Abstract
We investigated the feasibility of bioremediation as a treatment option for a chronically diesel-oil-polluted soil in an alpine glacier area at an altitude of 2,875 m above sea level. To examine the efficiencies of natural attenuation and biostimulation, we used field-incubated lysimeters (mesocosms) with unfertilized and fertilized (N-P-K) soil. For three summer seasons (July 1997 to September 1999), we monitored changes in hydrocarbon concentrations in soil and soil leachate and the accompanying changes in soil microbial counts and activity. A significant reduction in the diesel oil level could be achieved. At the end of the third summer season (after 780 days), the initial level of contamination (2,612 ± 70 μg of hydrocarbons g [dry weight] of soil−1) was reduced by (50 ± 4)% and (70 ± 2)% in the unfertilized and fertilized soil, respectively. Nonetheless, the residual levels of contamination (1,296 ± 110 and 774 ± 52 μg of hydrocarbons g [dry weight] of soil−1 in the unfertilized and fertilized soil, respectively) were still high. Most of the hydrocarbon loss occurred during the first summer season ([42 ± 6]% loss) in the fertilized soil and during the second summer season ([41 ± 4]% loss) in the unfertilized soil. In the fertilized soil, all biological parameters (microbial numbers, soil respiration, catalase and lipase activities) were significantly enhanced and correlated significantly with each other, as well as with the residual hydrocarbon concentration, pointing to the importance of biodegradation. The effect of biostimulation of the indigenous soil microorganisms declined with time. The microbial activities in the unfertilized soil fluctuated around background levels during the whole study.
Bioremediation of hydrocarbon-contaminated soils, which exploits the ability of microorganisms to degrade and/or detoxify organic contamination, has been established as an efficient, economic, versatile, and environmentally sound treatment (27). On-site–off-site and in situ systems may be used. Decontamination of polluted sites in cold climates has received increasing interest recently. Considerable oil bioremediation potential has been reported for a variety of terrestrial and marine cold ecosystems, including arctic, alpine, and antarctic soils; Alaskan groundwater; and antarctic seawater and sea ice (reviewed in references 3 and 19). Field temperatures play a significant role in controlling the nature and extent of hydrocarbon metabolism. Temperature affects the rate of biodegradation, as well as the physical nature and chemical composition of hydrocarbons (4, 36).
Monitored natural attenuation (intrinsic bioremediation) is becoming the accepted option for low-risk oil-contaminated sites and is a cost-effective remediation alternative (13) as it has few costs other than monitoring costs and the time required for natural processes to proceed (27). Biodegradation is most often the primary mechanism for contaminant destruction; however, physical and chemical processes, such as dispersion, dilution, sorption, volatilization, and abiotic transformations, are also important (33). The most widely used bioremediation procedure is biostimulation of the indigenous microorganisms by addition of nutrients, as input of large quantities of carbon sources (i.e., contamination) tends to result in rapid depletion of the available pools of major inorganic nutrients, such as N and P (26). Several studies of the effects of biostimulation with mainly N-P-K or oleophilic fertilizers have reported positive effects on oil decontamination in cold ecosystems (reviewed in references 3 and 19).
The objective of our study was to determine the feasibility of bioremediation as a treatment option for a chronically diesel-oil-polluted soil in an alpine glacier area at an altitude of 2,875 m above sea level. Oil pollution in ski resorts is caused by the use of motor vehicles for preparation of ski runs and also by leaks and storage tank ruptures. To examine the efficiencies of natural attenuation and biostimulation, we used field-incubated lysimeters (mesocosms) with unfertilized and fertilized soil. For three summer seasons (July 1997 to September 1999), we monitored changes in hydrocarbon concentrations in soil and soil leachate and the accompanying changes in soil microbial counts and activity.
MATERIALS AND METHODS
Study site.
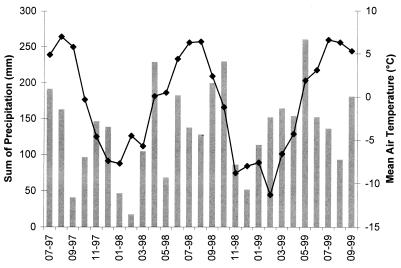
The field study was performed on the Eisgratferner Glacier in the Tyrolean Stubai Alps at an altitude of 2,875 m above sea level. The mean annual air temperatures in this area were 0.6, −1.3, and −1.8°C in 1997, 1998, and 1999, respectively; the annual levels of precipitation were 1,256, 1,472, and 1,780 mm, respectively. The annual soil thaw season is very short (between June or July and September). Summer temperatures vary greatly from near freezing to more than 20°C at the soil surface (Fig. 1 and Table 1).
FIG. 1.
Monthly precipitation (bars) and mean air temperatures (line) at the field study site.
TABLE 1.
Sampling dates and temperatures recorded in air and in lysimeters during the field study
| Date | No. of days | Soil dry wt (%) | Air temp (°C) | Soil temp (°C)a
|
Leachate temp (°C) | Leachate vol (liters) | |
|---|---|---|---|---|---|---|---|
| Surface | Bottom | ||||||
| 4 July 1997 | 0 | 91.2 | 5 | 8 | 10 | 3 | 0 |
| 26 July 1997 | 22 | 88.1 | 12 | 15 | 16 | 10 | 13 |
| 26 August 1997 | 53 | 88.8 | 15 | 21 | 23 | 14 | 6–8 |
| 22 September 1997 | 78 | 97.4 | 4 | 11 | 13 | 4 | 6–7 |
| 5 June 1998 | 336 | 91.2 | 10 | 22 | 20 | 9 | 20 |
| 4 July 1998 | 365 | 88.4 | 8 | 15 | 10 | 4 | 20 |
| 27 July 1998 | 388 | 91.0 | 2 | 8 | 10 | 1 | 5–6 |
| 12 August 1998 | 404 | 99.0 | 18 | 20 | 24 | 15 | 3 |
| 28 August 1998b | 420 | 89.1 | −1 | 0 | 1 | 0 | 10 |
| 24 September 1998b | 447 | 84.2 | 4 | 9 | 11 | 2 | 15 |
| 2 July 1999 | 697 | 92.1 | 7 | 5.5 | 17 | 6 | >20 |
| 23 September 1999 | 780 | 88.4 | 6 | 3 | 3 | 3 | >20 |
The soil temperature was measured at the surface and at the bottom (depth, 10 cm) of each lysimeter.
There were snow cover on the soil and ice cover on the leachates.
Soil.
The soil investigated was a mixture of carbonaceous gravel and sand; the geological underground (C horizon) was central alpine gneiss. The soil had a pH of 8.0 (measured in 10 mM CaCl2) and contained 0.014% total N (Kjeldahl), 0.34% organic C, 2.3% inorganic C, and 19.2% carbonate. The P2O5 and K2O contents (calcium lactate extract) and the Fe and Mn contents (EDTA extract) were 20, 430, 580, and 40 μg g of soil−1, respectively. The soil contained 2,612 ± 70 μg of hydrocarbons g (dry weight) of soil−1 at the beginning of the investigation (determined as described below); the contamination consisted of biodiesel oil.
Field lysimeters (mesocosms).
On 4 July 1997, about 150 kg of soil was removed from the contaminated zone in the motor pool area (i.e., in front of the garages and the petrol station for the motor vehicles used for preparation of ski runs), 50 m north of the Eisgrat station of the Stubaier Gletscherbahn. The soil was collected from an approximately 20-m2 area from the surface to a depth of about 0.1 m. After thorough mixing, about 22 kg of soil (density, 1.7 g cm−3) was placed into each of six lysimeters. Each lysimeter consisted of two polyethylene pans (length, 0.4 m; width, 0.32 m; fill height, 0.1 m), and one pan was mounted on top of the other; the bottom of the upper pan, which contained the soil, had drain holes so that the aqueous soil leachate could be collected in the lower pan.
In order to simulate natural attenuation (sum of abiotic elimination plus natural biodegradation), the soil in three lysimeters (replicates) was not treated. To determine the effect of biostimulation, the soil in the other three lysimeters (replicates) was mixed with a water-soluble N-P-K fertilizer (15-15-15; Agrolinz Melamin GmbH) containing 15% inorganic N (9.5% NH3-N, 5.5% NO3-N), 15% P2O5, and 15% K2O; the C/N ratio was adjusted to 20:1, and the C concentration was related to the measured initial contamination (the calculated concentrations were 71 μg of NH3-N g [dry weight] of soil−1, 41 μg of NO3-N g [dry weight] of soil−1, and 41 μg of P g [dry weight] of soil−1). One year later, after the first winter, nutrients were added again at a C/N ratio of 20:1, and the C concentration was related to the measured contamination (the calculated concentrations were 33 μg of NH3-N g [dry weight] of soil−1, 19 μg of NO3-N g [dry weight] of soil−1, and 19 μg of P g [dry weight] of soil−1).
The lysimeters were transported to an undisturbed area (where visitors were not allowed) about 50 m south of the soil sampling area and incubated until 23 September 1999 in the field under natural conditions (i.e., without any intervention, such as correction of the water content, etc.). Sampling was done immediately after the lysimeters were set up and at regular intervals during the summer seasons (Table 1) but was not possible between October and May, when the soil was frozen. The soil in the lysimeters was thoroughly mixed, and approximately 700-g samples, equally distributed, were taken from each pan. The volume of the soil leachates was recorded; 3 liters of leachate was collected from each lysimeter. Soil and leachate samples were transported in cooled boxes to the laboratory in order to perform the measurements described below. Each sample from each lysimeter was analyzed in triplicate.
Physical and chemical analyses.
Soil dry weight was determined from the weight loss after heat treatment (20 h at 80°C). Soil pH was determined with a glass electrode; 1 part of soil was mixed with 2.5 parts of 10 mM CaCl2. The available inorganic soil nutrient contents were determined as described in detail by Schinner et al. (30). Available nitrogen was extracted from soil by shaking the soil with 2 M KCl for 1 h. The ammonium-N in a filtrate was quantified colorimetrically by measuring the salicylic acid analogue of indophenol blue. The nitrate-N content was determined by measuring UV absorption at 210 nm; a correction for interfering substances was made by reducing nitrate with copper-sheathed, granulated zinc. The available phosphorus was extracted from soil by shaking the soil with 0.4 M LiCl and quantified colorimetrically in the filtrate by the molybdenum blue method.
Total petroleum hydrocarbons (TPH) were measured by the German standard method (10). Ten grams of soil was dehydrated with Na2SO4 and mixed for 30 min with 10 ml of 1,1,2-trichlorotrifluoroethane; the TPH content of the filtrate was quantified by infrared spectroscopy.
Soil biological analyses.
The following analyses were carried out as described in detail previously (30). Catalase activity was determined by measuring the amount of O2 that evolved from hydrogen peroxide in a phosphate-buffered (pH 6.8) soil suspension. To determine lipase activity, the butyric acid released from tributyrin at 25°C was extracted with ethyl acetate and quantified titrametrically by using 5 mM NaOH. Soil respiration (CO2 evolution) was determined by the Isermeyer technique; the CO2 produced during incubation for 48 h at 10°C was quantified by titration. Soil microbial counts were determined by the plate count method for viable cells (21) on agar plates that contained purified agar and no antifungal agents. R2A agar plates (29) were used to enumerate heterotrophic microorganisms. Hydrocarbon utilizers were quantified on oil agar plates (21) that contained a phosphate-buffered neutral-pH mineral medium and diesel oil as the sole carbon source. CFU were counted after incubation for 14 days at 10°C. No significant growth was observed on control plates without diesel oil.
Soil leachates (hydrocarbon quantification, inhibition of bioluminescence).
The TPH contents of soil leachates were determined as described previously (20). Hydrocarbons were extracted from 900 ml of leachate by 10 min of vigorous shaking with 20 ml of 1,1,2-trichlorotrifluoroethane and were quantified by infrared spectroscopy (10).
Inhibition of bioluminescence of Vibrio fischeri (Photobacterium phosphoreum), which is reduced in the presence of toxic compounds (the degree of inhibition depends on the toxicity), was determined by the German standard method (11). The soil leachate was amended with NaCl (2%). Light emission by V. fischeri (BioOrbit, 1243-500 BioTox kit) was measured luminometrically at 15°C immediately and 30 min after various dilutions of the leachates were added. NaCl (2%) was used as the control. Inhibition of bioluminescence was expressed as a GL value, the smallest dilution factor (G) for the test solution (leachate) which resulted in inhibition of light emission of ≤20%. The test solution was not toxic at a GL value of 1 or 2, while toxicity was indicated by GL values greater than 8 (15).
Statistical analyses.
Normal distribution of the data was tested by the Kholmogorov-Smirnov test. Whether a treatment had a significant effect on the measured parameters was analyzed by the t test for independent samples (P < 0.05) for data with a normal distribution, while nonparametric data were analyzed by the Mann-Whitney two-sample U test (P < 0.05). Correlations between the measured parameters were analyzed by the Pearson product-moment correlation technique (data with a normal distribution) or the Spearman rank order correlation technique (nonparametric data).
RESULTS AND DISCUSSION
Soil hydrocarbon decontamination.
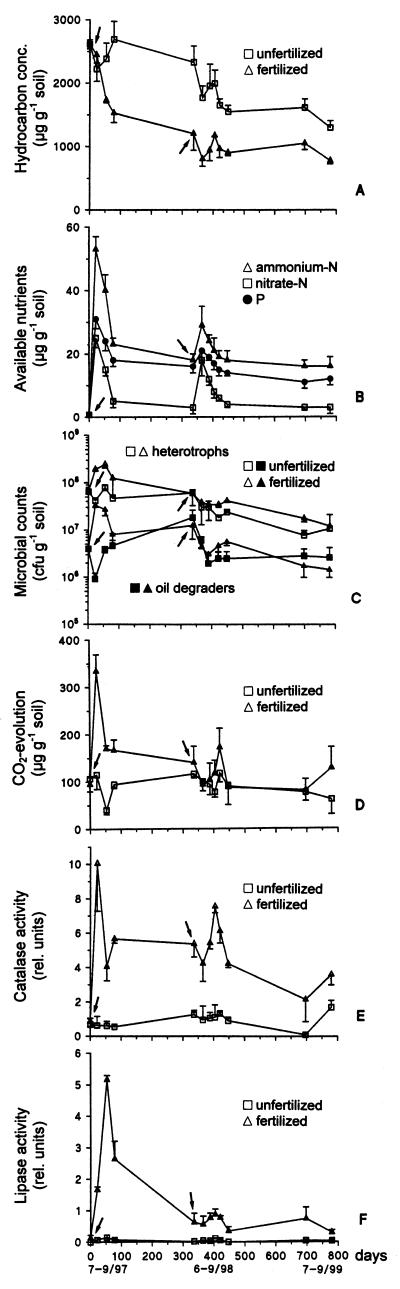
Figure 2A shows the effect of treatment on the time course of hydrocarbon disappearance. At the beginning of the study (2,612 ± 70 μg of hydrocarbons g [dry weight] of soil−1), chemical oxidation processes and metabolic activities, including biodegradation, were stimulated by manipulation (soil sampling, mixing), and thus the level of hydrocarbon loss was high with both treatments during the first 3 weeks. The main difference between treatments occurred during the next 8 weeks, when biostimulation resulted in significantly increased hydrocarbon loss. At the end of the first summer season (after 78 days), a significantly reduced hydrocarbon content, corresponding to a decontamination level of (42 ± 6)%, was measured for the fertilized soil (most of the hydrocarbon loss in this soil occurred during the first summer season), whereas no hydrocarbon loss was noticed in the unfertilized (i.e., naturally attenuated) soil due to an apparent significant increase in the hydrocarbon level accompanied by significant decreases in microbial counts and soil respiration (Fig. 2C and D) after an initial significant decrease. Such a release of hydrocarbons has been attributed to enhanced hydrocarbon mobilization by microbial biosurfactant production (20). We assume that the mobilized hydrocarbons were biodegraded due to the favorable nutrient conditions in the fertilized soil but not in the unfertilized soil.
FIG. 2.
Effect of treatment on hydrocarbon loss (A), available soil nutrients (B), heterotrophic and oil-degrading microorganisms (C), soil respiration (D), soil catalase activity (E), and lipase activity (F) during three summer seasons (means ± standard deviations [n − 1] for three replicate lysimeters). The arrows indicate when fertilization occurred.
Significant decontamination occurred at the beginning of the second summer season (June 1998), when the first manipulation after the long winter may have accelerated hydrocarbon loss. After the initial hydrocarbon loss, we observed apparent but insignificant hydrocarbon releases during the next 39 days with both treatments. At the end of the second summer season (after 447 days), the initial level of contamination had been reduced by (41 ± 4)% and (66 ± 2)% in the unfertilized and fertilized soils, respectively. At the end of the third summer season (after 780 days), biostimulation had decreased the hydrocarbon concentration to 774 ± 52 μg g (dry weight) of soil−1 ([70 ± 2]% reduction), while at the same time the hydrocarbon concentration was 1,296 ± 110 μg g (dry weight) of soil−1 ([50 ± 4]% reduction) with natural attenuation. Thus, the level of contamination was still high with both treatments, but the initial fertilization treatment was an appropriate treatment in terms of accelerated hydrocarbon loss. Results comparable to those obtained in our study were obtained in a field study of bioremediation of aged diesel fuel conducted during two successive summers in an arctic tundra soil (28).
The most direct way to measure bioremediation efficacy is to monitor hydrocarbon disappearance rates (4). In our study, the mean hydrocarbon content in all three summer seasons was significantly lower in the fertilized soil (Table 2). Biostimulation was most effective in the first summer (the rate of hydrocarbon disappearance was 13.9 μg of hydrocarbons g [dry weight] of soil−1 day−1); however, the positive effect did not last for the whole study. Despite addition of nutrients after the first winter, the biostimulation effect decreased. Both in the second summer and in the third summer natural attenuation resulted in higher rates of hydrocarbon disappearance (7.0 and 3.8 μg g−1 day−1, respectively) than of biostimulation (2.7 and 3.3 μg g−1 day−1, respectively). The natural attenuation process was slower, but nevertheless it was effective over a longer period of time.
TABLE 2.
Effect of treatment on soil hydrocarbon loss, soil pH, dry weight, and soil biological parameters (mean or median values) during three summer seasonsa
| Season | No. of samplesb | Treatment | Hydrocarbon concn (μg g of soil−1) | pHc | Dry wt (%) | No. of heterotrophs (CFU g [dry wt] of soil−1)c | No. of oil degraders (CFU g [dry wt] of soil−1)c | Respiration (μg of CO2 g [dry wt] of soil−1 48 h−1) | Catalase activity (relative units)c | Lipase activity (relative units)c |
|---|---|---|---|---|---|---|---|---|---|---|
| 26 July to 22 September 1997 | 9 | Natural attenuation | 2,432 | 8.10 | 91.53 | 5.56 × 107 | 3.09 × 106 | 83 | 0.52 | 0.60 |
| Biostimulation | 1,899d | 7.90d | 91.37e | 1.87 × 108d | 1.27 × 107d | 223d | 5.53d | 2.60d | ||
| 5 June to 24 September 1998 | 18 | Natural attenuation | 1,873 | 7.45 | 90.82 | 3.29 × 107 | 6.26 × 106 | 99 | 1.12 | 0.36 |
| Biostimulation | 995d | 7.30d | 90.18e | 4.19 × 107e | 6.03 × 106e | 119e | 5.30d | 0.72d | ||
| 2 July to 23 September 1999 | 6 | Natural attenuation | 1,453 | 7.45 | 90.30 | 9.50 × 106 | 2.75 × 106 | 69 | 0.86 | 0.04 |
| Biostimulation | 910d | 7.45e | 90.30e | 1.50 × 107e | 1.60 × 106e | 104e | 3.25d | 0.42d |
All values obtained for each summer season were analyzed with the t test for independent samples (data with normal distribution) or with the Mann-Whitney two-sample U test (nonparametric data) to determine whether biostimulation had a significant effect (95% confidence level).
Three replicate lysimeters were used for each treatment and sampling date.
Nonparametric data (median values). All other values are means.
The value is significantly different from the natural attenuation value for the same season.
The value is not significantly different from the natural attenuation value for the same season.
Several studies have reported favorable effects of fertilization on oil biodegradation at low temperatures in arctic soils (7, 25, 28, 37), alpine soils (17–19), and antarctic soils (1, 35). An understanding of nutrient effects at a specific site is essential for successful bioremediation (7, 8, 27). For old contaminations it is not clear that fertilization has a beneficial effect (13, 20). Hydrocarbon loss is known to decrease with time. At concentrations below possible threshold concentrations (which may depend on the soil structure and on the composition of the contaminant), biodegradation rates are low or negligible (2); this can be attributed to the formation of persistent polar compounds (24). Residual contamination is obviously greater in old contaminations than in fresh contaminations; in laboratory studies we obtained a level of decontamination of about 90% for experimentally diesel-oil-contaminated soils (17).
Soil water content and soil pH.
Low moisture content is an important limiting factor in biodegradation. The soil water content varied according to the weather and ranged from 1 to 16% at the time of soil sampling (Table 1). The soil buffering capacity was sufficient to maintain the soil pH in the neutral range, which is favorable for biodegradation (4, 27). The initially measured soil pH (pH 8.0 ± 0.06) decreased significantly in the fertilized soil (pH 7.7 ± 0.06) during the first 22 days of the study and then increased again (pH 7.9 ± 0.1). It was significantly lower (pH 7.5 ± 0.1) after the first winter season, and it decreased further to pH 7.3 ± 0.06 (unfertilized soil) or pH 7.2 ± 0.1 (fertilized soil) by the end of the second summer season. For both treatments, a pH of 7.4 ± 0.06 was obtained at the end of the study. A significantly lower pH in fertilized soil samples than in corresponding unfertilized soil samples has been described previously (20). In our study, the effect of biostimulation on soil pH decreased with time; the pH was significantly decreased by biostimulation in the summer in 1997 and 1998, while no effect was observed in the summer in 1999 (Table 2).
Available nutrients (ammonium-N, nitrate-N, P).
The soil investigated was nutrient deficient. The extractable available nutrient contents were around 1 μg g (dry weight) of soil−1 and did not change significantly during the field study (data not shown). The recommended C/N ratios for soil hydrocarbon bioremediation vary greatly and range at least from 100:1 to 10:1 (4, 26). It is important to add both N and P (1, 7, 25), although N has been shown to be the major limiting nutrient in arctic soils (7). In our study, during the whole study period fertilization resulted in significantly increased ammonium-N, nitrate-N, and P contents (Fig. 2B). Nutrient contents decreased considerably with time. At the end of the first summer period, the ammonium-N, nitrate-N, and P contents were 23 ± 2, 5 ± 2, and 18 ± 2 μg g (dry weight) of soil−1, respectively. Also, after the second fertilization, which was applied after the first winter period, the nutrient contents decreased with time. No significant changes were observed in the third summer. The disappearance of available nutrients over time can be attributed to metabolism, immobilization in biomass, immobilization on soil colloids, and washing out (the latter was confirmed by soil conductivity data obtained with soil leachates). The nitrate-N content decreased faster than the ammonium-N content because the level of immobilization of nitrate-N on soil matrix compounds is low (30). The decrease in the P content was caused by microbial metabolism and also by immobilization as apatite (calcium phosphate). The P content decreased much less than the N content, possibly because of P remobilization from apatite during microbial metabolism (14).
Soil leachates.
Aqueous soil leachates correspond to natural washing out (Table 1). The hydrocarbon content of soil leachates represents the water-mobilizable components of contamination and is an important parameter in regulations. During our entire study, the hydrocarbon content was close to or less than 0.1 mg liter of leachate−1, independent of the treatment. The pH remained in the neutral range (pH 7.0 to 7.5), and there was no inhibition of bioluminescence, which is frequently used for toxicity assessment (5). These results indicate that there was no mobilization of toxic hydrocarbon fractions.
Soil microbial counts.
At the beginning of our study, we counted (6.5 ± 0.4) × 107 culturable heterotrophic microorganisms g (dry weight) of soil−1. In the unfertilized soil, the counts remained almost unchanged in the summer of 1997 and decreased in the summer of 1998. Biostimulation resulted in a significant increase in the heterotroph counts during the first summer (the counts increased considerably in July and August and decreased in September) but did not have a significant effect in the second and third summer seasons, when the number of heterotrophs decreased. At the end of the study, the counts were comparable (1.2 × 107 CFU g [dry weight] of soil−1) for the two treatments (Fig. 2C and Table 2). A considerable portion of the heterotrophs ([4 ± 0.2] × 106 CFU g [dry weight] of soil−1) was able to utilize diesel oil as a sole carbon source. During the entire study, the time course for culturable oil degraders was almost comparable to that for heterotrophs; however, the fluctuations were greater, and the number of culturable oil degraders was significantly lower than the number of heterotrophs (Fig. 2C). Biostimulation resulted in significantly increased counts of oil degraders in the first summer, while no effect was detected in the second and third summer seasons (Table 2). At the end of the study, (2.7 ± 1.7) × 106 and (1.5 ± 0.5) × 106 CFU g (dry weight) of soil−1 were present in the unfertilized and fertilized soil, respectively.
It has been shown that fertilization increases the number of indigenous microorganisms in cold environments (6, 16, 28). We attribute the decreases in microbial counts with time, independent of treatment, to the decreases in the hydrocarbon concentrations. A downward trend for microbial abundance well in advance of the exhaustion of all hydrocarbons has also been noted in another study (32). Remarkably, no significant decreases in microbial counts were observed after the winter seasons. This could have been the result of microbial adaptation to a natural environment that is characterized by widely fluctuating temperatures and frost periods. During the winter, snow cover protects the soil against low air temperatures and freeze-thaw events by trapping residual heat (9). An alternative explanation is that population sizes decrease in winter but rapidly increase in spring. In cold ecosystems, the microbial community consists mainly of psychrotrophs (12) that grow over a wide temperature range (from 0 to >25°C) which parallels the ambient temperature range. Psychrotrophic microorganisms able to degrade a wide range of hydrocarbons, including aliphatic, aromatic, and polycyclic aromatic hydrocarbons, have been found in cold arctic soils (6, 7, 16, 25, 28, 37), alpine soils (17–20), and antarctic soils (1, 35). The presence of active diesel oil degraders in an alpine glacier environment points to the ubiquity of these organisms.
Soil microbial activities.
As the use of poisoned controls is precluded in the field, it was of interest to measure the activity of the soil microorganisms in response to treatment. Flat or depressed microbial activity signifies little or no microbial involvement, while strong positive responses indicate that the biodegradative contribution of the indigenous microorganisms is significant (34). Soil respiration and enzyme activities are measures of microbial activity in soil (30) and are indicative of the onset of hydrocarbon biodegradation (21, 34).
The levels of microbial activity in the unfertilized soil fluctuated around the background levels during the whole study. They were at or below the detection limit of the methods used; however, the presence of viable microorganisms was shown by microbial counts. Fertilization resulted immediately in marked but short-lived increases in soil respiration and enzyme activities (Fig. 2D to F). This pattern was also observed in the second and third summer seasons, although the activities were much lower. Soil respiration was significantly enhanced in the first summer season, while catalase and lipase activities were significantly stimulated in all three summer seasons (Table 2). This may have been a side effect of the very low enzyme activities in the unfertilized soil. Over time, there was a tendency for microbial activities to decline to background levels. This decline was associated with the loss of more labile contaminant components due to biodegradation (23).
The increases in both microbial counts and activity after the initial fertilization were not repeated to the same extent after refertilization in the second year, despite the fact that our nutrient data (Fig. 2B) do not indicate that conditions were nutrient limiting. Interestingly, we observed that the period when maximum microbial activity occurred in the second summer was not identical to the period when the nutrient level was highest, as was the case in the first year. It also did not correlate with the highest soil surface temperatures (Table 1). The possibility that nitrite toxicity occurred can be excluded since nitrite-N was not detected at any time (data not shown). We can also exclude the possibility that overfertilization occurred, since the nutrient concentrations added were well below concentrations that were found to inhibit microbial activity and hydrocarbon loss in cold soils (25; reference 7 and references therein). It has been suggested that bioremediation is not likely to be effective with extensively degraded oil (8). Aged hydrocarbon residues were not bioavailable to metabolically competent degrading microorganisms (31).
We have found that monitoring of soil lipase activity is a valuable indicator of diesel oil biodegradation in freshly contaminated, unfertilized and fertilized soils (22). In artificially contaminated laboratory microcosms, lipase activity remained stable even when the rates of hydrocarbon loss were considerably decreased (21, 22). However, in our field study, lipase activity was negligible in the unfertilized soil and decreased rapidly in the fertilized soil after an initial increase. This pattern was observed in all three summer seasons (Fig. 2F). Interfering factors, such as the composition of the contaminant components and the level of recalcitrant compounds, may influence lipase activity in soils in which the contamination is aged.
Correlations.
Correlations between parameters measured in the field study are presented in Table 3. In the fertilized soil, all biological parameters (microbial counts, soil respiration, enzyme activities) correlated significantly positively with each other, as well as with the residual hydrocarbon concentration, indicating the importance of biodegradation. The positive correlations of the available nutrient content with the hydrocarbon concentration, the microbial counts, and the activities in the fertilized soil indicate the relevance of nutrients. The low microbial activities in the unfertilized soil (Fig. 2D to F), as well as the lack of correlation with the residual hydrocarbon concentration, led to the conclusion that a considerable part of the hydrocarbon loss due to natural attenuation probably had to be attributed to abiotic processes.
TABLE 3.
Correlation matrix (coefficients and significance levels) for parameters investigated during the field study (July 1997 to September 1999)a
| Treatment | Parameter | Correlation coefficientb
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | Dry wt | NH4-N | NO3-N | P | Heterotrophs | Oil degraders | Respiration | Catalase activity | Lipase activity | ||
| Natural attenuation | Hydrocarbon concn | 0.8234∗∗∗ | 0.4242∗ | −0.8030∗∗∗ | 0.1276 NS | −0.5760∗∗ | 0.7920∗∗∗ | 0.2394 NS | 0.1049 NS | −0.1869 NS | 0.2434 NS |
| pH | 0.4742∗∗ | −0.6825∗∗∗ | −0.1242 NS | −0.4198∗ | 0.6232∗∗∗ | −0.0040 NS | −0.1240 NS | −0.3677∗ | 0.5152∗∗ | ||
| Dry wt | −0.2287 NS | −0.0473 NS | 0.0628 NS | 0.1381 NS | 0.1689 NS | −0.0359 NS | −0.1651 NS | 0.3539∗ | |||
| NH4-N | 0.0945 NS | 0.6672∗∗∗ | −0.7345∗∗∗ | −0.2332 NS | −0.2028 NS | 0.1797 NS | −0.0236 NS | ||||
| NO3-N | −0.1159 NS | 0.2895 NS | 0.4856∗∗ | 0.2709 NS | 0.2126 NS | −0.2808 NS | |||||
| P | −0.7735∗∗∗ | −0.1921 NS | −0.1333 NS | −0.0126 NS | 0.1417 NS | ||||||
| Heterotrophs | 0.3303 | 0.0972 NS | −0.1420 NS | 0.1346 NS | |||||||
| Oil degraders | 0.0418 NS | 0.0624 NS | −0.1715 NS | ||||||||
| Respiration | 0.1820 NS | −0.4393∗ | |||||||||
| Catalase activity | −0.2616 NS | ||||||||||
| Biostimulation | Hydrocarbon concn | 0.6860∗∗∗ | 0.0867 NS | 0.4709∗∗ | 0.3101 NS | 0.4685∗∗ | 0.7776∗∗∗ | 0.7663∗∗∗ | 0.8506∗∗∗ | 0.4421∗∗ | 0.8368∗∗∗ |
| pH | 0.1774 NS | 0.3860∗ | 0.1849 NS | 0.3991∗ | 0.5357∗∗ | 0.5372∗∗ | 0.4391∗ | 0.0283 NS | 0.6489∗∗∗ | ||
| Dry wt | −0.1870 NS | −0.2377 NS | −0.1347 NS | −0.1973 NS | −0.2771 NS | −0.0375 NS | 0.1896 NS | 0.1962 NS | |||
| NH4-N | −0.7785∗∗∗ | 0.8375∗∗∗ | 0.6469∗∗∗ | 0.6113∗∗∗ | 0.3464∗ | 0.3727∗ | 0.5510∗∗∗ | ||||
| NO3-N | 0.8497∗∗∗ | 0.4793∗∗ | 0.4405∗∗ | 0.3709∗ | 0.3936∗ | 0.4333∗ | |||||
| P | 0.6455∗∗∗ | 0.6416∗∗∗ | 0.3966∗ | 0.4729∗∗ | 0.5172∗∗ | ||||||
| Heterotrophs | 0.9087∗∗∗ | 0.5105∗∗ | 0.4473∗ | 0.7305∗∗∗ | |||||||
| Oil degraders | 0.5909∗∗∗ | 0.4754∗∗ | 0.5876∗∗∗ | ||||||||
| Respiration | 0.5582∗∗∗ | 0.5409∗ | |||||||||
| Catalase activity | 0.4020∗ | ||||||||||
A total of 33 samples were used in the analysis (three replicate lysimeters were used for each treatment and sampling date).
NS, not significant; ∗, P > 0.05; ∗∗, P > 0.01; ∗∗∗, P < 0.001.
In conclusion, we demonstrated that a significant reduction in the level of one of the most common pollutants, diesel oil, can be achieved even under unfavorable conditions, such as those present in an alpine glacier area at about 3,000 m above sea level, an environment in which to our knowledge bioremediation studies have never been performed before. Nonetheless, the rate of contaminant removal slowed drastically before the desired clean-up was complete.
ACKNOWLEDGMENTS
This work was supported by the Land Tyrol and by the Austrian Federal Ministries of Science and Environment.
We are especially grateful to H. Klier (Wintersport Tirol AG & Co. Stubaier Bergbahnen KG) for giving us permission to conduct the field experiment. We thank M. Neuner (Amt der Tiroler Landesregierung, Hydrogeographie) for providing the climate data and P. Thurnbichler for technical assistance.
REFERENCES
- 1.Aislabie J, McLeod M, Fraser R. Potential for biodegradation of hydrocarbons in soil from the Ross Dependency, Antarctica. Appl Microbiol Biotechnol. 1998;49:210–214. [Google Scholar]
- 2.Allard A S, Neilson A H. Bioremediation of organic waste sites: a critical review of microbiological aspects. Int Biodeterior Biodegrad. 1997;39:253–285. [Google Scholar]
- 3.Atlas R M. Microbial degradation of petroleum hydrocarbons: an environmental perspective. Microbiol Rev. 1981;45:180–209. doi: 10.1128/mr.45.1.180-209.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atlas R M, Bartha R. Hydrocarbon biodegradation and oil spill bioremediation. Adv Microb Ecol. 1992;12:287–338. [Google Scholar]
- 5.Bitton G, Koopman B. Bacterial and enzymatic bioassays for toxicity testing in the environment. Rev Environ Contam Toxicol. 1992;125:1–22. doi: 10.1007/978-1-4612-2890-5_1. [DOI] [PubMed] [Google Scholar]
- 6.Braddock J F, Lindstrom J E, Brown E J. Distribution of hydrocarbon-degrading microorganisms in sediments from Prince William Sound, Alaska, following the Exxon-Valdez oil spill. J Mar Pollut Bull. 1995;30:125–132. [Google Scholar]
- 7.Braddock J F, Ruth M L, Walworth J L, McCarthy K A. Enhancement and inhibition of microbial activity in hydrocarbon-contaminated arctic soils: implications for nutrient-amended bioremediation. Environ Sci Technol. 1997;31:2078–2084. [Google Scholar]
- 8.Bragg J R, Prince R C, Harner E J, Atlas R M. Effectiveness of bioremediation for the Exxon Valdez oil spill. Nature. 1994;368:413–418. [Google Scholar]
- 9.Coulson S J, Hodkinson I D, Strathdee A T, Block W, Webb N R, Bale J S, Worland M R. Thermal environments of Arctic soil organisms during winter. Arct Alp Res. 1995;27:364–370. [Google Scholar]
- 10.Deutsches Institut für Normung e.V. DIN 38 409-H18. Deutsche Einheitsverfahren zur Wasser-, Abwasser- and Schlammuntersuchung. Bestimmung von Kohlenwasserstoffen (H18). Berlin, Germany: Deutsches Institut für Normung e.V.; 1981. [Google Scholar]
- 11.Deutsches Institut für Normung e.V. DIN 38412-L34. Testverfahren mit Wasserorganismen (Gruppe L); Bestimmung der Hemmwirkung von Abwasser auf die Lichtemission von Photobacterium phosphoreum-Leuchtbakterien-Abwassertest mit konservierten Bakterien. Berlin, Germany: Deutsches Institut für Normung e.V.; 1991. [Google Scholar]
- 12.Gounot A M. Microbial life in permanently cold soils. In: Margesin R, Schinner F, editors. Cold-adapted organisms. Berlin, Germany: Springer; 1999. pp. 3–15. [Google Scholar]
- 13.Hinchee R E. In situ bioremediation: practices and challenges. In: Serra R, editor. Biotechnology for soil remediation. Milan, Italy: CIPA; 1998. pp. 17–20. [Google Scholar]
- 14.Illmer P, Schinner F. Solubilization of inorganic calcium phosphates—solubilization mechanisms. Soil Biol Biochem. 1995;27:257–263. [Google Scholar]
- 15.Kreysa G, Wiesner J, editors. Bioassays for soils. Frankfurt, Germany: Dechema e.V.; 1995. [Google Scholar]
- 16.Lindstrom J E, Prince R C, Clark J C, Grossmann M J, Yeager T R, Braddock J F, Brown E J. Microbial populations and hydrocarbon biodegradation potentials in fertilized shoreline sediments affected by the T/V Exxon Valdez oil spill. Appl Environ Microbiol. 1991;57:2514–2522. doi: 10.1128/aem.57.9.2514-2522.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margesin R, Schinner F. Bioremediation of diesel-oil contaminated alpine soils at low temperatures. Appl Microbiol Biotechnol. 1997;47:462–468. [Google Scholar]
- 18.Margesin R, Schinner F. Efficiency of indigenous and inoculated cold-adapted soil microorganisms for biodegradation of diesel oil in alpine soils. Appl Environ Microbiol. 1997;63:2660–2664. doi: 10.1128/aem.63.7.2660-2664.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Margesin R, Schinner F. Biological decontamination of oil spills in cold environments. J Chem Technol Biotechnol. 1999;74:1–9. [Google Scholar]
- 20.Margesin R, Schinner F. A feasibility study for the in situ remediation of a former tank farm. World J Microbiol Biotechnol. 1999;15:615–622. [Google Scholar]
- 21.Margesin R, Walder G, Schinner F. The impact of hydrocarbon remediation (diesel oil and polycyclic aromatic hydrocarbons) on enzyme activities and microbial properties of soil. Acta Biotechnol. 2000;20:313–333. [Google Scholar]
- 22.Margesin R, Zimmerbauer A, Schinner F. Soil lipase activity—a useful indicator of oil biodegradation. Biotechnol Tech. 1999;13:859–863. [Google Scholar]
- 23.McGill W B, Rowell M J, Westlake D W S. Biochemistry, ecology, and microbiology of petroleum components in soil. In: Paul E A, Ladd J N, editors. Soil biochemistry. Vol. 5. New York, N.Y: Marcel Dekker, Inc; 1981. pp. 229–296. [Google Scholar]
- 24.Miethe D, Riis V, Babel W. Zum Problem der Restkonzentration beim mikrobiellen Abbau von Mineralölen. Hamburger Ber. 1996;10:289–302. [Google Scholar]
- 25.Mohn W W, Stewart G R. Limiting factors for hydrocarbon biodegradation at low temperature in Arctic soils. Soil Biol Biochem. 2000;32:1161–1172. [Google Scholar]
- 26.Morgan P, Watkinson R J. Hydrocarbon degradation in soils and methods for soil biotreatment. Crit Rev Biotechnol. 1989;8:305–333. doi: 10.3109/07388558909148196. [DOI] [PubMed] [Google Scholar]
- 27.Norris R D. Handbook of bioremediation. Boca Raton, Fla: CRC Press; 1994. [Google Scholar]
- 28.Piotrowski M R, Aaserude R G. Bioremediation of diesel contaminated soil and tundra in an Arctic environment. In: Kostecki P T, Calabrese E J, editors. Contaminated soils—diesel fuel contamination. Chelsea, Mich: Lewis Publishers Inc.; 1992. pp. 115–141. [Google Scholar]
- 29.Reasoner D J, Geldreich E E. A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol. 1985;49:1–7. doi: 10.1128/aem.49.1.1-7.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schinner F, Öhlinger R, Kandeler E, Margesin R, editors. Methods in soil biology. Berlin, Germany: Springer; 1996. [Google Scholar]
- 31.Smith M J, Lethbridge G, Burns R G. Fate of phenanthrene, pyrene and benzo(a)pyrene during biodegradation of crude oil added to two soils. FEMS Microbiol Lett. 1999;173:445–452. doi: 10.1111/j.1574-6968.1999.tb13537.x. [DOI] [PubMed] [Google Scholar]
- 32.Song H G, Bartha R. Effects of jet fuel on the microbial community of soil. Appl Environ Microbiol. 1990;56:646–651. doi: 10.1128/aem.56.3.646-651.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.U.S. Environmental Protection Agency. Use of monitored natural attenuation at Superfund RCRA corrective action, and underground storage tank sites. OSWER Directive 9200.4–17. U.S. Washington, D.C.: Environmental Protection Agency; 1999. [Google Scholar]
- 34.Wang X, Bartha R. Effects of bioremediation on residues, activity and toxicity in soil contaminated by fuel spills. Soil Biol Biochem. 1990;22:501–505. [Google Scholar]
- 35.Wardell L J. Potential for bioremediation of fuel-contaminated soil in Antarctica. J Soil Contam. 1995;4:111–121. [Google Scholar]
- 36.Whyte L G, Hawari J, Zhou E, Bourbonnière L, Inniss W E, Greer C W. Biodegradation of variable-chain-length alkanes at low temperatures by a psychrotrophic Rhodococcus sp. Appl Environ Microbiol. 1998;64:2578–2584. doi: 10.1128/aem.64.7.2578-2584.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whyte L G, Bourbonnière L, Bellerose C, Greer C W. Bioremediation assessment of hydrocarbon-contaminated soils from the High Arctic. Bioremed J. 1999;3:69–79. [Google Scholar]