Abstract
Circulating nucleic acids and extracellular vesicles (EV) represent novel biomarkers to diagnose cancer. The non‐invasive nature of these so‐called liquid biopsies provides an attractive alternative to tissue biopsy‐based cancer diagnostics. This study aimed to investigate if circulating cell cycle‐related E2F target transcripts can be used to diagnose tumours in canine tumour patients with different types of tumours. Furthermore, we assessed if these mRNAs are localised within circulating EV. We isolated total RNA from the plasma of 20 canine tumour patients and 20 healthy controls. Four E2F target genes (CDC6, DHFR, H2AFZ and ATAD2) were selected based on the analysis of published data of tumour samples available in public databases. We performed reverse transcription and quantitative real‐time PCR to analyse the plasma levels of selected E2F target transcripts. All four E2F target transcripts were detectable in the plasma of canine tumour patients. CDC6 mRNA levels were significantly higher in the plasma of canine tumour patients compared to healthy controls. A subset of canine tumour patient and healthy control plasma samples (n = 7) were subjected to size exclusion chromatography in order to validate association of the E2F target transcripts to circulating EV. For CDC6, EV analysis enhanced their detectability compared to total plasma analysis. In conclusion, our study reveals circulating CDC6 as a promising non‐invasive biomarker to diagnose canine tumours.
Keywords: biomarkers, cell cycle, dogs, extracellular vesicles, liquid biopsy, tumour
1. INTRODUCTION
For over 10 centuries veterinary and human clinicians have used tissue biopsies as tools to diagnose cancer. 1 Even if tissue biopsies remain the test of choice for the definitive diagnosis of most types of cancer, they show several disadvantages. A common feature of tissue biopsies is that they provide the clinician with a snapshot of tumour tissue. 2 It would require the acquisition of multiple tumour biopsies to monitor the status of a tumour over time. As the procedure of tumour tissue biopsy acquirement is accompanied by the risk of surgical complications and discomfort for the patient, they often turn out to be solely applicable in the diagnostic stage of cancer. 2 To monitor cancer progression and treatment response, tumour tissue biopsies are simply impractical. Additionally, in metastatic cancer there exists a considerable amount of intratumour heterogeneity, leading to an unrepresentative view of the disease during the analysis of single tumour biopsies. 3 , 4 In some cancer patients, tumour tissue biopsies might not even be an option due to inaccessibility of the tumour tissue. 2 In light of the constraints of tumour tissue biopsies, the concept of liquid biopsies began to arise. Liquid biopsies consist of the analysis of body fluids, mainly blood samples, for the detection of circulating tumour cells, circulating tumour nucleic acids and extracellular vesicles (EV) as a source of genomic and proteomic information, therefore reflecting the global molecular status of cancer patients. 5 Moreover, less invasive sampling allows serial sampling and thereby enables monitoring of tumour features over the course of an anti‐cancer treatment more easily. 6 The final, largest advantage of liquid biopsies is the fact that they can be employed as a tool for routine screenings and therefore potentially enable detection of cancer at an early stage, 2 before the tumour is large enough to cause clinical symptoms or dense enough to show up on diagnostic imaging procedures.
Tumour cells release DNA and microRNA/mRNA into the extracellular environment, which end up in the circulation. 7 , 8 For this reason, circulating nucleic acids are being investigated for their potential to aid diagnosis of cancer, predict treatment response, and to monitor therapy response. 6 Especially extracellular RNA molecules appear to be promising with regard to the development of highly sensitive and specific diagnostic tests. 9 A proportion of circulating RNA is contained within EV. 9 EV are membrane‐enclosed structures of variable sizes, which are secreted by many cell types and released into the extracellular space. 10 , 11 Based on size and mode of biogenesis, EV are divided into two main classes. Exosomes (Exo) are small EV (30–100 nm) which arise in the endosomal system and are released upon fusion of multivesicular bodies with the plasma membrane. 12 Microvesicles (MV), also reported as ectosomes, are larger EV of 100–1000 nm which are released into the extracellular space by direct budding from the plasma membrane. 12 Both Exo and MV are released by cells in physiological and pathological states, including cancer. 13 The EV lipid bilayer membrane protects RNA from degradation by RNAses and consequently enables stabilised transport and functional transfer of bioactive molecules between cell and tissue compartments. 14 Therefore, EV‐enclosed RNA may be enriched in specific tumour‐associated RNA transcripts and potentially provide a more advantageous source of tumour biomarkers compared to the total plasma RNA pool.
In 2017, a study from Conley and colleagues 15 reported high levels of mRNAs encoding transcriptional targets of the E2F family of transcription factors in EV isolated from the plasma of human breast cancer patients. The E2F family of transcription factors are involved in the Rb‐E2F pathway, which is frequently deregulated in many different types of cancer. 16 E2F transcription factors play a key role in cell proliferation by regulating genes encoding proteins necessary for S phase entry and cell cycle progression. 17 Measurement of these cell cycle‐related genes in EV could potentially provide a blood‐based alternative to the current tissue‐based methods to quantify tumour proliferation rate, which can be used to detect and monitor tumour growth. As uncontrolled and increased proliferation are key features of many cancers, E2F target genes could potentially be employed as biomarkers in different types of cancer.
In this study, we demonstrate the presence of circulating E2F target transcripts in a cohort of canine tumour patients with different types of tumours. Furthermore, we show that these mRNAs are localised within EVs. To our knowledge, this is the first study to investigate EV‐enclosed mRNAs as tumour biomarkers in the blood of canine tumour patients. The results of this study shed light on potential advantages of measuring tumour biomarkers in EVs instead of total plasma.
2. EXPERIMENTAL PROCEDURES
2.1. In vitro experiments
2.1.1. Cell line validation statement and culture conditions
Canine melanoma cell line CMM12 was provided by N. Sasaki, T. Nakagawa, K. Saeki (University of Tokyo, Japan), and R. de Maria (University of Turin, Italy). This cell line has been previously established and validated by Yoshitake et al. 18 Additionally, the cell line has been regularly tested and confirmed to be mycoplasma‐free. CMM12 cells had been in culture for >70 passages at the time of this study. Cells were cultured in Dulbecco's Modified Eagle Medium/Ham's F‐12 (DMEM/F‐12, Invitrogen, Waltham, United States) supplemented with 10% fetal bovine serum (FBS, Invitrogen) and 1% penicillin/streptomycin (P/S, Invitrogen). Cells grew as monolayer cultures in a humidified atmosphere containing 5% CO2 at 37°C, and were maintained by passage when they reached over 90% confluence.
2.1.2. EV isolation by differential centrifugation
For melanoma‐derived EV isolation, two p150 petri dishes were seeded with each 6 × 105 CMM12 cells. After 24 h, cell culture media were removed, cells were washed once with phosphate buffered saline (PBS), and EV‐depleted cell culture medium was added. EV‐depleted cell culture medium was prepared by overnight centrifugation of a 30% medium/FBS solution at 100 000g (SW32 rotor; 4°C). After diluting to a 10% medium/FBS solution, P/S was added and the medium was passed through a 0.22 μm filter (Merck KGaA, Darmstadt, Germany). Conditioned media were collected after 48 h of culture. EVs were isolated from conditioned cell culture media by differential centrifugation (DC; Beckman Coulter Optima XPN‐80 Ultracentrifuge). Briefly, conditioned cell culture media were depleted from cells and cellular debris by centrifuging twice at 200g and 500g for 10 min. Large EVs were then pelleted by ultracentrifugation for 30 min at 10 000g (SW32 rotor; 4°C). Thereafter, the remaining supernatant was centrifuged at 100 000g for 65 min (SW32 rotor; 4°C) to pellet small EVs. Non‐conditioned EV‐depleted cell culture medium was subjected to the same protocol as a negative control.
2.1.3. Density gradient centrifugation
To separate EVs from protein contaminants in the differential centrifugation (DC) pellets, DC was followed by sucrose density gradient centrifugation as described previously. 19 In brief, EV pellets were resuspended in 20 μl PBS containing 0.2% BSA (depleted from aggregates by overnight ultracentrifugation at 100 000g). Subsequently, the vesicles were mixed with a 2.5 M sucrose solution and overlaid with 15 sucrose solutions with decreasing molarities (Table S1). Sucrose gradients were then spun for 16 h at 192 000g (SW40 rotor; 4°C), after which twelve 1‐ml sucrose fractions (F1‐F12 from bottom to top) were collected. Fractions with densities ranging from 1.12 to 1.16 g/cm3 (EV) were pooled in SW40 tubes, diluted with PBS and spun at 270 000g for 65 min (SW40 rotor; 4°C) to pellet EV.
2.1.4. RNA isolation
Cultured CMM12 cells and conditioned medium‐derived EV pellets were lysed in RLT buffer (Qiagen, Hilden, Germany) containing 10% ß‐Mercaptoethanol (Merck). RNA was isolated from cells and EV with the RNeasy micro kit (Qiagen) according to the manufacturer's procedure. The optional DNase digestion step was also included. Cellular RNA quantity and quality were assessed with a Nanodrop ND‐1000 spectrophotometer. EV pellet‐derived RNA was quantified using Agilent 2100 Bioanalyzer pico chips.
2.1.5. Reverse transcription and quantitative real‐time PCR
cDNA was synthesised using the RevertAid First Strand cDNA Synthesis Kit (ThermoScientific, Cat. # K1622) with random hexameric primers. In the qPCR reactions, an equivalent of 125 pg RNA was used with 3 μl 1.5 μM primermix (Biolegio, Nijmegen, the Netherlands), containing both forward and reverse primers, and 12.5 μl SYBR Green mastermix (Bio‐Rad, California, United States) in a 25 μl reaction. The following, most abundantly expressed E2F target genes were selected based on the analysis of published data and transcriptome profiles of tumour samples available in public databases: CDC6, DHFR, H2AFZ and ATAD2. Primers for these canine E2F target gene transcripts (Table 1) were designed using the Primer3 primer design tool and were based on coding mRNA sequences. For all primer sets, optimum melting temperatures were determined. Additionally, PCR amplification efficiencies were tested using 10‐fold dilution series of cDNA.
TABLE 1.
Canine E2F target gene and housekeeping gene primer sequences
| Primer | Sequence (5′‐3′) |
|---|---|
| CDC6 forward | TAACGTCTGTCGCAAACAGC |
| CDC6 reverse | AAGCGGGTTTCCTTGTTTTT |
| DHFR forward | GACCTCAAGGAACCTCCACA |
| DHFR reverse | GAACTGCCTCCCACTATCCA |
| H2AFZ forward | GGTAAGGCTGGGAAGGACTC |
| H2AFZ reverse | TCAGGTGTCGATGAATACGG |
| ATAD2 forward | ATGCCAAAGCAAAATTCCAC |
| ATAD2 reverse | CAGGAGTACTGCAAGCCACA |
Using the BioRad CFX Connect real‐time PCR detection system (Bio‐Rad), an initial denaturation step was performed at 95°C for 3 min, followed by 40 cycles of denaturation at 95°C for 10 s, and annealing/extension at 61°C for 30 s. All reactions were performed in duplicate and negative controls (Milli‐Q) were included. To date, reference genes for qPCR analysis of EV mRNAs are unknown. Therefore, all EV samples were normalised to the total input quantity of RNA (125 pg).
2.1.6. Western blot
EV and CMM12 cell pellets were lysed in respectively 100 and 150 μl lysis buffer containing 0.625 M Tris/HCl (pH 6.8), 2.5% SDS and 10% glycerol. The cell lysate was depleted of nuclei by centrifugation (13 000 rpm for 1 min) and collection of the supernatant. EV and cell lysates were incubated for 5 min at 95°C before running equal volumes (23 μl for the EV protein lysate and 45 μg for the cellular protein lysate) on a 12% polyacrylamide gel under non‐reducing conditions. Separated proteins were transferred onto PVDF membranes, which were then blocked in TBS‐T containing 2.5% BSA. Membranes were incubated with primary antibodies at 4°C overnight to detect CD9 and Calnexin. The following primary antibodies were used: mouse anti‐CD9 (1:1000, BioLegend, Cat. # 312102) and rabbit anti‐Calnexin (1:1000, Abcam, Cat. # ab75801). The following day, membranes were washed 3x with TBS‐T and incubated with horseradish peroxidase‐coupled secondary antibodies (1:5000, Cell Signalling, Cat. # 7074 + 7076) for 60 min at room temperature. After washing three times with TBS‐T and a short wash in PBS, the developer solution (SuperSignal West Dura, ThermoScientific, Cat. # 34076) was added. Labelled proteins were visualised by the Chemidoc Touch Imaging System (Biorad, Hercules, CA).
2.1.7. Statistical analysis
Statistical analysis of reverse transcription and quantitative real‐time PCR (RT‐qPCR) data was performed in SPSS Statistics 24 using the Kruskal–Wallis test, based on the analysis on data distribution. The significance threshold was set at p < .05.
2.2. In vivo experiments
2.2.1. Specimen collection, processing and storage
Canine tumour patients diagnosed with several types of tumours at the Department of Clinical Sciences of the Faculty of Veterinary Medicine at Utrecht University were enrolled in this study (Table 2 in Section 3). Each patient owner had to provide written consent for the collection and analysis of blood samples. Furthermore, 20 healthy dogs were included as controls (Table 3 in Section 3). Specimen collection from dogs was reviewed and approved by the Animal Welfare Body Utrecht (approval ethical number AVD1080020184847).
TABLE 2.
Patient description including tumour type, histopathological diagnosis and disease stage
| Tumour type | Histopathological diagnosis | N | Anatomic site | Disease stage | Age a (range) | Sex | ||
|---|---|---|---|---|---|---|---|---|
| Primary tumour | Recurrence | Metastasis | ||||||
| Overall | N = 20 | N = 17 | N = 2 | N = 1 |
8.50 (5–14) |
F: 10 M: 10 |
||
| Malignant epithelial tumours (carcinoma) | 6 | 6 |
8.50 (5–11) |
F: 4 M: 4 |
||||
| Adrenocortical carcinoma | 3 | Adrenal glands | 3 |
7 (5–8) |
F: 0 M: 3 | |||
| Urothelial carcinoma | 1 | Bladder | 1 | 11 |
F: 1 M: 0 |
|||
| Lung carcinoma | 1 | Lung | 1 | 8 |
F: 1 M: 0 |
|||
| Neuroendocrine | C cell carcinoma | 1 | Thyroid glands | 1 | 7 |
F: 1 M: 0 |
||
| Malignant non‐epithelial tumours | 10 | 7 | 2 | 1 |
8.80 (6–14) |
F: 5 M: 5 |
||
| Sarcoma | Fibrosarcoma | 3 | Skin/subcutis | 3 |
10 (9–11) |
F: 2 M: 1 |
||
| Hemangiopericytoma | 2 | Skin/subcutis | 1 | 1 | 11 |
F: 1 M: 1 |
||
| Osteosarcoma | 1 | Bone | 1 | 7 |
F: 0 M: 1 |
|||
| Pigmented tumour | Melanoma | 1 | Skin/subcutis | 1 | 8 |
F: 0 M: 1 |
||
| Melanoma | 1 | Skin/subcutis | 1 | 14 |
F: 0 M: 1 |
|||
| Round cell tumours | Mast cell tumour | 1 | Skin/subcutis | 1 | 6 |
F: 1 M: 0 |
||
| Plasma cell tumour | 1 | Skin/subcutis | 1 | 12 |
F: 1 M: 0 |
|||
| Benign epithelial tumours (adenoma) | 4 | 4 | 7 |
F: 1 M: 1 |
||||
| Pituitary adenoma | 2 | Pituitary gland | 2 | 7 |
F: 1 M: 1 |
|||
| Pheochromocytoma | 1 | Adrenal glands | 1 | 11 |
F: 1 M: 0 |
|||
| Insulinoma | 1 | Pancreas | 1 | 10 |
F: 0 M: 1 |
|||
Note: Bold values represent the total number of tumors for each tumor type.
Abbreviations: F, female; M, male.
Average age in years followed by the age range in brackets.
TABLE 3.
Healthy control description including breed, age and sex
| Breed | N | Age a (range) | Sex |
|---|---|---|---|
| Overall | 20 | 4.6 (1–11) |
F:16 M: 4 |
| Beagle | 18 | 4.8 (1–11) |
F: 15 M: 3 |
| Greyhound | 2 | 2 |
F: 1 M: 1 |
Note: Bold values represent the total number of tumors for each tumor type.
Abbreviations: F, female; M, male.
Average age in years followed by the age range in brackets.
Six millilitres of blood was collected from tumour patients and healthy controls in citrate anti‐coagulant tubes (BD Vacutainer) and processed within 2 h after collection (Figure 1). Blood samples were collected from tumour patients and healthy dogs in a fasted, conscious state by standardised venepuncture. In tumour patients, sampling was performed at the time of anaesthesia induction for tumour removal surgery. Plasma was separated from peripheral blood mononuclear cells by centrifugation for 15 min at 2500g (RT). Next, platelet free plasma (PFP) was obtained by applying another 15‐min centrifugation step at 2500g (RT). PFP was snap‐frozen in liquid nitrogen and stored at −80°C until RNA isolation.
FIGURE 1.
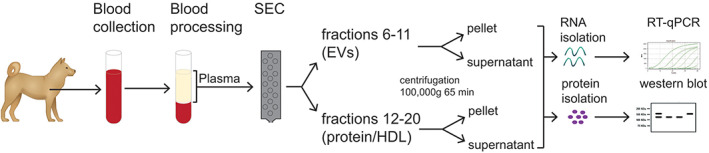
Scheme of experimental set‐up to analyse canine plasma‐derived extracellular vesicles (EV). Blood samples were collected and processed to platelet‐free plasma (PFP). PFP was fractionated by size exclusion chromatography (SEC). Early (F6‐11; containing EVs) and late (F12‐20; containing protein/HDL) SEC fractions were pooled and centrifuged at 100 000g for 65 min. RNA and protein were extracted from pellets and supernatants, after which RNA analysis was performed by reverse transcription and quantitative real‐time PCR (RT‐qPCR) and protein analysis by western blot
2.2.2. Size exclusion chromatography
Size exclusion chromatography (SEC) was performed using a qEV Classic size exclusion column (Izon Science, Christchurch, New Zealand). Immediately after applying plasma samples (500 μl) to the SEC column, the column was eluted with 1X PBS and 25 fractions of 500 μl were collected manually. The void volume (F1‐5) was discarded. Early fractions (F6‐11; containing EVs and large lipoprotein particles) and late fractions (F12‐20; containing protein complexes and small lipoprotein particles) were pooled into two SW41 tubes and subsequently centrifuged at 100 000g for 65 min (SW41 rotor; 4°C). After decanting the supernatant, 700 μl Qiazol (Qiagen) was immediately added to pellets for RNA isolation. For the analysis of non‐pelletable structures, 4 ml of supernatant was retained for RNA isolation. Prior to being subjected to RNA isolation, the samples were concentrated using Amicon Ultra 100 kDa spin filters (3000 g for 15 min), even if RNA‐binding proteins smaller than 100 kDA eventually present in the supernatant might not have been retained in our samples.
2.2.3. RNA isolation
One millilitre of banked PFP was used to isolate total plasma RNA using TRIzol LS Reagent (Invitrogen) following the manufacturer's instructions with minor modifications. During isopropanol RNA precipitation, 1 μl of 15 mg/ml GlycoBlue Coprecipitant (Invitrogen) was added to visualise the pelleted precipitate. The optional DNase digestion step was also included. Additionally, RNA sample quality was optimised by applying a second washing step with 75% ethanol. Washed RNA pellets were dissolved in 14 μl nuclease‐free water (=total plasma RNA). RNA was isolated from pellets and supernatants obtained from early and late SEC fractions using the miRNeasy micro kit (Qiagen) following the manufacturer's instructions.
2.2.4. Reverse transcription and quantitative real‐time PCR
Eleven microlitres of dissolved total plasma and eluted plasma‐derived EV RNA was used for cDNA synthesis with the RevertAid First Strand cDNA synthesis Kit (Invitrogen) with random hexameric primers. All samples were normalised to original PFP volumes. To this end, total plasma and plasma‐derived EV cDNA was diluted respectively 20 and 10 times for qPCR analysis, in order to compensate for the 2‐fold difference in input material.
To perform qPCR analysis of the E2F target genes, 2 μl of diluted cDNA was combined with 1.2 μl 1.5 μM primermix and 5 μl SYBR Green mastermix to generate a PCR reaction of 10 μl total volume. RT‐qPCR reactions were performed on the BioRad CFX384 real‐time system (Bio‐Rad), with the cycling conditions as described under Section 2.1.4. All reactions were performed in triplicate and negative controls were included, which confirmed the absence of non‐specific amplification.
2.2.5. Western blot
For western blot analysis, EVs were isolated from canine plasma as described in Section 2.2.2, after which SEC pellets and 30 μl of supernatants were resuspended in lysis buffer (end volume 100 μl) containing 0.625 M Tris/HCl (pH 6.8), 2.5% SDS and 10% glycerol. Then, 23 μl of the resulting samples was loaded onto a 12% polyacrylamide gel and CD9 was detected as described in Section 2.1.5.
2.2.6. Statistical analysis
Statistical analyses were performed in SPSS Statistics 24 or R Studio with ggplot2 and plyr. Data distribution was checked in order to decide which statistical test to use (parametric vs. nonparametric). The Mann–Whitney U test was used to compare plasma E2F target gene mRNA levels between tumour patients and controls. Additionally, the Mann–Whitney U test was used to compare EV RNA analysis with total plasma RNA analysis in both groups combined (tumour patients and healthy controls). The significance threshold was set at p < .05.
3. RESULTS
3.1. Canine melanoma cells produce EVs that contain E2F target gene transcripts
To identify candidate biomarkers, we analysed previously published RNA‐sequencing data from tumour cell lines 20 and tumour tissue samples 21 for E2F target genes that are abundantly expressed in tumours. Solely genes that had been validated as classical E2F target genes using ChIP‐sequencing were considered. 17 We discovered a high number of reads for CDC6, DHFR, H2AFZ and ATAD2. Moreover, these E2F target genes have been previously reported to be associated with poor prognosis in different types of cancer. 22 , 23 , 24 , 25
Next, we verified whether these candidate biomarkers could be identified in cultured canine tumour cell‐derived EVs. To this end, canine melanoma cell line CMM12 was used. Melanoma‐derived large EVs and small EVs were isolated by differential centrifugation (DC), followed by sucrose density gradient centrifugation to separate large EVs and small EVs from proteins and non‐floating ribonucleoprotein complexes in the DC pellets. Western blotting of small EV protein marker CD9 and non‐EV protein marker calnexin was performed to confirm purity of the isolated EVs. Non‐conditioned cell culture medium, which was subjected to the same EV isolation steps as conditioned medium, was included as a negative control. Small EV fractions had detectable levels of CD9, while the cellular protein marker calnexin was not detected in both small EVs and large EVs, indicating we have successfully isolated EVs with minimal co‐isolation of cellular contaminants. As expected, CD9 was not detectable in large EVs, due to the less abundant expression of the marker in this EV subpopulation together with the low sensitivity of the technique applied (Figure 2A).
FIGURE 2.
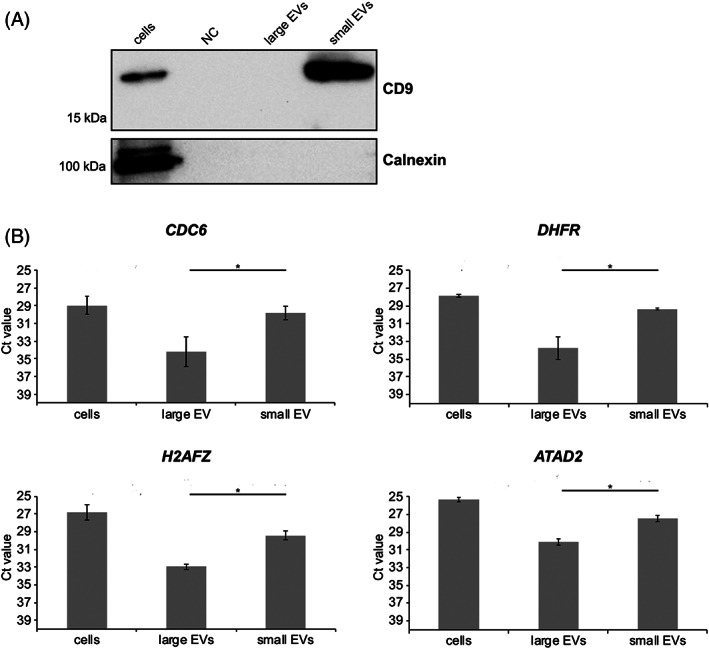
(A) Western blot analysis of extracellular vesicle (EV) marker CD9 and a non‐EV marker calnexin in isolated EVs and CMM12 donor cells. Non‐conditioned cell culture medium, which was subjected to the same EV isolation steps as conditioned medium, was included as a negative control (NC). (B) Reverse transcription and quantitative real‐time PCR (RT‐qPCR) analysis of E2F target gene transcripts in EV‐containing sucrose gradient fractions obtained after density gradient centrifugation of 10 000g (large EVs) and 100 000g (small EVs) DC pellets. Cellular RNA was quantified by a Nanodrop ND‐1000 spectrophotometer and EV pellet‐derived RNA was quantified using Agilent 2100 Bioanalyzer pico chips. All samples were normalised to the total input quantity of RNA (125 pg). Bars represent raw Ct values. Error bars represent the SD based on two biological replicates including two technical replicates each. Statistical analysis was performed to compare small and large EVs expression of the selected genes using the Kruskal–Wallis test. *p value <.05
Then, RNA was isolated from sucrose fractions containing large EVs and small EVs (densities 1.12–1.16 g/cm3; Figure S1), and expression levels of E2F target genes CDC6, DHFR, H2AFZ and ATAD2 were determined by RT‐qPCR. For further data analysis, solely Ct values equal to or below 35 were included. All four E2F target transcripts were detected (Ct ≤ 35) in the EV‐containing sucrose fractions (large EVs and small EVs; Figure 2B). The level of E2F target gene transcripts in the small EVs were similar to the levels of E2F target gene transcripts detected in the CMM12 donor cells. These results show that E2F target gene mRNAs are present in canine melanoma‐derived EVs.
3.2. Dogs enrolled in this study
Next, we verified whether CDC6, DHFR, H2AFZ and ATAD2 could be identified in the plasma of canine tumour patients, and if their levels differ from those of healthy controls. In total, 20 canine tumour patients were included in this study. The tumour patients were grouped according to their tumour type as having either malignant epithelial tumours (including neuroendocrine tumours, N = 6), malignant non‐epithelial tumours (N = 10), or benign epithelial tumours (N = 4, Table 2). Furthermore, these tumour types were grouped based on their histopathological diagnosis and anatomical sites. To evaluate the stage of the disease, tumours were classified as being primary tumours (N = 17), recurrent tumours (N = 2) or tumours where metastasis was present (N = 1). The ages of the tumour patients ranged from 5 to 14 years, and the sex was equally distributed among the group (10 females, 10 males).
Furthermore, we included a control group consisting of dogs where clinical absence of disease was confirmed. We grouped these dogs according to breed (18 Beagles, 2 Greyhounds, Table 3). The age range of these healthy dogs was large, as the youngest healthy dog was 1 year old and the eldest dog was 11 years old.
3.3. CDC6 mRNA levels are elevated in the plasma of canine tumour patients compared with healthy controls
We first investigated whether CDC6, DHFR, H2AFZ and ATAD2 could be detected in unfractionated plasma (total plasma RNA). Ct values were compared between the tumour patient group and the control group. mRNA transcripts with Ct values >35 were considered to be below the detection limit.
CDC6, ATAD2 and DHFR mRNA was detectable (Ct ≤ 35) in the plasma of respectively 11, 12 and 16 out of 20 tumour patients (Figure 3, Table S2). CDC6 was undetectable (Ct > 35 or n/a) in the plasma of most healthy controls (Figure 3, Table S3). ATAD2 and DHFR, in contrast, were detectable in the plasma of over half of the healthy controls. H2AFZ mRNA was detected in the plasma of all tumour patients and healthy controls.
FIGURE 3.
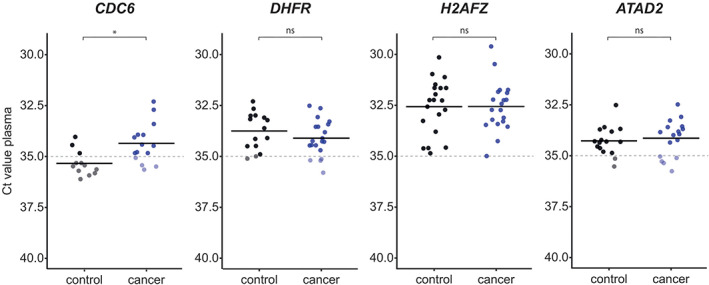
Plasma mRNA transcript levels were determined by reverse transcription and quantitative real‐time PCR (RT‐qPCR). Dots display the Ct values of the tumour patient/control samples. Each bar shows the average Ct value of the gene in the indicated group. Dashed lines represent the RT‐qPCR detection threshold, which was set at Ct = 35. Ct values <35 are highlighted. Statistical analysis was performed using the Mann–Whitney U test. *p value <.05; ns, no statistically significant difference
The average level of CDC6 mRNA in tumour patient samples was significantly higher compared to the healthy control group (p = .006) (Figure 4). The average plasma mRNA expression of DHFR, H2AFZ, and ATAD2 was comparable between the tumour patient and healthy control group.
FIGURE 4.
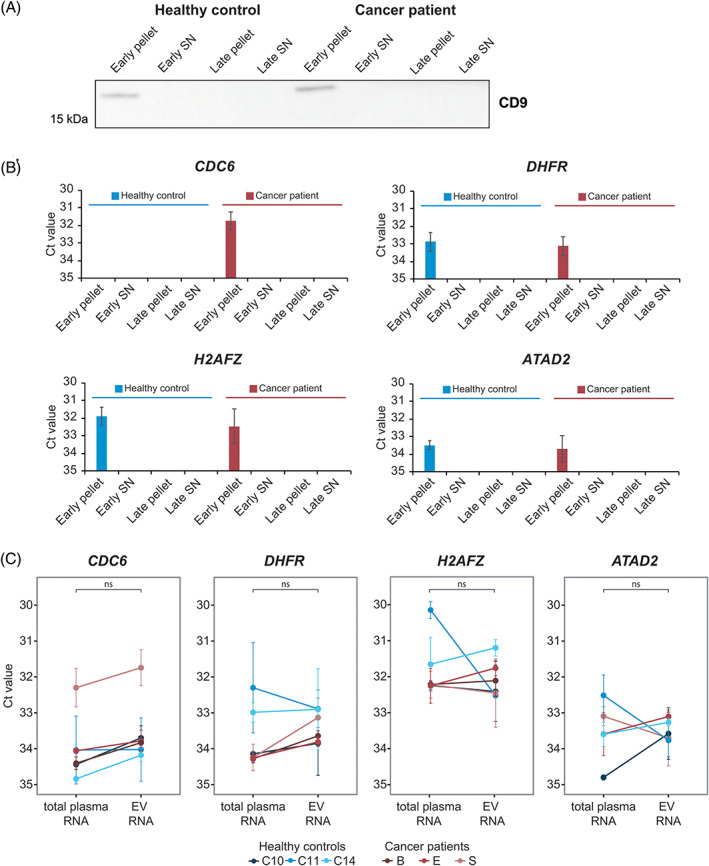
(A) Western blot analysis of the common extracellular vesicle (EV) protein CD9 in pellets and supernatants of spun‐down early (EV‐enriched) and late (protein/HDL enriched) size exclusion chromatography (SEC) fractions from a tumour patient (Patient E) and a healthy control (C11). Early pellet, 100 000g pellet of early SEC fractions (F6‐11; =EVs); Early SN, supernatant of early SEC fractions; Late pellet, 100 000g pellet of late SEC fractions (F12‐20; =protein/HDL); Late SN, supernatant of late SEC fractions. (B) Reverse transcription and quantitative real‐time PCR (RT‐qPCR) analysis of the E2F target genes in the pellets and supernatants of spun‐down early and late SEC fractions from a tumour patient (Patient S) and a healthy control (C1). Bars represent raw Ct values. Error bars represent the SD based on three technical replicates. Early pellet, 100 000g pellet of early SEC fractions (F6‐11; =EVs); Early SN, supernatant of early SEC fractions; Late pellet, 100 000g pellet of late SEC fractions (F12‐20; =protein/HDL); Late SN, supernatant of late SEC fractions. (C) RT‐qPCR analysis of CDC6, DHFR, H2AFZ and ATAD2 in EVs (early pellets) and total plasma RNA of tumour patients (n = 3) and healthy controls (n = 3). All samples were normalised to original plasma volumes. Dots display the raw Ct values in tumour patient and control samples. Lines connect total plasma RNA and EV RNA data from the same individual. Error bars represent the SE from the mean based on three technical replicates. The Mann–Whitney U test was used to statistically compare EV RNA analysis with total plasma RNA analysis in both groups combined (healthy controls + tumour patients). ns, no statistically significant difference
3.4. Circulating E2F target gene transcripts are associated to EVs
Finally, we selected a subset of tumour patients (Patients B, E and S) and healthy controls (C1, C10, C11 and C14) to validate association of the circulating E2F target genes to EVs. EVs were isolated from plasma samples of selected patients by SEC. This technique separates structures in plasma based on size: large structures such as EVs and large lipoprotein particles elute in the early fractions, while small lipoprotein particles and soluble proteins elute in the late fractions. Ultracentrifugation on the early and late fractions separates heavy structures (such as EVs) from light structures (such as lipoproteins). This procedure successfully separates EVs from other RNA carriers in plasma. 26
Early (F6‐11) and late (F12‐20) SEC fractions were ultracentrifuged, after which pellets and supernatants were collected for western blotting of the common small EV protein CD9 (n = 2).CD9 was detectable in the pellets of early SEC fractions, but not in the non‐concentrated supernatants of early SEC fractions and in pellets/supernatants of late SEC fractions (Figure 4A). For one tumour patient (Patient S) and one healthy control (C1) the supernatant was concentrated by ultrafiltration. RT‐qPCR was used to assess the presence of E2F target genes in pellets of early and late SEC fractions and concentrated supernatants (n = 2). The E2F target genes were solely detectable (Ct ≤35) in the pellets of early SEC fractions (Figure 4B). CDC6 was not detectable (Ct = n/a) in early SEC fractions of the healthy control, which was in line with our findings on the total plasma of this individual (Figure 3, Table S3). Together, these data clearly demonstrate that circulating E2F target genes are associated to the pellet of early SEC fractions, which are the fractions that are enriched in EVs.
To assess whether it is important to analyse E2F target genes in plasma‐derived EVs rather than in total plasma, we compared both approaches for CDC6, DHFR, H2AFZ and ATAD2. In a side‐by‐side comparison of the same sample set consisting of three tumour patients and three healthy controls, we showed that for CDC6 the Ct value decreased in the EV‐enriched fractions (pellets of early SEC fractions) compared to total plasma, although this difference did not reach statistical significance (Figure 4C). However, the fact that Ct values from the RT‐qPCR analysis of the tested E2F target genes are highly similar between total plasma RNA and EV RNA, supports our finding that CDC6, DHFR, H2AFZ and ATAD2 mRNA is predominantly contained within circulating EVs.
4. DISCUSSION
Tumour‐derived EVs represent a potent source of tumour biomarkers due to their protected, and therefore relatively stable cargo, and their presence in the circulation. This study provides novel insights into the utility of cell cycle‐related E2F target genes in circulating EVs as tumour biomarkers. We applied RT‐qPCR on total plasma and EVs of canine tumour patients and identified the presence of a small subset of cell cycle‐related mRNA transcripts (CDC6, DHFR, H2AFZ and ATAD2). Comparison with their expression levels in the plasma of healthy dogs unveiled CDC6 as a potential biomarker to diagnose canine tumours. Our study suggests that the analysis of some mRNAs in EVs might be superior to their analysis in total plasma.
Our in vitro data demonstrates that transcripts of E2F target genes CDC6, DHFR, H2AFZ and ATAD2 are present in multiple subclasses of EVs isolated from the conditioned medium of cultured canine melanoma cells. Density gradient centrifugation of 10 000g and 100 000g ultracentrifugation pellets confirmed that E2F target gene transcripts were associated to large EVs and small EVs and not to co‐pelleting contaminants of the ultracentrifugation pellets. The distribution of E2F target gene transcripts among small EVs and large EVs, however, differed. All four E2F target gene transcripts were more abundant in small EVs compared to large EVs. These findings contradict the study of Conley et al., 15 where RNA sequencing revealed similar levels of E2F target gene transcripts in small and large EV isolated from the conditioned medium of human breast cancer cells. Possibly the distribution of E2F target transcripts among different subclasses of EV varies per type of cancer, per cell line, or per species. These findings are surprising considering that the selected E2F transcriptional targets are relatively large mRNA molecules (500–5000 bp), 27 and they were therefore expected to be primarily present in larger‐sized EVs. However, our data do not exclude the possibility that merely fragments of the E2F target gene transcripts, instead of their full‐length forms, are present within tumour‐derived EVs. To validate the presence of full‐length E2F target gene mRNA transcripts, full‐length mRNA‐Seq needs to be performed. Furthermore, it would be interesting to study the role of these mRNA transcripts in tumour‐derived EVs. A growing body of evidence indicates that RNA molecules are specifically incorporated in EVs. 28 As mRNA can be translated into protein in the target cells, they can act as functional regulators of target cell behaviour. 29 Therefore, these EV‐enclosed mRNA transcripts may not only represent diagnostic biomarkers, but also functional biomarkers.
RT‐qPCR analysis of CDC6, DHFR, H2AFZ and ATAD2 in plasma samples revealed their presence in the circulation of canine tumour patients. We discovered CDC6 as a potential candidate tumour biomarker, as it was significantly increased in the plasma of canine tumour patients. For DHFR, H2AFZ and ATAD2 no statistically significant differences were found in the plasma mRNA levels between the tumour patient group versus the control group. There are different strategies for normalisation of RNA expression in plasma samples. 30 We have chosen to use the same amount of plasma volume for each patient, which is a well‐accepted way of normalising plasma EV data. 31 Furthermore we have measured the expression of multiple E2F targets, and since DHFR, H2AFZ and ATAD2 displayed no difference between tumour and healthy samples they served as additional controls. This approach lets us not distinguish whether the higher expression of CDC6 was related to more EVs with same amount of CDC6 or EVs with higher amount of CDC6. The substantial differences in circulating CDC6 expression levels between patients with different tumour types suggests that this biomarker could be of differential utility within different tumour types. Furthermore, the results of our study indicate that CDC6, DHFR, H2AFZ and ATAD2 mRNA transcripts are associated to circulating EVs and that the analysis of mRNA biomarkers in EVs might be more advantageous than the analysis of mRNA in unfractionated total plasma. Although no statistically significant difference was reached, our data show that for CDC6 the Ct value decreases when analysing EVs instead of total plasma. A similar finding was reported in a study by van Eijndhoven and colleagues, who studied miRNA biomarkers in the plasma of human lymphoma patients. 32 It could indeed be that purification of EVs increases the signal‐to‐noise ratio, resulting in enhanced biomarker detectability.
CDC6 is a protein that is essential in coordinating DNA replication during S phase of the cell cycle. 33 Given its important role in the process of cell division, it is not surprising that in several types of human cancers, such non‐small cell lung carcinomas and cervical cancer, increased tissue levels of CDC6 expression have been reported. 34 , 35 In canine mammary tumours, high CDC6 expression has been associated with metastasis. 36 , 37 Interestingly, the tumour patient with the highest CDC6 level in the plasma was a pituitary adenoma patient. Pituitary tumours in the dog generally do not display invasive growth and rarely metastasise, 38 however, these tumours sometimes regrow after surgery and Ki67 proliferation index represents an independent predictor of progression. 39 These high plasma E2F target gene levels could be related to the location of the tumour mass in proximity to the blood stream, the corticotroph nature of this tumour, and also to the typical histological features of this tumour type, as some pituitary adenomas contain endothelial‐lined blood sinusoids of considerable size (sinusoidal subclass). 40 , 41 These features are likely to influence how easily molecules secreted from the tumour cells reach the circulation. The hormone‐secreting function of the pituitary gland requires close connection to the circulatory system, which might make the blood stream relatively more accessible to molecular markers secreted by pituitary tumour cells. Due to the inaccessibility of these types of tumours for tissue biopsies, the use of liquid biopsies would represent a valid and precious alternative to obtain information about tumour features, behaviour and prognosis. The clinical management of brain tumours is challenging, which is why specifically for brain tumours there is an urgent need to identify biomarkers for diagnosis and post‐treatment monitoring. The reason for such high CDC6 mRNA levels in the plasma of this patient is unknown, but it would be worthwhile to further investigate CDC6 as a biomarker in patients with pituitary adenomas or other brain tumours.
One last matter that should be addressed is the fact that other factors exist that could influence the presence and quantity of E2F target genes in the circulation. Highly proliferative cells have high cellular levels of E2F target genes, so the proliferative status of the animal could influence the plasma levels of E2F target genes. An important physiological circumstance that influences the proliferative status of an animal is, for example, age, as the process of ageing is characterised by decreased cellular capacity to proliferate and regenerate. 42 We do not know if the age of an animal alters the baseline levels of E2F target genes in the plasma. It is possible that during the process of ageing, baseline levels of E2F target genes decrease. The average age of our control group was substantially lower than the average age of our tumour patient group, which could have resulted into a relatively high baseline level of the E2F target genes in the plasma of our control group. This could have muted the differences in plasma E2F target gene expression levels between our tumour patient and control groups. In our study, however, it seems unlikely that age has affected E2F target gene levels in the plasma, as plasma E2F target gene mRNA levels do not correlate with the age of the dogs included in our study (Figures S2 and S3). Additionally, other factors that determine the proliferative status of an animal and that are accompanied by high E2F expression, such as the presence of inflammation, other proliferative diseases (e.g., rheumatoid arthritis), or tissue regeneration after trauma might contribute to E2F target gene levels in the circulation. 43 , 44 , 45 Finally, it is important to note that the size of tumours and the number of tumour cells could influence the quantity of E2F target gene levels in the circulation. A small tumour with very high E2F target gene expression per tumour cell might release a similar amount of mRNA into the circulation as a larger tumour with low E2F target gene expression per tumour cell. The individual attributions of the described factors to the plasma E2F target gene mRNA levels remain to be further investigated.
In conclusion, in this study we have discovered CDC6 as a potential tumour biomarker candidate. Using RT‐qPCR, we found evidence that CDC6 is associated to circulating EVs. The measurement of E2F target gene mRNA could potentially provide clinicians with a tool to use in routine blood screenings. The results from our study warrant further investigations with additional canine tumour patients, selecting paired control dogs, perhaps focusing on specific tumour types in order to reduce interpatient variability.
CONFLICT OF INTEREST
The authors declare no conflict of interest to report.
Supporting information
Appendix S1: Supplementary Information
ACKNOWLEDGEMENTS
The authors would like to thank Takayuki Nakagawa, Nobuo Sasaki, Kohei Saeki and Raffaella De Maria for providing the canine melanoma cell line; Guy C. M. Grinwis and the Veterinary Pathology Diagnostic Centre for diagnosing the canine tumours; and Harry G. H. van Engelen and Inge I. M. van Duiven for collecting the blood samples from healthy control dogs.
Andriessen A, Bongiovanni L, Driedonks TAP, et al. CDC6: A novel canine tumour biomarker detected in circulating extracellular vesicles. Vet Comp Oncol. 2022;20(2):381‐392. doi: 10.1111/vco.12781
Funding information None.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Diamantis A, Magiorkinis E, Koutselini H. Fine‐needle aspiration (FNA) biopsy: historical aspects. Folia Histochem Cytobiol. 2009;47(2):191‐197. doi: 10.2478/v10042-009-0027-x [DOI] [PubMed] [Google Scholar]
- 2. Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer‐genetics in the blood. Nat Rev Clin Oncol. 2013;10(8):472‐484. [DOI] [PubMed] [Google Scholar]
- 3. Lu Y, Zhang H, Liang R, et al. Colorectal cancer genetic heterogeneity delineated by multi‐region sequencing. PLoS One. 2016;11(3):e0152673. doi: 10.1371/journal.pone.0152673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883‐892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pantel K, Alix‐Panabieres C. Real‐time liquid biopsy in cancer patients: fact or fiction? Cancer Res. 2013;73(21):6384‐6388. doi: 10.1158/0008-5472.CAN-13-2030 [DOI] [PubMed] [Google Scholar]
- 6. Schwarzenbach H, Hoon DS, Pantel K. Cell‐free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11(6):426‐437. [DOI] [PubMed] [Google Scholar]
- 7. Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122(1):6‐7. [DOI] [PubMed] [Google Scholar]
- 8. Bettegowda CC. Detection of circulating tumor DNA in early‐ and late‐stage human malignancies. Sci Transl Med. 2014;6(224):224ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Redzic J, Balaj L, van der Vos KE, Breakefield X. Extracellular RNA mediates and marks cancer progression. Semin Cancer Biol. 2014;28:14‐23. doi: 10.1016/j.semcancer.2014.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164(6):1226‐1232. doi: 10.1016/j.cell.2016.01.043 [DOI] [PubMed] [Google Scholar]
- 11. Van Niel G, d'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213‐228. [DOI] [PubMed] [Google Scholar]
- 12. Ciardiello C, Cavallini L, Spinelli C, et al. Focus on extracellular vesicles: new frontiers of cell‐to‐cell communication in cancer. Int J Mol Sci. 2016;17(2):175. doi: 10.3390/ijms17020175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maas SLN, Breakefield X, Weaver A. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27(3):172‐188. doi: 10.1016/j.tcb.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O'Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol. 2020;21(10):585‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Conley A, Minciacchi V, Lee D, et al. High‐throughput sequencing of two populations of extracellular vesicles provides an mRNA signature that can be detected in the circulation of breast cancer patients. RNA Biol. 2017;14(3):305‐316. doi: 10.1080/15476286.2016.1259061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen H, Tsai S, Leone G. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev Cancer. 2009;9(11):785‐797. doi: 10.1038/nrc2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Westendorp B, Mokry M, Groot Koerkamp MJ, Holstege FC, Cuppen E, de Bruin A. E2F7 represses a network of oscillating cell cycle genes to control S‐phase progression. Nucleic Acids Res. 2012;40(8):3511‐3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshitake R, Saeki K, Watanabe M, et al. Molecular investigation of the direct anti‐tumour effects of nonsteroidal anti‐inflammatory drugs in a panel of canine cancer cell lines. Vet J. 2017;221:38‐47. [DOI] [PubMed] [Google Scholar]
- 19. Bach F, Libregts S, Creemers L, et al. Notochordal‐cell derived extracellular vesicles exert regenerative effects on canine and human nucleus pulposus cells. Oncotarget. 2017;8(51):88845‐88856. doi: 10.18632/oncotarget.21483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yuan R, Liu Q, Segeren HA, et al. Cyclin F‐dependent degradation of E2F7 is critical for DNA repair and G2‐phase progression. EMBO J. 2019;38(20):e101430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kent LN, Rakijas JB, Pandit SK, et al. E2f8 mediates tumor suppression in postnatal liver development. J Clin Invest. 2016;126(8):2955‐2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mahadevappa R, Neves H, Yuen S, et al. The prognostic significance of Cdc6 and Cdt1 in breast cancer. Sci Rep. 2017;7(1):985. doi: 10.1038/s41598-017-00998-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Organista Nava J, Gómez Gómez Y, Illades Aguiar B, Rivera Ramírez A, Saavedra Herrera M, Leyva VM. Overexpression of dihydrofolate reductase is a factor of poor survival in acute lymphoblastic leukemia. Oncol Lett. 2018;15(6):8405‐8411. doi: 10.3892/ol.2018.8429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qi L, Zhou B, Chen J, et al. Significant prognostic values of differentially expressed‐aberrantly methylated hub genes in breast cancer. J Cancer. 2019;10(26):6618‐6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang MJ, Du WJ, Yang XZ, Chen ZP. ATAD2 is overexpressed in gastric cancer and serves as an independent poor prognostic biomarker. Clin Transl Oncol. 2016;18(8):776‐781. doi: 10.1007/s12094-015-1430-8 [DOI] [PubMed] [Google Scholar]
- 26. Driedonks TA, Mol S, de Bruin S, et al. Y‐RNA subtype ratios in plasma extracellular vesicles are cell type‐specific and are candidate biomarkers for inflammatory diseases. J Extracell Vesicles. 2020;9(1):1764213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nucleotide [Internet]. National Library of Medicine (US), National Center for Biotechnology Information. Accessed September 27, 2020. https://www.ncbi.nlm.nih.gov/nucleotide/.
- 28. O'Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol. 2020;21(10):585‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mateescu B, Kowal EJK, van Balkom BWM, et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA—an ISEV position paper. J Extracell Vesicles. 2017;6(1):1286095. doi: 10.1080/20013078.2017.1286095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huggett J, Dheda K, Bustin S, Zumla A. Real‐time RT‐PCR normalisation; strategies and considerations. Genes Immun. 2005;6(4):279‐284. [DOI] [PubMed] [Google Scholar]
- 31. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Eijndhoven Monique Aj MA. Plasma vesicle miRNAs for therapy response monitoring in Hodgkin lymphoma patients. JCI Insight. 2016;1(19):e89631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Borlado LR, Méndez J. CDC6: from DNA replication to cell cycle checkpoints and oncogenesis. Carcinogenesis. 2008;29(2):237‐243. [DOI] [PubMed] [Google Scholar]
- 34. Karakaidos P, Taraviras S, Vassiliou LV, et al. Overexpression of the replication licensing regulators hCdt1 and hCdc6 characterizes a subset of non‐small‐cell lung carcinomas: synergistic effect with mutant p53 on tumor growth and chromosomal instability—evidence of E2F‐1 transcriptional control over hCdt1. Am J Pathol. 2004;165(4):1351‐1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Murphy N, Ring M, Heffron CC, et al. p16INK4A, CDC6, and MCM5: predictive biomarkers in cervical preinvasive neoplasia and cervical cancer. J Clin Pathol. 2005;58(5):525‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klopfleisch R, Lenze D, Hummel M, Gruber AD. Metastatic canine mammary carcinomas can be identified by a gene expression profile that partly overlaps with human breast cancer profiles. BMC Cancer. 2010;10(1):618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bulkowska M, Rybicka A, Senses KM, et al. MicroRNA expression patterns in canine mammary cancer show significant differences between metastatic and non‐metastatic tumours. BMC Cancer. 2017;17(1):728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Rijn SJ, Grinwis GCM, Penning LC, Meij BP. Expression of Ki‐67, PCNA, and p27kip1 in canine pituitary corticotroph adenomas. Domest Anim Endocrinol. 2010;38(4):244‐252. doi: 10.1016/j.domaniend.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 39. Gejman R, Swearingen B, Hedley‐Whyte ET. Role of Ki‐67 proliferation index and p53 expression in predicting progression of pituitary adenomas. Hum Pathol. 2008;39(5):758‐766. [DOI] [PubMed] [Google Scholar]
- 40. Rosol TJ, Meuten DJ. Tumors of the endocrine glands. Tumors in Domestic Animals. Vol 5; Wiley; 2017:809‐815. [Google Scholar]
- 41. Sanders K, Galac S, Meij B. Pituitary tumour types in dogs and cats. Vet J. 2021;270:105623. [DOI] [PubMed] [Google Scholar]
- 42. Yun M. Changes in regenerative capacity through lifespan. Int J Mol Sci. 2015;16(10):25392‐25432. doi: 10.3390/ijms161025392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang S, Wang L, Wu C, Sun S, Pan J. E2F2 directly regulates the STAT1 and PI3K/AKT/NF‐κB pathways to exacerbate the inflammatory phenotype in rheumatoid arthritis synovial fibroblasts and mouse embryonic fibroblasts. Arthritis Res Ther. 2018;20(1):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang R, Wang L, Pan J, Han J. A critical role of E2F transcription factor 2 in proinflammatory cytokines‐dependent proliferation and invasiveness of fibroblast‐like synoviocytes in rheumatoid arthritis. Sci Rep. 2018;8(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. D'Souza SJA, Vespa A, Murkherjee S, Maher A, Pajak A, Dagnino L. E2F‐1 is essential for normal epidermal wound repair. J Biol Chem. 2002;277(12):10626‐10632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


