Abstract
Carbon‐nanohoop structures featuring one or more round‐shaped cavities represent ideal supramolecular hosts for spherical fullerenes, with potential to form host‐guest complexes that perform as organic semiconductors in the solid state. Due to the tight complexation between the shape‐complementary hosts and guests, carbon nanohoops have the potential to shield fullerenes from water and oxygen, known to perturb the electron‐transport process. Many nanohoop receptors have been found to form host‐guest complexes with fullerenes. However, there is only a little or no control over the long‐range order of encapsulated fullerenes in the solid state. Consequently, the potential of these complexes to perform as organic semiconductors is rarely evaluated. Herein, we present a survey of all known nanohoop‐fullerene complexes, for which the solid‐state structures were obtained. We discuss and propose instances where the inclusion fullerene guests form discrete supramolecular wires, which might open up possibilities for their use in electronic devices.
Keywords: fullerenes, host-guest chemistry, nanostructures, semiconductors, supramolecular chemistry
The geometric complementarity between carbon nanohoops and fullerenes enables them to form strong supramolecular host‐guest complexes. This Review article presents a survey of all nanohoop‐fullerene complexes, where the inclusion fullerene guests are assembled into discrete supramolecular arrays, forming linear or zigzag one‐dimensional nanowires with potential to perform as organic semiconductors.

Introduction
The design of receptors with high affinity for binding fullerenes is a subject of fundamental research in the areas of supramolecular and materials chemistry. [1] Since the discovery of C60, [2] fullerenes have been investigated as potential components of organic field‐effect transistors [3] and solar cells, [4] owing to their ability to absorb visible light, accept electrons, and transport charge. Encapsulation of fullerenes inside macrocyclic hosts via π‐π interactions represents a convenient strategy for supramolecular manipulation of the electronic properties and solid‐state morphology of fullerenes. [5] Calixarenes, [6] π‐extended tetrathiafulvalene derivatives, [7] and metalloporphyrins [8] are commonly used as electron‐rich hosts for electron‐poor fullerenes, because of electronic and structural complementarity. Single‐walled carbon nanotubes[ 9 , 10 ] featuring a one‐dimensional hollow channel have also been extensively explored as π‐hosts toward fullerenes, forming the so‐called “nanopeapods”. [11] It was found that the orientation of fullerene molecules in the tubular hollow space is the key factor determining the electronic and charge‐transport properties of the nanopeapods. [12] From a supramolecular perspective, carbon nanotubes function as templates for the alignment of fullerenes inside the channels, enabling a controlled assembly of encapsulated fullerenes into one‐dimensional wires.
Cycloparaphenylenes (CPPs) are tube‐shaped radially π‐conjugated molecular loops comprised of distorted para‐linked phenylene rings. [13] They represent the shortest segments of armchair carbon nanotubes. [14] Their synthesis was elusive until the landmark achievement of [9]‐, [12]‐, and [18]CPP in 2008. [15] The development of advanced synthetic methodologies [16] has enabled size‐selective syntheses of [n]CPPs (n≥5) and more complex CPP‐derived architectures including lemniscates,[ 17 , 18 ] double nanohoops, [19] propellers, [20] cylinders, [21] cages, [22] and catenanes. [23] In addition to CPPs bearing phenylene as a repeated panel, π‐extended aromatics such as naphthalene, [24] anthracene, [25] phenanthrene, [26] dibenzopentalene, [27] pyrene, [28] chrysene, [29] anthanthrene, [30] dibenzochrysene, [31] rubicene [32] and hexabenzocoronene, [33] have been exploited to construct a variety of hydrocarbon nanohoops. [34] A unique structural feature of CPPs and their π‐extended analogs is the radial cavity that enables them to encapsulate fullerenes and small polycyclic arenes. [35] Their concave aromatic framework is complementary to the convex surface of fullerenes, which results in strong π‐π interactions. Additionally, the associated complexes are stabilized by means of electronic complementarity between the electron‐rich carbon nanohoops and electron‐deficient fullerenes. [10]CPP was the first carbon nanohoop shown to have a suitably sized cavity for binding C60. [36] The solid‐state structure of the 1 : 1 [10]CPP⊃C60 complex revealed a close concave‐convex π‐π interaction between the two components [37] and the quenching of fluorescence of [10]CPP indicated strong electronic communication between the host and the guest. [36] Compared to [10]CPP and C60, π‐π interactions are significantly enhanced in the complexes of π‐extended nanohoops and C60, as implied by the larger association constants.[ 38 , 39 ]
Fullerenes and their derivatives are typical n‐type semiconductors. Among all pristine fullerenes, only C60 and C70 are synthetically available in large quantities. C60 holds the record of the highest charge‐carrier mobility among fullerenes, with a single‐crystal mobility of 11 cm2 V−1 s−1[40] due to its high symmetry and isotropic properties in the solid state. Despite these desirable features, fullerene‐based semiconductor devices are not stable in air because electron transport produces free radicals that are reactive toward oxygen and water. As a result, rigorous exclusion of oxygen and water contamination is required for the device fabrication and measurements. In this regard, the host‐guest supramolecular assembly of carbon nanohoops and fullerenes offers an opportunity to 1) protect the active organic layer from oxygen and water and 2) improve the solubility, thus enabling solution‐processed fabrication of electronic devices. In this endeavor, the packing structure of the nanohoop‐fullerene complexes is of critical importance because an isolated or a random arrangement of fullerene molecules is not suitable for achieving effective charge transport. In this Review article, we discuss and propose all the examples of host‐guest complexes between hydrocarbon nanohoops and fullerenes, where the inclusion fullerene guests align into discrete supramolecular wires – potential materials for organic semiconductors with enhanced conductivity performance.
Single‐Nanohoop Systems
Since the discovery of the host function of [10]CPP toward C60,[ 36 , 37 ] [10]CPP has been investigated as a receptor in supramolecular chemistry. For example, [10]CPP acts well as a supramolecular template in the synthesis of [10]CPP‐fullerene [2]rotaxanes. [41] In contrast to C60, which displays a spherical shape with a diameter of 0.71 nm, its analog C70 has an ellipsoidal shape. The size of C70 with a long and short axis of 0.796 nm and 0.712 nm, respectively, makes it an ideal guest for encapsulation by [11]CPP (1 a, Figure 1a). The solid‐state structure of a 1 : 1 1 a⊃C70 complex (Figure 2) revealed that C70 adopted a “standing” orientation with its long axis aligned within the [11]CPP plane. [42] The large association constant of 4.7×104 M−1 in toluene indicates a strong concave‐convex π‐π interaction between 1 a and C70. Despite a herringbone packing arrangement of the complex, the encapsulated C70 molecules are highly ordered, forming zigzag C70 wires (Figure 2) with the shortest C−C distances between the neighboring C70 molecules of 3.187 Å and 3.253 Å. Such supramolecular array of C70 guests opens up the possibility employing this cocrystal in semiconductive materials.
Figure 1.

Structures of selected hydrocarbon nanohoops featuring a) one and b) two nanoring‐cycles as supramolecular hosts for fullerenes.
Figure 2.

Packing in the solid‐state structure of a 1 : 1 1 a⊃C70 complex, d 1=3.187 Å, d 2=3.253 Å (CCDC no. 941083). Disordered solvent molecules are omitted for clarity.
Given the cavity size of 1 a that allows accommodation of C70, a cyclic heptamer of naphthalene (1 b, Figure 1a) with a diameter (1.47 nm) similar to that of 1 a (1.51 nm) is another suitable host for C70. The extended π‐surface gives rise to a tighter complexation between 1 b and C70, as implied by the larger association constant of 1.6×108 M−1 in toluene. [43] A standing orientation of C70 is found also in the solid‐state structure of the 1 : 1 1 b⊃C70 complex (Figure 3). Similarly to the case of the 1 a⊃C70 complex, C70 molecules in 1 b⊃C70 are also aligned into one‐dimensional nanowires with the shortest C−C distances of 3.595 Å and 3.143 Å between the C70 molecules. The 1 b⊃C70 complex is therefore also a potential candidate for charge‐transport materials.
Figure 3.

Packing in the solid‐state structure of a 1 : 1 1 b⊃C70 complex, d 1=3.595 Å, d 2=3.143 Å (CCDC no. 1875818).
The void of [10]CPP was shown to have an ideal diameter (1.38 nm) for binding C60 with an association constant of 6.0×103 M−1 in 1,2‐dichlorobenzene. [36] Benefitting from a larger π‐surface of the aromatic panels and similar void shape (diameter of 1.39 nm), [4]cyclochrysenylene (1 c, Figure 1a) exhibited an incredibly strong affinity toward C60 with an association constant of 4.0×109 M−1 in 1,2‐dichlorobenzene. [39a] The molecules of the 1 : 1 1 c⊃C60 complex stack in a columnar assembly, resulting in a linear supramolecular wire of the inclusion C60 molecules (Figure 4). The large C−C distance of 6.340 Å between the neighboring C60 molecules impedes, however, an effective charge transport that is a decisive factor in organic semiconductors. The π‐extended analog of 1 c, [4]cycloanthanthrenylene (1 d, Figure 1a) has a cavity size identical to 1 c with a diameter of 1.40 nm. As expected, 1 d forms even a tighter complex with C60, with the association constant reaching 5.0×109 M−1 in 1,2‐dichlorobenzene. [44] Given the strong complexation between 1 c/1 d and C60, a dumbbell‐shaped C60 dimer, C120, [45] is a suitable double‐fullerene guest for encapsulation by two host molecules of 1 c or 1 d to form a 2 : 1 complex.[ 46 , 47 ] The inclusion C120 guests of these two 2 : 1 complexes are, however, arranged randomly in an isolated fashion. Interestingly, in the 1 : 1 1 d⊃C120 complex, C120 molecules adopted two orientations and were uniformly arranged in each column (Figure 5). Within one column, the C120 molecules are aligned to form zigzag C60 wires, where the C60 molecules are – in an alternating fashion – either connected by two C−C single bonds or in close van der Waals contact (d=3.280 Å).
Figure 4.
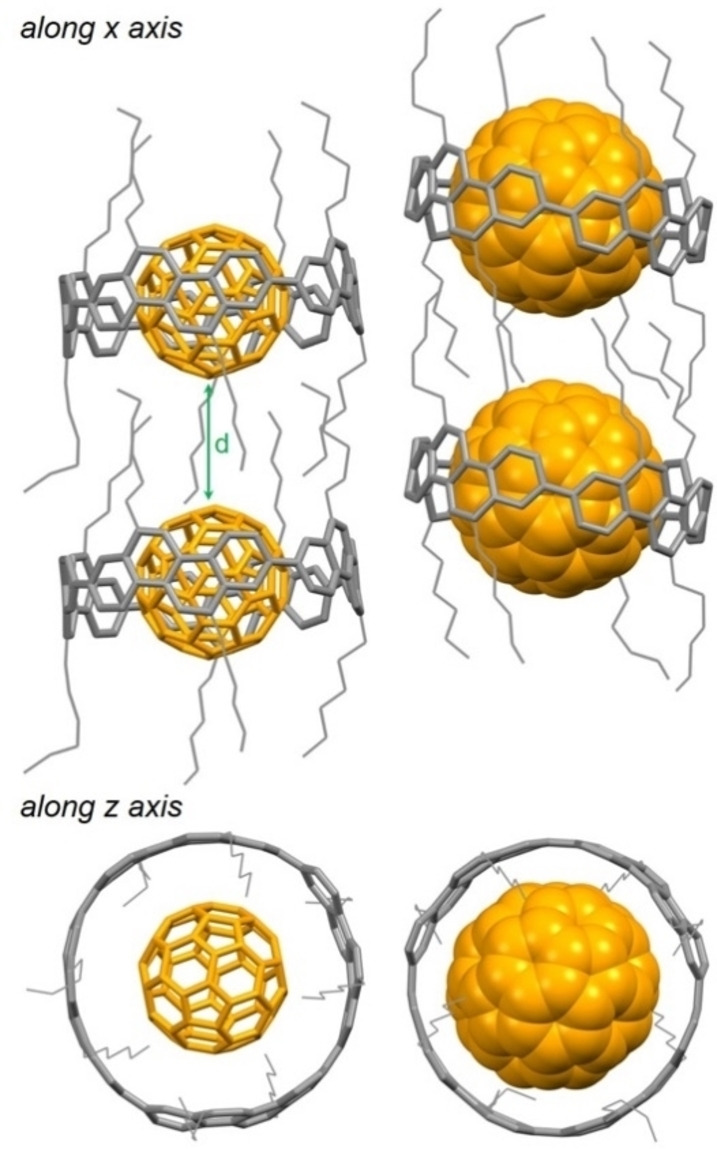
Packing in the solid‐state structure of a 1 : 1 1 c⊃C60 complex, d=6.340 Å (CCDC no. 993074).
Figure 5.

Packing in the solid‐state structures of a 1 : 1 1 d⊃C120 complex, d=3.280 Å (CCDC no. 1573516).
Double‐Nanohoop Systems
Nanohoop systems that feature two nanoring‐cycles are promising host candidates to form 1 : 2 complexes with two guest molecules.[ 17 , 18 , 19 ] To date, only two nanohoop hosts were found to form a 1 : 2 host‐guest complex with a fullerene guest: a nanotube‐like CPP dimer (2 a, Figure 1b) that incorporates two C60 molecules [19c] and a conjoint bis[10]CPP (2 b, Figure 1b) that interacts with two [6,6]‐phenyl‐C61‐butyric acid methyl ester (PCBM) molecules. [19d] Because, however, the solid‐state structures of these 1 : 2 complexes have not yet been validated by means of single‐crystal X‐ray diffraction (SC‐XRD), the spatial arrangement of the fullerene guests in the solid state is unknown.
Recently, we synthesized a peropyrene‐bridged double nanohoop system CPP‐PP (2 c, Figure 1b) and by means of SC‐XRD unequivocally confirmed its all‐sp2‐framework featuring two large round‐shaped cavities. [18a] The formation of host‐guest complexes with fullerenes could not, however, be observed, which indicates that the shape of the cavities is not a perfect match. Structural adjustment was implemented to modify these voids and a new member of the double‐nanohoop family, CPP‐ b PP (2 d, Figure 1b) was devised and synthesized. [18b] In this system, the nanoring‐cycles are connected at the bay‐regions of the central peropyrene segment and the two oval‐shaped voids have a slightly different shape in the solid state depending on the crystallization conditions. Unexpectedly, no binding interaction between 2 d and C60/C70 was detected in solution, possibly due to the cavity‐reorganization energy that counteracts the host‐guest complexation energy. Nevertheless, the binding behavior was clearly observed in the solid state. Surprisingly, a 1 : 1 2 d⊃C60 complex, instead of a 1 : 2 complex, was revealed by SC‐XRD. Strong concave‐convex π‐π interaction is found between 2 d and C60 as indicated by the shortest intermolecular C−C distance of 2.86 Å. The other interesting feature of the 2 d⊃C60 complex is its unique lamellar packing motif (Figure 6a) that is unprecedented for all nanohoop‐fullerene complexes, and nanohoops in general. The tight lamellar packing motif is the reason for the formation of the 1 : 1 complex and not 1 : 2. If a 1 : 2 2 d⊃C60 complex would form, the zigzag C60 wires would be assembled (Figure 6b). But in this manner, the close contacts between the neighboring C60 molecules (shortest C−C distance of 2.241 Å) would result in significant steric repulsion, assuming that the packing and interlayer distance would remain the same. To potentially achieve these zigzag supramolecular wires of the C60 guests, C120 represents an ideal alternative for encapsulation by 2 d because the length of the bridged C−C single bonds is 1.575 Å, shorter than the distance between C60 molecules in the packing structure of the hypothetical 1 : 2 2 d⊃C60 complex (Figure 6b). At the same time, C120 is able to accomplish the full occupancy of the two cavities of 2 d, thus leading to the formation of one‐dimensional zigzag C60 wires. On the other hand, the electronic communication between 2 d and C120 might help to reduce the interlayer distance between 2 d molecules and thus improve the change‐transport properties.
Figure 6.

a) Packing in the solid‐state structure of a 1 : 1 2 d⊃C60 complex (CCDC no. 2104609). Please note that the distribution of C60 shown here is only a representative example, and C60 can randomly reside in one of the two cavities, resulting in an average ratio of 1 : 1. Disordered solvent molecules are omitted for clarity. Hypothesized in this Article are the imaginary packing in the solid‐state structure of b) a 1 : 2 2 d⊃C60 complex, d1 and d2 indicate the shortest C−C distance between two fullerene molecules inside the green and red box, respectively, d 1=3.295 Å, d 2=2.241 Å, and c) a 1 : 1 2 d⊃C120 complex, bridged C−C single bond (highlighted in red) of 1.575 Å in C120, given the packing and interlayer distance would stay the same.
Even though the solid‐state structures of the surveyed nanohoop‐fullerene complexes endow them with a potential to be employed as organic semiconductors and components of solar cells, [48] their suitability for fabrication of semiconductive devices is scarcely assessed. In addition to the challenges associated with obtaining single crystals that are qualified for SC‐XRD, solvent molecules encapsulated in the crystals are another concern because the volatile behavior of inclusion solvents can disrupt the packing structure and thus the supramolecular arrangement of the fullerene wires. In a recent study, semiconductive performance of a [10]CPP polymer that can bind multiple C60 molecules was evaluated. [49] The solid‐state structure of this complex is, however, elusive, which impedes an in‐depth understanding of the structure‐property relationship. In addition, the norbornene‐embedded CPP polymers are potential hosts for fullerenes or metallofullerenes and the corresponding complexes are of interest as organic materials for applications in electronics and spintronics. [50]
Deposition Numbers 941083, 1875818, 993074, 1573516, and 2104609 contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
Conclusion and Outlook
The structural feature of CPPs and their π‐extended and more complex nanohoop analogs makes them ideal supramolecular hosts for encapsulation of fullerenes. The well‐developed synthetic tools contribute to the diversity of the available nanohoop architectures, which drives a considerable interest in their host‐guest chemistry. Although a variety of carbon nanohoops have been shown to bind fullerenes, the solid‐state structures of the complexes are not often available, presumably owing to the formidable challenge in obtaining single crystals qualified for XRD and high disorder of inclusion fullerenes. These obstacles impede the in‐depth understanding of the packing motif and materials application. Among some of the instances, where the solid‐state structures of the complexes were disclosed, the research focused on the dynamic behavior of the fullerene guests inside the carbon‐nanohoop hosts. However, applications of these complexes in functional materials have been far less exploited. In this Review, we summarized and proposed all the examples of nanohoop‐fullerene complexes, where the encapsulated fullerene guests align to form supramolecular linear or zigzag nanowires with close π‐π contacts between discrete fullerene molecules. Such fullerene‐based supramolecular wires hold potential for applications in organic semiconductors[ 5 , 51 ] and have rarely been explored within the nanohoop‐fullerene systems. The capability of carbon‐nanohoop hosts to act as templates that regulate the packing arrangement of the fullerene guests can aid and abet charge transfer between the components in close contact and thus enhance the semiconductive performance. In addition, the concave‐convex shape complementarity between carbon nanohoops and fullerenes can assist in excluding water and oxygen perturbations in the charge‐transport process. We look forward to the future development of nanohoop hosts that can form tight complexes with fullerenes aligned into supramolecular wires and their use in semiconducting and solar‐cell materials.
Conflict of interest
The authors declare no conflict of interest.
Biographical Information
Yong Yang received his Bachelor's degree (2012) from Soochow University and PhD (2017) from the Chinese University of Hong Kong under the direction of Professor Qian Miao. Then he worked with Professor Hiroyuki Isobe as a postdoctoral fellow at the University of Tokyo. In 2019, he moved to the University of Zurich and worked with Professor Michal Juríček as a postdoctoral fellow. As of 2022, he will work as a faculty member in Southeast University. His research interests focus on the design and synthesis of novel polycyclic aromatic hydrocarbons including curved π‐molecules, carbon nanohoops and nanobelts, and their functional applications in supramolecular chemistry and materials science.

Biographical Information
Michal Juríček received his Master's degree (2005) from the Comenius University in Bratislava and his PhD (2011) from the Radboud University Nijmegen mentored by Professor Alan Rowan. After a postdoctoral stay in the group of Professor Sir Fraser Stoddart at Northwestern University, United States, he started his independent research career at the University of Basel hosted in the group of Professor Marcel Mayor. From 2017, he is an assistant professor at the University of Zurich. His group designs, synthesizes, and investigates functional organic molecules derived from open‐shell graphene fragments for applications in spintronics.

Acknowledgements
This project received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (Grant Agreement No. 716139) and the Swiss National Science Foundation (SNSF, PP00P2_198900). We are grateful to Prof. Sota Sato (University of Tokyo) for his help with analyzing the disordered structure of the 1 : 1 1 d⊃C120 complex (Figure 5). Open access funding provided by Universitat Zurich.
Y. Yang, M. Juríček, ChemPlusChem 2022, 87, e202100468.
Contributor Information
Dr. Yong Yang, Email: yong.yang@chem.uzh.ch.
Prof. Michal Juríček, Email: michal.juricek@chem.uzh.ch.
References
- 1.
- 1a. Diederich F., Gómez-López M., Chem. Soc. Rev. 1999, 28, 263–277; [Google Scholar]
- 1b. Pérez E. M., Martín N., Chem. Soc. Rev. 2015, 44, 6425–6433; [DOI] [PubMed] [Google Scholar]
- 1c. Kawase T., Kurata H., Chem. Rev. 2006, 106, 5250–5273; [DOI] [PubMed] [Google Scholar]
- 1d. Toyota S., Tsurumaki E., Chem. Eur. J. 2019, 25, 6878–6890. [DOI] [PubMed] [Google Scholar]
- 2.
- 2a. Kroto H. W., Heath J. R., O'Brien S. C., Curl R. F., Smalley R. E., Nature 1985, 318, 162–163; [Google Scholar]
- 2b. Krätschmer W., Lamb L. D., Fostiropoulos K., Huffman D. R., Nature 1990, 347, 354–358. [Google Scholar]
- 3. Zhang Y., Murtaza I., Meng H., J. Mater. Chem. C 2018, 6, 3514–3537. [Google Scholar]
- 4. Ganesamoorthy R., Sathiyan G., Sakthivel P., Sol. Energy Mater. Sol. Cells 2017, 161, 102–148. [Google Scholar]
- 5. Barnes J. C., Dale E. J., Prokofjevs A., Narayanan A., Gibbs-Hall I. C., Juríček M., Stern C. L., Sarjeant A. A., Botros Y. Y., Stupp S. I., Stoddart J. F., J. Am. Chem. Soc. 2015, 137, 2392–2399. [DOI] [PubMed] [Google Scholar]
- 6. Georghiou P. E., in Calixarenes and Beyond (Eds.: Neri P., Sessler J. L., Wang M.-X.), Springer, Cham, 2016, Ch. 33, pp. 879–919. [Google Scholar]
- 7.
- 7a. Pérez E. M., Illescas B. M., Ángeles Herranz M., Martín N., New J. Chem. 2009, 33, 228–234; [Google Scholar]
- 7b. Jana A., Ishida M., Park J. S., Bähring S., Jeppesen J. O., Sessler J. L., Chem. Rev. 2017, 117, 2641–2710. [DOI] [PubMed] [Google Scholar]
- 8.
- 8a. Tashiro K., Aida T., Chem. Soc. Rev. 2007, 36, 189–197; [DOI] [PubMed] [Google Scholar]
- 8b. Zieleniewska A., Lodermeyer F., Roth A., Guldi D. M., Chem. Soc. Rev. 2018, 47, 702–714. [DOI] [PubMed] [Google Scholar]
- 9.
- 9a. Dai H., Acc. Chem. Res. 2002, 35, 1035–1044; [DOI] [PubMed] [Google Scholar]
- 9b. Carlson L. J., Krauss T. D., Acc. Chem. Res. 2008, 41, 235–243. [DOI] [PubMed] [Google Scholar]
- 10. Iijima S., Nature 1991, 354, 56–58. [Google Scholar]
- 11. Smith B. W., Monthioux M., Luzzi D. E., Nature 1998, 396, 323–324. [Google Scholar]
- 12. Kitaura R., Shinohara H., Chem. Asian J. 2006, 1, 646–655. [DOI] [PubMed] [Google Scholar]
- 13.Selected reviews of CPPs:
- 13a. Omachi H., Segawa Y., Itami K., Acc. Chem. Res. 2012, 45, 1378–1389; [DOI] [PubMed] [Google Scholar]
- 13b. Lewis S. E., Chem. Soc. Rev. 2015, 44, 2221–2304; [DOI] [PubMed] [Google Scholar]
- 13c. Golder M. R., Jasti R., Acc. Chem. Res. 2015, 48, 557–566; [DOI] [PubMed] [Google Scholar]
- 13d. Darzi E. R., Jasti R., Chem. Soc. Rev. 2015, 44, 6401–6410; [DOI] [PubMed] [Google Scholar]
- 13e. Wu D., Cheng W., Ban X., Xia J., Asian J. Org. Chem. 2018, 7, 2161–2181. [Google Scholar]
- 14.
- 14a. Omachi H., Nakayama T., Takahashi E., Segawa Y., Itami K., Nat. Chem. 2013, 5, 572–576; [DOI] [PubMed] [Google Scholar]
- 14b. Segawa Y., Yagi A., Matsui K., Itami K., Angew. Chem. Int. Ed. 2016, 55, 5136–5158; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 5222–5245. [Google Scholar]
- 15. Jasti R., Bhattacharjee J., Neaton J. B., Bertozzi C. R., J. Am. Chem. Soc. 2008, 130, 17646–17647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mirzaei S., Castro E., Sánchez R. H., Chem. Eur. J. 2021, 27, 8642–8655. [DOI] [PubMed] [Google Scholar]
- 17.
- 17a. Huang Z.-A., Chen C., Yang X.-D., Fan X.-B., Zhou W., Tung C.-H., Wu L.-Z., Cong H., J. Am. Chem. Soc. 2016, 138, 11144–11147; [DOI] [PubMed] [Google Scholar]
- 17b. Xu W., Yang X.-D., Fan X.-B., Wang X., Tung C.-H., Wu L.-Z., Cong H., Angew. Chem. Int. Ed. 2019, 58, 3943–3947; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 3983–3987; [Google Scholar]
- 17c. Senthilkumar K., Kondratowicz M., Lis T., Chmielewski P. J., Cybińska J., Zafra J. L., Casado J., Vives T., Crassous J., Favereau L., Stępień M., J. Am. Chem. Soc. 2019, 141, 7421–7427; [DOI] [PubMed] [Google Scholar]
- 17d. Schaub T. A., Prantl E. A., Kohn J., Bursch M., Marshall C. R., Leonhardt E. J., Lovell T. C., Zakharov L. N., Brozek C. K., Waldvogel S. R., Grimme S., Jasti R., J. Am. Chem. Soc. 2020, 142, 8763–8775. [DOI] [PubMed] [Google Scholar]
- 18.
- 18a. Yang Y., Blacque O., Sato S., Juríček M., Angew. Chem. Int. Ed. 2021, 60, 13529–13535; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18b. Yang Y., Huangfu S., Sato S., Juríček M., Org. Lett. 2021, 23, 7943–7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.
- 19a. Xia J., Golder M. R., Foster M. E., Wong B. M., Jasti R., J. Am. Chem. Soc. 2012, 134, 19709–19715; [DOI] [PubMed] [Google Scholar]
- 19b. Ishii Y., Matsuura S., Segawa Y., Itami K., Org. Lett. 2014, 16, 2174–2176; [DOI] [PubMed] [Google Scholar]
- 19c. Li K., Xu Z., Deng H., Zhou Z., Dang Y., Sun Z., Angew. Chem. Int. Ed. 2021, 61, 7649–7653; [DOI] [PubMed] [Google Scholar]
- 19d. Zhang X., Shi H., Zhuang G., Wang S., Wang J., Yang S., Shao X., Du P., Angew. Chem. Int. Ed. 2021, 60, 17368–17372. [DOI] [PubMed] [Google Scholar]
- 20. Li P., Zakharov L. N., Jasti R., Angew. Chem. Int. Ed. 2017, 56, 5237–5241; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 5321–5325. [Google Scholar]
- 21.
- 21a. Sun Z., Ikemoto K., Fukunaga T. M., Koretsune T., Arita R., Sato S., Isobe H., Science 2019, 363, 151–155; [DOI] [PubMed] [Google Scholar]
- 21b. Ikemoto K., Yang S., Naito H., Kotani M., Sato S., Isobe H., Nat. Commun. 2020, 11, 1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.
- 22a. Matsui K., Segawa Y., Namikawa T., Kamada K., Itami K., Chem. Sci. 2013, 4, 84–88; [Google Scholar]
- 22b. Matsui K., Segawa Y., Itami K., J. Am. Chem. Soc. 2014, 136, 16452–16458; [DOI] [PubMed] [Google Scholar]
- 22c. Kayahara E., Iwamoto T., Takaya H., Suzuki T., Fujitsuka M., Majima T., Yasuda N., Matsuyama N., Seki S., Yamago S., Nat. Commun. 2013, 4, 2694; [DOI] [PubMed] [Google Scholar]
- 22d. Cui S., Zhuang G., Lu D., Huang Q., Jia H., Wang Y., Yang S., Du P., Angew. Chem. Int. Ed. 2018, 57, 9330–9335; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 9474–9479. [Google Scholar]
- 23.
- 23a. Zhang W., Abdulkarim A., Golling F. E., Räder H. J., Müllen K., Angew. Chem. Int. Ed. 2017, 56, 2645–2648; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 2689–2692; [Google Scholar]
- 23b. Fan Y.-Y., Chen D., Huang Z.-A., Zhu J., Tung C.-H., Wu L.-Z., Cong H., Nat. Commun. 2018, 9, 3037; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23c. Segawa Y., Kuwayama M., Hijikata Y., Fushimi M., Nishihara T., Pirillo J., Shirasaki J., Kubota N., Itami K., Science 2019, 365, 272–276; [DOI] [PubMed] [Google Scholar]
- 23d. Segawa Y., Kuwayama M., Itami K., Org. Lett. 2020, 22, 1067–1070. [DOI] [PubMed] [Google Scholar]
- 24.
- 24a. Sun Z., Sarkar P., Suenaga T., Sato S., Isobe H., Angew. Chem. Int. Ed. 2015, 54, 12800–12804; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 12991–12995; [Google Scholar]
- 24b. Sun Z., Suenaga T., Sarkar P., Sato S., Kotani M., Isobe H., Proc. Natl. Acad. Sci. USA 2016, 113, 8109–8114; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24c. Yagi A., Segawa Y., Itami K., J. Am. Chem. Soc. 2012, 134, 2962–2965; [DOI] [PubMed] [Google Scholar]
- 24d. Okada K., Yagi A., Segawa Y., Itami K., Chem. Sci. 2017, 8, 661–667; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24e. Jia H., Gao Y., Huang Q., Cui S., Du P., Chem. Commun. 2018, 54, 988–991. [DOI] [PubMed] [Google Scholar]
- 25. Wang J., Zhuang G., Chen M., Lu D., Li Z., Huang Q., Jia H., Cui S., Shao X., Yang S., Du P., Angew. Chem. Int. Ed. 2020, 59, 1619–1626; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 1636–1643. [Google Scholar]
- 26. Sarkar P., Sun Z., Tokuhira T., Kotani M., Sato S., Isobe H., ACS Cent. Sci. 2016, 2, 740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wössner J. S., Wassy D., Weber A., Bovenkerk M., Hermann M., Schmidt M., Esser B., J. Am. Chem. Soc. 2021, 143, 12244–12252. [DOI] [PubMed] [Google Scholar]
- 28. Iwamoto T., Kayahara E., Yasuda N., Suzuki T., Yamago S., Angew. Chem. Int. Ed. 2014, 53, 6430–6434; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 6548–6552. [Google Scholar]
- 29.
- 29a. Hitosugi S., Nakanishi W., Yamasaki T., Isobe H., Nat. Commun. 2011, 2, 492; [Google Scholar]
- 29b. Hitosugi S., Yamasaki T., Isobe H., J. Am. Chem. Soc. 2012, 134, 12442–12445. [DOI] [PubMed] [Google Scholar]
- 30. Matsuno T., Kamata S., Hitosugi S., Isobe H., Chem. Sci. 2013, 4, 3179–3183. [Google Scholar]
- 31. Kogashi K., Matsuno T., Sato S., Isobe H., Angew. Chem. Int. Ed. 2019, 58, 7385–7389; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 7463–7467. [Google Scholar]
- 32. Hitosugi S., Sato S., Matsuno T., Koretsune T., Arita R., Isobe H., Angew. Chem. Int. Ed. 2017, 56, 9106–9110; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 9234–9238. [Google Scholar]
- 33. Lu D., Zhuang G., Wu H., Wang S., Yang S., Du P., Angew. Chem. Int. Ed. 2017, 56, 158–162; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 164–168. [Google Scholar]
- 34. Hermann M., Wassy D., Esser B., Angew. Chem. Int. Ed. 2021, 60, 15743–15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.
- 35a. Xu Y., von Delius M., Angew. Chem. Int. Ed. 2020, 59, 559–573; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 567–582; [Google Scholar]
- 35b. Lu D., Huang Q., Wang S., Wang J., Huang P., Du P., Front. Chem. 2019, 7, 668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iwamoto T., Watanabe Y., Sadahiro T., Haino T., Yamago S., Angew. Chem. Int. Ed. 2011, 50, 8342–8344; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 8492–8494. [Google Scholar]
- 37. Xia J., Bacon J. W., Jasti R., Chem. Sci. 2012, 3, 3018–3021. [Google Scholar]
- 38. Huang Q., Zhuang G., Jia H., Qian M., Cui S., Yang S., Du P., Angew. Chem. Int. Ed. 2019, 58, 6244–6249; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 6310–6315. [Google Scholar]
- 39.
- 39a. Isobe H., Hitosugi S., Yamasaki T., Iizuka R., Chem. Sci. 2013, 4, 1293–1297; [Google Scholar]
- 39b. Sato S., Yamasaki T., Isobe H., Proc. Natl. Acad. Sci. USA 2014, 111, 8374–8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li H., C.-K. Tee B., Cha J. J., Cui Y., Chung J. W., Lee S. Y., Bao Z., J. Am. Chem. Soc. 2012, 134, 2760–2765. [DOI] [PubMed] [Google Scholar]
- 41. Xu Y., Kaur R., Wang B., Minameyer M. B., Gsänger S., Meyer B., Drewello T., Guldi D. M., von Delius M., J. Am. Chem. Soc. 2018, 140, 13413–13420. [DOI] [PubMed] [Google Scholar]
- 42. Iwamoto T., Watanabe Y., Takaya H., Haino T., Yasuda N., Yamago S., Chem. Eur. J. 2013, 19, 14061–14068. [DOI] [PubMed] [Google Scholar]
- 43. Sun Z., Mio T., Okada T., Matsuno T., Sato S., Kono H., Isobe H., Angew. Chem. Int. Ed. 2019, 58, 2040–2044; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 2062–2066. [Google Scholar]
- 44. Matsuno T., Sato S., Iizuka R., Isobe H., Chem. Sci. 2015, 6, 909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang G.-W., Komatsu K., Murata Y., Shiro M., Nature 1997, 387, 583–586. [Google Scholar]
- 46. Matsuno T., Sato S., Yokoyama A., Kamata S., Isobe H., Angew. Chem. Int. Ed. 2016, 55, 15339–15343; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 15565–15569. [Google Scholar]
- 47. Matsuno T., Kamata S., Sato S., Yokoyama A., Sarkar P., Isobe H., Angew. Chem. Int. Ed. 2017, 56, 15020–15024; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 15216–15220. [Google Scholar]
- 48. Tang Y., Li J., Du P., Zhang H., Zheng C., Lin H., Du X., Tao S., Org. Electron. 2020, 83, 105747. [Google Scholar]
- 49. Wang S., Li X., Zhang X., Huang P., Fang P., Wang J., Yang S., Wu K., Du P., Chem. Sci. 2021, 12, 10506–10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Maust R. L., Li P., Shao B., Zeitler S. M., Sun P. B., Reid H. W., Zakharov L. N., Golder M. R., Jasti R., ACS Cent. Sci. 2021, 7, 1056–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yokoi H., Hiraoka Y., Hiroto S., Sakamaki D., Seki S., Shinokubo H., Nat. Commun. 2015, 6, 8215. [DOI] [PMC free article] [PubMed] [Google Scholar]


