Abstract
Introduction
Immune Tolerance Induction (ITI) is the first‐choice therapy to eradicate Factor VIII (FVIII) neutralizing antibodies in patients with haemophilia A (HA). There is limited published data on ITI from East Mediterranean countries.
Aim
To assess the effectiveness of a low‐dose ITI regimen to eradicate FVIII neutralizing antibodies in children with severe HA and high‐titre inhibitors.
Methods
A prospective, single‐arm study was conducted in children with HA (FVIII < 1 IU/dl), high‐titre inhibitors and poor prognostic factors for successful ITI. Patients were treated with ∼50 IU/kg plasma‐derived FVIII containing von Willebrand factor (pdFVIII/VWF) concentrate (Koate‐DVI, Grifols) three times a week. Time to achieve tolerance, total and partial success were analysed after ITI. Annual bleeding rate (ABR), number of target joints, FVIII recovery and school absence were compared before and after ITI.
Results
Twenty patients with median (range) age of 6.2 (3–12) years and pre‐ITI inhibitor titre of 36.5 (12–169) BU were enrolled. ITI lasted ≤12 months (early tolerization) in 45% of patients. Median follow‐up was 12 months (3–22) and total response rate was 80% (60% total success; 20% partial success). Patients with two and three poor prognosis factors achieved overall success rate of 60% and 50%, respectively. ABR, target joints and school absence were reduced after ITI by 60%, 50% and 44.1%, respectively. In successful ITI tolerized patients, FVIII recovery was 90 (60–100)%.
Conclusion
A low‐dose ITI therapy using a pdFVIII/VWF concentrate achieved at least partial tolerance in 80% of patients, and reduced annual bleeds in children with high inhibitor titres and at least one poor prognosis factor for ITI treatment success.
Keywords: bleeding, haemophilia A, inhibitors, low‐dose immune tolerance induction, plasma‐derived factor VIII containing von Willebrand factor, response rate
1. INTRODUCTION
Haemophilia A (HA) is a genetic bleeding disorder caused by the total or partial deficiency of clotting factor VIII (FVIII). 1 Approximately 20–30% of patients with severe HA develop neutralizing alloantibodies (inhibitors) against FVIII and they become resistant to FVIII replacement therapy, leading to an increased risk for severe bleeding, arthropathies, morbidity and mortality. 2 The risk for developing inhibitors is particularly high for previously untreated patients with severe HA during the first days of exposure, especially when they are exposed to high‐intensity treatment. 3
Immune tolerance induction (ITI) is the gold standard strategy to eradicate inhibitors with success rates of approximately 60–80%. 4 , 5 It is based on frequent and regular administration of either low or high doses of FVIII, to induce antigen tolerance and prevent the production of new neutralizing antibodies that results in partial or total lack of efficacy of the FVIII concentrate. 6 According to the most recent guidelines for the management of HA, all patients with inhibitors should undergo a trial of ITI and return to their previous FVIII concentrate replacement therapy. 7
The outcome of ITI therapy depends on the therapeutic regimen used, the type of FVIII concentrate, and patient‐related prognostic factors. Several therapeutic ITI regimens have been developed for ITI therapy, including the high‐dose (Bonn protocol), 8 high‐dose with immune modulation (Malmo protocol) 9 and low‐dose regimens (Van Creveld protocol) 10 . High ITI success rates have been achieved with plasma‐derived FVIII concentrates containing von Willebrand factor (pdFVIII/VWF) 8 , 11 , 12 and recombinant FVIII concentrates. 4 , 13
The outcome of ITI therapy is determined by risk factors for poor prognosis: high inhibitor titre immediately before ITI start (usually > 10 BU), age > 7 years at ITI start, time from inhibitor diagnosis to start of ITI, historical inhibitor peak > 200 BU. 6 , 8 , 14 Of these, showing high inhibitor titres before ITI, and a peak > 200BU during ITI are the most consistent predictors of ITI failure. 15 On the other hand, a low inhibitor titre peak during ITI is associated with higher ITI success rates. 16
A low‐dose ITI regimen in patients with good prognostic factors achieves long‐term success rate similar to high‐dose regimens, although times to achieve responses are longer. 6 However, the cost associated with ITI therapy is considered a major factor influencing the choice of ITI regimen, especially in regions with economic constraints. 17 Specifically, a low‐dose ITI regimen is a cost‐saving strategy compared to a high‐dose regimen 18 and studies with low‐dose ITI regimen in patients with severe HA, high inhibitor titres, and poor prognosis are limited. 6 , 19 Therefore, in this study, we hypothesized that a low‐dose ITI therapy with a highly purified pdFVIII/VWF concentrate might be effective in that cohort of patients. The aim of this study was to assess the effectiveness of low‐dose ITI regimen in children with severe HA suffering from high titre inhibitors.
2. MATERIALS AND METHODS
2.1. Study design and patient selection
A prospective, interventional, single‐arm study in children with severe HA (FVIII < 1 IU/dl), previously treated with either plasmatic or recombinant FVIII. Patients were recruited from three centres at the national referral Haemophilia Care Centre between June 2016 and December 2020. Historical data for recruited patients were collected retrospectively to 1 year before enrolment and data were confirmed by interviewing the parents. All prospective data were recorded in a diary and monthly follow‐up visits were scheduled during the study period.
The trial was performed according to the principles of the Declaration of Helsinki, the standards of Good Clinical Practice of the International Conference of Harmonization (ICH), and current legal regulations. The protocol was approved by the Ethical Committee of the participating centres and was explained in detail to the parents and children. Assent and consent forms were provided and signed by patients older than 6 years and by their parents or legal guardian
Patients eligible for the study were those with severe HA, between 2 and 18 years old, poor‐risk status for ITI treatment success (high inhibitor titres, > 10 BU), delay in ITI initiation (1–36 months), and willing to receive ITI. Exclusion criteria comprised previous ITI management within 12 months prior to the study.
2.2. ITI treatment regimen
All patients received an average of 50 IU/kg pdFVIII/VWF complex concentrate (Koate‐DVI, Grifols, Spain) three times a week. Koate‐DVI, a highly purified pdFVIII/VWF concentrate, contains functionally active FVIII and VWF with a FVIII:C/VWF:RCo ratio of .85. 20 This low‐dose ITI regimen was based on previously published studies and was in agreement with Van Creveld protocol. 6
The ITI regimen was not modified during all the study period. Bypassing agents, either recombinant coagulation factor activated VII; rFVIIa (NovoSeven, Novo‐Nordisk) or activated prothrombin complex concentrate; aPCC (FEIBA, Takeda) were administered on demand for breakthrough bleeding episodes during ITI. Once ITI was completed with success, patients were continued either under prophylactic treatment with 25 IU/Kg of pdFVIII/VWF two or three times per week or on‐demand treatment and followed‐up for an additional 3 months.
2.3. ITI outcomes
The primary endpoint of the study was the ITI overall outcome: total, partial response rates and failures after ITI therapy. ITI response rate was defined as complete success or tolerance when inhibitor levels were < .6 BU and a factor recovery of > 66% was achieved. A partial response was considered if inhibitor levels were < 5 BU but > .6 BU, and factor recovery < 66% for standard FVIII, and following guidelines for HA treatment. 7 Finally, if those levels were not reached after 24 months, ITI was considered failure and it was discontinued.
Patients were stratified according to well‐known risk factors for poor prognosis to ITI therapy: inhibitor titre > 10 BU at ITI start, peak titre > 200 BU during ITI, age at ITI start > 7 years and > 24 months from inhibitor diagnosis to ITI therapy. 21
Secondary endpoints included the time to achieve response, comparisons between before and after ITI therapy for the number of bleeding events per year, the number of target joints, FVIII recovery and school absences.
2.4. Data collection
Demographic and clinical variables assessed prior to ITI therapy were historic FVIII inhibitor titre (BU), titre at ITI start (BU), age at ITI initiation, the time from HA inhibitor diagnosis to initiation of ITI, and the type of FVIII used (plasmatic or recombinant).
The presence and severity of bleeding symptoms were assessed according to International Society of Thrombosis and Haemostasis Bleeding Assessment Tool (ISTH‐BAT) and classified as major, moderate or mild. Annual bleeding rate (ABR) was calculated for each patient during a 12‐month period before ITI initiation. During ITI, ABR was considered as the number of bleeding episodes that occurred in a time frame equivalent to ITI duration and adjusted to 12 months. Data on the number of days absent from school per year due to bleeding episodes were collected before and after ITI therapy from parents/guardians and the participants. Bleeding data were collected prospectively during ITI period by contemporaneous paper bleeding diary, and retrospectively collected by patients or parents at ITI initiation.
2.5. Laboratory measurements
FVIII inhibitors levels (BU) and FVIII recovery (%) were measured before ITI therapy, monthly for the first 3 months and quarterly until the end of ITI. FVIII inhibitor assay was carried out according to a Nijmegen modification of the Bethesda assay. 7 FVIII recovery was determined by measuring FVIII plasma levels by a standard method. 22 , 23 Once the inhibitor clearance was reached, patients were monitored for FVIII recovery 3 months after the end of ITI therapy.
2.6. Sample size and statistical analysis
The estimated sample size was the approximate number of patients who may have been treated with the drug during the study period. Results were summarized, and descriptive statistical analysis was conducted. For continuous data, median and ranges (minimum and maximum) values were calculated or reported as applicable. For qualitative variables, absolute or relative frequencies were calculated and expressed in percentages.
Median change before and after ITI therapy in ABR, number of target joints and school absence was analysed using Wilcoxon signed rank test. Shapiro‐Wilk normality test was used to test normality. All statistical tests were two sided and P value was set as < .05 statistically significant. GraphPad Prism version 9 (GraphPad Software, Inc) was used for statistical analysis calculations.
3. RESULTS
3.1. Baseline patient characteristics and disease activity
Twenty patients with median (range) age of 6.2 (3–12) years and pre‐ITI inhibitor titre of 36.5 (12–169) BU were enrolled and followed‐up for a median of 12 (3–22) months. All patients had severe HA (FVIII levels < 1 IU/dl). Patient baseline characteristics are shown in Table 1 including description of demographics, diagnosis, and serology data.
TABLE 1.
Baseline patient characteristics
| Parameters | ITI population, all patients, n (%) |
|---|---|
| Demographic | |
| Sex, male | 20 (100) |
| Age, years, median (range) | 6.2 (3–12) |
| Height, cm, median (range) | 114 (95–155) |
| Body weight, Kg/m2 | |
| ≤24 | 14 (70) |
| > 24 ‐ ≤28 | 5 (25) |
| > 28 | 1 (5) |
| Race, Arab | 20 (100) |
| Clinical data | |
| HA severity, FVIII < 1 IU/dl | 20 (100) |
| Blood group | |
| A | 4 (20) |
| B | 8 (40) |
| AB | 2 (10) |
| O | 6 (30) |
| Surgery | 1 (5) |
| Concomitant disease | |
| Cardiovascular disease | 0 (0) |
| Asthma | 2 (10) |
| Inhibitor antecedents | 2 (10) |
| Serology | |
| HIV+/HCV+ | 0 (0) |
| HCV+ | 2 (10) |
Abbreviations: ABR, annual bleeding rate; FVIII, Factor VIII; HA, haemophilia A; HCV, hepatitis C virus; HIV, Human immunodeficiency virus; ITI, immune tolerance induction.
3.2. Historical and pre‐ITI data
All patients were previously exposed to FVIII/VWF concentrates, either plasmatic or recombinant, at a median age of 5.5 (1–12) months. Before inhibitor detection, 11 patients were on‐demand treatment regimen and nine under prophylaxis, with a dosage regimen ranged from 30 to 50 U/Kg twice a week. The median historical peak inhibitor titre was 41 (8–320) BU.
The median age at ITI onset was 5 (3–12) years. Thirteen (65%) of them were under treatment with pdFVIII/VWF concentrate and the remaining 9 (35%) were treated with recombinant FVIII. The median time from inhibitor diagnosis to start low‐dose ITI was 8.5 (.5–33) months (Table 2, Table S1).
TABLE 2.
Historical and pre‐ITI data of study patients
| Data | Variable | All ITI population, n = 20 |
|---|---|---|
| Historical | Age at first exposure to FVIII, months | 5.5 (1–12) |
| FVIII treatment duration, months | 53 (24–139) | |
| Treatment, n (%) | ||
| Prophylaxis | 7 (35) | |
| On demand | 13 (65) | |
| Historical peak titre, BU | 41 (8–320) | |
| Pre‐ITI | Inhibitor titre at ITI start, BU | 36.5 (12–169) |
| Age at ITI start, years | 5 (3–12) | |
| Inhibitor diagnosis to start of ITI, months | 8.5 (.5–33) | |
| Type of FVIII at inhibitor detection | ||
| Plasma‐derived | 13 (65) | |
| Recombinant | 7 (35) | |
| FVIII recovery, % | 2 (1–4) |
Results are shown as number of patients and percentage or median (range), as applicable.
Abbreviations: ABR, annual bleeding rate; FVIII, Factor VIII; ITI, immune tolerance induction.
3.3. ITI treatment and outcome
All patients were undergoing a low‐dose ITI regimen for the first time. Treatment with the pdFVIII/VWF concentrate Koate‐DVI ranged from 44 IU/Kg to 65 IU/Kg, three times per week, although most patients were treated with 50 IU/Kg. Median peak inhibitor titre during ITI was 62 (2–412) BU. The total response rate was 80%, which included complete success in 60% of patients (n = 12) and partial success in 20% of patients (n = 4). The remaining four (20%) patients were rated as treatment failures, according to the international consensus recommendations for defining ITI outcome 24 (Table 3, Table S1).
TABLE 3.
Immune tolerance induction (ITI) outcomes
| Variable | All ITI population, n = 20 |
|---|---|
| Peak inhibitor level, BU | 62 (2–412) |
| pdFVIII/VWF dose, IU/kg | 50 (45–65) |
| Time to achieve success, months | 12 (3–22) |
| Response rate, n (%) | |
| Complete success | 12 (60) |
| Partial success | 4 (20) |
| Failure | 4 (20) |
| Concomitant medication | |
| Bypassing agents | 8 (40) |
| Immunomodulation | 0 (0) |
| Risk factor for poor ITI prognosis | |
| Inhibitor titre > 10 BU pre‐ITI | 20 (100) |
| Peak inhibitor during ITI > 200 BU | 5 (25) |
| Age at ITI start > 7 years old | 4 (20) |
| Time from inhibitor diagnosis to ITI initiation > 24 months | 3 (15) |
| FVIII recovery a , % | 90 (60–100) |
| Number of patients with FVIII recovery > 66% | 12 (60) |
Results are shown as number of patients and percentage or median (range), as applicable.
Abbreviations: FVIII, Factor VIII; ITI, immune tolerance induction; pdFVIII/VWF, plasma‐derived factor VIII containing von Willebrand factor.
In successful ITI tolerized patients, n = 16.
The median follow‐up time of ITI therapy until tolerization was 12 (3–22) months. In those patients with complete and partial successful ITI response, duration of ITI was ≤12 months in nine patients (45%), indicating an early tolerization with the low‐dose ITI strategy. In those patients with complete success, median FVIII recovery was 90 (60–100)%. By contrast, patients with partial success and failure did not achieve standard FVIII recovery levels (> 66%).
ITI success rate based on the number of risk factors for poor prognosis was assessed. All patients showed high‐risk factors related to high inhibitor titres (> 10 BU) at the onset of ITI. A total of eight (40%) patients had ≥ 2 risk factors for poor prognosis to ITI success. Complete and partial success were reached by 60% and 50% of the patients with two and three risk factors, respectively. The remaining patient had four poor prognostic risk factors and resulted in ITI failure (Table 4).
TABLE 4.
ITI response rate according to the number of poor prognosis factors of ITI population
| Number of poor prognosis factors | ||||
|---|---|---|---|---|
| Total response rate – ITI outcome, n (%) | 1 n = 20 | 2 n = 5 | 3 n = 2 | 4 n = 1 |
| Complete success | 12 (60) | 2 (40) | 1 (50) | 0 (0) |
| Partial success | 4 (20) | 1 (20) | 0 (0) | 0 (0) |
| Failure | 4 (20) | 2 (40) | 1 (50) | 1 (100) |
Results are expressed as n (%).
Abbreviation: ITI, immune tolerance induction.
Risk factors associated with a poor prognosis to ITI were further determined. All patients had one poor prognosis factor for ITI treatment success. Five (25%) patients had a peak titre > 200 BU during ITI, four (20%) were older than 7 years old at ITI start and three (15%) patients started ITI after > 24 months of diagnosis. Likewise, patients with cut‐off peak titres ≤200 BU during ITI exhibited higher success rate (93%) than those with peak titres > 200 BU (40%). For those aged ≤7 years, success rate was 87.6% compared with 50% of those aged > 7. Similarly, those patients who started ITI within 24 months after inhibitor diagnosis resulted in higher success rate (88.2%) versus those who started later (33.3%). Three out of four (75%) patients who achieved partial success were aged ≤7 years, and all of them started ITI ≤24 months from inhibitor diagnosis with cut‐off peak titres ≤200 BU during ITI. Two out of four (50%) patients who failed ITI therapy started their treatment after > 24 months of inhibitor diagnosis (Figure 1). In our study cohort, 93.75% of patients who had an inhibitor titre < 55 BU at the onset of ITI, achieved total and partial ITI success.
FIGURE 1.
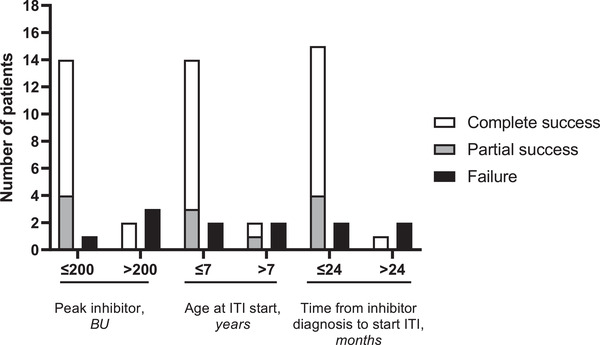
Patients stratification based on cut‐off values for the risk factors of poor immune tolerance induction response: peak inhibitor during ITI ≤200 BU (n = 15), > 200 BU (n = 5); age at ITI start ≤7 years (n = 16), > 7 years (n = 4); and time from inhibitor diagnosis to ITI initiation ≤24 months (n = 17), > 24 months (n = 3)
3.4. Effects of ITI on bleeding and school absence
Before ITI therapy, a total of 155 bleeding episodes were reported in a 12‐month period, and were classified by ISTH‐BAT as severe (n = 65, 42%), moderate (n = 45, 29%) and mild (n = 45, 29%). During ITI, the percentage of severe and moderate bleeding episodes were 12.5% (n = 11) and 25% (n = 22), respectively, whereas most of bleeding episodes were rated as mild (62.5%, n = 55). Concomitant treatment with bypassing agents for breakthrough bleeding was observed in eight treated patients during ITI. No patients were treated with immunomodulatory agents during ITI.
Overall, ABR was significantly reduced after ITI treatment median 3 [1–12]; 60% reduction (P < .001) compared with the pre‐ITI period with median of 7.5. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 Similarly, a significantly lower number of target joints was observed immediately after ITI therapy 1 [0–4] with 50% reduction (P < .001) compared with the pre‐ITI period median of 2 [1–4] (Figure 2).
FIGURE 2.
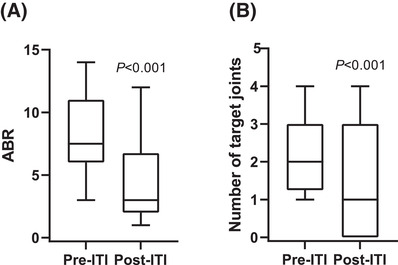
Effects of low‐dose immune tolerance induction (ITI) therapy on (A) annual bleeding rate (ABR) and (B) number of target joints of recruited patients. Data are displayed as median (interquartile range), whiskers are minimum and maximum values, n = 20 for each group. P < .001 versus pre‐ITI for ABR and number of target joints
To evaluate the effect of ITI strategy on health‐related quality of life, school absence was assessed. There was a statistically significant reduction in the median (range) number of missed school days in a year, from 17 (9–26) days, before ITI, to 9.5 (4–30) days, during ITI therapy (44.1% reduction) (P < .001).
4. DISCUSSION
This prospective single‐arm study investigated the effectiveness of low‐dose ITI regimen in children with severe HA, high titre inhibitors and poor risk factors for ITI success. Overall, we demonstrated FVIII inhibitor eradication in the cohort study of children treated with a pdFVIII/VWF concentrate.
According to most recent HA guidelines, all patients who develop neutralizing antibodies against FVIII should undergo a trial of ITI. 7 In our study, patients underwent ITI achieving a total response rate of 80%. This included complete success in 60% of patients and partial success in 20% of them. These results were aligned with the current guidelines for the management of HA, where ITI success in patients with severe HA is approximately 70–80%. 7 It is also observed that ITI success rate ranged from 60 to 80% throughout the literature, depending on the therapeutic dosing protocol used 25 , 26 ; the use of either plasmatic or recombinant FVIII concentrate 12 ; concomitant treatment with immunomodulatory agents, 19 as well as patients’ prognostic factors. 11
Our cohort of patients were treated with a low‐dose ITI therapeutic regimen because it uses the lowest effective dose, has a less aggressive dosing schedule, which may help compliance of patients and reduces the burden of more frequent vein puncture, and provides cost‐savings compared with a high‐dose protocol. 6 , 18 Therefore, by selecting a low‐dose ITI regimen would be of interest for countries where a high‐dose therapy may be more challenging for patient compliance and economic burden. Of note is that all our cohort of patients were Arabic. Although ethnicity has been shown not to affect ITI outcome, 27 it is important to emphasize that previous international ITI studies were mainly represented by other ethnicities. 6
Interestingly, the current study found that duration of ITI was 1‐year or less in nine patients (45%), indicating early tolerization in those patients. Likewise, the median time to achieve negative inhibitor titre, 12 (3–22) months, was shorter than the time reported in comparable high‐risk cohort studies 11 , 28 and similar to those patients receiving a low dose regimen, 9.2 (4.9–17) months. 6 As previously suggested, this rapid time to tolerization may reduce healthcare resource utilization and the costs associated with ITI. 29 Altogether, the results of this study may support the option of a low‐dose strategy for developing countries which cannot afford high‐dose regimens.
The use of a pdFVIII/VWF concentrate in our study may have partially influenced in the observed ITI success rate, since VWF may downregulate the immunogenicity of FVIII, and the inhibitor reactivity against FVIII concentrates is lower when VWF is present. 8 , 12 , 30 It is also acknowledged that several other factors determine success rate and a reduced immunogenicity may not be critical to achieve tolerance. Additionally, the pdFVIII/VWF concentrate used has a longer half‐life than other pdFVIII/VWF concentrates and standard half‐life recombinant products. The pdFVIII/VWF concentrate half‐life is close to that measured for extended half‐life FVIII products, 31 which not only is able to protect from breakthrough bleedings between infusions but also may be an effective option to obtain rapid time to tolerization. 29 , 32 Thus, we cannot disregard that this longer half‐life may have helped to achieve a shorter time to success for ITI. However, further investigations should be performed to confirm this hypothesis. Furthermore, this cohort of patients, who were successfully tolerized with a pdFVIII/VWF concentrate, were not concomitantly treated with any immunosuppressive agents.
During ITI, bleeding episodes were mostly mild in severity compared with the pre‐ITI period. Those patients who were successfully tolerized with the pdFVIII/VWF concentrate showed a normalized FVIII recovery and this was associated with a lower bleeding rate. The overall ABR and the number of target joints, decreased by 60% and 50%, respectively, after ITI therapy, thus reducing morbidity from HA disease. Besides the clinical benefits of the ITI therapy, the number of days that these children with severe HA did not attend the school were reduced (44.1%), suggesting an improvement in the patient's health‐related quality of life and contributing to normalise patients’ life.
This study was designed to assess the ITI effectiveness in patients with poor risk status, with at least one poor prognosis factor: pre‐ITI titre > 10BU. Traditionally, these patients had to wait until their titre had dropped below 10BU. 24 The Future of Immunotolerance Treatment (FIT) group recommended starting ITI as soon as inhibitor is detected and to stratify patients based on pre‐ITI titres. 33 Our data confirmed that it is plausible to start ITI regardless inhibitor levels at diagnosis. Remarkably, the low‐dose ITI response rate was 93,75% in patients with inhibitor titres below 55 BU at start of treatment. This result matches those observed in earlier studies where an increasing failure rate was reported in patients with titres > 50 BU at start of ITI. 11
Patients were also stratified according to an established cut‐off values predictors for ITI success, 6 , 8 , 11 and ITI outcome was analysed. A higher success rate was observed in the subgroup of patients aged < 7 years, in those who showed a peak threshold inhibitor titre < 200 BU during ITI, and when the time to diagnosis to ITI was less than 24 months. Interestingly, in half of the patients who failed ITI in our cohort, treatment was initiated after more than 2 years of inhibitor diagnosis. Likewise, patients with two poor prognosis factors showed a 60% overall success rate and in those patients with three prognostic factors, success rate was 50%, suggesting that the use of pdFVIII/VWF concentrate in low dose ITI therapy may be effective. However, these data must be interpreted with caution because of the small sample size of the cohort.
Our study has limitations. First, it was designed as a non‐controlled single arm cohort study and, therefore, it lacks a comparison group. Second, the sample size of patients after stratification according to the number of poor prognostic factors was small. Third, adverse events were not recorded during the study period and the follow up period after immune tolerance was short. Therefore, confirmatory studies in a larger cohort of patients would be needed for broader interpretation of these results.
5. CONCLUSION
In conclusion, these results confirmed that highly purified pdFVIII/VWF concentrates are effective in a low‐dose ITI strategy in patients with poor prognosis factors.
AUTHOR CONTRIBUTION
M. Elalfy: Conceptualization Ideas, methodology, study design, supervision, writing – review and editing, formal analysis, provision of resources; I. Elghamry: data curation, investigation, project administration; H. Hassab: data curation, investigation, project administration, supervision; O. Elalfy: data curation, investigation, project administration; N. G. Andrawes: data curation, investigation, project administration, follow‐up of patients; M. El‐Ekiaby: laboratory investigation, validation and provision of resources.
DISCLOSURES
The authors stated that they had no interests which might be perceived as posing a conflict or bias. None of the authors received any honoraria, travel grant or research income from Grifols.
Supporting information
Supporting information.
ACKNOWLEDGEMENTS
Eugenio Rosado, PhD and Jordi Bozzo, PhD CMPP (Grifols) are acknowledged for medical writing and editorial support in the preparation of this manuscript, under the direction of the authors. The authors wish to thank all the patients and their families who contributed to this study.
Elalfy M, Elghamry I, Hassab H, Elalfy O, Andrawes N, El‐Ekiaby M. Low‐dose immune tolerance induction therapy in children of Arab descent with severe haemophilia A, high inhibitor titres and poor prognostic factors for immune tolerance induction treatment success. Haemophilia. 2022;28:65–72. 10.1111/hae.14456
[Correction added on 17 December 2021, after first online publication: The copyright line was changed.]
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Mannucci PM, Tuddenham EG. The hemophilias–from royal genes to gene therapy. N Engl J Med. 2001;344(23):1773‐1779. [DOI] [PubMed] [Google Scholar]
- 2. Darby SC, Keeling DM, Spooner RJ, et al. The incidence of factor VIII and factor IX inhibitors in the hemophilia population of the UK and their effect on subsequent mortality, 1977–99. J Thromb Haemost. 2004;2(7):1047‐1054. [DOI] [PubMed] [Google Scholar]
- 3. Gouw SC, van den Berg HM, Fischer K, et al. Intensity of factor VIII treatment and inhibitor development in children with severe hemophilia A: the RODIN study. Blood. 2013;121(20):4046‐4055. [DOI] [PubMed] [Google Scholar]
- 4. Rocino A, Santagostino E, Mancuso ME, Mannucci PM. Immune tolerance induction with recombinant factor VIII in hemophilia A patients with high responding inhibitors. Haematologica. 2006;91(4):558‐561. [PubMed] [Google Scholar]
- 5. Kurth M, Puetz J, Kouides P, et al. The use of a single von Willebrand factor‐containing, plasma‐derived FVIII product in hemophilia A immune tolerance induction: the US experience. J Thromb Haemost. 2011;9(11):2229‐2234. [DOI] [PubMed] [Google Scholar]
- 6. Hay CR, DiMichele DM. The principal results of the International immune tolerance study: a randomized dose comparison. Blood. 2012;119(6):1335‐1344. [DOI] [PubMed] [Google Scholar]
- 7. Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26 Suppl 6:1‐158. [DOI] [PubMed] [Google Scholar]
- 8. Kreuz W, Escuriola Ettingshausen C, Vdovin V, et al. First prospective report on immune tolerance in poor risk haemophilia A inhibitor patients with a single factor VIII/von Willebrand factor concentrate in an observational immune tolerance induction study. Haemophilia. 2016;22(1):87‐95. [DOI] [PubMed] [Google Scholar]
- 9. Berntorp E, Astermark J, Carlborg E. Immune tolerance induction and the treatment of hemophilia. Malmö protocol update. Haematologica. 2000;85(10 Suppl):48‐50. [PubMed] [Google Scholar]
- 10. Ter Avest PC, Fischer K, Gouw SC, Van Dijk K, Mauser‐Bunschoten EP. Successful low dose immune tolerance induction in severe haemophilia A with inhibitors below 40 Bethesda Units. Haemophilia. 2010;16(102):71‐79. [DOI] [PubMed] [Google Scholar]
- 11. Oldenburg J, Jiménez‐Yuste V, Peiró‐Jordán R, Aledort LM, Santagostino E. Primary and rescue immune tolerance induction in children and adults: a multicentre international study with a VWF‐containing plasma‐derived FVIII concentrate. Haemophilia. 2014;20(1):83‐91. [DOI] [PubMed] [Google Scholar]
- 12. Bravo MI, Da Rocha‐Souto B, Grancha S, Jorquera JI. Native plasma‐derived FVIII/VWF complex has lower sensitivity to FVIII inhibitors than the combination of isolated FVIII and VWF proteins. Impact on Bethesda assay titration of FVIII inhibitors. Haemophilia. 2014;20(6):905‐911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carcao M, Shapiro A, Hwang N, et al. Real‐world data of immune tolerance induction using recombinant factor VIII Fc fusion protein in patients with severe haemophilia A with inhibitors at high risk for immune tolerance induction failure: a follow‐up retrospective analysis. Haemophilia. 2021;27(1):19‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Soto AV, Cortez SD, González SM. Immunotolerance induction effectivity in hemophilia A children and neutralizing alloantibodies. Rev Chil Pediatr. 2020;91(2):232‐238. [DOI] [PubMed] [Google Scholar]
- 15. Mariani G, Kroner B. Immune tolerance in hemophilia with factor VIII inhibitors: predictors of success. Haematologica. 2001;86(11):1186‐1193. [PubMed] [Google Scholar]
- 16. Haya S, Solano C, Cid AR, et al. Predictive factors of immune tolerance treatment response in severe haemophilia A patients with inhibitors: a real‐world report from a single centre, mixed retrospective‐prospective long‐term study. Haemophilia. 2019;25(2):e97‐e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rasekh HR, Imani A, Karimi M, Golestani M. Cost‐utility analysis of immune tolerance induction therapy versus on‐demand treatment with recombinant factor VII for hemophilia A with high titer inhibitors in Iran. Clinicoecon Outcomes Res. 2011:3207‐3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kenet G, Oladapo A, Epstein JD, Thompson C, Novack A, Nugent DJ. Estimating the potential cost of a high dose immune tolerance induction (ITI) therapy relative to the cost of a combined therapy of a low dose ITI therapy with bypassing agent prophylaxis. Haemophilia. 2017;23(5):e394‐e402. [DOI] [PubMed] [Google Scholar]
- 19. Li Z, Chen Z, Cheng X, et al. Low‐dose immune tolerance induction for children with hemophilia A with poor‐risk high‐titer inhibitors: a pilot study in China. Res Pract Thromb Haemost. 2019;3(4):741‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Viswabandya A, Mathews V, George B, et al. Successful surgical haemostasis in patients with von Willebrand disease with Koate DVI. Haemophilia. 2008;14(4):763‐767. [DOI] [PubMed] [Google Scholar]
- 21. Gringeri A, Musso R, Mazzucconi MG, et al. Immune tolerance induction with a high purity von Willebrand factor/VIII complex concentrate in haemophilia A patients with inhibitors at high risk of a poor response. Haemophilia. 2007;13(4):373‐379. [DOI] [PubMed] [Google Scholar]
- 22. Valke L, Bukkems LH, Barteling W, et al. Pharmacodynamic monitoring of factor VIII replacement therapy in hemophilia A: combining thrombin and plasmin generation. J Thromb Haemost. 2020;18(12):3222‐3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. El‐Ekiaby M, Goubran HA, Radosevich M, Abd‐Allah A, El‐Ekiaby A, Burnouf T. Pharmacokinetic study of minipooled solvent/detergent‐filtered cryoprecipitate factor VIII. Haemophilia. 2011;17(5):e884‐888. [DOI] [PubMed] [Google Scholar]
- 24. DiMichele DM, Hoots WK, Pipe SW, Rivard GE, Santagostino E. International workshop on immune tolerance induction: consensus recommendations. Haemophilia. 2007;13 Suppl 1:1‐22. [DOI] [PubMed] [Google Scholar]
- 25. Mauser‐Bunschoten EP, Nieuwenhuis HK, Roosendaal G, van den Berg HM. Low‐dose immune tolerance induction in hemophilia A patients with inhibitors. Blood. 1995;86(3):983‐988. [PubMed] [Google Scholar]
- 26. Ettingshausen CE, Kreuz W. The immune tolerance induction (ITI) dose debate: does the International ITI Study provide a clearer picture?. Haemophilia. 2013;19 Suppl 1:12‐17. [DOI] [PubMed] [Google Scholar]
- 27. DiMichele DM, Kroner BL. The North American immune tolerance registry: practices, outcomes, outcome predictors. Thromb Haemost. 2002;87(1):52‐57. [PubMed] [Google Scholar]
- 28. Wight J, Paisley S, Knight C. Immune tolerance induction in patients with haemophilia A with inhibitors: a systematic review. Haemophilia. 2003;9(4):436‐463. [DOI] [PubMed] [Google Scholar]
- 29. Carcao M, Shapiro A, Staber JM, et al. Recombinant factor VIII Fc fusion protein for immune tolerance induction in patients with severe haemophilia A with inhibitors‐A retrospective analysis. Haemophilia. 2018;24(2):245‐252. [DOI] [PubMed] [Google Scholar]
- 30. Oldenburg J, Lacroix‐Desmazes S, Lillicrap D. Alloantibodies to therapeutic factor VIII in hemophilia A: the role of von Willebrand factor in regulating factor VIII immunogenicity. Haematologica. 2015;100(2):149‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iorio A. Using pharmacokinetics to individualize hemophilia therapy. Hematology Am Soc Hematol Educ Program. 2017;2017(1):595‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Janbain M, Pipe S. What is the role of an extended half‐life product in immune tolerance induction in a patient with severe hemophilia A and high‐titer inhibitors?. Hematology Am Soc Hematol Educ Program. 2016;2016(1):648‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carcao M, Escuriola‐Ettingshausen C, Santagostino E, et al. The changing face of immune tolerance induction in haemophilia A with the advent of emicizumab. Haemophilia. 2019;25(4):676‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


