Abstract
Aims
To compare anthropometrics, and lipid and glucose metabolism in the 9‐year‐old offspring of mothers treated with metformin or insulin for gestational diabetes mellitus (GDM).
Materials and Methods
This was a Finnish two‐centre, 9‐year follow‐up study of two open‐label, randomized controlled trials comparing the effects observed in the offspring of mothers who received metformin and insulin treatment for GDM. Measurements included anthropometrics, blood pressure, lipoproteins, and oral glucose tolerance tests. This study was registered with ClinicalTrials.gov, number NCT02417090.
Results
At the age of 9 years 172 children (55% of the original study cohort, 82 from the metformin and 90 from the insulin group) participated in the study. No differences were found between the 9‐year‐old offspring groups in anthropometric variables, including body mass index and waist‐to‐height ratio. The offspring in the metformin group had higher high‐density lipoprotein (HDL) cholesterol concentrations (1.72 vs. 1.54 mmol/L; P = 0.039) but lower low‐density lipoprotein cholesterol (2.39 vs. 2.58 mmol/L; P = 0.046) and apolipoprotein B concentrations (0.63 vs. 0.67 g/L; P = 0.043) than the offspring in the insulin group. The difference in HDL cholesterol concentration was found to be significant only in boys (P = 0.003). The 2‐hour glucose value in the oral glucose tolerance test was 0.6‐mmol/L lower in boys from the metformin group than in those from the insulin group (P = 0.015).
Conclusions
Metformin treatment for GDM is associated with similar offspring growth and glucose metabolism but a more favourable lipid profile at the age of 9 years as compared to insulin treatment.
Keywords: anthropometry, gestational diabetes mellitus, glucose metabolism, lipid metabolism, metformin, offspring
1. INTRODUCTION
Gestational diabetes mellitus (GDM) is defined as a condition in which hyperglycaemia develops during pregnancy. 1 The prevalence of GDM has been increasing globally along with obesity, 1 and the overall prevalence of GDM is estimated at 12.8% according to the International Diabetes Federation. 2 Generally, GDM increases the risk of an adverse outcome during pregnancy and delivery, 3 as well as the risks of later development of type 2 diabetes in mothers and of long‐term metabolic sequelae in the offspring. 1 , 4 , 5
Adequate treatment of GDM reduces maternal and foetal adverse effects. 6 First‐line treatment includes combined nutritional therapy and exercise, and pharmacological treatment is added if optimal glucose levels are not obtained through lifestyle interventions. 1 , 7 Although insulin continues to be the first‐line therapy recommended by the American Diabetes Association, 7 metformin is increasingly used instead of insulin. The American College of Obstetricians and Gynecologists and the National Institute for Health and Care Excellence in the United Kingdom consider either insulin or metformin as a first‐line pharmacological choice. 1 , 8
Metformin has been documented to cross the placenta, with foetal levels similar to maternal concentrations. 9 , 10 However, no risk of teratogenicity has been observed when metformin is used from the first trimester of pregnancy onwards in humans or in animal models. 11 Two recent meta‐analyses 6 , 12 have indicated that the risks of adverse pregnancy outcomes, such as macrosomia, admission to a neonatal intensive care unit, neonatal hypoglycaemia and preeclampsia, were actually lower in the metformin group than in the group treated with insulin for GDM.
Long‐term data on the growth, anthropometrics, blood pressure (BP) and metabolism of the offspring of metformin‐treated patients with GDM are limited and the prepubertal metabolic effects are still poorly known and controversial. 13 , 14 One meta‐analysis 22 suggested that the offspring of metformin‐treated mothers have a lower birth weight but are heavier during infancy and mid‐childhood than the offspring of mothers who have been treated with insulin. However, in a recently published population‐based cohort study of patients with GDM who were treated with metformin or insulin, no differences regarding childhood growth were found. 23
To obtain evidence regarding the possible late effects of foetal exposure to metformin, therefore, we studied the growth and metabolism of the offspring, at 9 years of age, of mothers with GDM. Follow‐up data on mothers with GDM and their children were obtained from two Finnish randomized controlled trials. 16 , 17 The aim of the study was to compare the anthropometric variables as well as the glucose and lipid metabolism of the offspring of women with GDM who were treated with metformin or insulin.
2. MATERIALS AND METHODS
2.1. Study population
This was a longitudinal follow‐up study of two previously published Finnish randomized controlled trials with similar study designs16, 17 comparing metformin and insulin treatment for GDM. These two originally separate trials were combined, in a follow‐up setting, to obtain a larger study population (Figure 1). In the two original trials, a total of 321 women (221 women at Turku University Hospital and 100 women at Oulu University Hospital) with GDM were randomly assigned to receive either metformin (n = 161) or insulin (n = 160) between August 6, 2005 and October 14, 2010. Of these mothers, 314 completed the original trials. 16 , 17 Out of these participants altogether, 311 offspring were eligible for this follow‐up study and their mothers were contacted and invited for a study visit with their 9‐year‐old children between 2015 and 2019.
FIGURE 1.
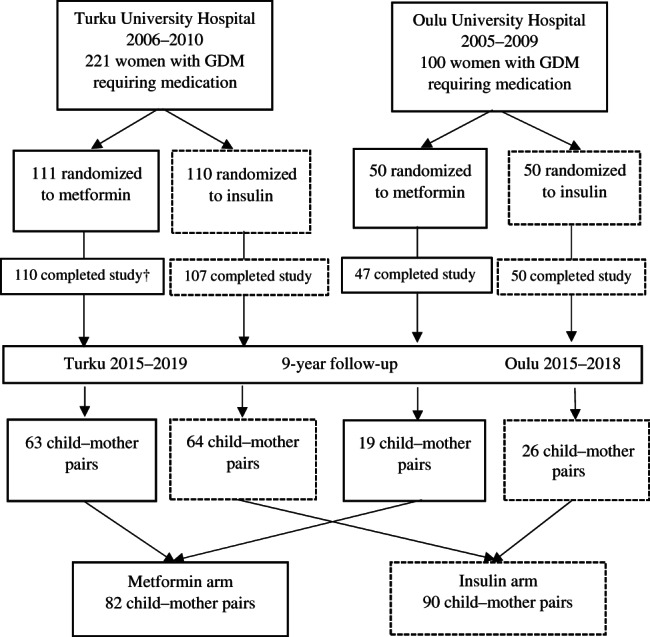
Participants of the two original randomized controlled trials and those of the 9‐year follow‐up study. † From 110 participants, who completed the original study in the metformin group in Turku, three offspring were excluded: one child had valproate syndrome, one child had Down syndrome and one child was stillborn. GDM, gestational diabetes mellitus
The age of 9 years, just before the onset of puberty, was regarded as the most appropriate age to compare the rates of growth and metabolism between the offspring of the two groups. The study visits were conducted at two sites: Turku University Hospital in Southwest Finland and Oulu University Hospital in Northern Finland. Written informed consent was obtained from each mother, child and father. The assessors were blinded to the treatment allocation of the mothers. This 9‐year follow‐up study was registered with the Clinical Trials Registry (NCT02417090) and approved by the Ethics Committee of the Hospital District of Southwest Finland (ETMK 31/2015, April 27, 2015).
2.2. Clinical examinations
Clinical examinations (BP, height, weight and waist circumference) were performed at both sites using well‐established methods, which are described in the Appendix. The Tanner stage (G1‐5, M1‐5, P1‐5) of pubertal development was assessed by a trained nurse or the study physician. Height was expressed as a standard deviation (SD) score that indicates how many SD units a child's height is above or below the average height value according to age and sex. 24 Body mass index (BMI) was calculated. To express the proportion of normal‐weight, overweight and obese children, we used the international cut‐off points for BMI for overweight and obesity by sex and age used by Cole et al. 25 At the age of 9 years, the cut‐off point of BMI for overweight was 19.10 kg/m2 for boys and 19.07 kg/m2 for girls, whereas the cut‐off point of BMI for obesity was 22.77 kg/m2 for boys and 22.81 kg/m2 for girls. The waist‐to‐height ratio (WHtR) was calculated and a cut‐off limit of 0.5 was used to describe the risk for mid‐body obesity. 26 The neonatal ponderal index was calculated as birth weight (g) × 100/crown‐heel length (cm)3. All maternal and paternal baseline demographic and lifestyle data as well as paternal height and weight values were collected using questionnaires.
2.3. Laboratory analysis
Venous blood was collected after an overnight fast. A 2‐hour oral glucose tolerance test (OGTT) with insulin and C‐peptide determinations was performed on both the children and their mothers. The oral glucose load was 75 g, except for children weighing less than 43 kg, for whom the glucose load was 1.75 g/kg. One child was found to have type 1 diabetes and was excluded from the laboratory analyses. Four children refused blood tests. The OGTT was discontinued for three children, because of difficulties in drinking the total amount of glucose in two children and vomiting in one child. For two children, the OGTT samples were lost. The laboratory tests (lipids, lipoproteins, glucose, insulin, C‐peptide and glycated haemoglobin [HbA1c]) performed are described in the Appendix (p 1). Insulin resistance was calculated using homeostatic model assessment (HOMA) based on fasting serum glucose and plasma insulin (HOMA‐IR = glucose [mmol/L] × insulin [mU/L]/22.5). 27 Maternal type 2 diabetes was defined as diagnosed previously or at the time of the 9‐year study by OGTT (fasting plasma glucose ≥7.0 mmol/L and/or 2‐hour glucose ≥11.0 mmol/L).
2.4. Statistical methods
Statistical power calculations were performed for two endpoints: offspring BMI and fasting serum glucose level at the age of 9 years. The mean (SD) BMI was assumed to be 18 (2.5) kg/m2 and the mean (SD) fasting glucose level was assumed to be 4.8 (0.2) mmol/L, based on average childhood values. A two‐sided test with a power of 80% and a significance level of 0.05 would, thus, detect a 1.0‐kg/m2 mean difference in BMI between the 110 metformin and 110 insulin group subjects (71% of the combined cohort). Similarly, a two‐sided test with a power of 80% and a significance level of 0.05 would detect a 0.12‐mmol/L difference in the fasting serum glucose level.
The Kolmogorov‐Smirnov test was used to analyse whether the variables were normally distributed and the Shapiro‐Wilk test was used to test the normality of the subgroups of boys and girls (n < 50). Between‐group comparisons were performed using Student's t‐test for normally distributed data and the Mann‐Whitney U‐test for skewed data. Most variables were not normally distributed in the subgroups. Fisher's exact test was used for categorical variables and the results are expressed as mean ± SD or median (interquartile range [IQR]) unless otherwise stated.
Potential differences in boys and girls between the treatment groups were explored using subgroup analysis. The IBM SPSS Statistics version 25.0 (IBM Corp., Armonk, NY, USA) statistical software package was used and a P value of <0.05 was taken to indicate statistical significance.
3. RESULTS
A total of 172 children participated in this follow‐up study, comprising 55% of all the eligible children (n = 311) from the two original trials 16 , 17 (Figure 1). The population was almost entirely of White ethnicity (99%). The participation rate was 59% (127/214) in Turku University Hospital and 46% (45/97) in Oulu University Hospital. In total 82 (48%) of the participating children were born to mothers with GDM who had been treated with metformin, and 90 (52%) were born to mothers treated with insulin. Furthermore, in the metformin group, 27% of the mothers (22/82) received additional insulin (17 in Turku University Hospital and five in Oulu University Hospital). In all analyses, children born to mothers originally randomized to receive metformin as initial drug therapy were handled as one group, including also those whose mothers needed additional insulin. The study was not powered to detect possible small differences between the offspring of mothers who had only metformin treatment (n = 60) and the offspring of mothers who were treated with metformin and needed additional insulin (n = 22). The anthropometry values, BP, serum lipids and markers of glucose metabolism of these two groups are presented in Table S1 (Appendix p 2).
Maternal baseline characteristics, such as prepregnancy BMI, glycaemic status, smoking habits, distribution of metformin and insulin treatment, duration of medication and gestational weeks at delivery, were similar between the participants and nonparticipants of the study (Table S2, Appendix p 3). Neonatal measures, such as birth weight, crown‐heel length, ponderal index and sex distribution, did not differ significantly between these two groups. Maternal baseline characteristics, pregnancy outcomes and neonatal measures were also found to be similar in the metformin‐ and insulin‐treated women who participated in the study (Table S3, Appendix p 4). Parental characteristics (ie, BMI, proportions of overweight and obese individuals, self‐reported smoking, educational level and perceived health status) were similar in the two treatment groups at the 9‐year follow‐up (Table S4, Appendix p 5). Only 15% of the mothers and 25% of the fathers had a normal BMI. The 9‐year overall prevalence of type 2 diabetes was 14% (24/172) in the mothers, 13% (11/82) in the metformin group and 14% (13/90) in the insulin group.
All children were prepubertal. There were no significant differences between the metformin and insulin groups in the offspring's weight, height, BMI, proportions of overweight or obese children, waist circumference, WHtR, distribution of WHtR over 0.5 or systolic or diastolic BP at the 9‐year assessment (Table 1). A slight tendency towards lower WHtR values (0.43 vs. 0.45; P = 0.06) was found in the boys of the metformin group compared to those of the insulin group.
TABLE 1.
Anthropometric measures and blood pressure at the age of 9 years in the offspring of mothers with gestational diabetes mellitus treated with metformin or insulin
| All children | Boys | Girls | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Metformin | Insulin | P value | Metformin | Insulin | P value | Metformin | Insulin | P value | |
| n = 82 | n = 90 | n = 42 | n = 44 | n = 40 | n = 46 | ||||
| Age, years | 9.0 (9.0‐9.1) | 9.0 (9.0‐9.1) | 0.50† | 9.0 (9.0‐9.1) | 9.0 (9.0‐9.1) | 0.27† | 9.0 (9.0‐9.1) | 9.1 (9.0‐9.1) | 0.87† |
| Height, cm | 136.7 ± 6.01 | 136.4 ± 6.66 | 0.74 | 137.5 ± 6.12 | 137.9 ± 7.43 | 0.80 | 135.9 ± 5.86 | 135.0 ± 5.56 | 0.46 |
| Relative height, SD | 0.11 ± 1.06 | 0.09 ± 1.12 | 0.90 | 0.14 ± 1.11 | 0.26 ± 1.31 | 0.66 | 0.09 ± 1.01 | ‐0.06 ± 0.90 | 0.47 |
| Weight, kg | 32.6 (29.5‐37.9) | 32.8 (28.6‐40.5) | 0.92† | 32.3 (29.0‐39.1) | 33.8 (28.5‐40.6) | 0.62† | 33.5 (29.8‐37.0) | 32.0 (28.6‐39.5) | 0.49† |
| BMI, kg/m2 | 17.5 (16.3‐19.4) | 17.7 (16.1‐20.1) | 0.78† | 17.3 (16.0‐18.8) | 17.9 (16.3‐20.8) | 0.46† | 17.8 (16.9‐19.6) | 17.6 (16.0‐20.0) | 0.79† |
| Normal weight§ (adjusted BMI < 25.0 kg/m2) | 60 (73.2) | 60 (66.7) | 0.35‡ | 32 (76.2) | 31 (70.5) | 0.55‡ | 28 (70.0) | 29 (63.0) | 0.50‡ |
| Overweight or obese§ (adjusted BMI ≥ 25.0 kg/m2) | 22 (26.8) | 30 (33.3) | 10 (23.8) | 13 (29.5) | 12 (30.0) | 17 (37.0) | |||
| Waist circumference, cm | 59.3 (56.2‐64.5) | 59.7 (55.9‐66.9) | 0.55† | 59.3 (56.1‐64.1) | 61.7 (58.2‐69.9) | 0.17† | 59.4 (56.2‐64.8) | 58.2 (54.8‐65.5) | 0.69† |
| Waist: height ratio | 0.43 (0.4‐0.5) | 0.44 (0.4‐0.5) | 0.20† | 0.43 (0.4‐0.5) | 0.45 (0.4‐0.50) | 0.06† | 0.44 (0.4‐0.5) | 0.44 (0.4‐0.5) | 0.92† |
| Waist: height ratio < 0.5 | 69 (84.1) | 76 (84.4) | 0.96‡ | 36 (85.7) | 36 (81.8) | 0.63‡ | 33 (82.5) | 40 (87.0) | 0.57‡ |
| Waist: height ratio > 0.5 | 13 (15.9) | 14 (15.6) | 6 (14.3) | 8 (18.2) | 7 (17.5) | 6 (13.0) | |||
| Systolic BP, mmHg | 106.0 ± 9.38 | 104.8 ± 9.84 | 0.43 | 106.5 ± 9.22 | 107.2 ± 9.97 | 0.76 | 105.3 ± 9.62 | 102.5 ± 9.24 | 0.42† |
| Diastolic BP, mmHg | 59.4 ± 7.35 | 60.2 ± 7.57 | 0.45 | 61.9 ± 7.62 | 60.1 ± 7.53 | 0.23† | 58.7 ± 7.10 | 58.7 ± 7.36 | 1.00 |
Note: Data are expressed as mean ± standard deviation, median (interquartile range) or n (%). The t‐test was used unless stated otherwise (†Mann‐Whitney U‐test, chi‐squared or ‡Fisher's exact test). §Age‐ and sex‐specific adjusted BMI cut‐off points are used according to Cole et al. 25
Abbreviations: BP, blood pressure; BMI, body mass index.
The offspring of the metformin group were found to have a more favourable lipid profile than the offspring of the insulin group (Table 2). That is, their high‐density lipoprotein (HDL) cholesterol concentration was higher (1.72 vs. 1.54 mmol/L; P = 0.039), whereas their low‐density lipoprotein (LDL) cholesterol (2.39 vs 2.58 mmol/L; P = 0.046) and apolipoprotein B (0.63 vs 0.67 g/L; P = 0.043) concentrations were lower than those of the children in the insulin group. In a detailed analysis, the difference in the HDL cholesterol concentration was evident in the boys (1.85 vs 1.54 mmol/L; P = 0.003 [Table 2, Figure 2]), but not in the girls (Table 2, Figure 2). The median (IQR) serum triglyceride concentration of the boys was 0.52 (0.4‐0.7) mmol/L in the metformin group and 0.63 (0.5‐0.8) mmol/L in the insulin group (P = 0.059). The glucose metabolism values (fasting glucose, fasting insulin, fasting C‐peptide, HbA1c, 0.5‐hour and 2‐hour glucose, insulin and C‐peptide in OGTT) were similar in the two treatment groups. The glucose values in the OGTT were within the normal reference range in both sexes, but the 2‐hour glucose value was 0.6 mmol/L lower (P = 0.015) in the boys of the metformin group (5.3 mmol/L) than in the boys of the insulin group (5.9 mmol/L [Table 2]).
TABLE 2.
Lipid, glucose and insulin values at the age of 9 years in the offspring of mothers with gestational diabetes mellitus treated with metformin or insulin
| All children | Boys | Girls | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Metformin | Insulin | P value | Metformin | Insulin | P value | Metformin | Insulin | P value | |
| n = 78 | n = 89 | n = 39 | n = 43 | n = 39 | n = 46 | ||||
| Total cholesterol, mmol/L | 4.45 ± 0.71 | 4.56 ± 0.69 | 0.35 | 4.48 ± 0.67 | 4.55 ± 0.65 | 0.62 | 4.43 ± 0.76 | 4.56 ± 0.72 | 0.34† |
| LDL cholesterol, mmol/L | 2.39 (2.1‐2.8) | 2.58 (2.2‐2.9) | 0.046† | 2.41 (2.1‐2.8) | 2.57 (2.2‐2.9) | 0.21 | 2.38 (2.1‐2.7) | 2.64 (2.3‐2.9) | 0.12† |
| HDL cholesterol, mmol/L | 1.72 (1.5‐1.9) | 1.54 (1.4‐1.8) | 0.039† | 1.85 (1.6‐2.0) | 1.54 (1.5‐1.8) | 0.003† | 1.55 (1.3‐1.8) | 1.55 (1.4‐1.8) | 0.89 |
| Triglycerides, mmol/L | 0.58 (0.4‐0.8) | 0.61 (0.5‐0.7) | 0.24† | 0.52 (0.4‐0.7) | 0.63 (0.5‐0.8) | 0.059† | 0.63 (0.5‐0.8) | 0.61 (0.5‐0.7) | 0.73† |
| HDL/total cholesterol ratio | 0.39 ± 0.09 | 0.36 ± 0.07 | 0.015 | 0.41 ± 0.09 | 0.36 ± 0.07 | 0.002† | 0.36 ± 0.09 | 0.35 ± 0.07 | 0.59 |
| HDL/LDL ratio | 0.73 (0.6‐0.9) | 0.61 (0.5‐0.7) | 0.006† | 0.76 (0.6‐0.9) | 0.59 (0.5‐0.7) | 0.003† | 0.66 (0.5‐0.8) | 0.63 (0.5‐0.7) | 0.32 |
| Non‐HDL cholesterol, mmol/L | 2.66 (2.4‐3.1) | 2.97 (2.5‐3.4) | 0.028† | 2.57 (2.3‐3.1) | 2.96 (2.5‐3.4) | 0.07 | 2.75 (2.5‐3.0) | 3.00 (2.4‐3.3) | 0.21† |
| ApoA1, g/L | 1.46 ± 0.17 | 1.47 ± 0.18 | 0.69 | 1.50 ± 0.15 | 1.50 ± 0.17 | 0.95 | 1.42 ± 0.17 | 1.44 ± 0.18 | 0.59 |
| ApoB, g/L | 0.63 (0.6‐0.7) | 0.67 (0.6‐0.8) | 0.043† | 0.62 (0.5‐0.7) | 0.66 (0.6‐0.8) | 0.19 | 0.64 (0.6‐0.7) | 0.68 (0.6‐0.8) | 0.15† |
| ApoA1/ApoB ratio | 2.39 ± 0.59 | 2.27 ± 0.55 | 0.17 | 2.53 ± 0.58 | 2.37 ± 0.59 | 0.13† | 2.25 ± 0.57 | 2.18 ± 0.50 | 0.52 |
| HbA1c, mmol/mol | 36.2 (34.5‐37.6) | 35.7 (34.7‐37.6) | 0.40† | 36.7 (34.7‐37.6) | 36.7 (34.5‐37.6) | 0.84† | 35.7 (33.8‐37.1) | 35.7 (35.7‐37.6) | 0.32† |
| HbA1c, % | 5.40 (5.2‐5.5) | 5.40 (5.3‐5.5) | 0.41† | 5.40 (5.2‐5.5) | 5.40 (5.2‐5.5) | 0.90† | 5.40 (5.2‐5.5) | 5.40 (5.3‐5.5) | 0.32† |
| HOMA‐IR | 1.71 (1.0‐2.7) | 1.86 (1.1‐3.1) | 0.45† | 1.55 (0.9‐2.5) | 1.73 (1.0‐3.0) | 0.51† | 1.92 (1.2‐2.8) | 2.06 (1.2‐3.2) | 0.70† |
| OGTT result | |||||||||
| Fasting glucose, mmol/L | 5.0 (4.8‐5.0) | 5.1 (4.8‐5.3) | 0.32† | 5.0 (4.8‐5.3) | 5.2 (4.9‐5.4) | 0.13† | 5.0 (4.8‐5.2) | 4.9 (4.8‐5.3) | 0.96 |
| 0.5 ‐h glucose, mmol/L | 8.3 ± 1.44 | 8.5 ± 1.67 | 0.54 | 8.4 ± 1.78 | 8.4 ± 1.57 | 0.92 | 8.2 ± 1.00 | 8.5 ± 1.78 | 0.40 |
| 2‐h glucose, mmol/L | 5.5 ± 1.08 | 5.7 ± 0.98 | 0.23 | 5.3 ± 1.02 | 5.9 ± 1.12 | 0.015† | 5.8 ± 1.09 | 5.6 ± 0.83 | 0.32 |
| Fasting insulin, mU/L | 7.5 (4.8‐12.1) | 8.5 (5.3‐13.6) | 0.52† | 6.6 (4.5‐10.6) | 7.2 (4.4‐13.0) | 0.57† | 9.0 (5.6‐13.0) | 9.5 (5.7‐14.9) | 0.76† |
| 0.5‐h insulin, mU/L | 57.4 (43.0‐84.3) | 61.7 (38.0‐88.4) | 0.93† | 54.2 (43.9‐75.8) | 61.6 (36.6‐79.2) | 0.86† | 57.9 (42.9‐90.5) | 63.2 (47.5‐101.6) | 0.79† |
| 2‐h insulin, mU/L | 29.8 (20.2‐44.3) | 31.8 (20.8‐47.4) | 0.62† | 25.9 (14.4‐36.6) | 29.1 (20.0‐38.3) | 0.16† | 37.9 (28.4‐61.4) | 33.4 (25.9‐52.6) | 0.47† |
| Fasting C‐peptide, ng/mL | 1.17 (1.0‐1.6) | 1.23 (0.9‐1.7) | 0.69† | 1.16 (0.9‐1.4) | 1.23 (0.9‐1.7) | 0.41† | 1.17 (1.0‐1.7) | 1.22 (0.9‐1.7) | 0.85† |
| 0.5‐h C‐peptide, ng/mL | 5.03 (3.9‐7.1) | 5.51 (4.0‐6.7) | 0.93† | 4.95 (3.9‐6.8) | 5.27 (3.7‐6.7) | 0.90 | 5.08 (4.3‐7.3) | 5.58 (4.5‐7.2) | 0.88† |
| 2‐h C‐peptide, ng/mL | 4.44 (3.3‐6.0) | 4.55 (3.3‐6.0) | 0.91† | 4.12 (3.0‐5.1) | 4.49 (3.5‐5.7) | 0.13† | 5.55 (4.0‐6.9) | 4.55 (3.3‐6.3) | 0.20† |
Note: Data are expressed as mean ± standard deviation, median (interquartile range) or n (%). The t‐test was used unless stated otherwise (†Mann‐Whitney U‐test).
Abbreviations: ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; HbA1c, glycated haemoglobin; HDL, high‐density lipoprotein; HOMA‐IR, homeostatic model assessment of insulin resistance; LDL, low‐density lipoprotein; OGTT, oral glucose tolerance test.
FIGURE 2.
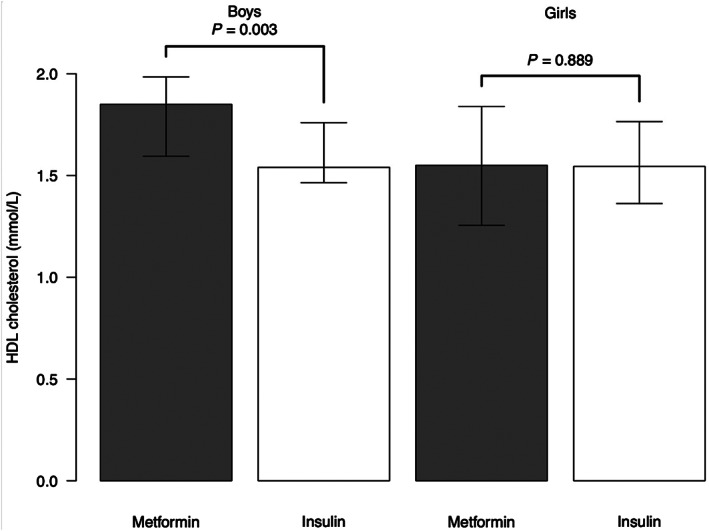
Offspring high‐density lipoprotein (HDL) cholesterol concentrations. Data are expressed as median (interquartile range)
4. DISCUSSION
In the present follow‐up study, which comprised 172 prepubertal 9‐year‐old children, we found no differences in anthropometric measurements, BP values or growth levels between the offspring of mothers who were treated with metformin or insulin for GDM. The boys who were exposed to metformin during pregnancy were found to have a more favourable lipid profile and better 2‐hour serum glucose values compared with the boys of insulin‐treated mothers.
In a meta‐analysis of 17 trials, Tarry‐Adkins et al 22 reported that the birth weight of the offspring of metformin‐treated mothers with GDM was on average 108‐g lower than that of the offspring of insulin‐treated mothers. Moreover, as reported in three studies, the neonatal ponderal index was 0.13 kg/m3 lower in the metformin group than in the insulin group. However, in the present study, both the ponderal index and the birth weight were similar in the two treatment groups. In contrast, several studies examining body weight and BMI in the metformin‐exposed offspring of mothers with GDM during later infancy and mid‐childhood have reported inconsistent results. 18 , 19 , 20 , 21
To our knowledge, only three original randomized controlled trials 15 , 16 , 17 that have compared metformin and insulin treatment for mothers with GDM have also reported longitudinal offspring anthropometric measures, 18 , 19 , 20 , 21 with only one of these reports using the metabolic follow‐up data. 21 Ijäs et al 19 found that the children exposed to metformin prenatally were taller and heavier at the age of 18 months than those whose mothers were treated with insulin. However, their body composition as defined by the ponderal index did not differ between the treatment groups. Furthermore, the mean weight for length and the proportion of overweight and obese children did not differ between the groups at the ages of 6, 12 and 18 months. 19 Tertti et al 20 compared the testicular size of 52 prepubertal boys at the age of 33‐85 months who were born to the mothers who participated in a trial 17 comparing metformin and insulin for GDM. They found no differences between the study groups in the size of the testicles or in height, weight, BMI, BMI z‐score or waist‐to‐hip ratio. Rowan et al 18 found no differences in weight, height or waist circumference between the offspring of the metformin and insulin treatment groups at the ages of 2 and 7 years. 21 However, at the age of 9 years, 21 the offspring (n = 45) of the metformin‐treated mothers were found to be heavier and had a greater waist circumference, WHtR and BMI than the offspring (n = 54) of the insulin‐treated mothers. The authors also reported that the 9‐year‐old study group was ethnically more heterogenous than the total Metformin in Gestational diabetes (MiG) cohort and that this might have influenced these results. In the present study, we observed no significant differences in the anthropometric variables between the two homogenous treatment groups (n = 172) at 9 years of age. Our study results are in line with those of a large population‐based cohort study by Landi et al, 23 who found no differences in the weight, height or BMI, also viewed by z‐scores and percentiles, in 3928 children aged 4 years whose mothers had been treated with metformin or insulin for GDM.
Aceti et al 28 reported that the offspring of mothers with GDM had 1.39‐mmHg higher systolic (P = 0.05) but similar diastolic BP to the offspring of mothers without GDM.
Battin et al 29 studied BP values in 170 offspring from the MiG cohort 15 at the age of 2 years and found no differences between the offspring of the metformin and insulin groups. In the present study, we found no difference in BP between the offspring of the metformin and insulin groups at the age of 9 years.
Rowan et al 21 measured the concentrations of LDL cholesterol, HDL cholesterol and triglycerides in 94 children aged 9 years and found no differences between the groups, even though the children in the metformin group were heavier than those in the insulin group. They also adjusted the results for the offspring's gender without any change in the significance. In the present study, we found that the HDL cholesterol concentration was higher, and the LDL cholesterol and apolipoprotein B concentrations were lower in the metformin group than in the insulin group, suggesting a more advantageous lipid profile in the offspring of the metformin group. However, the significantly higher HDL cholesterol values in the metformin group were found only in the boys.
Rowan et al 21 found no differences either in the fasting glucose levels between the metformin and insulin groups in 7‐year‐old offspring or in fasting glucose, fasting insulin values, HbA1c and homeostatic model assessment of insulin resistance (HOMA‐IR) in 9‐year‐old offspring. In the present study, we found that HOMA‐IR, 2‐hour glucose, insulin, and C‐peptide values during the OGTT tended to be slightly lower in the metformin group than in the insulin group. When compared with the boys of the insulin group, the boys of the metformin group had lower 2‐hour glucose values.
Brawerman et al 30 reported that metformin as a treatment for GDM within the third trimester may influence the offspring not only through interactions affecting foetal growth but also through epigenetic mechanisms that may possibly modify the long‐term metabolic health of the offspring. As discussed earlier, metformin treatment for GDM has been suggested to result in a lower birth weight of the offspring, 22 which may lead to the offspring having a higher weight and BMI during infancy and mid‐childhood. 19 , 21 However, in the present study, we did not find evidence of lower birth weights or higher BMI values at the age of 9 years in the offspring who had been prenatally exposed to metformin.
It should also be noted that the percentage of parental overweight and obesity was similar in the two groups, which may denote a similar lifestyle in the offspring in the two groups. Only 15% of the mothers and 25% of the fathers had a normal weight. Despite this, 70% (120/172) of the offspring had a normal weight at the age of 9 years. Indeed, we observed a slight tendency towards a higher percentage of normal‐weight offspring in the metformin group, particularly among boys.
An interesting question is whether metformin exposure during pregnancy can have a different long‐term influence on lipid and glucose metabolism of the boys and the girls of mothers with GDM, either independently or mediated by the anthropometric variables. Although statistically insignificant, the WHtR as an adiposity‐related variable was more favourable in the boys of the metformin group than in those of the insulin group. Among the girls, however, there was no such tendency. These findings might partly explain why a higher HDL cholesterol level was found only in the boys of the metformin group. In addition to adiposity variables, inherited genetic factors, other postnatal environmental factors and epigenetics might also have been involved in the sex‐associated difference in the serum lipids of the offspring of metformin‐ and insulin‐treated mothers with GDM.
Regarding epigenetics, the non‐coding RNA 866 (nc866) epiallele methylation status might be one possible explanation for our findings of the HDL cholesterol concentration differences between sexes. Marttila et al 31 studied the role of the nc866 epiallele methylation status in metabolic traits in childhood and early adulthood. In general, the methylation status of a nc866 epiallele in the offspring is dependent on the maternal nc866 epiallele methylation and the ambient conditions during gestation. Indeed, they found that boys, but not girls, with a nonmethylated nc866 epiallele had higher (P < 0.05) estimated HDL cholesterol levels during childhood (ages 6‐12 years) and adolescence than those of boys with a hemimethylated nc866 epiallele. Thus, differences in epigenetic regulation might be a possible mechanism behind the observed sex‐associated difference in HDL cholesterol between the offspring of metformin‐ and insulin‐treated mothers.
The major strength of the present follow‐up study is that the 9‐year‐old offspring provided a favourable representation of the original cohort, allowing valid comparisons between the treatment groups. Moreover, the baseline data were similar between the 9‐year study participants and the group of nonparticipants. Among the participating children, both sexes and medication groups were evenly distributed, and all the children were prepubertal. All measurements were performed using strict procedures and all blood samples were stored under similar conditions and analysed at the same time in one laboratory. In addition, the study protocol was similar at the two study sites as well as at the baseline and follow‐up. Currently, this follow‐up cohort of 172 9‐year‐old offspring whose mothers received either metformin or insulin treatment for GDM is the largest published cohort. Power calculations were performed before the study was performed. Notably, the follow‐up rate of 55% obtained for the total cohort was relatively favourable, considering the long period of 9 years between birth and follow‐up, although it was slightly lower than expected in the power calculation. This may have led to some potential differences not being detected between the treatment groups. Lastly, the participants were mainly White, which may affect the applicability of the results to other ethnic groups.
Our results, however, leave open an interesting question as to whether exposure to metformin during pregnancy may influence the offspring sexes differently with regard to lipid and glucose metabolism.
In conclusion, treating GDM with metformin or insulin was found not to lead in differences in the anthropometric values, BP, or glucose metabolism of the offspring at the age of 9 years. Lipid profiles were similar in the two treatment groups in the girls but more favourable in the boys in the metformin group than in those in the insulin group. These results support the hypothesis that using metformin for the treatment of GDM has no negative effects on the growth or glucose and lipid metabolism of the offspring during the prepubertal period.
CONFLICT OF INTEREST
There are no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Tapani Rönnemaa, Kristiina Tertti, Harri Niinikoski and Elisa Paavilainen designed the follow‐up study and Britt‐Marie Loo designed the laboratory analyses. Elisa Paavilainen recruited the participants and organized study visits in Turku University Hospital. Marja Vääräsmäki, Hilkka Nikkinen and Riitta Veijola contributed to the study design and organization regarding Oulu University Hospital. Hilkka Nikkinen recruited the participants and Päivi Tossavainen studied the children in Oulu University Hospital. Elisa Paavilainen studied, interpreted and analysed the data and was responsible for writing the first draft of the manuscript. Elisa Paavilainen and Harri Niinikoski verified the underlying data. All authors contributed to the writing of the manuscript. All authors have seen and approved the final text.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14589.
Supporting information
Appendix S1. Supporting Information
ACKNOWLEDGMENTS
This study was financially supported by the Diabetes Research Foundation (Finland), the Finnish Foundation for Paediatric Research, Special Governmental Grants for Health Sciences (Turku University Hospital), Yrjö Jahnsson Foundation and the Turku University Hospital Research Foundation. The funders of the study had no role in the study design, in the collection, analysis and interpretation of the data, in the writing of the report, or in the decision to submit the paper for publication. We would like to thank Tommi Kauko for the graphics (Figure 2), statistical advice, and support. We would also like to especially thank study nurse Ulla Torkko and study nurse Tiina Latva‐Aho for organizing the study visits and making the visits comfortable for the families. We would also like to acknowledge the expert work of the laboratory technicians Minna Romo and Elina Åkerblom. Finally, thanks are due to all the children and parents who agreed to take part in this study.
Paavilainen E, Tertti K, Nikkinen H, et al. Metformin versus insulin therapy for gestational diabetes: Effects on offspring anthropometrics and metabolism at the age of 9 years: A follow‐up study of two open‐label, randomized controlled trials. Diabetes Obes Metab. 2022;24(3):402‐410. doi: 10.1111/dom.14589
Funding information Diabetestutkimussäätiö; Lastentautien Tutkimussäätiö; Turun Yliopistollisen Keskussairaalan Koulutus‐ ja Tutkimussäätiö; Varsinais‐Suomen Sairaanhoitopiiri; Yrjö Jahnssonin Säätiö
DATA AVAILABILITY STATEMENT
Anonymised patient data will be available upon reasonable request from the publication date of manuscript for the following 24 months. Data will be provided following the review and approval of research proposal (including statistical analysis plan) and completion of data sharing agreement. Proposals should be directed to corresponding author (elisa.paavilainen@tyks.fi). Responses to the request for the raw data will be judged by a committee including HaN, TR, KT and EP.
REFERENCES
- 1. Committee on Practice Bulletins ‐ Obstetrics . ACOG practice bulletin no. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49‐e64. [DOI] [PubMed] [Google Scholar]
- 2. Yuen L, Saeedi P, Riaz M, et al. Projections of relevance of hyperglycaemia in pregnancy in 2019 and beyond: results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107841. [DOI] [PubMed] [Google Scholar]
- 3. Metzger BE, Lowe LP, Dyer AR, et al. HAPO study cooperative research group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991‐2002. [DOI] [PubMed] [Google Scholar]
- 4. Burguet A. Long‐term outcome in children of mothers with gestational diabetes. Diabetes Metab. 2010;36:682‐694. [DOI] [PubMed] [Google Scholar]
- 5. Dabelea D. The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care. 2007;30:S169‐S174. [DOI] [PubMed] [Google Scholar]
- 6. Farrar D, Simmonds M, Bryant M, et al. Treatments for gestational diabetes: a systematic review and meta‐analysis. BMJ Open. 2017;7:e015557. doi: 10.1136/bmjopen-2016-015557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Diabetes Association . 14. Management of diabetes in pregnancy: standards of medical care in diabetes–2019. Diabetes Care. 2019;42:S165‐S172. [DOI] [PubMed] [Google Scholar]
- 8. National Institute for Health and Clinical Excellence (NICE) . Diabetes in pregnancy: management from preconception to the postnatal period. NICE guidance. London 2015. https://www.nice.org.uk/guidance/ng3 (Accessed February 25, 2015). [PubMed]
- 9. Tertti K, Ekblad U, Heikkinen T, Rahi M, Rönnemaa T, Laine K. The role of organic cation transporters (OCTs) in the transfer of metformin in the dually perfused human placenta. Eur J Pharm Sci. 2010;39:76‐81. [DOI] [PubMed] [Google Scholar]
- 10. Vanky E, Zahlsen K, Spigset O, Carlsen SM. Placental passage of metformin in women with polycystic ovary syndrome. Fertil Steril. 2005;83:1575‐1578. [DOI] [PubMed] [Google Scholar]
- 11. Feig DS, Briggs GG, Koren G. Oral antidiabetic agents in pregnancy and lactation: a paradigm shift? Ann Pharmacother. 2007;41:1174‐1180. [DOI] [PubMed] [Google Scholar]
- 12. Guo L, Ma J, Tang J, Hu D, Zhang W, Zhao X. Comparative efficacy and safety of metformin, glyburideand insulin in treating gestational diabetes mellitus: a meta‐analysis. J Diabetes Res. 2019;2019:29. doi: 10.1155/2019/9804708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jorquera G, Echiburú B, Crisosto N, Sotomayor‐Zárate R, Maliqueo M, Cruz G. Metformin during pregnancy: effects on offspring development and metabolic function. Front Pharmacol. 2020;11:653. doi: 10.3389/fphar.2020.00653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Finneran MM, Landon MB. Oral agents for the treatment of gestational diabetes. Curr Diabetes Rep. 2018;18:119. doi: 10.1007/s11892-018-1093-2 [DOI] [PubMed] [Google Scholar]
- 15. Rowan JA, Hague WM, Gao W, Battin MR, Moore MP. MiG trial investigators. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003‐2015. [DOI] [PubMed] [Google Scholar]
- 16. Ijäs H, Vääräsmäki M, Morin‐Papunen L, et al. Metformin should be considered in the treatment of gestational diabetes: a prospective randomised study. BJOG. 2011;118:880‐885. [DOI] [PubMed] [Google Scholar]
- 17. Tertti K, Ekblad U, Koskinen P, Vahlberg T, Rönnemaa T. Metformin vs insulin in gestational diabetes. A randomized study characterizing metformin patients needing additional insulin. Diabetes Obes Metab. 2013;15:246‐251. [DOI] [PubMed] [Google Scholar]
- 18. Rowan JA, Rush EC, Obolonkin V, Battin M, Wouldes T, Hague WM. Metformin in gestational diabetes: the offspring follow‐up (MiG TOFU): body composition at 2 years of age. Diabetes Care. 2011;34:2279‐2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ijäs H, Vääräsmäki M, Saarela T, Keravuo R, Raudaskoski T. A follow‐up of a randomised study of metformin and insulin in gestational diabetes mellitus: growth and development of the children at the age of 18 months. BJOG. 2015;122:994‐1000. [DOI] [PubMed] [Google Scholar]
- 20. Tertti K, Toppari J, Virtanen HE, Sadov S, Rönnemaa T. Metformin treatment does not affect testicular size in offspring born to mothers with gestational diabetes. Rev Diabetes Stud. 2016;13:59‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow‐up (MiG TOFU): body composition and metabolic outcomes at 7–9 years of age. BMJ Open Diab Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tarry‐Adkins JL, Aiken CE, Ozanne SE. Neonatal, infantand childhood growth following metformin versus insulin treatment for gestational diabetes: a systematic review and meta‐analysis. PLoS Med. 2019;16:e1002848. doi: 10.1371/journal.pmed.1002848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Landi SN, Radke S, Engel SM, et al. Association of long‐term child growth and developmental outcomes with metformin vs insulin treatment for gestational diabetes. JAMA Pediatr. 2019;173:160‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sorva R, Perheentupa J, Tolppanen EM. A novel formal for a growth chart. Acta Paediatr Scand. 1984;73:527‐529. [DOI] [PubMed] [Google Scholar]
- 25. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Browning LM, Hsieh SD, Ashwell M. A systematic review of waist‐to‐height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0·5 could be a suitable global boundary value. Nutr Res Rev. 2010;23:247‐269. [DOI] [PubMed] [Google Scholar]
- 27. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487‐1495. [DOI] [PubMed] [Google Scholar]
- 28. Aceti A, Santhakumaran S, Logan KM, et al. The diabetic pregnancy and offspring blood pressure in childhood: a systematic review and meta‐analysis. Diabetologia. 2012;55:3114‐3127. [DOI] [PubMed] [Google Scholar]
- 29. Battin MR, Obolonkin V, Rush E, Hague W, Coat S, Rowan J. Blood pressure measurement at two years in offspring of women randomized to a trial of metformin for GDM: follow up data from the MiG trial. BMC Pediatr. 2015;15:54. doi: 10.1186/s12887-015-0372-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brawerman GM, Dolinsky VW. Therapies for gestational diabetes and their implications for maternal and offspring health: evidence from human and animal studies. Pharmacol Res. 2018;130:52‐73. [DOI] [PubMed] [Google Scholar]
- 31. Marttila S, Viiri LE, Mishra PP, et al. Methylation status of nc886 epiallele reflects periconceptional conditions and is associated with glucose metabolism through nc866 RNAs. Clin Epigenetics. 2021;13:143. doi: 10.1186/s13148-021-01132-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Data Availability Statement
Anonymised patient data will be available upon reasonable request from the publication date of manuscript for the following 24 months. Data will be provided following the review and approval of research proposal (including statistical analysis plan) and completion of data sharing agreement. Proposals should be directed to corresponding author (elisa.paavilainen@tyks.fi). Responses to the request for the raw data will be judged by a committee including HaN, TR, KT and EP.


