Abstract
Biomarkers of neuronal damage in status epilepticus (SE) would be of great relevance for clinical and research purposes. In a retrospective cross‐sectional study, serum neurofilament light chain (NfL) levels were measured in patients with SE (30 subjects), patients with drug‐resistant epilepsy (30 subjects), and healthy controls (30 subjects). Serum NfL levels were higher in patients with SE (median = 26.15 pg/ml) compared to both epilepsy patients (median = 7.35 pg/ml) and healthy controls (median = 6.81 pg/ml; p < .001). In patients with SE, serum NfL levels showed a high correlation with cerebrospinal fluid (CSF) NfL (τ = .68, p < .001) as well as with CSF total tau (t‐tau) levels (τ = .627, p < .001); they were higher in SE lasting >24 h (p = .013), in refractory/superrefractory SE (p = .004), and in patients who died within 30 days or who presented a worsening of clinical conditions (p = .001). Values of >28.8 pg/ml predicted 30‐day clinical worsening or death (odds ratio [OR] = 10.83, 95% confidence interval [CI] = 1.96–59.83, p = .006) and SE refractoriness (OR = 9.33, 95% CI = 1.51–57.65, p = .016). In conclusion, serum NfL levels are increased in SE and correlate with SE treatment response, duration, and outcomes, therefore representing a promising biomarker of seizure‐related neuronal damage.
Keywords: biomarkers, neurofilaments, status epilepticus
1. INTRODUCTION
In 2015, a new operational definition of status epilepticus (SE) was proposed, emphasizing that SE is a time‐dependent neurologic emergency requiring prompt treatment. 1 In approximately 30% of cases, SE is refractory to benzodiazepines and antiseizure medications (ASMs) and may lead to brain injury with cellular and molecular alterations (inflammation, or neuronal and astroglial injury) that could induce subsequent irreversible neurological impairment and further development of epilepsy, with a mortality rate ranging from 7% to 39%. 2 For a better stratification of patients in terms of response to treatment, severity of damage, and possible sequelae, having a diagnostic and prognostic biomarker would be of great support for both clinical practice and research. In SE, although cerebrospinal fluid (CSF) biomarkers have been proposed, none has yet been validated in clinical use to diagnose SE or to predict its clinical outcome. 3 , 4
In the past decade, neurofilaments (Nfs) have been the subject of numerous preclinical and clinical studies, proving to be biomarkers of degeneration or acute damage in several neurological disorders. 5 , 6 Nfs, further distinguished as Nf light (NfL), middle, or heavy based on their relative apparent molecular masses, are particularly expressed in the myelinated axons of neurons, where they have a structural function in the cytoskeleton establishing cross‐bridging with other filaments. Importantly, NfL might be released in significant amounts into blood when neuroaxonal damaged is present, therefore representing a promising and minimally invasive biomarker of neuronal damage. 4 , 5 Notably, although Nfs have been viewed traditionally as structural components primarily of axons and dendrites, besides structural functions, recent evidence has shown that distinctive assemblies of Nf subunits are also integral components of synapses. 5 , 7
To date, no study has investigated whether NfL in peripheral blood may represent a biomarker of neuronal damage due to SE. For this aim, we measured and compared serum NfL (sNfL) levels in adult patients with SE, patients with chronic epilepsy, and healthy controls.
2. MATERIALS AND METHODS
This is a retrospective, monocentric, cross‐sectional study. We analyzed serum and CSF samples of patients and healthy controls collected at the Ospedale Civile Baggiovara Hospital in Modena, Italy, and stored (at −80°C in polypropylene storage tubes) in our biobank starting from October 2018.
2.1. SE group
Patients with SE in whom a blood sample was collected soon after SE diagnosis were included. We excluded all SE patients with conditions known to cause Nf elevation per se, such as acute structural brain lesion (e.g., acute strokes, hemorrhages, postanoxic encephalopathy, autoimmune or infectious encephalitis) and inflammatory/neurodegenerative diseases (e.g., multiple sclerosis, dementias, Parkinson disease), as reported in Figure S1. Specific etiologies retained in our study are detailed in the notes of Table 1. At our center, all consecutive patients with SE have been prospectively collected (since 2013) using a specific SE form to collect demographic and clinical information, including age, gender, semiology, etiology, and dosage of ASMs, anesthetic drugs, and other therapies used. All SE episodes were reviewed by two authors (S.M. and G.G.), and met the International League Against Epilepsy diagnostic criteria. 1 Treatment responsiveness was defined as SE cessation after first‐line therapy with benzodiazepines alone or followed by second‐line treatment with one ASM administered intravenously. Refractory SE (RSE) was defined as a failure of first‐line therapy with benzodiazepines and one second‐line treatment with ASMs. In superrefractory SE (SRSE), SE continued or recurred despite the use of anesthetics for >24 h. The 30‐day functional outcome was assessed using the modified Rankin Scale (mRS), and we defined worsening of the clinical condition as an increase of at least 1 point on the mRS compared to the baseline score before SE developed. We chose an increment of 1 point as compared to baseline and not the absolute Rankin score as it allows better defining the worsening of clinical conditions (e.g., a patient with mRS of 3 at baseline who had 3 at 30 days from SE onset is defined as recovered, thus with a good outcome, independently from the absolute mRS value).
TABLE 1.
Clinical and demographic features and biomarkers in the different groups
| Feature/biomarker |
SE, n = 30 |
Epilepsy, n = 30 |
Healthy controls, n = 30 |
Significance |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Male | 16 (54%) | 17 (57%) | 10 (30%) | p = .147 |
| Female | 14 (46%) | 13 (43%) | 20 (60%) | |
| Age, years | ||||
| Mean (±SD) | 45 (±19.9) | 39 (±13.6) | 40 (±14.7) | p = .817 |
| Range | 11–79 | 20–65 | 17–75 | |
| sNfL, pg/ml | ||||
| Mean | 101.14 | 8.54 | 13.14 | |
| Median | 26.15 | 7.35 | 6.81 | p < .001 a |
| IQR | 97.07 | 6.41 | 9.29 | |
| CSF NfL, pg/ml, n = 17 | ||||
| Mean | 2410 | – | – | |
| Median | 752 | – | – | |
| IQR | 4392 | – | – | |
| t‐tau, pg/ml | ||||
| Mean | 21 997 | – | – | |
| Median | 512 | – | – | |
| IQR | 2217 | – | – | |
| Duration of SE, n (%) | ||||
| ≤24 h | 18 (60%) | – | – | |
| >24 h | 12 (40%) | – | – | |
| Time between SE onset and sample, n (%) | ||||
| ≤24 h | 14 (47%) | – | – | |
| >24 h | 16 (53%) | – | – | |
| Etiology classification, n (%) a | ||||
| Acute symptomatic | 10 (34%) | – | – | |
| Remote symptomatic | 6 (20%) | – | – | |
| Progressive symptomatic | 4 (13%) | – | – | |
| Unknown | 6 (20%) | – | – | |
| In definite epileptic syndrome | 4 (13%) | – | – | |
| Clinical manifestations, n (%) | ||||
| Motor symptoms | 21 (70%) | – | – | |
| GCSE | 7 | – | – | |
| GCSE‐NCSE | 4 | – | – | |
| FCSE | 8 | – | – | |
| FCSE‐NCSE | 1 | – | – | |
| MSE‐NCSE | 1 | – | – | |
| Nonconvulsive symptoms only | 9 (30%) | – | – | |
| Treatment response, n (%) | ||||
| Responsive SE | 20 (66%) | – | – | |
| RSE | 5 (17%) | – | – | |
| SRSE | 5 (17%) | – | – | |
| 30‐day outcome, n (%) | ||||
| Return to baseline conditions | 17 (57%) | – | – | |
| Worsening of clinical conditions or death | 13 (43%) | – | – |
RSE was defined as failure of first‐line therapy with benzodiazepines and one second‐line treatment with antiseizure medications. In SRSE, SE continued or recurred despite the use of anesthetics for >24 h.
Abbreviations: CSF, cerebrospinal fluid; FCSE, focal convulsive SE; GCSE, generalized convulsive SE; IQR, interquartile range; MSE, myoclonic SE; NCSE, nonconvulsive SE; RSE, refractory SE; SE, status epilepticus; sNfL, serum neurofilament light; SRSE, superrefractory SE; t‐tau, total tau.
Statistically significant.
Regarding the specific etiologies, the acute symptomatic group included four with toxic etiology, two with metabolic etiology due to hyponatremia, and four with precipitating factors in epilepsy; the remote symptomatic group included four with vascular etiology and two epileptic patients without an acute precipitating cause; the progressive symptomatic group included four low‐grade tumors; the specific epilepsy syndrome group included three cases of genetic generalized epilepsy and one case of sleep‐related hypermotor epilepsy.
2.2. Epilepsy group
This group consisted of patients with drug‐resistant epilepsy. In these patients, blood samples were collected during hospitalization in the epilepsy monitoring unit, where they presented isolated seizures. Blood sample was collected <24 h from the last recorded seizure in all patients; the median delay was 3 h. Clinical details and etiologies are reported in Table S1.
2.3. Healthy controls
This group consisted of age‐matched healthy volunteers without any neuropsychiatric condition, and a negative family history for neurodegenerative diseases.
The local ethics committee approved the study (protocol. 967/2017 and 556/2018 Azienda Ospedaliera‐Universitaria di Modena, Italy), and informed consent was obtained from all participants included in the study. Anonymized data will be shared upon request by any qualified investigator.
2.4. Procedures
sNfL concentrations and, whenever available, CSF NfL were determined on Simple Plex NfL Assay (ProteinSimple) on an Ella instrument, according to the manufacturers' instructions. Ella was calibrated using the in‐cartridge factory standard curve. The Ella instrument allows rapid and ultrasensitive measurement of biomarkers. This platform allows quantification of an analyte from 72 samples in a single disposable microfluidic cartridge, within 90 min.
2.5. Statistical analysis
Comparison of clinical data and sNfL between groups was performed using parametric or not parametric statistics as appropriate. Correlations analysis (Spearman and Kendall correlation) was applied to study the relationship between sNfL and demographic and clinical characteristics of SE patients as well as levels of t‐tau.
A receiver operating characteristic (ROC) curve was plotted to calculate the cutoff point for sNfL level with the best sensitivity and specificity in predicting clinical outcome and treatment refractoriness (using the maximum value of Youden index).
A univariate analysis was performed to identify factors associated with SE‐related clinical worsening and drug refractoriness. For all the analyses, the p‐value was set at <.05. All analyses were conducted using IBM SPSS Statistics 26.
3. RESULTS
Ninety subjects were included in the study: 30 patients with SE (median age = 39 years), 30 patients with epilepsy (median age = 36 years), and 30 healthy controls (median age = 41 years; one‐way analysis of variance with Bonferroni post hoc test, p = .817). Clinical characteristics of patients with SE are reported in Table 1.
Levels of sNfL in patients with SE showed great variability, from values in the range of the control groups up to 50‐fold. Kruskal–Wallis test with Dunn post hoc test showed that sNfL levels were markedly higher (p < .001) in patients with SE (median = 26.15 pg/ml) compared to both epilepsy patients (median = 7.35 pg/ml) and healthy controls (median = 6.81 pg/ml), whereas no differences were found between sNfL levels in patients with epilepsy and in healthy controls (p = .91; Table 1, Figure 1A).
FIGURE 1.
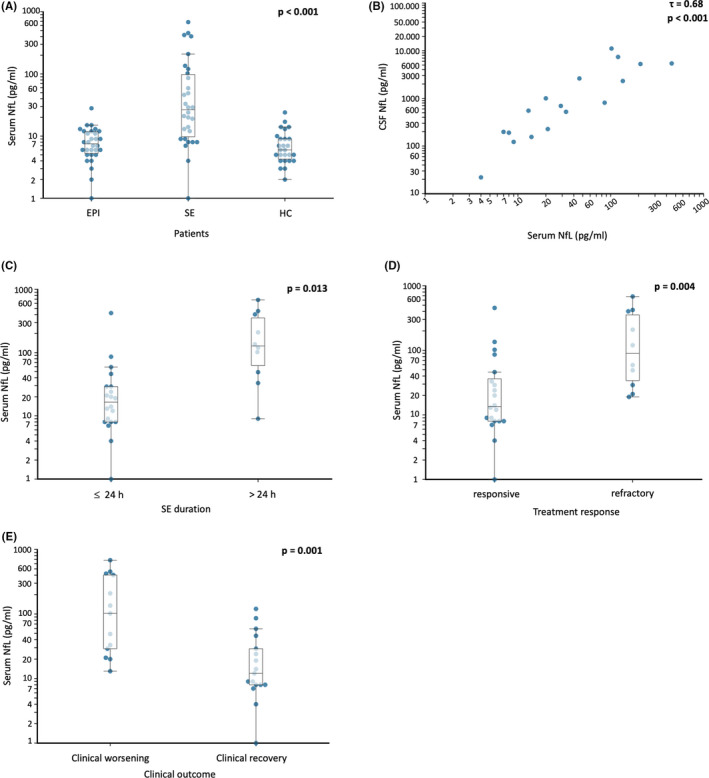
Neurofilaments across groups and in status epilepticus. (A) Log scale values of serum neurofilament light (NfL) across groups. EPI, epilepsy group; HC, healthy controls; SE, status epilepticus group. See text for groups details. (B) Serum–cerebrospinal fluid (CSF) correlation of NfL levels (log scale; p < .001). (C) Serum NfL levels in patients with status epilepticus resolved within 24 h (≤24 h) or with more prolonged duration (>24 h). (D) Serum NfL levels in patients with responsive or refractory status epilepticus. (E) Serum NfL in patients returning to baseline clinical condition and in patients who died or showed a clinical worsening after SE. Functional outcome was measured using the modified Rankin Scale
For 17 SE patients, CSF was also available (collected at the same time as blood sampling), and a high correlation was found between sNfL and CSF NfL levels (τ = .68, p < .001; Figure 1B). For these SE cases, a high correlation was also observed between sNfL and CSF t‐tau levels (τ = .63, p < .001; data not shown).
When analyzing sNfL in relation to treatment outcomes, sNfL levels were higher in patients with RSE and SRSE (responsive SE: median = 13.35 pg/ml, RSE/SRSE: median = 89.7 pg/ml, p = .004; Figure 1D). Moreover, considering the duration of SE, we observed that sNfL levels were increased in SE lasting >24 h compared to SE lasting ≤24 h (p = .013; Figure 1C). Notably, we did not find any correlation between the serum levels of NfL and the time elapsed between the estimated SE onset and sample collection (τ = .13, p = .352). Patients presenting worsening of clinical conditions (increment of at least 1 point of the mRS compared to baseline condition) had higher levels of sNfL compared to those who recovered (median = 102 and 11.70 pg/ml, respectively; p = .001; Figure 1E).
Finally, in patients with SE, we evaluated sNfL values to predict the development of treatment refractoriness (RSE and SRSE) and the 30‐day clinical worsening. To find the best cutoff point, we calculated ROC curves with Youden index for treatment response and for the 30‐day outcome. The value of 28.80 pg/ml was the best cutoff both for treatment refractoriness (sensitivity = 80%, specificity = 70%) and for 30‐day clinical worsening (sensitivity = 77%, specificity = 76%). sNfL values of >28.8 pg/ml predicted 30‐day clinical worsening (odds ratio [OR] = 10.83, 95% confidence interval [CI] = 1.96–59.83, p = .006) and SE refractoriness (OR = 9.33, 95% CI = 1.51–57.65, p = .016; Table S2).
4. DISCUSSION
In this study, we found that in patients with SE, sNfL levels were, on average, higher compared to both healthy subjects and patients with chronic epilepsy. As we excluded from the study all episodes of SE caused by acute structural damage, it is conceivable that sNfL values for the most part reflect seizure‐induced neuronal damage. The strict correlation observed between peripheral and central compartments (CSF) in patients with SE, where both samples were available, although expected, confirms the reliability of the measurement of NfL in serum in SE. Such a high correlation may be a consequence of the frequent blood–brain barrier damage present during SE. Importantly, sNfL showed a high correlation with CSF t‐tau, which represents an established biomarker of neural damage already demonstrated to be elevated in patients with SE. 8 NfL levels also correlated with duration of SE, response to first‐/second‐line treatments, and functional outcomes. On the contrary, we did not observe a correlation between the serum levels of NfL and the time elapsed between the estimated SE onset and sample collection.
The samples were collected within the first 48 h in all patients. Our explanation for this lack of correlation is that in this context the level of sNfL probably primarily reflects the intrinsic severity of the SE. Nevertheless, we strong believe that this important aspect deserves further studies with a strict ad hoc design (e.g., multiple repeated samplings at different defined time points in the same patient both during SE and after its end). Notably, electrographic SE in patients with postanoxic encephalopathy was recently found to be an independent predictor of high sNfL levels at 72 h after cardiac arrest. 9
Regarding NfL in epilepsy patients, we observed sNfL levels comparable to those of healthy volunteers, supporting the idea that only repetitive seizure activity results in neuronal damage that can be indexed by sNfL levels. In SE, blood–brain barrier disruption may contribute to an increased release of CSF NfL into blood, making sNfL a particularly efficient biomarker in indexing neuroaxonal damage in this context. A few recent studies investigated sNfL levels in drug‐resistant epilepsy patients and after a single afebrile or febrile seizure, reporting NfL levels in the range observed in this study, although obtained with single molecule array. 10 , 11 , 12 In the only study that explored the variation of sNfL levels longitudinally after a single seizure, only a very small increment in sNfL sampled immediately after the seizure (7.9 pg/ml), compared to baseline values (7.2 pg/ml) and compared to values obtained thereafter, was found. 10
In conclusion, although the retrospective design and limited study cohort do not allow the prediction of short‐term disability and these results cannot, therefore, drive therapeutic decisions, we believe that our preliminary results advocate a possible role for blood NfL as a promising biomarker in SE, deserving further studies for both clinical and research purposes. Finally, although speculative, these results could support the role and importance that in SE Nfs play at the synaptic level and in particular at the level of glutamatergic synapses, which certainly have a fundamental role in the genesis and maintenance of ictal activity during SE. 7
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose..
AUTHOR CONTRIBUTIONS
G.G.: Conception and design of the study, collected and interpreted the data, performed statistical analysis. R.B.: Major role in the acquisition of data, revised the manuscript for intellectual content. D.F.: Conception and design of the study, performed statistical analysis. A.E.V.: Interpreted the data, revised the manuscript for intellectual content. J.M.: Designed and conceptualized study, revised the manuscript for intellectual content, performed statistical analysis. S.M.: Designed and conceptualized study, drafted and revised the manuscript for intellectual content.
Supporting information
Supplementary Material
ACKNOWLEDGMENT
This study was funded by the grant "Dipartimenti di eccellenza" of the Ministero della Università e Ricerca, Italy, to the Dipartimento di Scienze Biomediche Metaboliche e Neuroscienze, University of Modena e Reggio Emilia, Italy. Open Access Funding provided by Universita degli Studi di Modena e Reggio Emilia. [Correction added on 7 June 2022, after first online publication CRUI funding statement has been added.]
Giovannini G, Bedin R, Ferraro D, Vaudano AE, Mandrioli J, Meletti S. Serum neurofilament light as biomarker of seizure‐related neuronal injury in status epilepticus. Epilepsia. 2022;63:e23–e29. doi: 10.1111/epi.17132
REFERENCES
- 1. Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus—report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015;56(10):1515–23. [DOI] [PubMed] [Google Scholar]
- 2. Leitinger M, Trinka E, Giovannini G, Zimmermann G, Florea C, Rohracher A, et al. Epidemiology of status epilepticus in adults: a population‐based study on incidence, causes, and outcomes. Epilepsia. 2019;60(1):53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanin A, Lambrecq V, Denis JA, Imbert‐Bismut F, Rucheton B, Lamari F, et al. Cerebrospinal fluid and blood biomarkers of status epilepticus. Epilepsia. 2020;61(1):6–18. [DOI] [PubMed] [Google Scholar]
- 4. Rejdak K, Kuhle J, Rüegg S, Lindberg RLP, Petzold A, Sulejczak D, et al. Neurofilament heavy chain and heat shock protein 70 as markers of seizure‐related brain injury: seizure‐related neurodegeneration. Epilepsia. 2012;53(5):922–7. [DOI] [PubMed] [Google Scholar]
- 5. Yuan A, Rao MV, Veeranna, Nixon RA. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol. 2017;9(4):a018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gafson AR, Barthélemy NR, Bomont P, Carare RO, Durham HD, Julien J‐P, et al. Neurofilaments: neurobiological foundations for biomarker applications. Brain. 2020;143(7):1975–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yuan A, Veeranna SH, Sershen H, Basavarajappa BS, Smiley JF, Hashim A, et al. Neurofilament light interaction with GluN1 modulates neurotransmission and schizophrenia‐associated behaviors. Transl Psychiatry. 2018;8(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Monti G, Tondelli M, Giovannini G, Bedin R, Nichelli PF, Trenti T, et al. Cerebrospinal fluid tau proteins in status epilepticus. Epilepsy Behav. 2015;49:150–4. [DOI] [PubMed] [Google Scholar]
- 9. Lybeck A, Friberg H, Nielsen N, Rundgren M, Ullén S, Zetterberg H, et al. Postanoxic electrographic status epilepticus and serum biomarkers of brain injury. Resuscitation. 2021;158:253–7. [DOI] [PubMed] [Google Scholar]
- 10. Evers KS, Hügli M, Fouzas S, Kasser S, Pohl C, Stoecklin B, et al. Serum neurofilament levels in children with febrile seizures and in controls. Front Neurosci. 2020;14:579958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nass RD, Akgün K, Elger C, Reichmann H, Wagner M, Surges R, et al. Serum biomarkers of cerebral cellular stress after self‐limiting tonic clonic seizures: an exploratory study. Seizure. 2021;85:1–5. [DOI] [PubMed] [Google Scholar]
- 12. Ouédraogo O, Rébillard R, Jamann H, Mamane VH, Clénet M, Daigneault A, et al. Increased frequency of proinflammatory CD4 T cells and pathological levels of serum neurofilament light chain in adult drug‐resistant epilepsy. Epilepsia. 2021;62(1):176–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


