Abstract
Lenvatinib is an oral multikinase inhibitor approved for use as first‐line treatment for patients with advanced hepatocellular carcinoma (HCC). However, like other agents in this drug class, lenvatinib is associated with clinically important adverse events (AEs) that could adversely affect patient outcomes. Hypertension, diarrhea, decreased appetite/weight, hand–foot skin reaction, and proteinuria are among the most common AEs associated with lenvatinib therapy. This article provides strategies for the effective management of lenvatinib‐associated AEs based on the expert opinion of authors and currently available literature. Due to the high risk of AEs in patients receiving lenvatinib, prophylactic measures and regular monitoring for AEs are recommended. Lenvatinib dose interruption, adjustment, or discontinuation of treatment may be required for patients who develop AEs. For grade 1 or 2 AEs, dose interruption is generally not required. For persistent or intolerable grade 2 or 3 AEs, lenvatinib treatment should be interrupted until symptoms improve/resolve to grade 0–1 or baseline levels. Thereafter, treatment should be resumed at the same or a lower dose. Disease progression may occur in patients who do not initially respond to treatment or receive a suboptimal lenvatinib dose following dose reduction, resulting in lack of efficacy. Therefore, to derive maximum treatment benefit and ensure long‐term disease control, lenvatinib should be maintained at the highest possible dose when managing AEs. To conclude, lenvatinib‐associated AEs can be managed with prophylactic measures, regular monitoring and symptomatic management, which can ensure continued treatment and maximum survival benefit in patients with advanced HCC receiving first‐line lenvatinib therapy.
Keywords: adverse events, hepatocellular carcinoma, lenvatinib, management
Introduction
Hepatocellular carcinoma (HCC) accounts for 90% of primary liver cancers and is the second leading cause of cancer deaths worldwide. 1 Advanced HCC has a poor prognosis, with an estimated median survival time of 6–8 months. 1 Sorafenib, a multikinase inhibitor, is the standard‐of‐care for patients with advanced HCC. 1 , 2 , 3 Until recently, it was the only first‐line systemic treatment available for patients with advanced disease. In 2018, another multikinase inhibitor, lenvatinib, was approved for the first‐line treatment of patients with unresectable HCC. 4 , 5
Lenvatinib inhibits the kinase activities of vascular endothelial growth factor (VEGF) receptors VEGFR1, VEGFR2, and VEGFR3, as well as other proangiogenic and oncogenic pathway‐related kinases, including fibroblast growth factor receptors 1, 2, 3, and 4; platelet‐derived growth factor receptor alpha; KIT; and RET. 4 , 5 An early phase 1/2 study in patients with advanced HCC demonstrated the clinical activity and acceptable safety of lenvatinib. 6 , 7 Pharmacokinetic and exposure‐response analyses based on pooled data from this study and studies in healthy adults and patients with mixed tumor types determined the optimal starting dose for lenvatinib. In patients with HCC Child–Pugh class A, the recommended initial dose of lenvatinib is 12 mg once daily in patients with body weight ≥ 60 kg and 8 mg once daily in those with body weight < 60 kg. 4 , 5 , 8
The clinical efficacy of lenvatinib was confirmed in the Phase 3, open‐label, multicenter, noninferiority REFLECT study (NCT01761266), which compared the efficacy and safety of lenvatinib versus that of sorafenib as first‐line treatment for advanced unresectable HCC. 9 Patients who had not received systemic treatment for advanced unresectable HCC were randomized to receive oral lenvatinib (12 mg once daily for body weight ≥ 60 kg or 8 mg once daily for body weight < 60 kg; n = 478) or sorafenib 400 mg twice‐daily (n = 476) in 4‐week cycles. Median overall survival (OS; primary endpoint) in the lenvatinib group was noninferior to that in the sorafenib group (13.6 vs 12.3 months; hazard ratio [HR] 0.92; 95% confidence interval [CI] 0.79–1.06), as the upper limit of the two‐sided 95% CI for HR was less than the predefined margin of 1.08. The median duration of follow‐up at the time of primary analysis was 27.7 months in the lenvatinib group and 27.2 months in the sorafenib group. Secondary endpoints were also significantly (all P < 0.0001) improved with lenvatinib compared with sorafenib, including median progression‐free survival (7.4 vs 3.7 months), median time to progression (8.9 vs 3.7 months) and objective response rate (24.1% vs 9.2%). 9
Other than sorafenib, lenvatinib is the only first‐line multikinase inhibitor that has demonstrated an OS benefit in patients with HCC. 1 However, like other multikinase inhibitors, lenvatinib is associated with clinically important adverse events (AEs) that could adversely affect patient outcomes. 9 This article provides up‐to‐date strategies for the prompt and effective management of lenvatinib‐associated AEs in patients with advanced HCC based on the expert opinion of authors and currently available literature.
Methods and search strategy
Consensus generation
Specialists in the management of HCC, who have experience of treating HCC patients with lenvatinib in Korea, provided their opinions on the management of lenvatinib‐associated AEs. Consensus was derived after several rounds of discussion, including an online virtual meeting that was held in February 2021.
Literature review
PubMed was searched on January 5, 2021 using the search terms: lenvatinib, HCC, and management to identify articles supporting the use of lenvatinib for the treatment of HCC. Articles reporting the safety and tolerability of lenvatinib and strategies for the management of lenvatinib‐associated AEs were selected for the review.
Tolerability and safety profile of lenvatinib in hepatocellular carcinoma
In the pivotal REFLECT study, treatment‐emergent adverse events (TEAEs) occurred in 99% of patients in the lenvatinib group (18.9 episodes per patient‐year), with 94% of patients experiencing treatment‐related AEs. 9 TEAEs of grade≥ 3 severity were reported in 75% of lenvatinib recipients (3.2 episodes per patient‐year), most of which were considered treatment‐related (57% of patients). 9
Hypertension was the most common TEAE in the lenvatinib group (42%), with the majority of events of grade ≥ 3 severity (23%). 9 Other commonly (incidence > 30%) occurring TEAEs with lenvatinib were diarrhea (39%), decreased appetite (34%) and decreased weight (31%); the most commonly (incidence > 5%) reported grade ≥ 3 AEs with lenvatinib were increased blood bilirubin (7%) and proteinuria (6%). 9
Serious treatment‐emergent or treatment‐related AEs occurred in 43% and 18% of patients in the lenvatinib group. 9 The most common serious AE in the lenvatinib group was hepatic encephalopathy (4.4%). 10 Treatment‐related fatal AEs occurred in 11 (2%) patients receiving lenvatinib (including hepatic failure [n = 3], cerebral hemorrhage [n = 3], and respiratory failure [n = 2]). 9 Treatment‐related AEs led to dose interruption in 40% of lenvatinib recipients, dose reduction in 37% of patients, and treatment withdrawal in 9% of patients. 9
The tolerability and safety profile of lenvatinib in real‐world studies 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 was generally similar to that seen in the REFLECT trial.
Interestingly, a post‐hoc analysis of the REFLECT study found that in patients treated with lenvatinib, some AEs were associated with longer OS. 21 Median OS was significantly longer in patients with than in those without hypertension (18.0 vs 11.5 months; HR 0.64; 95% CI 0.52–0.80; P < 0.001), diarrhea (17.7 vs 11.6 months; HR 0.72; 95% CI 0.58–0.90; P = 0.003), proteinuria (18.6 vs 12.3 months; HR 0.76; 95% CI 0.60–0.98; P = 0.03), or hypothyroidism (19.8 vs 13.4 months; HR 0.72; 95% CI 0.54–0.96; P = 0.024). 21 A retrospective, real‐world study also found a significant association between longer OS and occurrence of hypertension (P = 0.01) or hand–foot syndrome (P = 0.04); on the other hand, appetite loss was significantly (P = 0.01) correlated with shorter OS. 13 This study also showed that the median survival time was significantly (P < 0.001) longer in patients who did not require discontinuation of lenvatinib treatment due to serious AEs compared with those who did require discontinuation of treatment. 13 In a recent real‐world study in Korea, anorexia was the only factor associated with poor OS (HR 2.15; 95% CI 1.01–4.58) after adjustment for factors related to tumor burden and hepatic reserve function. 19 Moreover, diarrhea and HFSR were shown to have the potential to predict longer PFS during lenvatinib therapy. Diarrhea was the only favorable prognostic factor for disease progression in multivariable analysis (HR 0.37; 95% CI 0.17–0.84, P = 0.02), while HFSR was only marginally associated (HR 0.51; 95% CI 0.24–1.08, P = 0.08). 19 These results suggest that AEs may predict benefit with lenvatinib and careful management of AEs to avoid related treatment discontinuations may improve survival. However, further studies are required to confirm the correlation between AEs and clinical outcomes and to assess the role of baseline patient characteristics.
Dosage and administration of lenvatinib
It is recommended that health care professionals experienced in the use of anticancer therapies initiate lenvatinib therapy. The recommended starting dose of lenvatinib for patients with HCC is 8 mg once daily for those with a body weight of < 60 kg and 12 mg once daily for patients with a body weight of ≥ 60 kg. Lenvatinib is to be taken orally regardless of food. Dose interruption, adjustment, or discontinuation may be required to manage AEs associated with lenvatinib treatment. For mild to moderate adverse events, dose interruptions are generally not required unless AEs are intolerable despite optimal management. Recommended lenvatinib dose modifications are summarized in Table 1 and Common Terminology Criteria for Adverse Events (Version 5.0) definitions of grades 1–5 AEs are presented in Table S1.
Table 1.
General recommendations for lenvatinib dose modifications in hepatocellular carcinoma patients 4 , 5
| Persistent and intolerable grade 2 or 3 toxicities † | Grade 4 | ||||
|---|---|---|---|---|---|
| Body weight | Starting dose | Adverse reaction | Dose modification | Adjusted dose ‡ | |
| ≥ 60 kg | 12 mg od | 1st occurrence § | Interrupt until resolved to Grade 0 or 1, or baseline †† | 8 mg od | Discontinue ¶ |
| 2nd occurrence | 4 mg od | ||||
| 3rd occurrence | 4 mg qod | ||||
| < 60 kg | 8 mg od | 1st occurrence § | 4 mg od | ||
| 2nd occurrence | 4 mg qod | ||||
| 3rd occurrence | Discontinue | ||||
od, once daily; qod, every other day.
Medical management for nausea, vomiting, or diarrhea should be initiated prior to dose interruption or reduction.
Lenvatinib dose is to be reduced in succession based on the previous dose level (12, 8, 4, or 4 mg every other day).
No dose adjustment required for first occurrence of hematological toxicity or proteinuria.
For hematologic toxicity, dosing can restart when resolved to grade 2 and, for proteinuria, dosing can resume when resolved to less than 2 g/24 h.
Excluding laboratory abnormalities judged to be non–life‐threatening, which should be managed as grade 3.
Management of lenvatinib‐associated adverse events
Based on available data from clinical studies, the majority of AEs in lenvatinib‐treated patients with HCC occur in the first 1 to 2 months after starting treatment (Fig. 1). 9 , 10 In addition to providing recommendations regarding the management of specific AEs in the following sections, Table 2 provides a summary of important monitoring tests that should be conducted before and during lenvatinib therapy.
Figure 1.
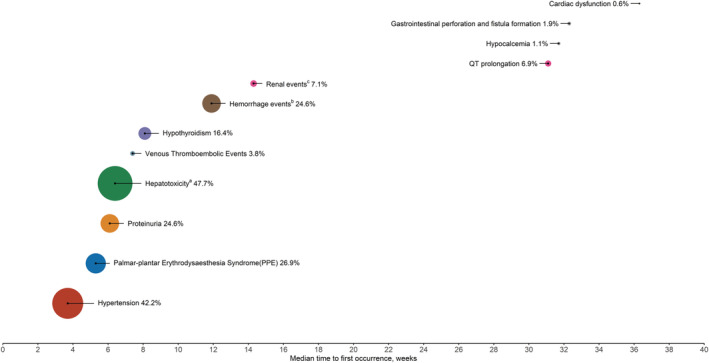
Incidence (%) and median time to onset of selected adverse events in patients with hepatocellular carcinoma treated with lenvatinib. Based on available data from clinical trials, 9 , 10 the size of the circle is proportional to the incidence. aHepatotoxicity includes blood bilirubin increase, ascites, AST/ALT increased, hypoalbuminemia, hepatic encephalopathy, gamma‐glutamyltransferase increase, hepatic failure, hepatic function abnormal, hyperbilirubinemia, hyperammonemia, jaundice cholestatic, hepatic pain, jaundice, urine bilirubin increased, hepatic cirrhosis, coma hepatic, edema due to hepatic disease, varices esophageal, and portal hypertensive gastropathy. bMost frequently reported hemorrhage events were epistaxis, hematuria, and gingival bleeding. Grade ≥ 3 events occurred in 24 subjects (5.0%) in the lenvatinib arm. cThe most frequently reported renal events were blood creatinine increased, acute kidney injury, and renal impairment. Grade ≥ 3 events occurred in 9 subjects (1.9%) in the lenvatinib arm.
Table 2.
Adverse event monitoring before and during lenvatinib treatment
| Baseline | Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Test | Weeks | |||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Regular follow‐up visits | |
| Liver function test (e.g., AST, ALT, ALP, bilirubin, and GGT) | ● | ● | ● | ● | ● | |||||
| Thyroid function test (TSH) | ● | ● | ● | ● | ||||||
| Hematology and clinical chemistry | ● | ● | ● | ● | ||||||
| Urinalysis | ● | ● | ● | ● | ● | |||||
| 12‐Lead ECG | ● | |||||||||
| Blood pressure † | ● | ● | ● | ● | ● | ● | ● | |||
| Vital signs and weight (e.g., fatigue and appetite) | ● | ● | ● | ● | ● | |||||
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ECG, electrocardiogram; GGT, gamma‐glutamyltransferase.
Blood pressure should be monitored after 1 week, then every 2 weeks for the first 2 months, and at least monthly thereafter during treatment.
Hypertension
Hypertension is the most frequently reported AE with lenvatinib therapy, usually occurring early in the course of treatment. In the REFLECT study, hypertension occurred in 42% of patients receiving lenvatinib, with the majority of events of grade ≥ 3 severity (23%). 9 The median time to first onset was 3.7 weeks. 10 Hypertension events led to dose interruption in 3.6% of lenvatinib recipients and dose reduction in 3.4% of patients; one (0.2%) patient discontinued lenvatinib due to hypertension. 10 Subgroup analyses suggested that the incidence of grade ≥ 3 hypertension events was higher in patients aged ≥ 75 years and in female patients. 10
Given the early onset and high rate of hypertension, and to avoid complications associated with excessive/prolonged elevation of blood pressure (BP), it is crucial that hypertension be managed aggressively. Prior to initiating lenvatinib therapy, it is recommended that a formal risk assessment for potential cardiovascular complications be conducted. 22 Patients known to be hypertensive should be on a stable dose of antihypertensive therapy for at least 1 week prior to lenvatinib treatment. 4 , 5 The Cardiovascular Toxicities Panel recommended a BP treatment goal of < 140/90 mmHg in most patients receiving VEGF inhibitor therapy, and a lower BP target in patients with specific pre‐existing cardiovascular risk factors (e.g. diabetes and/or chronic kidney disease). 22 The panel recommended that BP be actively monitored throughout treatment with VEGF inhibitors, with more frequent assessments during the first treatment cycle. 22 Based on this guidance, it is recommended that in patients receiving lenvatinib, BP should be monitored after 1 week of treatment, then every 2 weeks for the first 2 months, and monthly thereafter either at the clinic or at home. 4 , 5
For patients with systolic BP (SBP) ≥ 140 mmHg to < 160 mmHg or diastolic BP (DBP) ≥ 90 mmHg to < 100 mmHg (grade 2 hypertension), lenvatinib treatment should be continued (Fig. 2). Antihypertensive therapy should be initiated in these patients (if not already receiving it), or the dose of current antihypertensive therapy should be increased or an additional antihypertensive therapy added. 4 , 5 , 24 In patients presenting with SBP ≥ 160 mmHg or diastolic BP ≥ 100 mmHg (grade 3 hypertension) despite optimal antihypertensive therapy, treatment with lenvatinib should be withheld and subsequently resumed at a lower dose when SBP is ≤ 150 mmHg and DBP is ≤ 95 mmHg, and the patient has been on a stable dose of antihypertensive therapy for at least 48 h (refer to Table 1 for dosage adjustment). 4 , 5 , 24 In the event of life‐threatening consequences of hypertension (grade 4 hypertension; e.g. malignant hypertension, neurological deficit, or hypertensive crisis), urgent intervention is required; treatment with lenvatinib should be discontinued and appropriate medical management instituted. 4 , 5
Figure 2.
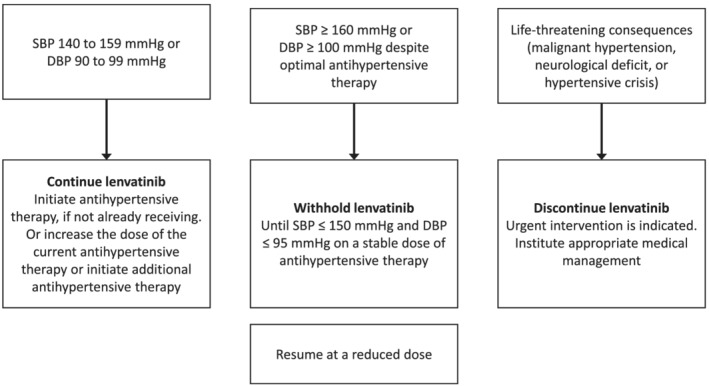
Management of hypertension.
There is no robust evidence for the choice of antihypertensive agents in patients who are receiving lenvatinib. 25 , 26 , 27 , 28 Angiotensin‐converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), calcium channel blockers (CCBs), and beta blockers can be used. 27 However, diuretics are not recommended as first‐line agents as they may worsen dehydration and electrolyte imbalance when diarrhea, a frequent adverse effect of lenvatinib, occurs. 26 An ACEI or ARB may be preferred if proteinuria is present 25 , 26 ; however, clear recommendations cannot be made, in view of earlier conflicting reports. 29 , 30 Non‐dihydropyridine CCBs such as verapamil and diltiazem are generally not recommended for patients receiving chemotherapy because they are cytochrome P450 (CYP) 3A4 inhibitors and may lead to drug–drug interactions. 25 , 28 However, they do not necessarily have to be avoided in patients receiving lenvatinib because CYP3A4 inducers or inhibitors have a minimal effect on lenvatinib exposure. 4 , 5 Nifedipine, a dihydropyridine CCB, should also be avoided, because of a possible effect on inducing VEGF secretion. 30
Expert recommendations
Hypertension must be managed aggressively.
BP should be monitored after 1 week of lenvatinib treatment, then every 2 weeks for the first 2 months, and monthly thereafter either at the clinic or at home.
Although there is no robust evidence for choice of antihypertensive, ACEIs, ARBs, CCBs, and beta blockers can be used depending on the patient's clinical circumstances and standard medical practice.
Lenvatinib dose interruption, reduction, or discontinuation may be necessary if treated hypertension remains uncontrolled.
Proteinuria
Proteinuria has been reported in patients receiving lenvatinib treatment, which occurs early in the course of treatment (Fig. 1). Proteinuria is a common AE associated with VEGF inhibitors, and the postulated pathogenesis involves VEGF inhibition‐derived changes in glomerular architecture and impairments in filtration function resulting from reduced nephrin production. 31 Hypertension is a risk factor for the development of proteinuria; moreover, proteinuria is associated with increased risk of mortality, myocardial infarction, and progression to kidney failure. 33 , 34
In the pivotal REFLECT study, proteinuria occurred in 25% of lenvatinib recipients, with AEs of grade 3 severity reported in 6% of patients in the lenvatinib group. 9 The median time to first onset was 6.1 weeks in patients receiving lenvatinib. 10 Serious AEs of proteinuria occurred in 0.6% of lenvatinib recipients. Proteinuria events led to dose interruption in 6.9% and dose reduction in 2.5% of lenvatinib recipients, and three patients (0.6%) discontinued lenvatinib treatment due to proteinuria. 10 The incidence of grade ≥ 3 AEs appeared to be higher in Asian patients compared with Caucasians and in patients aged ≥ 65 years compared with patients aged < 65 years. 10
Patients should be assessed for proteinuria prior to initiating treatment and monitored regularly. 23 Based on our clinical experience, patients are generally monitored every 2 weeks during the first month, then monthly thereafter, with regular follow‐up visits. Lenvatinib dose interruptions, adjustments (Table 1), or discontinuation of treatment, may be required if proteinuria of ≥ 2+ is detected (Fig. 3). 4 , 5 Proteinuria can be assessed by dipstick test, random urine collection, 24‐h urine collection, and/or urine protein‐to‐creatinine ratio (UPCR), depending on the judgment of clinicians. However, because 24‐h urine collection is inconvenient for patients, and can be misleading if urine collection is incomplete, 34 UPCR is the preferred method in our experience. In real‐world clinical practice, if urine dipstick proteinuria is ≥ 2+, random UPCR can be checked. A UPCR cut‐off value of 2.4 shows a high level of correlation with Grade 3 proteinuria measured by 24‐h urine collection (≥ 3.5 g/24 h). 35 Therefore, if UPCR is ≥ 2.4, a 24‐h urine collection can be considered to determine the grade of proteinuria. In patients with proteinuria of ≥ 2 g/24 h, 4 , 5 or if proteinuria of < 2 g/24 h is accompanied by edema, plural effusion, ascites, or increased serum creatinine, lenvatinib dose interruption is recommended. 23 Once proteinuria resolves to < 2 g/24 h, treatment can be resumed at the same dose (first occurrence) or after dose reduction (second occurrence; Table 1). 4 , 5 In the event of nephrotic syndrome (> 3.5 g/24 h), treatment with lenvatinib should be discontinued and not resumed. 4 , 5
Figure 3.
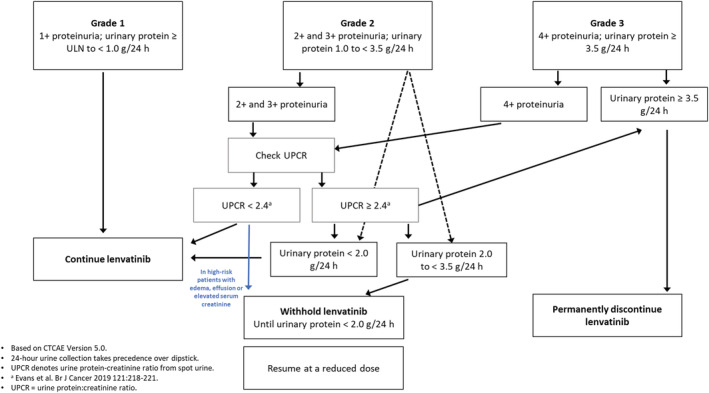
Management of proteinuria to enable continuation of lenvatinib treatment.
Expert recommendations
Based on our clinical experience, patients are generally monitored every 2 weeks during the first month, then monthly thereafter, with regular follow‐up visits. Lenvatinib dose interruptions, adjustments, or discontinuation of treatment may be required if proteinuria of ≥ 2+ is detected.
Diarrhea
Diarrhea occurs frequently and usually early during treatment with lenvatinib. In the REFLECT study, diarrhea occurred in 39% of lenvatinib recipients, with most events of mild or moderate severity (Grade ≥ 3 events occurred in 4% of patients). 9 Medical management for diarrhea should be instituted promptly to prevent dehydration, electrolyte imbalance, poor quality of life, and intolerance to cancer treatment and started prior to lenvatinib dose interruption or reduction (Table 1). 4 , 5 The treatment approach for diarrhea generally includes dietary adjustments such as avoiding caffeine, dairy, and greasy foods (which can worsen gastrointestinal distress); symptomatic control of diarrhea; and monitoring/managing electrolytes. For patients who are taking lactulose, the dose may be decreased. Patients receiving a diuretic for the treatment of hypertension should be monitored for the development of dehydration associated with diarrhea. 23 Supportive therapy with an antidiarrheal agent, such as loperamide, should be considered on an as‐needed basis. 36 mg (two tablets) initially, followed by 2 mg (one tablet) after every loose stool, not earlier than 2–3 h after the initial dose. The maximum daily dose should not exceed 12 mg (six tablets) in adults and 8 mg (four tablets) in adolescents. 37 Other useful treatments for diarrhea include dioctahedral smectite, 38 budesonide, and the combination of diphenoxylate and atropine. 36 Lenvatinib dose interruption and subsequent dose reduction is required for patients with persistent or intolerable grade 2 or 3 diarrhea, and lenvatinib treatment should be discontinued if grade 4 diarrhea persists despite medical management. 4 , 5 , 37
Expert recommendations
Medical management for diarrhea should be instituted promptly to prevent dehydration, electrolyte imbalance, poor quality of life, and intolerance to cancer treatment and started prior to lenvatinib dose interruption or reduction.
Palmar plantar erythrodysesthesia syndrome/hand–foot skin reaction
Palmar plantar erythrodysesthesia syndrome/hand–foot skin reaction (HFSR) is a cutaneous adverse event characterized by redness, marked discomfort, swelling, and tingling in the palms of the hands or the soles of the feet. 39 In the REFLECT study, HFSR occurred in 27% of lenvatinib recipients, with grade ≥ 3 AEs reported in 3% of patients. 9 The median time to first onset was 5.3 weeks in patients receiving lenvatinib. 10 No serious AEs of HFSR or discontinuations because of HFSR were reported in lenvatinib recipients. 10 The incidence of grade ≥ 3 HFSR appeared to be higher in Asian compared with White subjects and the incidence of HFSR appeared to be lower in patients aged ≥ 75 years. 10
Based on recommendations for managing HFSR caused by multikinase inhibitors, 41 , 42 , 43 patients receiving lenvatinib should be advised to take prophylactic measures before HFSR symptoms develop, such as emollients and urea‐containing prescription agents, and regularly monitored during lenvatinib treatment (Table 3). If grade 1 HFSR develops, lenvatinib treatment can be continued, as long as there is no pain (Table 3). If pain occurs (grade 2 symptoms), treatment with lenvatinib treatment should be interrupted and patients treated with steroids. After pain is alleviated, lenvatinib treatment should be resumed at the same dose if pain had occurred after several weeks of pain‐free treatment and at a reduced dose if dose interruption was required within 1 week of initiating treatment (Table 1).
Table 3.
Recommendations for the management of HFSR
| HFSR severity | Intervention |
|---|---|
|
No HFSR Therapy initiation |
Maintain frequent contact with physician to ensure early diagnosis of HFSR Full‐body skin examination, pedicure; wear thick cotton gloves and/or socks; avoid hot water, constrictive footwear, and excessive friction If symptoms develop at 2‐week clinical evaluation or within first month, proceed to next step … |
Grade 1
|
Maintain current dose of lenvatinib; monitor patient for change in severity Avoid hot water; use moisturizing creams for relief; wear thick cotton gloves and/or socks; use a 20–40% urea‐based cream to aid exfoliation If symptoms worsen after clinical evaluation at 2 weeks, proceed to next step … |
Grade 2
|
Dose reduction to 50% of dose for 7–28 days Treat as with grade 1 toxicity, with the following additions: topical steroid ointment, codeine, pregabalin for pain; follow dose modifications listed in Table 1 If symptoms worsen after clinical evaluation at 2 weeks, proceed to next step … |
Grade 3
|
Interrupt treatment for 7 days and until improvement to grade 0–1 Treat as with Grades 1 and 2; follow dose modifications listed in Table 1 |
ADL, activities of daily living; HFSR, hand–foot skin reaction.
Expert recommendations
Patients should be advised to take prophylactic measures before HFSR symptoms develop, such as emollients and urea‐containing prescription agents, and regularly monitored during lenvatinib treatment.
Lenvatinib interruption or dose reduction may be necessary if grade 2 or 3 symptoms are present.
Decreased appetite and weight loss
Decreased appetite and weight loss are also common occurrences in patients receiving lenvatinib. In the REFLECT study, 34% of lenvatinib recipients reported a decrease in appetite (grade ≥ 3 AEs in 5% of patients), and 31% of patients reported a decrease in body weight (grade ≥ 3 AEs 8% of patients). 9 Decreased body weight led to dose interruption or reduction in 4.2% of lenvatinib recipients. 10
Appetite and body weight should be monitored regularly in patients receiving lenvatinib. In patients with decreased appetite or weight loss of grade 1 or 2 severity, dose reduction or interruption may be needed temporarily, and, if so, subsequently resumed at the same dose when symptoms have been alleviated. 23 If decreased appetite or weight loss of grade 3 severity occurs early during treatment, dose reduction or interruption may be required. 23 Patients can also be offered an antiemetic agent or oral nutrition 23 ; in our clinical experience, patients may also benefit from the use of an appetite‐stimulating drug such as megestrol acetate.
Expert recommendations
Regularly monitor appetite and body weight.
Temporary (grade 1 or 2) or longer term (grade 3) lenvatinib dose reduction or interruption may be required.
Antiemetics, oral nutrition, or appetite stimulants may be helpful.
Fatigue
In the REFLECT study, fatigue was reported in 30% of patients in the lenvatinib group, with 4% of patients experiencing AEs of grade ≥ 3 severity. 9 Dose interruption or dose reduction because of fatigue was required in 5.7% of patients in the lenvatinib group. 10 Fatigue resulted in treatment discontinuation in seven patients (1.5%) in the lenvatinib group. 10
Although fatigue is a common non‐specific symptom in patients with advanced HCC, treatable causes of fatigue should be looked for during lenvatinib therapy. As an example, an association between fatigue and a rise in thyroid stimulating hormone (TSH), weight loss/anorexia, or disease progression should be taken into consideration. 34 In the first instance, all potential causes of AEs need to be evaluated thoroughly by checking complete blood cell counts and TSH levels, prior to lenvatinib dose reduction/interruption (depending on AE severity).
Patients with grade 1 fatigue can be managed with rest and do not require dose interruption or adjustment. 23 A healthy and active lifestyle should be encouraged in patients receiving lenvatinib. Physical exercise has been shown to improve cancer‐related fatigue effectively. 44 , 45 In patients with grade ≥ 2 fatigue, lenvatinib treatment can be interrupted if symptoms are intolerable. 23 Once symptoms are alleviated, treatment can be resumed at the same dose (if fatigue had occurred later during treatment) or at a reduced dose (if fatigue had been reported early during treatment). 23
Expert recommendations
Initially, thoroughly evaluate all treatable causes of fatigue.
For patients with grade ≥ 2 fatigue, interrupt lenvatinib treatment if symptoms are intolerable, resume at the same or a reduced dose once symptoms are alleviated.
Hepatic disorder/hepatotoxicity
Hepatotoxicity was common with lenvatinib in the REFLECT study, occurring in 47.7% of patients (grade ≥ 3 AEs 26.1%). 10 The median time to the first hepatotoxic event was 6.4 weeks in patients receiving lenvatinib. The most common (incidence ≥ 5%) hepatotoxic events in lenvatinib recipients were increased blood bilirubin (14.9%), increased aspartate aminotransferase (AST; 13.7%), increased alanine aminotransferase (ALT; 11.1%), hypoalbuminemia (9.2%), hepatic encephalopathy (8.0%), increased gamma‐glutamyltransferase (GGT; 7.8%), and increased blood alkaline phosphatase (ALP; 6.7%). Grade 3 or 4 increases in laboratory parameters in lenvatinib recipients included increases in blood bilirubin (13.2%), ALT (7.9%), AST (12.1%), GGT (31.4%), and ALP (6.6%). 10
Hepatic encephalopathy occurred in 8.4% of lenvatinib recipients; 5.5% of hepatic encephalopathy events in lenvatinib recipients were of grade ≥ 3 severity, and four patients had fatal events. 10 Hepatic failure occurred in 3.6% of lenvatinib recipients; all hepatic failure events with lenvatinib were of grade ≥ 3 severity, and 12 patients had fatal events. Hepatotoxic events led to treatment discontinuation in 5.5% of patients in the lenvatinib group, dose interruption in 7.4%, and dose reduction in 12.2% of patients; 17 deaths (3.6%) in the lenvatinib group were because of hepatotoxicity AEs. The risk of developing hepatic encephalopathy or hepatic failure was higher in patients with worse hepatic impairment and/or tumor burden at baseline and in patients aged ≥ 75 years. 10
Any underlying causes of hepatotoxicity (e.g. cirrhosis or hepatitis) need to be checked. Liver function tests should be performed prior to initiating lenvatinib therapy, and patients should be monitored regularly during treatment. Based on our clinical experience, we recommend that monitoring should occur every 2 weeks for the first month and monthly thereafter, and at every regular follow‐up visit, depending on the judgment of the individual clinician. Importantly, to reduce the burden of testing on patients and clinics, monitoring intervals for AEs (e.g. proteinuria and hepatotoxicity) should be aligned.
Table 4 provides an overview of the management of hepatotoxicity during lenvatinib treatment. Lenvatinib treatment can be continued in patients who develop a tolerable grade 2 hepatotoxic event and in patients with ALT, AST, or GGT levels 10‐fold lower than the normal range if considered appropriate. 23 In patients who develop an intolerable grade 2, 23 or grade 3 event, 4 , 5 , 24 treatment should be interrupted and resumed at lower dosage (Table 1) after improvement in liver function is confirmed. Patients with non–life‐threatening grade 4 hepatotoxic events can be managed in the same way as patients with grade 3 events; however, lenvatinib treatment should be permanently discontinued if hepatic failure occurs. 4 , 5 , 24
Table 4.
General recommendations for the management of hepatotoxicity during lenvatinib treatment
| Liver function test (CTCAE ver. 5.0) | Recommended management during lenvatinib therapy | |
|---|---|---|
| Baseline < ULN | Baseline > ULN | |
| ALT increased |
(1) Continue treatment in patients who develop a tolerable grade 2 event. (2) In case of 10‐fold decreases in ALT, AST, or GGT levels lower than the normal ranges, lenvatinib can be continued when the physician deems it appropriate. (3) For patients who develop an intolerable grade 2 event or a grade 3 event, resume treatment at a dose reduced by one dose level after an improvement in liver dysfunction is confirmed. (4) Patients with a non–life‐threatening grade 4 event can be managed in the same way as patients with a grade 3 event. However, treatment should be permanently discontinued in patients who develop hepatic failure. |
|
| Grade 1: > ULN to 3.0 × ULN | Grade 1: 1.5 to 3.0 × BL | |
| Grade 2: > 3.0 to 5.0 × ULN | Grade 2: > 3.0 to 5.0 × BL | |
| Grade 3: > 5.0 to 20.0 × ULN | Grade 3: > 5.0 to 20.0 × BL | |
| Grade 4: > 20.0 × ULN | Grade 4: > 20.0 × BL | |
| AST increased | ||
| Grade 1: > ULN to 3.0 × ULN | Grade 1: 1.5 to 3.0 × BL | |
| Grade 2: > 3.0 to 5.0 × ULN | Grade 2: > 3.0 to 5.0 × BL | |
| Grade 3: > 5.0 to 20.0 × ULN | Grade 3: > 5.0 to 20.0 × BL | |
| Grade 4: > 20.0 × ULN | Grade 4: > 20.0 × BL | |
| ALP increased | ||
| Grade 1: > ULN to 2.5 × ULN | Grade 1: 2.0 to 2.5 × BL | |
| Grade 2: > 2.5 to 5.0 × ULN | Grade 2: > 2.5 to 5.0 × BL | |
| Grade 3: > 5.0 to 20.0 × ULN | Grade 3: > 5.0 to 20.0 × BL | |
| Grade 4: > 20.0 × ULN | Grade 4: > 20.0 × BL | |
| Blood bilirubin increased | ||
| Grade 1: > ULN to 1.5 × ULN | Grade 1: > 1.0 to 1.5 × BL | |
| Grade 2: > 1.5 to 3.0 × ULN | Grade 2: > 1.5 to 3.0 × BL | |
| Grade 3: > 3.0 to 10.0 × ULN | Grade 3: > 3.0 to 10.0 × BL | |
| Grade 4: > 10.0 × ULN | Grade 4: > 10.0 × BL | |
| GGT increased | ||
| Grade 1: > ULN to 2.5 × ULN | Grade 1: 2.0 to 2.5 × BL | |
| Grade 2: > 2.5 to 5.0 × ULN | Grade 2: > 2.5 to 5.0 × BL | |
| Grade 3: > 5.0 to 20.0 × ULN | Grade 3: > 5.0 to 20.0 × BL | |
| Grade 4: > 20.0 × ULN | Grade 4: > 20.0 × BL | |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma‐glutamyltransferase; ULN, upper limit of normal.
Expert recommendations
Lenvatinib treatment can be continued in patients who develop a tolerable grade 2 hepatotoxic event.
Treatment should be interrupted for intolerable grade 2 or 3 events; after liver function improvement, resume lenvatinib at a lower dosage.
Discontinue lenvatinib if hepatic failure occurs.
Hypothyroidism
Treatment with lenvatinib has been associated with hypothyroidism. In the REFLECT study, hypothyroidism occurred in 16% of patients receiving lenvatinib; no grade ≥ 3 AEs were reported. 9 Prior to initiating treatment in this study, 89.6% of patients in the lenvatinib group had TSH levels of less than or equal to the upper limit of normal. 10 After treatment, TSH levels higher than the upper limit of normal were observed in 69.6% of lenvatinib recipients. Thyroid function should be monitored prior to initiating treatment, monthly during the first 2 months of treatment and regularly thereafter during treatment with lenvatinib. 23 Hypothyroidism, if detected, should be addressed with standard medical care to maintain euthyroid state. 23 However, based on our clinical experience, asymptomatic slight elevations in TSH levels do not necessarily require medical intervention for hypothyroidism. However, if TSH is > 10 mIU/L or is 5–10 mIU/L on two assays, 46 , 47 we recommend consulting with an endocrinologist as thyroid replacement therapy may be necessary.
Expert recommendations
Monitor thyroid function prior to treatment, monthly during the first 2 months and regularly thereafter.
Consult with an endocrinologist if TSH is > 10 mIU/L or 5–10 mIU/L on two assays.
Cardiovascular/cerebrovascular events
In the REFLECT study, cardiac dysfunction (including congestive cardiac failure, cardiogenic shock, and cardiopulmonary failure) occurred in 0.6% of lenvatinib recipients. 10 Arterial thromboembolic events were reported in 2.3% of lenvatinib recipients (serious AEs in 10 patients), with the most frequent events being myocardial infarction (four events) and cerebral infarction (one events). Posterior reversible encephalopathy syndrome (PRES)/reversible posterior leukoencephalopathy syndrome (RPLS) of grade 2 severity occurred in one patient in the lenvatinib group. 10
Patients on lenvatinib treatment should be monitored for clinical signs of cardiac decompensation. 34 If grade 3 cardiac dysfunction occurs, treatment should be interrupted and subsequently resumed at a lower dose once it resolves to grade 0–1 severity or baseline level; treatment should be discontinued for a grade 4 event. 4 , 5 If PRES/RPLS of any grade occurs, treatment should be interrupted; treatment resumption can be considered if the event resolves to grade 0–1 severity. Following an arterial thromboembolic event, treatment with lenvatinib should be discontinued. 4 , 5 In high‐risk patients (e.g. those with coronary artery disease or heart failure), medications known to prolong QT interval, such as class Ia and III anti‐arrhythmics, should be avoided where possible. 34 Prior to treatment initiation in these patients, echocardiography should be performed and cardiac troponin and natriuretic peptide levels measured; thereafter, patients should be monitored regularly with electrocardiograms. 34
Expert recommendations
Monitor patients for clinical signs of cardiac decompensation; interrupt lenvatinib (grade 3 cardiac dysfunction) then resume at a lower dose once grade 0–1 or baseline levels achieved. Discontinue lenvatinib if grade 4 symptoms occur.
Interrupt treatment if PRES/RPLS occurs (any grade); resume when severity resolves to grade 0–1.
Other adverse events
Dysphonia
Dysphonia is a known side effect of anti‐angiogenic drugs. 48 , 49 , 50 In the REFLECT study, dysphonia occurred in 24% of lenvatinib recipients, with < 1% of patients experiencing grade ≥ 3 dysphonia. 9 The only treatment for dysphonia due to angiogenesis inhibitors is discontinuation of therapy. 47
Expert recommendation
As dysphonia is not life‐threatening, therapy discontinuation must be weighed against the benefits of continued treatment.
Impaired wound healing
Although there is currently a lack of published clinical evidence, there are limited reports of impaired wound healing in patients receiving lenvatinib. 10 Recently, updated guidance proposes withholding lenvatinib for at least 1 week prior to elective surgery and not administering lenvatinib for at least 2 weeks following major surgery and until adequate wound healing. 4
Expert recommendation
As the safety of resuming lenvatinib after the resolution of wound healing complications has not been established, clinical decisions should be made after adequately considering the risks and benefits for each patient.
Osteonecrosis of the jaw
Although there is currently a lack of published clinical evidence, there are limited reports of osteonecrosis of the jaw (ONJ) in patients receiving lenvatinib. 4 , 51 Factors that may increase the risk of jaw bone problems include concomitant use of bisphosphonates, denosumab, presence of dental disease, or undergoing an invasive dental procedure. Clinicians should perform an oral examination prior to, and periodically during, treatment with lenvatinib. Patients should be advised about good oral hygiene practices and, where possible, invasive dental procedures should be avoided during lenvatinib treatment, particularly in high‐risk patients. If possible, lenvatinib should be stopped for at least 1 week prior to scheduled dental surgery or invasive dental procedures. For patients requiring invasive dental procedures, discontinuation of bisphosphonate treatment may reduce the risk of ONJ. If ONJ develops, lenvatinib should be withheld and restarted based on clinical judgment of adequate resolution. 4
Expert recommendation
Monitor patients for clinical signs of ONJ, consider oral examination prior to, and periodically during, lenvatinib treatment.
Summary
Lenvatinib provides a significant survival benefit in patients with advanced HCC. However, it is associated with high rates of AEs, which may adversely affect clinical outcomes. Patients receiving lenvatinib should be advised about prophylactic measures and monitored regularly for AEs. Patients who develop AEs can be managed with lenvatinib dose interruption, adjustment or discontinuation of treatment. For mild or moderate (grade 1 or 2) AEs, dose interruption is generally not required, unless AEs are intolerable despite optimal management. 4 , 5 For persistent or intolerable grade 2 or 3 AEs, lenvatinib treatment should be interrupted until symptoms improve/resolve to grade 0–1 or baseline levels. Thereafter, treatment should be resumed at the same or a lower dose. 4 , 5 There have been reports suggesting that occurrence of some AEs may predict a survival benefit, and patients who discontinue lenvatinib treatment because of AEs may have a poor survival prognosis. Progression may occur in patients who do not initially respond to treatment or receive a suboptimal dose following dosage reduction, resulting in lack of efficacy. Therefore, to derive maximum treatment benefit and ensure long‐term disease control, lenvatinib should be maintained at the highest possible dose when managing AEs. 23
Recently, immune checkpoint inhibitors have shown antitumor activity against HCC. 11 , 52 , 53 , 54 , 55 The overall incidence of any treatment‐related AEs tends to be higher in patients treated with multikinase inhibitors (93–95%) compared with PD‐1 inhibitor monotherapy (61–74%). 9 , 52 , 53 , 55 , 56 , 57 Treatment‐related AEs of ≥ grade 3 occurred more frequently with multikinase inhibitors than with PD‐1 inhibitor monotherapy (49–57% vs 18–26%). Frequently reported treatment‐related AEs ≥ grade 3 with multikinase inhibitors were hand–foot skin reaction, hypertension, anorexia, weight loss, and proteinuria with an incidence of > 5%, which are mostly symptomatic, but manageable. 9 , 56 For PD‐1 inhibitors, the most frequent treatment‐related AEs were AST or ALT elevation with an incidence of 4–7%, which are usually asymptomatic. 52 , 53 , 55 However, immune‐related AEs, a unique spectrum of AEs associated with immune checkpoint inhibitors, may happen in some patients treated with immune checkpoint inhibitors. Immune‐related AEs are generally manageable, but they are sometimes life‐threatening.
To conclude, effective management of lenvatinib‐associated AEs using prophylactic measures, regular monitoring and symptomatic management, can ensure continued treatment and maximum survival benefit in patients with advanced HCC receiving first‐line lenvatinib therapy.
[Correction added on 06 December 2021, after first online publication: references 46 and 56 have been swapped.]
Supporting information
Table S1 Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.
Acknowledgments
Under the direction of the authors, medical writing support was provided by David P. Figgitt, PhD, ISMPP CMPP™, Content Ed Net, and this support was funded by Eisai Korea Inc. Eisai Korea Inc. did not contribute to or influence the conceptual development of the manuscript and all decisions regarding content were the responsibility of the authors.
Kim, B. H. , Yu, S. J. , Kang, W. , Cho, S. B. , Park, S. Y. , Kim, S. U. , and Kim, D. Y. (2022) Expert consensus on the management of adverse events in patients receiving lenvatinib for hepatocellular carcinoma. Journal of Gastroenterology and Hepatology, 37: 428–439. 10.1111/jgh.15727.
Declaration of conflicts of interest: BHK has served in an advisory role for Eisai and Roche, has received honoraria from AbbVie, and has participated in research sponsored by Ono BMS. The other authors declare no conflict of interest.
Financial support: Eisai Korea.
References
- 1. European Association for the Study of the Liver . EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J. Hepatol. 2018; 69: 182–236. [DOI] [PubMed] [Google Scholar]
- 2. Vogel A, Cervantes A, Chau I et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann. Oncol. 2018; 29: iv238–iv255. [DOI] [PubMed] [Google Scholar]
- 3. NCCN (2020) NCCN clinical practice guidelines: hepatobiliary cancers (version 5.2020). https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf (Accessed 12 Jan 2021).
- 4. Lenvima US Prescribing information (2020). Available from: http://www.lenvima.com/pdfs/prescribing‐information.pdf (Accessed 5 March 2021).
- 5. European Medicines Agency (2020). LENVIMA (lenvatinib): summary of product characteristics. https://www.ema.europa.eu/en/documents/product‐information/lenvima‐epar‐product‐information_en.pdf. Accessed 2 March 2021.
- 6. Ikeda M, Okusaka T, Mitsunaga S et al. Safety and pharmacokinetics of lenvatinib in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 2016; 22: 1385–1394. [DOI] [PubMed] [Google Scholar]
- 7. Ikeda K, Kudo M, Kawazoe S et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J. Gastroenterol. 2017; 52: 512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tamai T, Hayato S, Hojo S et al. Dose finding of lenvatinib in subjects with advanced hepatocellular carcinoma based on population pharmacokinetic and exposure‐response analyses. J. Clin. Pharmacol. 2017; 57: 1138–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kudo M, Finn RS, Qin S et al. Lenvatinib versus sorafenib in first‐line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non‐inferiority trial. Lancet 2018; 391: 1163–1173. [DOI] [PubMed] [Google Scholar]
- 10. European Medicines Agency (2018). LENVIMA (lenvatinib): assessment report. https://www.ema.europa.eu/en/documents/variation‐report/lenvima‐h‐c‐3727‐ii‐0011‐g‐epar‐assessment‐report‐variation_en.pdf. Accessed 2 March 2021.
- 11. Ohki T, Sato K, Kondo M et al. Impact of adverse events on the progression‐free survival of patients with advanced hepatocellular carcinoma treated with lenvatinib: a multicenter retrospective study. Drugs Real World Outcomes 2020; 7: 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sho T, Suda G, Ogawa K et al. Early response and safety of lenvatinib for patients with advanced hepatocellular carcinoma in a real‐world setting. JGH Open. 2019; 4: 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shimose S, Iwamoto H, Niizeki T et al. Clinical significance of adverse events for patients with unresectable hepatocellular carcinoma treated with lenvatinib: a multicenter retrospective study. Cancers (Basel) 2020; 12: 1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang DX, Yang X, Lin JZ et al. Efficacy and safety of lenvatinib for patients with advanced hepatocellular carcinoma: a retrospective, real‐world study conducted in China. World J. Gastroenterol. 2020; 26: 4465–4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheon J, Chon HJ, Bang Y et al. Real‐world efficacy and safety of lenvatinib in Korean patients with advanced hepatocellular carcinoma: a multicenter retrospective analysis. Liver Cancer. 2020; 9: 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Celermajer Y, Prince DS, Stratton E et al. Real‐world safety and tolerability data for lenvatinib use in unresectable hepatocellular cancer. Presented at: American Association for the Study of Liver Diseases ‐ The Liver Meeting Digital Experience; November 13–16, 2020; virtual. Abstract 1167. Accessed 2 March 2021. https://www.aasld.org/posters/25569070/
- 17. Kim S, Kim KH, Kim BK et al. Lenvatinib is independently associated with the reduced risk of progressive disease when compared with sorafenib in patients with advanced hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2021; 36: 1317–1325. [DOI] [PubMed] [Google Scholar]
- 18. Amaro CP, Allen MJ, Knox JJ et al. Efficacy and safety of lenvatinib in the real‐world treatment of hepatocellular carcinoma: results from a Canadian multicenter database (HCC CHORD). J. Clin. Oncol. 2021; 39: 275. [Google Scholar]
- 19. Goh MJ, Oh JH, Park Y et al. Efficacy and safety of lenvatinib therapy for unresectable hepatocellular carcinoma in a real‐world practice in Korea. Liver Cancer. 2021; 10: 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rauthan A, Somashekhar AP, Patil P, Zaveri S. Real‐world experience with lenvatinib in the management of hepatocellular carcinoma: a single‐center Indian experience. J. Clin. Oncol. 2020; 38: e16596. [Google Scholar]
- 21. Sung MW, Finn RS, Qin S et al. Association between overall survival and adverse events with lenvatinib treatment in patients with hepatocellular carcinoma (REFLECT) [abstract and presentation]. J. Clin. Oncol. 2019; 37: 317. [Google Scholar]
- 22. Maitland ML, Bakris GL, Black HR et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J. Natl. Cancer Inst. 2010; 102: 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ikeda M, Kobayashi M, Tahara M, Kaneko S. Optimal management of patients with hepatocellular carcinoma treated with lenvatinib. Expert Opin. Drug Saf. 2018; 17: 1095–1105. [DOI] [PubMed] [Google Scholar]
- 24. Agarwal M, Thareja N, Benjamin M, Akhondi A, Mitchell GD. Tyrosine kinase inhibitor‐induced hypertension. Curr. Oncol. Rep. 2018; 20: 65. [DOI] [PubMed] [Google Scholar]
- 25. Cohen JB, Geara AS, Hogan JJ, Townsend RR. Hypertension in cancer Patients and survivors: epidemiology, diagnosis, and management. JACC CardioOncol. 2019; 1: 238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Versmissen J, Mirabito Colafella KM, Koolen SLW, Danser AHJ. Vascular cardio‐oncology: vascular endothelial growth factor inhibitors and hypertension. Cardiovasc. Res. 2019; 115: 904–914. [DOI] [PubMed] [Google Scholar]
- 27. Patel S, Dushenkov A, Jungsuwadee P, Krishnaswami A, Barac A. Team‐based approach to management of hypertension associated with angiogenesis inhibitors. J. Cardiovasc. Transl. Res. 2020; 13: 463–477. [DOI] [PubMed] [Google Scholar]
- 28. Lankhorst S, Kappers MH, van Esch JH et al. Treatment of hypertension and renal injury induced by the angiogenesis inhibitor sunitinib: preclinical study. Hypertension 2014; 64: 1282–1289. [DOI] [PubMed] [Google Scholar]
- 29. Emile G, Pujade‐Lauraine E, Alexandre J. Should we use the angiotensin‐converting enzyme inhibitors for the treatment of anti‐VEGF‐induced hypertension? Ann. Oncol. 2014; 25: 1669–1670. [DOI] [PubMed] [Google Scholar]
- 30. Miura S, Fujino M, Matsuo Y, Tanigawa H, Saku K. Nifedipine‐induced vascular endothelial growth factor secretion from coronary smooth muscle cells promotes endothelial tube formation via the kinase insert domain‐containing receptor/fetal liver kinase‐1/NO pathway. Hypertens. Res. 2005; 28: 147–153. [DOI] [PubMed] [Google Scholar]
- 31. Kandula P, Agarwal R. Proteinuria and hypertension with tyrosine kinase inhibitors. Kidney Int. 2011; 80: 1271–1277. [DOI] [PubMed] [Google Scholar]
- 32. Hemmelgarn BR, Manns BJ, Lloyd A et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010; 303: 423–429. [DOI] [PubMed] [Google Scholar]
- 33. Chronic Kidney Disease Prognosis Consortium , Matsushita K, van der Velde M et al. Association of estimated glomerular filtration rate and albuminuria with all‐cause and cardiovascular mortality in general population cohorts: a collaborative meta‐analysis. Lancet 2010; 375: 2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reed N, Glen H, Gerrard G et al. Expert consensus on the management of adverse events during treatment with lenvatinib for thyroid cancer. Clin. Oncol. (R. Coll. Radiol.) 2020; 32: e145–e153. [DOI] [PubMed] [Google Scholar]
- 35. Evans TRJ, Kudo M, Finn RS et al. Urine protein:creatinine ratio vs 24‐hour urine protein for proteinuria management: analysis from the phase 3 REFLECT study of lenvatinib vs sorafenib in hepatocellular carcinoma. Br. J. Cancer 2019; 121: 218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takahashi S, Kiyota N, Tahara M. Optimal use of lenvatinib in the treatment of advanced thyroid cancer. Cancers Head Neck. 2017; 2: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Janssen‐Cilag Pvt Ltd . (2021). Imodium (loperamide): package insert. https://www.medicines.org.uk/emc/files/pil.558.pdf. Accessed 2 March 2021.
- 38. Khediri F, Mrad AI, Azzouz M et al. Efficacy of diosmectite (smecta) in the treatment of acute watery diarrhoea in adults: a multicentre, randomized, double‐blind, placebo‐controlled, parallel group study. Gastroenterol. Res. Pract. 2011; 2011: 783196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Manchen E, Robert C, Porta C. Management of tyrosine kinase inhibitor‐induced hand‐foot skin reaction: viewpoints from the medical oncologist, dermatologist, and oncology nurse. J. Support. Oncol. 2011; 9: 13–23. [DOI] [PubMed] [Google Scholar]
- 40. Lacouture ME, Wu S, Robert C et al. Evolving strategies for the management of hand‐foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist 2008; 13: 1001–1011. [DOI] [PubMed] [Google Scholar]
- 41. Anderson R, Jatoi A, Robert C, Wood LS, Keating KN, Lacouture ME. Search for evidence‐based approaches for the prevention and palliation of hand‐foot skin reaction (HFSR) caused by the multikinase inhibitors (MKIs). Oncologist 2009; 14: 291–302. [DOI] [PubMed] [Google Scholar]
- 42. Wood LS, Lemont H, Jatoi A et al. Practical considerations in the management of hand‐foot skin reaction caused by multikinase inhibitors. Community Oncology. 2010; 7: 23–29. [Google Scholar]
- 43. Mustian KM, Alfano CM, Heckler C et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer‐related fatigue: a meta‐analysis. JAMA Oncol. 2017; 3: 961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hilfiker R, Meichtry A, Eicher M et al. Exercise and other non‐pharmaceutical interventions for cancer‐related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect‐comparisons meta‐analysis. Br. J. Sports Med. 2018; 52: 651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bhattacharya S, Goyal A, Kaur P, Singh R, Kalra S. Anticancer drug‐induced thyroid dysfunction. Eur. Endocrinol. 2020; 16: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Drui D, Illouz F, Do Cao C, Caron P. Expert opinion on thyroid complications of new anti‐cancer therapies: tyrosine kinase inhibitors. Ann. Endocrinol. (Paris) 2018; 79: 569–573. [DOI] [PubMed] [Google Scholar]
- 47. Saavedra E, Hollebecque A, Soria JC, Hartl DM. Dysphonia induced by anti‐angiogenic compounds. Invest. New Drugs 2014; 32: 774–782. [DOI] [PubMed] [Google Scholar]
- 48. Wong H, Yau T, Man CW, Epstein RJ. Hoarseness during treatment with bevacizumab and other vascular endothelial growth factor signalling inhibitors. Acta Oncol. 2009; 48: 1213–1215. [DOI] [PubMed] [Google Scholar]
- 49. Hartl DM, Ferté C, Loriot Y et al. Dysphonia induced by vascular endothelium growth factor/vascular endothelium growth factor receptor inhibitors. Invest. New Drugs 2010; 28: 884–886. [DOI] [PubMed] [Google Scholar]
- 50. Mauceri R, Panzarella V, Morreale I, Campisi G. Medication‐related osteonecrosis of the jaw in a cancer patient receiving lenvatinib. Int. J. Oral Maxillofac. Surg. 2019; 48: 1530–1532. [DOI] [PubMed] [Google Scholar]
- 51. El‐Khoueiry AB, Sangro B, Yau T et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open‐label, non‐comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017; 389: 2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhu AX, Finn RS, Edeline J et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE‐224): a non‐randomised, open‐label phase 2 trial. Lancet Oncol. 2018; 19: 940–952. [DOI] [PubMed] [Google Scholar]
- 53. Finn RS, Qin S, Ikeda M et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020; 382: 1894–1905. [DOI] [PubMed] [Google Scholar]
- 54. Finn RS, Ryoo BY, Merle P et al. Pembrolizumab as second‐line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE‐240: a randomized, double‐blind, phase III trial. J. Clin. Oncol. 2020; 38: 193–202. [DOI] [PubMed] [Google Scholar]
- 55. Yau T, Kang YK, Kim TY et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020; 6: e204564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bruix J, Qin S, Merle P et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet 2017; 389: 56–66. [DOI] [PubMed] [Google Scholar]
- 57. Kim BH, Park J‐W. Systemic therapy for advanced hepatocellular carcinoma: consideration for selecting second‐line treatment. J. Liver Cancer. 2021; 21: 124–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.


