABLE S1 Risk‐of‐bias assessment according to RoB 2 tool for controlled randomized trials
TABLE S2 Ris‐of‐bias assessment for non‐controlled trials
TABLE S3 Risk‐of‐bias assessment for before and after studies
TABLE S4 Synthesis of the results
TABLE S5 Synthesis of the results involved in the meta‐analyses
FIGURE S1 Search strategy
ACKNOWLEDGEMENTS
Open Access Funding provided by Universita degli Studi di Torino within the CRUI‐CARE Agreement. [Correction added on 25 May 2022, after first online publication: CRUI funding statement has been added.]
Citterio, F. , Gualini, G. , Chang, M. , Piccoli, G. M. , Giraudi, M. , Manavella, V. , Baima, G. , Mariani, G. M. , Romano, F. , & Aimetti, M. (2022). Pocket closure and residual pockets after non‐surgical periodontal therapy: A systematic review and meta‐analysis. Journal of Clinical Periodontology, 49(1), 2–14. 10.1111/jcpe.13547
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Badersten, A. , Nilveus, R. , & Egelberg, J. (1984). Effect of nonsurgical periodontal therapy. II. Severely advanced periodontitis. Journal of Clinical Periodontology, 11(1), 63–76. 10.1111/j.1600-051x.1984.tb01309.x [DOI] [PubMed] [Google Scholar]
- Baelum, V. , & Lopez, R. (2016). Defining and predicting outcomes of non‐surgical periodontal treatment: A 1‐yr follow‐up study. European Journal of Oral Sciences, 124(1), 33–44. 10.1111/eos.12240 [DOI] [PubMed] [Google Scholar]
- Becker, W. , Becker, B. E. , Caffesse, R. , Kerry, G. , Ochsenbein, C. , Morrison, E. , & Prichard, J. (2001). A longitudinal study comparing scaling, osseous surgery, and modified Widman procedures: Results after 5 years. Journal of Periodontology, 72(12), 1675–1684. 10.1902/jop.2001.72.12.1675 [DOI] [PubMed] [Google Scholar]
- Becker, W. , Becker, B. E. , Ochsenbein, C. , Kerry, G. , Caffesse, R. , Morrison, E. C. , & Prichard, J. (1988). A longitudinal study comparing scaling, osseous surgery and modified Widman procedures. Results after one year. Journal of Periodontology, 59(6), 351–365. 10.1902/jop.1988.59.6.351 [DOI] [PubMed] [Google Scholar]
- Borges, I. , Faveri, M. , Figueiredo, L. C. , Duarte, P. M. , Retamal‐Valdes, B. , Montenegro, S. C. L. , & Feres, M. (2017). Different antibiotic protocols in the treatment of severe chronic periodontitis: A 1‐year randomized trial. Journal of Clinical Periodontology, 44(8), 822–832. 10.1111/jcpe.12721 [DOI] [PubMed] [Google Scholar]
- Brayer, W. K. , Mellonig, J. T. , Dunlap, R. M. , Marinak, K. W. , & Carson, R. E. (1989). Scaling and root planing effectiveness: The effect of root surface access and operator experience. Journal of Periodontology, 60(1), 67–72. 10.1902/jop.1989.60.1.67 [DOI] [PubMed] [Google Scholar]
- Buchanan, S. A. , & Robertson, P. B. (1987). Calculus Removal by Scaling/Root Planing with and without Surgical Access. Journal of periodontology, 58, 159–163. 10.1902/jop.1987.58.3.159 [DOI] [PubMed] [Google Scholar]
- Chapple, I. L. C. , Mealey, B. L. , van Dyke, T. E. , Bartold, P. M. , Dommisch, H. , Eickholz, P. , Geisinger, M. L. , Genco, R. J. , Glogauer, M. , Goldstein, M. , Griffin, T. J. , Holmstrup, P. , Johnson, G. K. , Kapila, Y. , Lang, N. P. , Meyle, J. , Murakami, S. , Plemons, J. , Romito, G. A. , … Yoshie, H. (2018). Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 world workshop on the classification of periodontal and Peri‐implant diseases and conditions. Journal of Clinical Periodontology, 45(Suppl 20), S68–S77. 10.1111/jcpe.12940 [DOI] [PubMed] [Google Scholar]
- Claffey, N. , & Egelberg, J. (1995). Clinical indicators of probing attachment loss following initial periodontal treatment in advanced periodontitis patients. Journal of Clinical Periodontology, 22(9), 690–696. 10.1111/j.1600-051x.1995.tb00828.x [DOI] [PubMed] [Google Scholar]
- Claffey, N. , Nylund, K. , Kiger, R. , Garrett, S. , & Egelberg, J. (1990). Diagnostic predictability of scores of plaque, bleeding, suppuration and probing depth for probing attachment loss. 3 1/2 years of observation following initial periodontal therapy. Journal of Clinical Periodontology, 17(2), 108–114. 10.1111/j.1600-051x.1990.tb01071.x [DOI] [PubMed] [Google Scholar]
- Cobb, C. M. (2002). Clinical significance of non‐surgical periodontal therapy: an evidence‐based perspective of scaling and root planing. Journal of Clinical Periodontology, 29, 22–32. 10.1034/j.1600-051X.29.s2.4.x [DOI] [PubMed] [Google Scholar]
- Cosgarea, R. , Heumann, C. , Juncar, R. , Tristiu, R. , Lascu, L. , Salvi, G. E. , Arweiler, N. B. , & Sculean, A. (2017). One year results of a randomized controlled clinical study evaluating the effects of non‐surgical periodontal therapy of chronic periodontitis in conjunction with three or seven days systemic administration of amoxicillin/metronidazole. PLoS One, 12(6), e0179592. 10.1371/journal.pone.0179592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aiuto, F. , Ready, D. , Parkar, M. , & Tonetti, M. S. (2005). Relative contribution of patient‐, tooth‐, and site‐associated variability on the clinical outcomes of subgingival debridement. I. Probing depths. Journal of Periodontology, 76(3), 398–405. 10.1902/jop.2005.76.3.398 [DOI] [PubMed] [Google Scholar]
- Feres, M. , Soares, G. M. , Mendes, J. A. , Silva, M. P. , Faveri, M. , Teles, R. , Socransky, S. S. , & Figueiredo, L. C. (2012). Metronidazole alone or with amoxicillin as adjuncts to non‐surgical treatment of chronic periodontitis: A 1‐year double‐blinded, placebo‐controlled, randomized clinical trial. Journal of Clinical Periodontology, 39(12), 1149–1158. 10.1111/jcpe.12004 [DOI] [PubMed] [Google Scholar]
- Giannelli, M. , Formigli, L. , Lorenzini, L. , & Bani, D. (2015). Efficacy of combined photoablative‐photodynamic diode laser therapy adjunctive to scaling and root planing in periodontitis: Randomized split‐mouth trial with 4‐year follow‐up. Photomedicine and Laser Surgery, 33(9), 473–480. 10.1089/pho.2015.3955 [DOI] [PubMed] [Google Scholar]
- Giannelli, M. , Materassi, F. , Fossi, T. , Lorenzini, L. , & Bani, D. (2018). Treatment of severe periodontitis with a laser and light‐emitting diode (LED) procedure adjunctive to scaling and root planing: A double‐blind, randomized, single‐center, split‐mouth clinical trial investigating its efficacy and patient‐reported outcomes at 1 year. Lasers in Medical Science, 33(5), 991–1002. 10.1007/s10103-018-2441-9 [DOI] [PubMed] [Google Scholar]
- Goncalves, T. E. , Feres, M. , Zimmermann, G. S. , Faveri, M. , Figueiredo, L. C. , Braga, P. G. , & Duarte, P. M. (2015). Effects of scaling and root planing on clinical response and serum levels of adipocytokines in patients with obesity and chronic periodontitis. Journal of Periodontology, 86(1), 53–61. 10.1902/jop.2014.140266 [DOI] [PubMed] [Google Scholar]
- Graziani, F. , Karapetsa, D. , Mardas, N. , Leow, N. , & Donos, N. (2018). Surgical treatment of the residual periodontal pocket. Periodontology 2000, 76(1), 150–163. 10.1111/prd.12156 [DOI] [PubMed] [Google Scholar]
- Griffiths, G. S. , Smart, G. J. , Bulman, J. S. , Weiss, G. , Shrowder, J. , & Newman, H. N. (2000). Comparison of clinical outcomes following treatment of chronic adult periodontitis with subgingival scaling or subgingival scaling plus metronidazole gel. Journal of Clinical Periodontology, 27(12), 910–917. 10.1034/j.1600-051x.2000.027012910.x [DOI] [PubMed] [Google Scholar]
- Higgins, J. P. T. , & Thompson, S. G. (2002). Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine, 21, 1539–1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- İnce, G. , Gürsoy, H. , İpçi, Ş. D. , Cakar, G. , Emekli‐Alturfan, E. , & Yılmaz, S. (2015). Clinical and Biochemical Evaluation of Lozenges Containing Lactobacillus reuteri as an Adjunct to Non‐Surgical Periodontal Therapy in Chronic Periodontitis. Journal of Periodontology, 88(6), 746–754. 10.1902/jop.2015.140612 [DOI] [PubMed] [Google Scholar]
- Jiao, J. , Shi, D. , Cao, Z. Q. , Meng, H. X. , Lu, R. F. , Zhang, L. , Song, Y. , & Zhao, J. R. (2017). Effectiveness of non‐surgical periodontal therapy in a large Chinese population with chronic periodontitis. Journal of Clinical Periodontology, 44(1), 42–50. 10.1111/jcpe.12637 [DOI] [PubMed] [Google Scholar]
- Jönsson, B. , Ohrn, K. , Lindberg, P. , & Oscarson, N. (2010). Evaluation of an individually tailored oral health educational programme on periodontal health. Journal of Clinical Periodontology, 37(10), 912–919. 10.1111/j.1600-051X.2010.01590.x [DOI] [PubMed] [Google Scholar]
- Kaldahl, W. B. , Kalkwarf, K. L. , Patil, K. D. , Molvar, M. P. , & Dyer, J. K. (1996). Long‐term evaluation of periodontal therapy: I. Response to 4 therapeutic modalities. Journal of Periodontology, 67(2), 93–102. 10.1902/jop.1996.67.2.93 [DOI] [PubMed] [Google Scholar]
- Laleman, I. , Cortellini, S. , De Winter, S. , Rodriguez Herrero, E. , Dekeyser, C. , Quirynen, M. , & Teughels, W. (2017). Subgingival debridement: End point, methods and how often? Periodontology 2000, 75(1), 189–204. 10.1111/prd.12204 [DOI] [PubMed] [Google Scholar]
- Lindhe, J. , & Nyman, S. (1985). Scaling and granulation tissue removal in periodontal therapy. Journal of Clinical Periodontology, 12(5), 374–388. 10.1111/j.1600-051x.1985.tb00928.x [DOI] [PubMed] [Google Scholar]
- Lindhe, J. , Socransky, S. S. , Nyman, S. , Haffajee, A. , & Westfelt, E. (1982). "Critical probing depths" in periodontal therapy. Journal of Clinical Periodontology, 9(4), 323–336. 10.1111/j.1600-051x.1982.tb02099.x [DOI] [PubMed] [Google Scholar]
- Matuliene, G. , Pjetursson, B. E. , Salvi, G. E. , Schmidlin, K. , Bragger, U. , Zwahlen, M. , & Lang, N. P. (2008). Influence of residual pockets on progression of periodontitis and tooth loss: Results after 11 years of maintenance. Journal of Clinical Periodontology, 35(8), 685–695. 10.1111/j.1600-051X.2008.01245.x [DOI] [PubMed] [Google Scholar]
- Mestnik, M. J. , Feres, M. , Figueiredo, L. C. , Soares, G. , Teles, R. P. , Fermiano, D. , Duarte, P. M. , & Faveri, M. (2012). The effects of adjunctive metronidazole plus amoxicillin in the treatment of generalized aggressive periodontitis: A 1‐year double‐blinded, placebo‐controlled, randomized clinical trial. Journal of Clinical Periodontology, 39(10), 955–961. 10.1111/j.1600-051X.2012.01932.x [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. Journal of Clinical Epidemiology, 62(10), 1006–1012. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- Moher, D. , Schulz, K. F. , & Altman, D. G. (2001). The CONSORT statement: Revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet, 357(9263), 1191–1194. [PubMed] [Google Scholar]
- Morales, A. , Carvajal, P. , Silva, N. , Hernandez, M. , Godoy, C. , Rodriguez, G. , Cabello, R. , Garcia‐Sesnich, J. , Hoare, A. , Diaz, P. I. , & Gamonal, J. (2016). Clinical effects of lactobacillus rhamnosus in non‐surgical treatment of chronic periodontitis: A randomized placebo‐controlled trial with 1‐year follow‐up. Journal of Periodontology, 87(8), 944–952. 10.1902/jop.2016.150665 [DOI] [PubMed] [Google Scholar]
- Rabbani, G. M. , Ash, M. M., Jr. , & Caffesse, R. G. (1981). The effectiveness of subgingival scaling and root planing in calculus removal. Journal of Periodontology, 52(3), 119–123. 10.1902/jop.1981.52.3.119 [DOI] [PubMed] [Google Scholar]
- Ramberg, P. , Rosling, B. , Serino, G. , Hellström, M. , Socransky, S. S. , & Lindhe, J. (2001). The long term effect of systemic tetracycline used as an adjunct to non‐surgical treatment of advanced periodontitis. Journal of Clinical Periodontology, 28, 446–452. 10.1034/j.1600-051x.2001.028005446.x [DOI] [PubMed] [Google Scholar]
- Ramseier, C. A. , Nydegger, M. , Walter, C. , Fischer, G. , Sculean, A. , Lang, N. P. , & Salvi, G. E. (2019). Time between recall visits and residual probing depths predict long‐term stability in patients enrolled in supportive periodontal therapy. Journal of Clinical Periodontology, 46, 218–230. 10.1111/jcpe.13041 [DOI] [PubMed] [Google Scholar]
- Rateitschak‐Pluss, E. M. , Schwarz, J. P. , Guggenheim, R. , Duggelin, M. , & Rateitschak, K. H. (1992). Non‐surgical periodontal treatment: Where are the limits? An SEM study. Journal of Clinical Periodontology, 19(4), 240–244. 10.1111/j.1600-051x.1992.tb00460.x [DOI] [PubMed] [Google Scholar]
- Rosa, E. F. , Corraini, P. , de Carvalho, V. F. , Inoue, G. , Gomes, E. F. , Lotufo, J. P. , De Micheli, G. , & Pannuti, C. M. (2011). A prospective 12‐month study of the effect of smoking cessation on periodontal clinical parameters. Journal of Clinical Periodontology, 38(6), 562–571. 10.1111/j.1600-051X.2011.01723.x [DOI] [PubMed] [Google Scholar]
- Rosling, B. , Hellstrom, M. K. , Ramberg, P. , Socransky, S. S. , & Lindhe, J. (2001). The use of PVP‐iodine as an adjunct to non‐surgical treatment of chronic periodontitis. Journal of Clinical Periodontology, 28(11), 1023–1031. 10.1034/j.1600-051x.2001.281106.x [DOI] [PubMed] [Google Scholar]
- Salvi, G. E. , Mischler, D. C. , Schmidlin, K. , Matuliene, G. , Pjetursson, B. E. , Bragger, U. , & Lang, N. P. (2014). Risk factors associated with the longevity of multi‐rooted teeth. Long‐term outcomes after active and supportive periodontal therapy. Journal of Clinical Periodontology, 41(7), 701–707. 10.1111/jcpe.12266 [DOI] [PubMed] [Google Scholar]
- Sampaio, E. , Rocha, M. , Figueiredo, L. C. , Faveri, M. , Duarte, P. M. , Gomes Lira, E. A. , & Feres, M. (2011). Clinical and microbiological effects of azithromycin in the treatment of generalized chronic periodontitis: A randomized placebo‐controlled clinical trial. Journal of Clinical Periodontology, 38(9), 838–846. 10.1111/j.1600-051X.2011.01766.x [DOI] [PubMed] [Google Scholar]
- Sanz‐Sanchez, I. , Montero, E. , Citterio, F. , Romano, F. , Molina, A. , & Aimetti, M. (2020). Efficacy of access flap procedures compared to subgingival debridement in the treatment of periodontitis. A systematic review and meta‐analysis. Journal of Clinical Periodontology, 47, 282–302. 10.1111/jcpe.13259 [DOI] [PubMed] [Google Scholar]
- Sanz‐Sanchez, I. , Alonso, B. , Carasol, M. , Herrera, D. , & Sanz, M. (2012). Nonsurgical treatment of periodontitis. The Journal of Evidence‐Based Dental Practice, 12(3 Suppl), 76–86. 10.1016/s1532-3382(12)70019-2 [DOI] [PubMed] [Google Scholar]
- Sanz‐Sánchez, I. , Ortiz‐Vigón, A. , Matos, R. , Herrera, D. , & Sanz, M. (2015). Clinical efficacy of subgingival debridement with adjunctive erbium:yttrium‐aluminum‐garnet laser treatment in patients with chronic periodontitis: a randomized clinical trial. Journal of periodontology, 86(4), 527–535. 10.1902/jop.2014.140258 [DOI] [PubMed] [Google Scholar]
- Sato, K. , Yoneyama, T. , Okamoto, H. , Dahlen, G. , & Lindhe, J. (1993). The effect of subgingival debridement on periodontal disease parameters and the subgingival microbiota. Journal of Clinical Periodontology, 20, 359–365. [DOI] [PubMed] [Google Scholar]
- Serino, G. , Rosling, B. , Ramberg, P. , Socransky, S. S. , & Lindhe, J. (2001). Initial outcome and long‐term effect of surgical and non‐surgical treatment of advanced periodontal disease. Journal of Clinical Periodontology, 28(10), 910–916. 10.1034/j.1600-051x.2001.028010910.x [DOI] [PubMed] [Google Scholar]
- Shiloah, J. , Bland, P. S. , Scarbecz, M. , Patters, M. R. , Stein, S. H. , & Tipton, D. A. (2014). The effect of long‐term aspirin intake on the outcome of non‐surgical periodontal therapy in smokers: A double‐blind, randomized pilot study. Journal of Periodontal Research, 49(1), 102–109. 10.1111/jre.12085 [DOI] [PubMed] [Google Scholar]
- Smiley, C. J. , Tracy, S. L. , Abt, E. , Michalowicz, B. S. , John, M. T. , Gunsolley, J. , Cobb, C. M. , Rossmann, J. , Harrel, S. K. , Forrest, J. L. , Hujoel, P. P. , Noraian, K. W. , Greenwell, H. , Frantsve‐Hawley, J. , Estrich, C. , & Hanson, N. (2015). Evidence‐based clinical practice guideline on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. Journal of the American Dental Association (1939), 146(7), 525–535. 10.1016/j.adaj.2015.01.026 [DOI] [PubMed] [Google Scholar]
- Soares, G. M. , Mendes, J. A. , Silva, M. P. , Faveri, M. , Teles, R. , Socransky, S. S. , Wang, X. , Figueiredo, L. C. , & Feres, M. (2014). Metronidazole alone or with amoxicillin as adjuncts to non‐surgical treatment of chronic periodontitis: a secondary analysis of microbiological results from a randomized clinical trial. Journal of Clinical Periodontology, 41(4), 366–376. 10.1111/jcpe.12217 [DOI] [PubMed] [Google Scholar]
- Sterne, J. A. C. , Savovic, J. , Page, M. J. , Elbers, R. G. , Blencowe, N. S. , Boutron, I. , Cates, C. J. , Cheng, H.‐Y. , Corbett, M. S. , Eldridge, S. M. , Emberson, J. R. , Hernán, M. A. , Hopewell, S. , Hróbjartsson, A. , Junqueira, D. R. , Jüni, P. , Kirkham, J. J. , Lasserson, T. , Li, T. , … Higgins, J. P. T. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ, 366, l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- Suvan, J. , Leira, Y. , Moreno, F. , Graziani, F. , Derks, J. , & Tomasi, C. (2019). Subgingival instrumentation for treatment of periodontitis. A systematic review. Journal of Clinical Periodontology, 47, 155–175. 10.1111/jcpe.13245 [DOI] [PubMed] [Google Scholar]
- Tekce, M. , Ince, G. , Gursoy, H. , Dirikan Ipci, S. , Cakar, G. , Kadir, T. , & Yilmaz, S. (2015). Clinical and microbiological effects of probiotic lozenges in the treatment of chronic periodontitis: A 1‐year follow‐up study. Journal of Clinical Periodontology, 42(4), 363–372. 10.1111/jcpe.12387 [DOI] [PubMed] [Google Scholar]
- Timmerman, M. F. , van der Weijden, G. A. , van Steenbergen, T. J. M. , Mantel, M. S. , de Gractff, J. , & van der Veldern, U. (1996). Evaluation of the long‐term efficacy and safety of locally‐applied minocvcline in adult periodontitis patients. Journal of Clinical Periodontology, 23, 707–716. [DOI] [PubMed] [Google Scholar]
- Tomasi, C. , & Wennström, J. L. (2017). Is the use of differences in the magnitude of CAL gain appropriate for making conclusions on the efficacy of non‐surgical therapeutic means? Journal of Clinical Periodontology, 44(6), 601–602. 10.1111/jcpe.12733 [DOI] [PubMed] [Google Scholar]
- Tomasi, C. , Leyland, A. H. , & Wennstrom, J. L. (2007). Factors influencing the outcome of non surgical periodontal treatment: A multilevel approach. Journal of Clinical Periodontology, 34(8), 682–690. 10.1111/j.1600-051X.2007.01111.x [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , Greenwell, H. , & Kornman, K. S. (2018). Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. Journal of Clinical Periodontology, 45(Suppl 20), S149–s161. 10.1111/jcpe.12945 [DOI] [PubMed] [Google Scholar]
- Van der Weijden, G. A. , & Timmerman, M. F. (2002). A systematic review on the clinical efficacy of subgingival debridement in the treatment of chronic periodontitis. Journal of Clinical Periodontology, 29(Suppl 3), 55–71; discussion 90–51. [DOI] [PubMed] [Google Scholar]
- Wan, C. P. , Leung, W. K. , Wong, M. C. M. , Wong, R. M. S. , Wan, P. , Lo, E. C. M. , & Corbet, E. F. (2009). Effects of smoking on healing response to non‐surgical periodontal therapy: A multilevel modelling analysis. Journal of Clinical Periodontology, 36, 229–239. 10.1111/j.1600-051X.2008.01371.x [DOI] [PubMed] [Google Scholar]
- Westfeld, E. , Rylander, H. , Dahlen, G. , & Lindhe, J. (1998). The effect of supragingival plaque control on the progression of advanced periodontal disease. Journal of Clinical Periodontology, 25(7), 536–541. 10.1111/j.1600-051x.1998.tb02484.x [DOI] [PubMed] [Google Scholar]
- Wong, R. M. , Ng, S. K. , Corbet, E. F. , & Keung Leung, W. (2012). Non‐surgical periodontal therapy improves oral health‐related quality of life. Journal of Clinical Periodontology, 39(1), 53–61. 10.1111/j.1600-051X.2011.01797.x [DOI] [PubMed] [Google Scholar]
- Yen, C. A. , Damoulis, P. D. , Stark, P. C. , Hibberd, P. L. , Singh, M. , & Papas, A. S. (2008). The effect of a selective cyclooxygenase‐2 inhibitor (celecoxib) on chronic periodontitis. Journal of Periodontology, 79(1), 104–113. 10.1902/jop.2008.070271 [DOI] [PubMed] [Google Scholar]
- Zafar, F. , Romano, F. , Citterio, F. , Ferrarotti, F. , Dellavia, C. , Chang, M. , & Aimetti, M. (2021). Chemical cleansing as an adjunct to subgingival instrumentation with ultrasonic and hand devices in deep periodontal pockets: A randomized controlled study. Journal of Periodontal & Implant Science, 51, e21.276–e21.284. 10.5051/jpis.2007080354 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Risk‐of‐bias assessment according to RoB 2 tool for controlled randomized trials
TABLE S2 Ris‐of‐bias assessment for non‐controlled trials
TABLE S3 Risk‐of‐bias assessment for before and after studies
TABLE S4 Synthesis of the results
TABLE S5 Synthesis of the results involved in the meta‐analyses
FIGURE S1 Search strategy
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


