Abstract
Background
The effect of being born late preterm (34–36 weeks gestation) on cardiometabolic outcomes across the life course is unclear.
Objectives
To systematically review the association between being born late preterm (spontaneous or indicated), compared to the term and cardiometabolic outcomes in children and adults.
Data sources
EMBASE(Ovid), MEDLINE(Ovid), CINAHL.
Study selection and data extraction
Observational studies up to July 2021 were included. Study characteristics, gestational age, cardiometabolic outcomes, risk ratios (RRs), odds ratios (ORs), hazard ratios (HRs), mean differences and 95% confidence intervals (CIs) were extracted.
Synthesis
We pooled converted RRs using random‐effects meta‐analyses for diabetes, hypertension, ischemic heart disease (IHD) and body mass index (BMI) with subgroups for children and adults. The risk of bias was assessed using the Newcastle‐Ottawa scale and certainty of the evidence was assessed using the grading of recommendations, assessment, development and evaluation (GRADE) approach.
Results
Forty‐one studies were included (41,203,468 total participants; median: 5.0% late preterm). Late preterm birth was associated with increased diabetes (RR 1.24, 95% CI 1.17, 1.32; nine studies; n = 6,056,511; incidence 0.9%; I 2 51%; low certainty) and hypertension (RR 1.21, 95% CI 1.13, 1.30; 11 studies; n = 3,983,141; incidence 3.4%; I 2 64%; low certainty) in children and adults combined. Late preterm birth was associated with decreased BMI z‐scores in children (standard mean difference −0.38; 95% CI −0.67, −0.09; five studies; n = 32,602; proportion late preterm 8.3%; I 2 96%; very low certainty). There was insufficient evidence that late preterm birth was associated with increased IHD risk in adults (HR 1.20, 95% CI 0.89, 1.62; four studies; n = 2,706,806; incidence 0.3%; I 2 87%; very low certainty).
Conclusions
Late preterm birth was associated with an increased risk of diabetes and hypertension. The certainty of the evidence was low or very low. Inconsistencies in late preterm and term definitions, confounding variables and outcome age limited the comparability of studies.
Keywords: cardiovascular, diabetes, late preterm, meta‐analysis, systematic review
Synopsis
Study question
Does being born late preterm (34–36 weeks), compared to full‐term birth (≥37 weeks), confer greater risk to cardiometabolic outcomes across the life course?
What is already known
Late preterm birth is now the largest subset of preterm neonates, but most research on early life exposures has focused on very preterm birth (<32 weeks) or any preterm birth defined as (<37 weeks). Recent systematic reviews of the long‐term health outcomes of children born preterm have found consistently worse cardiometabolic outcomes in adulthood.
What this study adds
Late preterm birth was associated with an increased risk of cardiometabolic outcomes, specifically diabetes and hypertension. These results may support guidelines for managing medically indicated late preterm deliveries, as well as targeted screening to improve child cardiometabolic health and minimise cardiometabolic disease in adults born late preterm.
1. BACKGROUND
Preterm birth, defined as birth <37 weeks’ gestation, affects 10.6% of pregnancies globally, such that 14.9 million children are born preterm annually. 1 , 2 Late preterm birth, which is generally defined as birth between 34 and 36 weeks’ gestation, has been increasing steadily since 1990 3 and accounts for 75% of all preterm births. 4 Much of this increase is thought to be due to medically indicated early delivery as a part of an interventionist approach to pregnancy management for maternal, foetal and placental complications. 5 , 6 , 7 However, risk factors for spontaneous preterm delivery including older maternal age, multiple gestation births, maternal obesity and diabetes and have also been increasing. 8 , 9
Early life exposures during both the prenatal and early postnatal period can impact the predisposition to adult‐onset chronic diseases. 10 Most research on early life exposures has focused on very preterm birth defined as <32 weeks’ gestation or any preterm birth defined as <37 weeks’ gestation, but there has been comparatively little work evaluating the impact of late preterm birth. Very preterm infants experience a high rate of morbidity and mortality in the first year of life and developmental and cardiovascular adversity later in life. 11 , 12 , 13 Systematic reviews have revealed that overall preterm birth (<37 weeks) is associated with increased blood pressure 14 , 15 , 16 and diabetes risk 14 , 17 , 18 in adults, but results for fat mass or body mass index (BMI) 14 , 15 , 19 and cholesterol 14 , 15 , 19 have been inconsistent or null. Late preterm birth may also confer negative consequences across the life course, 20 but there is limited evidence from the systematic reviews focused on this group. A 2019 narrative review of 15 studies (all Scandinavian) found some evidence that late preterm birth was associated with metabolic syndrome, obesity, hypertension and type 2 diabetes, but there was insufficient evidence for cardiovascular disease. 21 The associations between late preterm birth and cardiometabolic outcomes such as diabetes, hypertension and ischemic heart disease (IHD) have not been systematically reviewed and quantified through a meta‐analysis.
The primary objective of this study was to systematically review and evaluate the certainty of the association between being born late preterm, compared to full‐term birth, and cardiometabolic outcomes across the life course, including childhood and adult outcomes. We hypothesised that late preterm birth is associated with increased cardiometabolic conditions across the life course.
2. METHODS
We conducted a systematic review and meta‐analysis, and the protocol was registered (PROSPERO registration number: CRD42020198870). Results of the study were reported following the Preferred Reporting Items for systematic reviews and meta‐analyses (PRISMA) statement recommendations and guidelines for meta‐analyses and systematic reviews of observational studies (MOOSE). 22 , 23 Ethics approval was not required for this study.
2.1. Literature search
We searched the following electronic databases, EMBASE, MEDLINE and CINAHL, in June 2020 and updated the search on 5 July 2021. All databases were searched from the date of inception of the database. Health research librarians at McMaster University were consulted to develop the search strategy. The search captured two broad concepts: late preterm birth and cardiometabolic conditions. The following terms were used and revised as appropriate depending on the database: late preterm birth, premature, prematurity, gestation, gestational age, cardiometabolic conditions, cardiometabolic, cardiovascular disease, BMI, body mass index, high blood pressure, hypertension, diabetes mellitus, diabetes, heart disease, myocardial infarction, heart infarction, obesity, overweight, lipids, cholesterol blood level and glucose blood level. The specific search strategies for EMBASE, MEDLINE and CINAHL can be found in Tables S1–S3. Reference lists of eligible studies were hand‐searched to identify additional articles.
2.2. Study selection
We included studies published in any year that assessed the association between late preterm birth and cardiometabolic conditions, were conducted in human participants, written in English and had available in full texts. Studies were excluded if they evaluated only very preterm (<32 weeks) or overall preterm birth without distinguishing a late preterm group. Further, studies were excluded if they evaluated cardiometabolic outcomes in the first year of life only, or if they evaluated non‐cardiometabolic outcomes, evaluated the maternal risk of preterm birth, included only infants with congenital anomalies or children born with cardiometabolic diseases. Cross‐sectional studies, case series, qualitative research, review articles, theses, conference abstracts and brief reports without sufficient information were also excluded. Following the database searches, studies were screened by two independent reviewers at both title and abstract level, and at the full text (pairs of SI, ES, YYM, VD, ATA, LNA). In the case of conflicts, a third reviewer determined consensus regarding the eligibility of the study.
2.3. Data extraction
Data were extracted by two independent abstractors (SI, ES, YYM, VD, ATA, LNA). The standardised data extraction form included the following study characteristics: year of publication, study design, country of publication, sample size, length of follow‐up, number and type of confounders controlled for, and details of the specific measures regarding the exposure (defined weeks of gestation) and cardiometabolic outcomes of each study. Effect measures including unadjusted and adjusted risk ratios (RRs), odds ratios (ORs), hazard ratios (HRs), mean differences and corresponding 95% confidence intervals (CIs) were extracted for all outcomes.
2.4. Quality assessment
Study quality was assessed using the Newcastle‐Ottawa Scale (NOS) where up to 9 stars were awarded based on how well cohort selection, comparability and ascertainment of the outcome was implemented. 24 For comparability, we awarded one star if authors controlled for at least one sociodemographic variable, one maternal variable and one maternal cardiometabolic variable because these variables are associated with the exposure and outcome and are not on the causal pathway. Two stars were awarded if authors controlled for more than the three aforementioned variables and did not control for variables on the causal pathway (such as birthweight and child BMI variables). If the authors controlled for variables that were on the causal pathway and thus did not meet the standard definition of a confounder (over‐adjusted), then a maximum of one star was awarded. The NOS was chosen due to its applicability for non‐randomised studies, specifically cohort and case‐control studies. Each assessment was conducted independently by two reviewers and a third reviewer was consulted for any discrepancies (LNA).
2.5. Statistical analysis
To obtain the summary estimates, we natural log‐transformed and pooled the RRs using the generic inverse variance method with random ‐effects models. We extracted unadjusted and adjusted RRs, ORs, HRs and mean differences. Maximally adjusted estimates, i.e. estimates from full models, were preferred if variables on the causal pathway (such as birthweight, birthweight for gestational age, and child BMI) were not included in this model. If they were the most‐adjusted model that did not include these variables was selected as ‘maximally adjusted’. For binary data, we converted ORs to RRs using the equation described in section 15.4.4.4 of the Cochrane handbook and HRs to RRs using the equation described by Shor et al., 2017 25 , 26 For continuous data, we extracted data on the z‐score standardised mean differences and calculated the standardised z‐score if required. The Standardised mean estimates were also pooled using the generic inverse variance method with random‐effects models. The I 2 statistic was used to assess the heterogeneity of each meta‐analysis. If I 2 was 50%–74%, we interpreted this as indicating substantial heterogeneity, and if I 2 was 75%–100% we interpreted this as indicating considerable heterogenity. 27 For the outcomes of diabetes and hypertension, the subgroup analyses were conducted to determine if outcomes differed between adults (≥18) and children (<18) since a sufficient number of studies were available. If 10 or more cohort comparisons were available, we investigated publication bias using Egger's regression test in R version 4.0.5. 28 Data were analysed using Review Manager (RevMan) version 5.4.1. Synthesis without meta‐analysis (SWiM) in systematic reviews reporting guidelines were used to narratively summarise the outcomes which could not be pooled statistically. 29 If more than one study used data from the same cohort, we reviewed the study to ensure authors investigated a different outcome.
2.6. Sensitivity analyses
To assess the potential for the measurement error based on the definition of the reference group and the potential for confounding by indication, sensitivity analyses were conducted where possible (two or more studies per outcome) by restricting pooled analyses to studies that used a strict definition of term births (39–40 weeks) and studies that controlled for maternal cardiometabolic factors.
2.7. Grading of the evidence
The grading of recommendations, assessment, development and evaluations (GRADE) approach was used to assess the certainty of the evidence. 27 , 30 Included observational studies started at low‐certainty evidence by default and then were down or upgraded dependent on pre‐specified criteria. Criteria to downgrade the evidence was determined based on the NOS, inconsistency (wide variance in point estimates encompassing different magnitudes of risk, minimal or no overlap in 95% CIs and I 2 > 75%), indirectness (factors that reduce the applicability of evidence to the target population, or not directly measuring either the exposure or outcome of interest), imprecision (95% CIs that include 1.0, and a small sample size of <4000 participants) and publication bias (based on Egger's test when ≥10 studies were available). Criteria to upgrade included any one of large effect size (RR > 2.0 or RR < 0.5), a dose‐response gradient or attenuation by plausible confounding effects. 27
3. RESULTS
A total of 20,910 studies were identified from which 15,329 studies were screened at the title and abstract level after the removal of duplicates. The full‐text screening was conducted for 312 studies of which 41 studies were eligible for inclusion into the review. Figure 1 outlines the complete PRISMA flowchart. Figure 2 displays a bubble plot of included studies.
FIGURE 1.
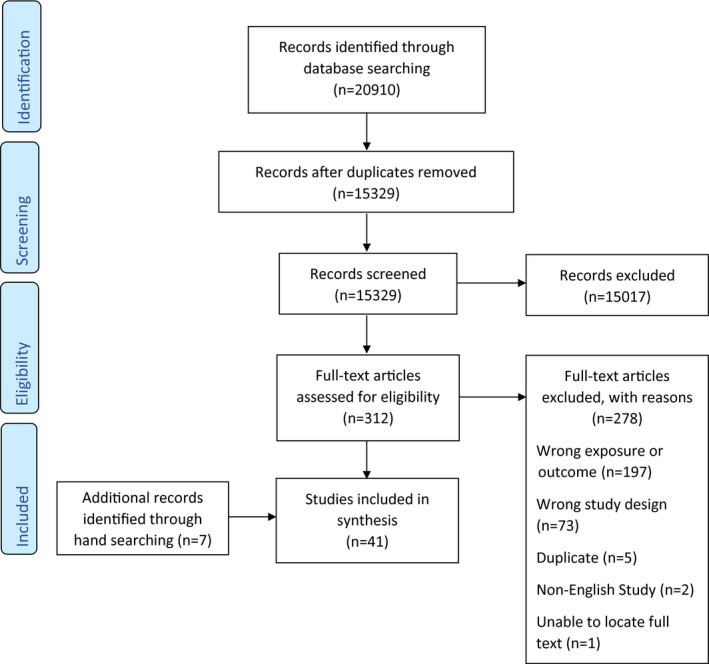
PRISMA flow chart
FIGURE 2.
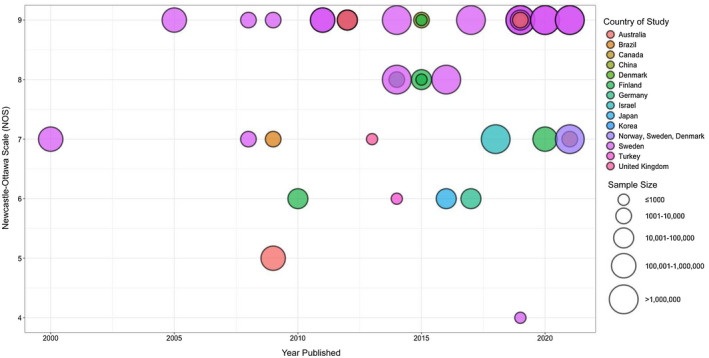
Bubble plot of included studies (n = 41). The colour of the bubble represents the country. The size of the bubble represents the sample size. The x‐axis represents the year published whilst the y‐axis represents the Newcastle‐Ottawa Scale (NOS) score
3.1. Description of studies
A summary of the included articles is provided in Table 1 and the entire data extraction table is provided in Table S4. Participants were from 14 countries and had a median age at the outcome of 18.5 years (Interquartile range [IQR] 8–30 years). Cardiometabolic outcomes assessed were hypertension and blood pressure (32%), diabetes (24%), BMI (17%), IHD (10%) and others (44%). 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 Twenty‐nine studies which could be pooled by the outcome and effect measure were meta‐analysed to create four pooled estimates for diabetes, hypertension, IHD and BMI.
TABLE 1.
Characteristics of included studies reporting cardiometabolic conditions among children born late preterm (n = 41)
| Characteristic | n (%) |
|---|---|
| Country | |
| Sweden | 19 (46) |
| Finland | 6 (15) |
| Brazil | 3 (7) |
| Australia | 2 (5) |
| United Kingdom | 2 (5) |
| Other a | 9 (22) |
| Year of publication | |
| Median (Q1, Q3) | 2015 (2011, 2019) |
| 2000–2004 | 1 (2) |
| 2005–2010 | 7 (17) |
| 2011–2015 | 15 (37) |
| 2016–2021 | 18 (44) |
| Study design | |
| Retrospective Cohort | 24 (59) |
| Prospective Cohort | 15 (37) |
| Case‐Cohort | 1 (2) |
| Case‐Control | 1 (2) |
| Sample size | |
| Median (Q1, Q3) | 35,733 (5334, 2,140,493) |
| <1000 | 6 (15) |
| 1000–10,000 | 9 (22) |
| 10,000–1,000,000 | 14 (34) |
| >1,000,000 | 12 (29) |
| Age at measurement of CMR b | |
| Median (Q1, Q3) | 18.5 (7.9, 30.0) |
| <18 years | 26 (63) |
| ≥18 years | 24 (59) |
| Definition of late preterm | |
| 34–36 weeks | 22 (54) |
| 32–36 weeks | 8 (20) |
| 33–36 weeks | 6 (15) |
| 35–36 weeks | 4 (10) |
| 35–37 weeks | 1 (2) |
| Definition of full term | |
| ≥37 weeks | 11 (27) |
| 37–42 weeks | 9 (22) |
| 39–41 weeks | 9 (22) |
| 37–41 weeks | 4 (10) |
| Other c | 8 (19) |
| Cardiometabolic outcome d | |
| Blood pressure/hypertension | 13 (32) |
| Diabetes | 10 (24) |
| Body Mass Index (BMI) | 7 (17) |
| Ischemic Heart Disease (IHD) | 4 (10) |
| Other e | 18 (44) |
Including Canada, China, Denmark, Germany, Israel, Japan, Korea, Norway and Turkey.
Percentages do not sum to 100% because some studies included participants both less than and greater than 18.
Including 37–39, 39–40, 39 and undefined weeks of gestation.
Percentages do not sum to 100% because some studies included multiple cardiometabolic outcomes.
Including blood lipids, blood glucose, other forms of heart disease, stroke and cerebrovascular disease, metabolic syndrome, etc.
3.2. Quality assessment
The number of stars from the NOS for included studies ranged from 4 to 9 with a median of 7. All studies provided a valid ascertainment of exposure and appropriately assessed the outcome. Some studies did not provide adequate follow‐up or control for appropriate confounders, reducing the quality of studies and cohort comparability. A table describing the confounders in each study is provided in Table S5. The quality assessment of included studies is provided in Table S6.
3.3. Meta‐analysis
There was an increased risk of diabetes, hypertension and IHD for those born late preterm compared to term. Late preterm birth was associated with a reduction in mean BMI z‐scores compared to the term birth. For diabetes and hypertension, there was no evidence of a difference between children and adults (I 2 = 0%). The associations between late preterm birth and diabetes, hypertension, IHD and BMI are in Figures 3, 4, 5, 6. Egger's regression test did not show evidence of publication bias for hypertension. Publication bias was not assessed for BMI, diabetes or IHD because there were <10 comparisons.
FIGURE 3.
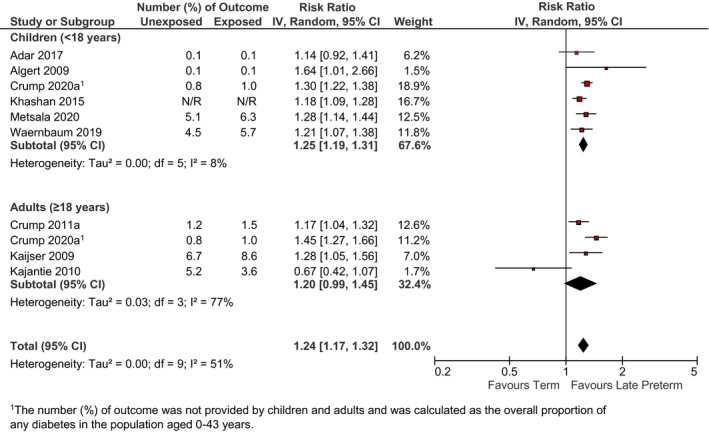
Relation between late preterm birth and incidence of diabetes (late preterm birth vs. term birth) in children <18 years and adults ≥18 years. Pooled risk estimate is represented by the black diamond. Values of I 2 > 50% indicate substantial heterogeneity. Values >1 indicate an adverse association in those born late preterm. CI, confidence interval; RR, risk ratio
FIGURE 4.
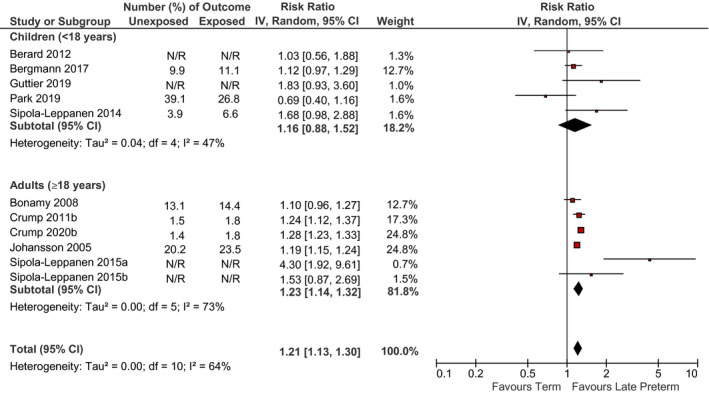
Relation between late preterm birth and incidence of hypertension (late preterm birth vs. term birth) in children <18 years and adults ≥18 years. Pooled risk estimate is represented by the black diamond. Values of I 2 > 50% indicate substantial heterogeneity. Values >1 indicate an adverse association in those born late preterm. CI, confidence interval; RR, risk ratio
FIGURE 5.
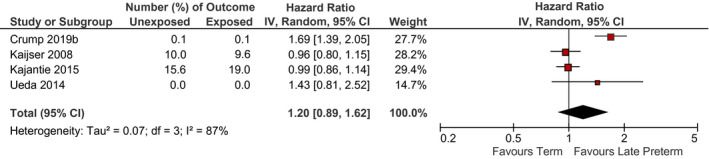
Relation between late preterm birth and incidence of ischemic heart disease (late preterm birth vs. term birth) for all ages (15 years and older). Pooled risk estimate is represented by the black diamond. Values of I 2 > 50% indicate substantial heterogeneity. Values >1 indicate an adverse association in those born late preterm. CI, confidence interval; RR, risk ratio
FIGURE 6.
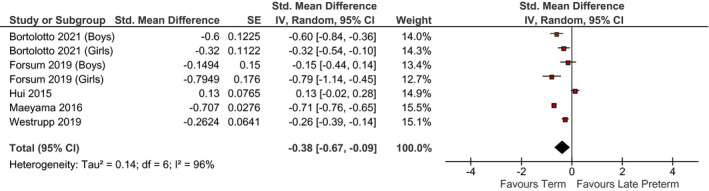
Relation between late preterm birth and z‐score standardised mean difference in BMI (late preterm birth vs. term birth) in children <18 years. Pooled risk estimate is represented by the black diamond. Values of I 2 > 50% indicate substantial heterogeneity. Values >0 indicate an adverse association in those born late preterm. CI, confidence interval; Std, standardised
The GRADE summary of findings for pooled studies is provided in Table S7. The evidence for diabetes and hypertension was rated low quality, and very low quality for BMI and IHD due to downgrades for serious inconsistency for both and also serious imprecision for IHD.
3.4. Sensitivity analyses
When we restricted the meta‐analyses for diabetes to only studies that defined term births as 39–40 weeks, results were slightly attenuated and the evidence of heterogeneity increased, but this was only based on two eligible studies and only possible for the one outcome (Figure S1). When we restricted the meta‐analyses to studies that controlled for maternal cardiometabolic factors, the associations between late preterm birth and diabetes, hypertension and IHD were strengthened but attenuated for BMI (Figure S2–5).
3.5. Other cardiometabolic outcomes
There were 21 studies with cardiometabolic outcomes that were not pooled because of outcome heterogeneity. 33 , 34 , 35 , 37 , 38 , 39 , 42 , 45 , 46 , 47 , 49 , 51 , 56 , 58 , 59 , 63 , 64 , 65 , 67 , 68 , 71 Eight studies investigated cardiometabolic outcomes in children, five of which found an increased association between late preterm birth and cardiometabolic outcomes. 34 , 49 , 51 , 58 , 65 In early childhood, there was generally no evidence of an association between weight or height with late preterm birth, 38 , 64 but higher waist‐hip and waist‐height ratio z‐score were found at 14 years. 51 Late preterm birth was associated with higher mean systolic blood pressure in children and adolescents. 49 , 58 Results for lipids (total cholesterol, low‐density lipoprotein, cholesterol and triglycerides) as well as insulin and glucose markers were null or mixed. 35 , 65 The association between late preterm birth and cardiovascular and cerebrovascular disease in the first three years of life were increased. 34
In adults, five studies investigated cardiometabolic outcomes, four of which found an association between late preterm birth and measures of increased cardiometabolic risk. 33 , 42 , 59 , 67 These included increased metabolic syndrome, obesity and greater waist circumferences. 67 Further, late preterm birth was associated with lipid disorders in early to mid‐adulthood 42 and gestational diabetes in adult women 33 but not BMI differences. 37
Eight studies investigated incident cardiovascular disease outcomes 39 , 45 , 46 , 47 , 56 , 68 , 71 and mortality, 63 of which six studies found late preterm birth conferred greater risk. 39 , 45 , 46 , 47 , 63 , 71 This included increased risk of heart failure from 1 year until the age of death 39 and from 0 to 43 years, 46 increased risk of other major cardiovascular disease, 45 death from cardiovascular disease 63 and risk of venous thromboembolism in adulthood only. 71 There was no increased risk of cerebrovascular disease 68 among young adults. One study found an increased risk of stroke 47 whilst another did not 56 among adults born late preterm.
4. COMMENT
4.1. Principal findings
This systematic review found low certainty evidence that being born late preterm is a risk factor for both diabetes and hypertension in children and adults. For the association between late preterm birth and risk of IHD, the pooled HR was 1.20, possibly suggestive of increased risk, but the 95% CI was wide and included 1.0. Evidence was assessed as very low quality for IHD since the estimate was inconsistent and imprecise. Late preterm birth was associated with decreased BMI z‐scores in children and evidence was assessed at very low quality since the estimate was inconsistent; however, insufficient studies were identified to conduct a meta‐analysis of BMI in adults. Lastly, 5 out of 8 (63%) and 10 out of 13 (77%) narratively synthesised studies found associations between late preterm birth and other cardiometabolic outcomes in children and adults, respectively.
4.2. Strengths of the study
A major strength of our study was that we used a comprehensive search strategy by working with research librarians to identify available studies investigating the association between late preterm and included a broad range of cardiometabolic outcomes, including outcomes in both children and adults. Additional strengths of our study were that we performed quantitative synthesis, using meta‐analysis where appropriate and possible, and assessed the certainty of the evidence using GRADE.
4.3. Limitations of the data
Despite the inclusion of several large, high‐quality cohort studies with a long duration of follow up, the inability to rule out residual confounding is an inherent limitation in all observational studies and the reason that observational studies start at low quality in GRADE. Further, the lack of adjustment for confounders known to be associated with late preterm birth and cardiometabolic risk, especially maternal cardiometabolic conditions (such as maternal diabetes, hypertension and obesity) and family medical history was a major source of bias resulting in confounding by indication. Mothers with diabetes, hypertension and obesity are more likely to have indicated preterm delivery. 72 Children born to mothers with cardiometabolic conditions are more likely to have these conditions themselves due to maternal genetic and lifestyle factors regardless of the term they are delivered. 73 Another major source of bias was over‐adjusting for inappropriate confounders on the causal pathway such as birthweight. Models that were not over‐adjusted were used for the meta‐analysis when possible, for all but two studies. 34 , 71
High heterogeneity was observed for all meta‐analyses. One important source of heterogeneity is the reason for preterm delivery (such as spontaneous versus medically indicated) which was not distinguished in most studies. Studies that were able to distinguish between the two, found no difference in diabetes 44 or stroke risk 47 but a greater risk of hypertension 45 and heart failure 46 in medically indicated preterm births. Another source of heterogeneity is variation in the definition of full term. Some studies defined the term as >37 weeks, whereas others were more specific and included only 39–42 weeks in the term definition. Including early‐term children who were born at 37–38 weeks may have attenuated effect sizes. Due to the relatively small number of studies, we were not able to evaluate the sources of heterogeneity or conduct subgroup analyses investigating the impact of the type of preterm delivery, term definition or family medical history.
Lastly, of the 41 reviewed studies, 27 were conducted in Scandinavian countries, 5 were from upper middle‐income countries (Brazil, China and Turkey), and the remaining nine studies were from high‐income countries (Australia, Canada, Germany, Israel, Japan, Korean and the United Kingdom). Although it is a strength of the Scandinavian healthcare system that linked administrative data are available over long time periods which are necessary for this type of life‐course research study, it should be recognised that our results, which are primarily from Scandinavian studies, may not be generalizable globally.
4.4. Interpretation
Our meta‐analysis demonstrated that late preterm birth was associated with an increased risk of hypertension in adults. This is consistent with reviews in preterm infants of any gestational age 14 , 19 and late preterm infants 21 and supported by the proposed biological mechanisms. Preterm infants are born whilst nephrogenesis is still ongoing, leading to abnormal renal development due to accelerated renal maturation. 74 Increased arterial stiffness has also been proposed as a mechanism for the association of preterm birth with intrauterine growth restriction and hypertension. 75 Consequently, altered kidney function in preterm infants may account for the observed increased risk for high blood pressure and ultimately for cardiovascular disease. 76 However, late preterm birth was not associated with high blood pressure in children, but this was only evaluated in five studies. 34 , 35 , 50 , 62 , 65
There was insufficient evidence that late preterm birth was associated with increased IHD risk. There were four studies included in the meta‐analysis for IHD, and the two studies which suggested no increased risk were both conducted among older adults (with cohorts born prior to 1950), 53 , 56 whilst the two studies which suggested an increased risk of IHD were among younger cohorts born after 1970. 68 , 77 It is possible that changes in the reason for preterm birth over the past decade contribute to this heterogeneity in findings, but we do not have sufficient data to fully investigate this hypothesis. However, this is consistent with the research in all preterm infants, whereby coronary heart disease has been associated with size at birth, rather than length of gestation. 78 Only one study found an increased risk of stroke among adults born late preterm in narratively synthesised studies. 47 However, after preterm birth, the arterial tree undergoes altered development which could have implications for stroke and heart disease later in life if vessel size does not increase in proportion with the rest of the growing body and arteries continue to narrow. 79 There appeared to be a relation between late preterm birth and other cardiovascular outcomes including heart failure, venous thromboembolism, right ventricular mass and other major cardiovascular diseases mainly in adults but these were only investigated by few studies which highlight the need for more research. 39 , 45 , 46 , 59 , 71
Late preterm birth was associated with an increased risk of diabetes in children and adults. All six studies identified for children were consistent with an increased risk. Whereas in the meta‐analysis among adults, one of the four studies observed an association in the opposite direction, suggesting a decreased risk of diabetes. 55 Several proposed mechanisms may account for the increased risk of diabetes observed in late preterm children. Preterm birth interrupts the development of pancreatic beta cells which could lead to a permanent reduction in their number and function. 44 Physiological changes following preterm birth may also account for long‐term insulin sensitivity, such as structural changes to organ systems, changes to endocrine feedback signalling, cellular ageing and epigenetic modifications. 18 Increased visceral adiposity and insulin resistance may also result from exposure to antenatal corticosteroids and rapid catch‐up growth in infancy. 44 These biological mechanisms may also explain the increased measures of cardiometabolic risk found in children and adults born late preterm in narratively synthesised studies. 42 , 51 , 65 , 67 Mixed findings were observed, however, for some cardiometabolic risk factors such as triglycerides with no association for cholesterol and lipoproteins in children. 35 , 65 This is consistent with systematic reviews investigating any preterm birth and cardiometabolic risk where no differences were found between adolescents born preterm compared to the term for most risk factors other than blood pressure. 15
Late preterm birth was inversely associated with BMI in children which were not consistent with our hypothesis of increased cardiometabolic risk across the life course, but this may be explained by the young age at the outcome for the BMI studies (most were among children <7 years). It is possible that an association between late preterm birth and obesity might emerge in later life but we only identified two studies of adults. 37 , 67
5. CONCLUSIONS
The overall findings of this review indicate that late preterm birth is associated with an increased risk for cardiometabolic outcomes, specifically hypertension and diabetes. Additionally, these associations were observed in both childhood and adulthood, demonstrating an increased lifelong risk for adverse health consequences following late preterm birth. Future research is needed to explain the mechanisms underlying the observed associations, including factors that accelerate or impede foetal organ maturation, as well as other risk factors that may be experienced in utero that increase the risk for both late preterm birth and alter cardiometabolic disease outcomes. Our findings highlight the need for high‐quality studies to delineate medically indicated versus spontaneous preterm, define term birth as 39–40 weeks’ gestation and control for maternal cardiometabolic factors. Such studies would provide a better understanding of how late preterm birth contributes to cardiometabolic disease risk, reduce residual confounding and rule out confounding by indication, respectively. The findings from this review will support guidelines for managing medically indicated late preterm deliveries and future interventions, as well as targeted screening to improve child cardiometabolic health and minimise cardiometabolic disease in adults born late preterm.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTION
Yulika Yoshida‐Montezuma, Erica Stone, and Saman Iftikhar conceptualized and designed the study, contributed to the analysis and interpretation of data, drafted the initial manuscript, and reviewed and revised the manuscript. Vanessa De Rubeis, Alessandra T. Andreacchi, and Charles Keown‐Stoneman conceptualized and designed the study, contributed to the analysis and interpretation of data, and reviewed the manuscript for important intellectual content. Lawrence Mbuagbaw, Hilary K. Brown, and Russell J. de Souza contributed to the analysis and interpretation of data and reviewed the manuscript for important intellectual content. Laura N. Anderson conceptualized and designed the study, contributed to the analysis and interpretation of data, and critically reviewed the manuscript for important intellectual content.
Supporting information
Supplementary
ACKNOWLEDGEMENTS
None.
Yoshida‐Montezuma Y, Stone E, Iftikhar S, et al. The association between late preterm birth and cardiometabolic conditions across the life course: A systematic review and meta‐analysis. Paediatr Perinat Epidemiol. 2022;36:264–275. 10.1111/ppe.12831
Funding information
This work was funded by a Canadian Institutes of Health Research (CIHR) Project Grant (#PJT‐168955)
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162‐2172. [DOI] [PubMed] [Google Scholar]
- 2. Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7:e37‐e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martin JA, Kirmeyer S, Osterman M, Shepherd RA. Born a bit too early: recent trends in late preterm births. NCHS Data Brief. 2009;(24):1‐8. [PubMed] [Google Scholar]
- 4. Dong Y, Yu JL. An overview of morbidity, mortality and long‐term outcome of late preterm birth. World J Pediatr. 2011;7:199‐204. [DOI] [PubMed] [Google Scholar]
- 5. Holland MG, Refuerzo JS, Ramin SM, Saade GR, Blackwell SC. Late preterm birth: how often is it avoidable? Am J Obstet Gynecol. 2009;201:404.e1‐404.e4. [DOI] [PubMed] [Google Scholar]
- 6. Bassil KL, Yasseen AS, Walker M, et al. The association between obstetrical interventions and late preterm birth. Am J Obstetr Gynecol. 2014;210:538.e1‐538.e9. [DOI] [PubMed] [Google Scholar]
- 7. Laughon SK, Reddy UM, Sun L, Zhang J. Precursors for late preterm birth in singleton gestations. Obstetr Gynecol. 2010;116:1047‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cobo T, Kacerovsky M, Jacobsson B. Risk factors for spontaneous preterm delivery. Int J Gynecol Obstetr. 2020;150:17‐23. [DOI] [PubMed] [Google Scholar]
- 9. Brown H, Speechley K, Macnab J, Natale R, Campbell M. Biological determinants of spontaneous late preterm and early term birth: a retrospective cohort study. BJOG. 2015;122:491‐499. [DOI] [PubMed] [Google Scholar]
- 10. Sullivan MC, Hawes K, Winchester SB, Miller RJ. Developmental origins theory from prematurity to adult disease. J Obstet Gynecol Neonatal Nurs. 2008;37:158‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chehade H, Simeoni U, Guignard J‐P, Boubred F. Preterm birth: long term cardiovascular and renal consequences. Curr Pediatr Rev. 2018;14:219‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baron IS, Rey‐Casserly C. Extremely preterm birth outcome: a review of four decades of cognitive research. Neuropsychol Rev. 2010;20:430‐452. [DOI] [PubMed] [Google Scholar]
- 13. Glass HC, Costarino AT, Stayer SA, Brett CM, Cladis F, Davis PJ. Outcomes for extremely premature infants. Anesth Analg. 2015;120:1337‐1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Markopoulou P, Papanikolaou E, Analytis A, Zoumakis E, Siahanidou T. Preterm birth as a risk factor for metabolic syndrome and cardiovascular disease in adult life: a systematic review and meta‐analysis. J Pediatr. 2019;210:69‐80.e5. [DOI] [PubMed] [Google Scholar]
- 15. Andraweera PH, Condon B, Collett G, Gentilcore S, Lassi ZS. Cardiovascular risk factors in those born preterm – systematic review and meta‐analysis. J Dev Orig Health Dis. 2021;12:539‐554. [DOI] [PubMed] [Google Scholar]
- 16. De Jong F, Monuteaux MC, Van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta‐analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li S, Zhang M, Tian H, Liu Z, Yin X, Xi B. Preterm birth and risk of type 1 and type 2 diabetes: systematic review and meta‐analysis. Obes Rev. 2014;15:804‐811. [DOI] [PubMed] [Google Scholar]
- 18. Tinnion R, Gillone J, Cheetham T, Embleton N. Preterm birth and subsequent insulin sensitivity: a systematic review. Arch Dis Child. 2014;99:362‐368. [DOI] [PubMed] [Google Scholar]
- 19. Parkinson JRC, Hyde MJ, Gale C, Santhakumaran S, Modi N. Preterm birth and the metabolic syndrome in adult life: a systematic review and meta‐analysis. Pediatrics. 2013;131:e1240‐e1263. [DOI] [PubMed] [Google Scholar]
- 20. Loftin RW, Habli M, Snyder CC, Cormier CM, Lewis DF, Defranco EA. Late preterm birth. Rev Obstetr Gynecol. 2010;3:10‐19. [PMC free article] [PubMed] [Google Scholar]
- 21. Kajantie E, Strang‐Karlsson S, Evensen KAI, Haaramo P. Adult outcomes of being born late preterm or early term – what do we know? Semin Fetal Neonatal Med. 2019;24:66‐83. [DOI] [PubMed] [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. J Am Med Assoc. 2000;283:2008‐2012. [DOI] [PubMed] [Google Scholar]
- 24. Wells G, Shea B, O’Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Accessed December 11, 2020. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 25. Shor E, Roelfs D, Vang ZM. The “Hispanic mortality paradox” revisited: meta‐analysis and meta‐regression of life‐course differentials in Latin American and Caribbean immigrants’ mortality. Soc Sci Med. 2017;186:20‐33. [DOI] [PubMed] [Google Scholar]
- 26. Schünemann HJ, Visit GE, Higgins JP, et al. Chapter 15: interpreting results and drawing conclusions. Accessed July 21, 2021. https://training.cochrane.org/handbook/current/chapter‐15#section‐15‐4‐4
- 27. Schunemann H, Brozek J, Guyatt G & Oxman A. GRADE handbook. Accessed December 11, 2020. https://gdt.gradepro.org/app/handbook/handbook.html
- 28. Higgins JP & Green S. 10.4.3.1 Recommendations on testing for funnel plot asymmetry. Accessed April 20, 2021. https://handbook‐5‐1.cochrane.org/chapter_10/10_4_3_0_introductory_text.htm
- 29. Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta‐analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. GRADE Working Group . Grading quality of evidence and strength of recommendations. Br Med J. 2004;328:1490‐1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adar A, Shalitin S, Eyal O, et al. Prevalence of early and late prematurity is similar among pediatric type 1 diabetes patients and the general population. Diabetes Metab Res Rev. 2018;34:1‐8. [DOI] [PubMed] [Google Scholar]
- 32. Algert CS, McElduff A, Morris JM, Roberts CL. Perinatal risk factors for early onset of Type 1 diabetes in a 2000–2005 birth cohort. Diabet Med. 2009;26:1193‐1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. á Rogvi R, Forman JL, Damm P, Greisen G. Women born preterm or with inappropriate weight for gestational age are at risk of subsequent gestational diabetes and pre‐eclampsia. PLoS One. 2012;7:e34001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bérard A, Le Tiec M, De Vera MA. Study of the costs and morbidities of late‐preterm birth. Arch Dis Child Fetal Neonatal Ed. 2012;97:F329‐F334. [DOI] [PubMed] [Google Scholar]
- 35. Bergmann RL, Bergmann KE, Richter R, Schlaud M, Henrich W, Weichert A. Growth attainment in German children born preterm, and cardiovascular risk factors in adolescence. Analysis of the population representative KiGGS data. J Perinat Med. 2017;45:619‐626. [DOI] [PubMed] [Google Scholar]
- 36. Bonamy AKE, Norman M, Kaijser M. Being born too small, too early, or both: does it matter for risk of hypertension in the elderly? Am J Hypertens. 2008;21:1107‐1110. [DOI] [PubMed] [Google Scholar]
- 37. Bortolotto CC, Santos IS, dos Santos Vaz J, et al. Prematurity and body composition at 6, 18, and 30 years of age: Pelotas (Brazil) 2004, 1993, and 1982 birth cohorts. BMC Public Health. 2021;21:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boyle EM, Poulsen G, Field DJ, et al. Effects of gestational age at birth on health outcomes at 3 and 5 years of age: Population based cohort study. BMJ (Online). 2012;344:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carr H, Cnattingius S, Granath F, Ludvigsson JF, Edstedt Bonamy AK. Preterm birth and risk of heart failure up to early adulthood. J Am Coll Cardiol. 2017;69:2634‐2642. [DOI] [PubMed] [Google Scholar]
- 40. Crump C, Winkleby MA, Sundquist K, Sundquist J. Risk of diabetes among young adults born preterm in Sweden (a). Diabetes Care. 2011;34:1109‐1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Crump C, Winkleby MA, Sundquist K, Sundquist J. Risk of hypertension among young adults who were born preterm: a swedish national study of 636,000 births (b). Am J Epidemiol. 2011;173:797‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Crump C, Sundquist J, Sundquist K. Association of preterm birth with lipid disorders in early adulthood: a Swedish cohort study (a). PLoS Medicine. 2019;16:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Crump C, Howell EA, Stroustrup A, McLaughlin MA, Sundquist J, Sundquist K. Association of preterm birth with risk of ischemic heart disease in adulthood (b). JAMA Pediatr. 2019;173:736‐743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Crump C, Sundquist J, Sundquist K. Preterm birth and risk of type 1 and type 2 diabetes: a national cohort study (a). Diabetologia. 2020;63:508‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Crump C, Sundquist J, Sundquist K. Risk of hypertension into adulthood in persons born prematurely: a national cohort study (b). Eur Heart J. 2020;41:1542‐1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Crump C, Groves A, Sundquist J, Sundquist K. Association of preterm birth with long‐term risk of heart failure into adulthood. JAMA Pediatr. 2021;175:689‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Crump C, Sundquist J, Sundquist K. Stroke risks in adult survivors of preterm birth: national cohort and cosibling study (b). Stroke. 2021;52:2609‐2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Forsum EK, Flinke E, Olhager E. Premature birth was not associated with increased body fatness in four‐year‐old boys and girls. Acta Paediatr. 2020;109:327‐331. [DOI] [PubMed] [Google Scholar]
- 49. Gunay F, Alpay H, Gokce I, Bilgen H. Is late‐preterm birth a risk factor for hypertension in childhood? Eur J Pediatr. 2014;173:751‐756. [DOI] [PubMed] [Google Scholar]
- 50. Guttier MC, Barcelos RS, Ferreira RW, et al. Repeated high blood pressure at 6 and 11 years at the Pelotas 2004 birth cohort study. BMC Public Health. 2019;19:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hui LL, Lam HS, Leung GM, Schooling CM. Late prematurity and adiposity in adolescents: evidence from “children of 1997” birth cohort. Obesity. 2015;23:2309‐2314. [DOI] [PubMed] [Google Scholar]
- 52. Johansson S, Iliadou A, Bergvall N, Tuvemo T, Norman M, Cnattingius S. Risk of high blood pressure among young men increases with the degree of immaturity at birth. Circulation. 2005;112:3430‐3436. [DOI] [PubMed] [Google Scholar]
- 53. Kaijser M, Bonamy AKE, Akre O, et al. Perinatal risk factors for ischemic heart disease: disentangling the roles of birth weight and preterm birth. Circulation. 2008;117:405‐410. [DOI] [PubMed] [Google Scholar]
- 54. Kaijser M, Bonamy AKE, Akre O, et al. Perinatal risk factors for diabetes in later life. Diabetes. 2009;58:523‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kajantie E, Osmond C, Barker DJP, Eriksson JG. Preterm birth – a risk factor for type 2 diabetes? The Helsinki Birth Cohort study. Diabetes Care. 2010;33:2623‐2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kajantie E, Osmond C, Eriksson JG. Coronary heart disease and stroke in adults born preterm – the Helsinki Birth Cohort Study. Paediatr Perinat Epidemiol. 2015;29:515‐519. [DOI] [PubMed] [Google Scholar]
- 57. Khashan AS, Kenny LC, Lundholm C, et al. Gestational age and birth weight and the risk of childhood type 1 diabetes: a population‐based cohort and sibling design study. Diabetes Care. 2015;38:2308‐2315. [DOI] [PubMed] [Google Scholar]
- 58. Leon DA, Johansson M, Rasmussen F. Gestational age and growth rate of fetal mass are inversely associated with Swedish men aged 18 years. Am J Epidemiol. 2000;152:597‐604. [DOI] [PubMed] [Google Scholar]
- 59. Lewandowski AJ, Bradlow WM, Augustine D, et al. Right ventricular systolic dysfunction in young adults born preterm. Circulation. 2013;128:713‐720. [DOI] [PubMed] [Google Scholar]
- 60. Maeyama K, Morioka I, Iwatani S, et al. Gestational age‐dependency of height and body mass index trajectories during the first 3 years in Japanese small‐for‐gestational age children. Sci Rep. 2016;6:38659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Metsälä J, Hakola L, Lundqvist A, Virta LJ, Gissler M, Virtanen SM. Perinatal factors and the risk of type 1 diabetes in childhood and adolescence—a register‐based case‐cohort study in Finland, years 1987 to 2009. Pediatr Diabetes. 2020;21:586‐596. [DOI] [PubMed] [Google Scholar]
- 62. Park B, Lee JW, Kim HS, Park EA, Cho SJ, Park H. Effects of prenatal growth status on subsequent childhood renal function related to high blood pressure. J Korean Med Sci. 2019;34:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Risnes K, Bilsteen JF, Brown P, et al. Mortality among young adults born preterm and early term in 4 Nordic Nations. JAMA Netw Open. 2021;4:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Santos IS, Matijasevich A, Domingues MR, Barros AJD, Victora CG, Barros FC. Late preterm birth is a risk factor for growth faltering in early childhood: a cohort study. BMC Pediatr. 2009;9:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sipola‐Leppänen M, Vääräsmäki CM, Tikanmäki M, et al. Cardiovascular risk factors in adolescents born preterm. Pediatrics. 2014;134:e1072‐e1081. [DOI] [PubMed] [Google Scholar]
- 66. Sipola‐Leppänen M, Karvonen R, Tikanmäki M, et al. Ambulatory blood pressure and its variability in adults born preterm (a). Hypertension. 2015;65:615‐621. [DOI] [PubMed] [Google Scholar]
- 67. Sipola‐Leppänen M, Vääräsmäki M, Tikanmäki M, et al. Cardiometabolic risk factors in young adults who were born preterm (b). Am J Epidemiol. 2015;181:861‐873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ueda P, Cnattingius S, Stephansson O, Ingelsson E, Ludvigsson JF, Bonamy AKE. Cerebrovascular and ischemic heart disease in young adults born preterm: a population‐based Swedish cohort study. Eur J Epidemiol. 2014;29:253‐260. [DOI] [PubMed] [Google Scholar]
- 69. Waernbaum I, Dahlquist G, Lind T. Perinatal risk factors for type 1 diabetes revisited: a population‐based register study. Diabetologia. 2019;62:1173‐1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Westrupp EM, D’Esposito F, Freemantle J, Mensah FK, Nicholson JM. Health outcomes for Australian Aboriginal and Torres Strait Islander children born preterm, low birthweight or small for gestational age: a nationwide cohort study. PLoS One. 2019;14:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zöller B, Li X, Sundquist J, Sundquist K, Crump C. Gestational age and risk of venous thromboembolism from birth through young adulthood. Pediatrics. 2014;134:e473‐e480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Catalano PM. The impact of gestational diabetes and maternal obesity on the mother and her offspring. J Dev Orig Health Dis. 2010;1:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zohdi V, Sutherland MR, Lim K, Gubhaju L, Zimanyi MA, Black MJ. Low birth weight due to intrauterine growth restriction and/or preterm birth: effects on nephron number and long‐term renal health. Int J Nephrol. 2012;2012:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cheung YF, Wong KY, Barbara CC, Lam BCC, Tsoi NS. Relation of arterial stiffness with gestational age and birth weight. Arch Dis Child. 2004;89:217‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Abitbol CL, DeFreitas MJ, Strauss J. Assessment of kidney function in preterm infants: lifelong implications. Pediatr Nephrol. 2016;31:2213‐2222. [DOI] [PubMed] [Google Scholar]
- 77. Crump C, Howell EA, Stroustrup A, McLaughlin MA, Sundquist J, Sundquist K. Association of preterm birth with risk of ischemic heart disease in adulthood. JAMA Pediatr. 2019;173:736‐743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Osmond C, Barker DJP. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ Health Perspect. 2000;108:545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Norman M. Premature birth: implications for cardiovascular health. Future Cardiol. 2013;9:293‐295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


