Abstract
Aim
Globally, evidence from short‐term studies is insufficient for the guidelines to uniformly recommend a particular antipsychotic(s) for the maintenance treatment of schizophrenia. Therefore, long‐term comprehensive evaluation of antipsychotics is required from a social rehabilitation perspective, especially for drugs that have not yet been studied. The Japan Useful Medication Program for Schizophrenia (JUMPs) is a large‐scale, long‐term naturalistic study to present pivotal 52‐week data on the continuity of second‐generation antipsychotics (SGA: aripiprazole, blonanserin, and paliperidone).
Methods
JUMPs was an open‐label, three‐arm, randomized, parallel‐group, 52‐week study. Enrolled patients had schizophrenia, were ≥20 years old, and required antipsychotic treatment or switched from previous therapy. The primary endpoint was treatment discontinuation rate over 52 weeks. Secondary outcomes included remission rate, social functioning, and quality‐of‐life scores [Personal and Social Performance Scale (PSP) and EuroQol‐5 dimensions], and safety.
Results
In total, 251 patients received aripiprazole (n = 82), blonanserin (n = 85), or paliperidone (n = 84). The discontinuation rate (P = 0.9771) and remission rates (P > 0.05) over 52 weeks did not differ significantly between the three treatment groups. The discontinuation rates were 68.3%, 68.2%, and 65.5% in the aripiprazole, blonanserin, and paliperidone groups, respectively. Significant improvements (all P < 0.05) from baseline in PSP scores were observed at start of monotherapy, week 26, and week 52 in the overall cohort and blonanserin group and at week 26 in the aripiprazole group. The adverse event profile favored blonanserin.
Conclusion
All three SGAs evaluated in this study showed similar treatment discontinuation rates in patients with chronic schizophrenia in Japan.
Keywords: discontinuation rate, long‐term effectiveness, remission rate, second generation antipsychotics
The global age‐standardized point prevalence of schizophrenia in 2016 was estimated to be 0.28% and did not vary widely across countries or regions. Globally, the prevalence of schizophrenia increased from 13.1 million cases in 1990 to 20.9 million cases in 2016. 1 According to the 2015 report of the Ministry of Health, Labour and Welfare, 773 000 patients in Japan were being prescribed treatment for schizophrenia, schizotypal disorders, and delusional disorders in 2014. 2
Globally, both first‐generation antipsychotics (FGAs) and second‐generation antipsychotics (SGAs) are recommended for preventing relapse, and drug selection is based on the risk–benefit profile of each drug. 3 , 4 , 5 , 6 Because of the insufficiency and heterogeneity of available evidence, definitive recommendations for SGAs are not available in the guidelines outside Japan 3 , 4 , 6 from the viewpoint of both effectiveness or efficacy and safety. However, advantages with respect to the occurrence of extrapyramidal symptoms (EPS) or evidence showing superiority of certain SGAs in terms of treatment persistence is supported by both the Japanese Society of Neuropsychopharmacology (JSNP) and the World Federation of the Societies of Biological Psychiatry (WFSBP) guidelines, 5 , 6 although no specific SGA is recommended.
Overall, the aim of treatment is to achieve symptom remission and recovery, which should also result in improved social functioning. 7 As the clinical efficacy and safety of SGAs have been largely derived from short‐term randomized controlled trials (RCTs) evaluating a limited number of SGAs for comparison, it is often difficult to generalize the results of RCTs to routine clinical practice, which often lasts longer. On the other hand, the assessments of core outcomes of antipsychotic treatment, such as all‐cause discontinuation, quality of life (QOL), and psychopathology, have historically been often evaluated using a naturalistic study design—recently it has also been called a ‘pragmatic trial’ design—so that the observations from these trials closely resemble the real‐world outcomes in usual practice. Large‐scale naturalistic studies that evaluated the long‐term effectiveness of SGAs in routine clinical practice have been conducted. 8 , 9 , 10 , 11 Notably, the endpoints used in these naturalistic studies—discontinuation rates, remission rates, and improvement in QOL—were able to provide a clear assessment of the effectiveness of a drug.
In this study, three common SGAs (aripiprazole, blonanserin, and paliperidone) were selected because they were approved in Japan after olanzapine and risperidone, and their discontinuation has not been evaluated in long‐term naturalistic RCTs such as CATIE and EUFEST. 8 , 9 Aripiprazole is a partial agonist of the dopamine D2 receptor and does not interfere with the normal functioning of the striatum and pituitary gland. 12 Blonanserin has a higher affinity for dopamine D2 receptors than for serotonin 2A (5‐HT2A) receptors. 13 These two drugs are less likely to cause weight gain or metabolic abnormalities. Paliperidone, the major active metabolite of risperidone, is a type of serotonin–dopamine antagonist with potent 5‐HT2A receptor–blocking activity but is relatively more likely to cause EPS and elevated blood prolactin levels. 14
Blonanserin was specifically included because it is approved and used only in Asia and its long‐term effectiveness has not been previously evaluated. For comparison with preceding naturalistic studies, we primarily evaluated the treatment discontinuation rate as a comprehensive indicator of effectiveness and safety. 15 , 16 , 17 , 18 In addition, remission rates were adopted as an essential indicator of effectiveness. Therefore, the Japan Useful Medication Program for Schizophrenia (JUMPs) undertook to compare the effectiveness and safety of three SGAs (aripiprazole, blonanserin, and paliperidone) in patients with schizophrenia, as assessed by discontinuation rates, remission rates, and also improvements in social functioning over 2 years. JUMPs was designed to have a broad eligibility to showcase standard investigator‐patient‐based decisions for continuation or discontinuation of treatment in a flexible maintenance dose setting. Here, we present the 52‐week pivotal results of JUMPs, the first long‐term naturalistic study assessing continuity of SGA treatment in patients with schizophrenia.
Methods
Study design
This open‐label, three‐arm, multicenter, randomized, parallel‐group study enrolled patients at 75 of 110 participating sites in Japan. The study was approved by the appropriate institutional review boards 19 and was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Tripartite Guideline for Good Clinical Practice, and the Ethics Guidelines for Clinical Research. 2 Written informed consent was obtained from all patients after they received a complete description of the study. The study is registered on UMIN‐CTR: https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000009273.
Patients
Outpatients with schizophrenia aged ≥20 years diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM‐IV‐TR); requiring new antipsychotic treatment (treatment naïve) or switching from the current antipsychotic medication to the other because of lack of efficacy, intolerability, or other reasons; and capable of providing written consent were included in the study. Patients with a history of allergy to the test drugs or risperidone; under strong influence of central nervous system suppressants such as barbiturate derivatives; receiving adrenaline, azole antifungal agents (excluding topical preparations), or human immunodeficiency virus (HIV) protease inhibitors; with moderately or severely compromised renal function (creatinine clearance <50 mL/min); with poorly controlled diabetes mellitus [glycated hemoglobin (HbA1c) (NGSP) >8.4%]; with severe symptoms despite sufficient antipsychotic treatment or a history of clozapine therapy; with other psychiatric illness in addition to schizophrenia; with complication of Parkinson's disease; with a history of alcohol or drug abuse; or likely to attempt suicide [Clinical Global Impression of Severity of Suicidality (CGI‐SS) class 4 or higher (severe tendency for suicide)] were excluded. The full exclusion criteria have been published previously by Ishigooka and colleagues. 19
Randomization and masking
Patients were randomized in a 1:1:1 ratio to receive aripiprazole, blonanserin, or paliperidone as monotherapy within 4 weeks after eligibility assessment (Fig. 1). Randomization was achieved using the ‘variable permuted block randomization’ method (35 blocks each with block sizes of 12 and 15) for treatment groups, and no stratification factor was set. A computer‐based random allocation was performed by Mebix Inc., a third‐party clinical research organization (Minato‐ku, Tokyo; Akasaka Intercity).
Fig. 1.
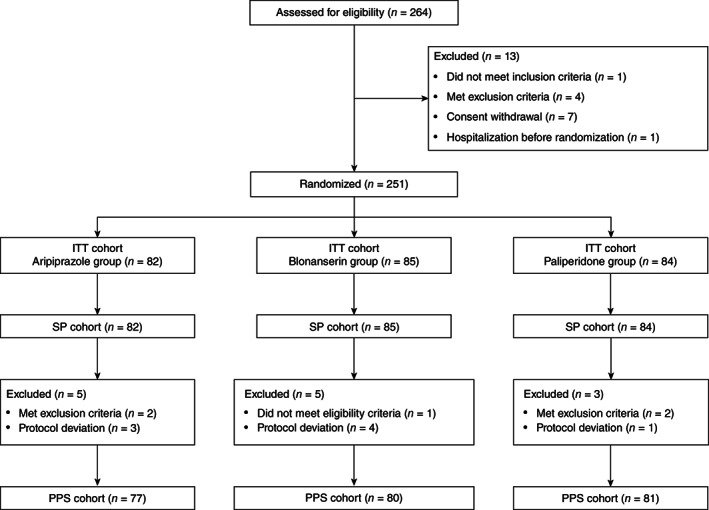
Patient disposition (CONSORT flow chart). ITT, intent to treat; PPS, per protocol set; SP, safety population.
Procedures
Patients requiring a switch from prior antipsychotics were switched to the protocol treatment during the dose adjustment period for 4 weeks (maximum 8 weeks). The initial dose and maximum oral maintenance dose during the titration/dose adjustment period were ≤12 mg/day and 30 mg/day for aripiprazole, ≤8 mg/day and 24 mg/day for blonanserin, and ≤6 mg/day and 12 mg/day once daily after breakfast for paliperidone, respectively. For patients with mild renal impairment (creatinine clearance 50 to <80 mL/min), the initial dose of paliperidone was 3 mg/day and was not to exceed 6 mg/day. During the study period, except for the dose adjustment period, the dosage of each study drug could be adjusted within the approved dosage range (aripiprazole 12–30 mg/day, blonanserin 8–24 mg/day, and paliperidone 6–12 mg/day), depending on the patients' clinical condition. The chlorpromazine (CP)‐equivalent dose [dose equivalence of antipsychotics (2012 version)] 20 was evaluated from the number of prior antipsychotic medications taken before starting the study treatment and the total doses of the study medication used (Table S1). 20 The CP‐equivalent dose was calculated for every medication, excluding rescue medications, using the following formula:
The conditions for use of concomitant medications are presented in Table S2. Reasons for treatment discontinuation were lack of efficacy, intolerance to the study drug, adverse events (AEs) or worsening of schizophrenia complications, diagnosis of pregnancy, patient unwilling to follow treatment protocol, and investigator's discretion. There were no restrictions to alternative treatment after treatment discontinuation, and treatment with any one of the other two study drugs was permitted. Thereafter, patients were observed for the entire duration of the study period. Patients were withdrawn from the study if they required hospitalization for worsened psychiatric symptoms or significant adverse reactions due to study treatment, owing to patient‐related reasons (e.g. change of address or hospital), and at the patient's discretion. No follow‐up assessments were scheduled after withdrawal from the study.
Outcomes
The primary endpoint was the all‐cause treatment discontinuation rate over 52 weeks. Secondary endpoints were remission rate {defined by Andreasen [rating below mild on all eight items of the Positive and Negative Syndrome Scale (PANSS) for 6 consecutive months or more]}, which was evaluated from baseline to 12 weeks as determined by a physician (investigator) using the results of the postbaseline PANSS assessment and historical information according to Andreasen's criteria 21 , 22 ; social functioning and QOL scores [determined by the Personal and Social Performance Scale (PSP) 23 and the EuroQol‐5 dimensions (EQ‐5D)] 24 ; alleviation of psychiatric symptoms (using the eight items in the PANSS score); psychiatric symptom aggravation and recurrence rate based on PANSS, Clinical Global Impression of Improvement (CGI‐I), 25 and CGI‐SS scores 26 ; incidence of AEs; and Drug‐Induced Extrapyramidal Symptoms Scale (DIEPSS) score. 27 The eight items in the PANSS score were delusions, unusual thought content, hallucinatory behavior, conceptual disorganization, mannerisms and posturing, blunted affect, social withdrawal, and lack of spontaneity. 21 Observations and tests were conducted at eligibility determination (baseline); at the start of monotherapy and at weeks 8, 12, 26, and 52; or at the time of treatment discontinuation. Further, a post hoc analysis was performed to evaluate the inter‐group differences in the proportion of patients with AEs and endocrine disorder/related side effects or AEs.
Statistical analysis
Data were evaluated from the intent‐to‐treat (ITT) population (all randomized patients who received study medication), per protocol set (patients in the ITT population who had no relevant deviations from the study protocol), and safety population (all patients with available safety data). On the basis that data from approximately 100 patients are recommended for safety evaluations of >1‐year duration, 28 and that previous clinical trials assessing aripiprazole, blonanserin, and paliperidone randomized at least 100 patients per active drug group, a total target number of 300 patients was set for this 2‐year naturalistic study. For the primary endpoint analysis, treatment discontinuation was estimated using the Kaplan–Meier method along with corresponding 95% confidence intervals (CIs). For effectiveness evaluations, the last observation carried forward or mixed‐effect model repeated measure method was used to impute values for missing data. Data obtained after treatment discontinuation were not analyzed. The Kruskal–Wallis test and one‐way analysis of variance were used for significant differences on a continuous dependent variable by a categorical independent variable (with two or more groups). Continuous and categorical variables at baseline were compared between treatment groups by an independent t test and the Chi‐square test, respectively.
In the exploratory analysis to identify other factors that may affect the continuity of treatment, Cox proportional‐hazards regression models were used to evaluate the risk factors for treatment discontinuation and determine the hazard ratios (HRs) and corresponding 95% CIs. Separate multivariate Cox proportional‐hazards regression analyses were conducted using variables such as CP‐equivalent dose <1000 mg and ≥1000 mg (model 1) and number of prior medications (1 and ≥2; model 2); each was adjusted for sex, duration of illness (≥6 years), and PANSS total scores (≥80). The 1000‐mg CP‐equivalent dose was considered the cutoff point because the recommended standard daily dose of antipsychotic drugs is less than this dose. 29 Furthermore, the duration of illness of 6 years was determined according to the critical period, 30 and the PANSS score of 80 was set between ‘moderately ill’ and ‘markedly ill’. 31 Separate analyses were conducted by including and excluding patients without prior medication at baseline. The Tukey's test was used for post hoc analyses of inter‐group differences in the proportion of patients with AEs and endocrine disorder/related side effects or AEs.
The statistical analysis was contracted to Mebix (Minato‐ku, Tokyo; Akasaka Intercity) and performed using SAS version 9.4 (SAS Institute Inc., Cary, NC); a P‐value of < 0.05 was considered statistically significant for all tests.
Results
Of the 264 patients assessed for eligibility between July 2012 and December 2013, 251 were randomized in a 1:1:1 ratio to receive aripiprazole (n = 82), blonanserin (n = 85), or paliperidone (n = 84; ITT cohort) (Fig. 1). Overall, patient demographics and baseline characteristics were similar among the three groups, and 6% of patients were treatment naïve (Table 1).
Table 1.
Patient demographics and baseline characteristics (ITT cohort)
| Characteristics, mean (SD) | Overall N = 251 | Aripiprazole group n = 82 | Blonanserin group n = 85 | Paliperidone group n = 84 | Statistical test (P value) |
|---|---|---|---|---|---|
| Age, years | 46.5 (13.3) | 48.0 (14.1) | 46.7 (12.6) | 45.0 (13.2) | 0.3626 a |
| Sex, male, n (%) | 133 (53.0) | 42 (51.2) | 48 (56.5) | 43 (51.2) | 0.7313 b |
| Height, cm | 162.9 (9.0) | 161.5 (9.6) | 163.7 (9.0) | 163.4 (8.1) | 0.2212 a |
| Body weight, kg | 67.7 (14.9) | 66.5 (17.4) | 69.1 (14.5) | 67.3 (12.5) | 0.5174 a |
| Disease type (DSM‐IV classifications), n (%) | |||||
| Paranoid | 161 (64.1) | 55 (67.1) | 51 (60.0) | 55 (65.5) | 0.5029 b |
| Disorganized | 15 (6.0) | 3 (3.7) | 6 (7.1) | 6 (7.1) | |
| Catatonic | 9 (3.6) | 5 (6.1) | 2 (2.4) | 2 (2.4) | |
| Undifferentiated | 22 (8.8) | 5 (6.1) | 7 (8.2) | 10 (11.9) | |
| Residual | 44 (17.5) | 14 (17.1) | 19 (22.4) | 11 (13.1) | |
| Clinical picture (selected parameters), n (%) | |||||
| Hallucinatory/delusion state | 76 (30.3) | 28 (34.1) | 25 (29.4) | 23 (27.4) | |
| Delusions in foreground | 27 (10.8) | 10 (12.2) | 9 (10.6) | 8 (9.5) | |
| Loss of initiative/apathy in foreground I | 14 (5.6) | 4 (4.9) | 4 (4.7) | 6 (7.1) | |
| Loss of initiative/apathy in foreground II | 123 (49.0) | 42 (51.2) | 44 (51.8) | 37 (44.0) | |
| Neurosis‐like state in foreground | 16 (6.4) | 3 (3.7) | 4 (4.7) | 9 (10.7) | |
| Disease duration, years | 17.1 (12.3) | 18.9 (13.3) | 16.5 (11.7) | 15.8 (11.9) | 0.2392 a |
| <6, n (%) | 57 (22.7) | 19 (23.2) | 18 (21.2) | 20 (23.8) | 0.9132 b |
| ≥6, n (%) | 194 (77.3) | 63 (76.8) | 67 (78.8) | 64 (76.2) | |
| Prior treatment, n (%) | |||||
| No | 15 (6.0) | 5 (6.1) | 4 (4.7) | 6 (7.1) | 0.7986 b |
| Yes | 236 (94.0) | 77 (93.9) | 81 (95.3) | 78 (92.9) | |
| Reason for switching from prior medications, n (%) | |||||
| Lack of efficacy of prior medications | 99 (41.9) | 32 (41.6) | 34 (42.0) | 33 (42.3) | 0.4430 b |
| Treated with polypharmacy | 23 (9.7) | 8 (10.4) | 10 (12.3) | 5 (6.4) | |
| Lowered tolerability | 54 (22.9) | 19 (24.7) | 13 (16.0) | 22 (28.2) | |
| Patient's reason | 30 (12.7) | 7 (9.1) | 11 (13.6) | 12 (15.4) | |
| Others | 30 (12.7) | 11 (14.3) | 13 (16.0) | 6 (7.7) | |
| Monotherapy/polypharmacy, n (%) | |||||
| Monotherapy (1) | 182 (77.1) | 53 (68.8) | 62 (76.5) | 67 (85.9) | 0.0404 b |
| Polypharmacy (≥2) | 54 (22.9) | 24 (31.2) | 19 (23.5) | 11 (14.1) | |
| Chlorpromazine‐equivalent dose, mean (SD) and n (%) | |||||
| Mean (SD) | 442.5 (321.7) | 453.6 (335.1) | 436.4 (304.0) | 438.0 (329.8) | 0.9347 a |
| <1000 mg | 221 (93.6) | 71 (92.2) | 76 (93.8) | 74 (94.9) | 0.7910 b |
| ≥1000 mg | 15 (6.4) | 6 (7.8) | 5 (6.2) | 4 (5.1) | |
| <400 mg | 116 (49.2) | 38 (49.4) | 39 (48.1) | 39 (50.0) | 0.9722 b |
| ≥400 mg | 120 (50.8) | 39 (50.6) | 42 (51.9) | 39 (50.0) | |
| Comorbidities, n (%) | 167 (66.5) | 63 (76.8) | 52 (61.2) | 52 (61.9) | 0.0548 b |
| Hypertension | 29 (11.6) | 13 (15.9) | 9 (10.6) | 7 (8.3) | 0.2991 b |
| Diabetes | 24 (9.6) | 9 (11.0) | 8 (9.4) | 7 (8.3) | 0.8444 b |
| Hyperlipidemia | 38 (15.1) | 14 (17.1) | 13 (15.3) | 11 (13.1) | 0.7736 b |
| Others | 142 (56.6) | 55 (67.1) | 45 (52.9) | 42 (50.0) | 0.0604 b |
| Social functioning (PSP) total score | 56.3 (20.8) | 59.0 (21.0) | 53.6 (21.1) | 56.5 (20.4) | 0.2487 a |
| Social functioning (EQ‐5D utility value) | 0.788 (0.167) c | 0.779 (0.168) | 0.768 (0.176) | 0.818 (0.153) d | 0.1258 a |
| PANSS total score | 73.8 (21.1) | 75.2 (22.2) | 75.7 (18.4) | 70.5 (22.4) | 0.2094 a |
| DIEPSS overall severity, n (%) | |||||
| None, normal | 163 (64.9) | 44 (53.7) | 60 (70.6) | 59 (70.2) | 0.0199 e |
| Minimal, questionable | 60 (23.9) | 24 (29.3) | 17 (20.0) | 19 (22.6) | |
| Mild | 20 (8.0) | 8 (9.8) | 7 (8.2) | 5 (6.0) | |
| Moderate | 7 (2.8) | 5 (6.1) | 1 (1.2) | 1 (1.2) | |
| Severe | 1 (0.4) | 1 (1.2) | 0 (0.0) | 0 (0.0) | |
| Per protocol switch to monotherapy within 8 weeks from randomization | 224 (89.2) | 75 (91.5) | 76 (89.4) | 73 (86.9) | 0.6370 b |
| Days elapsed until switch to monotherapy f | 20.0 (19.0) g | 20.4 (19.5) h | 20.5 (20.5) i | 19.2 (17.2) j | 0.8962 a |
| Days elapsed until switch to monotherapy among patients with prior medication | 21.3 (19.0) k | 21.5 (19.5) l | 21.3 (20.5) j | 20.9 (16.9) m | 0.9795 a |
ANOVA.
χ2 test.
n = 250.
n = 83.
Kruskal‐Wallis test.
Elapsed days for patients without prior medication was defined as zero.
n = 224.
n = 75.
n = 76.
n = 73.
n = 210.
n = 70.
n = 67.
ANOVA, analysis of variance; DIEPSS, Drug‐Induced Extrapyramidal Symptoms Scale; DSM, Diagnostic and Statistical Manual of Mental Disorders; EQ‐5D, EuroQol‐5 dimensions; ITT, intent to treat; PANSS, Positive and Negative Syndrome Scale; PSP, Personal and Social Performance Scale; SD, standard deviation.
By Kaplan–Meier analysis, the discontinuation rate (primary endpoint) over 52 weeks following randomization did not differ among the three treatment groups (log‐rank test, P = 0.9771) (Fig. 2). The percentage/proportion of patients discontinuing treatment over 52 weeks was 68.3%, 68.2%, and 65.5% in the aripiprazole, blonanserin, and paliperidone groups, respectively, with no significant differences reported among the treatment groups (P = 0.9060). The most commonly reported reasons for treatment discontinuation in the aripiprazole, blonanserin, and paliperidone groups were lack of efficacy (34.1% vs 32.9% vs 22.6%, respectively), intolerance to study treatment (19.5% vs 12.9% vs 25.0%, respectively), withdrawal at patient's discretion (8.5% vs 14.1% vs 10.7%, respectively), and other reasons (6.1% vs 8.2% vs 7.1%, respectively). The median (95% CI) time to treatment discontinuation was 144.5 (91.0–210.0), 144.0 (81.0–238.0), and 129.5 (84.0–252.0) days in the aripiprazole, blonanserin, and paliperidone groups, respectively.
Fig. 2.
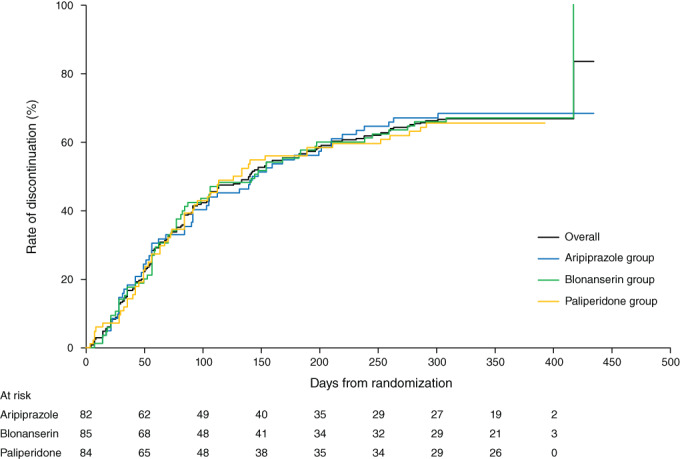
Rates of treatment discontinuation (primary endpoint) from the time of randomization. Log‐rank test: P = 0.9771.
Exploratory analyses excluding patients without prior medication identified few variables associated with treatment discontinuation over 52 weeks. Discontinuation was positively affected by prior medication of a ≥1000‐mg CP‐equivalent dose (HR, 1.84; 95% CI, 1.02–3.29; P = 0.0412) and negatively by disease duration ≥6 years (HR, 0.67; 95% CI, 0.46–0.96; P = 0.0289) in model 1, and negatively by disease duration ≥6 years (HR, 0.67; 95% CI, 0.47–0.97; P = 0.0318) and positively by ≥2 prior medications (HR, 1.43; 95% CI, 1.00–2.05; P = 0.0499) in model 2. This suggests that patients taking a relatively high dosage and multiple medications with a short disease duration tend to discontinue treatment after switching the drugs.
Similarly, exploratory analyses including patients without prior medication demonstrated that CP‐equivalent dose ≥1000 mg (HR, 1.85; 95% CI, 1.03–3.32; P = 0.0382) in model 1 and ≥2 prior medications (HR, 1.48; 95% CI, 1.03–2.11; P = 0.0321) in model 2 were significantly associated with treatment discontinuation over 52 weeks.
There was a gradual increase in the percentage of patients achieving remission over 52 weeks, which did not differ significantly between the treatment groups (Table 2). A significant improvement in the total PANSS scores (including all subscales) was observed from the time of initiating monotherapy to week 26 in all three treatment groups (Fig. S1). However, at week 52, significant improvements were observed in the overall cohort, aripiprazole group, and blonanserin group, but not in the paliperidone group (Fig. S1 and Table S3). Approximately 95% of patients were not at all suicidal, and the remaining patients were mildly suicidal at the baseline assessment. There was no significant difference in the CGI‐SS scores in any treatment group and among groups over 52 weeks (Tables S4 and S5). There was no significant difference in the CGI‐I scores in any treatment group and among groups over 52 weeks; the proportion of patients showing no change versus improvement versus worsening remained constant at weeks 8, 12, 26, and 52 (Table S6).
Table 2.
Rates of remission over 52 weeks
| Overall | Aripiprazole group | Blonanserin group | Paliperidone group | χ2 test | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Evaluation point | Total | Remission rate, n (%) | 95% CI | Total | Remission rate, n (%) | 95% CI | Total | Remission rate, n (%) | 95% CI | Total | Remission rate, n (%) | 95% CI | P value |
| Before the start of protocol treatment | 251 | 74 (29.5) | 23.9–35.5 | 82 | 23 (28.0) | 18.7–39.1 | 85 | 23 (27.1) | 18.0–37.8 | 84 | 28 (33.3) | 23.4–44.5 | 0.6312 |
| Time of monotherapy start | 224 | 61 (27.2) | 21.5–33.6 | 75 | 23 (30.7) | 20.5–42.4 | 76 | 18 (23.7) | 14.7–34.8 | 73 | 20 (27.4) | 17.6–39.1 | 0.6281 |
| Week 8 | 186 | 37 (19.9) | 14.4–26.4 | 62 | 14 (22.6) | 12.9–35.0 | 62 | 10 (16.1) | 8.0–27.7 | 62 | 13 (21.0) | 11.7–33.2 | 0.6449 |
| Week 12 | 157 | 37 (23.6) | 17.2–31.0 | 53 | 13 (24.5) | 13.8–38.3 | 50 | 11 (22.0) | 11.5–36.0 | 54 | 13 (24.1) | 13.5–37.6 | 0.9498 |
| Week 26 | 112 | 31 (27.7) | 19.6–36.9 | 38 | 11 (28.9) | 15.4–45.9 | 37 | 7 (18.9) | 8.0–35.2 | 37 | 13 (35.1) | 20.2–52.5 | 0.2899 |
| Week 52 | 82 | 34 (41.5) | 30.7–52.9 | 26 | 11 (42.3) | 23.4–63.1 | 27 | 11 (40.7) | 22.4–61.2 | 29 | 12 (41.4) | 23.5–61.1 | 0.9933 |
CI, confidence interval.
The personal and social functioning (as measured by PSP) also improved over 52 weeks, which did not differ significantly between the treatment groups. In comparison to baseline assessments, a significant improvement (P < 0.05) was observed at the start of monotherapy, week 26, and week 52 in the overall cohort and blonanserin group and at week 26 in the aripiprazole group. No significant improvement in personal and social functioning was observed at any time point in the paliperidone group (Table 3). Similarly, in comparison to baseline assessments, a significant improvement (P < 0.05) in QOL (as assessed by EQ‐5D) was observed at the start of monotherapy, week 26, and week 52 in the overall cohort; at the start of monotherapy in the aripiprazole and paliperidone groups; and at week 26 in the blonanserin group (Table 3).
Table 3.
Social functioning
| PSP; comparison with the time before the start of protocol treatment (ITT cohort) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Baseline assessment | Time of monotherapy start | Week 26 | Week 52 | |||||
| Treatment group | Total score or utility value | Total score or utility value | Change | Total score or utility value | Change | Total score or utility value | Change | |
| PSP total score | ||||||||
| Overall | N | 251 | 216 | 216 | 105 | 105 | 77 | 77 |
| Mean (SD) | 56.3 (20.8) | 58.8 (21.0) | 2.4 (14.2) † | 61.5 (21.5) | 5.4 (16.8) † | 61.4 (21.8) | 7.8 (17.7) † | |
| Median (minimum, maximum) | 60.0 (6, 100) | 63.0 (4, 100) | 0.0 (−58, 82) | 65.0 (4, 95) | 0.0 (−71, 73) | 65.0 (6, 95) | 0.0 (−25, 74) | |
| Aripiprazole group | N | 82 | 72 | 72 | 34 | 34 | 23 | 23 |
| Mean (SD) | 59.0 (21.0) | 60.7 (22.7) | 0.4 (14.5) | 67.5 (21.7) | 6.4 (15.7) † | 61.9 (25.8) | 5.9 (14.4) | |
| Median (minimum, maximum) | 65.0 (6, 100) | 65.0 (6, 100) | 0.0 (−58, 55) | 68.0 (11, 95) | 0.0 (−21, 56) | 70.0 (6, 95) | 0.0 (−19, 42) | |
| Blonanserin group | N | 85 | 74 | 74 | 37 | 37 | 27 | 27 |
| Mean (SD) | 53.6 (21.1) | 56.7 (20.4) | 3.7 (14.4) † | 60.0 (18.8) | 7.8 (16.9) † | 59.9 (23.2) | 9.5 (18.7) † | |
| Median (minimum, maximum) | 60.0 (7, 85) | 62.0 (4, 91) | 0.0 (−55, 62) | 65.0 (10, 90) | 1.0 (−25, 73) | 65.0 (6, 95) | 1.0 (−25, 66) | |
| Paliperidone group | N | 84 | 70 | 70 | 34 | 34 | 27 | 27 |
| Mean (SD) | 56.5 (20.4) | 58.9 (19.8) | 3.2 (13.5) | 57.3 (23.3) | 1.9 (17.5) | 62.4 (16.7) | 7.7 (19.5) | |
| Median (minimum, maximum) | 60.0 (6, 95) | 60.0 (7, 95) | 0.0 (−10, 82) | 60.0 (4, 95) | 0.0 (−71, 52) | 65.0 (20, 95) | 0.0 (−7, 74) | |
| EQ‐5D utility value | ||||||||
| Overall | N | 250 | 218 | 218 | 106 | 106 | 79 | 79 |
| Mean (SD) | 0.79 (0.17) | 0.82 (0.17) | 0.03 (0.12) † | 0.83 (0.17) | 0.04 (0.17) † | 0.84 (0.16) | 0.05 (0.15) † | |
| Median (minimum, maximum) | 0.77 (0.18, 1.00) | 0.79 (−0.06, 1.00) | 0.00 (−0.43, 0.61) | 0.79 (0.12, 1.00) | 0.00 (−0.35, 0.52) | 0.80 (0.41, 1.00) | 0.00 (−0.31, 0.53) | |
| Aripiprazole group | N | 82 | 74 | 74 | 34 | 34 | 25 | 25 |
| Mean (SD) | 0.78 (0.17) | 0.82 (0.16) | 0.04 (0.13) † | 0.83 (0.17) | 0.04 (0.20) | 0.83 (0.16) | 0.07 (0.18) | |
| Median (minimum, maximum) | 0.77 (0.39, 1.00) | 0.79 (0.42, 1.00) | 0.00 (−0.21, 0.61) | 0.79 (0.47, 1.00) | 0.00 (−0.34, 0.47) | 0.77 (0.59, 1.00) | 0.00 (−0.31, 0.53) | |
| Blonanserin group | N | 85 | 73 | 73 | 36 | 36 | 27 | 27 |
| Mean (SD) | 0.77 (0.18) | 0.79 (0.20) | 0.03 (0.13) | 0.81 (0.19) | 0.05 (0.13) † | 0.81 (0.17) | 0.05 (0.13) | |
| Median (minimum, maximum) | 0.77 (0.18, 1.00) | 0.77 (−0.06, 1.00) | 0.00 (−0.43, 0.34) | 0.79 (0.12, 1.00) | 0.00 (−0.23, 0.34) | 0.79 (0.41, 1.00) | 0.00 (−0.25, 0.34) | |
| Paliperidone group | N | 83 | 71 | 71 | 36 | 36 | 27 | 27 |
| Mean (SD) | 0.82 (0.15) | 0.84 (0.15) | 0.03 (0.11) † | 0.86 (0.15) | 0.03 (0.17) | 0.88 (0.16) | 0.03 (0.13) | |
| Median (minimum, maximum) | 0.79 (0.48, 1.00) | 0.79 (0.48, 1.00) | 0.00 (−0.26, 0.37) | 0.90 (0.59, 1.00) | 0.00 (−0.35, 0.52) | 1.00 (0.48, 1.00) | 0.00 (−0.30, 0.29) | |
There was no significant difference over 52 weeks between the groups (analysis of variance).
EQ‐5D, EuroQol‐5 dimensions; ITT, intent to treat; PSP, Personal and Social Performance Scale; SD, standard deviation.
P < 0.05 for change from baseline assessment (paired t test).
All AEs occurring over 52 weeks in the three treatment groups are reported in Table S7. There was a significant difference in the aripiprazole, blonanserin, and paliperidone groups in the total number of patients with AEs (52.4% vs 37.6% vs 58.3%, respectively; P = 0.0215) and endocrine disorder/related side effects (1.2% vs 0.0% vs 9.5%, respectively; P = 0.0015). The most frequent AEs of any grade in the aripiprazole, blonanserin, and paliperidone groups were related to psychiatric disorders (26.8% vs 18.8% vs 23.8%, respectively) and nervous system disorders (15.9% vs 9.4% vs 19.0%, respectively). In the inter‐group multiple Tukey test conducted as a post hoc analysis (P < 0.05 was considered significant) for the proportion of total number of patients with AEs, a significant difference was found in the blonanserin group versus the paliperidone group (P = 0.032). Likewise, in the multiple Tukey test for the proportion of patients with endocrine disorder/related side effects, a significant difference was found in the blonanserin group versus the paliperidone group (P < 0.001) and in the aripiprazole group versus the paliperidone group (P = 0.014). Differences in these proportions did not reach a significant level in the blonanserin group versus the aripiprazole group.
Incidence of serious adverse drug reactions (ADRs; grades 3 and 4) in the aripiprazole, blonanserin, and paliperidone groups was not significantly different (11.0% vs 3.5% vs 10.7%, respectively). The most frequent serious ADRs in the aripiprazole, blonanserin, and paliperidone groups were psychiatric disorders (8.5% vs 3.5% vs 8.3%, respectively), followed by nervous system disorders (2.4% vs 0.0% vs 1.2%, respectively) (Table S8). In comparison to baseline assessments, a significant decrease in the DIEPSS total score was observed at the start of monotherapy in the aripiprazole, blonanserin, and overall cohort; however, at week 52, a significant decrease in the DIEPSS total score was noted only in the paliperidone group. A significant decrease was also observed at week 12 in the overall group, but inter‐group differences were not significant (Table S9).
Discussion
JUMPs is a large‐scale, long‐term naturalistic study to assess three SGAs (aripiprazole, blonanserin, and paliperidone) with a low sedative effect for the treatment of schizophrenia in routine clinical practice. The long‐term comparative outcome of these three SGAs has not been evaluated in previous overseas studies, and evidence from short‐term clinical trials has formed the basis of the global guidelines. On the other hand, long‐term naturalistic studies capture the change in outcome with change in drug use over time, thereby highlighting the importance of the current results from a real‐world perspective. Long‐term effectiveness in JUMPs was measured using the remission rate along with the discontinuation rate, with the latter being an orthodox yet essential indicator of effectiveness.
Patients enrolled in JUMPs reflected the average Japanese population in terms of their age, sex, height, and body weight. Findings from this study suggest that the clinical utility of the three SGAs was equivalent in this study and could partially be attributed to the fact that in large‐scale, naturalistic studies, it is difficult to observe between‐drug differences when discontinuation rate is set as an outcome. 32 , 33
In the current study, a small proportion of patients (6.4%; n = 15) were prescribed the ≥1000‐mg CP‐equivalent dose, of whom more patients (7.8%; n = 6) were switched to aripiprazole compared with other drugs (blonanserin, 6.2%, n = 5; paliperidone, 5.1%, n = 4). However, aripiprazole was discontinued over 52 weeks in a similar proportion of patients due to lack of efficacy (34.1%) and intolerance to treatment (19.5%) to other drugs.
While results from this study cannot be directly compared with other large‐scale, naturalistic, observational studies due to differences in drug class, race, and study duration, some similarities and differences were noted. The CATIE study in 1493 patients reported similar discontinuation rates over 18 months in other antipsychotics, such as olanzapine (64%), ziprasidone (79%), perphenazine (75%), quetiapine (82%), and risperidone (74%). 8 The SOHO study in 6642 patients reported lower discontinuation rates compared with this study over 36 months with olanzapine (36%), risperidone (43%), quetiapine (69%), amisulpride (54%), clozapine (33%), oral typical antipsychotics (53%), and depot typical antipsychotics (51%). 11 The EUFEST study in 498 patients also reported lower discontinuation rates compared with this study with haloperidol (72%), amisulpride (40%), olanzapine (33%), quetiapine (53%), and ziprasidone (45%) over 52 weeks. 9 Furthermore, the CUtLASS study in 227 patients using FGAs or non‐clozapine SGAs showed no disadvantage of FGAs over 52 weeks in terms of QOL and clinical symptoms. 34 Overall, CATIE 8 and SOHO 11 showed better persistence rates for olanzapine compared with risperidone or quetiapine, while no significant difference in the discontinuation rates was observed among SGAs in CUtLASS 34 and EUFEST. 9 Similar to this study, the reasons for treatment discontinuation in all studies were mostly related to inefficacy or intolerable side effects. The tendency for paliperidone to have a high discontinuation rate may be attributed to its tolerability and safety, and that for aripiprazole and blonanserin could be due to their inadequate efficacy, which was generally predicted from previous data. 17 , 35 , 36 Considering the objective of this study, evaluation of the discontinuation rate for any reason remains an overall measure of effectiveness of antipsychotics in long‐term use. 37 While antipsychotic‐naïve patients are considered more responsive to antipsychotics, the proportion of these patients was small (6%) in this study compared with the CATIE (28%) 8 and EUFEST (33%) 9 studies. Furthermore, the differences in the duration of illness and treatment history may have influenced drug discontinuation.
A recent meta‐analysis of 10 RCTs (n = 1521) in 2019 concluded that the short‐term efficacy of blonanserin for schizophrenia is comparable to that of other antipsychotics such as risperidone and paliperidone, but to assure its clinical usefulness, evidence from long‐term RCTs is essential, which is currently unavailable. 38
Both CATIE (olanzapine, perphenazine, quetiapine, risperidone, or ziprasidone) and EUFEST (haloperidol, amisulpride, olanzapine, quetiapine, and ziprasidone) studies did not evaluate aripiprazole and paliperidone, and a meta‐analysis of the two studies reported a treatment continuity of approximately 45% and 65% at day 380, respectively. 39 Another meta‐analysis of 48 studies concluded that patients receiving continuous treatment had a reduced risk of relapse and a longer relapse‐free duration compared with those receiving placebo and intermittent treatment strategies, thus highlighting the importance of treatment continuity. 40
The three SGAs used in this study are expected to have a lower risk of EPS onset and less sedative effect compared with FGAs. SGAs are expected to improve social functioning and QOL in patients with schizophrenia. 41 , 42 , 43 Interestingly, only blonanserin showed improvements in the PSP score from the start of monotherapy through week 52 in the present study. Furthermore, significant improvements in health‐related QOL from baseline were observed at the start of monotherapy in the aripiprazole and paliperidone groups and at week 26 in the blonanserin group. A significant decrease in the DIEPSS total score from baseline was observed only at the start of monotherapy and week 12 in the overall group and at the start of monotherapy in the aripiprazole and blonanserin groups. However, at week 52, a significant decrease in the DIEPSS total score from baseline was noted only with long‐term use of paliperidone. Overall, no new safety signal was identified, and the safety profile was similar to that reported in the literature. 35 Comparatively, this unique treatment response (side effect vs efficacy profile) of blonanserin could be attributed to its higher affinity for dopamine D2 than for serotonin 5‐HT2A receptors and lower affinity for adrenaline α1, serotonin 5‐HT2C, histamine H1, and muscarinic M1 receptors. 13 Additionally, blonanserin is also shown to have high affinity for dopamine D3 receptor, which may play an important role as well. 44
Furthermore, managing the risk of developing metabolic syndrome is one of the challenges of SGA treatment for patients with schizophrenia. In our study, only three (1%) patients had metabolic abnormalities, and four (2%) had weight gain (Table S7), which is consistent with a report by Khalil R, 2012, indicating that East Asian countries, including Japan, have a small risk of metabolic syndrome. 45
The trend in QOL results could be attributed to a switch from polypharmacy and high‐dose antipsychotics at baseline to SGA monotherapy, thereby improving the dimensions of anxiety/depression, pain/discomfort, and usual activities in EQ‐5D in this study. 46 Therefore, the switch from polypharmacy to monotherapy and the lower dose of SGA initiated may have contributed to improved DIEPSS scores and QOL at the start of monotherapy. However, QOL may have been merely influenced by patients' preference for dose reduction and extraneous variables as potential study limitations.
The generalizability of the results is limited by the fact that this study was performed in a medical environment under the Japanese healthcare system and treatment policy. Compared with other countries, the universal healthcare system in Japan allows patients to see psychiatrists directly, which may provide more opportunities for treatment changes to be considered. There are also differences in the approved treatments between countries. Patients with chronic schizophrenia in Japan discontinued their SGAs at a rate approximately in line with previously published naturalistic evidence for antipsychotics. There were no observed differences in effectiveness between aripiprazole, blonanserin, and paliperidone. The observed discontinuation rate indicates the limitations of the effectiveness of these SGAs. However, the improvements in QOL noted at the start of SGA treatment indicate that the switch was justified. Apart from the significance of drug types, we found that patients taking higher dosage and polypharmacy as well as those with a short duration of illness showed a high tendency to discontinue the drugs after switching. More care is needed when switching drugs in such patients as the switch may need to be done more gradually.
Disclosure statement
J. Ishigooka reports a grant from The Waksman Foundation of Japan Inc. for the work under consideration for publication and personal fees from Dainippon Sumitomo, Eisai, Eli Lilly, MSD, Novartis, Otsuka, Pfizer, Shionogi, and Takeda, outside the submitted work.
K. Nakagome reports grants from The Waksman Foundation of Japan Inc. during the conduct of this study and from Meiji Seika Pharma, Otsuka Pharmaceutical, Dainippon Sumitomo, Mochida Pharmaceutical, Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Astellas Pharma, Asahi Kasei Pharma, and Shionogi and personal fees from Meiji Seika Pharma, MSD K.K., Otsuka, Kyowa Hakko Kirin, Dainippon Sumitomo, Takeda Pharmaceutical, Eli Lilly Japan K.K., Pfizer Japan, Nippon Boehringer Ingelheim, Toyama Chemical, Mochida, Janssen Pharmaceutical, Yoshitomiyakuhin Corporation, Taisho Toyama Pharma, and Kowa, outside the submitted work.
T. Ohmori reports personal fees from Astellas, Daiichi Sankyo, Sumitomo Dainippon, Eisai, Eli Lilly, GlaxoSmithKline, Meiji Seika Pharma, Mochida, MSD, Novartis, Otsuka, Pfizer, Takeda, Mitsubishi Tanabe, and Yoshitomi and grants from Astellas, Dainippon Sumitomo, Eisai, Meiji Seika Pharma, Otsuka, and Pfizer.
N. Iwata reports personal fees from Dainippon Sumitomo, Eli Lilly, Janssen, Otsuka, Meiji Seika Pharma, and Pfizer and a grant from Otsuka, outside the submitted work.
K. Inada reports personal fees from Dainippon Sumitomo, Eisai, Eli Lilly, Janssen, Meiji Seika Pharma, Mochida, MSD, Novartis, Otsuka, Shionogi, Tanabe‐Mitsubishi, and Yoshitomi and a grant from MSD, outside the submitted work.
J. Iga reports personal fees from Dainippon Sumitomo, Eli Lilly, Janssen, Meiji Seika Pharma, Mochida, MSD, Mylan, Novartis, and Otsuka, outside the submitted work.
T. Kishi has received speaker's honoraria from Daiichi Sankyo, Dainippon Sumitomo, Eisai, Janssen, Otsuka, Meiji, MSD, Yoshitomi, and Tanabe‐Mitsubishi and has received a Health Labour Sciences Research Grant, Grant‐in‐Aid for Scientific Research (C), and Fujita Health University School of Medicine research grant.
H. Tabuse reports personal fees from Dainippon Sumitomo, Eli Lilly, GlaxoSmithKline, Janssen, Otsuka, Meiji Seika Pharma, Pfizer, Tanabe‐Mitsubishi, and Yoshitomi, outside the submitted work.
Hiroshi Terada reports personal fees from Eli Lilly Japan K.K., Janssen Pharmaceuticals K.K., Otsuka, Meiji Seika Pharma, Dainippon Sumitomo, Tanabe‐Mitsubishi, and Yoshitomiyakuhin, outside the submitted work.
T. Kishimoto reports personal fees from Sumitomo Dainippon Pharma, Otsuka, Janssen Pharmaceutical, Meiji Seika Pharma, Eisai, MSD, Yoshitomiyakuhin, Mochida, and Takeda and grants from Eisai, Meiji Seika Pharma, Sumitomo Dainippon Pharma, Yoshitomiyakuhin, Shionogi, Mochida, MSD, Pfizer, Eli Lilly, Mitsubishi Tanabe Pharma, Otsuka, Nippon Boehringer Ingelheim, and Janssen Pharmaceutical, outside the submitted work.
Y. Tsutsumi reports personal fees from Otsuka and Janssen, outside the submitted work.
Y. Kanda reports personal fees from Eli Lilly Japan K.K. and Janssen Pharmaceutical K.K. during the conduct of the study and from Eli Lilly Japan K.K., outside the submitted work.
K. Sekiyama reports personal fees from Otsuka, outside the submitted work.
K. Fujita, Y. Kikuchi, T. Shichijo, S. Koretsune, Haruko Terada, and K. Ohi have no conflicts of interest.
Author contributions
J. Ishigooka, K. N., T. O., and N. I. contributed toward conception, design, and planning of the study and interpretation of the results.
N. I. and T. Kishi contributed toward analysis of data.
K. N., K. I., J. I., and T. Kishi contributed toward drafting of the manuscript.
All authors contributed toward acquisition of data, critical revision of the manuscript for important intellectual content, and approval of the final version of the manuscript.
All authors agree to be accountable for all aspects of the work and ensure that any questions related to the accuracy or integrity of any part of the work will be appropriately investigated and resolved.
Data sharing statement
The datasets analyzed during the current study are not available because data sharing with third parties was not included in the contract with study sites.
Supporting information
Table S1. Chlorpromazine equivalents of oral antipsychoticsa
Table S2. Use of concomitant medications.
Table S3. Mean (SD) change in PANSS subscale scores over 52 weeks (ITT cohort).
Table S4. Severity of suicidality: CGI‐SS (part 1).
Table S5. Improvement of suicidality: CGI‐SS (part 2).
Table S6. Overall improvement: CGI‐I.
Table S7. Incidence of adverse events listed by type (SP cohort).
Table S8. Incidence of serious adverse drug reactions listed by type (SP cohort).
Table S9. Drug‐induced extrapyramidal symptoms scale total scores compared with score before the start of protocol treatment (SP cohort).
Figure S1. Mean (SD) change in PANSS total score over 52 weeks.
Acknowledgments
Editorial support, in the form of medical writing, assembling tables and creating high‐resolution images based on the authors' detailed directions, collating author comments, copyediting, fact checking, and referencing, was provided by Annirudha Chillar, MD, PhD, of Cactus Life Sciences (part of Cactus Communications) and funded by The Waksman Foundation of Japan Inc. This work was supported by a research grant from The Waksman Foundation of Japan Inc. The funding source had no role in study planning, implementation, and reporting of study results or interpretation; the study implementation does not conflict with patients' rights or benefits. The Waksman Foundation of Japan Inc. funded the writing support and approved the publication for submission. The corresponding author confirms that he had full access to all the data in the study and takes final responsibility for the decision to submit for publication.
Trial Registration: UMIN‐Clinical Trials Registry 000007942.
References
- 1. Charlson FJ, Ferrari AJ, Santomauro DF et al. Global epidemiology and burden of schizophrenia: Findings from the global burden of disease study 2016. Schizophr. Bull. 2018; 44: 1195–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. The Ministry of Health, Labour and Welfare. Statistics by Ministry of Health, Labour and Welfare, as of 2008. [Cited 3 November 2020] Available from URL: http://www.mhlw.go.jp/kokoro/speciality/detail_into.html.
- 3. National Institute for Health and Care Excellence. NICE guideline 2014. Psychosis and schizophrenia in adults: prevention and management. [Cited 3 November 2020] Available from URL: https://www.nice.org.uk/guidance/cg178/chapter/1‐Recommendations#promoting‐recovery‐and‐possible‐future‐care‐2. [PubMed]
- 4. American Psychiatric Association . APA practical guideline 2019. [Cited 3 November 2020] Available from URL: https://ajp.psychiatryonline.org/doi/10.1176/appi.ajp.2020.177901. https://www.psychiatry.org/psychiatrists/practice/clinical‐practice‐guidelines
- 5. The Japanese Society of Neuropsychopharmacology. “Guideline for Pharmacological Therapy of Schizophrenia” [Cited 3 November 2020] Available from URL: http://www.asas.or.jp/jsnp/csrinfo/03.html. [DOI] [PMC free article] [PubMed]
- 6. Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Thibaut F, Moller H‐J. World Federation of Societies of Biological Psychiatry Guidelines 2013. [Cited 3 November 2020] Available from URL: https://www.wfsbp.org/fileadmin/user_upload/Treatment_Guidelines/WFBSP_SZ_Guidelines_Part2_2013.pdf.
- 7. Brissos S, Dias VV, Balanzá‐Martinez V, Carita AI, Figueira ML. Symptomatic remission in schizophrenia patients: Relationship with social functioning, quality of life, and neurocognitive performance. Schizophr. Res. 2011; 129: 133–136. [DOI] [PubMed] [Google Scholar]
- 8. Lieberman JA, Stroup TS, McEvoy JP et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 2005; 353: 1209–1223. [DOI] [PubMed] [Google Scholar]
- 9. Kahn RS, Fleischhacker WW, Boter H et al. Effectiveness of antipsychotic drugs in first‐episode schizophrenia and schizophreniform disorder: An open randomised clinical trial. Lancet 2008; 371: 1085–1097. [DOI] [PubMed] [Google Scholar]
- 10. McEvoy JP, Lieberman JA, Perkins DO et al. Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: A randomized, double‐blind 52‐week comparison. Am. J. Psychiatry 2007; 164: 1050–1060. [DOI] [PubMed] [Google Scholar]
- 11. Novick D, Haro JM, Suarez D, Vieta E, Naber D. Recovery in the outpatient setting: 36‐month results from the Schizophrenia Outpatients Health Outcomes (SOHO) study. Schizophr. Res. 2009; 108: 223–230. [DOI] [PubMed] [Google Scholar]
- 12. de Bartolomeis A, Tomasetti C, Iasevoli F. Update on the mechanism of action of aripiprazole: Translational insights into antipsychotic strategies beyond dopamine receptor antagonism. CNS Drugs 2015; 29: 773–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deeks ED, Keating GM. Blonanserin: A review of its use in the management of schizophrenia. CNS Drugs 2010; 24: 65–84. [DOI] [PubMed] [Google Scholar]
- 14. Food and Drug Administration . Highlights of prescribing information (paliperidone palmitate). [Cited 31 August 2021] Available from URL: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/022264Orig1s015.pdf
- 15. Ishigooka J. Selection of antipsychotics taking effectiveness into consideration with dropout rate and remission rate as two major indicators. Jpn. J. Clin. Psychopharmacol. 2007; 10: 1639–1649 (in Japanese). [Google Scholar]
- 16. Miyake N, Miyamoto S. Long‐term effectiveness outcome study. Jpn. J. Clin. Psychopharmacol. 2010; 13: 273–283 (in Japanese). [Google Scholar]
- 17. Takeuchi H, Uchida H, Suzuki T, Watanabe K, Kashima H. Predictors of clinical worsening after a switch to aripiprazole in patients with schizophrenia: A 1‐year naturalistic follow‐up study. J. Clin. Psychopharmacol. 2009; 29: 394–395. [DOI] [PubMed] [Google Scholar]
- 18. Ye W, Ascher‐Svanum H, Tanji Y, Flynn JA, Takahashi M. Predictors of continuation with olanzapine during the 1‐year naturalistic treatment of patients with schizophrenia in Japan. Patient Prefer. Adherence 2011; 5: 611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ishigooka J, Nakagome K, Ohmori T, Iwata N. Japan useful medication program for schizophrenia (JUMPs)‐long‐term study on discontinuation rate, resolution and remission, and improvement in social functioning rate associated with atypical antipsychotic medications in patients with schizophrenia. BMC Psychiatry 2013; 13: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Japan Psychiatry Evaluation Scale Study Group. Dose equivalence of antipsychotics (2012 version)” by The Japanese Society of Psychiatric Rating Scales. [Cited 31 August 2021] Available from URL: http://jsprs.org/toukakansan/2012ver/index.php.
- 21. Andreasen NC, Carpenter WT Jr, Kane JM, Lasser RA, Marder SR, Wenberger DR. Remission in schizophrenia: Proposed criteria and rationale for consensus. Am. J. Psychiatry 2005; 162: 441–449. [DOI] [PubMed] [Google Scholar]
- 22. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987; 13: 261–276. [DOI] [PubMed] [Google Scholar]
- 23. Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM‐IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr. Scand. 2000; 101: 323–329. [PubMed] [Google Scholar]
- 24. EuroQol Group . EuroQol—a new facility for the measurement of health‐related quality of life. Health Policy 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 25. Guy W. ECDEU Assessment Manual for Psychopharmacology, Revised. US Department of Health, Education, and Welfare Publication (ADM). National Institute of Mental Health, Rockville, MD, 1976; 76–338. [Google Scholar]
- 26. Lindenmayer JP, Czobor P, Alphs L et al. The InterSePT scale for suicidal thinking reliability and validity. Schizophr. Res. 2003; 63: 161–170. [DOI] [PubMed] [Google Scholar]
- 27. Inada T. Recent research trends in diagnosis, treatment, and prevention of drug‐induced extrapyramidal symptoms seen in psychiatric patients. Nihon Shinkei Seishin Yakurigaku Zasshi 1996; 16: 181–185. [PubMed] [Google Scholar]
- 28.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. The extent of population exposure to assess clinical safety for drugs intended for long‐term treatment of non‐life‐threatening conditions. E1., 1994. [Cited 23 January 2018.] Available from URL: https://www.ich.org/products/guidelines/efficacy/article/efficacy-guidelines.html.
- 29. Baldessarini RJ, Cohen BM, Teicher MH. Significance of neuroleptic dose and plasma level in the pharmacological treatment of psychoses. Arch. Gen. Psychiatry 1988; 45: 79–91. [DOI] [PubMed] [Google Scholar]
- 30. Chan V. Schizophrenia and psychosis: Diagnosis, current research trends, and model treatment approaches with implications for transitional age youth. Child Adolesc. Psychiatr. Clin. N. Am. 2017; 26: 341–366. [DOI] [PubMed] [Google Scholar]
- 31. Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophr. Res. 2005; 79: 231–238. [DOI] [PubMed] [Google Scholar]
- 32. Glick ID, Berg PH. Time to study discontinuation, relapse, and compliance with atypical or conventional antipsychotics in schizophrenia and related disorders. Int. Clin. Psychopharmacol. 2002; 17: 65–68. [DOI] [PubMed] [Google Scholar]
- 33. Guo X, Fang M, Zhai J et al. Effectiveness of maintenance treatments with atypical and typical antipsychotics in stable schizophrenia with early stage: 1‐year naturalistic study. Psychopharmacology (Berl) 2011; 216: 475–484. [DOI] [PubMed] [Google Scholar]
- 34. Jones PB, Barnes TR, Davies L et al. Randomized controlled trial of the effect on Quality of Life of second‐ vs first‐generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch. Gen. Psychiatry 2006; 63: 1079–1087. [DOI] [PubMed] [Google Scholar]
- 35. Solmi M, Murru A, Pacchiarotti I et al. Safety, tolerability, and risks associated with first‐ and second‐generation antipsychotics: A state‐of‐the‐art clinical review. Ther. Clin. Risk Manag. 2017; 13: 757–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kishi T, Matsuda Y, Matsunaga S et al. A randomized trial of aripiprazole vs blonanserin for the treatment of acute schizophrenia and related disorders. Neuropsychiatr. Dis. Treat. 2016; 12: 3041–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huhn M, Nikolakopoulou A, Schneider‐Thoma J et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi‐episode schizophrenia: A systematic review and network meta‐analysis. Lancet 2019; 394: 939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kishi T, Matsui Y, Matsuda Y et al. Efficacy, tolerability, and safety of blonanserin in schizophrenia: An updated and extended systematic review and meta‐analysis of randomized controlled trials. Pharmacopsychiatry 2019; 52: 52–62. [DOI] [PubMed] [Google Scholar]
- 39. Czobor P, Van Dorn RA, Citrome L, Kahn RS, Fleischhaker WW, Volavka J. Treatment adherence in schizophrenia: A patient‐level meta‐analysis of combined CATIE and EUFEST studies. Eur. Neuropsychopharmacol. 2015; 25: 1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De Hert M, Sermon J, Geerts P, Vansteelandt K, Peuskens J, Detraux J. The use of continuous treatment versus placebo or intermittent treatment strategies in stabilized patients with schizophrenia: A systematic review and meta‐analysis of randomized controlled trials with first‐ and second‐generation antipsychotics. CNS Drugs 2015; 29: 637–658. [DOI] [PubMed] [Google Scholar]
- 41. Awad AG, Voruganti LN. The impact of newer atypical antipsychotics on patient‐reported outcomes in schizophrenia. CNS Drugs 2013; 27: 625–636. [DOI] [PubMed] [Google Scholar]
- 42. Voruganti L, Cortese L, Oyewumi L, Cernovsky Z, Zirul S, Awad A. Comparative evaluation of conventional and novel antipsychotic drugs with reference to their subjective tolerability, side‐effect profile and impact on quality of life. Schizophr. Res. 2000; 43: 135–145. [DOI] [PubMed] [Google Scholar]
- 43. Voruganti L, Cortese L, Owyeumi L et al. Switching from conventional to novel antipsychotic drugs: Results of a prospective naturalistic study. Schizophr. Res. 2002; 57: 201–208. [DOI] [PubMed] [Google Scholar]
- 44. Baba S, Enomoto T, Horisawa T, Hashimoto T, Ono M. Blonanserin extensively occupies rat dopamine D3 receptors at antipsychotic dose range. J. Pharmacol. Sci. 2015; 127: 326–331. [DOI] [PubMed] [Google Scholar]
- 45. Bou KR. Atypical antipsychotic drugs, schizophrenia, and metabolic syndrome in non‐Euro‐American societies. Clin. Neuropharmacol. 2012; 35: 141–147. [DOI] [PubMed] [Google Scholar]
- 46. Millier A, Amri I, Boyer L, Toumi M. Utility decrements associated with side effects in schizophrenia. J. Med. Econ. 2014; 17: 853–861. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Chlorpromazine equivalents of oral antipsychoticsa
Table S2. Use of concomitant medications.
Table S3. Mean (SD) change in PANSS subscale scores over 52 weeks (ITT cohort).
Table S4. Severity of suicidality: CGI‐SS (part 1).
Table S5. Improvement of suicidality: CGI‐SS (part 2).
Table S6. Overall improvement: CGI‐I.
Table S7. Incidence of adverse events listed by type (SP cohort).
Table S8. Incidence of serious adverse drug reactions listed by type (SP cohort).
Table S9. Drug‐induced extrapyramidal symptoms scale total scores compared with score before the start of protocol treatment (SP cohort).
Figure S1. Mean (SD) change in PANSS total score over 52 weeks.


