Abstract
Essential thrombocythemia (ET) is a myeloproliferative neoplasm characterized by enhanced platelet production and thrombotic complications. The inhibition of platelet cyclooxygenase (COX) activity by the standard once‐daily aspirin is mostly incomplete due to accelerated thrombopoiesis. The phase II Aspirin Regimens in EsSential thrombocythemia (ARES) trial has recently compared the efficacy of once‐ vs. twice‐ or three‐times daily low‐dose aspirin in inhibiting platelet thromboxane (TX) A2 production, as reflected by serum (s) TXB2 measurements. The present substudy characterized the determinants of the highly variable response to the standard aspirin 100 mg once‐daily regimen in fully compliant patients with ET and the effects of the experimental dosing regimens on response variability. By multivariable analysis, the platelet count (directly) and cytoreductive treatment (inversely) were significantly associated with sTXB2 values in 218 patients with ET. However, the platelet count positively correlated with sTXB2 in patients not being treated with cytoreductive drugs (ρ = 0.51, P < 0.01, n = 84), but not in patients on cytoreduction. Patients in the lowest sTXB2 quartile were older, more often on cytoreductive drugs, had lower platelet count and Janus‐Associated Kinase2 (JAK2)‐V617F allele frequency as compared with patients in the upper sTXB2 quartiles. After 2 weeks of a twice‐ or 3‐times daily aspirin regimen, the association between the platelet count and sTXB2 became similar in cytoreduced and non‐cytoreduced patients. In conclusion, the platelet count appears the strongest determinant of TXA2 inhibition by once‐daily low‐dose aspirin in ET, with different patterns depending of cytoreductive treatment. More frequent aspirin dosing restores adequate platelet inhibition and reduces interindividual variability, independently of cytoreduction.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Essential thrombocythemia (ET) is characterized by clonal overproduction of platelets, increased thrombo‐hemorrhagic complications, and incomplete and widely variable inhibition of platelet cyclooxygenase (COX) activity by once‐daily low‐dose aspirin, most likely due to accelerated thrombopoiesis.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ This prespecified Aspirin Regimens in EsSential thrombocythemia (ARES) trial substudy characterized the determinants of the highly variable platelet inhibition by aspirin (100 mg once‐daily) in a large cohort of fully‐compliant patients with ET and the effects of different experimental regimens on variability.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Platelet count is differentially associated with platelet COX inhibition by once‐daily aspirin in cytoreduced vs. non‐cytoreduced patients. A twice‐ or three‐times daily aspirin regimen restores adequate platelet inhibition substantially restraining interindividual variability, independently of cytoreduction.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ The present study highlights the importance of potential changes in platelet COX renewal rate and platelet count in less extreme clinical conditions than ET, and identifies serum thromboxane B2 as the biomarker to characterize the determinants of aspirin response variability.
Abnormal megakaryopoiesis, increased platelet generation, microvascular symptoms, and thrombotic complications are the main hematological and clinical hallmarks of essential thrombocythemia (ET), a myeloproliferative neoplasm (MPN). 1 Arterial and venous thromboses significantly impact on life quality and expectancy of patients with ET, 2 thus antiplatelet therapy is key to ET management. Low‐dose aspirin (75–100 mg, once daily (q.d.)), alone or combined with cytoreductive drugs (mostly hydroxyurea (HU)), is currently used to prevent thrombosis and improve microvascular symptoms in ET. 3 , 4 The bleeding risk may outweigh the expected benefits in patients at low risk for thrombosis. 5 , 6 , 7 Therefore, aspirin is recommended in patients with ET deemed at medium to high thrombotic risk, according to the International Prognostic Score for Thrombosis in Essential Thrombocythemia (IPSET‐thrombosis) based on the following criteria: age (> 60 years), previous thrombosis, presence of traditional cardiovascular risk factors, or Janus‐Associated Kinase (JAK)2‐V617F point mutation, 5 , 6 that is observed in ~ 60% of patients with ET. Somatic mutations of the endoplasmic reticulum calreticulin (CALR) protein and/or of the thrombopoietin receptor (MPL) are observed in ~ 25% and 5% of patients with ET, respectively. All somatic mutations constitutively activate the MPL through JAK/Signal Transducers and Activators of Transcription (STAT) pathway, inducing megakaryocyte commitment, proliferation, and differentiation with pro‐platelet formation. 8 The JAK2‐V617 seems to increase platelet activation 9 , 10 and to worsen thrombotic diathesis 11 as compared with CALR mutations. 11 , 12 , 13
Aspirin permanently acetylates cyclooxygenase (COX)‐1 and ‐2 in peripheral platelets and their bone‐marrow precursors, 14 (i.e., the megakaryocytes, pre‐ and pro‐platelets), 15 thus blocking thromboxane (TX)A2 biosynthesis. 16 The COX‐protein turnover rate is a known variable affecting the antiplatelet pharmacodynamics (PDs) of a standard low‐dose aspirin od regimen, in particular the extent of TXA2 suppression and duration of the antiplatelet effect. 17 , 18 The Aspirin Regimens in EsSential Thrombocythemia (ARES) dose‐finding, phase II trial was designed to ascertain the most effective regimen of low‐dose aspirin to fully suppress platelet TXA2 biosynthesis throughout the dosing interval. 19 Serum (s)TXB2 provides a highly specific index of the platelet biosynthetic capacity in response to the endogenously formed thrombin during whole blood clotting. 20 In the ARES trial, 254 patients with ET, already on aspirin 100 mg q.d. were randomized to a 2‐week course of aspirin 100 mg q.d., twice‐ (b.i.d.) or 3‐times (t.i.d.) daily. 19 Before randomization, patients showed a high median value and large interindividual variability in sTXB2 that spanned across three orders of magnitude (i.e., from < 1 ng/mL up to > 1,000 ng/mL), thereby ranging from a fully suppressed to an aspirin‐naïve phenotype. As compared with the standard q.d. regimen, the b.i.d. and t.i.d. regimens significantly reduced median sTXB2 values by 80% and 90%, respectively, and reduced interindividual variability. 21
The present prespecified ARES substudy aimed at characterizing the main determinants of the striking interindividual variability in aspirin response observed in patients with ET, and their interaction with the randomized aspirin regimens.
METHODS
Design of the study
The rationale, main objectives, design, inclusion, and exclusion criteria of the ARES trial (EudraCT 2016‐002885‐30) were previously published 19 ( Supplementary Material ). In particular, a platelet count > 1,000 × 109/L over repeated measurements was an exclusion criterion due to the potentially associated bleeding risk. 12 The study flow in relation to the present analyses is depicted in Figure 1 . Briefly, eligible patients with ET on low‐dose aspirin (100 mg q.d.) for primary or secondary cardiovascular prevention underwent a 10‐day run‐in period during which they took their daily aspirin tablet at breakfast. At the end of the run‐in (visit 2), patients were randomized 1:1:1 to enteric‐coated aspirin (Cardioaspirin, Bayer Italy) 100 mg q.d., b.i.d., or t.i.d. for 2 weeks and were sampled again at visit 3 (Figure 1 ). 21 Matching placebo was administered so that all patients took pills three times per day, which included aspirin with or without placebo tablets according to the randomized treatment.
Figure 1.
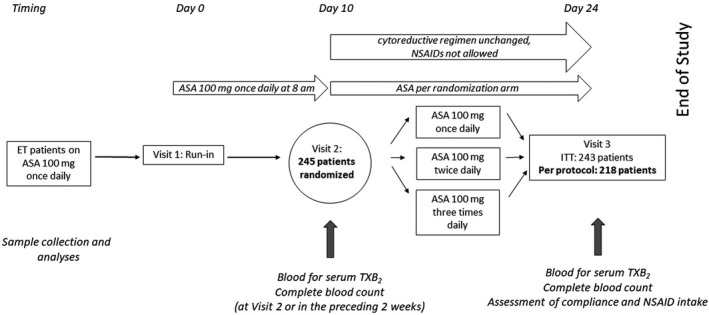
Study design and patients’ flow. Two hundred eighteen fully compliant patients with ET were included in the analyses of this study. Patients underwent visits for blood collection and biomarker measurements as depicted in the figure. ASA, acetylsalicylic acid; ET, essential thrombocythemia; ITT, intention to treat; NSAIDs, non‐steroidal anti‐inflammatory drugs; TX, thromboxane.
The cytoreductive regimen (if any) was left to the standard practice of each center, but had to remain unchanged (both drug and daily dose) over the 2 study weeks. Nonsteroidal anti‐inflammatory drugs (NSAIDs) were prohibited during the study, but paracetamol was allowed for fever or pain (Figure 1 ). The Ethics Committee of the Fondazione Policlinico Universitario “Agostino Gemelli” IRCCS and all participating institutions reviewed and approved the study protocol and the informed consent form which was signed by all patients.
The extent of platelet TXA2 suppression by the different aspirin regimens, as reflected by sTXB2, was the primary end point of the trial, as a surrogate of clinical efficacy. 22 The urinary excretion rate of 11‐dehydro‐TXB2 (TXM), a major enzymatic metabolite of TXA2/TXB2 reflecting in vivo platelet activation, 23 was a secondary end point.
The present prespecified analyses aimed at investigating the determinants of the variable platelet inhibition by the q.d. aspirin regimen as reflected by sTXB2 at visit two (randomization visit; Figure 1 ), as well as the influence of the experimental regimens, as reflected by sTXB2 at visit three (end of study visit; Figure 1 ).
Methods
Routine hematochemical parameters and the mutational profile were analyzed in each participating center. Clinical and laboratory characteristics were collected through Research Electronic Data Capture (REDCap), and patients were randomized, as previously described. 21 Compliance was assessed by pill counting and by reviewing the patient’s daily diary, where patients recorded daily the timing of each tablet intake, any drug other than their usual therapy, and any symptom or comment that they deemed relevant while on the study. Full compliance was defined if patients took all 9 scheduled pills in the 3 days preceding visit 3. Although NSAIDs were prohibited during the entire study duration, occasional NSAID intake was further inquired over the 3 days before visit 3 to exclude drug‐drug interaction 24 and reported in an ad hoc form.
For sTXB2 measurements, peripheral venous blood was collected without anticoagulant, incubated within 5 minutes for 1 hour at 37°C, centrifuged 10 minutes at 1,200 g, and the supernatant serum was stored at −40°C until assayed. 19 , 20 The sTXB2 was measured by a previously described and liquid chromatography/tandem mass spectrometry‐validated immunoassay 20 , 25 , 26 in the core laboratory of the ARES trial, under the supervision of authors B.R. and G.P. The feasibility and reproducibility of the sTXB2 measurements in serum samples obtained from different hematological centers was tested before starting patient enrollment. 19 No major adverse events, either thrombotic or hemorrhagic, were recorded during the 2‐week study period.
Statistical analysis
As this prespecified substudy was aimed at evaluating the determinants of platelet thromboxane inhibition by aspirin, we analyzed data of fully compliant patients, as defined above. We selected a priori some disease‐specific parameters and variables known to be associated with the risk of thrombosis. 12 We evaluated the potential associations of sTXB2 with seven variables: leukocyte and platelet counts, sex, age, JAK2‐V617F mutational status, and body mass index at study enrollment, and ongoing cytoreductive therapy. These potential associations were tested by univariate linear regression, using log‐transformation for sTXB2 and platelet count, or Kruskal‐Wallis test for the association between continuous and dichotomous variables (e.g., sTXB2 levels with cytoreductive therapy). 27 Only variables associated with sTXB2 with a univariate P value < 0.007 (corresponding to a univariate P value < 0.05 after Bonferroni adjustment for 7 variables) were considered for further analysis.
As a second step of the variable selection process, we visually analyzed the reciprocal association using a correlogram plot for those variables having a paired significant association. A correlogram plot consists of a matrix of scatterplots, histograms, and bar charts for all possible variable‐to‐variable combinations. This analysis was done in addition to the multiple linear regression, in order to avoid missing potentially significant biological correlations, as multivariate analysis may exclude variables with higher variance and/or lower effect. To further improve the presentation of the influence of independent variables on sTXB2, we analyzed the distribution of the latter in quartiles in some analyses presented in the corresponding tables and figures. Nonparametric median test and bootstrapped 95% confidence intervals were calculated for all quantitative variables. The R version 4.0.0 software package 28 was used for data analysis and plotting.
A serum TXB2 level < 10 ng/mL was considered the threshold of optimal platelet inhibition in non‐MPN subjects based on the following evidence: (i) this concentration corresponds to > 97% inhibition of pre‐aspirin serum TXB2 in aspirin‐treated healthy subjects 29 and therefore can be considered as a reference limit for platelet inhibition 30 ; (ii) it also corresponds to the upper limit of serum TXB2 measured 24 hours after the last aspirin intake in non‐diabetic patients on low‐dose aspirin for primary or secondary cardiovascular prophylaxis. 25
Data sharing
De‐identified individual participant data that underlie the reported results will be made available 3 months after publication for a period of 3 years after the publication date. The study dataset is kept available at www.osf.io, upon request. Requests for access should be sent to alberto.tosetto@aulss8.veneto.it, and will be subjected to review and approval by the steering committee of the study. The study protocol is included as a data supplement available with the online version of this article.
RESULTS
Determinants of sTXB2 on a standard once‐daily aspirin regimen
Out of 243 enrolled patients with ET, the present analyses included the 218 patients who were considered fully compliant. The clinical characteristics of the overall trial population as well as of the compliant vs. non‐compliant subgroups are shown in Table 1 . No statistically significant differences in clinical and hematological features were found between the groups. Moreover, no statistically significant differences were observed among patients with ET recruited in different centers in relation to the sTXB2 values measured at visit two. Platelet counts were relatively stable during the 2‐week study duration (Figure S1 ).
Table 1.
Characteristics of the aspirin‐compliant and non‐compliant ET subgroups and of the overall study population at randomization
|
ALL N = 243 |
Compliant N = 218 |
Non‐compliant N = 25 |
P value a | |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 112 (46.1) | 104 (47.7) | 8 (32.0) | 0.20 |
| Female | 131 (53.9) | 114 (52.3) | 17 (68.0) | |
| Age at enrollment | 60.0 [51.0; 67.0] | 60.0 [52.0; 67.0] | 54.0 [45.0; 66.0] | 0.07 |
| Time since ET diagnosis | 5.0 [2–10] | 5.0 [2–10] | 5.0 [3–12] | 0.60 |
| BMI, kg/m2 | 25.0 [22.9; 27.3] | 24.9 [22.8; 27.0] | 25.5 [23.4; 29.0] | 0.71 |
| Leukocytes, ×109/L | 7.00 [5.7; 8.5] | 7.00 [5.70; 8.4] | 7.67 [5.6; 9.7] | 0.39 |
| Hematocrit, % | 41.7 [39.2; 44.3] | 41.7 [39.0; 44.3] | 41.8 [39.9; 44.6] | 0.72 |
| Platelet count, ×109/L | 521 [422; 641] | 516 [422; 629] | 573 [434; 712] | 0.16 |
| TXB2 (ng/mL serum) | 19.0 [9.20; 42.6] | 18.6 [8.93; 42.9] | 23.3 [13.6; 38.8] | 0.54 |
| TXM (pg/mg creatinine) | 428 [318; 618] | 434 [319; 628] | 386 [310; 575] | 0.80 |
| JAK2‐V617F, n (%) b | ||||
| Wild type | 99 (40.9) | 88 (40.6) | 11 (44.0) | 0.91 |
| Mutated | 143 (59.1) | 129 (59.4) | 14 (56.0) | |
| CALR mutation, n (%) c | ||||
| Type 1 | 19 (14.8) | 19 (16.4) | 0 (0) | 0.34 |
| Type 2 | 16 (12.5) | 15 (12.9) | 1 (8.3) | |
| Other c | 93 (72.7) | 82 (70.7) | 11 (91.7) | |
| Cytoreductive therapy, n (%) d | ||||
| No | 98 (40.3) | 84 (38.5) | 14 (56.0) | 0.14 |
| Yes | 145 (59.7) | 134 (61.5) | 11 (44.0) | |
| Microvascular symptoms | ||||
| No | 218 (89.7) | 194 (89.0) | 24 (96.0) | 0.49 |
| Yes | 25 (10.3) | 24 (11.0) | 1 (4.00) | |
| Previous thrombosis, n (%) e | ||||
| No | 234 (96.3) | 209 (95.9) | 25 (100) | 0.60 |
| Yes | 9 (3.70) | 9 (4.13) | 0 (0.00) | |
Out of 218 fully compliant patients, 134 (61%) were on cytoreductive treatment (110 hydroxyurea (HU), 19 anagrelide, one interferon‐2alpha, 3 interferon‐2beta, and one a non‐specified agent) and they were analyzed together.
BMI, body mass index; ET, essential thrombocythemia; TX, thromboxane.
P value: Quantitative values are reported as median (interquartile range), unless otherwise specified. There were no significant differences between the different groups, based on the Kruskal‐Wallis test or chi‐square test for continuous or discrete variables.
One patient had no JAK2‐V617F genotype (in the compliant group).
Includes the following genotypes: CALR‐negative, CALR mutations other than type I or type II and MPL mutations. CALR was not genotyped in 115 patients (102 in the compliant group, 13 in the not compliant group).
In the 218 fully compliant patients, 110 were taking HU, 19 anagrelide, one interferon‐2alpha, three interferon‐2beta, and one a non‐specified agent.
Defined as any major thrombosis occurring within 2 years before diagnosis and at any time afterward.
Upon univariate analysis, platelet and leukocyte counts (directly), age (inversely), and cytoreductive treatment (inversely) were all significantly associated with sTXB2 values measured at visit two, when all patients were on chronic, low‐dose aspirin (100 mg q.d.) therapy. The reciprocal correlations between these variables and sTXB2 are shown in the correlogram plot (Figure 2 ) and in Table S1 . By multivariable analysis, only platelet count (directly, P < 0.001) and cytoreductive therapy (inversely, P = 0.017) were independently and significantly associated with sTXB2 levels.
Figure 2.
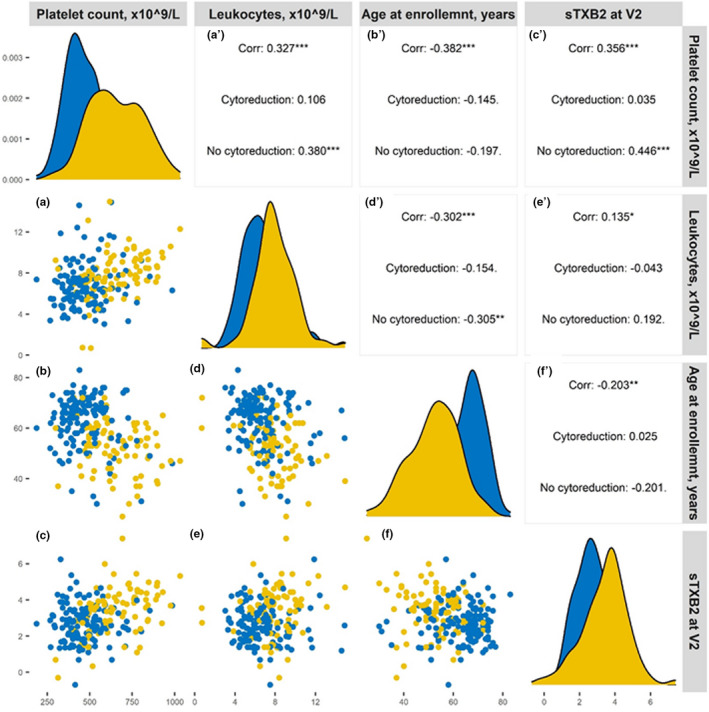
Correlogram plot of variables influencing residual sTXB2. The figure represents the mutual correlation of all those variables associated with sTXB2 at univariate analysis. In each panel, data from patients on cytoreduction are shown in yellow and data on non‐cytoreduced patients are shown in blue. The legends for the x axes are reported in the upper line, the legends for the y axis are reported on the left. The panels on the diagonal line represent the frequency histograms (from the upper left to the bottom right panel: platelet count, leukocyte count, age at enrollment, and sTXB2, respectively). The panels a–f represent reciprocal bivariate scatterplots (lower left corner of the figure) and the corresponding Pearson correlation (rho) coefficients for the all, cytoreduced and non‐cytoreduced cohorts are reported in the panels a′–f′ (upper right corner). For instance, a presents the scatterplot of platelet count (x‐axis) vs. sTXB2 (y‐axis) and a′ reports the corresponding rhos. *P < 0.05; **P < 0.001; ***P < 0.0001.
We then examined the clinical and hematological variables across quartiles of sTXB2 values, as shown in Table 2 . As compared to the 55 patients in the highest sTXB2 quartile (sTXB2 > 42 ng/mL), the 57 patients in the lowest quartile (sTXB2 < 9 ng/mL) were older (63 vs. 56 years), had lower platelet count (451 × 109/L vs. 641 × 109/L), lower frequency of mutated JAK2‐V617F (47% vs. 73%), were more often on cytoreductive drugs (77% vs. 34%), and displayed a lower urinary TXM excretion (436 vs. 680 pg/mg creatinine; Table 2 ).
Table 2.
Hematological, biochemical, and clinical variables of 218 patients with ET according to sTXB2 quartiles
| Quartiles | P value | ||||
|---|---|---|---|---|---|
|
1st < 9.1 ng/mL N = 57 |
2nd 9.1–19 ng/mL N = 56 |
3rd 19.3–42 ng/mL N = 50 |
4th > 42 ng/mL N = 55 |
||
| Sex | |||||
| Male, n (%) | 23 (40.4) | 27 (48.2) | 25 (50.0) | 29 (52.7) | 0.593 |
| Female, n (%) | 34 (59.6) | 29 (51.8) | 25 (50.0) | 26 (47.3) | |
| Age at enrollment, years | 63.0 [54.0; 69.0] | 64.0 [56.0; 69.0] | 58.0 [50.0; 66.8] | 56.5 [49.0; 62.0] | 0.006 |
| Body mass index, kg/m2 | 24.5 [22.1; 27.7] | 25.5 [23.1; 26.9] | 25.0 [23.0; 27.0] | 24.6 [21.7; 27.0] | 0.626 |
| Leukocytes, ×109/L | 6.93 [5.5; 8.2] | 6.90 [5.8; 8.2] | 6.54 [5.5; 7.6] | 7.69 [6.8; 9.0] | 0.019 |
| Platelets, ×109/L | 451 [397; 500] | 520 [405; 598] | 528 [460; 625] | 641 [482; 772] | < 0.001 |
| Hematocrit, % | 40.3 [38.4; 43.6] | 41.7 [39.3; 44.2] | 40.7 [38.7; 43.6] | 43.8 [41.5; 46.0] | < 0.001 |
| JAK2‐V617F | |||||
| Wild type, n (%) | 30 (52.6) | 26 (46.4) | 18 (36.0) | 15 (27.3) | 0.034 |
| Mutated, n (%) | 27 (47.4) | 30 (53.6) | 32 (64.0) | 40 (72.7) | |
| CALR | |||||
| Type 1, n (%) | 7 (21.9) | 7 (21.2) | 2 (7.69) | 3 (12.0) | 0.127 |
| Type 2, n (%) | 4 (12.5) | 6 (18.2) | 5 (19.2) | 0 (0.00) | |
| Other, a n (%) | 21 (65.6) | 20 (60.6) | 19 (73.1) | 22 (88.0) | |
| Cytoreduction | |||||
| No, n (%) | 13 (22.8) | 16 (28.6) | 19 (38.0) | 36 (65.5) | <0.001 |
| Yes, n (%) | 44 (77.2) | 40 (71.4) | 31 (62.0) | 19 (34.5) | |
| Microvascular symptoms | |||||
| No, n (%) | 52 (91.2) | 51 (91.1) | 45 (90.0) | 46 (83.6) | 0.532 |
| Yes, n (%) | 5 (8.8) | 5 (8.9) | 5 (10.0) | 9 (16.4) | |
| Thrombosis | |||||
| No, n (%) | 55 (96.5) | 53 (94.6) | 49 (98.0) | 52 (94.5) | 0.833 |
| Yes, n (%) | 2 (3.5) | 3 (5.4) | 1 (2.00) | 3 (5.5) | |
| IPSET score | |||||
| 0, n (%) | 8 (14.0) | 7 (12.5) | 7 (14.0) | 6 (10.9) | |
| 1, n (%) | 15 (26.3) | 12 (21.4) | 6 (12.0) | 5 (9.09) | |
| 2, n (%) | 15 (26.3) | 13 (23.2) | 17 (34.0) | 23 (41.8) | |
| 3, n (%) | 3 (5.26) | 12 (21.4) | 13 (26.0) | 12 (21.8) | |
| 4, n (%) | 15 (26.3) | 10 (17.9) | 7 (14.0) | 7 (12.7) | |
| 5, n (%) | 1 (1.8) | 2 (3.4) | 0 (0.0) | 1 (1.8) | |
| 6, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.8) | |
| Primary prophylaxis | |||||
| No, n (%) | 7 (12.3) | 7 (12.5) | 6 (12.0) | 11 (20.0) | 0.568 |
| Yes, n (%) | 50 (87.7) | 49 (87.5) | 44 (88.0) | 44 (80.0) | |
| Urinary TXM, pg/mg creatinine | 436 (176) | 492 (232) | 443 (223) | 680 (555) | < 0.001 |
Values are median and [IQR] or frequency and (percentages). P values are from median and chi‐square test, respectively.
IPSET, International Prognostic Score of Thrombosis for Essential Thrombocythemia; TXM, urinary 11‐dehydro‐TxB2.
Includes the following detected genotypes: CALR‐negative, CALR mutations other than type I or type II and MPL mutations. CALR was not genotyped in 115 patients (102 in the compliant group, 13 in the not compliant group).
As expected, both platelet and leukocyte counts were significantly lower in association with cytoreduction as compared with no cytoreduction (median (95% confidence interval) platelet and leukocyte counts: 454 (426–481) and 6.4 (5.99–6.74) × 109/L vs. 631 (600–703) and 7.8 (7.4–8.3) × 109/L, respectively; other differential characteristics of the two subgroups are detailed in Table S2 ).
Although the association between the platelet count and sTXB2 was significant in the entire study cohort (n = 218, ρ = 0.42, P = 0.001), nevertheless this association was significantly different in patients on vs. off cytoreductive treatment (P for interaction < 0.001). A significant and direct association was detectable only in patients not on cytoreductive drugs (ρ = 0.446, P < 0.01), whereas it was absent in patients on cytoreduction (ρ = 0.035, P = ns; Figure 3a ). This association was observed also when data were analyzed according to the type of cytoreductive agent (HU: P for interaction = 0.004; anagrelide: P for interaction = 0.009, data not shown).
Figure 3.
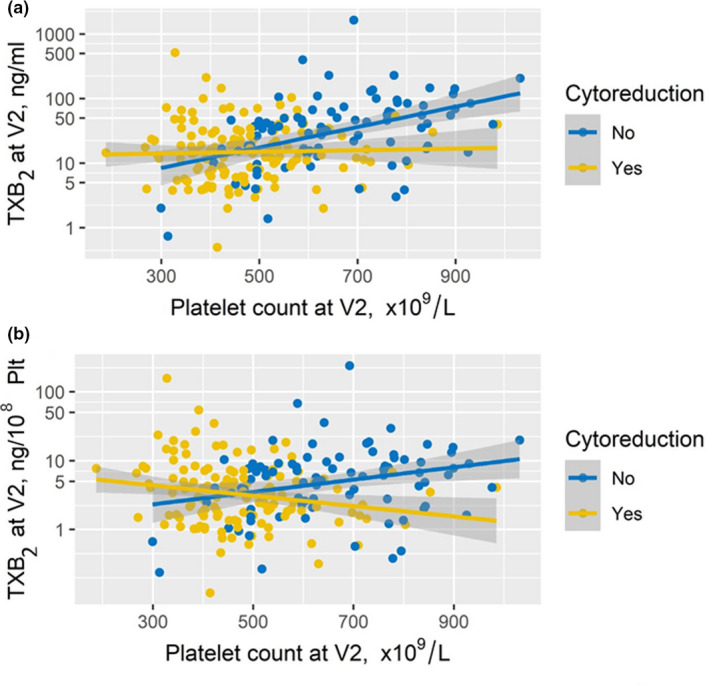
Effect of platelet count and cytoreductive treatment on sTXB2. The figure depicts the correlation between platelet counts and sTXB2 levels at visit 2 in 218 patients with ET while on aspirin 100 mg once daily; data are expressed as ng/mL (panel a) or ng/105 platelets (panel b) in patients on (yellow) or off (blue) cytoreductive treatment.
Moreover, in order to exclude a simple effect of the platelet number or mass, we corrected the sTXB2 values for the corresponding platelet count/mL and sTXB2 values were expressed as ng/105 platelets. The same association was observed after correcting sTXB2 values per platelet count (Figure 3b ).
Effects of randomized aspirin regimens
We then analyzed the associations of the platelet count and cytoreductive treatment with sTXB2 measured at the end of the 2‐week randomized treatment (Figure 4 ). The determinants of sTXB2 values observed in the entire cohort of 218 patients while on aspirin 100 mg q.d. at visit 2 were consistently reproduced in the 73 patients randomized to continue the q.d. regimen.
Figure 4.
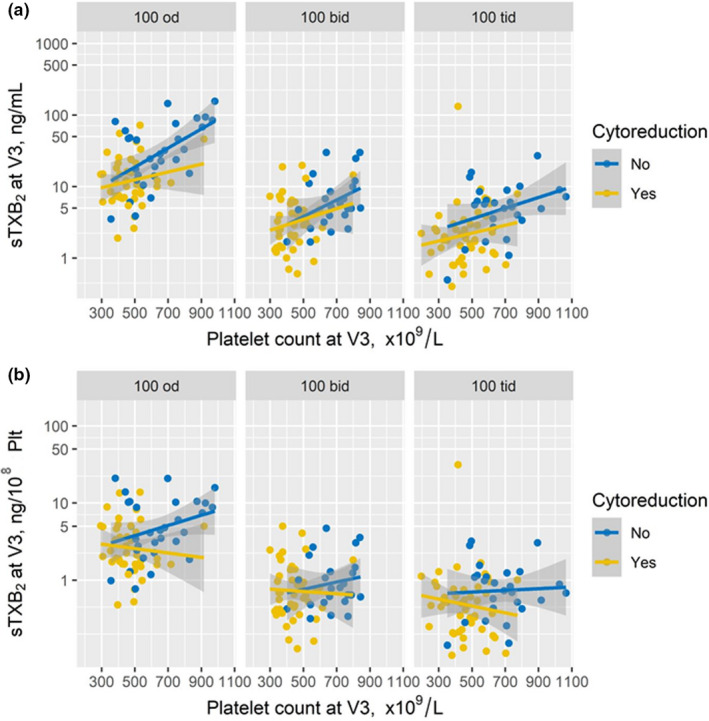
Correlations between platelet count and sTXB2 at the end of the randomized phase of the ARES trial according to cytoreduction. The plots depict the correlations between platelet count and sTXB2 at visit 3 (i.e., at the end of each randomized treatment; i.e., aspirin once‐ n = 73, twice‐daily n = 71, or three times‐daily n = 74). The sTXB2 levels are expressed as ng/mL of serum (panel a) or as ng/105 platelets (panel b).
Both in the b.i.d. and t.i.d. experimental arms, sTXB2 values at visit 3 were one log lower and showed a reduced inter‐individual variability as compared to the q.d. control arm (Figure 4 ). Furthermore, both platelet count and cytoreduction correlated with sTXB2 in the q.d. arm (multivariate ρ = 0.17, P = 0.005; P for interaction = 0.02), but not in the b.i.d. and t.i.d. arms (multivariate ρ = 0.074 and 0.071, P for interaction = 0.23 and 0.24, respectively). A higher degree of sTXB2 suppression was observed in both experimental arms, irrespective of cytoreductive treatment or platelet count (Figure 4a ). Similar results were obtained when sTXB2 values were expressed as ng/108 platelets (Figure 4b ).
DISCUSSION
The present substudy of the recently completed ARES trial was aimed at characterizing the main variables associated with the extent of platelet COX activity suppression by low‐dose aspirin, as reflected by sTXB2, in a contemporary cohort of 218 patients with ET. Overall, this study demonstrates that a high platelet count is significantly associated with incomplete suppression of platelet COX activity, and this pharmacological phenotype characterizes about three‐quarters of fully compliant patients with ET receiving a standard once‐daily low‐dose aspirin regimen. On the other hand, the minority of patients with adequate platelet COX inhibition (i.e., in the lower quartile of sTXB2), were characterized by a lower platelet count, lower frequency of JAK2‐V617F, older age, and more frequent use of cytoreductive treatment as compared to patients with inadequate sTXB2 suppression (Table 2 ).
In a previous study of 173 fully compliant non‐ET patients on long‐term low‐dose aspirin (100 mg q.d.) for cardiovascular prevention, the rate of sTXB2 recovery during the 24‐hour dosing interval was inversely associated with age and directly associated with the mean platelet volume, an indirect index of platelet turnover. 25 In ET, it is conceivable that abnormal megakaryopoiesis and its modulation by cytoreductive treatment may influence both the platelet count and the renewal rate of the drug target (i.e., platelet COX), thus explaining the apparent direct association between the platelet count and sTXB2, as well as its modulation by cytoreductive treatment. Given the very short half‐life of aspirin in the systemic circulation (15–20 minutes), its typically long‐lasting antiplatelet effect has been adequately modeled and predicted in silico 17 by integrating the irreversible nature of COX‐1 and COX‐2 acetylation, the substantial lack of de novo protein synthesis in peripheral platelets, and the limited renewal of the drug target during the 24‐hour dosing interval because of COX acetylation in the platelet progenitors. Changes in the latter process, rather than so‐called aspirin “resistance,” most likely account for a shorter duration of the antiplatelet effect of low‐dose aspirin in patients with ET. Consistent with this hypothesis, platelet TXA2 biosynthesis in ET was not fully inhibited by a conventional q.d. regimen of low‐dose aspirin, as measured at the end of the 24‐hour dosing interval 31 , 32 ; moreover, shortening the dosing interval, but not doubling the q.d. dose, rescued platelet COX inhibition in this setting. 18 Interestingly, the increasing percentage of JAK2‐V617F mutation across the sTXB2 quartiles (Table 2 ) would be consistent with a biologically plausible contribution to enhancing platelet turnover and activation, as suggested by animal and human data. 9 , 10 , 11 The number of CALR‐mutated patients in this study was too low to allow any meaningful inference.
In the vast majority of patients with ET, a standard once‐daily low‐dose aspirin regimen only partially inhibits platelet COX activity, whereas the inhibition is virtually complete with the b.i.d. regimen, independently of cytoreduction, as indicated by the significantly lower values of sTXB2 with the b.i.d. vs. q.d. dosing regimen. Interestingly, sTXB2 values that we measured in patients with ET on b.i.d. aspirin (4.0 (2.1–6.7) ng/mL) are comparable to those we reported in non‐MPN cardiovascular patients on once‐daily low‐dose aspirin (3.0 ± 3.0 ng/mL). 25 Moreover, the clinical relevance of a steady and virtually complete vs. partial inhibition of platelet COX activity is indirectly suggested by the evidence for the cardiovascular toxicity of NSAIDs. 33 In fact, most traditional NSAIDs, with the exception of high‐dose (500 mg b.i.d.) naproxen, 34 only incompletely inhibit platelet COX‐1 activity throughout the dosing interval 34 , 35 and are associated with increased risk of serious vascular events. 33 Therefore, incomplete and short‐lived inhibition of platelet TXA2 production seems unable to counteract a prothrombotic milieu associated with COX‐2 inhibition. 27 When applied to aspirin‐treated patients with ET, a similar conceptual paradigm would predict that a b.i.d. regimen is associated with cardioprotection, whereas a q.d. regimen is not. In this context, it should be emphasized that the cardiovascular benefit of low‐dose aspirin in ET is based on observational, retrospective studies, 36 , 37 and on extrapolation of the evidence from a randomized trial performed in polycythemia vera, 38 a distinct MPN not usually characterized by thrombocytosis, in which the antiplatelet PD of low‐dose aspirin has been poorly investigated. Although the efficacy and safety of antiplatelet therapy in patients with ET remains to be established, 3 , 39 it is interesting to note that this is currently recommended 12 and all the patients in the ARES trial were recruited while on long‐term low‐dose aspirin therapy. 19
As for the apparent modifying effect of cytoreductive treatment on the correlation between the platelet count and the extent of sTXB2 suppression, this could not be adequately explained by the present study. It can be hypothesized that the pro‐apoptotic effect of HU, 40 , 41 especially on pathologic megakaryocytes, 42 may only in part correct the quality of released platelets. However, HU is unable to re‐establish a normal platelet phenotype and turnover and an adequate aspirin PD. A properly designed randomized trial and mechanistic studies will be needed to test this hypothesis.
According to current recommendations, cytoreductive treatment to lower platelet counts is indicated in patients with ET with additional risk factors for thrombosis, such as age > 60 years, previous thrombosis, and JAK2 V617F mutation. 4 However, the relationship between platelet count and thrombotic risk as well as the optimal platelet count target to prevent thrombosis remain controversial. 43 In our study, the b.i.d. aspirin regimen seemed to blunt the variable impact of cytoreduction on platelet count and sTXB2.
Altogether, our findings may have clinically relevant implications. First, in patients with ET on cytoreductive treatment, the standard low‐dose aspirin regimen is largely inadequate to inhibit platelet TXA2 production throughout the 24‐hour dosing interval, irrespective of the achieved platelet count. 44 , 45 Second, in non‐cytoreduced patients with ET, the platelet biosynthetic capacity of producing TXA2 in response to a standard aspirin regimen increases as a function of the platelet count. Thus, the majority of non‐cytoreduced patients with platelet counts > 600 × 109/L display inadequate and widely variable platelet COX‐1 inhibition in response to q.d. low‐dose aspirin. These trends cannot be simply accounted for by the increase in platelet count/mass because COX activity per platelet was also increased. Third, our study confirmed that only a minority (≈ 25%) of fully compliant patients with ET achieve adequate platelet COX inhibition in response to a standard low‐dose aspirin regimen. However, their main clinical characteristics are scarcely discriminative (Table 2 ) and thus hardly applicable to predict the adequacy of drug effect. Fourth, the experimental b.i.d. and t.i.d. aspirin regimens were highly effective in restraining the large interindividual variability in response to q.d. low‐dose aspirin, substantially improving the inhibition of platelet COX activity, independently of the platelet count and cytoreductive treatment (Figure 4 ). Although the assessment of sTXB2 as a PD biomarker to guide aspirin dosing in patients with ET was proven effective by the ARES feasibility study, 19 such measurement may not be routinely available in hematological centers. Therefore, given that ~ 75% of patients with ET display inadequate TXA2 inhibition in response to a standard antiplatelet regimen, and that cytoreduction may not fully restore adequate platelet inhibition, it would be advisable—in principle—to treat all patients for whom antiplatelet therapy is clearly indicated with a b.i.d. aspirin regimen. 12 The results of the ARES trial extension 19 will provide relevant information on the long‐term tolerability, safety, and persistence of biochemical superiority of such an experimental approach vs. the current standard of aspirin therapy. Finally, although not tested in the ARES trial, we suggest that the same determinants of antiplatelet PD may apply to the thienopyridine class of P2Y12 blockers (i.e., clopidogrel and prasugrel) that share with aspirin an irreversible mechanism of action involving permanent inactivation of a platelet protein that cannot be resynthesized within the dosing interval. 46 A similarly accelerated renewal of platelet P2Y12 would be expected in a setting such as ET and, possibly, in less extreme clinical paradigms of faster platelet turnover. This working hypothesis needs to be tested by ad hoc studies in the appropriate clinical setting.
Some limitations of the present study should also be acknowledged. First, it was based on subgroup analyses of the ARES trial, with the intrinsic limitations of this type of analyses. 47 Second, the platelet count target and choice of cytoreductive treatment was not standardized, but was left to the current practice of each participating center.
In conclusion, in a large, contemporary cohort of patients with ET participating in a controlled randomized clinical trial, the platelet count was the independent variable with the strongest association with the (in)adequacy of platelet TXA2 suppression by a standard regimen of low‐dose aspirin therapy, although with a different pattern in cytoreduced vs. non‐cytoreduced patients. A more frequent dosing regimen restored adequate platelet inhibition and reduced interindividual variability in response to low‐dose aspirin, independent of cytoreduction.
FUNDING
The ARES trial was funded by the Italian Medicines Agency (AIFA), study code FARM12Y8H.
CONFLICTS OF INTEREST
A.T. has received consultant and speaker fees from Bayer AG, Novo‐Nordisk, and Werfen. B.R. has received consultant and speaker fees from Bayer AG, and MedScape. A.I. has received speaker fees from Novartis, Pfizer, and Incyte. A.M.V. has received consultant and speaker fees from AOP Orphan Pharmaceuticals, Celgene, Novartis, Takeda and BMS. V.D.S. has received consultant and speaker fees from Alexion, Amgen, AOP Orphan Pharmaceuticals Celgene, Grifols, Novartis, Takeda, Abbvie, SOBI, and research grants from Novartis. C.P. reports receiving consultant and speaker fees from Acticor Biotech, Amgen, Bayer, GlaxoSmithKline, Eli Lilly, Tremeau, and Zambon; he chairs the Scientific Advisory Board of the International Aspirin Foundation. All other authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
C.P., A.To., B.R., and V.D.S. wrote the manuscript. C.P., V.D.S., and F.R. designed the research. S.B., D.S., E.R., A.Ti., A.I., D.C., C.B., A.D., M.D.I., P.R., F.P., N.V., E.B., G.L., M.R., G.C., E.M.E., S.P., M.L.R., I.B., G.G.L., A.R., G.S., A.M.V., V.C., and B.P. performed the research. A.To. B.R., and G.P. analyzed the data.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
Aspirin and placebo used in the trial were generously provided by Bayer AG, through the courtesy of Drs. Elmar Detering and Paolo Montanari. Open Access Funding provided by Universita Cattolica del Sacro Cuore within the CRUI‐CARE Agreement.
[Correction added on 26 November 2021, after first online publication: Open Access funding statement has been added in acknowledgments section.]
Valerio De Stefano and Carlo Patrono are co‐last authors.
- 1. Tefferi, A. & Pardanani, A. Essential thrombocythemia. N. Engl. J. Med. 381, 2135–2144 (2019). [DOI] [PubMed] [Google Scholar]
- 2. Ahlstrand, E. et al. Highly reduced survival in essential thrombocythemia and polycythemia vera patients with vascular complications during follow‐up. Eur. J. Haematol. 104, 271–278 (2020). [DOI] [PubMed] [Google Scholar]
- 3. Patrono, C. , Rocca, B. & De Stefano, V. Platelet activation and inhibition in polycythemia vera and essential thrombocythemia. Blood 121, 1701–1711 (2013). [DOI] [PubMed] [Google Scholar]
- 4. Barbui, T. et al. Philadelphia chromosome‐negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia 32, 1057–1069 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barbui, T. et al. Development and validation of an International Prognostic Score of thrombosis in World Health Organization‐essential thrombocythemia (IPSET‐thrombosis). Blood 120, 5128–5133; quiz 5252 (2012). [DOI] [PubMed] [Google Scholar]
- 6. Barbui, T. et al. Practice‐relevant revision of IPSET‐thrombosis based on 1019 patients with WHO‐defined essential thrombocythemia. Blood Cancer J. 5, e369 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alvarez‐Larran, A. et al. Antiplatelet therapy versus observation in low‐risk essential thrombocythemia with a CALR mutation. Haematologica 101, 926–931 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Noetzli, L.J. , French, S.L. & Machlus, K.R. New insights into the differentiation of megakaryocytes from hematopoietic progenitors. Arterioscler. Thromb. Vasc. Biol. 39, 1288–1300 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hobbs, C.M. et al. JAK2V617F leads to intrinsic changes in platelet formation and reactivity in a knock‐in mouse model of essential thrombocythemia. Blood 122, 3787–3797 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hauschner, H. et al. Platelets from Calreticulin mutated essential thrombocythemia patients are less reactive than JAK2 V617F mutated platelets. Am. J. Hematol. 95, 379–386 (2020). [DOI] [PubMed] [Google Scholar]
- 11. Venugopal, S. & Mascarenhas, J. Novel therapeutics in myeloproliferative neoplasms. J. Hematol. Oncol. 13, 162 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tefferi, A. & Barbui, T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk‐stratification and management. Am. J. Hematol. 95, 1599–1613 (2020). [DOI] [PubMed] [Google Scholar]
- 13. Klampfl, T. et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N. Engl. J. Med. 369, 2379–2390 (2013). [DOI] [PubMed] [Google Scholar]
- 14. Burch, J.W. , Stanford, N. & Majerus, P.W. Inhibition of platelet prostaglandin synthetase by oral aspirin. J. Clin. Invest. 61, 314–319 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Machlus, K.R. & Italiano, J.E. Jr The incredible journey: From megakaryocyte development to platelet formation. J. Cell Biol. 201, 785–796 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patrignani, P. , Filabozzi, P. & Patrono, C. Selective cumulative inhibition of platelet thromboxane production by low‐dose aspirin in healthy subjects. J. Clin. Invest. 69, 1366–1372 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giaretta, A. , Rocca, B. , Di Camillo, B. , Toffolo, G.M. & Patrono, C. In silico modeling of the antiplatelet pharmacodynamics of low‐dose aspirin in health and disease. Clin. Pharmacol. Ther. 102, 823–831 (2017). [DOI] [PubMed] [Google Scholar]
- 18. Pascale, S. et al. Aspirin‐insensitive thromboxane biosynthesis in essential thrombocythemia is explained by accelerated renewal of the drug target. Blood 119, 3595–3603 (2012). [DOI] [PubMed] [Google Scholar]
- 19. De Stefano, V. et al. The Aspirin Regimens in Essential Thrombocythemia (ARES) phase II randomized trial design: implementation of the serum thromboxane B2 assay as an evaluation tool of different aspirin dosing regimens in the clinical setting. Blood Cancer J. 8, 49 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patrono, C. et al. Low dose aspirin and inhibition of thromboxane B2 production in healthy subjects. Thromb. Res. 17, 317–327 (1980). [DOI] [PubMed] [Google Scholar]
- 21. Rocca, B. et al. A randomized double‐blind trial of 3 aspirin regimens to optimize antiplatelet therapy in essential thrombocythemia. Blood 136, 171–182 (2020). [DOI] [PubMed] [Google Scholar]
- 22. European Medicines Agency (EMA) . <http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003340.pdf>.
- 23. Patrono, C. & Rocca, B. Measurement of thromboxane biosynthesis in health and disease. Front. Pharmacol. 10, 1244 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Catella‐Lawson, F. et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N. Engl. J. Med. 345, 1809–1817 (2001). [DOI] [PubMed] [Google Scholar]
- 25. Rocca, B. et al. The recovery of platelet cyclooxygenase activity explains interindividual variability in responsiveness to low‐dose aspirin in patients with and without diabetes. J. Thromb. Haemost. 10, 1220–1230 (2012). [DOI] [PubMed] [Google Scholar]
- 26. Petrucci, G. et al. Patient‐independent variables affecting the assessment of aspirin responsiveness by serum thromboxane measurement. Thromb. Haemostasis 116, 891–896 (2016). [DOI] [PubMed] [Google Scholar]
- 27. Team, R.D.C. A Language and Environment for Statistical Computing (v 3.6.1). (R Foundation for Statistical Computing, Austria, Vienna, 2019). [Google Scholar]
- 28. R Core Team. R Foundation for Statistical Computing, V . A Language and Environment for Statistical Computing v 4.0.2 (R Foundation for Statistical Computing, Austria, Vienna, 2020). [Google Scholar]
- 29. Santilli, F. et al. Platelet cyclooxygenase inhibition by low‐dose aspirin is not reflected consistently by platelet function assays: implications for aspirin “resistance”. J. Am. Coll. Cardiol. 53, 667–677 (2009). [DOI] [PubMed] [Google Scholar]
- 30. Solberg, H.E. The theory of reference values Part 5. Statistical treatment of collected reference values. Determination of reference limits. J. Clin. Chem. Clin. Biochem. 21, 749–760 (1983). [PubMed] [Google Scholar]
- 31. Dragani, A. et al. The contribution of cyclooxygenase‐1 and ‐2 to persistent thromboxane biosynthesis in aspirin‐treated essential thrombocythemia: implications for antiplatelet therapy. Blood 115, 1054–1061 (2010). [DOI] [PubMed] [Google Scholar]
- 32. Dillinger, J.G. et al. Twice daily aspirin to improve biological aspirin efficacy in patients with essential thrombocythemia. Thromb. Res. 129, 91–94 (2012). [DOI] [PubMed] [Google Scholar]
- 33. Bhala, N. et al. Vascular and upper gastrointestinal effects of non‐steroidal anti‐inflammatory drugs: meta‐analyses of individual participant data from randomised trials. Lancet 382, 769–779 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Capone, M.L. et al. Clinical pharmacology of platelet, monocyte, and vascular cyclooxygenase inhibition by naproxen and low‐dose aspirin in healthy subjects. Circulation 109, 1468–1471 (2004). [DOI] [PubMed] [Google Scholar]
- 35. Patrono, C. et al. Clinical pharmacology of platelet cyclooxygenase inhibition. Circulation 72, 1177–1184 (1985). [DOI] [PubMed] [Google Scholar]
- 36. Alvarez‐Larrán, A. et al. Observation versus antiplatelet therapy as primary prophylaxis for thrombosis in low‐risk essential thrombocythemia. Blood 116, 1205–1210 (2010), quiz 1387. [DOI] [PubMed] [Google Scholar]
- 37. Alvarez‐Larran, A. , Pereira, A. , Arellano‐Rodrigo, E. , Hernandez‐Boluda, J.C. , Cervantes, F. & Besses, C. Cytoreduction plus low‐dose aspirin versus cytoreduction alone as primary prophylaxis of thrombosis in patients with high‐risk essential thrombocythaemia: an observational study. Br. J. Haematol. 161, 865–871 (2013). [DOI] [PubMed] [Google Scholar]
- 38. Landolfi, R. et al. Efficacy and safety of low‐dose aspirin in polycythemia vera. N. Engl. J. Med. 350, 114–124 (2004). [DOI] [PubMed] [Google Scholar]
- 39. Chu, D.K. , Hillis, C.M. , Leong, D.P. , Anand, S.S. & Siegal, D.M. Benefits and risks of antithrombotic therapy in essential thrombocythemia: a systematic review. Ann. Intern. Med. 167, 170–180 (2017). [DOI] [PubMed] [Google Scholar]
- 40. Andréasson, B. , Swolin, B. & Kutti, J. Hydroxyurea treatment reduces haematopoietic progenitor growth and CD34 positive cells in polycythaemia vera and essential thrombocythaemia. Eur. J. Haematol. 64, 188–193 (2000). [DOI] [PubMed] [Google Scholar]
- 41. Hong, Y. , Wang, G. , Del Arroyo, A.G. , Hernandez, J. , Skene, C. & Erusalimsky, J.D. Comparison between anagrelide and hydroxycarbamide in their activities against haematopoietic progenitor cell growth and differentiation: selectivity of anagrelide for the megakaryocytic lineage. Leukemia 20, 1117–1122 (2006). [DOI] [PubMed] [Google Scholar]
- 42. Tefferi, A. , Elliott, M.A. , Kao, P.C. , Yoon, S. , El‐Hemaidi, I. & Pearson, T.C. Hydroxyurea‐induced marked oscillations of platelet counts in patients with polycythemia vera. Blood 96, 1582–1584 (2000). [PubMed] [Google Scholar]
- 43. Kuykendall, A.T. & Komrokji, R. What’s in a number? Examining the prognostic and predictive importance of platelet count in patients with essential thrombocythemia. J. Natl. Compr. Canc. Netw. 18, 1279–1284 (2020). [DOI] [PubMed] [Google Scholar]
- 44. Barosi, G. et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG‐MRT consensus project. Blood 121, 4778–4781 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Harrison, C. Rethinking disease definitions and therapeutic strategies in essential thrombocythemia and polycythemia vera. Hematol. Am. Soc. Hematol. Educ. Program 2010, 129–134 (2010). [DOI] [PubMed] [Google Scholar]
- 46. Patrono, C. et al. Antiplatelet agents for the treatment and prevention of coronary atherothrombosis. J. Am. Coll. Cardiol. 70, 1760–1776 (2017). [DOI] [PubMed] [Google Scholar]
- 47. Assmann, S.F. , Pocock, S.J. , Enos, L.E. & Kasten, L.E. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet 355, 1064–1069 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


