Abstract
Background
Milvexian (BMS‐986177/JNJ‐70033093) is an orally bioavailable factor XIa (FXIa) inhibitor currently in phase 2 clinical trials.
Objectives
To evaluate in vitro properties and in vivo characteristics of milvexian.
Methods
In vitro properties of milvexian were evaluated with coagulation and enzyme assays, and in vivo profiles were characterized with rabbit models of electrolytic‐induced carotid arterial thrombosis and cuticle bleeding time (BT).
Results
Milvexian is an active‐site, reversible inhibitor of human and rabbit FXIa (Ki 0.11 and 0.38 nM, respectively). Milvexian increased activated partial thromboplastin time (APTT) without changing prothrombin time and potently prolonged plasma APTT in humans and rabbits. Milvexian did not alter platelet aggregation to ADP, arachidonic acid, or collagen. Milvexian was evaluated for in vivo prevention and treatment of thrombosis. For prevention, milvexian 0.063 + 0.04, 0.25 + 0.17, and 1 + 0.67 mg/kg+mg/kg/h preserved 32 ± 6*, 54 ± 10*, and 76 ± 5%* of carotid blood flow (CBF) and reduced thrombus weight by 15 ± 10*, 45 ± 2*, and 70 ± 4%*, respectively (*p < .05; n = 6/dose). For treatment, thrombosis was initiated for 15 min and CBF decreased to 40% of control. Seventy‐five minutes after milvexian administration, CBF averaged 1 ± 0.3, 39 ± 10, and 66 ± 2%* in groups treated with vehicle and milvexian 0.25 + 0.17 and 1 + 0.67 mg/kg+mg/kg/h, respectively (*p < .05 vs. vehicle; n = 6/group). The combination of milvexian 1 + 0.67 mg/kg+mg/kg/h and aspirin 4 mg/kg/h intravenous did not increase BT versus aspirin monotherapy.
Conclusions
Milvexian is an effective antithrombotic agent with limited impact on hemostasis, even when combined with aspirin in rabbits. This study supports inhibition of FXIa with milvexian as a promising antithrombotic therapy with a wide therapeutic window.
Keywords: antithrombotic, blood coagulation, factor XIa inhibitor, hemostasis, thrombosis
Essentials.
Milvexian is a high affinity and selective factor XIa (FXIa) inhibitor.
In vitro studies and antithrombotic and bleeding studies in rabbits were conducted with milvexian.
Milvexian had robust efficacy in preventing and treating thrombosis.
Milvexian preserved hemostasis at the doses studied.
1. INTRODUCTION
Factor XI (FXI) is a component of the blood coagulation system. 1 FXI is activated by factor XIIa or thrombin in response to injury of a blood vessel, producing factor XIa (FXIa). 2 FXIa participates in a cascade of proteases by activating factor IX, a function partially redundant with factor VIIa, ultimately leading to the formation of a fibrin clot, via the action of thrombin, which along with platelets seals the injured blood vessel. Intact, healthy blood vessels are not reactive toward the coagulation system, but in certain disease states, such as atherosclerosis, activation of the coagulation system can cause thrombosis, which is the partial or total occlusion of a blood vessel. When thrombosis occurs, inadequate blood flow reduces the distribution of oxygen and nutrients to the surrounding tissue and allows metabolic products such as carbon dioxide and lactic acid to accumulate. Effective treatment of thrombosis requires modulation of the pathologic process while maintaining sufficient hemostatic function to prevent blood loss.
Deficiency of FXI, known as hemophilia C, is associated with a mild bleeding disorder that occurs mostly in the context of surgery or trauma. 3 This phenotype is distinct from hemophilia A and B, which are deficiencies in factors VIII and IX, respectively, and where spontaneous hemorrhage is observed. In a recent study of patients undergoing total knee arthroplasty, a 80% reduction in circulating FXI by an antisense oligonucleotide proved more effective than enoxaparin in preventing postoperative venous thromboembolism and appeared to be safe with regard to bleeding risk, providing a proof of concept in humans for FXIa inhibition. 4 Given the preclinical and early clinical evidence for robust antithrombotic efficacy with limited hemostatic liability upon genetic disruption or pharmacologic inhibition, FXIa is an attractive target for antithrombotic therapy. 4 , 5 , 6 , 7 , 8 , 9 , 10
Milvexian (formerly referred to as BMS‐986177/JNJ‐70033093; Figure 1A), an orally bioavailable small molecule that inhibits FXIa with high affinity and selectivity, 11 is being developed to prevent thrombotic events in diverse patient populations. In a phase 1 study, milvexian given orally either as a single dose or multiple ascending doses for 14 days was orally bioavailable, and generally safe and well tolerated in healthy volunteers. 12 In addition, the pharmacodynamic effect of milvexian, as measured by increases in activated partial thromboplastin time (APTT), was closely correlated with milvexian plasma concentration. 12
FIGURE 1.
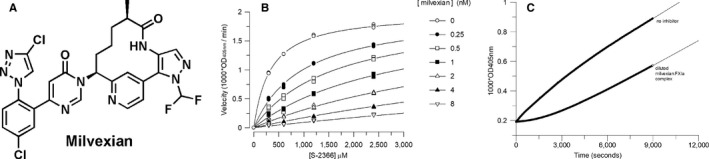
(A) Chemical structure of the FXIa inhibitor milvexian. (B) Milvexian competitive inhibition of human FXIa peptide substrate hydrolysis. (C) Dissociation of human FXIa–milvexian complex following 2000‐fold dilution into 2 mM S‐2366. FXIa, factor XIa
In this study, the in vitro properties and in vivo profiles of milvexian were characterized. The in vivo antithrombotic efficacy of milvexian was characterized in a rabbit model of electrically mediated carotid arterial thrombosis (ECAT), focusing on both the prevention of thrombosis and the treatment of preformed thrombus. In addition, therapeutic index of milvexian was examined in vivo by evaluating the antihemostatic effect in a rabbit model of cuticle bleeding time (BT). Because aspirin (ASA) is widely used in patients for the prevention and treatment of acute and chronic arterial diseases, it is likely that milvexian will be combined with ASA in the clinical setting. Therefore, the BT effects of the combination of ASA and milvexian were studied in rabbits. These rabbit models have been well characterized with reference agents. 13 , 14 , 15 , 16 , 17
2. MATERIALS AND METHODS
Full details on the methods and materials used can be found in the Appendix S1.
2.1. In vitro affinity assays
The affinity of milvexian for human FXIa (Haematologic Technologies) was determined using purified human enzyme and synthetic substrate from commercial sources. The affinity of milvexian for various enzymes in preclinical animal species was determined using recombinant enzymes, enzymes purified from plasma, or plasma kallikrein activated in situ and synthetic substrates from commercial sources. Rabbit thrombin and factor Xa were obtained from Enzyme Research Laboratories. Recombinant cynomolgus monkey thrombin was obtained from Cambridge Protein Works. Assays were conducted with simultaneous measurement of enzyme activities in control and inhibitor‐containing solutions. The inhibition constant (Ki) for milvexian was evaluated for FXIa under a range of substrate and inhibitor concentrations in both human and preclinical species.
Milvexian inhibition of human FXIa was evaluated to calculate an observed association rate constant (kobs). Association (kon) and dissociation (koff) rates were fitted by linear regression from a secondary plot of kobs versus inhibitor concentration at multiple substrate concentrations. The association rate constant for binding of milvexian to human FXIa was also calculated from nonlinear time courses of inhibition in the presence of S‐2366 using stopped‐flow spectrophotometry.
2.2. In vitro coagulation assays
The anticoagulant activity of milvexian in pooled human plasma (Affinity Biologicals) as measured by APTT was conducted against several different reagents (Table S1). FXI clotting activity, prothrombin time (PT), and thrombin time (TT) were measured. The anticoagulant activity of milvexian in preclinical species plasma as measured by APTT was also examined, as well as PT and ecarin clotting time. Cynomolgus monkey plasma was obtained from Affinity Biologicals. Blood was drawn from male New Zealand white rabbits, male beagle dogs, and male Sprague‐Dawley rats.
2.3. In vitro platelet aggregation
In vitro platelet aggregation was measured in hirudin‐treated rabbit platelet‐rich plasma with a platelet aggregometer, and peak platelet aggregation response to adenosine 5′‐diphosphate (ADP), arachidonic acid, and collagen was assessed.
2.4. In vivo assays
2.4.1. Animals and ethics
For in vivo characterization studies, male New Zealand white rabbits were obtained from Covance. Animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the regulations of the Animal Care and Use Committee of the Bristol Myers Squibb Company.
2.4.2. Pharmacokinetics
Pharmacokinetics were assessed as described by Zhang et al. 18 Briefly, milvexian was administered intravenously and orally and blood samples were collected at the following time intervals: predose and then 10, 15, 30, and 45 min and 1, 2, 4, 6, and 8 h relative to the start of the infusion; predose and then 1, 2, 3, 4, 8, and 24 h after administration of the oral dose.
2.4.3. Rabbit ECAT
The rabbit ECAT model as described by Wong et al 16 was used in this study to assess the effects of vehicle and milvexian on carotid blood flow after thrombus induction. For the prevention ECAT study, plasma concentrations of milvexian at 30 and 120 min after the start of the infusion were determined. The average value of plasma concentrations of milvexian at 30 and 120 min after the start of infusion in each animal was then used for the analysis of the concentration‐response curve and the determination of antithrombotic the concentration that gives 50% maximal response (EC50). Integrated blood flow, thrombus weight, APTT, TT, and PT were also measured. In the treatment ECAT study, only the plasma concentrations of milvexian at the end of the study were determined.
2.4.4. Rabbit cuticle BT model
The rabbit cuticle BT model as described by Wong et al 14 was used in this study. BT was defined as the time after cuticle transection when bleeding ceased. Milvexian or its vehicle and ASA or its vehicle (saline) were given at 30 and 60 min before cuticle transection, respectively. BT was measured before and after treatment in opposite hind limbs and was expressed as a ratio of treated over the control value. The dose of ASA used in this study was a maximal effective antiplatelet dose in rabbits. 19
2.4.5. Ex vivo coagulation assays
Arterial blood samples for the determination of ex vivo APTT, TT, and PT were collected before the start of the infusion of milvexian and at 120 min after the start of the infusion. Clotting times were measured as previously described by Wong et al. 13
2.5. Bioanalytical assays
Concentrations of milvexian in plasma samples were determined by the BMS Bioanalytical Research group and were measured by a specific and sensitive liquid chromatographic tandem mass spectrometry method.
2.6. Statistical analyses
Statistical analyses used were analysis of variance and Tukey's multiple comparison test using the GraphPad Prism version 5.0 for Windows (GraphPad Software). A value of p < .05 was considered statistically significant. All data are mean ± SEM.
For human and preclinical species in vitro affinity assays, data were analyzed using linear and nonlinear fitting routines from GraFit (Erithracus Software).
For in vitro anticoagulant activity in plasma, the plasma milvexian concentration required to cause a specified increase in relative clotting time or a specified decrease in clotting activity was calculated by interpolation.
3. RESULTS
3.1. In vitro affinity for human enzymes
Because milvexian exhibited high affinity for FXIa, determination of the inhibition constant, or Ki, was conducted using a range of substrate and inhibitor concentrations. Competitive inhibition of human FXIa by milvexian is shown in Figure 1B as a representative plot of steady‐state reaction velocity versus synthetic peptide substrate concentration. The Ki of milvexian for human FXIa is 0.11 nM, as determined from the average of three independent experiments (Table S2).
3.1.1. Microscopic association rate constant
Nonlinear time courses for milvexian inhibition of FXIa were observed at several concentrations of the peptide substrate used to generate the competitive inhibition plot illustrated in Figure 1C. An example of the curved time courses at one substrate concentration is depicted in Figure S1. These data were fitted to the slow binding inhibition equation to obtain kobs values.
A representative plot of kobs versus milvexian concentration at different fixed concentrations of substrate is shown in Figure S2. The kobs data were fit to an equation modified for competitive inhibition to obtain values for the milvexian kon and koff. From three independent experiments, the average value for kon was 2 µM−1 s−1 and the average value for koff was 0.0002 s−1 (Table S3).
The milvexian association rate with FXIa was also determined in rapid mixing, stopped‐flow experiments to confirm the association rate derived from the 2‐h, 96‐well microtiter plate time course data. A plot of kobs versus milvexian concentration at different fixed concentrations of substrate is shown in Figure S3. The kobs data was fit to an equation modified for competitive inhibition to obtain values for the milvexian kon and koff. Results from triplicate experiments are shown in Table S4.
3.1.2. Microscopic dissociation rate constant
Reversibility of FXIa inhibition is demonstrated by the recovery of FXIa activity upon 2000‐fold dilution of a FXIa:milvexian complex into 2 mM peptide substrate as shown in Figure 1C. Analysis of the nonlinear time course of this reaction revealed a milvexian koff of 0.00041 ± 0.000001 s−1. This value is only 2‐fold higher than the koff derived from the y‐intercept of kobs versus inhibitor concentration from the 96‐well microtiter plate time course experiments described previously. By either measurement, the milvexian dissociation rate from FXIa is slow with a half‐life for dissociation of 28–58 min.
3.2. In vitro affinity for preclinical species enzymes
Competitive inhibition was also observed for milvexian inhibition of recombinant FXIa catalytic domain from cynomolgus monkeys, dogs, rats, and mice, and the results are summarized in Table 1.
TABLE 1.
Affinity of milvexian for recombinant FXIa from nonclinical species
| Species | Ki (nM) |
|---|---|
| Cynomolgus monkey | 0.15 ± 0.01 |
| Rabbit | 0.38 ± 0.01 |
| Dog | 0.64 ± 0.03 |
| Rat | 490 ± 40 |
| Mouse | 350 ± 15 |
Abbreviations: FXIa, factor XIa; Ki, inhibitory constant.
3.2.1. Activated plasma kallikrein, thrombin, and factor Xa (FXa)
Milvexian inhibits purified human plasma kallikrein with a Ki of 44 nM. For comparison with activated plasma kallikrein from nonclinical species, in situ activated human plasma kallikrein was assessed using the same method. The results are summarized in Table S5.
Milvexian does not bind to rabbit FXa (Ki: >18 000 nM) and has 4000‐fold selectivity for rabbit FXIa (Ki: 0.38 nM) over rabbit thrombin (Ki: 1700 nM). Milvexian inhibits cynomolgus monkey thrombin with a Ki of 670 ± 40 nM.
3.3. In vitro anticoagulant activity in human plasma
The anticoagulant activity of milvexian in pooled human plasma as measured by APTT using different reagents is depicted in Figure 2. The graphs demonstrate the reagent‐dependent differences in APTT as a function of milvexian concentration. The upper left panel is Actin FS, a reagent with high relative sensitivity to milvexian. The upper right panel is Actin FSL, a reagent with low relative sensitivity to milvexian. The milvexian plasma concentrations required to produce specified increases in APTT relative to baseline are summarized in Table S6.
FIGURE 2.
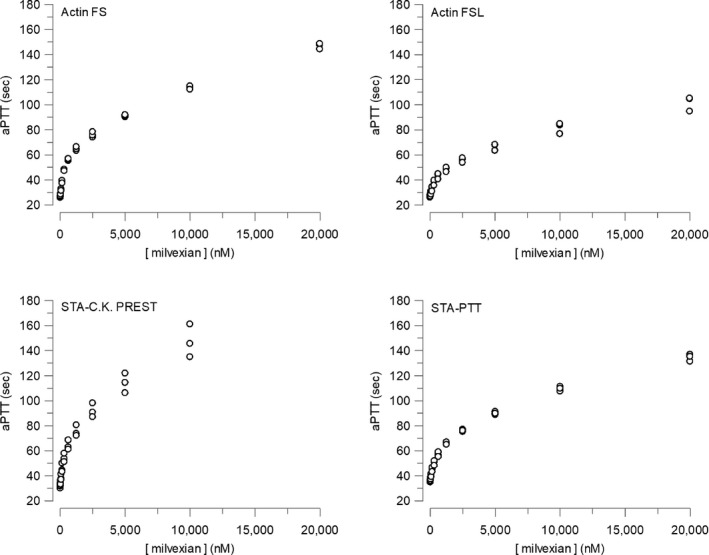
APTT as a function of milvexian concentration in pooled human plasma. APTT, activated partial thromboplastin time
Extended contact activation time assays decreased the milvexian plasma concentration required to produce changes in the APTT. The results for the extended contact activation assays are summarized in Table S7.
Milvexian causes an apparent reduction in FXI clotting activity, as assessed by the standard specialty coagulation assay, with lower percentages of normal FXI clotting activity occurring at higher milvexian concentrations (Table 2). The reduction is classified as apparent because milvexian does not change the FXI protein concentration in this in vitro test, but rather inhibits the FXIa enzymatic activity produced by the contact activation phase of the assay. As expected for a FXIa inhibitor, the fold‐dilution of plasma affected the observed FXI clotting activity with dilutions >1:10 producing higher FXI clotting activity and dilutions <1:10 producing lower FXI clotting activity (data not shown).
TABLE 2.
Apparent reduction of FXI clotting activity by milvexian in pooled normal human plasma
| Percentage of normal FXI clotting activity | Milvexian plasma concentration (nM) |
|---|---|
| 50 | 540 |
| 20 | 1800 |
| 10 | 3600 |
Abbreviation: FXI, factor XI.
In pooled human plasma, baseline PT values averaged 10.3 ± 0.1 s. At a total plasma concentration of 40 μM milvexian, PT values averaged 12.9 ± 0.4 s and the international normalized ratio was <1.3. Thrombin clotting time baseline values averaged 16.6 s. At total plasma concentrations of 20 μM and 40 μM milvexian, TT values averaged 24.8 and 28.4 s, respectively. For comparison, at 20 μM milvexian total plasma concentration the PT and TT are increased <50% relative to baseline and the aPTT is increased more than 3 times the baseline value.
3.4. In vitro anticoagulant activity in preclinical species plasma
Milvexian was associated with concentration‐dependent prolongation of the APTT in plasma from cynomolgus monkeys, rabbits, and dogs, with reduced potency in plasma from rats. The milvexian plasma concentrations required to produce 1.5×, 2×, and 2.5× increases on APTT relative to baseline are summarized in Table S8.
Milvexian was associated with only minimal increases in PT at concentrations up to 40 μM in plasma from rats, rabbits, and dogs. In cynomolgus monkey plasma, milvexian was associated with concentration‐dependent increases in PT, with an approximately 2× increase over baseline at a plasma concentration of 40 μM. The effects on PT, expressed as fold‐increase over baseline, are summarized in Table S9.
In cynomolgus monkey plasma, milvexian prolonged the ecarin clotting time in a linear, concentration‐dependent manner with a 1.9× increase over baseline observed at a concentration of 40 μM.
3.4.1. In vitro platelet aggregation
In vitro platelet aggregation responses to ADP (10 µM), arachidonic acid (250 µM), and collagen (10 µg/ml) averaged 59 ± 1%, 67 ± 1%, and 66 ± 1%, respectively, in rabbit platelet‐rich plasma (n = 4 per group). These platelet responses were not significantly altered by milvexian at 1 μM (58 ± 2%, 65 ± 1%, and 67 ± 2%, respectively) and 10 μM (55 ± 2%, 66 ± 2%, and 69 ± 1%, respectively; n = 4 per group). We showed previously that these platelet aggregation responses were almost completely inhibited by the GPIIb/IIIa antagonist DMP802 at 3µM. 13
3.5. In vivo characterization studies
3.5.1. Pharmacokinetics studies
Figure S4 shows a plot of milvexian concentration versus time following an IV (0.8 mg/kg) or PO (20 mg/kg) dose in rabbits. Milvexian at 0.8 mg/kg IV produced average plasma concentrations of 2000 and 100 nM at 10 min and 8 h after dosing, respectively. Milvexian at 20 mg/kg PO produced average plasma concentrations of 2000 and 40 nM at 1 and 24 h after dosing, respectively. The pharmacokinetic parameters for the IV and PO studies are shown in Table S10.
3.5.2. Rabbit ECAT model
Figure 3A shows the effects of vehicle and milvexian on carotid blood flow after thrombus induction in the prevention ECAT rabbit model. Following electric current stimulation, thrombus formation was induced, blood flow was decreased to zero, and the artery was occluded in about 40–45 min in vehicle‐treated animals. Milvexian was associated with a dose‐dependent increase in duration of the patency of the carotid artery. Figure 3B shows the effects of vehicle and milvexian on integrated blood flow in the prevention ECAT rabbit model. Integrated blood flow averaged 11 ± 2% in vehicle‐treated animals following thrombus induction. By blocking the formation of thrombus, milvexian was associated with a dose‐dependent increase in integrated blood flow. At the highest dose, integrated blood flow was 76 ± 5% of the control level.
FIGURE 3.
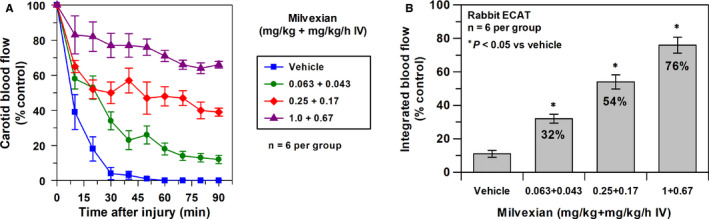
(A) Effects of vehicle and milvexian given IV on carotid blood flow after thrombus induction in ECAT rabbits. Means ± SEM and n = 6 per group. (B) Effects of vehicle and milvexian on integrated blood flow expressed as % control carotid blood flow (i.e., preinjury blood flow) in ECAT rabbits. *p < .05 compared with vehicle. Means ± SEM and n = 6 per group. (C) Relationship between total plasma concentrations of milvexian and antithrombotic effects expressed as % reduction of thrombus weight in prevention ECAT rabbits. ECAT, electrically mediated carotid arterial thrombosis; IV, intravenous; SEM, standard error of the mean
Milvexian was associated with a dose‐dependent reduction in thrombus weight. At the highest dose, thrombus weight was reduced by 70 ± 2% of the control level. Figure 3C shows the concentration‐response curve for milvexian in the prevention ECAT model. Milvexian was associated with a concentration‐dependent antithrombotic effect with a 20% maximal effective concentration of 55 nM (95% confidence interval [CI], 23–128), a EC50 of 375 nM (95% CI, 250–561), and a Hill slope of 0.7 (95% CI, 0.4–1).
Figure 4A shows ex vivo effects of milvexian on APTT, TT, and PT. Milvexian elevated APTT significantly at the highest two doses and did not alter TT and PT significantly, consistent with the mechanism of FXIa inhibition. Figure 4B shows a good correlation (r 2 = 0.83) between the antithrombotic effect of milvexian in the rabbit ECAT and its ex vivo APTT activity. Figure 4B also shows that a 1.6‐fold prolongation of APTT may be needed to achieve 50% thrombus weight reduction in this model.
FIGURE 4.
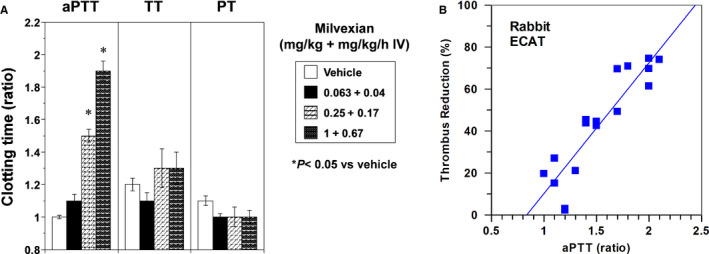
(A) Effects of vehicle and milvexian on APTT, TT, and PT in ECAT rabbits. *p < .05 compared with vehicle. Means ± SEM and n = 6 per group. (B) Tracking antithrombotic activity of milvexian with ex vivo APTT in rabbit ECAT. APTT, activated partial thromboplastin time; ECAT, electrically mediated carotid arterial thrombosis; PT, prothrombin time; TT, thrombin time; SEM, standard error of the mean
Figure 5A shows antithrombotic effects of vehicle and milvexian in the treatment ECAT. After the initiation of thrombosis, blood flow was gradually decreased to similar levels at 15 min among different groups. Administration of milvexian at 15 min improved the patency of the injured artery in a dose‐dependent manner, and blood flow at 90 min averaged 1 ± 0.3, 39 ± 10, and 66 ± 2%* in the groups treated with the vehicle and with milvexian 0.25 + 0.17 and 1 + 0.67 mg/kg ± mg/kg/h, respectively (*p < .05 vs. vehicle; Figure 5A). It also produced a significant dose‐dependent reduction in thrombus weight by 25 ± 7% and 61 ± 6% at doses of 0.25 + 0.17 and 1 + 0.67 (mg/kg +mg/kg/h, respectively). EC50 of milvexian in the treatment ECAT is 1.06 μM (95% CI =0.76–1.47), which was about 2.8‐fold higher than the EC50 obtained in the prevention ECAT (Figure 3).
FIGURE 5.
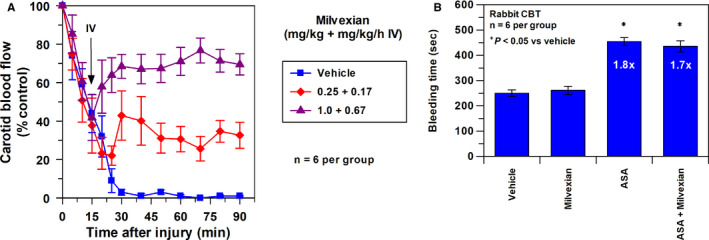
(A) Effects of vehicle and milvexian on carotid blood flow (expressed as % of control carotid blood flow). In the treatment protocol, vehicle or milvexian was given IV (bolus + infusion) at 15 min after the initiation of arterial thrombosis. Means ± SEM and n = 6 per group. (B) Effects of ASA, milvexian, and combination of ASA and milvexian on CBT model in rabbits. ASA (4 mg/kg/h IV), milvexian (1 mg/kg + 0.67 mg/kg/h IV). Means ± SEM and n = 6 per group. ASA, aspirin; CBT, cuticle bleeding time; SEM, standard error of the mean
3.5.3. Rabbit model of cuticle bleeding time
Figure 5B shows the effects of ASA (4 mg/kg/h IV), milvexian (1 mg/kg + 0.67 mg/kg/h IV), and the combination of ASA (4 mg/kg/h IV) and milvexian (1 mg/kg + 0.67 mg/kg/h IV) on BT in the rabbit model of cuticle BT. BT in the vehicle group averaged 250 ± 13 s. At the doses studied, ASA alone increased BT by 1.8‐fold, whereas milvexian did not prolong BT. The combination of ASA and milvexian increased BT by 1.7‐fold, approximately the same BT as ASA alone (Figure 5B).
4. DISCUSSION
This study shows that milvexian is a selective, reversible, orally active FXIa inhibitor. Milvexian demonstrated robust efficacy in both the prevention and treatment of arterial thrombosis in rabbit models. Limited impact on hemostasis was observed even when combined with ASA in rabbits.
Milvexian binds rapidly and reversibly to FXIa and exhibits competitive inhibition versus a peptide substrate. Milvexian inhibits human FXIa with a Ki of 0.11 nM and has >5000‐fold selectivity over FXIa‐related serine proteases, except chymotrypsin and plasma kallikrein where the Ki values are 35 nM and 44 nM, respectively. 11 The in vitro FXIa affinity and selectivity over related serine proteases support evaluation of milvexian as an antithrombotic agent.
The enzyme most closely related to FXI is plasma pre‐kallikrein, a zymogen of the protease α‐kallikrein. 20 Plasma kallikrein participates in the contact activation pathway of coagulation through reciprocal activation of factor XII. Plasma kallikrein releases the vasodilator bradykinin from high molecular weight kininogen. Milvexian was found to have considerable in vitro potency against plasma kallikrein in humans and rabbits. Recent animal studies reported that inhibition of plasma kallikrein activity might contribute to the antithrombic efficacy. 21 , 22 , 23 Reductions in either venous or arterial thrombosis have been observed in plasma kallikrein–deficient mice 21 and in response to pre‐kallikrein knock‐down by either antisense oligonucleotide 22 or by short‐chain interfering RNA. 23
We reasoned that the contribution of plasma kallikrein inhibition to the antithrombic effect of milvexian may be minimal in the rabbit ECAT. We showed that the antithrombic EC50 of milvexian was achieved at 375 nM. With the plasma protein free fraction of approximately 0.05 in rabbits, the free plasma concentration of milvexian relative to rabbit plasma kallikrein Ki is ~1 or less. In comparison, the ratio of free plasma concentration of milvexian to rabbit FXIa Ki is ~50, which is many folds higher than the plasma kallikrein Ki. We therefore hypothesized that the contribution of FXIa inhibition was higher than that of plasma kallikrein inhibition because of the antithrombic effect of milvexian. Further studies are needed to evaluate this hypothesis. The plasma kallikrein disruption as reported in the animal studies 21 , 22 , 23 were associated with low impact on BT. Deficiency of plasma pre‐kallikrein in humans is also not associated with bleeding or manifested in other phenotypes. 20
Milvexian is a high‐affinity inhibitor of FXIa in cynomolgus monkeys, rabbits, and dogs. Milvexian exhibits ≥100‐fold selectivity for FXIa over activated plasma kallikrein in cynomolgus monkeys and rabbits, species used in toxicologic and pharmacologic studies, respectively. Milvexian does not inhibit FXa in rabbits and is a weak inhibitor of thrombin in rabbits and cynomolgus monkeys. Milvexian has affinity for activated plasma kallikrein in rats that is comparable to other species but is a weaker inhibitor of FXIa in rats. Because milvexian has weak affinity for FXIa in rats and mice, it is anticipated to exhibit lower potential to produce FXIa‐mediated pharmacologic or toxicologic effects in rats and mice unless the milvexian exposures are very high.
Milvexian produced a concentration dependent prolongation of the APTT, with potency dependent on the composition of the APTT reagent. The concentration of milvexian in human plasma that produced a 1.5‐fold (50%) increase in APTT relative to baseline ranged from 0.16 µM to 0.43 µM. The concentration of milvexian in human plasma that produced a 2‐fold (100%) increase in APTT relative to baseline ranged from 0.44 µM to 2.1 µM. Extending the contact activation time within the APTT assay resulted in improved sensitivity to plasma concentrations of milvexian as low as 0.03 µM. The PT and TT were minimally increased at milvexian plasma concentrations that produced a doubling of the APTT, indicating high specificity for FXIa inhibition. These in vitro studies show that the effects of milvexian on standard coagulation assays in human plasma are consistent with specific inhibition of FXIa. In the clinical setting, assuming no pharmacologically active metabolites are formed, the ex vivo anticoagulant activity for milvexian is anticipated to correlate with the in vitro anticoagulant activity.
Consistent with its mechanism of action as an FXIa inhibitor, milvexian increased plasma clotting times, which are known to be dependent on FXIa functional activity. The APTT, where initiation of coagulation occurs via the intrinsic (contact activation) pathway, which includes FXIa, was increased in a milvexian concentration–dependent manner. The milvexian concentration, which produced a 2× increase in APTT relative to baseline, ranged from 0.5 μM to 2.4 μM in plasma from cynomolgus monkeys, rabbits, and dogs. In rat plasma, higher concentrations of milvexian were required to increase APTT, but even at 40 μM, the APTT was <2× increased relative to baseline. Milvexian had minimal effects on APTT in rats, likely because of a >1000‐fold reduced affinity for rat FXIa versus cynomolgus monkey, rabbit, and dog FXIa. As shown in experiments with milvexian in human plasma, some differences in the sensitivity of APTT reagents to milvexian were observed.
The PT, where initiation of coagulation occurs via the extrinsic (tissue factor) pathway, was not significantly increased compared with baseline in plasma from rabbits, dogs, and rats, even at a milvexian concentration of 40 μM. In cynomolgus monkey plasma, milvexian at a concentration of 40 µM increased PT approximately 2× relative to baseline. Although milvexian is a selective FXIa inhibitor, it does exhibit weak affinity for cynomolgus monkey thrombin where the Ki is 670 nM; at milvexian plasma concentrations of 10 μM or greater, it is plausible that thrombin inhibition leads to an increase in the PT. To substantiate this hypothesis, ecarin clotting time was performed. Ecarin directly activates prothrombin to thrombin, bypassing other coagulation enzymes, to cleave fibrinogen and form a fibrin clot. Milvexian increased the ecarin clotting time in a concentration‐dependent manner that paralleled increases in PT. Therefore, it is likely that increases in PT are due solely to thrombin inhibition at these high milvexian concentrations. These results may be used to aid the interpretation of experiments conducted in cynomolgus monkeys, rabbits, dogs, and rats for purposes of pharmacology or toxicology evaluations.
Milvexian exhibited robust antithrombotic efficacy in the prevention of arterial thrombosis in the rabbit ECAT model. It was also effective in the treatment of existing arterial thrombosis, suggesting a potential clinical utility of FXIa inhibitors for treatment of arterial thrombosis. It is not likely that the antithrombotic activity of milvexian is related to direct antiplatelet effects because the platelet aggregation responses to several platelet agonists were not altered by milvexian in vitro. However, by inhibiting FXIa, milvexian is expected to inhibit thrombin generation in vivo, thus reducing thrombin‐induced platelet activation and producing an indirect antiplatelet effect.
The increase in APTT provided a reasonable biomarker for the antithrombotic activity of milvexian, whereas PT and TT were unaffected, consistent with the mechanism of FXIa inhibition. A 1.6‐fold prolongation of APTT was shown to be needed to achieve 50% thrombus weight reduction in in the rabbit ECAT model.
This study demonstrates that at a full antithrombotic dose of milvexian there were no changes in BT in the rabbit cuticle BT model. In contrast, at equivalent antithrombotic doses, anticoagulants such as warfarin and antiplatelet agents such as clopidogrel and prasugrel produced 4‐ to 6‐fold increases in BT in the same model. 13 , 14 We also reported similar results in the ECAT model in nonhuman primates, showing that a small‐molecule FXIa inhibitor, BMS‐724296, at doses with equivalent antithrombotic efficacy produced no changes in kidney BT, whereas apixaban and dabigatran were associated with moderate to greater increase in kidney BT. 24 Theses result confirms and extends previously published findings, showing robust antithrombotic effects of FXIa inhibitors with a lower risk of bleeding.
ASA is widely used to prevent cardiovascular events in patients with established coronary artery disease. In some clinical states, milvexian may be combined with ASA; therefore, the BT effects of the combination of ASA and milvexian were evaluated in rabbits. This study showed that ASA alone resulted in a 1.8‐fold increase in BT. When ASA was combined with the highest antithrombotic dose of milvexian, the increase in BT was only 1.7‐fold and similar to ASA alone. Together, these results suggest that milvexian has a wide therapeutic index in rabbits. People with FXI deficiency are cautioned to avoid ASA use because of a perceived increased risk of bleeding. One study in patients with FXI deficiency concluded that antithrombotic therapy, including ASA, was not associated with an increased risk of major bleeding. 25 Clinical studies are needed to explore the therapeutic index of milvexian when combined with ASA.
In summary, this study showed that milvexian prevented arterial thrombosis, demonstrated antithrombotic efficacy without an increase in BT, and was efficacious in the treatment of existing arterial thrombosis in rabbit models. Taken together, this study supports inhibition of FXIa with milvexian as a promising antithrombotic therapy with a wide therapeutic index. Milvexian may provide an improved risk/benefit profile compared with current antithrombotic agents by preventing and treating thrombosis while preserving hemostasis. Milvexian is currently in phase 2 clinical trials for the secondary prevention of major cardiovascular events in patients with acute ischemic stroke and for the prevention of total venous thromboembolism events in patients undergoing total knee replacement surgery. 26 , 27 Antisense oligonucleotides targeting FXI synthesis and antibodies targeting either FXI or FXIa are in clinical development. These agents offer exquisite selectivity for their target but the parenteral route of administration and long duration of action may impact their clinical utility. Orally administered FXIa inhibitors may be more convenient for patients and offer a rapid onset and offset of action as compared to an ASO or an antibody.
CONFLICT OF INTEREST
Dr. Wong, Dr. Dilger, Dr. Wexler and Dr. Ewing are former employees, and Mr. Crain, Mr. Bozarth, Ms. Wu, Dr. Gordon and Mr. Luettgen are employees and stockholders of Bristol Myers Squibb.
AUTHOR CONTRIBUTION
Pancras C. Wong designed and analyzed the in vivo data, supervised Earl J. Crain to perform the platelet aggregation study, as well as the efficacy and bleeding time studies of milvexian in rabbits, and wrote the manuscript. Andrew K. Dilger and William R. Ewing contributed to the FXIa discovery chemistry. Joseph M. Luettgen designed and analyzed the in vitro data, supervised Jeffrey M. Bozarth and Yiming Wu to perform in vitro studies, and wrote the manuscript. David A. Gordon and Ruth R. Wexler provided program guidance and support and critically reviewed the manuscript. All authors read and approved the final version of the manuscript to be submitted to the Journal of Thrombosis and Haemostasis.
Supporting information
App S1
ACKNOWLEDGMENTS
The authors thank Dr. William Schumacher for his pioneer effort, collaboration, and helpful discussion during the course of the FXIa drug discovery program and would like to dedicate this paper to Dr. Schumacher, who passed away in early 2019. The authors also thank Mojgan Abousleiman and Chi Sum for performing in vitro studies. Editorial support was provided by Alana Simorellis, PhD, of Cello Health Communications/MedErgy, supported by Bristol Myers Squibb and Janssen.
Wong PC, Crain EJ, Bozarth JM, et al. Milvexian, an orally bioavailable, small‐molecule, reversible, direct inhibitor of factor XIa: In vitro studies and in vivo evaluation in experimental thrombosis in rabbits. J Thromb Haemost. 2022;20:399–408. doi: 10.1111/jth.15588
Manuscript Handled by: Joost Meijers
Final decision: Joost Meijers, 05 November 2021
Footnote: Presented in part at the 2020 International Society on Thrombosis and Haemostasis Congress; July 12–14, 2020. Abstract number: PB0121.
Funding information
This work was supported by Bristol Myers Squibb.
REFERENCES
- 1. Emsley J, McEwan PA, Gailani D. Structure and function of factor XI. Blood. 2010;115:2569‐2577. 10.1182/blood-2009-09-199182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matafonov A, Sarilla S, Sun MF, et al. Activation of factor XI by products of prothrombin activation. Blood. 2011;118:437‐445. 10.1182/blood-2010-10-312983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seligsohn U. Factor XI deficiency in humans. J Thromb Haemost. 2009;7(Suppl 1):84‐87. 10.1111/j.1538-7836.2009.03395.x [DOI] [PubMed] [Google Scholar]
- 4. Buller HR, Bethune C, Bhanot S, et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372:232‐240. 10.1056/NEJMoa1405760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schumacher WA, Luettgen JM, Quan ML, Seiffert DA. Inhibition of factor XIa as a new approach to anticoagulation. Arterioscler Thromb Vasc Biol. 2010;30:388‐392. 10.1161/ATVBAHA.109.197178 [DOI] [PubMed] [Google Scholar]
- 6. Gailani D, Gruber A. Factor XI as a therapeutic target. Arterioscler Thromb Vasc Biol. 2016;36:1316‐1322. 10.1161/ATVBAHA.116.306925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weitz JI, Fredenburgh JC. 2017 scientific sessions sol sherry distinguished lecture in thrombosis: factor XI as a target for new anticoagulants. Arterioscler Thromb Vasc Biol. 2018;38:304‐310. 10.1161/ATVBAHA.117.309664 [DOI] [PubMed] [Google Scholar]
- 8. Wong PC, Quan ML, Watson CA, et al. In vitro, antithrombotic and bleeding time studies of BMS‐654457, a small‐molecule, reversible and direct inhibitor of factor XIa. J Thromb Thrombolysis. 2015;40:416‐423. 10.1007/s11239-015-1258-7 [DOI] [PubMed] [Google Scholar]
- 9. Quan ML, Pinto DJP, Smallheer JM, et al. Factor XIa inhibitors as new anticoagulants. J Med Chem. 2018;61:7425‐7447. 10.1021/acs.jmedchem.8b00173 [DOI] [PubMed] [Google Scholar]
- 10. Weitz JI, Bauersachs R, Becker B, et al. Effect of osocimab in preventing venous thromboembolism among patients undergoing knee arthroplasty: the FOXTROT randomized clinical trial. JAMA. 2020;323:130‐139. 10.1001/jama.2019.20687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dilger AK, Pabbisetty KB, Corte JR, et al. Discovery of milvexian, a high‐affinity, orally bioavailable inhibitor of factor XIa in clinical studies for antithrombotic therapy. J Med Chem. 2021. 10.1021/acs.jmedchem.1c00613 [DOI] [PubMed] [Google Scholar]
- 12. Perera V, Wang Z, Luettgen J, et al. First‐in‐human study of milvexian, an oral, direct, small molecule factor XIa inhibitor. Clin Transl Sci. 2021. 10.1111/cts.13148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong PC, Crain EJ, Xin B, et al. Apixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, antithrombotic and antihemostatic studies. J Thromb Haemost. 2008;6:820‐829. 10.1111/j.1538-7836.2008.02939.x [DOI] [PubMed] [Google Scholar]
- 14. Wong PC, Crain EJ, Watson CA, Hua J, Schumacher WA, Rehfuss R. Clopidogrel versus prasugrel in rabbits. Effects on thrombosis, haemostasis, platelet function and response variability. Thromb Haemost. 2009;101:108‐115. [PubMed] [Google Scholar]
- 15. Wong PC, Luettgen JM, Rendina AR, et al. BMS‐593214, an active site‐directed factor VIIa inhibitor: enzyme kinetics, antithrombotic and antihaemostatic studies. Thromb Haemost. 2010;104:261‐269. 10.1160/TH10-01-0025 [DOI] [PubMed] [Google Scholar]
- 16. Wong PC, Crain EJ, Knabb RM, et al. Nonpeptide factor Xa inhibitors: II. Antithrombotic evaluation in a rabbit model of electrically induced carotid artery thrombosis. J Pharmacol Exp Ther. 2000;295:212‐218. [PubMed] [Google Scholar]
- 17. Wong PC, Crain EJ, Watson CA, Schumacher WA. A small‐molecule factor XIa inhibitor produces antithrombotic efficacy with minimal bleeding time prolongation in rabbits. J Thromb Thrombolysis. 2011;32:129‐137. 10.1007/s11239-011-0599-0 [DOI] [PubMed] [Google Scholar]
- 18. Zhang D, He K, Raghavan N, et al. Metabolism, pharmacokinetics and pharmacodynamics of the factor Xa inhibitor apixaban in rabbits. J Thromb Thrombolysis. 2010;29:70‐80. 10.1007/s11239-009-0401-8 [DOI] [PubMed] [Google Scholar]
- 19. Wong PC, Crain EJ, Watson CA, et al. Platelet aggregometry and receptor binding to predict the magnitude of antithrombotic and bleeding time effects of clopidogrel in rabbits. J Cardiovasc Pharmacol. 2007;49:316‐324. 10.1097/FJC.0b013e31803e8772 [DOI] [PubMed] [Google Scholar]
- 20. Schmaier AH, McCrae KR. The plasma kallikrein‐kinin system: its evolution from contact activation. J Thromb Haemost. 2007;5:2323‐2329. 10.1111/j.1538-7836.2007.02770.x [DOI] [PubMed] [Google Scholar]
- 21. Bird JE, Smith PL, Wang X, et al. Effects of plasma kallikrein deficiency on haemostasis and thrombosis in mice: murine ortholog of the Fletcher trait. Thromb Haemost. 2012;107:1141‐1150. 10.1160/TH-11-10-0682 [DOI] [PubMed] [Google Scholar]
- 22. Revenko AS, Gao D, Crosby JR, et al. Selective depletion of plasma prekallikrein or coagulation factor XII inhibits thrombosis in mice without increased risk of bleeding. Blood. 2011;118:5302‐5311. 10.1182/blood-2011-05-355248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen Z, Luo B, Cai TQ, et al. Proof‐of‐concept studies for siRNA‐mediated gene silencing for coagulation factors in rat and rabbit. Mol Ther Nucleic Acids. 2015;4:e224. 10.1038/mtna.2014.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wong PC, Quan ML. Improved efficacy/safety profile of factor XIa inhibitor BMS‐724296 versus factor Xa inhibitor apixaban and thrombin inhibitor dabigatran in cynomolgus monkeys. Res Pract Thromb Haemost. 2021;5:e12524. 10.1002/rth2.12524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chapin J, Klute K, Christos P, DeSancho M. The safety of chronic antithrombotic therapy in patients with factor XI deficiency. Br J Haematol. 2017;179:506‐508. 10.1111/bjh.14227 [DOI] [PubMed] [Google Scholar]
- 26. ClinicalTrials.gov . A study on BMS‐986177 for the prevention of stroke in patients receiving aspirin and clopidogrel (AXIOMATIC‐SSP).
- 27. ClinicalTrials.gov . NCT03891524. A study of JNJ‐70033093 (BMS‐986177) versus subcutaneous enoxaparin in participants undergoing elective total knee replacement surgery (AXIOMATIC‐TKR).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
App S1


