Abstract
Purpose of Review:
The benefits of intensive diabetes management have been established by the Diabetes Control and Complications Trial. However, challenges with optimizing glycemic management in youth with type 1 diabetes (T1D) remain across pediatric clinics in the United States. This article will review our Teamwork, Targets, Technology, and Tight Control (4T) study that implements emerging diabetes technology into clinical practice with a team approach to sustain tight glycemic control from the onset of T1D and beyond to optimize clinical outcomes.
Recent Findings:
During the 4T Pilot study and study 1, our team-based approach to intensive target setting, education, and remote data review has led to significant improvements in hemoglobin A1c throughout the first year of T1D diagnosis in youth, as well as family and provider satisfaction.
Summary:
The next steps include refinement of the current 4T study 1, developing a business case, and broader implementation of the 4T study. In study 2, we are including a more pragmatic cadence of remote data review and disseminating exercise education and activity tracking to both English- and Spanish-speaking families. The overall goal is to create and implement a translatable program that can facilitate better outcomes for pediatric clinics across the United States.
Keywords: Type 1 diabetes, continuous glucose monitoring, diabetes technology, glycemic control
Introduction
The efficacy of intensive diabetes management to reduce vascular complications associated with type 1 diabetes (T1D) has been well established by The Diabetes Control and Complications Trial (DCCT) (1, 2). However, almost 30 years after the DCCT, pediatric diabetes clinics in the United States do not achieve glycemic control achieved in the DCCT (3, 4). The challenges associated with achieving and maintaining tight glycemic control in adolescents with T1D are often multifactorial. Some of these factors include fatigue associated with the management of a chronic condition (i.e., diabetes burnout), concerns about social context and peers, and physiological changes such as pubertal changes that may lead to increased insulin resistance (5). Systems and processes of care also present challenges for diabetes care teams in the US healthcare system (6).
International pediatric diabetes clinics, in contrast to the United States, have documented a significant decrease in mean hemoglobin A1c (HbA1c) in youth with T1D (7–10). Clinics outside of the United States have also reduced their mean HbA1c (11–19) through a number of common themes including i) a unified and consistent team approach; ii) communicating clear glucose targets to patients and their families; iii) flexibility in supporting patients and families with technology; iv) timely detection of increasing glucose trends followed by rapid intensification of therapy to regain tight control. As such, we have developed the 4T (Teamwork, Targets, Technology, and Tight Control) program at Stanford that implements proven methods and emerging diabetes technology into our clinical practice to sustain tight glucose control from the onset of T1D diagnosis and optimizes patient reported, psychosocial, and health outcomes in a cost-effective manner that enables team members to work at the top of their license (20). The Covid-19 pandemic has also accelerated the adoption of telehealth for both clinical care and research visits (21). With the clear demonstration of improved glycemic and quality of life (QoL) outcomes with continuous glucose monitoring (CGM) (22–26), we have made this a priority to support and start CGM for all children, independent of insurance status, early after T1D diagnosis (10, 21, 27). The aim of this review is to describe the current state of the 4T study as well as the challenges and future directions of this program, especially the second stage of the 4T study.
4T Study Overview
The 4T study is a pragmatic research study that utilizes a multi-disciplinary diabetes team approach and a revised diabetes education program that clearly defines tighter glucose targets at T1D diagnosis and individualizes intensification of control as insulin needs and care demands increase (25).
In the Pilot 4T study, youth with new onset T1D were offered to start on CGM shortly following diabetes diagnosis. A total of 135 youth enrolled and 124 initiated CGM within the first month of diagnosis and 11 initiated CGM after 30 days of diagnosis. We have previously developed a system for transmitting data from an individual’s CGM into our electronic medical records using Apple HealthKit on iOS devices (28). The Stanford SURF (Systems Utilization Research Force) team has also developed a glucose data dashboard to display relevant CGM data for review by a Certified Diabetes Care and Education Specialist (CDCES) (29). Our team has continued to iterate and improve on the features of the dashboard. This Timely Interventions for Diabetes Excellence (TIDE) tool is a unique, open-source dashboard that has algorithm-enabled prioritization of participants who would benefit from remote CGM data review and dose adjustments by a CDCES to apply precision medicine on a population health basis (29, 30). It serves as an automated decision-support tool that uses CGM data to support setting personalized goals and identify a need for insulin dose adjustments in between quarterly visits.
During the Pilot 4T study, remote glucose monitoring was developed and then initiated for the next 89 (out of the 135) children and families who consented to participate in remote data review (i.e., remote monitoring). HbA1c at 12 months post-diagnosis in the Pilot 4T cohort was 0.58% lower than the historic cohort and those Pilot 4T patients receiving remote monitoring had a HbA1c 0.14% lower than patients in the Pilot 4T cohort not receiving remote monitoring at 12 months post-diagnosis (21). In addition, our 4T program demonstrates that CGM initiation during the new-onset period is feasible and well accepted by youth and their families (31). Focus groups and individual interviews have been conducted and show that parents find early CGM initiation valuable and an essential part of living and adjusting to a new diagnosis (32). During the Covid-19 pandemic, we also held focus groups and interviews with parents of T1D youth who initiated CGM over telehealth during the first few weeks of diagnosis (33). Overall, most parents appreciated receiving the CGM initiation over telehealth and stated the importance to offer the choice of telehealth or in-person visits to all families in the future.
Following the Pilot 4T study, all newly diagnosed clinic patients with T1D in study 1 are exposed to the clinic-wide 4T program consisting of the opportunity to start a CGM in the weeks after diagnosis, a 1-week follow-up CGM education visit, 1-month post-diagnosis visit, and routine diabetes follow-up visits every 3 months thereafter (Figure 1). The Covid-19 pandemic ushered in the rapid adoption and digital transformation of diabetes care delivery in a telehealth model. All Study 1 participants are followed with remote monitoring and the automated-support TIDE tool (29) within 2–4 weeks of CGM initiation. Psychosocial and patient reported outcome surveys (PROs) are collected at baseline, 1, 3, 6, 9, and 12 months and incorporated into the clinic’s psychosocial screening and treatment program. The 4T study recruits all patients seen at the Pediatric Endocrinology clinics at Stanford Children’s Health that present with a T1D or possible T1D diagnosis regardless of insurance status, language spoken, or provider referral in recognition of health disparities and inequities in diabetes technology access (20, 21, 34–36). The 4T study provides CGM supplies to patients with insurance coverage barriers such as a lapse in insurance coverage or insurance claim denial (i.e., common in public insurance) until insurance can be obtained for the duration of the 12-month study. The 4T study 1 is currently underway and we anticipate recruitment to be completed with approximately 130 youth participants by April 2022 at which point, 4T study 2 will commence.
Figure 1:
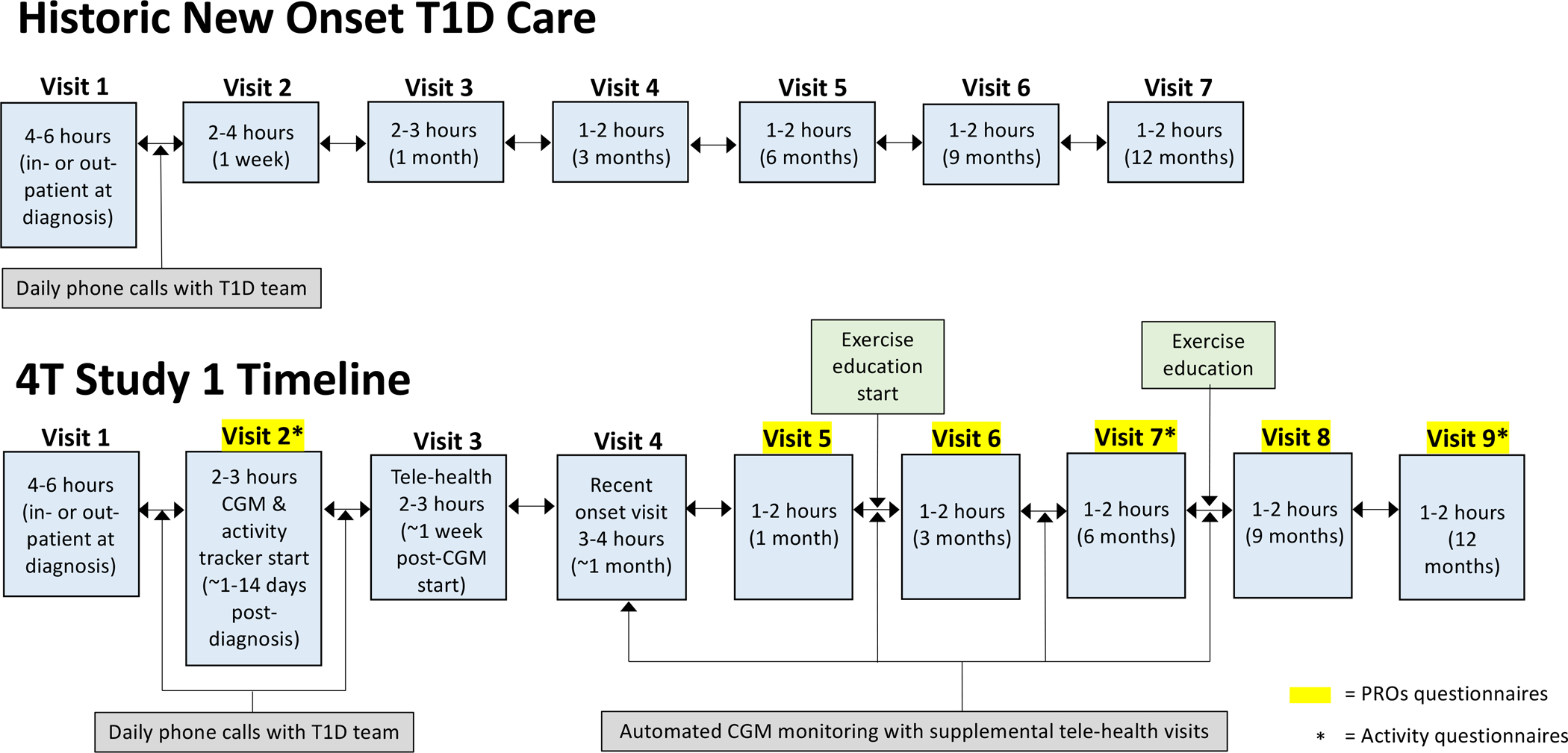
The 4T study 1 timeline including usual clinical visits and specific 4T study 1 components. CGM = continuous glucose monitoring; T1D = type 1 diabetes; PROs = patient reported outcomes survey.
In addition to sustaining tight glycemic control and psychosocial support for youth with newly diagnosed T1D, our team has also developed and initiated an ancillary study focusing on exercise and physical activity education and tracking in this population. The aim is to assess real-world physical activity data and to develop and disseminate structured exercise education materials for youth with recent T1D diagnosis and their families.
Exercise and regular physical activity play a vital role in improving psychosocial well-being, controlling weight, and minimizing long-term complications in youth (37–39). However, many youth with T1D are not meeting physical activity recommendations (40), with a particularly worse impact throughout the Covid-19 pandemic (41). In adults with T1D, evidence suggests that active days tend to increase the CGM percent time-in-range (TIR), but also increase the percent time spent in hypoglycemia compared to sedentary days (42). In addition to barriers such as lack of motivation and fear of hypoglycemia, exercise interventions in adults with T1D have been shown to be acceptable and feasible to deliver (43). Individuals with T1D and healthcare professionals have identified ‘exercising safely’ as one of the most challenging aspects of diabetes, and many choose not to partake in activity because of the associated risks (44). Our 4T exercise ancillary study includes four telehealth-based exercise education sessions with parent(s) and youth (≥11 years), led by a Certified Exercise Physiologist, over the 12-month study duration (Figure 1). Some of the topics covered in the education modules include various types, intensities, and durations of exercise and how each of these may impact glycemia. Parent and child education sessions are conducted separately to create a safe space to educate and address any fears to ‘exercising safely’ and associated strategies tailored to the parent and child. With increasing adoption of CGM use, patients can use the detailed information (e.g., trend arrows, patterns) to learn how different factors may impact glycemia around physical activity and exercise (40). In addition, with advancements in automated insulin delivery (AID) systems, improvements in overnight glycemia are apparent (45); but exercise and glycemic management remain a challenge (46, 47). In study 2, we will encourage patients to initiate the use of an insulin pump or AID system during the first 3-month period post-diagnosis and continue to offer exercise education to increase the knowledge and education around safe exercise.
Together in 4T study 1 and study 2, the goals include lowering HbA1c, reducing the fear of hypoglycemia around exercise, supporting the psychosocial challenges of T1D, and developing CGM data management and communication systems in a program that is practical, sustainable, and translatable to other pediatric diabetes clinics across the United States.
Future Directions
The Teamwork, Targets, Technology, and Tight Control (4T) study 2 will be a single-arm design of the remote monitoring and automated-support CGM tool (i.e., TIDE) using a step-down approach (weekly versus monthly) to the cadence of remote data reviews (Figure 2). By focusing on the cadence of reviews, study 2 will help to determine whether a more pragmatic and generalizable cadence of reviews yields comparable outcomes while increasing remote data review capacity by clinical team members and testing for non-inferiority. Assessing a more pragmatic cadence of review will define how TIDE can be translated to other clinical settings in a time and cost-efficient manner (21, 22). We will also compare outcomes to historic controls in our program and contemporaneous outcomes in other T1D populations. Future integrations of the TIDE dashboard also aim to include physical activity data for review.
Figure 2:
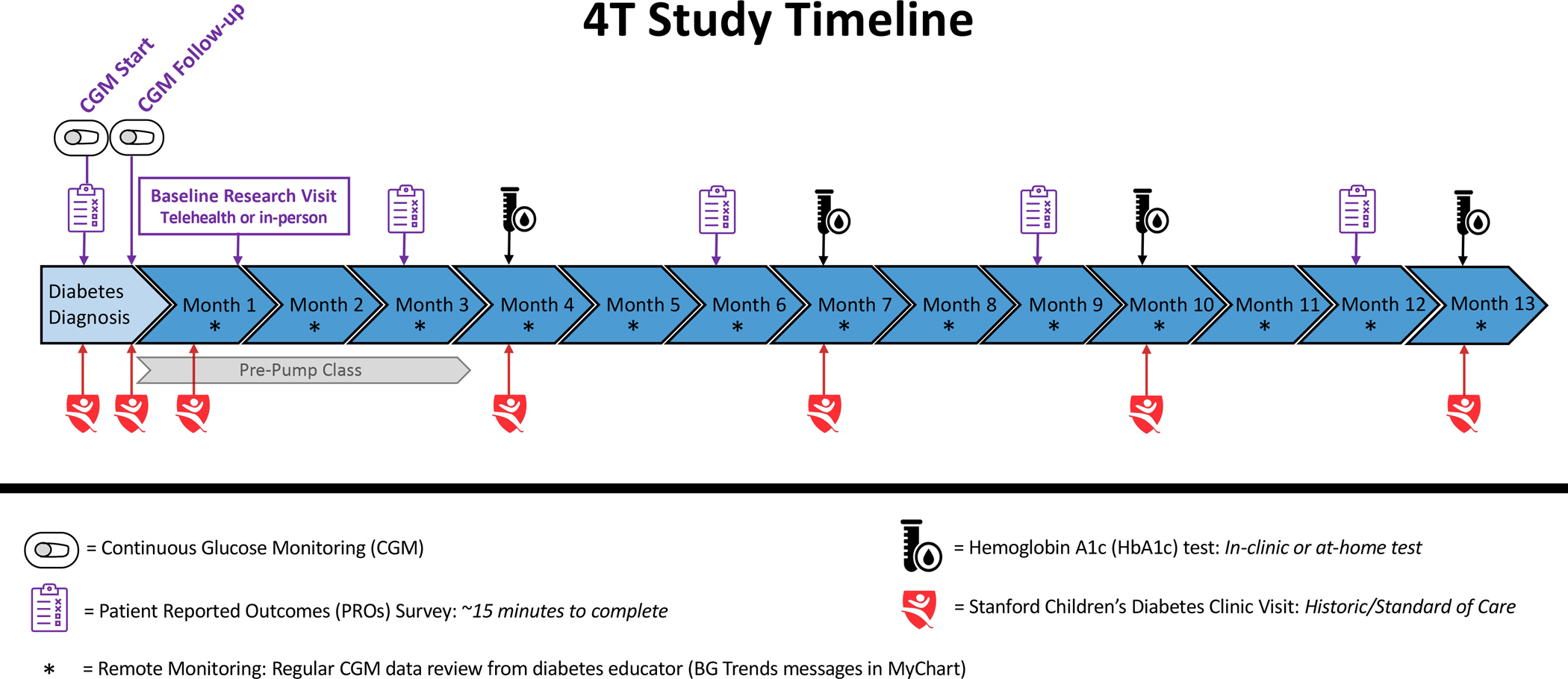
The 4T study 2 timeline including usual clinical visits and specific 4T study 2 components. Study 2 will encourage the use of an insulin pump or AID system during the initial 3-month period post-diagnosis and will incorporate a step-down approach (weekly versus monthly) to the cadence of remote data reviews.
Additionally, during study 2, youth participants will also be encouraged to initiate the use of an insulin pump or AID system during the initial 3-month period post-diagnosis. The use of AID systems as a standard of care for all participants will be encouraged, but participants can also choose an open loop insulin pump or multiple daily injections. Participants that do not want to use an insulin pump or AID system will be encouraged to use a smart insulin pen (e.g., InPen™ Smart Insulin Pen, Medtronic, Minneapolis, MN) which tracks insulin doses in real-time. Participants do not have to initiate an insulin pump, AID system, or InPen™ to participate in the study, but will be encouraged to do so to enhance diabetes management by integrating all devices into the TIDE dashboard.
The integration of psychology into the existing program allows the team to respond to referrals, and the addition of systematic screening through PROs adds another layer of safety monitoring in case positive screens were not observed as needing a referral. The psychosocial screening system will be designed so that it is sustainable and translatable to centers with fewer psychology team resources or alternative resources, such as a social work team member. This is in line with current American Diabetes Association (ADA) recommendations on psychosocial care and needs to be modeled for broader implementation (48–50). We will assess factors that inform sustainability, acceptability, and feasibility. We will also continue with weekly diabetes team meetings at which feedback from team members will lead to iterations of the overall 4T study plan.
For study 2, integrating the physical activity trackers and disseminating of exercise education modules will be expanded to include both English- and Spanish-speaking families (Figure 3). Our aim is to be more inclusive of the general patient population and therefore, including Spanish-speaking families in the study 2 exercise study. By including Spanish-speaking families in study 2, this will also improve the overall generalizability of study findings for future dissemination.
Figure 3:
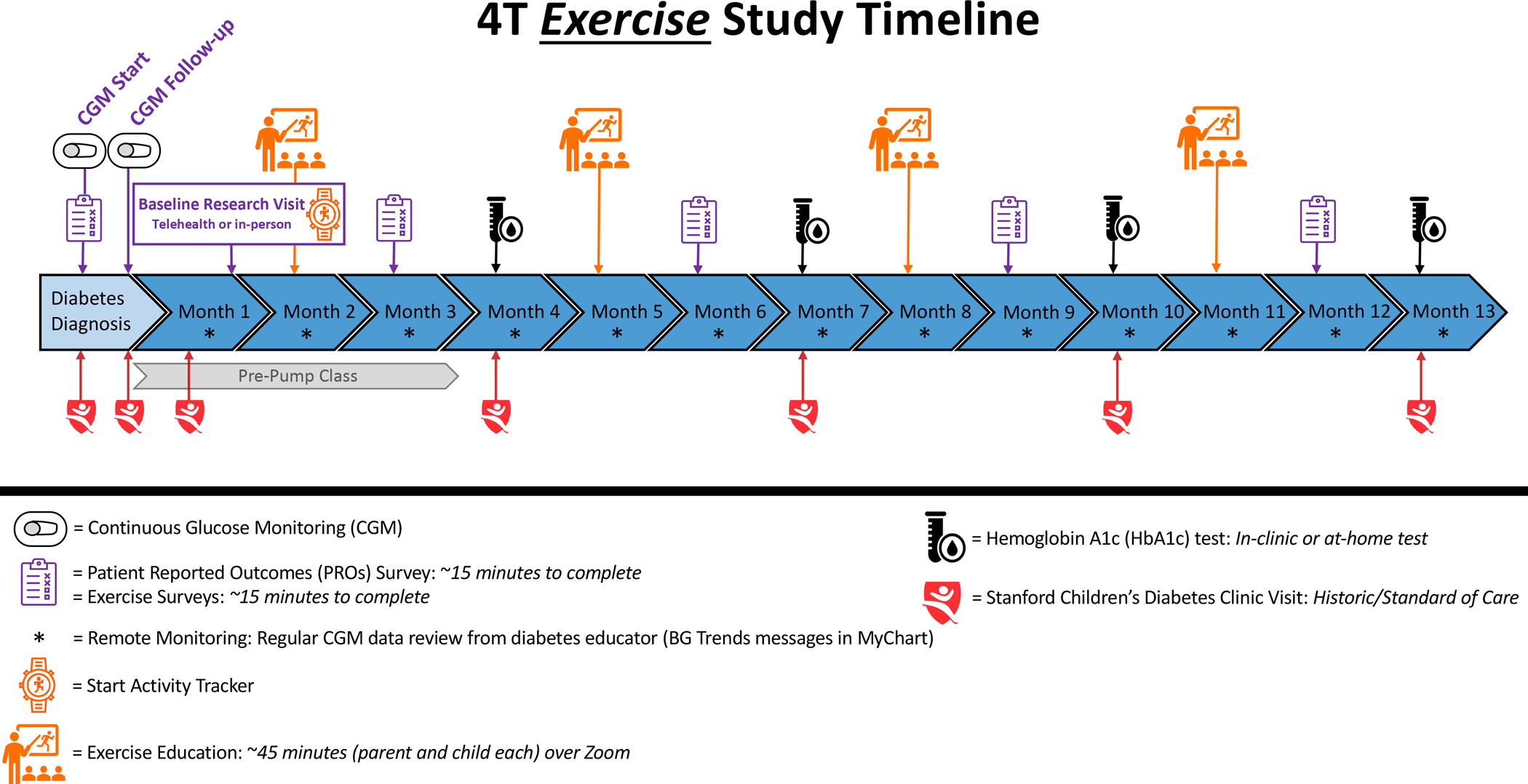
The 4T study 2 timeline with the exercise intervention including usual clinical visits, 4T study 2 components and specific 4T study 2 exercise components.
An important focus for study 2 is the expansion and growth of the 4T study beyond the Stanford clinic. To do so, we are focusing on scaling the 4T program at three levels. First, is to document the current workflow including constraints and frictions for CDCES to review data and provide patient education at the clinic level. For example, an initial deep dive into time utilization of CDCES’ suggests additional opportunities continue to exist for algorithmic improvements in workflow, that would free up more time for them to focus on “top-of-license” tasks (29). Second, are the operational improvements. For expansion both at Stanford and beyond, we need to develop scalable telehealth workflows, and our technology platform needs to account for heterogeneity in clinic staff and available resources, as well as heterogeneity in patient needs over time (20). Finally, we need to quantify costs and efficiency gains to arrive at an understanding of economic feasibility (20). This is especially critical for deployment beyond the Stanford clinic. This requires a careful inspection of the billing models available for telemedicine (e.g., how will the asynchronous review of patient data be reimbursed?) and the costs of clinic resources, compared against the gains in quality of patient care provided.
4T Study Challenges
Based on Pilot 4T and study 1, strategies and improvements have been made and iterated on including advancements to the TIDE dashboard for study 2. However, a few recurring themes and challenges remain including missing (both CGM and physical activity) data and limitations with capturing specific variables (e.g., insulin data). With insulin pump downloads, specific and individualized insulin data can be captured in a subset of participants using this technology, but for participants in the Pilot 4T and study 1 using MDI (not Smart Pen technology), there are barriers to collecting insulin data. As such, for study 2, we have discussed the importance of encouraging the use of an insulin pump or Smart Insulin Pen technology. Another challenge for translation of the 4T program to other clinics is the coverage of CGM early after diagnosis before insurance approval. Similarly, most pediatric diabetes programs are understaffed and implementation of a new program without additional resources may be a barrier and more data on financial feasibility are needed. Moreover, any disruptions in access to CGM data or new barriers to access of CGM data would negatively impact the 4T program (51). Finally, a primary component of the 4T study involves CGM glucose and physical activity data sharing which raises a recurring concern in the clinical and academic setting around the privacy and ownership of data (52).
Conclusion
In summary, the 4T study has leveraged CGM remote data review and novel telemedicine workflows to improve T1D patient care. Novel, open-source dashboards deployed through the 4T study has allowed CDCES’ to remotely monitor patients’ CGM data, and an algorithmic backend prioritizes these patients for review and follow-up. This tool has not only improved patient care by dramatically speeding up the typical once-quarterly cadence of CGM data review, but it has increased CDCES efficiency by reducing the time spent on reviewing patient data (20, 29, 30). Overall, our Pilot 4T data demonstrate a 0.5% reduction in HbA1c at 1-year, improved QoL, and family and provider satisfaction and the challenges described above are being explored and addressed in current and future iterations of the 4T program.
Key Bullet Points.
The 4T study utilizes an open-source dashboard with algorithm-enabled prioritization of participants who would benefit from remote CGM data review and dose adjustments by the diabetes care team to apply precision medicine on a population health basis
The 4T study with early CGM initiation and intensified early education have shown success in improving glycemic outcomes in youth with T1D
With future iterations of the 4T study, our aim is to create and implement a translatable program that can facilitate better outcomes for pediatric clinics across the United States
Acknowledgements
The authors would like to acknowledge all participants and families involved in the 4T Study. A special thank you to all staff and team members that are involved in the 4T Study including Priya Prahalad, David Scheinker, Korey Hood, Ananta Addala, Ramesh Johari, Manisha Desai, Victoria Ding, Nora Arrizon-Ruiz, Liz Heckard, Dom Mitchell, Barry Conrad, Ana Cortes, Andrea Ospina Bonilla, Rebecca Gardner, Carolyn Herrera, Julie Hooper, Brianna Leverenz, Jeannine Leverenz, Alex Freeman, Esli Osmanlliu, Erica Pang, Natalie Pageler, Piper Sagan, Annette Chmielewski, Julie Senaldi, Molly Tanenbaum, and Anjoli Martinez-Singh. Also, thank you to the 4T Exercise team collaborators including Michael Riddell, Mark Clements, and Avinash Kollu.
An additional thank you to the Stanford Systems Utilization Research Force (SURF) students who contributed to the development and deployment of the dashboard that facilitated remote data review: Oseas Oyardi, Angela Gu, Josh Grossman, Jacqualine Jil Valon, Anastasiya Vitko, Dianelys Perez Morales, Daniel Jun, Ryan Leonard, Michael Zikai Gao, Annie Chang, Prashant Yadav, Isha Thapa, and Johannes Opsahl Ferstad.
Financial Support and Sponsorship
This work was supported in part by the National Institutes of Health P30DK116074 via the Stanford Diabetes Research Center and R18DK122422 to D.M.M. CGM supplies for the first month (transmitter, 3 sensors, and receiver per patient) were donated by Dexcom. Funding for iOS devices and some CGM supplies was provided by a grant through the Lucile Packard Children’s Hospital Auxiliaries Endowment. The 4T Exercise study is supported by the Leona M. and Harry B. Helmsley Charitable Trust and the ISPAD-JDRF Research Fellowship to D.P.Z.
Conflicts of Interest
D.P.Z. has received speaker’s honoraria from Medtronic Diabetes, Ascensia Diabetes, and Insulet Canada; and research support from the Helmsley Charitable Trust and ISPAD-JDRF Research Fellowship. She is also on the Dexcom Advisory board. D.M.M. has received research support from the National Institutes of Health, JDRF, NSF, and the Helmsley Charitable Trust; and his institution has received research support from Medtronic, Dexcom, Insulet, Bigfoot Biomedical, Tandem, and Roche. He has consulted for Abbott, the Helmsley Charitable Trust, Sanofi, Novo Nordisk, Eli Lilly, and Insulet, and is supported by grant number P30DK116074. F.K.B. has no conflicts of interest.
References:
Papers of particular interest, published within the annual period of review, have been highlighted as:
* = of special interest
** = of outstanding interest
- 1.Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes control and complications trial. Diabetes control and complications trial research group. J Pediatr 1994;125(2):177–88. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 2.Diabetes C, Complications Trial Research G, Nathan DM, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329(14):977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the t1d exchange in 2016–2018. Diabetes Technol Ther 2019;21(2):66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the u.S.: Updated data from the t1d exchange clinic registry. Diabetes Care 2015;38(6):971–8. doi: 10.2337/dc15-0078. [DOI] [PubMed] [Google Scholar]
- 5.Borus JS and Laffel L. Adherence challenges in the management of type 1 diabetes in adolescents: Prevention and intervention. Curr Opin Pediatr 2010;22(4):405–11. doi: 10.1097/MOP.0b013e32833a46a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pihoker C, Forsander G, Fantahun B, et al. Ispad clinical practice consensus guidelines 2018: The delivery of ambulatory diabetes care to children and adolescents with diabetes. Pediatr Diabetes 2018;19 Suppl 27:84–104. doi: 10.1111/pedi.12757. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbauer J, Dost A, Karges B, et al. Improved metabolic control in children and adolescents with type 1 diabetes: A trend analysis using prospective multicenter data from germany and austria. Diabetes Care 2012;35(1):80–6. doi: 10.2337/dc11-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermann JM, Miller KM, Hofer SE, et al. International comparison of hba1c across lifespan in males and females with type 1 diabetes. in American Diabetes Association. 2020. Chicago, IL. [Google Scholar]
- 9.Danne T and Hanas R. The mission of sweet: Harmonize care to optimize outcomes of children with diabetes worldwide. Pediatr Diabetes 2016;17 Suppl 23:3–6. doi: 10.1111/pedi.12411. [DOI] [PubMed] [Google Scholar]
- 10.Prahalad P, Tanenbaum M, Hood K, et al. Diabetes technology: Improving care, improving patient-reported outcomes and preventing complications in young people with type 1 diabetes. Diabet Med 2018;35(4):419–429. doi: 10.1111/dme.13588. [DOI] [PubMed] [Google Scholar]
- 11.Cameron FJ, de Beaufort C, Aanstoot HJ, et al. Lessons from the hvidoere international study group on childhood diabetes: Be dogmatic about outcome and flexible in approach. Pediatr Diabetes 2013;14(7):473–80. doi: 10.1111/pedi.12036. [DOI] [PubMed] [Google Scholar]
- 12.Skinner TC, Lange KS, Hoey H, et al. Targets and teamwork: Understanding differences in pediatric diabetes centers treatment outcomes. Pediatr Diabetes 2018;19(3):559–565. doi: 10.1111/pedi.12606. [DOI] [PubMed] [Google Scholar]
- 13.Hofer SE, Raile K, Frohlich-Reiterer E, et al. Tracking of metabolic control from childhood to young adulthood in type 1 diabetes. J Pediatr 2014;165(5):956–61 e1–2. doi: 10.1016/j.jpeds.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Svensson J, Johannesen J, Mortensen HB, et al. Improved metabolic outcome in a danish diabetic paediatric population aged 0–18 yr: Results from a nationwide continuous registration. Pediatr Diabetes 2009;10(7):461–7. doi: 10.1111/j.1399-5448.2008.00460.x. [DOI] [PubMed] [Google Scholar]
- 15.Samuelsson U, Akesson K, Peterson A, et al. Continued improvement of metabolic control in swedish pediatric diabetes care. Pediatr Diabetes 2018;19(1):150–157. doi: 10.1111/pedi.12467. [DOI] [PubMed] [Google Scholar]
- 16.Hanberger L, Samuelsson U, Bertero C, et al. The influence of structure, process, and policy on hba(1c) levels in treatment of children and adolescents with type 1 diabetes. Diabetes Res Clin Pract 2012;96(3):331–8. doi: 10.1016/j.diabres.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Charalampopoulos D, Hermann JM, Svensson J, et al. Exploring variation in glycemic control across and within eight high-income countries: A cross-sectional analysis of 64,666 children and adolescents with type 1 diabetes. Diabetes Care 2018;41(6):1180–1187. doi: 10.2337/dc17-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherr JL, Hermann JM, Campbell F, et al. Use of insulin pump therapy in children and adolescents with type 1 diabetes and its impact on metabolic control: Comparison of results from three large, transatlantic paediatric registries. Diabetologia 2016;59(1):87–91. doi: 10.1007/s00125-015-3790-6. [DOI] [PubMed] [Google Scholar]
- 19.Szypowska A, Schwandt A, Svensson J, et al. Insulin pump therapy in children with type 1 diabetes: Analysis of data from the sweet registry. Pediatr Diabetes 2016;17 Suppl 23:38–45. doi: 10.1111/pedi.12416. [DOI] [PubMed] [Google Scholar]
- 20.Scheinker D, Prahalad P, Johari R, et al. How a technology-enabled care model for type one diabetes improves quality while enhancing provider satisfaction. NEJM Catalyst 2022. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prahalad P, Ding VY, Zaharieva DP, et al. Teamwork, targets, technology, and tight control in newly diagnosed type 1 diabetes: Pilot 4t study. J Clin Endocrinol Metab 2021. doi: 10.1210/clinem/dgab859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laffel LM, Kanapka LG, Beck RW, et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: A randomized clinical trial. JAMA 2020;323(23):2388–2396. doi: 10.1001/jama.2020.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study G. Effectiveness of continuous glucose monitoring in a clinical care environment: Evidence from the juvenile diabetes research foundation continuous glucose monitoring (jdrf-cgm) trial. Diabetes Care 2010;33(1):17–22. doi: 10.2337/dc09-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patton SR, Noser AE, Youngkin EM, et al. Early initiation of diabetes devices relates to improved glycemic control in children with recent-onset type 1 diabetes mellitus. Diabetes Technol Ther 2019;21(7):379–384. doi: 10.1089/dia.2019.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prahalad P, Zaharieva DP, Addala A, et al. Improving clinical outcomes in newly diagnosed pediatric type 1 diabetes: Teamwork, targets, technology, and tight control-the 4t study. Front Endocrinol (Lausanne) 2020;11:360. doi: 10.3389/fendo.2020.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burckhardt MA, Roberts A, Smith GJ, et al. The use of continuous glucose monitoring with remote monitoring improves psychosocial measures in parents of children with type 1 diabetes: A randomized crossover trial. Diabetes Care 2018;41(12):2641–2643. doi: 10.2337/dc18-0938. [DOI] [PubMed] [Google Scholar]
- **27.Addala A, Zaharieva DP, Gu AJ, et al. Clinically serious hypoglycemia is rare and not associated with time-in-range in youth with newly diagnosed type 1 diabetes. JCEM 2021;106(11). [DOI] [PMC free article] [PubMed] [Google Scholar]; A recent publication highlighting hypoglycemia rates do not differ in a clinically meaningful way by partial clinical remission status (PCR). This study also highlights the importance of a larger trial in the year post-diagnosis to determine whether a lower threshold (e.g., 65 mg/dL) is safe and may in turn, reduce hypoglycemia- related alarms without increasing the risk of clinically significant or severe hypoglycemia.
- 28.Kumar RB, Goren ND, Stark DE, et al. Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J Am Med Inform Assoc 2016;23(3):532–7. doi: 10.1093/jamia/ocv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *29.Ferstad JO, Vallon JJ, Jun D, et al. Population-level management of type 1 diabetes via continuous glucose monitoring and algorithm-enabled patient prioritization: Precision health meets population health. Pediatr Diabetes 2021;22(7):982–991. doi: 10.1111/pedi.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrates the work and advancements with the remote data review dashboard and prioritization of patient contact.
- 30.Scheinker D, Gu A, Grossman J, et al. Algorithm-enabled, personalized glucose management for type 1 diabetes at the population scale: A prospective evaluation in clinical practice. JMIR Diabetes 2022. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prahalad P, Addala A, Scheinker D, et al. Cgm initiation soon after type 1 diabetes diagnosis results in sustained cgm use and wear time. Diabetes Care 2020;43(1):e3–e4. doi: 10.2337/dc19-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanenbaum ML, Zaharieva DP, Addala A, et al. “I was ready for it at the beginning”: Parent experiences with early introduction of continuous glucose monitoring following their child’s type 1 diabetes diagnosis. Diabetic Med 2021. doi: 10.1111/dme.14567 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanenbaum M, Zaharieva DP, Addala A, et al. Initiating cgm over telehealth is well accepted by parents of newly diagnosed youth with t1d (#as06). in Advanced Technologies & Treatments for Diabetes (ATTD). 2022. Barcelona, Spain. [Google Scholar]
- 34.Addala A, Hanes S, Naranjo D, et al. Provider implicit bias impacts pediatric type 1 diabetes technology recommendations in the united states: Findings from the gatekeeper study. J Diabetes Sci Technol 2021;15(5):1027–1033. doi: 10.1177/19322968211006476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker AF, Hood KK, Gurka MJ, et al. Barriers to technology use and endocrinology care for underserved communities with type 1 diabetes. Diabetes Care 2021;44(7):1480–1490. doi: 10.2337/dc20-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **36.Addala A, Auzanneau M, Miller K, et al. A decade of disparities in diabetes technology use and hba1c in pediatric type 1 diabetes: A transatlantic comparison. Diabetes Care 2021;44(1):133–140. doi: 10.2337/dc20-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]; This important publication demonstrates that the use of diabetes technology is lowest and HbA1c is highest in those of the lowest socioeconomic status quintile in the United States. In addition, this difference for HbA1c has broadened in the past decade. The reasons for this increasing HbA1c despite increasing diabetes technology use in the US underscores the critical need for additional research on this topic.
- 37.Riddell MC, Gallen IW, Smart CE, et al. Exercise management in type 1 diabetes: A consensus statement. Lancet Diabetes Endocrinol 2017;5(5):377–390. doi: 10.1016/S2213-8587(17)30014-1. [DOI] [PubMed] [Google Scholar]
- 38.Adolfsson P, Riddell MC, Taplin CE, et al. Ispad clinical practice consensus guidelines 2018: Exercise in children and adolescents with diabetes. Pediatr Diabetes 2018;19 Suppl 27:205–226. doi: 10.1111/pedi.12755. [DOI] [PubMed] [Google Scholar]
- 39.Corbin KD, Driscoll KA, Pratley RE, et al. Obesity in type 1 diabetes: Pathophysiology, clinical impact, and mechanisms. Endocr Rev 2018;39(5):629–663. doi: 10.1210/er.2017-00191. [DOI] [PubMed] [Google Scholar]
- 40.Chetty T, Shetty V, Fournier PA, et al. Exercise management for young people with type 1 diabetes: A structured approach to the exercise consultation. Front Endocrinol (Lausanne) 2019;10:326. doi: 10.3389/fendo.2019.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tulchin-Francis K, Stevens W Jr., Gu X, et al. The impact of the coronavirus disease 2019 pandemic on physical activity in u.S. Children. J Sport Health Sci 2021;10(3):323–332. doi: 10.1016/j.jshs.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riddell MC, Li Z, Beck RW, et al. More time in glucose range during exercise days than sedentary days in adults living with type 1 diabetes. Diabetes Technol Ther 2021;23(5):376–383. doi: 10.1089/dia.2020.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brennan MC, Albrecht MA, Brown JA, et al. Self-management group education to reduce fear of hypoglycemia as a barrier to physical activity in adults living with type 1 diabetes: A pilot randomized controlled trial. Can J Diabetes 2021;45(7):619–628. doi: 10.1016/j.jcjd.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Litchfield I, Andrews RC, Narendran P, et al. Patient and healthcare professionals perspectives on the delivery of exercise education for patients with type 1 diabetes. Front Endocrinol (Lausanne) 2019;10:76. doi: 10.3389/fendo.2019.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Isganaitis E, Raghinaru D, Ambler-Osborn L, et al. Closed-loop insulin therapy improves glycemic control in adolescents and young adults: Outcomes from the international diabetes closed-loop trial. Diabetes Technol Ther 2021;23(5):342–349. doi: 10.1089/dia.2020.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaharieva DP, Messer L, Paldus B, et al. Glucose control during physical activity and exercise using closed loop technology in type 1 diabetes. Can J Diabetes 2020;44(8):740–749. [DOI] [PubMed] [Google Scholar]
- 47.Paldus B, Morrison D, Lee M, et al. Strengths and challenges of closed loop insulin delivery during exercise in people with type 1 diabetes: Potential future directions. Journal of Diabetes Science and Technology 2022. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.American Diabetes Association Professional Practice C, American Diabetes Association Professional Practice C, Draznin B, et al. 5. Facilitating behavior change and well-being to improve health outcomes: Standards of medical care in diabetes-2022. Diabetes Care 2022;45(Suppl 1):S60–S82. doi: 10.2337/dc22-S005. [DOI] [PubMed] [Google Scholar]
- 49.Marrero DG, Hilliard ME, Maahs DM, et al. Using patient reported outcomes in diabetes research and practice: Recommendations from a national workshop. Diabetes Res Clin Pract 2019;153:23–29. doi: 10.1016/j.diabres.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 50.Young-Hyman D, de Groot M, Hill-Briggs F, et al. Psychosocial care for people with diabetes: A position statement of the american diabetes association. Diabetes Care 2016;39(12):2126–2140. doi: 10.2337/dc16-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Addala A, Maahs DM, Scheinker D, et al. Uninterrupted continuous glucose monitoring access is associated with a decrease in hba1c in youth with type 1 diabetes and public insurance. Pediatr Diabetes 2020. doi: 10.1111/pedi.13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kostkova P, Brewer H, de Lusignan S, et al. Who owns the data? Open data for healthcare. Front Public Health 2016;4:7. doi: 10.3389/fpubh.2016.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]


