Abstract
Background
Female genital schistosomiasis (FGS) constitutes four different lesions known to be caused by Schistosoma haematobium ova deposited in the genital tract. Schistosoma mansoni ova may also be found in the genital tract. However, it is not known if S. mansoni causes lower genital tract lesions characteristic of FGS.
Methodology
This study was conducted in 8 villages along the shores of Lake Victoria, western Kenya. Stool and urine samples, collected from women of reproductive age on three consecutive days, were analysed for S. mansoni and S. haematobium infection. S. mansoni positive and S. haematobium negative willing participants, aged 18–50 years were invited to answer a questionnaire (demographics, symptoms), undergo a gynaecological examination and cytology specimen collection by an FGS expert.
Principal findings
Gynaecologic investigations were conducted in 147 S. mansoni-positive women who had a mean infection intensity of 253.3 epg (95% CI: 194.8–311.9 epg). Nearly 90% of them used Lake Victoria as their main water source. None were found to have cervicovaginal grainy sandy patches or rubbery papules. Homogenous yellow patches were found in 12/147 (8.2%) women. Women with homogenous yellow patches were significantly older (47 years) than the rest (34 years, p = 0.001). No association was found between intensity of S. mansoni infection and homogenous yellow patches (p = 0.70) or abnormal blood vessels (p = 0.14). S. mansoni infection intensity was not associated with genital itch, bloody or malodorous vaginal discharge.
Conclusion
S. mansoni infection was neither associated with lower genital tract lesions nor symptoms typically found in women with FGS.
Author summary
Women who have fresh water contact in rivers or lakes may get infected with the waterborne parasites, that can cause Bilharzia, also called schistosomiasis. In Africa, Schistosoma (S.) haematobium is associated with HIV acquisition, genital tract lesions, bloody and malodorous discharge. There is also decreased clearance of human papillomavirus (HPV) in women with genital schistosomiasis. There are occasional case reports showing eggs of S. mansoni (intestinal schistosomiasis) in the genitals. However, no study has systematically looked for the genital tract lesions of S. mansoni and the impact on gynaecological health has never been explored. We sought to explore if S. mansoni causes the lower genital tract genital lesions. In women living near Lake Victoria, Kenya, we included 147 women who had S. mansoni but not S. haematobium infection. We found that the few women who had signs of sequelae of schistosomiasis infection in the genitals, so-called homogenous yellow patches had all potentially been exposed to S. haematobium in their youth. In this study population we found no lower genital tract lesions or genital symptoms to be associated with S. mansoni.
Introduction
Schistosomiasis is endemic in sub-Saharan Africa where close to 205 million people are infected and many more are at risk of infection [1]. The most vulnerable are children and women who frequently come into contact with snail-infested water while playing, or in the course of their daily chores [2]. Despite current treatment efforts with mass drug administration using praziquantel, re-infections and chronic infection occur leading to morbidity that may last for decades, including Female Genital Schistosomiasis (FGS) [3–5].
All the human Schistosoma species may deposit ova in the genitals, sometimes denoted “ectopic schistosomiasis”. S. mansoni ova, usually located in the lower gastrointestinal tract, are sometimes also found as far as the urinary tract and it is hypothesized that ova travel from the intestinal tract to the genital organs through blood vessel anastomoses in the pelvis [6,7]. However, lower genital tract lesions have almost exclusively been reported from S. haematobium infected women [8–14]. FGS affects an estimated 56 million women worldwide [15].
Kenya is endemic for both the intestinal form (caused by S. mansoni) and the urogenital form (caused by S. haematobium) of schistosomiasis, with an estimate of approximately 9 million people infected and approximately 17.4 million at risk [16]. S. haematobium infection manifests itself with bloody urine, pain on urination, and higher risk of bladder cancer, genital symptoms and lesions [13,17]. The lower genital tract manifestations in women are grainy sandy patches, homogenous yellow patches, rubbery papules, abnormal blood vessels and mucosal bleeding of the surfaces, both in the vagina wall and on the cervix [18]. FGS is associated with decreased fertility, ectopic pregnancies, abdomino-pelvic pain, genital itch, dyspareunia, foul smelling discharge, and has been found to be associated with HIV [19–23].
There are a number of case reports of S. mansoni ova in the genital tract and it has been hypothesized that it could lead to similar lower genital tract morbidity [24–26]. However, only a paucity of studies have explored this. A study in Brazil, where only S. mansoni is endemic, found no genital tract morbidity due to schistosomiasis [27], but S. mansoni infection intensity was low and participants had received repeated praziquantel treatment. A second study by Downs and others in an area endemic for both S. haematobium and S. mansoni in Tanzania reported that 5% of the women had Schistosoma ova in cytology smears, but the authors did not differentiate the cases according to Schistosoma species and genitalia were not inspected for lesions [28]. Interestingly, the prevalence of HIV was higher in the S. mansoni villages than in the S. haematobium villages [28]. A qualitative study in western Kenya found that genital health problems were reported among women exposed to S. mansoni, but no diagnostic tests were done and there was no control group [29]. Cunin et al found that people with very high infection intensity of dual infection with S. mansoni and S. haematobium made it more likely to find S. mansoni ova in urine, possibly as a result of “worm over-crowding” [7].
HIV/AIDS is a leading cause of death in Kenya and is disproportionately high in Nyanza, western Kenya along the shores of Lake Victoria [30,31], an area that also has a high prevalence of S. mansoni [32–35]. Based on these observations and the hypothesis that S. mansoni may also be a risk factor for HIV [28], we sought to explore the prevalence of FGS in a S. mansoni-endemic area, investigate if S. mansoni causes lesions in the lower genital tract, and characterize the gynaecological symptoms in women with S. mansoni infection.
Methods
Ethics statement
Approval for the study was obtained from the Kenya Medical Research Institute (KEMRI) Scientific Steering Committees as well as the KEMRI National Ethical Review Committee (KEMRI SSC # 2937). Consent documents and participation information were approved and provided to potential study participants in English or the local language, Dholuo. The local authorities and health professionals were informed about the study. Informed written consent was obtained from each participant. A unique coded identification number was assigned to each subject and was used for sample tracking. All identifiers were delinked from data for the analysis. Privacy and confidentiality were strictly maintained. The risks of the study were minimal, with only the temporary discomfort and brief embarrassment associated with the gynaecological examination. All participants were treated with 40 mg/kg praziquantel (PZQ) and those infected with soil-transmitted helminths (STHs) were treated with 400 mg albendazole. In addition, treatment was provided in accordance with the Kenyan syndromic management protocol for sexually transmitted infections (STI). Results and treatment were delivered privately to the participant by a local professional nurse immediately after the study procedures.
Study sites
The study was conducted in Rachuonyo North and Kisumu West sub-counties (Fig 1), western Kenya in November 2015. The area is characterized by a modified equatorial climate. It is generally warm and humid with the long rains falling from March to May and the short rains between August and November/December, with an average annual temperature of 25°C. In this study, we focused on villages that are within a 5 km distance from Lake Victoria, where S. mansoni prevalence is the highest [36]. Together with other sub-counties bordering the lake, the region has the highest prevalence of HIV/AIDS (15.1%) in Kenya amongst persons aged 15–64 years [30]. The exact HIV prevalence in the selected communities was not known, however the overall prevalence of HIV in Kisumu was 16.3% [37]. The chief mode of HIV transmission is thought to be via heterosexual intercourse [38,39].
Fig 1. Study sites in western Kenya.
(A) Kisumu West and (B) Rachuonyo North sub-counties, https://opensource.com/.
Study population
The majority of the population consisted of the Luo ethnic community who were at risk of infection with S. mansoni through their occupational or recreational activities. This cross-sectional study focused on 18–50 year old women. At the time of the study, mass drug administration for schistosomiasis control was unavailable for adults in this endemic area.
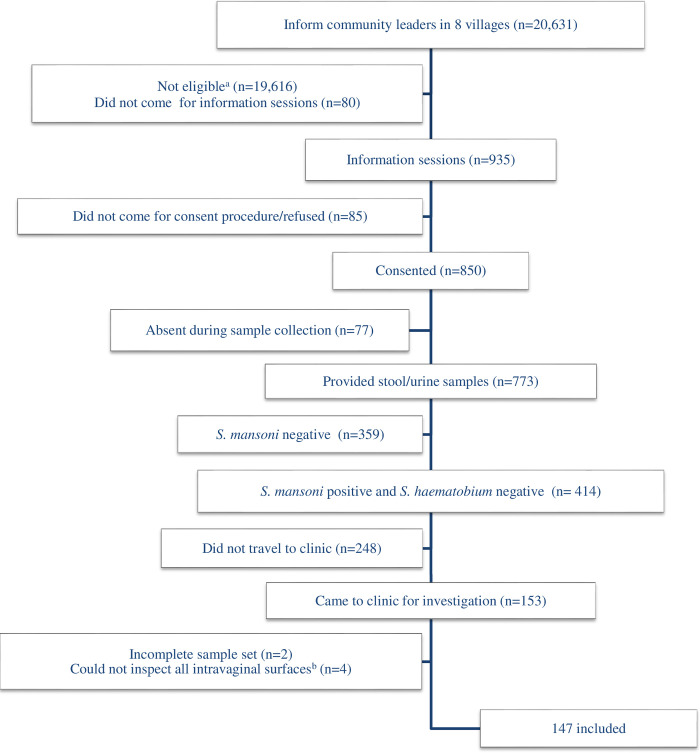
Women were approached at home by a community health worker to introduce the study and invite potential participants on a specific date to a specified health facility in their community. The investigators held information meetings and sought consent. Women who were not observably ill, were willing to take part in the study, and to provide the requested samples (stool, urine, blood, and intra-vaginal examination) were invited to participate. Only women who were S. mansoni egg-positive and S. haematobium egg-negative were invited for gynaecological examination. Fig 2 provides an overview of the participant recruitment process.
Fig 2. Participant recruitment.
aMales, children, outside age range. bCervix, fornices, lateral, anterior and/or posterior vaginal walls.
Laboratory investigations
Stool and urine samples from three consecutive days were collected at the health facility, transported to the KEMRI laboratory and investigated microscopically for S. mansoni and S. haematobium eggs. Two Kato-Katz smears were prepared from each stool sample and read by trained microscopists. Eggs were enumerated to determine eggs per gram of faeces (epg) and the presence or absence of the STHs (Ascaris lumbricoides, Trichuris trichiura, hookworm) was noted. Intensity of infection for schistosomiasis was calculated based on arithmetic mean egg counts and categorized according to the WHO classification as negative for no detectable eggs; light S. mansoni infection for 1–99 epg; moderate for 100–399 epg and ≥400 epg for heavy [40]. Urine samples were thoroughly mixed and a 10 ml aliquot of urine filtered through 12 micrometres, 13 millimetres Polycarbonate (PCTE) membrane filters (Sterlitech; Kent, WA). The filter paper was then placed on a labelled slide and a drop of Lugol’s solution added. The slides were then examined under a microscope for S. haematobium and S. mansoni eggs [40].
Questionnaires and clinical examinations
A questionnaire on water contact behaviour, reproductive history, water sources, water exposure, genital and abdominal symptoms was administered individually to S. mansoni egg-positive/S. haematobium egg-negative participants in the local language (Dholuo) prior to gynaecological examination. The clinician performing the exams was blinded to the childhood origin of the women and the intensity of S. mansoni infection. Examination was commenced by cervico-vaginal lavage. Saline (10 ml) was sprayed on the vaginal wall and cervix twice, whereupon it was drawn back into a syringe and deposited into four tubes. This was followed by photocolposcopic examination (Leisegang Photocolposcope, Germany, Magnifications 7.5; 15; 30) using an autoclaved metal speculum after which Pap (Papanicolau) smears were collected from all consenting women [41]. The cervix, the fornices, the entire vaginal wall and vulval surfaces were inspected section by section according to a predefined protocol [41]. Acetic acid and/or iodine application for colposcopic examination was always done last. The grainy sandy patch diagnosis was defined as observing grains approximately 0.05 mm by 0.2 mm long, shaped as minuscule rice grains, appearing singly or in clusters of up to 300 grains [42]. The homogeneous yellow patches were defined as sandy looking areas with no visible grains when using the x15 magnification setting on the colposcope. Rubbery papules were defined as papulous lesions, firm as hard rubber, that had only been seen in Madagascan women by the same clinician who had previously investigated women for FGS in four Southern African countries [43]. Abnormal blood vessels were defined as pathological convoluted (cork-screw), reticular, circular and/ or branched, uneven-calibre blood vessels visible (by x15 magnification) on the mucosal surface. Contact bleeding was defined as fresh blood originating from the mucosal surface. Pre-contact bleeding was defined as darkened blood on the mucosal surface in the absence of recent or present menstruation. A polyp was defined as a single, smooth pedunculated mass originating from the endocervix or from the mucosal surface. Leucoplakia was defined as white plaque on the mucosal surface, visible with or without acetic acid. Papilloma was defined as a sessile mass either on the mucosa or vulva, whitish in colour, often with a cauliflower appearance.
Data management and statistical analyses
Data were entered into Excel, and analysed using SPSS version 12 and Graphpad Prism 5. Prevalence of S. mansoni and soil-transmitted helminths are presented for all who underwent gynaecological examination. S. mansoni data is also presented for those who submitted stool but did not attend the research clinic. Univariate and multivariate analyses were used to determine associations between the clinical manifestations and the intensity of S. mansoni infection. P values < 0.05 were considered statistically significant.
Results
Out of the 773 women who provided stool samples, 414 (53.6%) had S. mansoni infection with a mean intensity of 185 epg (95%CI: 155–216 epg). A total of 72 women from Kisumu West and 79 from Rachuonyo North sub-counties came for gynaecological investigations. We included 147 women as 2 had recently delivered a baby and 2 had undergone hysterectomies. The mean age of menarche was 14 years (SD = 1.96).
Among the 147 women who underwent gynaecological examinations, the mean intensity of S. mansoni infection was 253 epg (95% CI: 195–312 epg). Table 1 shows that almost all these women had contact with Lake Victoria waters at least once per day, more than 90% lived less than 900 metres from the lake, and most used the lake as their main water source for domestic chores. Table 2 shows that more than half of the included population had heavy or moderate infections. The prevalence of the soil-transmitted helminths was 4.5% (95%CI: 3.3–6.3) for T. trichiura, 2.2% (95%CI: 1.4–3.5) for A. lumbricoides, and 0.3% (95%CI: 0.01–1) for hookworm respectively. No S. mansoni eggs were detected in the urine of any of the participants. None of the women had Schistosoma ova in their Pap smears.
Table 1. Characteristics of 147 women in Schistosoma mansoni endemic villages near Lake Victoria, Kenya.
| Variable | Statistic | % |
|---|---|---|
| Age (years) | ||
| 18–19 | 5/147 | 3.4 |
| 20–24 | 29/147 | 19.7 |
| 25–29 | 20/147 | 13.6 |
| 30–39 | 45/147 | 30.6 |
| 40 and above | 48/147 | 32.7 |
| Number of children | ||
| Infertile | 2/147 | 1.4 |
| 1–3 | 69/147 | 46.9 |
| 4 and more | 76/147 | 51.7 |
| Distance between home and Lake Victoria (km) | ||
| 0–0.9 | 136/147 | 92.5 |
| 1–1.9 | 6/147 | 4.1 |
| 2–2.9 | 2/147 | 1.4 |
| > 3 | 3/147 | 2.0 |
| Main water source | ||
| Lake Victoria | 131/147 | 89.1 |
| River | 13/147 | 8.8 |
| Borehole | 3/147 | 2.0 |
| Frequency of contact with Lake Victoria (times/week) | ||
| None | 3/147 | 2.0 |
| 1 to 7 | 83/147 | 56.4 |
| 8–27 | 40/147 | 27.2 |
| 28–41 | 14/147 | 9.5 |
| 42–56 | 7/147 | 4.8 |
| Main source of income | ||
| Farming | 34/147 | 23.1 |
| Fish trade | 35/147 | 23.8 |
| Housewife | 21/147 | 14.3 |
| Other | 57/147 | 38.8 |
Table 2. Prevalence and intensity of Schistosoma mansoni infection in included and non-included women of reproductive age in Kisumu West and Rachuonyo North sub-Counties.
| Sub-County | Category | N | Prevalence (CI)1 | Intensity prevalence (%) Light Moderate Heavy | Infection intensity (epg)2 (CI)1 | ||
|---|---|---|---|---|---|---|---|
| Kisumu West | Screened | 262 | 45.8 (39.9–51.9) | 54.2 | 27.5 | 18.3 | 243.3 (173.3–313.2) |
| Included | 70 | 100c (93.7–100) | 48.6 | 28.6 | 22.9 | 311.1 (201.1–421.1) | |
| Rachuonyo North | Screened | 511 | 57.5 (53.2–61.8) | 61.2 | 27.5 | 11.2 | 161.6 (129.5–193.7) |
| Included | 77 | 100c (94.3–100) | 37.7 | 49.4 | 12.9 | 200.8 (150.8–250.9) | |
| Overall | Screened | 773 | 53.6 (50–57) | 59.2 | 27.5 | 13.3 | 185.3 (154.7–215.8) |
| Included | 147 | 100c (96.9–100) | 42.8 | 39.5 | 17.7 | 253.3 (194.8–311.9) | |
a95% Confidence Interval.
bArithmetic mean infection intensity of eggs found in stool.
cAll included for gynae examination were S. mansoni positive
Gynaecological examinations
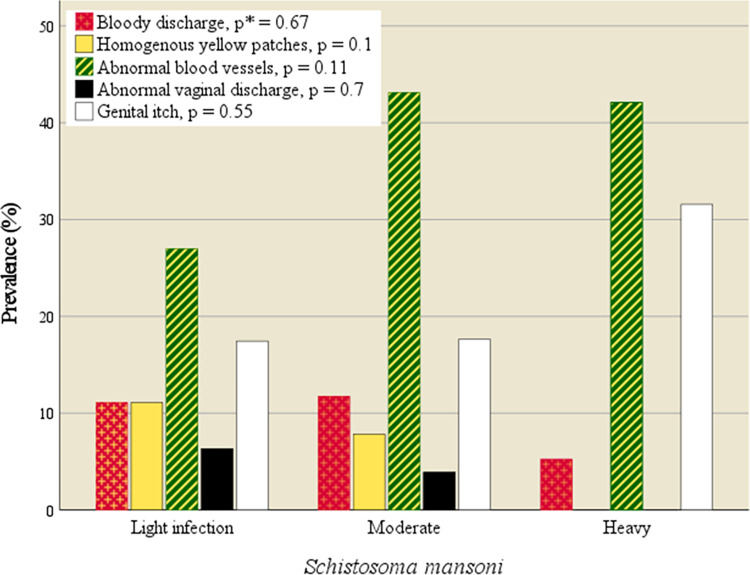
Homogenous yellow patches were found in 12/147 (8.2%) of the study population. No participants were found to have grainy sandy patches or rubbery papules. The women with homogenous yellow patches were significantly older than the remaining population (OR 1.1, 95% CI: 1.0–1.2, p = 0.001), mean age 47 years (SD 11.2) versus 34 years (SD 10.7). Homogenous yellow patches were not associated with the intensity of S. mansoni infection (age-Adjusted Odds Ratio (AOR) 0.6, 95% CI: 0.1–6.7, p = 0.70) (Fig 3). Likewise, having abnormal blood vessels on the mucosal surface (49/147, 33%), was not associated with the intensity of S. mansoni infection (AOR 1.0, 95% CI: 1.0–1.0, p = 0.14). In 7 of the 147 women (4.8%), not all the surfaces of the fornices could be seen.
Fig 3. Genital symptoms and findings by S. mansoni intensity.
No significant association between symptoms and intensity of S. mansoni. *Likelihood ratio p-value.
Symptoms
More than half of the population had genital symptoms (58%). S. mansoni intensity was not associated with higher prevalence of abnormal vaginal discharge (AOR 0.8, 95% CI: 0.1–6.7, p = 0.81), bloody discharge (AOR 0.2, 95% CI: 0.1–0.8, p = 0.21), or genital itch (AOR 0.8, 95% CI: 0.2–3.2, p = 0.79), as shown in Fig 3. Homogenous yellow patches (n = 12) were not associated with any of the symptoms (p >0.39).
Potential S. haematobium exposure
In a selection of 140 women who were fully investigated gynecologically (all surfaces seen), 16 (11.4%) said they had grown up “far away” from Lake Victoria, although the exact distance could not be determined. They also indicated that they used other water sources in their childhood, such as rivers. These women had significantly more homogenous yellow patches (OR 5.6, 95% CI 1.4–21.8, p = 0.014). The age-adjusted odds ratio (AOR) was 3.9 (95% CI: 0.92–16.9, p = 0.065). Childhood residence or river contact did not influence the presence of abnormal blood vessels (p > 0.8).
Discussion
In an area highly endemic for Schistosoma mansoni, we found that S. mansoni infection was not associated with any of the FGS lesions [18]. To our knowledge, this is the first study in women with S. mansoni where the antero-posterior and lateral vaginal surfaces, fornices and cervix were all inspected by an FGS expert, using a colposcope [14]. We are therefore confident we would have detected FGS, if present. Underpinning this, there was no increase in bloody discharge, abnormal vaginal discharge or lesions with heavy intensity of S. mansoni infection.
Previous studies have shown there is a strong association between S. haematobium ova in urine or genitals and four different lesion types [18,41]. A small proportion of women were found to have homogenous yellow patches, however no grainy sandy patches or rubbery papules were seen in this S. mansoni endemic area [42]. Although none of our patients had detectable S. haematobium (in three urines) many reported to have had river contact “elsewhere” in their youth or childhood. Urine egg excretion is known to decrease with age and our study population may have ceased to excrete S. haematobium ova [44,45]. Furthermore, in S. haematobium endemic areas, it is worth noting that, even in the absence of excretion of ova in urine, 23–41% of women have one of the typical FGS lesions and ova in the genitals [41,46]. We cannot preclude that homogenous yellow patches are due to long-standing or prior S. haematobium infection, they may be a sign of “aged” FGS lesions [47].
There is often a wide overlap in the geographic distribution of S. haematobium and S. mansoni in sub-Saharan Africa, making it difficult for single species incrimination [48]. In the absence of detectable ova in urine, other hard-to-achieve techniques such as species differentiation in histological specimens may be indicated or S. haematobium-specific PCR in cervicovaginal lavage or swabs [8,43,49–52]. While we did not observe any S. mansoni eggs in urine, we cannot rule out the possibility that homogenous yellow patches may have been induced by a tendency of schistosomes to shift their intravascular positions late in chronic infection as occurs with liver fibrosis when stool ova excretion may be low or there may be abnormal migration patterns of adult worms triggered by “worm crowding” [7,53–55]. In this population, however, nothing was found to indicate genital deposition S. mansoniova and ensuing inflammation on the cervico-vaginal or vulval surfaces.
There is an increasing interest in the gender-specific manifestations of urogenital schistosomiasis with a growing appreciation of its multiple nexus as a chronic risk for HIV transmission. S. mansoni has been found in surgical specimens in Puerto Rico by Arean and others in the Fallopian tubes (18 cases), ovaries (10 cases); uterus or vulva (1 case), cervix (6 cases), and other case reports have also shown this [10,56–62]. However, most of these cases of S. mansoni were incidental findings in conjunction with malignancies, cervicitis or teratoma. In the cases with cervicitis differential diagnostic tests were not performed and hence S. mansoni cannot be ruled out as a cause.
FGS is a neglected disease and has previously been overlooked as a sexual and reproductive health rights issue [63,64], including its possible link with cervical cancer, HIV, and also mental health and social consequences [65,66]. S. haematobium infection is associated with abnormal vaginal discharge and bloody discharge, indicating intravaginal lesions or inflammation [13,21,67]. While S. mansoni ova can also be found in the genital tract, no study, including this one, has shown surface lesions of the intravaginal mucosa caused by S. mansoni. This study did not include S. mansoni negative women and we did not perform analyses for other diseases such as sexually transmitted diseases or endometriosis. However, the lack of increased number or extent of cervicovaginal lesions and symptoms in women with heavy intensity S. mansoni infections suggests that symptoms and findings were independent of S. mansoni. Furthermore, the same colposcope and colposcopist (EFK) that conducted the exams in this S. mansoni-endemic area had performed investigations and found ample FGS in four S. haematobium endemic countries prior to this study [68]. Thus, we feel confident that, had lower genital tract lesions been present, caused by S. mansoni, we would have detected them.
In the upper genital tract, reports from pathology laboratories in S. mansoni-endemic areas have, on occasion, found S. mansoni ova and we cannot preclude that our study participants may have been affected there [58,62]. Surgeries and laparoscopies cannot be done (for ethical reasons) as a research project, however upper genital tract should be explored when laparoscopy or surgery is done for other reasons. Alternatively, lesions of the upper genital tract can be explored in connection with post-mortems.
Conclusions
In this study population we found that S. mansoni was not associated with lower genital tract lesions or genital symptoms. However, further investigations are needed to explore the causes of genital symptoms around Lake Victoria to offer correct management. Furthermore, the possibility of lesions and pathology due to S. mansoni in the upper genital tract should be explored.
Supporting information
(XLSX)
Acknowledgments
We acknowledge Ministry of Health officers at Kisumu West and Rachuonyo North sub-Counties and their respective community health workers who helped us in reaching out to participants. The Kendu District Hospital provided the clinical space to conduct this study. We also thank participating staff from KEMRI and the University of Kwa Zulu Natal for their support. We thank the University of Georgia Research Foundation, Inc, USA, who were funded by the Bill and Melinda Gates Foundation for the Schistosomiasis Consortium for Operational Research and Evaluation (SCORE) project (https://score.uga.edu/) to PNNM (sub-award # RR374-053/4787596). We are grateful for the colposcope which was lent to the study by Centre for Bilharzia and Tropical Health Research—BRIGHT Academy, Ugu District, South Africa (https://brightresearch.org/about-us/sites/) and technical and practical assistance from R.F. Manyaira. We finally immensely appreciate Dr. Evan W. Secor (CDC, Division of Parasitic diseases and Malaria) for his helpful discussions. This work is published with permission of the Director, Kenya Medical Research Institute.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by a grant from the International Society for Infectious Diseases (ISID), 2014 for HCS (https://isid.org/research/#1571752454186-41d39e4c-1e5f) Support was also received for PP from the National Research Foundation, South Africa (SGD14052367807). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Schistosomiasis. 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis. [Google Scholar]
- 2.Hotez PJ, Harrison W, Fenwick A, Bustinduy AL, Ducker C, Mbabazi PS, et al. Female genital schistosomiasis and HIV/AIDS: Reversing the neglect of girls and women. PLoS Neglected Tropical Diseases. 2019. doi: 10.1371/journal.pntd.0007025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organisation. Accelerating work to overcome the global impact of neglected tropical diseases. A roadmap for implementation. Executive summary. 2012. [Google Scholar]
- 4.WHO urges increased access to praziquantel as schistosomiasis treatment coverage improves. March 2013. Geneva: WHO; 2016. Available from: http://www.who.int/neglected_diseases/news/WHO_urges_increased_access_to_praziquantel/en/. [Google Scholar]
- 5.Davis SM, Wiegand RE, Mulama F, Kareko EI, Harris R, Ochola E, et al. Morbidity associated with schistosomiasis before and after treatment in young children in Rusinga Island, western Kenya. Am J Trop Med Hyg. 2015/03/12. 2015;92: 952–958. doi: 10.4269/ajtmh.14-0346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldmeier H, Poggensee G, Krantz I. A synoptic inventory of needs for research on women and tropical parasitic diseases. II. Gender-related biases in the diagnosis and morbidity assessment of schistosomiasis in women. Acta Trop. 1993/11/01. 1993;55: 139–169. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7903838. doi: 10.1016/0001-706x(93)90074-l [DOI] [PubMed] [Google Scholar]
- 7.Cunin P, Tchuem Tchuenté LA, Poste B, Djibrilla K, Martin PMV. Interactions between Schistosoma haematobium and Schistosoma mansoni in humans in north Cameroon. Trop Med Int Heal. 2003;8: 1110–1117. doi: 10.1046/j.1360-2276.2003.01139.x [DOI] [PubMed] [Google Scholar]
- 8.Berry A. Multispecies schistosomal infections of the female genital tract detected in cytology smears. Acta Cytol. 1976;20: 361–365. [PubMed] [Google Scholar]
- 9.Sakamoto H. The influence of Schistosomiasis japonica from the gynecological aspect. Kurume Igakki Zasshi. 1951;21: 2383. [Google Scholar]
- 10.Arean VM. Manson’s schistosomiasis of the female genital tract. Am J Obs Gynecol. 1956/11/01. 1956;72: 1038–1053. Available from: http://www.ncbi.nlm.nih.gov/pubmed/13362416. [PubMed] [Google Scholar]
- 11.Carpenter CB, Lewis NG. Schistosomiasis japonica involvement in the female genital tract. JAMA. 1964;188: 647–650. doi: 10.1001/jama.1964.03060330027006 [DOI] [PubMed] [Google Scholar]
- 12.Richard-Lenoble D, Kombila M, Duong TH, Gendrel D. [Bilharziasis caused by Schistosoma intercalatum, a recent and forgotten form of schistosomiasis]. Rev Prat. 1993;43: 432–439. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8341906. [PubMed] [Google Scholar]
- 13.Kjetland EF, Leutscher PDC, Ndhlovu PD. A review of female genital schistosomiasis. Trends Parasitol. 2012;28: 58–65. doi: 10.1016/j.pt.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 14.Patel P, Rose CE, Kjetland EF, Downs JA, Mbabazi PS, Sabin K, et al. Association of schistosomiasis and HIV infections: a systematic review and meta-analysis. Int J Infect Dis. 2020;102: 544–553. doi: 10.1016/j.ijid.2020.10.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sturt AS, Webb EL, Phiri CR, Mweene T, Chola N, van Dam GJ, et al. Genital self-sampling compared with cervicovaginal lavage for the diagnosis of female genital schistosomiasis in zambian women: The BILHIV study. PLoS Negl Trop Dis. 2020;14: 1–18. doi: 10.1371/journal.pntd.0008337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.London Applied and Spatial Epidemiology Reseach Group (LASER). Global atlas of helminth infections. 2019. [cited 10 Mar 2019]. Available from: http://www.thiswormyworld.org/maps/create-a-map. [Google Scholar]
- 17.Zaghloul MS, Awwad HK, Akoush HH, Omar S, El Attar I. Postoperative Radiotherapy of Carcinaoma in Bilharzial Bladder: Improved Disease Free Survival Through Improving Local Control. IntJRadiation Oncol BiolPhys. 1992;23: 511–517. [DOI] [PubMed] [Google Scholar]
- 18.Kjetland EF, Norseth HM, Taylor M, Lillebø K, Kleppa E, Holmen SD, et al. Classification of the lesions observed in female genital schistosomiasis. Int J Gynaecol Obstet. 2014;127: 227–228. doi: 10.1016/j.ijgo.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 19.Kjetland EF, Kurewa EN, Mduluza T, Midzi N, Gomo E, Friis H, et al. The first community-based report on the effect of genital Schistosoma haematobium infection on female fertility. Fertil Steril. 2010/02/13. 2010;94: 1551–1553. doi: 10.1016/j.fertnstert.2009.12.050 [DOI] [PubMed] [Google Scholar]
- 20.Schanz A, Richter J, Beyer I, Baldus SE, Hess AP, Kruessel JS. Genital schistosomiasis as a cause of female sterility and acute abdomen. Fertil Steril. 2010;93: 2075.e7–9. doi: 10.1016/j.fertnstert.2009.05.043 [DOI] [PubMed] [Google Scholar]
- 21.Hegertun IEA, Sulheim Gundersen KM, Kleppa E, Zulu SG, Gundersen SG, Taylor M, et al. S. haematobium as a Common Cause of Genital Morbidity in Girls: A Cross-sectional Study of Children in South Africa. PLoS Negl Trop Dis. 2013;7: e2104. doi: 10.1371/journal.pntd.0002104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galappaththi-Arachchige HN, Hegertun IEA, Holmen S, Qvigstad E, Kleppa E, Sebitloane M, et al. Association of urogenital symptoms with history of water contact in young women in areas endemic for S. haematobium. a cross-sectional study in rural South Africa. Int J Environ Res Public Health. 2016;13. doi: 10.3390/ijerph13111135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller-Fellows SC, Howard L, Kramer R, Hildebrand V, Furin J, Mutuku FM, et al. Cross-sectional interview study of fertility, pregnancy, and urogenital schistosomiasis in coastal Kenya: Documented treatment in childhood is associated with reduced odds of subfertility among adult women. 2017. doi: 10.1371/journal.pntd.0006101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohammed AZ, Edino ST, Samaila AA. Surgical pathology of schistosomiasis. J Natl Med Assoc. 2007;99: 570–4. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2576076&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- 25.Lambertucci JR, Villamil QT, Savi D, Dias IC. Genital Schistosomiasis mansoni: tubal tumor and parietal peritoneum involvement diagnosed during laparoscopy. Rev Soc Bras Med Trop. 2009/12/08. 2009;42: 583–586. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19967244. doi: 10.1590/s0037-86822009000500019 [DOI] [PubMed] [Google Scholar]
- 26.Landry P, Favrat B, Raeber PA. Genital schistosomiasis after a missed diagnosis of Katayama syndrome. J Travel Med. 1996;3: 237–238. Available from: http://www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?db=m&form=6&dopt=r&uid=0009815465. doi: 10.1111/j.1708-8305.1996.tb00755.x [DOI] [PubMed] [Google Scholar]
- 27.Oliveira FA, Soares VL, Dacal AR, Cavalcante FG, Mesquita AM, Fraga F, et al. Absence of cervical schistosomiasis among women from two areas of north-eastern Brazil with endemic Schistosoma mansoni. Ann Trop Med Parasitol. 2006;100: 49–54. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16417713. doi: 10.1179/136485906X78490 [DOI] [PubMed] [Google Scholar]
- 28.Downs JA, van Dam GJ, Changalucha JM, Corstjens PL, Peck RN, de Dood CJ, et al. Association of schistosomiasis and HIV infection in Tanzania. Am J Trop Med Hyg. 2012/10/04. 2012;87: 868–873. doi: 10.4269/ajtmh.2012.12-0395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musuva RM, Awiti A, Omedo M, Ogutu M, Secor WE, Montgomery SP, et al. Community knowledge, attitudes and practices on schistosomiasis in western Kenya—the SCORE Project. Am J Trop Med Hyg. 2014/02/19. 2014;90: 646–652. doi: 10.4269/ajtmh.13-0488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.KAIS. Kenya AIDS indicator survey preliminary report. 2014. Available from: http://nascop.or.ke/library/3d/PreliminaryReportforKenyaAIDSindicatorsurvey2012pdf. [Google Scholar]
- 31.CDC. Centers for Disease Control and Prevention Kenya. Annual Report 2015. 2016. Available from: https://www.cdc.gov/globalhealth/countries/kenya/pdf/cdc_kenya2015report.pdf. [Google Scholar]
- 32.Odiere MR, Rawago FO, Ombok M, Secor WE, Karanja DM, Mwinzi PN, et al. High prevalence of schistosomiasis in Mbita and its adjacent islands of Lake Victoria, western Kenya. Parasit Vectors. 2012/12/05. 2012;5: 278. doi: 10.1186/1756-3305-5-278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Handzel T, Karanja DMS, Addiss DG, Hightower AW, Rosen DH, Colley DG, et al. Geographic distribution of schistosomiasis and soil-transmitted helminths in Western Kenya: implications for anthelminthic mass treatment. Am J Trop Med Hyg. 2003/11/25. 2003;69: 318–323. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14628951. [PubMed] [Google Scholar]
- 34.Verani JR, Abudho B, Montgomery SP, Mwinzi PN, Shane HL, Butler SE, et al. Schistosomiasis among young children in Usoma, Kenya. Am J Trop Med Hyg. 2011/05/05. 2011;84: 787–791. doi: 10.4269/ajtmh.2011.10-0685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ofulla A V, Adoka SO, Anyona DN, Abuom PO, Karanja D, Vulule JM, et al. Spatial distribution and habitat characterization of schistosomiasis host snails in lake and land habitats of Western Kenya. 2013 18: 197–215. Lakes Reserv Res Manag. 2013;18: 197–2015. [Google Scholar]
- 36.Woodhall DM, Wiegand RE, Wellman M, Matey E, Abudho B, Karanja DM, et al. Use of geospatial modeling to predict Schistosoma mansoni prevalence in Nyanza Province, Kenya. PLoS One. 2013/08/27. 2013;8: e71635. doi: 10.1371/journal.pone.0071635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.NACC. Kenya HIV County Profiles. Programme NA and STIC, editor. Ministry of Health Kenya; 2014. Available from: http://blog.opendata.go.ke/?p=40; https://www.opendata.go.ke/Health/2014-Kenya-HIV-County-Profiles/i4t2-smf8.
- 38.Chen L, Jha P, Stirling B, Sgaier SK, Daid T, Kaul R, et al. Sexual risk factors for HIV infection in early and advanced HIV epidemics in sub-Saharan Africa: systematic overview of 68 epidemiological studies. PLoS One. 2007;2: e1001. doi: 10.1371/journal.pone.0001001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iversen AK, Larsen AR, Jensen T, Fugger L, Balslev U, Wahl S, et al. Distinct determinants of human immunodeficiency virus type 1 RNA and DNA loads in vaginal and cervical secretions. J Infect Dis. 1998;177. Available from: http://www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?db=m&form=6&dopt=r&uid=0009593006. doi: 10.1086/515266 [DOI] [PubMed] [Google Scholar]
- 40.World Health Organisation. Helminth control in school-age children. A guide for managers of control programmes. Preventive Chemotherapy and Transmission Control (PCT), Department of Control of Neglected Tropical Diseases (NTD), World Health Organization, 20, Avenue Appia, 1211 Geneva 27, Switzerland; 2011. Available from: http://www.who.int/neglected_diseases/en. [Google Scholar]
- 41.Kjetland EF, Gwanzura F, Ndhlovu PD, Mduluza T, Gomo E, Mason PR, et al. Simple clinical manifestations of genital Schistosoma haematobium infection in rural Zimbabwean women. Am J Trop Med Hyg. 2005;72: 311–319. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15772328. [PubMed] [Google Scholar]
- 42.Mbabazi PS, Vwalika B, Randrianasolo BS, Roald B, Ledzinski D, Olowookorun F, et al. World Health Organisation Female genital schistosomiasis. A pocket atlas for clinical health-care professionals. WHO/HTM/NTD/2015.4. Geneva: WHO; 2015. Available from: http://apps.who.int/iris/bitstream/10665/180863/1/9789241509299_eng.pdf. [Google Scholar]
- 43.Randrianasolo BS, Jourdan PM, Ravoniarimbinina P, Ramarokoto CE, Rakotomanana F, Ravaoalimalala VE, et al. Gynecological manifestations, histopathological findings, and schistosoma-specific polymerase chain reaction results among women with Schistosoma haematobium infection: A cross-sectional study in Madagascar. J Infect Dis. 2015;212. doi: 10.1093/infdis/jiv035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fulford AJC, Webster M, Ouma JH, Kimani G, Dunne DW. Puberty and age-related changes in susceptibility to schistosome infection. Parasitol Today. 1998;14: 23–26. doi: 10.1016/s0169-4758(97)01168-x [DOI] [PubMed] [Google Scholar]
- 45.Kjetland EF, Ndhlovu PD, Kurewa EN, Midzi N, Gomo E, Mduluza T, et al. Prevention of gynecologic contact bleeding and genital sandy patches by childhood anti-schistosomal treatment. Am J Trop Med Hyg. 2008/07/09. 2008;79: 79–83. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18606767. [PubMed] [Google Scholar]
- 46.Poggensee G, Kiwelu I, Saria M, Richter J, Krantz I, Feldmeier H. Schistosomiasis of the lower reproductive tract without egg excretion in urine. Am J Trop Med Hyg. 1998;59: 782–783. Available from: http://www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?db=m&form=6&dopt=r&uid=0009840597. doi: 10.4269/ajtmh.1998.59.782 [DOI] [PubMed] [Google Scholar]
- 47.Nemungadi TG, Kleppa E, van Dam GJ, Corstjens PLAM, Galappaththi-Arachchige HN, Pillay P, et al. Female Genital Schistosomiasis Lesions Explored Using Circulating Anodic Antigen as an Indicator for Live Schistosoma Worms. Front Trop Dis. 2022;3: 821463. doi: 10.3389/fitd.2022.821463 [DOI] [Google Scholar]
- 48.Cunin P, Griffet A, Poste B, Djibrilla K, Martin PMV. Epidemic Schistosoma mansoni in a known S. haematobium area. Trans R Soc Trop Med Hyg. 2000;94: 657–660. doi: 10.1016/s0035-9203(00)90221-9 [DOI] [PubMed] [Google Scholar]
- 49.de Jonge N, Schommer G, Feldmeier H, Krijger FW, Dafalla AA, Bienzle U, et al. Mixed Schistosoma haematobium and S. mansoni infection: effect of different treatments on the serum level of circulating anodic antigen (CAA). Acta Trop. 1990;48: 25–35. Available from: http://www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?db=m&form=6&dopt=r&uid=0001980801. doi: 10.1016/0001-706x(90)90062-5 [DOI] [PubMed] [Google Scholar]
- 50.Feldmeier H, Zwingenberger K, Steiner A, Dietrich M. Diagnostic Value of Rectal Biopsy and Concentration Methods in Schistosomiasis intercalatum: Quantitative Comparison of Three Methods. TropenmedParasit. 1981;32: 243–246. [PubMed] [Google Scholar]
- 51.Galappaththi-Arachchige HN, Holmen S, Koukounari A, Kleppa E, Pillay P, Sebitloane M, et al. Evaluating diagnostic indicators of urogenital Schistosoma haematobium infection in young women: A cross sectional study in rural South Africa. PLoS One. 2018;13. doi: 10.1371/journal.pone.0191459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Søfteland S, Sebitloane MH, Taylor M, Roald BB, Holmen S, Galappaththi-Arachchige HN, et al. A systematic review of handheld tools in lieu of colposcopy for cervical neoplasia and female genital schistosomiasis. Int J Gynecol Obstet. 2021;153: 190–199. doi: 10.1002/ijgo.13538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feldmeier H, Poggensee G, Krantz I. Gender-related biases in diagnosis and morbidity assessment of schistosomiasis and their implications for control operations. IDRC / TDR / WHO. 1992; 1–36. [Google Scholar]
- 54.Von Lichtenberg F, Edington GM, Nwabuebo I, Taylor JR, Smith JH. Pathologic effects of schistomiasis in Ibadan Western State of Nigeria. II. Pathogenesis of lesions of the bladder and ureters. Am J Trop Med Hyg. 1971/03/01. 1971;20: 244–254. Available from: http://www.ncbi.nlm.nih.gov/pubmed/5553265. doi: 10.4269/ajtmh.1971.20.244 [DOI] [PubMed] [Google Scholar]
- 55.Fulford AJ, Feldmeier H, Poggensee G, Krantz I, Fulford AJ. Puberty and age intensity profiles in schistosome infections: another hypothesis. Parasitol Today. 1998;14: 435. doi: 10.1016/s0169-4758(98)01313-1 [DOI] [PubMed] [Google Scholar]
- 56.Lima JP. Study of the so-called “ectopical lesions” in Manson’s schistosomiasis. II. Myocardial schistosomiasis. Rev Inst Med Trop Sao Paulo. 1969/07/01. 1969;11: 290–293. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=5380217. [PubMed] [Google Scholar]
- 57.Poderoso WLS, de Santana WB, da Costa EF, Cipolotti R, Fafhouri R, Santana WB, et al. Ectopic schistosomiasis: description of five cases involving skin, one ovarian case and one adrenal case. Rev Soc Bras Med Trop. 2009/01/15. 2008;41: 668–671. doi: 10.1590/s0037-86822008000600021 [DOI] [PubMed] [Google Scholar]
- 58.Sedlis A. Manson’s schistosomiasis of the Fallpoian tube. A case report. Am J Obstet Gynecol. 1960;81: 254–255. [DOI] [PubMed] [Google Scholar]
- 59.Tiboldi T, Colfs B, De Smet M, van Soom H. Ovaries and Adrenals in Murine Schistosomiasis Mansoni. II. Some Observations of the Functions of the Ovaries in Acute Infection. Am J Trop Med Hyg. 1979;28: 871–872. [PubMed] [Google Scholar]
- 60.Tiboldi T. Ovaries and Adrenals in Murine Schistosomiasis Mansoni. I. Histopathological Changes of the Ovaries in Acute and Chronic Infection. Am J Trop Med Hyg. 1979;28: 670–676. [PubMed] [Google Scholar]
- 61.Roca C, Balanzo X, Gascon J, Fernandez-Roure JL, Vinuesa T, Valls ME, et al. Comparative, clinico-epidemiologic study of Schistosoma mansoni infections in travellers and immigrants in Spain. Eur J Clin Microbiol Infect Dis. 2002;21: 219–223. doi: 10.1007/s10096-001-0683-z [DOI] [PubMed] [Google Scholar]
- 62.Poggensee G, Krantz I, Feldmeier H. Presence of Schistosoma mansoni eggs in the cervix uteri of women in Mwanga District. Trans R Soc Trop Med Hyg. 2001;95: 299–300. doi: 10.1016/s0035-9203(01)90239-1 [DOI] [PubMed] [Google Scholar]
- 63.Ribeiro AR, Luis C, Fernandes R, Botelho MC. Schistosomiasis and Infertility: What Do We Know? Trends in Parasitology. 2019. pp. 964–971. doi: 10.1016/j.pt.2019.09.001 [DOI] [PubMed] [Google Scholar]
- 64.Engels D, Hotez PJ, Ducker C, Gyapong M, Bustinduy AL, Secor WE, et al. Integration of prevention and control measures for female genital schistosomiasis, hiv and cervical cancer. Bull World Health Organ. 2020;98: 615–624. doi: 10.2471/BLT.20.252270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hotez PJ, Engels D, Gyapong M, Ducker C, Malecela MN. Female genital schistosomiasis. N Engl J Med. 2019. pp. 2493–2495. doi: 10.1056/NEJMp1914709 [DOI] [PubMed] [Google Scholar]
- 66.UNAIDS World Health Organization. No more neglect. Female genital schistosomiasis and HIV. Integrating reproductive health interventions to improve women’s lives. Geneva, Switzerland; 2019. Available from: https://www.unaids.org/sites/default/files/media_asset/female_genital_schistosomiasis_and_hiv_en.pdf. [Google Scholar]
- 67.Ekpo UF, Odeyemi OM, Sam-Wobo SO, Onunkwor OB, Mogaji HO, Oluwole AS, et al. Female genital schistosomiasis (FGS) in Ogun State, Nigeria: a pilot survey on genital symptoms and clinical findings. Parasitol Open. 2017;3. [Google Scholar]
- 68.Norseth HM, Ndhlovu PD, Kleppa E, Randrianasolo BS, Jourdan PM, Roald B, et al. The colposcopic atlas of schistosomiasis in the lower female genital tract based on studies in Malawi, Zimbabwe, Madagascar and South Africa. PLoS Neglected Trop Dis. 2014;8: e3229. doi: 10.1371/journal.pntd.0003229 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.