ABSTRACT
Background and Aims:
Percutaneous dilational tracheostomy (PDT) is a common procedure in intensive care unit (ICU) patients requiring long-term mechanical ventilation. PDT has gradually replaced surgical tracheostomy because it is associated with minimal invasiveness, reduced bleeding and simplicity in technique.This study was conducted to compare ultrasound-guided PDT versus conventional tracheostomy in terms of duration of the procedure, number of passes and immediate peri-procedural complications.
Methods:
A total of 72 patients with clinical indications of tracheostomy were recruited. A total of 12 patients met the exclusion criteria. The remaining were randomly assigned into two groups of 30 each: Group A (Landmark) with traditional anatomical landmark and Group B (USG) with real-time ultrasound guidance. Puncture positions were recorded with bronchoscopy. Midline deviation was captured on a bronchoscopy image using a protractor. Data on procedural safety and efficacy were also collected.
Results:
Group B had significantly fewer cases of midline deviation (11.33 ± 9.51) in comparison to Group A (16.60 ± 12.31). Trials > 2 were equal to 11 in Group A and 2 in Group B. However, the duration of the procedure was higher in Group B (20.07 ± 3.25 min) as compared to Group A (15.20 ± 3.71 min). Peri-procedural and post-procedural complications were also higher in the Landmark group.
Conclusion:
Ultrasound-guided PDT showed superiority over landmark PDT in terms of less number of trials, midline puncture and fewer complications. However, it took a little longer to perform USG-guided PDT.
Keywords: Critical illness, tracheostomy, ultrasound
INTRODUCTION
Critically ill patients often receive tracheostomy for long-term ventilatory support. Percutaneous dilational tracheostomy (PDT) has now become a safe alternative to conventional surgical tracheostomy. PDT being a bedside procedure is safer, less invasive with fewer complications.[1] Largely, PDT is a safe procedure, but some complications like higher incidences of de-cannulation and obstruction, especially in the hands of an inexperienced operator have been observed. Major complications like bleeding and death though rare have also been reported with PDT.[2,3,4,5,6]
Many assisting tools have been suggested to guide the procedure of PDT and decrease the incidence of complications such as bleeding and malposition of the tube. The availability of bedside ultrasonography (USG) in modern intensive care settings seems to be a promising alternative. USG is nowadays becoming an important diagnostic, monitoring and guiding tool in the critical care unit.[7,8] Pre-procedural and real-time intra-procedural ultrasound guidance have been advocated as potential tools to further improve the safety and efficacy of the procedure.[7-10] The potential advantages of ultrasound include the ability to identify the cervical vasculature, help identify the most appropriate location for the tracheal puncture site, and guide the needle insertion into the trachea.[9] The visualisation of the needle path up to the anterior tracheal wall further decreases the risk of injury. USG for PDT is useful in technically difficult situations like morbid obesity, anticipated difficult airway and in patients with a cervical spine injury. The use of USG before, during, and after PDT plays an instrumental role in landmarking, identifying vulnerable structures, choosing the appropriate tracheostomy tube size, and providing real-time guidance for needle penetration.[11,12,13,14]
Bronchoscopic guidance during PDT is an additional safety adjunct, which enables the confirmation of midline puncture of the trachea and allows the visualisation of the posterior wall of the trachea for injuries.[15] Newer tools and methods would provide a better understanding of the anatomy of the neck to guide PDT procedures and improve their safety and would always be welcomed. Literature has insufficient high-quality evidence comparing landmark versus USG-guided PDT.
METHODS
The study was a prospective randomised controlled trial conducted over 18 months (April 2019 to December 2020) in a tertiary care institute. Critically ill patients aged more than 20 years, who needed tracheostomy after prolonged ventilator support for various types of illness were included. Non-consenting patients, in extremes of age (<20 years or >80 years), on ventilator support for less than 48 h, with neck swelling/mass/abnormal neck anatomy and those with coagulopathy were excluded. The study was conducted after ethics committee approval [468/IEC/IGIMS] and registration in the Clinical Trial Registry of India [CTRI/2019/04/018805]. The principles of the Declaration of Helsinki were followed during the conduct of the study.
Out of 72 eligible patients, 8 non-consented and for 4 patients, the plan of tracheostomy was cancelled/surgical tracheostomy was done after recruitment [Figure 1]. The patients were divided randomly using a computer-generated table into two groups of 30 each as Group-A: PDT was performed in patients with conventional anatomical landmarks; Group-B: PDT was performed in patients under ultrasonographic guidance.
Figure 1.
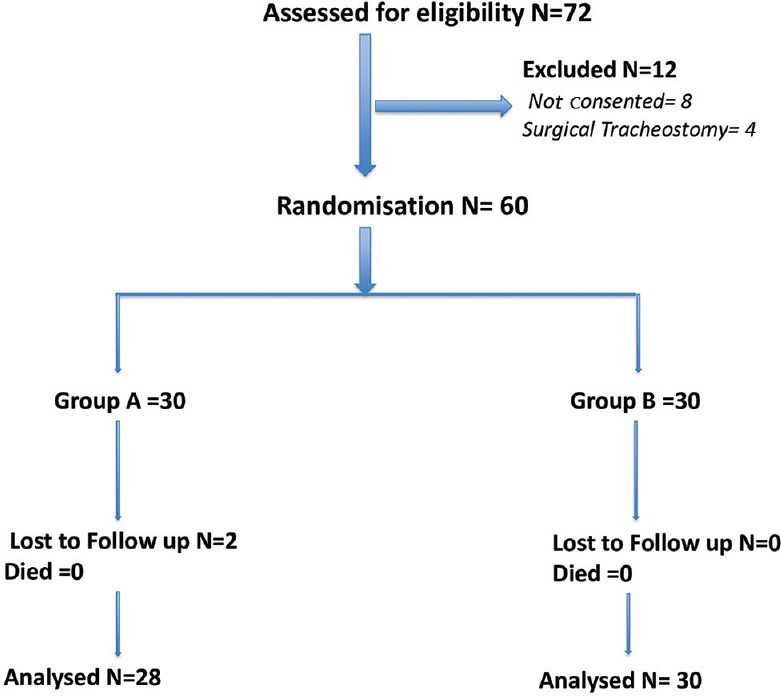
Consolidated standards of reporting trials (CONSORT): Flow Diagram showing recruitment of patients. N:Number
After obtaining written and informed consent from the next of kin, the selected patients in both the groups underwent PDT using the dilatation technique using the PortexBlue Line Ultra percutaneous tracheostomy kit. A portable ultrasound system (Sonosite Turbo M) with a linear array probe (5 Hz) was used. Transverse/axial real-time images of the upper airway starting from the hyoid bone down to the thyroid gland and its isthmus were obtained. Important structures such as blood vessels and the thyroid gland were identified in the tracheostomy field in all the patients. Sliding the USG probe (in transverse view) caudally, the cricoid cartilage was identified within the anterior wall of the larynx caudal to the cricothyroid membrane by its relatively large acoustic shadow seen as a large hump. The posterior surface of the anterior wall was identified by a bright air mucosal interface [Figure 2]. The linear probe was further moved caudally to identify the tracheal rings as a thin acoustic shadow within the anterior wall of the trachea. Then, the probe was moved 90° to obtain a longitudinal view of the cricoid cartilage, and the space between the first and second or second and third cartilage at the midline of the trachea was identified. The mode of imaging was set to maximal resolution, and the depth of imaging was adjusted to keep the trachea just within in-screen. Now, transverse/axial real-time imaging of the trachea was performed to permit clear visualisation of the needle path up to the middle of the anterior wall of the trachea. A 14-G needle was introduced perpendicularly to the skin, and the needle path was determined by the distinct acoustic shadow. Indentation of the anterior tracheal wall by the needle was also visible. As the needle passed through the anterior wall, a change in resistance was felt, and the lumen was entered followed by the aspiration of air-fluid into the attached syringe. Now, the ultrasound probe was set aside, and the syringe was disconnected. A guide-wire from a pre-assembled tracheostomy kit was then inserted through the needle into the trachea as a guide for further tracheostomy. Finally, a video bronchoscope (Ambu® aScope™) was used only after the insertion of a guidewire to confirm the intraluminal position before dilation and to document the puncture position for analysis. Once the adequate intraluminal position of the guidewire was confirmed, the initial stoma was created with a 14 French dilator followed by the single-stage ‘Rhino Horn’ dilator over the guidewire and guiding catheter. An appropriately sized tracheostomy tube was fitted over an appropriate loading tube, which was then passed through the stoma and secured. The placement of the tube was confirmed immediately using auscultation and appropriate breath delivery on the ventilator. The bronchoscope was then re-introduced through the tracheostomy tube as well as the oro-tracheal tube to look for any complications, such as airway injury, tube misplacement, and endotracheal tube (ETT) cuff rupture. The ETT was withdrawn after deflation of the ETT cuff. A chest radiogram was obtained in all patients to look for further complications. Similarly, in Group A, PDT was performed using the landmark technique by an intensivist of similar experience.
Figure 2.
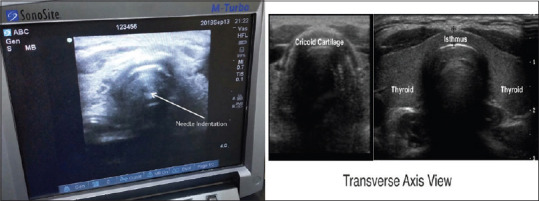
USG image showing posterior surface of anterior tracheal wall and needle site insertion and its confirmation on USG
Baseline demographic data were collected on admission. Post-procedure, the following data were collected: duration of the procedure, number of passes, with subsequent passes defined by the need to withdraw the needle completely from the skin and reinsert it, and immediate peri-procedural complications defined as complications arising during the procedure or the following 1 h. Video acquired during the bronchoscopy was recorded. For calculation of midline deviation, a protractor was aligned with the anterior curve of the trachea at the level of the puncture and aligning the transverse axis with the posterior tracheal wall [Figure 3]. The deviation from the midline in degrees was recorded with the aligned protractor. Follow-up of the patients for complications was carried out until day 30 or until de-cannulation, whichever occurred first. Time to wean from the ventilator, length of stay in the intensive care unit (ICU) and time to de-cannulation were recorded. Any tracheostomy-related adverse events during the follow-up period were documented. Our study primarily aimed to compare conventional anatomical landmark PDT versus ultrasonographic-guidance method of PDT in terms of deviation from the midline. Other secondary outcomes were the number of passes to cannulate, duration of procedure and rates of immediate peri-procedural complications. Lacking significant published data, the degrees of malposition to calculate sample size requirements were estimated. Presuming an average displacement of 25° from the midline in the landmark group and 15° in the ultrasound group with 15° and 10°, respective, standard deviations, a sample size of 50 was estimated.
Figure 3.
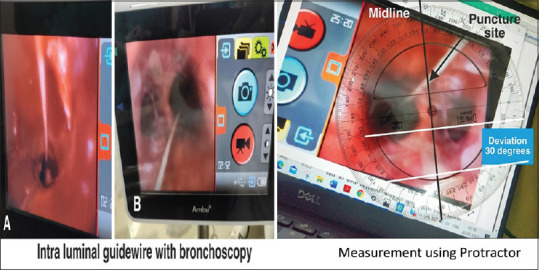
Intraluminal confirmation of guidewire by bronchoscopy and use of protractor on captured image for calculation of midline deviation
Statistical analyses of data were performed using Stata version 10 (Stata Corp, Texas, USA). Continuous variables were presented as mean with a 95% confidence interval (CI), and a t-test was applied for comparing the difference of means between the two groups after checking the normality condition. The Chi-square test was applied to test the independence of attributes of categorical variables. Power analysis was performed using software G*Power version 3.1.9.2 and for determining the effective sample size. A P value less than 0.05 was considered statistically significant.
RESULTS
The baseline characteristics were similar in both the groups in the study [Table 1]. After measurement with the protractor, the groups were compared with a midline deviation of >30° or less. It was found that Group B had significantly fewer cases of midline deviation 11.33 ± 9.51 in comparison to 16.60 ± 12.31 in Group A with a P value of 0.040. In Group A, only eight (26.67%) cases had midline puncture >30°, whereas only two (6.67%) cases in Group B had midline puncture >30°. Group A had a large number of cases of midline deviation than Group B, and it was significant [Table 2].
Table 1.
Comparison of baseline characteristics between the groups
| Group A (Mean±SD) | Group B (Mean±SD) | P | |
|---|---|---|---|
| BMI (kg/m2) | 23.07±2.58 | 22.96±2.45 | 0.698 |
| APACHE 2 Score | 17.07±0.907 | 17.00±0.871 | 0.486 |
| Days Prior To PDT (Days) | 9.20±0.887 | 9.30±0.837 | 0.559 |
| Score On Day Prior To Trachestomy (SOFA) | 3.90±0.803 | 3.73±0.785 | 0.815 |
| INR | 1.34±0.133 | 1.41±0.104 | 0.24 |
| PaO2/FiO2 ratio <=200 | 4/26 | 6/24 | 0.731 |
BMI- Body Mass Index, APACHE- Acute Physiology and Chronic Health Evaluation; SOFA- Sequential Organ Failure Assessment Score; INR- International Normalised Ratio; PaO2/FiO2- Partial pressure of oxygen/Fractional inspired oxygen; PDT-Percutaneous dilational tracheostomy; SD: Standard deviation
Table 2.
Comparison of outcomes
| Characteristics | Group A (Mean±SD) | Group B (Mean±SD) | P |
|---|---|---|---|
| Midline deviation (>30°) | 16.60±12.31 | 11.33±9.51 | 0.040 |
| Number of trials (>2) | 1.40±0.56 | 1.07±0.25 | 0.005 |
| Duration of Procedure (min) | 15.20±3.71 | 20.07±3.25 | <0.001 |
SD: Standard deviation
Statistical analysis showed that the mean number of trials for the puncture in Group A (1.40 ± 0.56) was significantly more than in Group B (1.07 ± 0.25). In Group A, 11 (36.67%) cases had a number of trials >2 and only 2 (6.67%) cases in Group B had a number of trials >2. The number of trials in Group A was larger as compared to Group B, and it was significant (P-value <0.05). The duration of the procedure in Group B (20.07 ± 3.25 min) was significantly higher in comparison to that in Group A (15.20 ± 3.71 min) due to the method applied in the USG group with a P value <0.001 [Table 2].
Our study showed complications such as bleeding requiring intervention in 13.33% in Group A as compared to 6.67% in Group B. No incidence of pneumothorax and desaturation in either of the groups occurred and ruptured cuff of ETT with 13.33% in the landmark group in comparison to 3.33% in the USG group was observed. Equal incidence of para-tracheal placement of tracheostomy tube was observed [Table 3].
Table 3.
Immediate complications
| Immediate complication | Group A Number (%) | Group B Number (%) | P |
|---|---|---|---|
| Minor bleeding and number of interventions | 04 (13.33%) | 02 (6.67%) | 0.389 |
| Bleeding requiring intervention | 04 (13.33%) | 02 (6.67%) | 0.389 |
| Pneumothorax | 0 (0%) | 0 (0%) | - |
| Paratracheal placement | 01 (3.33%) | 01 (3.33%) | 1 |
| Desaturation | 0 (0%) | 0 (0%) | - |
| Ruptured ETT Cuff | 04 (13.33%) | 01 (3.33%) | 0.353 |
ETT: Endotracheal tube
DISCUSSION
PDT has been accepted as a common treatment strategy for ICU patients requiring long-term mechanical ventilation. PDT has gradually replaced surgical tracheostomy because of its minimally invasive nature, its association with less bleeding and simplicity in technique. In our study, we proposed to compare the use of USG to perform PDT and its superiority over conventional anatomical landmark technique.
The use of real-time USG helps in the visualisation of the needle path for percutaneous tracheostomy. It has become a promising tool in reducing the rate of complications and hence has gained widespread acceptibility.[12,13] In addition to USG, our study showed the superiority of video bronchoscopes. It was used for real-time intraluminal confirmation of a guidewire, and the captured video and still images derived from videos were used for the calculation of the midline deviation of the puncture site. The deviations from the midline in degrees were recorded with the aligned protractor and deviations of >30° were noted. Moreover, the use of pre-procedural USG imaging in the front of the neck had enabled us to identify the position and anatomical relations of important landmarks like the thyroid and cricoid cartilage, tracheal rings, thyroid gland, and carotid and jugular vessels.
A study of thirteen patients who successfully underwent USG-guided PDT showed that three patients were morbidly obese, two had cervical spine precautions and one had a previous tracheostomy. In all the 13 patients, bronchoscopy confirmed that guidewire entry was through the anterior wall and between the first and fifth tracheal rings.[16] There was no case of tube misplacement, pneumothorax, posterior wall injury, significant bleeding or other complication during the procedure. Similarly, the use of pre-procedural USG imaging of the front of the neck had enabled the intensivist to safely visualise the neck anatomy, the presence of any blood vessels lying nearby and the depth of insertion of the needle. The use of USG-guided PDT offers other advantages apart from the reduced rate of complications. USG helps in the proper selection of the tracheostomy tube, especially in obese patients, children, and in situations where neck anatomy is difficult by gauging the pre-tracheal soft tissue diameter.[11,16,17,18] Intra-procedural identification of blood vessels and real-time guidance of needle puncture improve the safety of the procedure as well as the accuracy of puncture both in simple and difficult anatomy of the neck.[18] The duration of the procedure in Group B was significantly higher in comparison with Group A due to the method applied in the USG group with a P value <0.001. A study in 341 patients observed that the use of ultrasound guidance before and during PDT could render the procedure easier and safer, with fewer complications but with a slightly longer procedure time and could possibly be explained by the higher learning curve of the young physician doing USG by the bedside.[18] Our study showed complications such as bleeding requiring intervention in 13.33% of the cases in Group A as compared to 6.67% in Group B, with no incidence of pneumothorax and desaturation in either of the groups. There was accidental rupture of the cuff of ETT in 13.33% of the cases in Group A in comparison to 3.33% of the cases in Group B. Equal incidence of paratracheal placement of tracheostomy tube was seen in both groups.
Similarly, a meta-analysis showed lower rates of minor complications in the ultrasound-guided PDT group [pooled relative risk (RR): 0.55; 95% CI: 0.31–0.98, I2 ¼ 0%].[19] Inappropriate tracheostomy tube positions are also said to be associated with long-term complications like late bleeding, tracheal stenosis and difficulty weaning.[7,20,21] Though we did not observe these in our study, they can be minimised by appropriate midline tube position.
The strengths of this study are a strong methodology and protocol, which were well defined and followed by the intensivist. The outcomes were clearly defined and analysed. The study is associated with a few limitations. It was a single centre study with a limited sample size. It was a single-blinded study as only the patients remained blinded.
CONCLUSION
To conclude, the study highlights the supremacy of USG-assisted PDT over landmark PDT as real-time ultrasound guidance improves the accuracy of tracheal needle puncture. It also minimises the complications and improves accuracy in airway procedures in the ICU where time is critical. Along with the recommendations of the best available evidence, the study supports the use of USG before, during and after the PDT.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Alansari M, Alotair H, Al Aseri Z, Elhoseny MA. Use of ultrasound guidance to improve the safety of percutaneous dilatational tracheostomy: A literature review. Crit Care. 2015;19:229–31. doi: 10.1186/s13054-015-0942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delaney A, Bagshaw SM, Nalos M. Percutaneous dilatational tracheostomy versus surgical tracheostomy in critically ill patients: A systematic review and meta-analysis. Crit Care. 2006;10:R55. doi: 10.1186/cc4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon M, Metschke M, Braune SA, Püschel K, Kluge S. Death after percutaneous dilatational tracheostomy: A systematic review and analysis of risk factors. Crit Care. 2013;17:R258. doi: 10.1186/cc13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dennis BM, Eckert MJ, Gunter OL, Morris JA, Jr, May AK. Safety of bedside percutaneous tracheostomy in the critically ill: Evaluation of more than 3,000 procedures. J Am Coll Surg. 2013;216:858–65. doi: 10.1016/j.jamcollsurg.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Shlugman D, Satya-Krishna R, Loh L. Acute fatal haemorrhage during percutaneous dilatational tracheostomy. Br J Anaesth. 2003;90:517–20. doi: 10.1093/bja/aeg074. [DOI] [PubMed] [Google Scholar]
- 6.Grant CA, Dempsey G, Harrison J, Jones T. Tracheo-innominate artery fistula after percutaneous tracheostomy: Three case reports and a clinical review. Br J Anaesth. 2006;96:127–31. doi: 10.1093/bja/aei282. [DOI] [PubMed] [Google Scholar]
- 7.Rastogi A, Mandelia A, Priya V. Ultrasound guided central venous cannulation. Missing climacteric step? Indian J Anaesth. 2021;65:770–2. doi: 10.4103/ija.ija_124_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehdiratta L, Kumar N, Bajwa SJ. Advancing, strengthening and reshaping obstetric critical care with point-of-care ultrasound (POCUS) Indian J Anaesth. 2021;65:711–5. doi: 10.4103/ija.ija_924_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flint AC, Midde R, Rao VA, Lasman TE, Ho PT. Bedside ultrasound screening for pretracheal vascular structures may minimize the risks of percutaneous dilatational tracheostomy. Neurocrit Care. 2009;11:372–6. doi: 10.1007/s12028-009-9259-z. [DOI] [PubMed] [Google Scholar]
- 10.Rudas M. The role of ultrasound in percutaneous dilatational tracheostomy. Australas J Ultrasound Med. 2012;15:143–8. doi: 10.1002/j.2205-0140.2012.tb00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardee PSGF, Ng SY, Cashman M. Ultrasound imaging in the preoperative estimation of the size of tracheostomy tube required in specialised operations in children. Br J Oral Maxillofac Surg. 2003;41:312–31. doi: 10.1016/s0266-4356(03)00125-6. [DOI] [PubMed] [Google Scholar]
- 12.Dinsmore J, Heard AM, Green RJ. The use of ultrasound to guide time-critical cannula tracheotomy when anterior neck airway anatomy is unidentifiable. Eur J Anaesthesiol. 2011;28:506–10. doi: 10.1097/EJA.0b013e328344b4e1. [DOI] [PubMed] [Google Scholar]
- 13.Sustić A, Zupan Z, Eskinja N, Dirlić A, Bajek G. Ultrasonographically guided percutaneous dilatational tracheostomy after anterior cervical spine fixation. Acta Anaesthesiol Scand. 1999;43:1078–80. doi: 10.1034/j.1399-6576.1999.431019.x. [DOI] [PubMed] [Google Scholar]
- 14.Sustić A, Zupan Z, Antoncić I. Ultrasound-guided percutaneous dilatational tracheostomy with laryngeal mask airway control in a morbidly obese patient. J Clin Anesth. 2004;16:121–3. doi: 10.1016/j.jclinane.2003.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Kollig E, Heydenreich U, Roetman B, Hopf F, Muhr G. Ultrasound and bronchoscopic controlled percutaneous tracheostomy on trauma ICU. Injury. 2000;31:663–8. doi: 10.1016/s0020-1383(00)00094-2. [DOI] [PubMed] [Google Scholar]
- 16.Rajajee V, Williamson CA, West BT. Impact of real-time ultrasound guidance on complications of percutaneous dilatational tracheostomy: A propensity score analysis. Crit Care. 2005;191:98–201. doi: 10.1186/s13054-015-0924-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott DSJ, Baker PA, Scott MR, Birch CW, Thompson JMD. Accuracy of surface landmark identification for cannula cricothyroidotomy. Anaesthesia. 2010;65:889–94. doi: 10.1111/j.1365-2044.2010.06425.x. [DOI] [PubMed] [Google Scholar]
- 18.Yavuz A, Yılmaz M, Göya C, Alimoglu E, Kabaalioglu A. Advantages of US in percutaneous dilatational tracheostomy: Randomised controlled trial and review of the literature. Radiology. 2014;273:927–36. doi: 10.1148/radiol.14140088. [DOI] [PubMed] [Google Scholar]
- 19.Gobatto ALN, Besen BAMP, Cestari M, Pelosi P, Malbouisson LMS. Ultrasound-guided percutaneous dilational tracheostomy: A systematic review of randomised controlled trials and meta-analysis. J Intensive Care Med. 2020;35:445–52. doi: 10.1177/0885066618755334. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt U, Hess D, Kwo J, Lagambina S, Gettings E, Khandwala F, et al. Tracheostomy tube malposition in patients admitted to a respiratory acute care unit following prolonged ventilation. Chest. 2008;134:288–94. doi: 10.1378/chest.07-3011. [DOI] [PubMed] [Google Scholar]
- 21.Sarper A, Ayten A, Eser I, Ozbudak O, Demircan A. Tracheal stenosis after tracheostomy or intubation: Review with special regard to cause and management. Tex Heart Inst J. 2005;32:154–8. [PMC free article] [PubMed] [Google Scholar]


