Abstract
BACKGROUND
Excessive nitrogen (N) fertilization in glasshouse fields greatly increases N loss and fossil‐fuel energy consumption resulting in serious environmental risks. Microbial inoculants are strongly emerging as potential alternatives to agrochemicals and offer an eco‐friendly fertilization strategy to reduce our dependence on synthetic chemical fertilizers. Effects of a N‐fixing strain Pseudomonas protegens CHA0‐ΔretS‐nif on ginger plant growth, yield, and nutrient uptake, and on earthworm biomass and the microbial community were investigated in glasshouse fields in Shandong Province, northern China.
RESULTS
Application of CHA0‐ΔretS‐nif could promote ginger plant development, and significantly increased rhizome yields, by 12.93% and 7.09%, respectively, when compared to uninoculated plants and plants treated with the wild‐type bacterial strain. Inoculation of CHA0‐ΔretS‐nif had little impact on plant phosphorus (P) acquisition, whereas it was associated with enhanced N and potassium (K) acquisition by ginger plants. Moreover, inoculation of CHA0‐ΔretS‐nif had positive effects on the bacteria population size and the number of earthworms in the rhizosphere. Similar enhanced performances were also found in CHA0‐ΔretS‐nif‐inoculated ginger plants even when the N‐fertilizer application rate was reduced by 15%. A chemical N input of 573.8 kg ha−1 with a ginger rhizome yield of 1.31 × 105 kg ha−1 was feasible.
CONCLUSIONS
The combined application of CHA0‐ΔretS‐nif and a reduced level of N‐fertilizers can be employed in glasshouse ginger production for the purpose of achieving high yields while at the same time reducing the inorganic‐N pollution from traditional farming practices. © 2021 The Authors. Journal of The Science of Food and Agriculture published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: heavy nitrogen fertilization, biological nitrogen fixation, biofertilizer, ginger, glasshouse vegetable fields
INTRODUCTION
China is now home to 1.411 billion people according to data issued by the National Bureau of Statistics. 1 , 2 As the most populous nation in the world, excessive input of fossil fuel‐based fertilizers in soils, especially in glasshouse fields, remains the most convenient fertilization strategy to prevent future food scarcity. 3 However, excessive nitrogen (N) input from synthetic chemical fertilizers has exceeded crop demand, and only around 30–50% of the applied N‐fertilizer can be absorbed by crops. 4 , 5 The total land area for glasshouse vegetable cultivation covered 6.3 million ha in 2019 in China, and more than 1000 kg N ha−1 yr−1 from chemical fertilizers was applied to Chinese vegetable production; however, the N use efficiency was only about 19.7%. 6 , 7 Long‐term application of chemical N‐fertilizers with low use efficiency of N is neither cost‐effective nor eco‐friendly. 8
Inorganic‐N losses from agricultural fields pose an unprecedented threat to the soil environment, aquatic ecosystems, and the atmosphere globally. 9 , 10 , 11 , 12 More than half of the N is lost through nitrate leaching, surface runoff and gas emissions [e.g. ammonia (NH3) and nitrous oxide (N2O)] in major Chinese croplands contributing to climate change, water pollution, and soil acidification. 3 , 7 , 13 , 14 Substantial environmental damages, in turn, lead to adverse effects on humans, as well as potential ecological risks to sustainable food production. Therefore, there is an urgent need to reform traditional farming practices and exploit eco‐friendly strategies for reducing external fertilizer consumption.
Microbial inoculation offers an alternative approach to reducing our dependence on agrochemicals in agricultural practice. The application of plant growth‐promoting rhizobacteria (PGPR) has gained increasing attention for its potential in establishing sustainable agricultural systems and for reducing the need for agrochemicals. 15 , 16 , 17 Widespread application of PGPR has now become feasible, and some organisms have been used as biofertilizers, which are less harmful to the environment and less expensive than chemical fertilizers. 18
Several recent studies exploited genetically engineered Pseudomonas as inoculants to enhance plant productivity; for example, N‐fixing P. protegens pf‐5 mutants were employed for plant growth promotion in some pot experiments. 19 , 20 , 21 Pseudomonas protegens CHA0 is a well‐characterized biocontrol agent capable of colonizing the rhizosphere, promoting plant growth, and controlling plant pathogens. 22 , 23 Complete genomic data of CHA0 revealed that it has a genome of 6.87 Mbp but no N‐fixation genes, and furthermore, no nitrogenase activity was detected in this strain. 24 , 25 In a previous study, we successfully transferred a 49 kb nif gene island from P. stutzeri DSM 4166 to a retS deletion mutant of CHA0, resulting in a genetically engineered N‐fixing strain named P. protegens CHA0‐ΔretS‐nif. 26 The retS deletion mutant was used as the basis for this N‐fixing strain because the retS deletion can strongly enhance the expression of antimicrobial compounds in CHA0, thus providing additional useful properties. 26 CHA0‐ΔretS‐nif exhibited enhanced biocontrol activity and high levels of nitrogenase activity. 26
Ginger (Zingiber officinale Roscoe, Zingiberaceae) is one of the most widely used culinary spices worldwide and is a highly important commercial crop in China. 27 The annual ginger rhizome yield in China was estimated to be 540 million kilograms in 2017; however, soft rot disease causes ginger yield losses of up to 50 to 60%, and succession monocropping also resulted in significant yield reductions. 28 , 29 To improve economic benefits, excessive amounts of N‐fertilizers have been applied by farmers to agricultural fields, especially in glasshouse fields (over two times higher than those in open fields). 30 Hence, sustainable farming practices need to be further explored for the production of ginger and other crops.
The N‐fixing bacterium P. protegens CHA0‐ΔretS‐nif has strong potential for eco‐friendly and high‐yield glasshouse vegetable production. This study firstly aimed to assess the effects of CHA0‐ΔretS‐nif inoculation on the growth, yield, and nutrient levels of ginger, as well as on earthworm biomass and the microbial community. We also evaluated whether CHA0‐ΔretS‐nif inoculation was effective at supporting plant N requirements in glasshouse fields with reduced N‐fertilizer inputs. Understanding the benefits of applying N‐fixing rhizobacteria for glasshouse vegetable cultivation in China is still lacking, and our study should contribute to the further development of Pseudomonas inoculation as an improved method for glasshouse farming practices in China.
MATERIALS AND METHODS
Experimental area and plant species
Plastic glasshouse trials were conducted from April 2, 2018 to October 15, 2019 in Beimeng County, Changyi City, Shandong Province, China [36°64′ N, 119°54′ E, 40 m above sea level (a.s.l)] (Supporting Information Fig. S1). The experimental location is characterized as a seasonal temperate semi‐humid monsoon climate, and the mean annual temperature and precipitation are 12 °C and 650 mm, respectively. Precipitation mostly occurs from June to September. Chemical properties of the soil, primarily comprised of cinnamon soil, were measured prior to the initiation of the study, and are presented in Supporting Information Table S1. Ginger seeds utilized for experiments were Shandong mianjiang, a cultivar of ginger, reserved by the local farmers. Ginger has been grown continuously for many years in the experimental area.
Bacterial strains and experimental setup
Bacterial strains used in this study were the wild‐type P. protegens CHA0 strain and the genetically engineered N‐fixing strain CHA0‐ΔretS‐nif. 26 Inoculant products were mass‐produced in KB medium (pH 7.0; K2HPO4, 1.5 g L−1; MgSO4•7H2O, 1.5 g L−1; peptone, 20 g L−1; glycerin, 10 mL L−1) to obtain adequate quantities at 30 °C. 26 Groups were designed as follows: control, full dose of fertilizers without inoculation; WI‐N100%, full dose of fertilizers with CHA0 inoculation; MI‐N100%, full dose of fertilizers with CHA0‐ΔretS‐nif inoculation; MI‐N85%, CHA0‐ΔretS‐nif inoculation but with the N fertilizer input reduced by 15%; and MI‐N70%, CHA0‐ΔretS‐nif inoculation but with the N fertilizer input reduced by 30%. All groups were managed by the local farmers, and full doses of basal fertilizers were used based on the traditional tillage (Table S2), including 675 kg N ha−1, 375 kg P2O5 ha−1, and 1186.5 kg K2O ha−1. All groups included full fertilization with phosphorus (P) and potassium (K). Pre‐germinated ginger seeds were planted on April 2 at a depth of 25 cm with a spacing of 60 cm between plants. Liquid inoculants (5 × 1010 CFU mL−1 of viable bacteria) were applied on May 15, June 4, June 24 and July 24 with a volume of 60 L ha−1 at every time point. Each block had an area of 45 m2 (7.5 m × 6 m) with ten rows, and each group was set up in a completely randomized block with three replicates. Pesticides were applied based on conventional practices, and no pesticides were applied in the inoculant‐treated groups. Irrigation and herbicide applications were conducted as need.
Sampling and measurements
Samples aboveground or underground were collected by cutting plants at the soil surface, and roots were cleaned in sterile, distilled water to remove loosely adherent soil. Plant biomass (in g plant−1), plant height (in centimeters), stem diameter (in centimeters), and branch number were obtained on July 13, August 12, September 11, and October 15 from ten randomly collected plants per block. Total yields of ginger rhizome (in kg ha−1) were weighed after harvesting on October 15; and N, P, and K contents (g kg−1 dry weight) of the plants were calculated based on the Kjeldahl method, molybdenum blue method, and atomic absorption spectrophotometry method, respectively. 31 , 32 Total protein content (in g kg−1 dry weight) in the ginger rhizome was assayed based on the semi‐micro Kjeldahl method. 33 Soluble sugar content and starch content (in g kg−1 dry weight) in the ginger rhizome were assayed based on protocols GB 5009.9‐2016 and NY/T 1278‐2007, respectively, from the China National Standardization Management Committee. Polyphenol content and flavonoid content (in g kg−1 dry weight) in the rhizome were assayed by measuring the absorbance using a spectrophotometer at 735 nm and 415 nm, respectively, according to a previous report. 34
Earthworm surveys were conducted using cubes of soil (length, 1.0 m; width, 2.0 m; and height, 0.3 m) and excavated manually after the ginger rhizome harvest. All of the earthworms were counted, washed, blotted dry, and then their mass was determined. 35 Culturable bacteria, actinomycetes, and fungal strains, at depths of 0 to 20 cm and 20 to 40 cm, were determined by the dilution agar plating method on July 13. 36 , 37 , 38 Percent increases in ginger rhizome yield (%) associated with inoculation and different N‐fertilizer levels were assayed and compared to the uninoculated control. Prices in the current study are expressed in US dollars based on the exchange rate of 6.534 Chinese Yuan per dollar. The local market price of ginger rhizome was $0.62 kg−1 in 2019. The price of pure N was an average of $0.55 kg−1, in the last few years in China, according to the National Bureau of Statistics of China. Nitrogen production efficiency (in kg kg−1) was estimated as the ginger yield (in kg ha−1) divided by the N input (in kg ha−1) and then multiplied by 1000.
Data analysis
GraphPad Prism 6 software (GraphPad Software, Inc., San Diego, CA, USA) was used to generate the figures. Microsoft Excel software (Microsoft Corporation, Redmond, WA, USA) was used for data processing. Statistical analyses of the data were carried out using SPSS 25 software (SPSS Inc., Chicago, IL, USA). The differences between mean values (n = 3) were assessed by one‐way analysis of variance (ANOVA) according to Duncan's test at a P < 0.05 level of significance.
RESULTS
CHA0‐ΔretS‐nif inoculation enhanced ginger plant growth
Data collected on the harvest date (October 15) are shown in Fig. 1. Results showed that plants that were both inoculated with CHA0‐ΔretS‐nif and fertilized with full inorganic‐N (group MI‐N100%) achieved the highest fresh weight of whole plants compared to all other groups (Fig. 1(a)). The fresh weight of shoots of plants in group MI‐N100% was significantly increased by 21.79% and 17.98% compared with plants in the control and WI‐N100% groups, respectively, and root fresh weight increased by 11.33% and 8.93%. Moreover, the fresh weight of whole plants decreased with decreased levels of N‐fertilizer. Shoot and root fresh weights in group MI‐N85% reduced by 4.25% and 7.06%, respectively, when compared to group MI‐N100%. However, a higher fresh weight of whole plants was observed in group MI‐N85% compared to group WI‐N100%, with an increase of 12.97% in shoot weight and 1.24% in root weight. Among all groups, the lowest fresh weight of whole plants was observed in group MI‐N70%. With regard to plant height, stem diameter and branch number, group MI‐N100% was superior to all other groups, and group MI‐N70% had lower growth than groups received with abundant N (Fig. 1(b–d)). Variations in plant growth parameters in samples collected on July 13, August 12, September 11, and October 15 are shown in Fig. S2.
Figure 1.

Effects of different treatments on shoot and root weight (a), plant height (b), stem diameter (c) and branch number (d). Values are means with standard error bars (n = 3). Means sharing the same letter are not significantly different between treatments (P < 0.05).
CHA0‐ΔretS‐nif inoculation enhanced ginger yield
Total yields of fresh ginger rhizomes for each of the groups were ranked as follows: group MI‐N100% > WI‐N100% > MI‐N85% > control > MI‐N70%, as shown in Fig. 2. The yield for group MI‐N100% was significantly increased, by 12.93% and 7.09%, respectively, when compared to yields for the control and WI‐N100% groups. Group MI‐N85% also had increased yield, by 3.33%, in comparison to the control. The yield for group MI‐N85% was similar to that of group WI‐N100%, although the input of N‐fertilizer in the MI‐N85% group was reduced by 15%. However, the yield for group MI‐N70% was decreased by 6.48% when compared to the control. The total economic income for group MI‐N100% reached $88 870.8 ha−1, more than 10 000 dollars above the income from the control ($78 696.6 ha−1) (Table 1). Groups WI‐N100% and MI‐N85% gave similar increments of 5.45% and 3.33% of total income in comparison to the control. The lowest total economic benefit were obtained in group MI‐N70%, although this group had the highest N production efficiency due to a reduction of the chemical N supply by 30%, followed by the MI‐N85% and MI‐N100% groups.
Figure 2.
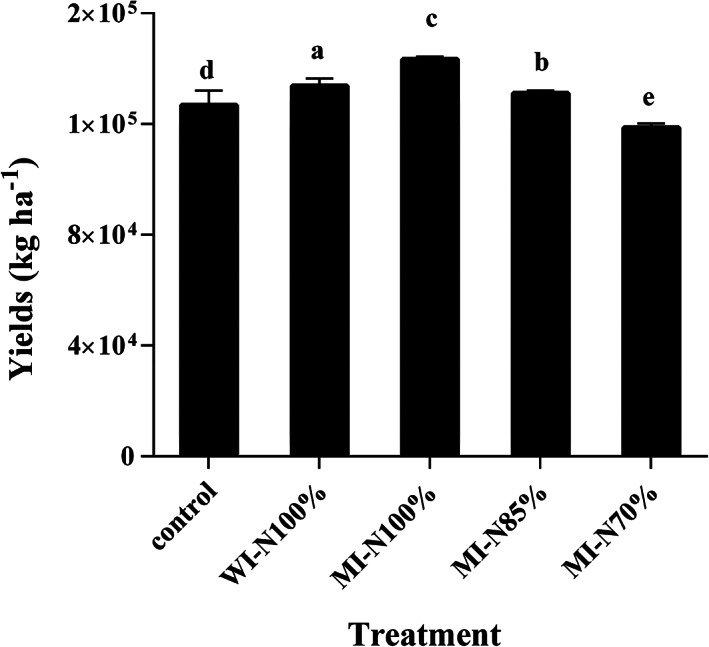
Effects of different treatments on ginger rhizome yields. Values are means with standard error bars (n = 3). Means sharing the same letter are not significantly different between treatments (P < 0.05).
Table 1.
Effects of different treatments on economic income
| Treatment | Nitrogen application (kg ha−1) | Plant nitrogen content (kg ha−1) | Yield (×105 kg ha−1) | Increase in yield (%) | Nitrogen production efficiency (kg kg−1) | Economic income ($ ha−1) |
|---|---|---|---|---|---|---|
| Control | 675.0 | 137.51 ± 8.54 b | 1.27 ± 0.05 d | — | 188.04 | 78 696.6 d |
| WI‐N100% | 675.0 | 145.14 ± 3.91 b | 1.34 ± 0.02 a | 5.45 | 198.30 | 82 987.0 a |
| MI‐N100% | 675.0 | 177.46 ± 10.52 a | 1.43 ± 0.01 c | 12.93 | 212.36 | 88 870.8 c |
| MI‐N85% | 573.8 | 162.50 ± 12.22 a | 1.31 ± 0.01 b | 3.33 | 228.58 | 81 319.2 b |
| MI‐N70% | 472.5 | 141.02 ± 3.60 b | 1.19 ± 0.02 e | −6.48 | 251.22 | 73 594.0 e |
Values presented are mean ± standard error (n = 3). Means sharing the same letter are not significantly different between treatments (P < 0.05). Plant nitrogen (N) content refers to the whole plant N acquisition measured on October 15.
Contribution of CHA0‐ΔretS‐nif inoculation to ginger plant nutrition
Plants inoculated with CHA0‐ΔretS‐nif had a higher N acquisition when compared to the uninoculated control and the wild‐type CHA0‐treated plants (Table 2). Plant N accumulation in group MI‐N100% was significantly increased by 22.04%, 7.77%, 28.29%, and 22.27% for July 13, August 12, September 11, and October 15, respectively, when compared to group WI‐N100%. Group MI‐N85% also had a markedly higher level of N accumulation, with increases of 45.38%, 3.49%, 19.06% and 11.96% when compared to group WI‐N100% for July 13, August 12, September 11, and October 15, respectively. Groups WI‐N100%, MI‐N100% and MI‐N85% showed significant increases in plant N accumulation in the August 12, September 11 and October 15 samples, compared to those of the control. However, a higher level of N accumulation was not observed in group MI‐N70% compared to the other groups.
Table 2.
Effects of different treatments on plant acquisition of nitrogen (N), phosphorus (P) and potassium (K)
| Treatment | Control (kg ha−1) | WI‐N100% (kg ha−1) | MI‐N100% (kg ha−1) | MI‐N85% (kg ha−1) | MI‐N70% (kg ha−1) |
|---|---|---|---|---|---|
| Nitrogen | |||||
| July 13 | 22.08 ± 0.44bC | 17.74 ± 0.99cC | 21.65 ± 2.07bC | 25.79 ± 1.45aC | 13.21 ± 2.32dC |
| August 12 | 49.66 ± 2.27dB | 64.10 ± 3.26bB | 69.08 ± 1.74aB | 66.34 ± 0.33abB | 59.62 ± 1.54cB |
| September 11 | 130.43 ± 16.36bA | 132.02 ± 5.69bA | 169.37 ± 12.01aA | 157.18 ± 14.84aA | 114.64 ± 9.40bA |
| October 15 | 137.51 ± 8.54bA | 145.14 ± 3.91bD | 177.46 ± 10.52aA | 162.50 ± 12.22aA | 141.02 ± 3.60bD |
| Phosphorus | |||||
| July 13 | 4.20 ± 1.11aC | 15.22 ± 1.34bC | 5.42 ± 1.25aC | 4.25 ± 0.36aC | 8.98 ± 1.63cC |
| August 12 | 13.98 ± 0.51bB | 18.17 ± 2.75aAC | 18.92 ± 1.05aB | 17.42 ± 0.44aB | 16.89 ± 0.93aB |
| September 11 | 23.60 ± 1.77aA | 28.93 ± 0.41bA | 25.39 ± 2.33aA | 27.22 ± 1.08aA | 27.98 ± 1.34bA |
| October 15 | 34.20 ± 1.87aD | 26.87 ± 1.93bB | 35.36 ± 1.57aD | 35.00 ± 1.69aD | 26.16 ± 1.81bD |
| Potassium | |||||
| July 13 | 68.96 ± 6.52dC | 196.31 ± 13.06bcC | 236.40 ± 8.98abC | 275.16 ± 33.16aC | 157.88 ± 36.05cC |
| August 12 | 240.96 ± 11.53cB | 424.25 ± 22.64abB | 467.06 ± 32.64abB | 476.03 ± 35.61aB | 415.11 ± 42.93bB |
| September 11 | 505.54 ± 44.37dA | 608.91 ± 10.41cA | 759.04 ± 61.02aA | 723.20 ± 75.91abA | 637.98 ± 24.90bcA |
| October 15 | 837.37 ± 51.98dD | 900.74 ± 45.83cdD | 1031.52 ± 27.75abD | 1072.19 ± 31.59aD | 948.80 ± 71.80bcD |
All values presented are mean ± standard error (n = 3). Means sharing the same lowercase letter are not significantly different between treatments (P < 0.05). The capital letters indicate significant differences for time effects (P < 0.05).
No significant differences in plant P accumulation were detected among the groups over time (Table 2). Plant K accumulation in group MI‐N100% increased compared to levels in the control and group WI‐N100% (Table 2). Plant K accumulation in group MI‐N100% increased by 20.42%, 10.09%, 24.66% and 14.52% for July 13, August 12, September 11 and October 15, respectively, when compared to group WI‐N100%. In October, the highest level of K accumulation was observed in group MI‐N85%, with increases of 28.04%, 19.03%, and 3.94%, respectively, compared to the control group, WI‐N100%, and MI‐N100%. Interestingly, plants in group MI‐N70% had a higher K accumulation in the October samples compared with those of the control group and group WI‐N100%.
CHA0‐ΔretS‐nif‐inoculated plants in group MI‐N100% had significantly higher N content in both shoots and roots when compared to the control and group WI‐N100% (Fig. 3(a)). On October 15, the N content of plants in group MI‐N100% was increased by 8.62% and 116.17% in shoots and roots, respectively, compared to levels in group WI‐N100%. Similar increments in plant N content were observed in group MI‐N85%, especially in plant roots, with a significant increase of 93.65% compared to the level in group WI‐N100%. However, these beneficial increments were not observed in group MI‐N70%. Significant increments in P content were only observed in plant root (Fig. 3(b)). In the October 15 samples, groups MI‐N100% and MI‐N85% had increases of 31.75% and 38.10%, respectively, in plant root P content, when compared to group WI‐N100%. CHA0‐ΔretS‐nif inoculation had no apparent effect on shoot P content.
Figure 3.

Effects of different treatments on plant contents of N (a), P (b) and K (c), and total protein (d) in the ginger rhizome. Values are means with standard error bars (n = 3). Means sharing the same letter are not significantly different between treatments (P < 0.05). DW, dry weight.
In contrast to the results for N and P contents, group MI‐N70% gave the highest K content in shoots on October 15, with an increase of 49.49% and 12.23% compared to the control and WI‐N100% groups, respectively, followed by group MI‐N85%, which had increases of 42.53% and 7.00% over those two groups (Fig. 3(c)). Group MI‐N85% had the highest K content in root, with levels 7.91% and 10.51% higher than those in the control and group WI‐N100%, respectively, followed by group MI‐N100%, which had increases of 1.81% and 4.26% over those two groups.
The total protein contents in ginger rhizomes in groups MI‐N100% and MI‐N85% were significantly increased, by 52.38% and 48.55%, respectively, when compared to levels in group WI‐N100% (Fig. 3(d)). However, similar positive effects were not noted in group MI‐N70%. The impacts of inoculation with CHA0‐ΔretS‐nif on the contents of polyphenol, flavonoid, starch, and soluble sugars in rhizomes are shown in Fig. S3, with no significant differences observed among the different groups.
CHA0‐ΔretS‐nif inoculation altered soil microbial diversity in rhizosphere
As shown in Table 3, the total microbial numbers at a depth of 0 to 20 cm were higher in all the CHA0‐ΔretS‐nif‐treated groups when compared to the uninoculated control and the wild‐type‐strain‐treated group. The maximum bacterial population size observed in group MI‐N85% was significantly increased by 75.86% and 95.08% in comparison to those of the control and group WI‐N100%, respectively, and the ratio of bacteria to fungi was increased by more than four times when compared to levels with the other two groups. The total numbers of microbes and bacteria were also increased in group MI‐N70% when compared to levels in the control, WI‐N100%, and MI‐N100% groups. The proportions of actinomycetes and fungi with the CHA0‐ΔretS‐nif‐treated groups exhibited decreases. The total numbers of microbes and bacteria were decreased at a depth of 20 to 40 cm in the ginger rhizosphere in all groups, compared with those found at a depth of 0 to 20 cm. Plants inoculated with the wild‐type strain CHA0 gave the lowest bacterial population in soils at a depth of 0 to 20 cm when compared with all groups; however, the bacterial population size for group WI‐N100% at a depth of 20 to 40 cm was a little higher than that of the control. Group MI‐N100% had the maximum bacterial population size at a depth of 20 to 40 cm, followed by groups MI‐N85% and MI‐N70%, with increases of 180.95%, 63.49%, and 53.96%, respectively, when compared with group WI‐N100%.
Table 3.
Effects of different treatments on microbial populations in ginger rhizosphere
| Treatment | Microbial biomass (×106 CFU g−1) | Bacteria (×106 CFU g−1) | Actinomycetes (×105 CFU g−1) | Fungi (×104 CFU g−1) | Percentage (%) | Bacteria/Fungi | ||
|---|---|---|---|---|---|---|---|---|
| Bacteria | Actinomycetes | Fungi | ||||||
| 0–20 cm | ||||||||
| Control | 2.23 ± 0.05d | 2.03 ± 0.05d | 1.83 ± 0.13b | 1.60 ± 0.20a | 91.07 | 8.21 | 0.72 | 126.49 |
| WI‐N100% | 2.13 ± 0.05d | 1.83 ± 0.06d | 2.83 ± 0.22a | 1.60 ± 0.13a | 85.96 | 13.29 | 0.75 | 114.61 |
| MI‐N100% | 2.57 ± 0.21c | 2.43 ± 0.21c | 1.30 ± 0.07c | 0.53 ± 0.06b | 94.73 | 5.06 | 0.21 | 451.10 |
| MI‐N85% | 3.67 ± 0.28a | 3.57 ± 0.29a | 0.93 ± 0.07d | 0.63 ± 0.11c | 97.28 | 2.55 | 0.17 | 572.24 |
| MI‐N70% | 3.09 ± 0.26b | 3.03 ± 0.17b | 0.50 ± 0.05e | 1.10 ± 0.14c | 98.03 | 1.62 | 0.36 | 272.31 |
| 20–40 cm | ||||||||
| Control | 0.53 ± 0.01d | 0.43 ± 0.02d | 0.80 ± 0.16c | 1.47 ± 0.18b | 82.07 | 15.15 | 2.78 | 29.52 |
| WI‐N100% | 0.78 ± 0.07c | 0.63 ± 0.07c | 1.37 ± 0.07a | 1.13 ± 0.08c | 80.77 | 17.56 | 1.45 | 55.70 |
| MI‐N100% | 1.89 ± 0.20a | 1.77 ± 0.21a | 1.17 ± 0.08b | 0.53 ± 0.04d | 93.54 | 6.18 | 0.28 | 334.07 |
| MI‐N85% | 1.06 ± 0.13b | 1.03 ± 0.13b | 0.23 ± 0.06d | 0.60 ± 0.08d | 97.24 | 2.20 | 0.56 | 173.64 |
| MI‐N70% | 1.10 ± 0.02b | 0.97 ± 0.09b | 1.10 ± 0.03b | 2.03 ± 0.07a | 88.12 | 10.03 | 1.85 | 47.63 |
Values presented are mean ± standard error (n = 3). Means sharing the same lowercase letter are not significantly different between treatments (P < 0.05).
An increase in the population density of earthworms was found in plots inoculated with the wild‐type strain CHA0, when compared to the control, but the increment was markedly lower than that of the groups with CHA0‐ΔretS‐nif inoculation, in which the number of earthworms ranged from 15 to 28 per m3 (Fig. 4). The greatest earthworm biomass was noted in group MI‐N70%, which had N‐fertilizer input reduced by 30%.
Figure 4.

Effects of different treatments on earthworm number (a) and weight (b) per m3. Values are means with standard error bars (n = 3). Means sharing the same letter are not significantly different between treatments (P < 0.05).
DISCUSSION
It has been previously reported that Pseudomonas inoculation resulted in significant increases in plant growth parameters, including root length, stem diameter, leaf area, and consequently, the total yield of the plants. 39 , 40 Several recent studies exploited genetically modified Pseudomonas as inoculants to enhance plant biomass production for example, N‐fixing P. protegens pf‐5 mutants were applied for plant growth promotion in pot experiments. 19 , 20 , 21
Results in this study showed that N‐fixing CHA0‐ΔretS‐nif inoculation could significantly enhance the growth and production of ginger plants when compared to the uninoculated control and the wild‐type strain‐treated plants, which suggested that inoculating with CHA0‐ΔretS‐nif was more effective than inoculating with wild‐type CHA0 for plant growth promotion. Moreover, these enhancements were maintained under low N‐fertilizer input conditions (approximately 85% of full N supply), indicating that the capacity of the mutant strain to fix biological N can partially compensate for the need for N‐fertilizers. This finding is in agreement with earlier investigations showing that reasonable use of captured N2‐fixation ability can largely improve N uptake in maize, wheat, and tomato plants. 19 , 41
The novelty of our study is that proposed an improved N‐fertilizer management strategy that can be applied to enhancing glasshouse vegetable production in northern China. Most previous studies applied microbial inoculants to benefit major cereal crop yields and to reduce N fertilization. 15 , 34 , 40 , 42 However, limited information has been available on the impact of inoculation on the glasshouse vegetable yields. Fast‐growing vegetables produced in glasshouses demand larger amounts of fertilizers and pesticides compared to cereal production. 3 Increased risk of N loss in vegetable fields is one of the major barriers to environmental protection and synthetic chemical N inputs can be reduced in many agricultural systems. 43 , 44 Results in this study demonstrated that a smaller reduction of N‐fertilization levels (by approximately 15%) might be more suitable for reducing the use of chemical fertilizer without compromising ginger yield. When the amount of chemical N‐fertilizers was reduced by 30%, ginger plant growth promotion was considerably suppressed despite inoculation with CHA0‐ΔretS‐nif, in contrast to the findings of Ju et al. in which a 40% reduction in chemical N application rate had no significant effect on glasshouse vegetable production. 6
Nitrogen availability positively correlates with ginger plant development. In contrast to the significant increase in N content within CHA0‐ΔretS‐nif‐treated ginger plants in full N supply, little effect was found on plants treated with the wild‐type strain, indicating that the significant increase in N uptake associated with the mutant was primarily due to the exogenous N fixed by this strain. Similar beneficial effects from inoculation on plant N uptake and food production have also been found in other commercial crops. 14 , 15 Enormous amounts of atmospheric N becomes available to plants annually through the process of biological N fixation. Biologically fixed N has been confirmed as the most suitable nitrogenous source for resolving the conflict between over‐production and environmental pollution. 45 Nitrogen‐fixing CHA0‐ΔretS‐nif has high potential for use as an inoculant to increase plant N uptake even under low N‐fertilizer input conditions (Fig. 3(a)). The combined application of CHA0‐ΔretS‐nif and suitable levels of N‐fertilizer could be a desirable and promising strategy to reduce over‐fertilization in vegetable systems, even though glasshouse vegetable planting generally demands more chemical fertilizers than do agricultural practices in open fields. 46 However, a higher level of N acquisition was not observed in group MI‐N70% compared to the other groups, indicating that CHA0‐ΔretS‐nif inoculation cannot completely replace chemical fertilizer in terms of promoting N acquisition. Moreover, inoculation with CHA0‐ΔretS‐nif enhanced the uptake and efficiency of K‐fertilizers. The enhanced K levels in CHA0‐ΔretS‐nif‐treated plants compared to those of the uninoculated control can be attributed to multiple factors, such as the K solubilizing ability and the higher root uptake surface stimulated by inoculants. 47
Application of reasonable amounts of synthetic chemical N‐fertilizer would be an effective way to achieve both yield stability and environmental protection. 48 Heavy N input from chemical fertilizers has exceeded crop demand, and more than half of the N is lost, particularly through gas emission and nitrate leaching, in Chinese croplands resulting in serious environmental risks. 7 , 14 Poor farming practices with high input of synthetic chemical fertilizers, in particular N‐fertilizer, has led to high fossil‐fuel energy consumption and resulted in a significant increase in carbon emission. 49 China has made a commitment to a peak of carbon emission by 2030 under the Pair Agreement, 50 and hence, there is an urgent need to reform traditional management practices. The results of our work showed that CHA0‐ΔretS‐nif inoculation can reduce the input of chemical nitrogenous fertilizers, and consequently control the ever‐increasing adverse effects of synthetic chemical N loss on ecosystems. Considering both environmental effects and yield, group MI‐N85% was optimal for glasshouse ginger production with the recommended N application rate in glasshouse fields of less than 600 kg ha−1 (Table 1), significantly lower than the average annual N‐fertilizer inputs for some vegetable glasshouses of northern China (about 1358 kg ha−1 according to Ti et al. 7 ).
A healthy rhizosphere microbial diversity is necessary for maintaining sustainable agricultural practices. 51 Pseudomonas protegens and its genetically modified derivatives have the ability to colonize a wide variety of crop plants and exert substantial effects on the microbial community structure in the rhizosphere. 36 , 52 , 53 , 54 Our results indicated that microbial community composition in the ginger rhizosphere was immensely affected by the inoculants and that CHA0‐ΔretS‐nif inoculation strongly increased the density of the bacteria population over that of fungi and actinomycetes; these findings may be attributable to the capacity of CHA0‐ΔretS‐nif to colonize plant roots and outcompete resident soil microflora and to enhance the biocontrol activities of this strain against the total fungal spectrum in the rhizosphere. Compared to levels in the soil at a depth of 0 to 20 cm, the total amount of microbial biomass decreased as soil depth increased, suggesting that soil depth had some effects on bacterial population size, as found in a previous report. 46 Significantly, group MI‐N85% harbored the maximum amount of microbial biomass at a depth of 0 to 20 cm, indicating that some reduction of chemical fertilizers could enhance the survival of microorganisms, which is in agreement with a similar study showing that high N fertilization could negatively impact the diversity of native rhizobacteria. 55
Likewise, agronomic practices can also affect the population of earthworms, since earthworms are capable of distinguishing and avoiding polluted environments. 35 , 56 , 57 Therefore, the earthworm population has been used as a biological indicator to assess soil health. Observations in this study demonstrated that CHA0‐ΔretS‐nif inoculation combined with reduction of N‐fertilizer use is much more efficient than conventional cropping methods at increasing earthworm biomass. Microbial inoculants provide an additional food source for earthworms, and earthworm movements in turn contribute to improved soil nutrients and porosity, and therefore result in longer‐term persistence of the microbial products. 58 Overall, earthworms prefer soils with low contents of chemicals.
CONCLUSIONS
Pseudomonas protegens CHA0‐ΔretS‐nif inoculation positively supported ginger plant growth, yields, and nutrient uptake, significantly increased the population size of rhizosphere bacteria and earthworms, and partially reduced inorganic‐N application in glasshouse fields. Overall, the application of CHA0‐ΔretS‐nif can mitigate environmental risks caused by the massive use of N‐fertilizers without compromising yield. The effects of the genetically engineered N‐fixing bacterium on various non‐leguminous crops and in different soil and climate conditions will be a part of future investigations, as the performance and efficacy of bacterial inoculants may be affected by plant cultivars, indigenous microbes, soil types, climate, and other environmental factors.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Supporting information
Figure S1. The location of the experimental region in China.
Table S1. The basic physical and chemical properties of the original soil.
Table S2. Full dose of fertilizers applied at different development stage.
Figure S2. Effect of different groups on shoot (a) and root fresh weight (b), plant height (c), stem diameter (d) and branch number (e) on July 13, August 12, and September 11.
Figure S3. Effects of different groups on levels of polyphenol (a), flavonoid (b), soluble sugars (c), and starch (d) in the ginger rhizome. Means sharing the same letter are not significantly different between treatments (P < 0.05). DW, dry weight.
ACKNOWLEDGEMENTS
This research was supported by funding from the National Key R&D Program of China (Grant no. 2019YFA0904000, 2018YFD0201207), the Recruitment Program of Global Experts (1000 Plan), the Shandong Key Research and Development Program (2019QYTPY002, 2019JZZY010724), the Shandong Agricultural Academy Innovation Engineering Fund (CXGC2016A06) and the Program of Introducing Talents of Discipline to Universities (B16030). The authors would like to thank Nannan Dong from the State Key Laboratory of Microbial Technology of Shandong University for help and guidance in fermentation.
Contributor Information
Youming Zhang, Email: zhangyouming@sdu.edu.cn.
Qiang Tu, Email: tuqiang1986@163.com.
REFERENCES
- 1. Normile D, China's population still growing, census shows‐but barely. Science 372:669 (2021). [DOI] [PubMed] [Google Scholar]
- 2. National Bureau of Statistics of China , The seventh national census . (2021). http://www.stats.gov.cn/ztjc/zdtjgz/zgrkpc/dqcrkpc/ggl/202105/t20210519_1817695.html.
- 3. Kalkhajeh YK, Huang B, Hu W, Ma C and Hansen H, Environmental soil quality and vegetable safety under current greenhouse vegetable production management in China. Agric Ecosyst Environ 307:107230 (2021). [Google Scholar]
- 4. Froussart E, Bonneau J, Franche C and Bogusz D, Recent advances in actinorhizal symbiosis signaling. Plant Mol Biol 90:613–622 (2016). [DOI] [PubMed] [Google Scholar]
- 5. Stokstad E, The nitrogen fix. Science 353:1225–1227 (2016). [DOI] [PubMed] [Google Scholar]
- 6. Ju M, Zhang H and Shi W, Optimizing nitrogen input to reduce nitrate leaching loss in greenhouse vegetable production. Agric Water Manag 111:53–59 (2012). [Google Scholar]
- 7. Ti C, Luo Y and Yan X, Characteristics of nitrogen balance in open‐air and greenhouse vegetable cropping systems of China. Environ Sci Pollut Res Int 22:18508–18518 (2015). [DOI] [PubMed] [Google Scholar]
- 8. Wang J, Cheng Y, Cai Z and Zhang J, Effects of long‐term fertilization on key processes of soil nitrogen cycling in agricultural soil: a review. Acta Pedol Sin 53:292–304 (2016). [Google Scholar]
- 9. Liu H, Wang Z, Yu R, Li F and Hui M, Optimal nitrogen input for higher efficiency and lower environmental impacts of winter wheat production in China. Agric Ecosyst Environ 224:1–11 (2016). [Google Scholar]
- 10. Zhou M, Zhu B, Brüggemann N, Dannenmann M, Wang Y and Butterbach‐Bahl K, Sustaining crop productivity while reducing environmental nitrogen losses in the subtropical wheat‐maize cropping systems: a comprehensive case study of nitrogen cycling and balance. Agric Ecosyst Environ 231:1–14 (2016). [Google Scholar]
- 11. Guo J, Hu X, Gao L, Xie K, Ling N, Shen Q et al., The rice production practices of high yield and high nitrogen use efficiency in Jiangsu, China. Sci Rep 7:2101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenblueth M, Ormeno‐Orrillo E, Lopez‐Lopez A, Rogel MA, Reyes‐Hernandez BJ, Martinez‐Romero JC et al., Nitrogen fixation in cereals. Front Microbiol 9:1794 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xia X, Zhang S, Li S, Zhang L, Wang G, Zhang L et al., The cycle of nitrogen in river systems: sources, transformation, and flux. Environ Sci Process Impacts 20:863–891 (2018). [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y, Wang H, Lei Q, Luo J, Lindsey S, Zhang J et al., Optimizing the nitrogen application rate for maize and wheat based on yield and environment on the northern China plain. Sci Total Environ 618:1173–1183 (2018). [DOI] [PubMed] [Google Scholar]
- 15. Kuan KB, Othman R, Abdul Rahim K and Shamsuddin ZH, Plant growth‐promoting rhizobacteria inoculation to enhance vegetative growth, nitrogen fixation and nitrogen remobilisation of maize under greenhouse conditions. PLoS One 11:e0152478 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Santos MS, Nogueira MA and Hungria M, Microbial inoculants: reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 9:205 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trivedi P, Leach JE, Tringe SG, Sa T and Singh BK, Plant‐microbiome interactions: from community assembly to plant health. Nat Rev Microbiol 18:607–621 (2020). [DOI] [PubMed] [Google Scholar]
- 18. Olanrewaju OS, Glick BR and Babalola OO, Mechanisms of action of plant growth promoting bacteria. World J Microbiol Biotechnol 33:197 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fox AR, Soto G, Valverde C, Russo D, Lagares A Jr, Zorreguieta A et al., Major cereal crops benefit from biological nitrogen fixation when inoculated with the nitrogen‐fixing bacterium Pseudomonas protegens Pf‐5 X940. Environ Microbiol 18:3522–3534 (2016). [DOI] [PubMed] [Google Scholar]
- 20. Jing X, Cui Q, Li X, Yin J, Ravichandran V, Pan D et al., Engineering Pseudomonas protegens Pf‐5 to improve its antifungal activity and nitrogen fixation. J Microbial Biotechnol 13:118–133 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Setten L, Soto G, Mozzicafreddo M, Fox AR, Lisi C, Cuccioloni M et al., Engineering Pseudomonas protegens Pf‐5 for nitrogen fixation and its application to improve plant growth under nitrogen‐deficient conditions. PLoS One 8:e63666 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Flury P, Vesga P, Dominguez‐Ferreras A, Tinguely C, Ullrich CI, Kleespies RG et al., Persistence of root‐colonizing Pseudomonas protegens in herbivorous insects throughout different developmental stages and dispersal to new host plants. ISME J 13:860–872 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vesga P, Flury P, Vacheron J, Keel C, Croll D and Maurhofer M, Transcriptome plasticity underlying plant root colonization and insect invasion by Pseudomonas protegens . ISME J 14:2766–2782 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takeuchi K, Noda N and Someya N, Complete genome sequence of the biocontrol strain Pseudomonas protegens Cab57 discovered in Japan reveals strain‐specific diversity of this species. PLoS One 9:e93683 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smits THM, Rezzonico F, Frasson D, Vesga P, Vacheron J, Blom J et al., Updated genome sequence and annotation for the full genome of Pseudomonas protegens CHA0. Microbiol Resour Announ 8:e01002‐19(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu F, Jing X, Li X, Wang H, Chen H, Zhong L et al., Recombineering Pseudomonas protegens CHA0: an innovative approach that improves nitrogen fixation with impressive bactericidal potency. Microbiol Res 218:58–65 (2019). [DOI] [PubMed] [Google Scholar]
- 27. Wang J, Ke W, Bao R, Hu X and Chen F, Beneficial effects of ginger Zingiber officinale Roscoe on obesity and metabolic syndrome: a review. Ann NY Acad Sci 1398:83–98 (2017). [DOI] [PubMed] [Google Scholar]
- 28. Rai M, Ingle AP, Paralikar P, Anasane N, Gade R and Ingle P, Effective management of soft rot of ginger caused by Pythium spp. and Fusarium spp.: emerging role of nanotechnology. Appl Microbiol Biotechnol 102:6827–6839 (2018). [DOI] [PubMed] [Google Scholar]
- 29. Yim B, Baumann A, Grunewaldt‐Stocker G, Liu B, Beerhues L, Zuhlke S et al., Rhizosphere microbial communities associated to rose replant disease: links to plant growth and root metabolites. Hortic Res 7:144 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duan P, Fan C, Zhang Q and Xiong Z, Overdose fertilization induced ammonia‐oxidizing archaea producing nitrous oxide in intensive vegetable fields. Sci Total Environ 650:1787–1794 (2019). [DOI] [PubMed] [Google Scholar]
- 31. Yoshida S, Forno DA, Cock JH and Gomez KA, Laboratory Manual for Physiological Studies of Rice. International Rice Research Institute, Laguna: (1971). [Google Scholar]
- 32. Burrowes JD and Ramer NJ, Changes in potassium content of different potato varieties after cooking. J Ren Nutr 18:530–534 (2008). [DOI] [PubMed] [Google Scholar]
- 33. Zhang X, Wang L, Ma F, Yang J and Su M, Effects of arbuscular mycorrhizal fungi inoculation on carbon and nitrogen distribution and grain yield and nutritional quality in rice (Oryza sativa L.). J Sci Food Agric 97:2919–2925 (2017). [DOI] [PubMed] [Google Scholar]
- 34. Lu FC, Lee CY and Wang CL, The influence of arbuscular mycorrhizal fungi inoculation on yam (Dioscorea spp.) tuber weights and secondary metabolite content. PeerJ 3:e1266 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sizmur T, Martin E, Wagner K, Parmentier E, Watts C and Whitmore AP, Milled cereal straw accelerates earthworm (Lumbricus terrestris) growth more than selected organic amendments. Appl Soil Ecol 113:166–177 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shaukat SS and Siddiqui IA, Impact of biocontrol agents Pseudomonas fluorescens CHA0 and its genetically modified derivatives on the diversity of culturable fungi in the rhizosphere of mungbean. J Appl Microbiol 95:1039–1048 (2003). [DOI] [PubMed] [Google Scholar]
- 37. Jamali F, Sharifi‐Tehrani A, Lutz MP and Maurhofer M, Influence of host plant genotype, presence of a pathogen, and coinoculation with Pseudomonas fluorescens strains on the rhizosphere expression of hydrogen cyanide‐ and 2,4‐diacetylphloroglucinol biosynthetic genes in P. fluorescens biocontrol strain CHA0. Microb Ecol 57:267–275 (2009). [DOI] [PubMed] [Google Scholar]
- 38. Trabelsi I, Oves D, Manteca A, Genilloud O, Altalhi A and Nour M, Antimicrobial activities of some actinomycetes isolated from different rhizospheric soils in Tunisia. Curr Microbiol 73:220–227 (2016). [DOI] [PubMed] [Google Scholar]
- 39. Maroniche GA, Rubio EJ, Consiglio A and Perticari A, Plant‐associated Pseudomonas fluorescent from red lateritic soil: beneficial characteristics and their impact on lettuce growth. J Gen Appl Microbiol 62:248–257 (2016). [DOI] [PubMed] [Google Scholar]
- 40. Ke X, Feng S, Wang J, Lu W, Zhang W, Chen M et al., Effect of inoculation with nitrogen‐fixing bacterium Pseudomonas stutzeri A1501 on maize plant growth and the microbiome indigenous to the rhizosphere. Syst Appl Microbiol 42:248–260 (2019). [DOI] [PubMed] [Google Scholar]
- 41. He Y, Pantigoso HA, Wu Z and Vivanco JM, Co‐inoculation of Bacillus sp. and Pseudomonas putida at different development stages acts as a biostimulant to promote growth, yield and nutrient uptake of tomato. J Appl Microbiol 127:196–207 (2019). [DOI] [PubMed] [Google Scholar]
- 42. Al‐Enazy AR, Al‐Oud SS, Al‐Barakah FN and Usman AR, Role of microbial inoculation and industrial by‐product phosphogypsum in growth and nutrient uptake of maize (Zea mays L.) grown in calcareous soil. J Sci Food Agric 97:3665–3674 (2017). [DOI] [PubMed] [Google Scholar]
- 43. Gai X, Liu H, Zhai L, Tan G and Wang H, Vegetable yields and soil biochemical properties as influenced by fertilization in southern China. Appl Soil Ecol 107:170–181 (2016). [Google Scholar]
- 44. Ed L and Smith G, And Siciliano, a comprehensive review of constraints to improved management of fertilizers in China and mitigation of diffuse water pollution from agriculture. Agric Ecosyst Environ 209:15–25 (2015). [Google Scholar]
- 45. Soumare A, Diedhiou AG, Thuita M, Hafidi M, Ouhdouch Y, Gopalakrishnan S et al., Exploiting biological nitrogen fixation: a route towards a sustainable agriculture. Plants (Basel) 9:1011 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang K, Mao H, Wang Z and Tian Y, Succession of organics metabolic function of bacterial community in swine manure composting. J Hazard Mater 360:471–480 (2018). [DOI] [PubMed] [Google Scholar]
- 47. Colla G, Rouphael Y, Di Mattia E, El‐Nakhel C and Cardarelli M, Co‐inoculation of Glomus intraradices and Trichoderma atroviride acts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops. J Sci Food Agric 95:1706–1715 (2015). [DOI] [PubMed] [Google Scholar]
- 48. Wang H, Zhang Y, Chen A, Liu H, Zhai L, Lei B et al., An optimal regional nitrogen application threshold for wheat in the north China plain considering yield and environmental effects. Field Crop Res 207:52–61 (2017). [Google Scholar]
- 49. Wang Y, Ma Q, Li Y, Sun T, Jin H, Zhao C et al., Energy consumption, carbon emissions and global warming potential of wolfberry production in Jingtai oasis, Gansu Province, China. Environ Manag 64:772–782 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang H, Lu X, Deng Y, Sun Y, Nielsen C, Liu Y et al., Peak before 2030 implied from characteristics and growth of cities. Nat Sustain 2:748–754 (2019). [Google Scholar]
- 51. Berg G, Koberl M, Rybakova D, Muller H, Grosch R and Smalla K, Plant microbial diversity is suggested as the key to future biocontrol and health trends. FEMS Microbiol Ecol 93:1‐24 (2017). [DOI] [PubMed] [Google Scholar]
- 52. Girlanda M, Perotto S, Moenne‐Loccoz Y, Bergero R, Lazzari A, Defago G et al., Impact of biocontrol Pseudomonas fluorescens CHA0 and a genetically modified derivative on the diversity of culturable fungi in the cucumber rhizosphere. Appl Environ Microbiol 67:1851–1864 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fliessbach A, Winkler M, Lutz MP, Oberholzer HR and Mader P, Soil amendment with Pseudomonas fluorescens CHA0: lasting effects on soil biological properties in soils low in microbial biomass and activity. Microb Ecol 57:611–623 (2009). [DOI] [PubMed] [Google Scholar]
- 54. Almario J, Muller D, Defago G and Moenne‐Loccoz Y, Rhizosphere ecology and phytoprotection in soils naturally suppressive to Thielaviopsis black root rot of tobacco. Environ Microbiol 16:1949–1960 (2014). [DOI] [PubMed] [Google Scholar]
- 55. Lian T, Yu Z, Liu J, Li Y, Wang G, Liu X et al., Rhizobacterial community structure in response to nitrogen addition varied between two Mollisols differing in soil organic carbon. Sci Rep 8:12280 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bouldin JL, Klasky JW and Green VS, Earthworm preference bioassays to evaluate land management practices. Bull Environ Contam Toxicol 96:767–772 (2016). [DOI] [PubMed] [Google Scholar]
- 57. Zhang Q, Saleem M and Wang C, Effects of biochar on the earthworm (Eisenia foetida) in soil contaminated with and/or without pesticide mesotrione. Sci Total Environ 671:52–58 (2019). [DOI] [PubMed] [Google Scholar]
- 58. Gong X, Wei L, Yu X, Li S, Sun X and Wang X, Effects of rhamnolipid and microbial inoculants on the vermicomposting of green waste with Eisenia fetida . PLoS One 12:e0170820 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The location of the experimental region in China.
Table S1. The basic physical and chemical properties of the original soil.
Table S2. Full dose of fertilizers applied at different development stage.
Figure S2. Effect of different groups on shoot (a) and root fresh weight (b), plant height (c), stem diameter (d) and branch number (e) on July 13, August 12, and September 11.
Figure S3. Effects of different groups on levels of polyphenol (a), flavonoid (b), soluble sugars (c), and starch (d) in the ginger rhizome. Means sharing the same letter are not significantly different between treatments (P < 0.05). DW, dry weight.


