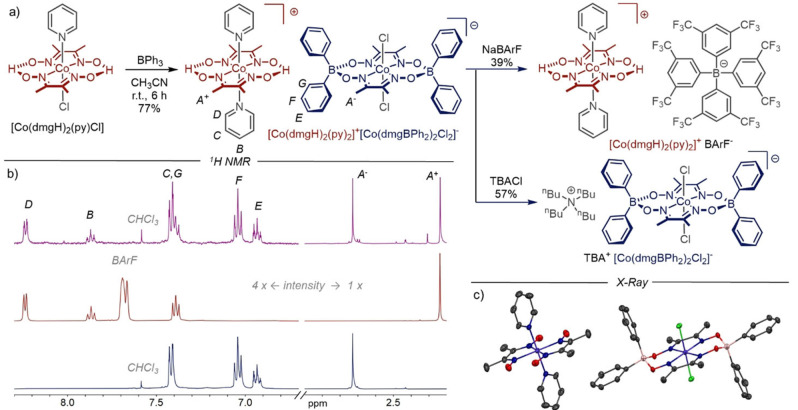
Figure 1.
a) Synthesis of [Co(dmgH)2(py)2]+[Co(dmgBPh2)2Cl2]−, [Co(dmgH)2(py)2]+ BArF− and TBA+ [Co(dmgBPh2)2Cl2]−. b) 1H NMR (CD3CN, 400 MHz, RT). Top: [Co(dmgH)2(py)2]+[Co(dmgBPh2)2Cl2]−; middle: [Co(dmgH)2(py)2]+ BArF−; bottom: TBA+ [Co(dmgBPh2)2Cl2]−. c) Solid state structure of [Co(dmgH)2(py)2]+[Co(dmgBPh2)2Cl2]−. Space group: P‐1, crystal system: triclinic. The average equatorial Co−Noxime distance is 1.90 Å in the cationic complex [Co(dmgH)2(py)2]+, 1.86 Å in the anionic complex [Co(dmgBPh2)2Cl2]− and 1.89 Å in neutral cobaloximes. [29] Solvent and H atoms are omitted for clarity.