ABSTRACT
Background and Aims:
Preschool age children are psycho-biologically vulnerable to all surgical procedures. In this study, we investigated the effect of nebulised dexmedetomidine, midazolam and ketamine as sedative premedication for alleviating parental separation anxiety, facilitating face mask acceptance and reducing emergence agitation in paediatric patients undergoing general anaesthesia.
Methods:
A prospective, randomised, double-blind study was done involving 96 children of age 3–7 years, randomly allocated into three equal groups and pre-medicated with either nebulised dexmedetomidine 2 mg/kg (GroupD), midazolam 0.2 mg/kg (GroupM) or ketamine 2 mg/kg (Group K). The scores of sedation scale, parental separation anxiety scale, mask acceptance scale and emergence agitation scale were recorded along with haemodynamic parameters. Two-way repeated measures analysis of variance (ANOVA), post hoc test and Kruskal–Wallis test were used for statistical analysis.
Results:
A statistically significant difference in sedation score was seen between the different study groups, χ2(2) = 8.561, P = 0.014 with mean rank sedation score of 56.50 for Group D, 38.92 for Group M and 43.84 for Group K. Parental separation anxiety scale score and Mask acceptance scale score also showed statistically significant difference between the different study groups, χ2(2) = 9.369, P = 0.009 and χ2(2) = 11.97, P = 0.003, respectively.
Conclusion:
Nebulisation with dexmedetomidine produced easy parental separation, more satisfactory sedation and face mask acceptance with less postoperative agitation than nebulisation with midazolam or ketamine.
Keywords: Children, dexmedetomidine, ketamine, midazolam, separation anxiety
INTRODUCTION
All surgical procedures are perturbing for children.[1] It will be worse if the children are of preschool age as they are a physiologically vulnerable age group and the preoperative period is quite distressing for them.[2] This mainly occurs because of parental separation, application of face mask for induction of anaesthesia, fear of needles and unfamiliar faces. Inadequate premedication can result in turbulent anaesthetic induction and adverse behavioural sequelae.[3]
Sedative premedicants such as midazolam, dexmedetomidine, clonidine and ketamine can be administered to alleviate pre-operative and parental separation anxiety.[4,5] An ideal premedicant for children should be easily available, cheaper and palatable with rapid onset and short duration of action. It should be able to curtail anaesthetic and analgesic requirements with minimal adverse effects and faster recovery time.[6]
Inhalation of nebulised drug is an alternative route of administration which does not require venipuncture and is associated with high bioavailability of the administered drug.[3,7] Nebulisation technique is simple and convenient for both child and parent.
In this study, a comparison between nebulised solution of three sedative premedicants, viz., dexmedetomidine, midazolam and ketamine was done to investigate their effect on preschool children undergoing general anaesthesia to achieve satisfactory sedation, reduce parental separation anxiety and alleviate emergence agitation.
METHODS
This prospective, randomised, double-blind, comparative study was conducted on 96 paediatric patients belonging to American Society of Anesthesiologists (ASA) physical status I and II, and aged 3–7 years, of either gender posted for elective surgical procedures under general anaesthesia, during March 2019–August 2020 at a tertiary care medical college hospital of Eastern India. Exclusion criteria for the study were parent refusal, emergency, children with known allergy to the study drugs, significant organ dysfunction, congenital disorders, behavioural disorders and children with >85th percentile of body mass index for age percentiles.
The primary objective was to evaluate the efficacy of nebulised dexmedetomidine, midazolam and ketamine as sedative premedication administered by nebuliser 30 min before the induction of general anaesthesia in the study patients. The secondary objective was to compare these premedicants in terms of achieving adequate sedation, alleviating parental separation anxiety and providing better face mask acceptance with least emergence reactions. In a previous study by Abdel-Gaffar et al.,[8] after operation, 40% patients in nebulised midazolam group and 20% patients in nebulised ketamine group were combative and disoriented (emergence agitation score of ≥2). Targeting the same difference, with 95% confidence level and 80% power, the sample size (n) was calculated as 32 in each group.
After approval from the Institutional Ethics Committee and informed written consent from the guardians of each patient, 96 children presenting for elective surgical procedures under general anaesthesia were randomised to three groups to receive premedication by nebulisation; Group D, Group M and Group K received inhalation of nebulised solution of dexmedetomidine (2 mg/kg), midazolam (0.2 mg/kg) and ketamine (2 mg/kg) respectively, diluted in 3 ml of 0.9% of normal saline 30 min before the induction of general anaesthesia. Randomisation was based on a computer-generated randomisation table, with group allocation concealed in sealed opaque envelopes. All the principles of the Declaration of Helsinki were followed during the course of the study.
All the selected patients were kept 2 hours nil per oral for clear fluids and anxiolytics or sedatives were stopped 12 hours before surgery. On the day of surgery, after confirmation of identity, the patients were shifted to the pre-operative room. Using the multiparameter monitor, basal heart rate (HR), mean blood pressure (MBP) and oxygen saturation were recorded. A good venous access site was identified and a eutectic mixture of local anaesthetic cream (prilox; 2.5% w/w prilocaine +2.5% w/w lidocaine) was applied. Venous catheters of varied sizes and emergency drugs were kept ready in all cases to help secure immediate venous access if needed.
An independent assistant with good clinical knowledge, but not a part of the study, was made available for opening the envelopes with details of the study drugs to be administered. The assistant prepared nebulised solutions in identical syringes with matching random codes 1 hour before the induction of anaesthesia and gave those medications via nebulisation 30-45 min before induction. Study drugs were diluted in 3 ml of 0.9% saline and were administrated in the pre-operative area by a standard hospital jet nebuliser ((Rossmax NA100 Piston nebuliser, Rossmax international Ltd. Taipei, Taiwan) via face mask with a continuous flow of 100% oxygen at 6 L/min for 10 to 15 min.
The attending anaesthesiologist, data collection personnel and the patient guardians were blinded to the patient group assignment. Each patient had to complete all the three phases of the study: pre-operative phase (30 min after end of administration of the nebulised study drug), intra-operative phase and the early postoperative phase (1 hour after operation).
The HR and MBP were assessed before (0 min, baseline) and at 5, 10, 15, 20 and 30 min after the end of study drug administration. Sedation level was assessed at the same time points mentioned above using a five-point sedation scale (FPSS) as follows: 1—agitated, 2—alert, 3—calm, 4—drowsy, and 5—asleep.[9] At the end of the preoperative phase, parental separation was assessed by a four-point parental separation anxiety scale (PSAS) as follows: 1—easy separation (excellent), 2—whimpers, but easily reassured, not clinging (good), 3—cries and cannot be easily reassured, not clinging (fair), 4—crying and clinging to parents (poor).[9]
Patients’ acceptance of the anaesthesia mask was assessed using a four-point mask acceptance scale (MAS) as follows: 1—excellent, unafraid, cooperative, accepts mask easily, 2—good, slight fear of mask, easily assured, 3—fair, moderate fear of mask, not calmed with reassurance, 4—poor, terrified, crying or combative.[9] HR and MBP were recorded before (0 min, baseline) and at 5, 10, 15 and 20 min after induction of general anaesthesia and also in the post-anaesthesia care unit. Recovery was assessed using the three-point emergence agitation scale as follows: 1—Calm, 2—Restless but calms in response to verbal instructions, 3—Combative and disoriented.[10]
The anaesthetic technique was standardised in all paediatric patients according to institutional protocol.
Data were entered in Microsoft Excel and analysis was performed using Statistical Package for the Social Sciences for Windows, Version 20.0 software (IBM, Bengaluru, India). Data were summarised as count and percentages for categorical variables and mean and standard deviation for numerical variables. Z test was used for comparing proportion. Changes in haemodynamic parameters (HR, MBP) with time were analysed with two-way repeated measures analysis of variance (ANOVA) and post hoc tests. Kruskal–Wallis test was used for the analysis of ordinal variables of the study groups. Mean rank obtained from this test was used for interpreting the results. Mean rank relied on scores being ranked from the lowest to highest. Therefore, the group with the lowest or the highest mean rank was the group with the greatest number of low or high scores respectively in it. P value less <0.05 was considered statistically significant.
RESULTS
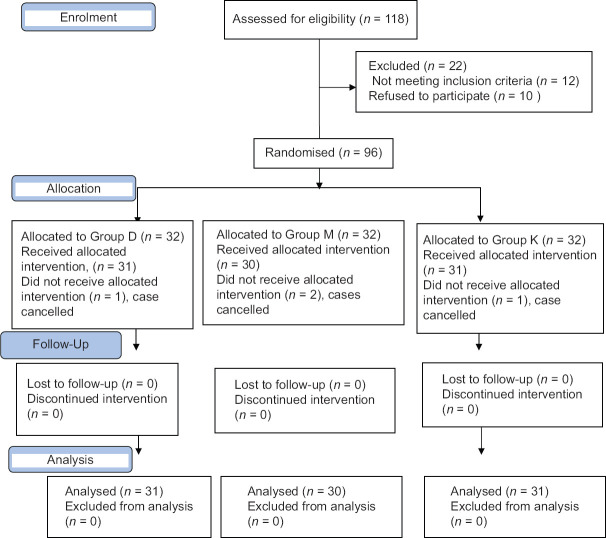
In total, 118 patients were screened and 96 patients meeting the inclusion criteria and willing to participate in the study were randomised into three groups. One each from group D and K and two from group M did not get allocation because of cancellation of case (total four patients) [Figure 1].
Figure 1.
Consolidated standards of reporting trials (CONSORT) flow chart showing division of patients at every stage of the trial
All three groups were comparable with no statistically significant difference in their demographic profile [Table 1].
Table 1.
Comparison of demographic parameters (age, height and weight) and gender among study groups
| Demographic Variables | Group-D (n=31) | Group-M (n=30) | Group-K (n=31) | P |
|---|---|---|---|---|
| Age (years) | 4.80±0.86 | 5.21±1.16 | 5.14±0.97 | 0.212 |
| Height (cm) | 105.06±6.3 | 107.6±8.5 | 106.5±7.3 | 0.363 |
| Weight (kg) | 16.3±2.9 | 17±2.9 | 16.5±2.1 | 0.561 |
| Gender (Male:Female) | 15:17 | 17:15 | 16:16 | 0.849 |
Data entered as mean±Standard deviation, n=number of patients, Gender expressed as count, P<0.05 considered statistically significant
When comparing sedation level and parental separation anxiety of patients, Group D produced statistically significant difference compared to Group M and K (FPSS: χ2(2) = 8.561, with mean rank sedation score of 56.50, 38.92 and 43.84 for Groups D, M and K, respectively, P = 0.014, PSAS: χ2(2) = 9.369, with mean rank anxiety score of 38.47, 43.75 and 56.92 for Groups D, M and K, respectively, P = 0.009) [Table 2].
Table 2.
Comparison of sedation, parental separation anxiety, mask acceptance and emergence agitation scores among the three groups
| Scale | Group D (n=31) | Group M (n=30) | Group K (n=31) | P |
|---|---|---|---|---|
| Five Point Sedation (mean rank) | 56.50 | 38.92 | 43.84 | 0.014 |
| Parental separation anxiety (mean rank) | 38.47 | 43.75 | 56.92 | 0.009 |
| Mask Acceptance (mean rank) | 34.48 | 51.73 | 53.45 | 0.003 |
| Emergence Agitation (mean rank) | 24.73 | 52.37 | 62.60 | 0.000 |
P<0.05 considered statistically significant
Acceptance to face mask was higher in Group D compared to Groups M and K (MAS: χ2(2) = 11.97, with mean rank mask acceptance score of 34.48 versus 51.73 and 53.45 for Groups D, M and K, respectively, P = 0.003) [Table 2].
Group D also showed least emergence agitation than Groups M and K (EAS: χ2(2) = 38.19, with mean rank emergence agitation score of 24.73 versus 52.37 and 62.60 for Groups D, M and K, respectively, P = 0.000) [Table 2].
The haemodynamic parameters (HR and MBP) showed statistically significant differences throughout the perioperative period in group D when compared with M and K [Table 3].
Table 3.
Comparison of mean blood pressure (MBP) and heart rate (HR) of patients among the three groups in the three phases; pre-operative (baseline), intra-operative and post-operative
| Parameters MBP & HR | Group D (n=31) Mean±SD | Group M (n=30) Mean±SD | Group K (n=31) Mean±SD | P | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Overall | D vs M | K vs D | M vs K | ||||
| Baseline | 81.46±8.22 | 82.75±8.64 | 83.90±7.91 | 0.501 | 0.810 | 0.468 | 0.842 |
| Intra-operative MBP (mm Hg) | |||||||
| 5 min | 77.77±7.88 | 81.80±8.53 | 85.22±7.41 | 0.002 | 0.124 | 0.001 | 0.218 |
| 10 min | 76.74±7.63 | 81.40±8.84 | 86.25±6.78 | 0.000 | 0.056 | 0.000 | 0.044 |
| 20 min | 77.32±7.94 | 81.10±8.21 | 86.29±6.95 | 0.000 | 0.142 | 0.000 | 0.027 |
| 30 min | 77.09±7.72 | 81.53±8.49 | 85.90±7.38 | 0.000 | 0.077 | 0.000 | 0.083 |
| Post-operative MBP (mm Hg) | |||||||
| 15 min | 77.32±7.94 | 81.10±8.21 | 86.29±6.95 | 0.000 | 0.142 | 0.000 | 0.027 |
| 30 min | 77.09±7.72 | 81.53±8.49 | 85.90±7.38 | 0.000 | 0.087 | 0.000 | 0.093 |
| 45 min | 76.74±7.63 | 81.40±8.84 | 86.25±6.78 | 0.000 | 0.059 | 0.000 | 0.048 |
| 60 min | 77.77±7.88 | 81.80±8.53 | 85.22±7.41 | 0.002 | 0.144 | 0.002 | 0.238 |
| HR (beats/min) | |||||||
| Baseline | 109.03±7.9 | 108.15±7.8 | 107.75±7.9 | 0.806 | 0.899 | 0.796 | 0.977 |
| Intra-operative | |||||||
| 5 min | 95.96±8.00 | 103.5±7.13 | 109.87±6.78 | 0.001 | 0.000 | 0.000 | 0.143 |
| 10 min | 94.64±6.25 | 101.83±6.39 | 109.8±6.71 | 0.000 | 0.000 | 0.000 | 0.089 |
| 20 min | 93.45±6.08 | 102.23±5.42 | 109.48±6.17 | 0.000 | 0.000 | 0.000 | 0.075 |
| 30 min | 93.06±6.95 | 100.13±5.70 | 110.03±6.36 | 0.000 | 0.000 | 0.000 | 0.063 |
| Post-operative | |||||||
| 15 min | 92.96±6.63 | 100.63±5.42 | 108.83±5.56 | 0.000 | 0.000 | 0.000 | 0.072 |
| 30 min | 94.32±6.25 | 101.23±5.69 | 106.87±6.83 | 0.000 | 0.000 | 0.000 | 0.168 |
| 45 min | 93.63±6.13 | 103.60±6.00 | 109.22±6.06 | 0.000 | 0.000 | 0.000 | 0.129 |
| 60 min | 94.25±6.43 | 102.43±7.08 | 109.35±6.33 | 0.000 | 0.000 | 0.000 | 0.084 |
Data entered as mean±Standard deviation (SD), n=number of patients, P<0.05 considered statistically significant. D= Dexmedetomidine group, M=Midazolam group, K=Ketamine group
DISCUSSION
This study demonstrated that inhalation was an easily acceptable and convenient route of administration of premedicants. Paediatric study patients premedicated with nebulised dexmedetomidine had satisfactory sedation score, more satisfactory parental separation and mask acceptance with calmer emergence than those who received nebulized ketamine or midazolam.
Children are not miniature adults. They should be considered as a special population.[11] Perioperative anxiety in children is associated with adverse clinical outcomes like emergence delirium, increased analgesic requirements and negative behavioural changes such as sleep disturbance, separation anxiety, eating problems, new onset enuresis and aggression towards authority.[12] Premedication plays an integral role in the practice of paediatric anaesthesia.
In this study, three sedative premedicants, viz., dexmedetomidine, midazolam and ketamine were compared. Drugs were administered in doses of 2 mg/kg, 0.2 mg/kg and 2 mg/kg, respectively, as nebulised solutions.[8] Data on administration of these drugs via nebulised route were limited. So, in our study, we decided the doses based on previous clinical studies that proved clinical effectiveness of these doses with minimal adverse events.[8,13] The inhalation of nebulised drugs used in this study offered an alternative mode of administration of sedative premedication that was relatively easy to set up, convenient for patients and painless as it did not require an intravenous (IV) cannulation or injection. Still it was associated with high bioavailability of the administered drug. Through jet nebuliser, a high velocity of gas is blown through a fine hole creating an area of negative pressure. Fluid is drawn from the nebulisation chamber by Bernoulli effect into the jet stream and is impacted on a baffle breaking the fluid into droplets. Large droplets of size >10 mm are most likely to deposit in the mouth and throat. Drops with diameter 5-10 mm get deposited from mouth to upper airway and those with size <5 mm are likely to get deposited in the lower airways.
The nebuliser generates a spray of drug that maximises surface area coverage with a thin layer of drug that enables rapid drug absorption through the nasal, buccal, and respiratory mucosa, which can help to achieve higher cerebrospinal fluid concentrations, better patient acceptability and improved clinical effectiveness.[4]
Various studies have compared the efficacy of midazolam, ketamine and dexmedetomidine as sedative premedication using different routes of administration and by using one drug or combination of the study drugs in different doses with varying results.
Dexmedetomidine is a newer selective alpha-2 agonist with a site of action at the locus coeruleus. It inhibits presynaptic release of norepinephrine that is responsible for its sedative and hypnotic effects.[14] Ketamine binds non-competitively to the phenylcyclohexyl piperidine of the N-methyl-D- aspartate receptor, inhibiting its activation by glutamate, and decreases presynaptic release of glutamate. Ketamine abolishes a rhythm, and there is dominance of q activity. It also produces burst suppression and increases cortical amplitude of somatosensory evoked potentials. Ketamine is used for sedation and analgesia at a dose of 0.2–0.8 mg/kg IV over 2–3 minutes or 2–4 mg/kg intramuscularly.
There are several studies which have compared the effectiveness of dexmedetomidine and ketamine as premedication as a single drug or a combination of both. In a previous study conducted by Abdel-Gaffar et al.,[8] a comparison of nebulised dexmedetomidine, ketamine, or midazolam as premedication in preschool children undergoing bone marrow biopsy and aspiration was made. They used a study population which was going for a single type of procedure, viz., bone marrow biopsy. In our study, we applied similar objectives to a wider group of study population which included pre-school children undergoing general anaesthesia. Findings of Abdel Gaffar study and our study were similar such that preschool children premedicated with nebulised dexmedetomidine had more satisfactory sedation, less parental separation anxiety and emergence agitation than those who received nebulised ketamine or midazolam. When given as premedication to children, intranasal dexmedetomidine was found to provide acceptable parental separation and decreased emergence agitation.[15] Even though most of the studies showed lack of additional benefits by combining dexmedetomidine and ketamine as premedication, Zanaty OM et al.[13] found that a nebulised combination of low-dose ketamine and dexmedetomidine produced more satisfactory sedation and provided a smoother induction than either of the nebulised drugs alone. Dexmedetomidine and ketamine were found to have comparable level of sedation, analgesia scores and haemodynamic stability in anaesthesia for paediatric dental surgery.[16]
Midazolam has been established as an effective oral premedicant in children.[17] At a dose of 0.5 mg/kg administered orally 30 minutes before the induction of anaesthesia, it provides reliable sedation and anxiolysis without producing delayed awakening. Studies have found that intranasal midazolam spray appears to be a near ideal premedicant having significant sedation and anxiolytic properties with no significant effects on haemodynamic and respiratory physiology.[18]
In a triple blinded randomised study, the authors concluded that dexmedetomidine, midazolam and ketamine could be used safely and effectively through the intra-nasal route for producing moderate sedation in uncooperative paediatric dental patients.[19] In contrast to our study finding, a prospective randomised double-blind controlled trial by Aynur Akin et al.[20] concluded that intranasal administration of dexmedetomidine and midazolam was equally effective in alleviating anxiety upon separation from parents; however, midazolam was superior in providing satisfactory conditions during face mask induction.
Most of the published studies have used intranasal route for premedicant administration in paediatric patients. The intranasal route may be less acceptable in this group of patients because of irritation on application, though studies have not documented it. The nebulised route for administering premedicant drugs in children is under-utilised and needs further studies. In the present study, the study population included 3 to 7 year old preschool children who were aware of the situation and were co-operative. However, we also had a minimum number of children who were initially uncooperative due to their fear of painful procedure. But when we allowed them to sit with their parents and gave nebulisation mask in their hands, we could manage to alleviate their anxiety with support of their loved ones.
Nevertheless, the assessment of scoring system needs adequate patient cooperation. This could be a limitation at times in the paediatric age group.
CONCLUSION
The study concludes that nebulised dexmedetomidine (2 mg/kg) diluted in 3 ml of 0.9% of normal saline when used as a premedicant in preschool children produces a better sedation score on arrival at the operation theatre, higher PSAS and MAS scores and less postoperative emergence agitation in comparison to nebulised midazolam (0.2 mg/kg) or ketamine (2 mg/kg).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kain ZN, Caldwell-Andrews AA, Maranets I, McClain B, Gaal D, Mayes LC, et al. Preoperative anxiety and emergence delirium and postoperative maladaptive behaviors. Anesth Analg. 2004;99:1648–54. doi: 10.1213/01.ANE.0000136471.36680.97. [DOI] [PubMed] [Google Scholar]
- 2.Kain ZN, Caldwell-Andrews AA, Krivutza DM, Weinberg ME, Wang S-M, Gaal D. Trends in the practice of parental presence during induction of anesthesia and the use of preoperative sedative premedication in the United States, 1995–2002: Result sofa follow-up national survey. Anesth Analg. 2004;98:1252–9. doi: 10.1213/01.ane.0000111183.38618.d8. [DOI] [PubMed] [Google Scholar]
- 3.Kumar L, Kumar A, Panikkaveetil R, Vasu BK, Rajan S, Nair SG. Efficiency of intranasal dexmedetomidine versus oral midazolam for paediatric premedication. Indian J Anaesth. 2017;61:125–30. doi: 10.4103/0019-5049.199850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCormick ASM, Thomas VL, Berry D, Thomas PW. Plasma concentrations and sedation scores after nebulized and intranasal midazolam in healthy volunteers. Br J Anaesth. 2008;100:63–6. doi: 10.1093/bja/aen072. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt AP, Valinetti EA, Bandeira D, Bertacchi MF, Simoes CM, Auler JO., Jr Effects of preanesthetic administration of midazolam, clonidine, or dexmedetomidine on postoperative pain and anxiety in children. Paediatr Anaesth. 2007;17:667–74. doi: 10.1111/j.1460-9592.2006.02185.x. [DOI] [PubMed] [Google Scholar]
- 6.Lapin SL, Auden SM, Goldsmith LJ, Reynolds AM. Effects of sevoflurane anaesthesia on recovery in children, a comparison with halothane. Paediatr Anaesth. 1999;9:299–304. doi: 10.1046/j.1460-9592.1999.00351.x. [DOI] [PubMed] [Google Scholar]
- 7.Peng K, Wu SR, Ji FH, Li J. Premedication with dexmedetomidine in pediatric patients: A systematic review and meta-analysis. Clinics. 2014;69:777–86. doi: 10.6061/clinics/2014(11)12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdel-Gaffar HS, Kamal SM, El Sherif FA, Mohamed SA. Comparison of nebulized dexmedetomidine, ketamine, or midazolam for premedication in preschool children undergoing bone marrow biopsy. Br J Anaesth. 2018;121:445–52. doi: 10.1016/j.bja.2018.03.039. [DOI] [PubMed] [Google Scholar]
- 9.Wilton NC, Leig J, Rosen DR, Pandit UA. Pre-anesthetic sedation of preschool children using intranasal midazolam. Anesthesiology. 1988;69:972–5. doi: 10.1097/00000542-198812000-00032. [DOI] [PubMed] [Google Scholar]
- 10.Bajwa SA, Costi D, Cyna AM. A comparison of emergence delirium scales following general anesthesia in children. Paediatr Anaesth. 2010;20:704–11. doi: 10.1111/j.1460-9592.2010.03328.x. [DOI] [PubMed] [Google Scholar]
- 11.Doctor JR, Malde A, Yaddanapudi S. The IJA postgraduate educational issue on current concept of paediatric anaesthesia. Indian J Anaesth. 2019;63:688–9. doi: 10.4103/ija.IJA_671_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dave NM. Premedication and induction of Anaesthesia in paediatric patients. Indian J Anaesth. 2019;63:713–20. doi: 10.4103/ija.IJA_491_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zanaty OM, Metainy ELSA. A comparative evaluation of nebulized dexmedetomidine, nebulized ketamine, and their combination for premedication for outpatient pediatric dental surgery. Anesth Analg. 2015;121:167–71. doi: 10.1213/ANE.0000000000000728. [DOI] [PubMed] [Google Scholar]
- 14.Grewal A. Dexmedetomidine: New avenues. J Anaesthesiol Clin Pharmacol. 2011;27:297–302. doi: 10.4103/0970-9185.83670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhat R, Santhosh MC, Annigeri VM, Rao RP. Comparison of intranasal dexmedetomidine and dexmedetomidine-ketamine for premedication in pediatrics patients: A randomized double-blind study. Anesth Essays Res. 2016;10:349–55. doi: 10.4103/0259-1162.172340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu J, Luo Z. The comparison of dexmedetomidine and ketamine for pediatric dental surgery: A meta-analysis of randomized control studies. Medicine (Baltimore) 2019;98:e15068. doi: 10.1097/MD.0000000000015068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radhika KP, Sreejith MS, Ramdas KT. Efficacy of midazolam as oral premedication in children in comparison to triclofos sodium. Indian J Anaesth. 2016;60:415–9. doi: 10.4103/0019-5049.183389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De A, Gupta PK, Singh RB. Midazolam as intranasal spray in paediatric surgical patients. A prospective, randomized double blinded placebo controlled comparative study. Int J Basic Appl Med Sci. 2013;3:208–17. [Google Scholar]
- 19.Surendar MN, Pandey RK, Saksena AK, Kumar R, Chandra G. A comparative evaluation of intranasal dexmedetomidine, midazolam and ketamine for their sedative and analgesic properties: A triple blind randomized study. J Clin Pediatr Dent. 2014;38:255–61. doi: 10.17796/jcpd.38.3.l828585807482966. [DOI] [PubMed] [Google Scholar]
- 20.Akin A, Bayram A, Esmaoglu A, Tosun Z, Aksu R, Altuntas R, et al. Dexmedetomidine vs midazolam for premedication of pediatric patients undergoing anaesthesia. Pediatric Anesthesia. 2012;22:871–6. doi: 10.1111/j.1460-9592.2012.03802.x. [DOI] [PubMed] [Google Scholar]