Abstract
In the last decade, several cases affected by Developmental Topographical Disorientation (DTD) have been described. DTD consists of a neurodevelopmental disorder affecting the ability to orient in the environment despite well-preserved cognitive functions, and in the absence of a brain lesion or other neurological or psychiatric conditions. Described cases showed different impairments in navigational skills ranging from topographic memory deficits to landmark agnosia. All cases lacked a mental representation of the environment that would allow them to use high-order spatial orientation strategies. In addition to the single case studies, a group study performed in Canada showed that the disorder is more widespread than imagined. The present work intends to investigate the occurrence of the disorder in 1,698 young Italian participants. The sample is deliberately composed of individuals aged between 18 and 35 years to exclude people who could manifest the loss of the ability to navigate as a result of an onset of cognitive decline. The sample was collected between 2016 and 2019 using the Qualtrics platform, by which the Familiarity and Spatial Cognitive Style Scale and anamnestic interview were administered. The data showed that the disorder is present in 3% of the sample and that the sense of direction is closely related to town knowledge, navigational strategies adopted, and gender. In general, males use more complex navigational strategies than females, although DTD is more prevalent in males than in females, in line with the already described cases. Finally, the paper discusses which protective factors can reduce DTD onset and which intervention measures should be implemented to prevent the spread of navigational disorders, which severely impact individuals’ autonomy and social relationships.
Introduction
Navigation is the ability to move from one location to the next, following habitual routes and avoiding getting lost [1] in new and familiar environments. Due to its importance, it is not surprising that many cognitive processes (i.e., memory, mental imagery, attention to landmarks and other features, and our perception of directions and distances as well as decision-making, planning, and problem-solving) [2–6], and multiple brain regions [7–11] are involved in successful navigation. Specifically, the success of this process also depends on internal and external factors to the individual. The internal factors are of greater interest because they directly affect navigational competence; by consequence, it is possible to better intervene on them in order to plan prevention programs related to navigational disorders. The most important internal factors for navigation are: a) the cognitive predisposition to grasp certain environmental information rather than others [12–16]; b) gender [17–24]; c) age [25–30]; d) professional experience [31–36]; e) familiarity with the environment [37–40], reflecting the result of repeated exposure to a stimulus or an environment [41, 42]; f) navigational strategies used during navigation [36, 43]; g) psychiatric (e.g., spatial anxiety, depression, agoraphobia: [44–46]) and neurologic diseases (Alzheimer’s disease and brain lesions in the navigational brain network: [47–50]).
In the present study we focused on an indirect measure of navigation, namely the Sense of Direction (SOD), which is our own perception about navigation ability, in order to understand the critical internal variables that affect it: the demographic factors (e.g., age, gender and education), the degree of familiarity with the environment (e.g., town knowledge), and navigational strategies, which include not only cognitive styles (e.g., landmark, route and survey), but also the preferential mean to explore the environment, that is to say, means of travelling (MoT: active, passive). In addition, we also considered the right-left confusion (RLC), with reflects a pathological condition that can be associated or not with navigational disorders.
At the aim, Siegel and White’s model [51] is used as the theoretical framework. This model suggests that environmental knowledge occurs in three distinct hierarchical steps, namely Landmark (i.e., figurative memory of environmental objects), Route (i.e., a sequence memory of the path connecting environmental objects in an egocentric perspective), and Survey (i.e., map-like representation in an allocentric perspective) knowledge. Following this model, people can be classified in three categories according to the navigational strategies they adopt: landmark (less skilled and get lost easily), route (more skilled at connecting landmarks by verbal labels, such as right, left, behind, ahead, etc.., but less able at finding shortcuts or changing routes after environmental changes), and survey (the most navigational skilled, excellent visual-spatial abilities) users [52, 53]. Notably, both egocentric (route representations) and allocentric (survey representations) frames of reference are needed to specify categorical (non-metric) and coordinate (metric) spatial encodings, which are two different but complementary aspects of spatial cognition [54–56]. According to Montello [5], even though familiarity (the repetitive exposure to a particular environment) is more associated with a survey format, spatial knowledge would be not hierarchically organized, but rather would occur in a parallel fashion, depending on the situation [57]. This means that navigation is very complex and that different difficulties can arise when moving through the environment. Furthermore, in the Environmental Knowledge Model [37] emerges as the environmental familiarity allows to perform navigational tasks requiring higher spatial cognitive strategies (e.g. environmental map; perspective changing) even in those individuals with poor spatial orientation skills. In this vein, familiarity with environment enhances wayfinding and reduces wandering and topographical disorientation in individuals suffering from neurocognitive decline [39, 58]. The effect due to familiarity emerges also in healthy ageing given that older people may be better than younger people [59].
Amongst others, the right-left confusion can explain same individual differences in term of SOD [60–62]. Indeed, the ability to discriminate left from right from one’s own perspective is useful when individuals explore and recall the environment [60]. In addition, the ways of travelling can also affect the ability to orient oneself in space. Specifically, active travel (e.g., moving by car or bike or on foot) often indicates a better SOD and leads to a better representation of one’s surroundings [63], whereas passive travel (e.g., moving as a passenger in a car, but, taxi, etc…) is likely related to less navigation ability [64]. Yet, Bocchi et al. [12] found that navigational strategies can also affect SOD. Indeed, landmark users can show more difficulties, whereas survey users are the most skilled in solving navigational problems and in travel planning [53, 65–67].
Most importantly for the purpose of the present study, navigational difficulties can be associated to a neurodevelopmental disorder that specifically undermines navigational skills. This disorder was described for the first time by Iaria et al. [68] and was named ‘Developmental Topographical Disorientation (DTD)’. Afterwards, numerous people worldwide have been identified as suffering from this condition [69–75]. Iaria and Barton [74] demonstrated that DTD is widespread in the Canadian population, finding 120 individuals fulfilling the criteria for a diagnosis of DTD. This led to conclude that DTD is rather widespread in the population and requires targeted interventions by clinical services. In general, people with DTD have normal memory and neuropsychological profiles but show a major cognitive deficit in spatial cognition, and complain of severe problems in navigating on an everyday basis. Specifically, they are unable to use cognitive maps or place-based navigation strategies to find their way around familiar and novel environments. In general, individuals with DTD show a higher impairment of metric (coordinates) than nonmetric (categorical) spatial encoding, and, basing on Siegel and White’s model, they hardly reach a level of route knowledge of the environment, often stopping at a landmark knowledge, highlighting a lack of allocentric representation capacity [76]. By analysing single cases reports, subjects are characterised by a different degree in terms of severity of illness and in terms of navigational impairment. Specifically, Case one had a severe deficit in the formation of the environment mental map [66]; FG had a normal acquisition of environmental information but a specific impairment in the retrieval with a loss of information after 5 minutes [69]; Dr. WAI and LA had both normal acquisition and retrieval of environmental information [70, 73]. FG, LA, and Dr. WAI had deficits in mental representation, mental rotation and mental generation of environmental images. Nobody had difficulty in landmark recognition. LG, instead, was the first case of Landmark Agnosia Development, showing a selective deficit in recognizing landmarks allowing spatial orientation [77]. CF [75] was fully effective in learning and following routes and in building up cognitive maps as well as in recognizing landmarks. However, she performed significantly worse than age and gender-matched controls on the map-following task, namely when she was required to use a map to navigate in a novel environment. In terms of neural correlates, the first studies on DTD cases showed no clear brain structural abnormalities (i.e., [68, 69]). However, using an fMRI landmark sequencing task, Nemmi and co-workers [71] demonstrated that DTD individuals did not show any activation in the navigation brain network, whereas prefrontal areas, known to be involved in processing the sequential order of everyday life actions [78, 79], were normally activated. The decreased functional connectivity between the hippocampus and the prefrontal cortex has also been described [80] and interpreted as a defective functioning of two crucial areas for navigation and decision making. In addition, the rs-fMRI experiment demonstrated aberrant functional connectivity between regions within the default-mode network (DMN), specifically between the medial prefrontal cortex and the posterior cingulate cortex, the medial parietal and temporal cortices.
Thus, the objective of the present study was: to estimate the percentage of the DTD among a convenience sample of Italian adults aged 18–35 years to define soon clinical lines of intervention and a protocol of investigation shared on the national network. To this purpose we investigated the SOD and its correlates. We hypothesize that some internal factors may be correlated to the presence of DTD. Specifically, we expect the gender distribution to be different between males and females: in line with previous described cases we expect to find more males suffering from DTD than females [69, 70, 73, 75, 77]. Moreover, we hypothesize that DTD correlates differently to navigational strategies: we expect that individuals with Survey abilities show higher navigational skills [36] and less probability to have DTD symptoms with respect to people with lower navigational skills (Landmark and Route users). In this vein, we also hypothesize that individuals with DTD show higher right/left confusion and a passive use of means of transport. Consistent with the familiarity effect on SOD [37, 39, 40, 58, 59], we also hypothesize that familiarity, measured as town knowledge, may affect SOD favouring navigation, and could be a protective factor for individuals with DTD who may be able to perform certain spatial orientation tasks in a familiar environment.
Methods
Study design
For this study we conducted an online survey among Italian young people in order to investigate the presence of the DTD and its individual correlates.
Study population
Participants
The eligible study population consisted of all those people without neurological disorders and with an age between 18 and 35 years. A sample of 1,698 participants took part in the experiment. Participants had a full-time education, ranging from 8 to 18 years (mean = 14.80 years, SD = 2.83 years). They were not all university students. Specifically, 81 (4.79%) participants achieved: a secondary school diploma; 842 (49.76%) a high school diploma; 769 (45.45%) a degree or post-degrees. Demographic data of all participants are reported in Table 1. The study was performed according to the ethical principles expressed in the Declaration of Helsinki and it was approved by the Local Ethics Committee (Department of Psychology, University of Bologna, Italy).
Table 1. Demographic data of participants.
| N. total | 1,698 |
| N. males | 635 |
| N. females | 1,063 |
| Age Total (years) | 24.89 (4.08) |
| Age males (years) | 25.56 (4.25) |
| Age females (years) | 24.48 (3.92) |
| Education Total (years) | 14.80 (2.83) |
| Males Education (years) | 14.39 (2.08) |
| Females Education (years) | 15.04 (2.82) |
Note: means (Standard Deviations).
Data collection
Participants from all Italian regions (from North to South, including the Islands) were recruited between 2016 and 2019 using notices on social networks and on bulletin boards of researchers. The advices about the survey were basically spread out by word of mouth and flyers, that were distributed in community meeting points, such as bookshops, cafeterias, public library, and sport clubs. The software Qualtrics (First release: 2005, Provo, Utah, USA, Available at: https://www.qualtrics.com) was used. All participants gave their informed consent before their inclusion in the study.
Measures
A. Anamnesis questionnaire
Participants had to fill in some questions about problems of spatial orientation from an early age, neurological or major psychiatric illness, previous traumatic brain injury, history of learning disabilities, alcohol or drug abuse.
Specifically, with regard to neurological outcomes, participants were asked to specify whether they had experienced head trauma, ischaemic attacks, encephalitis, brain infections, pre-perinatal complications. For the psychiatric side, whether they had suffered or were suffering from depression, anxiety, psychosis, obsessive-compulsive disorder, eating disorder, post-traumatic stress disorder, schizophrenia, phobias. When he/she suffered from it and whether he/she was treated pharmacologically and/or is currently on medication. For substance use, we investigated whether he or she uses alcohol, if so how often, and whether he or she uses or has used drugs (cannabis, amphetamines, cocaine etc.) if so when did he or she use them, how often and which substances.
B. Familiarity and spatial cognitive style scale [81, 82]
Participants had to fill in a series of questions: each item of the scale was a self-referential statement about some aspect of environmental spatial cognition. At the beginning of the scale participants were asked to report demographic information (age, gender and educational level), as well as how they moved around the environment, that is whether they used active or passive means of transport. Specifically, we investigated the use of means of transport, distinguishing active means of transport in which the participant actively drives and moves around the environment (i.e., driving a car; riding a moped; riding a bicycle; riding a motorbike; walking), and passive means of transport in which the participant is led around the environment by others (i.e., being a passenger in a car; using a taxi; using a bus; using a train). For each choice of means of transport, the participant indicated on a scale of 1 to 5 how often they used it. Based on the frequency of use of the various means, the prevalence of active or passive use of the means of transport was defined.
In addition, they were also asked to indicate in the section ‘town knowledge’ to think of a town they knew well even if it was different from the one they lived in.
The scale was divided into the following subscales:
Sense of Direction (SOD) was the summed rating for items concerning the sense of direction, Items 1, 2, 3, 4, 6, 7, 9, 10, 11, and 22 (e.g., Item 1: “How is your ability to read a map?”);
Town Knowledge (TK) was the summed ratings of Items 8, 12, 13, 14, 15, 16, 19, 20, and 21 [e.g., Item 12: “How well do you know (insert the name of the city where you live in)?”];
Spatial cognitive style. To evaluate individual spatial cognitive style, two items were used [Items 17 (a, b, c) and 18 (a, b, c)]. (e.g., given the following item “Try to imagine a route you usually take (e.g., home to work, college to cafeteria…”), participants were asked to evaluate each of the following strategies: a) Landmark—Do you visualize only the landmarks (e.g., your home, the cafeteria. . .)? b) Route—Do you visualize both the landmarks and the route leading to your destination? c) Survey—Do you ever imagine the route as if it were on a map?).
Right-left confusion (RLC). Confusion of right and left self-referents was obtained by Item 5, “In everyday life, do you confound right and left?”.
For each item, participants should circle a number from 1 to 5 to indicate their response: higher numbers correspond to higher ability. In previous works [82–84], the overall value of the Cronbach’s alpha for the total scale ranged between .79. and .74, which is good [84], as the test-retest reliability as reported by Nori and Piccardi [81]. In the present sample, Cronbach’s alpha for the total scale was = .71. In Piccardi, Risetti, Nori [82] the internal consistency of SOD and TK was estimated as a combined score (.74). In the present study, the internal consistencies of SOD (.73) and TK (.71) were estimated separately. The full Familiarity and Spatial Cognitive Style Scale is available in the appendix of Piccardi, Risetti and Nori [82].
In this study, the final scores were the means of the item scores for SOD, TK and spatial cognitive styles (landmark, route and survey), whereas ‘right-left confusion’ and the ‘ways of travelling’ were analysed as dichotomous variables. First of all, regarding the item ‘right-left confusion’ (hereafter RLC) we dichotomised as follows: ‘yes’ if the subject responded ‘sometimes’, ‘often’ or ‘always’; ‘no’ if the subject responded ‘never’ or ‘rarely’. Secondly, the ways of travelling, that is the Means of Transport (hereafter MoT) was computed from 5 items: 1) drove a car; 2) other MoT (e.g., bike); 3) only travelled as a passenger; 4) took public transport; 5) only moved on foot. Thus, a subject was defined as an ‘active traveller’ if he/she indicated to move mainly by car, motorbike, bicycle or on foot; a subject was defined as a ‘passive traveller’ if he/she indicated to move mainly as a passenger in a car, taxi, coach, bus, train, air, etc.
Specifically, we classified participants with DTD if they reported a total SOD of 2 Standard Deviations (SD) below the mean (95% CI), as calculated from data collected by Nori and Piccardi [79]. Furthermore, on the basis of the anamnesis questionnaire we also considered the four following diagnostic criteria suggested by Iaria and Barton in [74]: i) getting lost daily or often (1 to 5 times a week) in the most familiar environments; ii) the problem of spatial orientation must be present from an early age; iii) no other cognitive difficulties that may affect daily life activities, and as the last criterion; iv) no known brain lesions, malformation or any condition affecting the central nervous system, with the exception of migraine. In addition, we also took into account for two adding criteria: v) no psychiatric disorders and psychotropic drug use, and vi) substance abuse behaviour.
Statistical methods
Continuous variables were reported as means (Standard Deviation = SD), and categorical factors were reported as percentages. To identify the variables that were significantly related to SOD, the Spearman correlation coefficient was computed for variables measured at least on ordinal scale: Age, Educational Level (low: people with at most a high school diploma; high: degree or post-degrees), TK (score), Landmark (score), Route (score) and Survey (score); and the Point-Biserial Correlation Coefficient for the nominal variables: Gender, RLC (yes/no) and MoT (active/passive). When the correlation coefficients were statistically significant (p < .05) the variables were introduced into a Generalised Linear Regression model (Glm). For the Glm model, an identity link function and a normal family distribution were specified as a linear model, with SOD as the continuous dependent variable and the following model terms as independent variables: Age, TK (score), Landmark (score), Route (score) and Survey (score) as centred covariates; Gender (M/F), RLC (yes/no) and MoT (active/passive) as dummy factors. All interactions with categorical variables were introduced because they explained more variability of SOD (R2 = .337).
In addition, to study the associations between DTD and each socio-demographic variable and each different subscales of the Family and Spatial Cognitive Style Scale, the Chi-square test and One-way analysis of variance, for categorical or continuous data respectively, were used. Specifically, the univariate Logistic regression model, taking DTD as binary dependent variable (yes/no) and one independent variable at a time (Gender, Age, Educational level, RLC, MoT, TK, Landmark, Route, Survey) was carried out to estimate significant predictors, reporting the unadjusted odds ratio (OR) with their 95% Confidence Intervals (95% CI). The significant predictors (p < .05), estimated by univariate analysis, were introduced into a multivariable logistic model, in order to both estimate the odds ratio controlling for the other covariates (adjusted Odds ratios: ORadj), and identify significant protective or risk factors for DTD.
The analyses were performed by using STATA/MP14 software and the jamovi project (2021): jamovi (Version 1.6) Computer-Software, setting alpha to .05. All tests were two-tailed.
Results
General characteristics
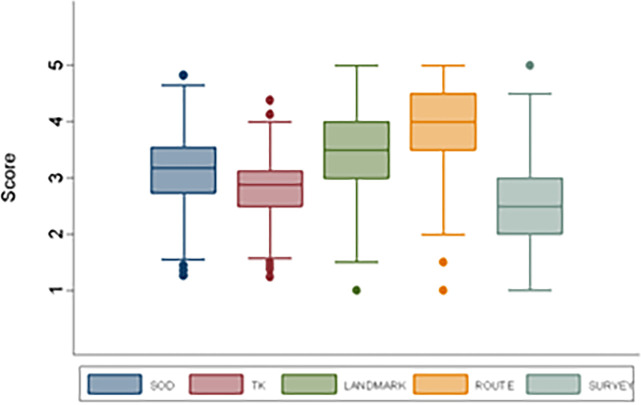
Participants reported higher means for the Route scale (3.87, SD = 4), the Landmark (3.58, SD = 3.5) scale, and the SOD scale (3.14, SD = .6), while for the TK scale and the Survey scale the mean values were 2.79, SD = 2.9 and 2.55, SD = 2.5 respectively. Fig 1 shows the different score levels measured by the different subscales.
Fig 1. Box plot of SOD scale and TK, Landmark, Route and Survey subscales.
Correlation between factors investigated and SOD
As reported in Table 2, Spearman correlation analysis showed that SOD was negatively correlated with a weak magnitude level to gender, RLC and Educational level. In addition, SOD was positively correlated with a magnitude ranging from weak to moderate level to Landmark, Route and Survey scores. Moreover, SOD was also positively correlated with a weak magnitude level to Age, MoT and TK. (see [85, 86] for correlation magnitude).
Table 2. Spearman correlation between SOD and investigated factors.
| Parameter | n | Correlation coefficients (rho) | degrees of freedom | p-value |
|---|---|---|---|---|
| Age (years) | 1698 | .069 | 1696 | .004 |
| Gender (M/F) | 1698 | -.289 | 1696 | < .001 |
| Educational Level (Low/High) | 1692 | -.045 | 1690 | .066 |
| RLC (yes/no) | 1697 | -.163 | 1695 | < .001 |
| MoT (active/passive) | 1392 | .064 | 1390 | .017 |
| TK (score) | 1697 | .290 | 1695 | < .001 |
| Landmark (score) | 1697 | .119 | 1695 | < .001 |
| Route (score) | 1697 | .276 | 1695 | < .001 |
| Survey (score) | 1698 | .506 | 1698 | < .001 |
Note: RLC = Right-Left confusion; MoT = Means of Travelling; TK = Town Knowledge.
Using the generalised linear model, the multivariable analysis showed that SOD was related to gender and spatial abilities. In particular, SOD increased because of Survey ability (Beta coefficient = .241; p < .01), whereas decreased in subjects with RCL (Beta coefficient = -.555; p = .009). The model showed that the investigated interactions were not significant (Table 3).
Table 3. Factors related with SOD (multivariable analysis).
| 95%CI | |||||||
|---|---|---|---|---|---|---|---|
| Independent variables | Estimate | SE | Beta | lower | upper | z | p |
| (Intercept) | 3.225 | .0636 | .098 | -.11 | .30 | 50.670 | < .001 |
| Gender (male) | -.179 | .0827 | -.293 | -.56 | -.03 | -2.157 | .031 |
| TK | .199 | .0310 | .147 | .10 | .19 | 6.424 | < .001 |
| Landmark | .055 | .0164 | .078 | .03 | .12 | 3.366 | < .001 |
| Route | .082 | .0195 | .104 | .06 | .15 | 4.227 | < .001 |
| Survey | .241 | .0152 | .389 | .34 | .44 | 15.823 | < .001 |
| MoT (active) | .082 | .0683 | .134 | -.08 | .35 | 1.200 | .230 |
| Age | .001 | .0034 | .002 | -.00 | .01 | .358 | .720 |
| RLC (no) | -.555 | .2122 | -.909 | -1.59 | -.23 | -2.610 | .009 |
| Gender * MoT | -.045 | .0888 | -.074 | -.36 | .21 | -.510 | .610 |
| Gender * RLC | .425 | .2343 | .697 | -.06 | 1.45 | 1.815 | .070 |
| MoT* RLC | .418 | .2312 | .684 | -.06 | 1.43 | 1.806 | .071 |
| Gender*MoT* RLC | -.777 | .2547 | -.617 | -1.43 | .20 | -1.479 | .139 |
Note: RLC = Right-Left confusion; MoT = Means of Travelling; TK = Town Knowledge.
Fifty-four participants met the criteria for DTD, then the percentage of DTD was 3% of our sample (54/1698: 95% CI: 2.4% - 4.0%). The logistic univariate analysis showed that DTD was associated with gender: the males showed a higher risk than females to have DTD (OR: 2.39; 95% CI: 1.2–4.7; p = .009). The risk of DTD was lower in people with higher scores in TK, Route, and Survey scales (Table 4).
Table 4. Factors related to DTD (univariate logistic regression).
| DTD | |||||
|---|---|---|---|---|---|
| No (n = 1644) | Yes (n = 54) | P* | OR | 95%CI | |
| Factors | N (%) or mean (SD) | N (%) or mean (SD) | |||
| Gender | |||||
| Females | 624 (98%) | 11 (2%) | 1 | ||
| Males | 1020 (96%) | 43 (4%) | .009 | 2.39 | 1.2–4.7 |
| Age (yrs.) | 24.9 (4.1) | 24.8 (3.9) | .9429 | 1.00 | .9–1.1 |
| Educational Level | |||||
| Low | 895 (97%) | 28 (3%) | 1 | ||
| High | 743 (97%) | 26 (3%) | .686 | 1.1 | .6–1.9 |
| RLC | |||||
| No | 1276 (97%) | 37 (3%) | 1 | ||
| yes | 367 (96%) | 17 (4%) | .114 | 1.6 | .9–2.9 |
| MoT | |||||
| Passive | 184 (96%) | 7 (4%) | 1 | ||
| Active | 1167 (97%) | 34 (3%) | .527 | .8 | .3–1.7 |
| TK (score) | -.01 (1.00) | -.42 (1.13) | .0058 | .4 | .2–0.8 |
| LANDMARK (score) | .03 (0.98) | -.11 (0.80) | .054 | .8 | .6–1.1 |
| ROUTE (score) | .06 (0.96) | -.51 (0.97) | < .001 | .5 | .3-.7 |
| SURVEY (score) | .02 (0.99) | -.99 (0.70) | < .001 | .2 | .1-.3 |
*Chi square or Anova test
Note: Sub-totals are not 1,698 because some participants did not fill in some questions. RLC = Right-Left confusion; MoT = Means of Travelling; TK = Town Knowledge.
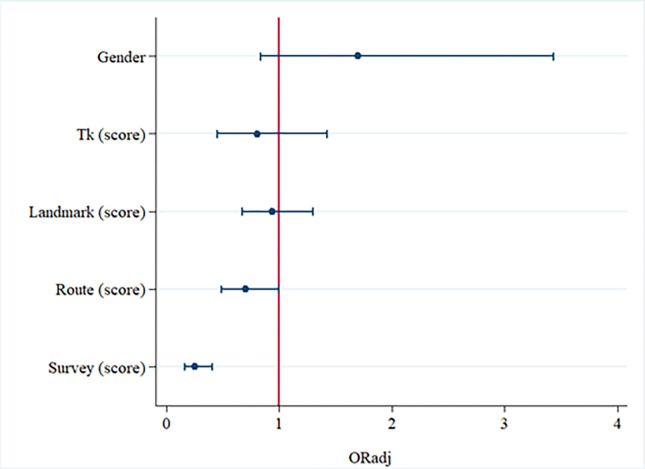
The multivariable logistic regression analysis confirmed that the risk of DTD was statistically lower in subjects with a higher Survey Ability (ORadj = .26; 95% CI: .16 - .40), adjusted for all factors investigated (Fig 2).
Fig 2. Multivariable association between five risk factors and DTD.
Note: error bars are 95% confidence intervals.
Discussion
The present online investigation aimed to estimate the presence of DTD in a large young Italian sample to better understand the spread of this condition in the population. Thus, we considered the percentage of the DTD in a sample of 1,698 participants, using as a basic criterion 2 standard deviations below the means of the SOD. Then, we also investigated the critical factors predictive of both the SOD and DTD. As in Iaria and Barton’s study [74], we confirmed that the rate of occurrence of the disorder is not a rare condition; rather, it affects 3% of young people undermining their autonomy and ability to work away from family boundaries.
As concerns the predictive factors of SOD, the analyses showed that gender more than educational level is strictly related to SOD. In particular, although females use more landmark-based navigational strategies and complain more difficulties in SOD, males show a higher risk of DTD than females. Indeed, given a glance at the DTD literature, the most of the single cases described are males; this means that although males have better visuospatial and navigational skills than females, they are also the most fragile [69, 70, 72, 75, 77]. Moreover, SOD was related to TK and Survey competencies. Iaria and co-workers [87] clarified that normal navigators can switch from one strategy to another by increasing their familiarity with the environment. This means that it is possible to implement higher navigational skills by acting on environmental knowledge. Also in Nori and Piccardi [37] emerged that when the individual was familiar with the environment, even if s/he generally preferred low navigational strategies, s/he was able to perform more complex navigational tasks in that specific environment, therefore, citizens of Bologna who were familiar with a neighbourhood of the city were able to recognize rotated monuments of the city even though their ability to mentally rotate an object was low [37, 84].
Consistent with these results, we found that TK and the Survey strategy are negatively related to DTD, suggesting that they can be protective factors in counteracting the onset of DTD. In this vein, Bartonek and co-workers [88–90] showed that children with cerebral palsy and motor disability manifested differences in topographic working memory as a function of the degree of autonomy to explore the environment, regardless of motor impairment. Our results also showed that the use of advanced navigational strategies (survey strategies) is not associated with the presence of DTD. In line with this finding, we also showed that individuals who achieve high spatial skills do not complain of RLC, which is generally involved in navigational disorders [60]. Consistent with this result, Giancola et al. [36] showed that survey strategies also characterise samples of experienced navigators, such as military pilots. According to Verde et al. [32, 33, 91, 92] this professional category is already selected on the basis of spatial and navigational skills. In addition, the military training would reinforce the survey strategy even more, including all those cognitive processes related to navigation, such as the ability to mentally rotate two and three-dimensional objects [93] or the ability to make directional and metric judgments.
Undoubtedly, the presence of a navigational deficit can also make the subject anxious and more reluctant to explore the environment, so it is difficult to determine how much one comes before the other. This result is particularly interesting in light of Lopez et al.’s [94] study, which showed that the role of the direct experience with exploring hometown on spatial mental representations appeared to be more important in the elderly than in young people. Our sample includes young people, therefore we can imagine that TK and the Survey strategy protects not only seniors from the detrimental effects of ageing on spatial mental representations but also young people in acquiring spatial competence by reducing the risk of navigational disorders.
Given the key role of spatial strategies, our results imply navigational training starting with pre-schoolers, in order to prevent the DTD, such as the one already used in Boccia et al. [95], which allows implementing spatial orientation and autonomy skills from the earliest years of life, starting in kindergarten. The introduction of navigational training in education settings may be useful not only for healthy children but also for children with different types of disabilities (e.g. sensory-motor impairments or acquired brain-damaged or ADHD: [96]), who show several navigational disorders. Furthermore, given that simply enhancing cognitive performance is insufficient to reduce a sense of inadequacy about one’s ability [97], the introduction of training activities specifically designed to improve metacognition would improve self-efficacy in individuals with respect to their SOD and related activities, and by consequence would reduce the risk of DTD. In this vein, De Lisi and Wolford [98] showed children improvements in mental rotation through the daily practice of the popular video game Tetris. This policy of intervention could have important spin-offs increasing also social life.
The current research is not without limitations. First, the study was conducted using an online self-reported survey. In the future it will be important to investigate DTD in presence using a battery of navigational tests. For example, no significant result was found in terms of MoT (active and/or passive movements in the environment). Probably this aspect should be investigated differently by quantifying more precisely the movements and their duration and stratifying the sample by strategies developed. Future work should investigate this component along with environmental characteristics (size of the place where one lives; the presence of distant landmarks; need to travel far to receive medical care or to use school services). Second, individuals with DTD were not tested for other cognitive deficits using a battery of neuropsychological tests, but were only asked to report if they suffered from cognitive disorders. Third, the diagnosis of DTD was not supported by structural imaging data in order to exclude the presence of any brain lesions.
In conclusion, the present study allowed us to identify in a large sample of young Italians the presence of DTD and its occurrence. It has also allowed us to observe protective factors that are associated with good navigational skills and that in the future can be used within protocols for the prevention of the development of spatial orientation disorders, as well as to promote these skills by reducing the gender gap that still emerged in this sample.
Data Availability
All data collected are available at the following link: https://osf.io/qwapm/?view_only=2da5876783c842779a4d1a9380784688 as reported also in the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Montello DR, Pick H L. Integrating knowledge of vertically aligned large-scale spaces. Environ. Behav. 1993; 25: 457–484. [Google Scholar]
- 2.Epstein RA, Patai EZ, Julian JB, Spiers HJ. The cognitive map in humans: spatial navigation and beyond. Nature Neuroscience 2017; 20:1504–1513. doi: 10.1038/nn.4656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekstrom AD, Arnold AE, Iaria G. A critical review of the allocentric spatial representation and its neural underpinnings: toward a network-based perspective. Front Hum Neurosci 2014; 8: 803, 2014. doi: 10.3389/fnhum.2014.00803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garling T, Book A, Lindberg E. Cognitive mapping of large-scale environments the interrelationship of action plans, acquisition, and orientation. Environ Behav 1984; 16: 3–34. doi: 10.1177/0013916584161001 [DOI] [Google Scholar]
- 5.Montello DR. A new framework for understanding the acquisition of spatial knowledge in large-scale environments. In: Spatial and Temporal Reasoning in Geographic Information Systems, edited by Engenhofer MJ and Golledge RG. New York: Oxford University Press, 1998; 143–154. [Google Scholar]
- 6.Spiers HJ, Gilbert SJ. Solving the detour problem in navigation: a model of prefrontal and hippocampal interactions. Front Hum Neurosci 2015; 9: 125, 2015. doi: 10.3389/fnhum.2015.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boccia M, Guariglia C, Sabatini U, Nemmi F. Navigating toward a novel environment from a route or survey perspective: neural correlates and context-dependent connectivity. Brain StructFunct. 2016; 221: 2005–21. doi: 10.1007/s00429-015-1021-z [DOI] [PubMed] [Google Scholar]
- 8.Boccia M, Nemmi F, Guariglia C. Neuropsychology of Environmental Navigation in Humans: Review and Meta-Analysis of fMRI Studies in Healthy Participants. Neuropsychol Rev. 2014; 24: 236–51. doi: 10.1007/s11065-014-9247-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boccia M, Sulpizio V, Nemmi F, Guariglia C, Galati G. Direct and indirect parieto-medial temporal pathways for spatial navigation in humans: evidence from resting-state functional connectivity. Brain Struct Funct. 2017; 222: 1945–1957. doi: 10.1007/s00429-016-1318-6 [DOI] [PubMed] [Google Scholar]
- 10.Sulpizio V, Boccia M, Guariglia C, Galati G. Functional Connectivity between Posterior Hippocampus and Retrosplenial Complex Predicts Individual Differences in Navigational Ability. Hippocampus 2016; 26: 841–7. doi: 10.1002/hipo.22592 [DOI] [PubMed] [Google Scholar]
- 11.Ekstrom AD, Huffman DJ, Starret M. Interacting networks of brain regions underlie human spatial navigation: a review and novel synthesis of the literature. J Neurophisiol. 2017; 118: 3328–3344. doi: 10.1152/jn.00531.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bocchi A, Giancola M, Piccardi L, Palmiero M, Nori R, et al. How would you describe a familiar route or put in order the landmarks along it? It depends on your cognitive style! Exp. Brain Res. 2018; 236: 3121–3129. doi: 10.1007/s00221-018-5367-3 [DOI] [PubMed] [Google Scholar]
- 13.Boccia M, Piccardi L, Di Marco M, Pizzamiglio, L, Guariglia C. Does field independence predict visuo-spatial abilities underpinning human navigation? Behavioural evidence. Exp. Brain Res. 2016; 234: 2799–2807. doi: 10.1007/s00221-016-4682-9 [DOI] [PubMed] [Google Scholar]
- 14.Boccia M, Piccardi L, D’Alessandro A, Nori R, Guariglia C. Restructuring the navigational field: Individual predisposition towards field independence predicts preferred navigational strategy. Exp. Brain Res. 2017; 235: 1741–1748. doi: 10.1007/s00221-017-4936-1 [DOI] [PubMed] [Google Scholar]
- 15.Boccia M, Vecchione F, Piccardi L, Guariglia C. Effect of Cognitive Style on Learning and Retrieval of Navigational Environments. Front. Pharmacol. 2017; 8: 496. doi: 10.3389/fphar.2017.00496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boccia M, Vecchione F, Di Vita A, D’Amico S, Guariglia C. et al. Effect of Cognitive Style on Topographical Learning Across Life Span: Insights From Normal Development. Child Dev. 2019, 90, 462–470. doi: 10.1111/cdev.13184 [DOI] [PubMed] [Google Scholar]
- 17.Nazareth A, Huang X, Voyer D, Newcombe N. A meta-analysis of sex differences in human navigation skills. Psychon. Bull.Rev. 2019; 5: 1503–1528. doi: 10.3758/s13423-019-01633-6 [DOI] [PubMed] [Google Scholar]
- 18.Lawton CA. Gender and regional differences in spatial referents used in direction giving. Sex Roles 2001; 44: 321–337. doi: 10.1023/A:1010981616842 [DOI] [Google Scholar]
- 19.Lawton C.A. Gender, spatial abilities and wayfinding. In Handbook of Gender Research in Psychology; Chrisler J., McCreary D. Eds.; Springer: New York, NY, USA, 2010; pp 317–341. [Google Scholar]
- 20.Piccardi L, Risetti M, Nori R, Tanzilli A, Bernardi L et al. Perspective changing in primary and secondary learning: A gender difference study. Learn. Individ. Diff. 2011; 21: 114–118. 10.1016/j.lindif.2010.11.003 [DOI] [Google Scholar]
- 21.Piccardi L, Bianchini F, Iasevoli L, Giannone G, Guariglia C. Sex differences in a landmark environmental re-orientation task only during the learning phase. Neurosc. Lett. 2011; 503: 181–185. doi: 10.1016/j.neulet.2011.08.031 [DOI] [PubMed] [Google Scholar]
- 22.Bianchini F, Verde P, Colangeli S, Boccia M, Strollo F, et al. Effects of oral contraceptives and natural menstrual cycling on environmental learning. BMC Womens Health 2018; 18: 179. doi: 10.1186/s12905-018-0671-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmiero M, Nori R, Rogolino C, D’Amico S, Piccardi L. Sex differences in visuospatial and navigational working memory: the role of mood induced by background music. Exp Brain Research 2016; 234: 2381–9. doi: 10.1007/s00221-016-4643-3 [DOI] [PubMed] [Google Scholar]
- 24.Nori R, Piccardi L, Migliori M, Guidazzoli A, Frasca F, et al. Sex differences in learning real and virtual environments. Computers Hum Behav 2015; 48: 72–77. [Google Scholar]
- 25.Ramanoël S, York E, Le Petit M, Lagrené K; Habas C, et al. Age-Related Differences in Functional and Structural Connectivity in the Spatial Navigation Brain Network. Front. Neural. Circuits 2019; 13: 69. doi: 10.3389/fncir.2019.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piccardi L, Nori R, Palermo L, Guariglia C. Age effect in generating mental images of building but not common objects. Neurosc. Lett. 2015; 602: 79–83. doi: 10.1016/j.neulet.2015.06.058 [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Baizan C, Diaz-Caceres E, Arias JL, Mendez M. Egocentric and allocentric spatial memory in healthy aging: Performance on real-world tasks. Braz. J. Med. Biol. Res. 2019; 52: e8041. doi: 10.1590/1414-431X20198041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muffato V, Hilton C, Meneghetti C, De Beni R, Wiener JM. Evidence for age-related deficits in object-location binding during place recognition. Hippocampus 2019; 29: 971–979. doi: 10.1002/hipo.23099 [DOI] [PubMed] [Google Scholar]
- 29.Li AWY, King J. Spatial memory and navigation in ageing: A systematic review of MRI and fMRI studies in healthy participants. Neurosci. Biobehav. Rev. 2019; 103: 33–49. doi: 10.1016/j.neubiorev.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 30.Moffat SD, Zonderman AB, Resnick SM. Age differences in spatial memory in a virtual environment navigation task. Neurobiol. Aging 2001; 22: 787–796. doi: 10.1016/s0197-4580(01)00251-2 [DOI] [PubMed] [Google Scholar]
- 31.Guzmán JF, Pablos AM, Pablos C. Perceptual-cognitive skills and performance in orienteering. Percept. Mot. Skills 2008; 107: 159–164. doi: 10.2466/pms.107.1.159-164 [DOI] [PubMed] [Google Scholar]
- 32.Verde P, Piccardi L, Bianchini F, Guariglia C, Carrozzo P, et al. Gender has no effect on pilots’ navigational memory. AMHP 2015; 86: 103–111. doi: 10.3357/AMHP.4024.2015 [DOI] [PubMed] [Google Scholar]
- 33.Verde P, Angelino G, Piccolo F, Carrozzo P, Bottiglieri A et al. Spatial orientation and directional judgments in pilots vs. nonpilots. AMHP 2018; 89: 857–862. doi: 10.3357/AMHP.5023.2018 [DOI] [PubMed] [Google Scholar]
- 34.Verde P, Angelino G, Piccolo F, Carrozzo P, Piccardi L et al. Engineers’ abilities influence spatial perspective changing. IJEE 2019; 1: 106–113. 10.14710/ijee.1.2.106-113 [DOI] [Google Scholar]
- 35.Weisberg SM, Newcombe NS, Chatterjee A. Everyday taxi drivers: Do better navigators have larger hippocampi? Cortex 2019; 115: 280–293. doi: 10.1016/j.cortex.2018.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giancola M, Verde P, Cacciapuoti L, Angelino G, Piccardi L, et al. Do Advanced Spatial Strategies Depend on the Number of Flight Hours? The Case of Military Pilots. Brain Sci.2021; 11: 851. doi: 10.3390/brainsci11070851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nori R, Piccardi L. Familiarity and spatial cognitive style: How important are they for spatial representation? In Spatial Memory: Visuospatial Processes, Cognitive Performance and Developmental Effects; Thomas J.B., Ed.; Nova Science: New York, NY, USA, 2011; pp. 103–124. [Google Scholar]
- 38.Piccardi L, Palmiero M, Bocchi A, Boccia M, Guariglia C. How does environmental knowledge allow us to come back home? Exp. Brain Res. 2019; 237: 1811–1820. doi: 10.1007/s00221-019-05552-9 [DOI] [PubMed] [Google Scholar]
- 39.Lopez A, Caffò AO, Bosco A. Topographical disorientation in aging. Familiarity with the environment does matter. Neurol. Sci. 2018; 39: 1519–1528. doi: 10.1007/s10072-018-3464-5 [DOI] [PubMed] [Google Scholar]
- 40.Nori R, Piccardi L, Maialetti A, Goro M, Rossetti A et al. No Gender Differences in Egocentric and Allocentric Environmental Transformation after Compensating for Male Advantage by Manipulating Familiarity. Front. Neurosci. 2018; 12: 204. doi: 10.3389/fnins.2018.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Craig T, Conniff A, Galan-Diaz C. The influences of actual and perceived familiarity on environmental preferences for the design of a proposed urban square. Urb Stud Res, 2012; 9 pages, doi: 10.1155/2012/767049 [DOI] [Google Scholar]
- 42.Zajonc RB. Feeling and thinking: preferences need no inferences, Am Psychol, 1980; 35: 151–175, [Google Scholar]
- 43.Bondi D, Verratti V, Nori R, Piccardi L, Prete G et al. Spatial Abilities at High Altitude Exploring the Role of Cultural Strategies and Hypoxia. High Alt Med Biol, 2021; 22: 157–165 doi: 10.1089/ham.2020.0115 [DOI] [PubMed] [Google Scholar]
- 44.Zucchelli MM, Piccardi L, Nori R. The fear to move in a crowded environment. Poor spatial memory related to agoraphobic disorder. Brain Sciences, 2021; 11 (6): 796. doi: 10.3390/brainsci11060796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kallai J, Karádi K, Bereczkei T, Rózsa S, Jacobs WJ et al. Spatial exploration behaviour in an extended labyrinth in patientswith panic disorder and agoraphobia. Psych Res 2007; 149: 223–30. doi: 10.1016/j.psychres.2003.12.032 [DOI] [PubMed] [Google Scholar]
- 46.Kállai J, Karádi K, Feldmann A. Anxiety-dependent spatial navigation strategies in virtual and real spaces. CognProcess. 2009; 10 Suppl 2:S229–32. doi: 10.1007/s10339-009-0288-5 [DOI] [PubMed] [Google Scholar]
- 47.Boccia M, Di Vita A, Diana S, Margiotta R, Imbriano L, et al. Is Losing One’s Way a Sign of Cognitive Decay? Topographical Memory Deficit as an Early Marker of Pathological Aging. J Alzheimers Dis. 2019; 68(2):679–693. doi: 10.3233/JAD-180890 [DOI] [PubMed] [Google Scholar]
- 48.Coughlan G, Laczo J, Hort J, Minihane AM, Hornberger M. Spatial navigation deficits—overlooked cognitive marker for preclinical Alzheimer disease? Nature Reviews Neurology, 2018; 14: 496–506. doi: 10.1038/s41582-018-0031-x [DOI] [PubMed] [Google Scholar]
- 49.Tu S, Wong S, Hodges JR, Irish M, Piguet O, Hornberger M. Lost in spatial translation—A novel tool to objectively assess spatial disorientation in Alzheimer’s disease and frontotemporal dementia. Cortex, 2015; 67, 83–94. doi: 10.1016/j.cortex.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 50.Guariglia C, Piccardi L, Iaria G, Nico D, Pizzamiglio L. Representational neglect and navigation in real space, Neuropsychologia, 2005; 43: 1138–1143. doi: 10.1016/j.neuropsychologia.2004.11.021 [DOI] [PubMed] [Google Scholar]
- 51.Siegel AW, White SH The development of spatial representations of large-scale environments, in Advances in Child Development & Behavior, Reese HW(Ed.) New York, NY: Academic Press, 1975; 9–55. [DOI] [PubMed] [Google Scholar]
- 52.Pazzaglia F, De Beni R. Strategies of processing spatial information in survey and landmark centered individuals. Eur. J. Cogn. Psychol. 2001; 13: 493–508. 10.1080/09541440125778 [DOI] [Google Scholar]
- 53.Shelton AL, Marchette SA, Furman AJ. A mechanistic approach to individual differences in spatial learning. Psychol. Learn. Motiv. 2013; 59: 223–258. 10.1016/B978-0-12-407187-2.00006-X [DOI] [Google Scholar]
- 54.Lopez A, Caffò AO, Postma A, Bosco A. How to separate coordinate and categorical spatial relation components in integrated spatial representations: A new methodology for analysing sketch maps. SJoP, 2020; 61: 607–615. doi: 10.1111/sjop.12633 [DOI] [PubMed] [Google Scholar]
- 55.Lopez A, Postma A, Bosco A. Categorical & coordinate spatial information: Can they be disentangled in sketch maps? J. Environ. Psychol., 2020; 68: 101392. 10.1016/j.jenvp.2020.101392 [DOI] [Google Scholar]
- 56.Ruotolo F, van der Ham IJM, Iachini T, Postma A The relationship between allocentric and egocentric frames of reference and categorical and coordinate spatial information processing. Q J Exp Psychol 2011; 64:1138–1156. doi: 10.1080/17470218.2010.539700 [DOI] [PubMed] [Google Scholar]
- 57.Ekstrom A, Ranganath C. Space, time and episodic memory: The hippocampus is all over the cognitive map. Hippocampus 2018; 28: 680–87. doi: 10.1002/hipo.22750 [DOI] [PubMed] [Google Scholar]
- 58.Lancioni GE, Perilli V, O’Reilly MF, Singh NN, Sigafoos J, et al. Technology-based orientation programs to support indoor travel by persons with moderate Alzheimer’s disease: Impact assessment and social validation. Res. Dev. Disabil., 2013; 34: 286–293. doi: 10.1016/j.ridd.2012.08.016 [DOI] [PubMed] [Google Scholar]
- 59.Lopez A, Caffò AO, Bosco A. The impact of age and familiarity with the environment on categorical and coordinate spatial relation representations. Sc J Psychol, 2021; 62: 125–133. doi: 10.1111/sjop.12703 [DOI] [PubMed] [Google Scholar]
- 60.Constant M, Mellet E. The Impact of Handedness, Sex, and Cognitive Abilities on Left–Right Discrimination: A Behavioral Study. Front. Psychol. 2018; 9:405. doi: 10.3389/fpsyg.2018.00405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hannay HJ, Ciaccia PJ, Kerr JW, Barrett D. Self-report of right-left confusion in college men and women. Percept. Mot. Skills 1990; 70: 451–457. doi: 10.2466/pms.1990.70.2.451 [DOI] [PubMed] [Google Scholar]
- 62.van der Ham IJ, Dijkerman HC, van Stralen HE Distinguishing left from right: A large-scale investigation of left–right confusion in healthy individuals. Q. J. Exp. Psychol., 2021; 74: 497–509. doi: 10.1177/1747021820968519 [DOI] [PubMed] [Google Scholar]
- 63.Nori R, Giusberti F. Predicting cognitive styles from spatial abilities. Am. J. Psychol., 2006; 119: 67–86. [PubMed] [Google Scholar]
- 64.Nori R, Palmiero M, Bocchi A, Giannini AM, Piccardi L. The specific role of spatial orientation skills in predicting driving behaviour. Transportation Research Part F: Traffic Psychological Behaviour, 2020; 71: 259–271. 10.1016/j.trf.2020.04.009 [DOI] [Google Scholar]
- 65.Bocchi A, Palmiero M, Boccia M, Di Vita A, Guariglia C et al. Travel planning ability in right brain-damaged patients: Two case reports. Front. Hum. Neurosci. 2020; 14: 117. doi: 10.3389/fnhum.2020.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bocchi A, Palmiero M, Piccardi L. Travel planning in men and women. Who is better? Curr. Psychol. 2021. 10.1007/s12144-021-01451-x [DOI] [Google Scholar]
- 67.Piccardi L, De Luca M, Nori R, Palermo L, Iachini F et al. Navigational Style Influences Eye Movement Pattern during Exploration and Learning of an Environmental Map. Front. Behav. Neurosci. 2016; 10:140. doi: 10.3389/fnbeh.2016.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iaria G, Bogod N, Fox CJ, Barton JJ. Developmental topographic disorientation: Case one. Neuropsychologia 2009; 47: 30–40. doi: 10.1016/j.neuropsychologia.2008.08.021 [DOI] [PubMed] [Google Scholar]
- 69.Bianchini F, Incoccia C, Palermo L, Piccardi L, Zompanti L et al. Developmental topographical disorientation in a healthy subject. Neuropsychologia 2010, 48, 1563–1573. doi: 10.1016/j.neuropsychologia.2010.01.025 [DOI] [PubMed] [Google Scholar]
- 70.Bianchini F, Palermo L, Piccardi L, Incoccia C, Nemmi F, et al. Where am I? A new case of Developmental Topographical Disorientation. J. Neuropsychol. 2014; 8: 107–124. doi: 10.1111/jnp.12007 [DOI] [PubMed] [Google Scholar]
- 71.Nemmi F, Bianchini F, Piras F, Péran P, Palermo L, et al. Finding my own way: An fMRI single case study of a subject with developmental topographical disorientation. Neurocase, 2015; 21: 573–583. doi: 10.1080/13554794.2014.960424 [DOI] [PubMed] [Google Scholar]
- 72.Palermo L, Foti F, Ferlazzo F, Guariglia C, Petrosini L. I find my way in a maze but not in my own territory! Navigational processing in developmental topographical disorientation. Neuropsychology, 2014; 28: 135–146. doi: 10.1037/neu0000021 [DOI] [PubMed] [Google Scholar]
- 73.Palermo L, Piccardi L, Bianchini F, Nemmi F, Giorgio V.et al. Looking for the compass in a case of developmental topographical disorientation: A behavioral and neuroimaging study. J Clin Exp Neuropsycho, 2014; 36: 464–81. doi: 10.1080/13803395.2014.904843 [DOI] [PubMed] [Google Scholar]
- 74.Iaria G, Barton JJ. Developmental topographical disorientation: A newly discovered cognitive disorder. Exp Brain Res, 2010; 206: 189–196. doi: 10.1007/s00221-010-2256-9 [DOI] [PubMed] [Google Scholar]
- 75.Conson M, Bianchini F, Quarantelli M, Boccia M, Salzano S, et al. Selective map-following navigation deficit: A new case of developmental topographical disorientation. J. Clin. Exp. Neuropsychol. 2018; 40 (9): 940–950. doi: 10.1080/13803395.2018.1451493 [DOI] [PubMed] [Google Scholar]
- 76.Guariglia C, Piccardi L Ariadne’s thread and the unravelling of navigational skills development In: Les arts de la mémoire et les images mentales [Berthoz A; Scheid J. Eds.]. Paris: Collège de France, 2018. (généré le 28 septembre 2018). ISBN: 9782722604988. [Google Scholar]
- 77.Piccardi L, De Luca M, Di Vita A, Palermo L, Tanzilli A, et al. Evidence of taxonomy for Developmental Topographical Disorientation: Developmental Landmark Agnosia Case 1. Appl Neuropsychol Child. 2019; 8(2):187–198. doi: 10.1080/21622965.2017.1401477 [DOI] [PubMed] [Google Scholar]
- 78.Schubotz RI, von Cramon Y. Sequences of abstract nonbiological stimuli share ventral premotor cortex with action observation and imagery. J. Neurosci. 2004; 24: 5467–5474. doi: 10.1523/JNEUROSCI.1169-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wildgruber D, Kischka U, Ackermann H, Klose U, Grodd W. Dynamic pattern of brain activation dur- ing sequencing of word strings evaluated by fMRI. Cogn. Brain Res. 1999; 7: 285–294. doi: 10.1016/S0926-6410(98)00031-7 [DOI] [PubMed] [Google Scholar]
- 80.Iaria G, Arnold AE, Burles F, Liu I, Slone E et al. Developmental Topographical Disorientation and decreased hippocampal functional connectivity. Hippocampus 2014; 24: 1364–74. doi: 10.1002/hipo.22317 [DOI] [PubMed] [Google Scholar]
- 81.Nori R, Piccardi L. Il senso dell’orientamento: quanto conta la familiarità con l’ambiente? GIP; 2012; 39: 343–368. [Google Scholar]
- 82.Piccardi L, Risetti M, Nori R (2011) How well do you know your city? Effects of familiarity on environmental representations: A self-report study. Psychol. Rep., 2011; 109: 309–326. [DOI] [PubMed] [Google Scholar]
- 83.Nori R., Giusberti F. Cognitive styles: Errors in the directional judgements. Perception, 2003; 32: 307–320. doi: 10.1068/p3380 [DOI] [PubMed] [Google Scholar]
- 84.Nori R, Piccardi L. I believe I’m good at orienting myself… But is that true? Cogn Process 2015; 16: 301–7. doi: 10.1007/s10339-015-0655-3 [DOI] [PubMed] [Google Scholar]
- 85.Nunnally JC, Bernstein IH. Psychometric tehory, 3rd edn., 1994; McGraw-Hill, New York. [Google Scholar]
- 86.Akoglu H. User’s guide to correlation coefficients. Turkish journal of emergency medicine, 2018; 18: 91–93. doi: 10.1016/j.tjem.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: variability and change with practice. J. Neurosci. 2003; 22: 5945–5952. 10.1523/JNEUROSCI.23-13-05945.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bartonek Å, Guariglia C, Piccardi L. Topographical working memory in children and adolescents with motor disabilities. Cogent Psychology, 2020; 7:1, doi: 10.1080/23311908.2020.1757855 [DOI] [Google Scholar]
- 89.Bartonek Å, Piccardi L, Guariglia C. Topographical Working Memory in Children with Cerebral Palsy, Journal of Motor Behavior, 2021; 53: 200–208– doi: 10.1080/00222895.2020.1748861 [DOI] [PubMed] [Google Scholar]
- 90.Bartonek Å, Guariglia C, Piccardi L. Locomotion and Topographical Working Memory in Children With Myelomeningocele and Arthrogryposis Multiplex Congenita. Front. Psychiatry 2021; 12:729859. doi: 10.3389/fpsyt.2021.729859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Verde P, Piccardi L, Bianchini F, Trivelloni P., Guariglia C et al. (2013). Gender effects on mental rotation in pilots vs. nonpilots. Aviat. Space Environ. Med. 84, 726–729. doi: 10.3357/asem.3466.2013 [DOI] [PubMed] [Google Scholar]
- 92.Verde P, Boccia M, Colangeli S, Barbetti S, Nori R, et al. Domain-Specific Interference Tests on Navigational Working Memory in Military Pilots. Aerosp Med Hum Perform, 2016; 87(6): 528–33. doi: 10.3357/AMHP.4521.2016 [DOI] [PubMed] [Google Scholar]
- 93.Palermo L, Iaria G, Guariglia C. Mental imagery skills and topographical orientation in humans: a correlation study. Behav. Brain Res. 2008; 192(2): 248–253. doi: 10.1016/j.bbr.2008.04.014 [DOI] [PubMed] [Google Scholar]
- 94.Lopez A, Germani A, Tinella L, Caffò AO, Postma A, et al. The Road More Travelled: The Differential Effects of Spatial Experience in Young and Elderly Participants. Int. J. Environ. Res. Public Health 2021; 18: 709. doi: 10.3390/ijerph18020709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boccia M, Rosella M, Vecchione F, Tanzilli A, Palermo L, et al. Enhancing Allocentric Spatial Recall in Pre-schoolers through Navigational Training Programme. Front Neurosci. 2017; 11:574. doi: 10.3389/fnins.2017.00574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Faedda N, Guariglia C, Piccardi L, Natalucci G, Rossetti S, et al. Link Between Topographic Memory and the Combined Presentation of ADHD (ADHD-C): A Pilot Study. Front. Psychiatry 2021; 12:647243. doi: 10.3389/fpsyt.2021.647243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carretti B, Borella E, Zavagnin M, De Beni R Impact of metacognition and motivation on the efficacy of strategic memory training in older adults: analysis of specific, transfer and maintenance effects. Arch Gerontol Geriatr 2011; 28:331–347. doi: 10.1016/j.archger.2010.11.004 [DOI] [PubMed] [Google Scholar]
- 98.De Lisi R, Wolford JL Improving children’s mental rotation accuracy with computer game playing. J Genet Psychol 2002; 163:272–282. doi: 10.1080/00221320209598683 [DOI] [PubMed] [Google Scholar]