Abstract
Vibrio harveyi VIB 645, which is very pathogenic towards salmonids and produces extracellular product with a high titer of hemolytic activity towards fish erythrocytes, was found to contain two closely related hemolysin genes (designated vhhA and vhhB), whereas the majority of strains examined (11 of 13) carried only a single hemolysin gene. Both genes from VIB 645 were cloned and sequenced. The open reading frames (ORFs) of vhhA and vhhB shared a high level of identity (98.8%) and were predicted to encode identical polypeptides comprising 418 amino acid residues. The VHH protein shows homology to the lecithinase of V. mimicus and V. cholerae. Transformants of Escherichia coli containing the ORF of either vhhA or vhhB displayed weak hemolytic activity in rainbow trout blood agar. The hemolytic activity was very high when the ORF of vhhB was cloned in E. coli together with the native promoter. Surprisingly, the level of vhh-specific RNA transcript produced by VIB 645 was found to be very low. We conclude that the hemolytic phenotype of VIB 645 is not due to increased expression of one or both copies of the vhh gene.
Vibrio harveyi is a gram-negative, luminous bacterium which is widely distributed in the marine environment (28, 29). Recently, this organism has emerged as a serious pathogen of marine animals. For example, the organism has been reported as a primary pathogen of cultured penaeid shrimp, especially in South America and Asia (1, 10, 18, 26, 33, 36). Additionally, V. harveyi has been associated with diseases in finfish (1, 8, 9, 15, 30) and pearl oysters (24). However, little is known about the pathogenicity mechanisms of V. harveyi. Liu et al. (19) considered that proteases, phospholipases, or hemolysins might be important for pathogenicity; cysteine protease has been reported as the major exotoxin to penaeid shrimp (16, 17, 20). Montero and Austin (21) suggested that the lipopolysaccharide might constitute the lethal toxin of V. harveyi E2 to penaeid shrimp. We have previously examined a large number of well-characterized V. harveyi cultures and found that the hemolysin activity in the extracellular product was involved in pathogenesis in salmonids. V. harveyi VIB 645, which was the most pathogenic isolate, produced extracellular product with the highest titer of hemolytic activity towards Atlantic salmon (1:256) and rainbow trout erythrocytes (1:32) (40).
In general, bacterial hemolysins have been suggested to be important factors of pathogenic vibrios by causing hemorrhagic septicemia and diarrhea in the host. Many of these hemolysins are well characterized, and genes encoding them have been cloned from V. parahaemolyticus (22, 35), V. cholerae (3, 27), V. hollisae (38), V. mimicus (12), V. vulnificus (38), and V. anguillarum (7).
In this study, we describe the cloning and characterization of the hemolysin gene of V. harveyi as a first step towards assessing the role of V. harveyi hemolysin in fish disease.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Thirteen V. harveyi isolates from a diverse range of hosts and geographical locations and one type strain of V. parahaemolyticus were used in this study (Table 1). The cultures were obtained from the Vibrio collection in the Department of Biological Sciences, Heriot-Watt University, and were previously identified and confirmed for authenticity as V. harveyi, that is, cluster 1 of Pedersen et al. (25). The cultures were routinely grown on TNA (tryptone soy agar [Oxoid, Basingstoke, United Kingdom] supplemented with 1% [wt/vol] NaCl) at 28°C, overnight. Escherichia coli NM522 was routinely employed for bacterial transformations and for the maintenance and amplification of recombinant plasmids. E. coli TOP10F′ (Invitrogen, Groningen, The Netherlands) was used as a host for transformation with the pCR2.1-TOPO vector and its derivatives. LB medium (31) was used for the routine culturing of E. coli at 37°C. E. coli transformants were maintained on LB-Ap (LB supplemented with 100 μg of ampicillin per ml) agar. The plasmid pUC19 (37) was used as a vector for the cloning of restriction enzyme-digested DNA, and pCR2.1-TOPO (Invitrogen) was used for the cloning of Taq polymerase-amplified PCR products. Plasmids constructed in this study are listed in Table 2.
TABLE 1.
Strains of V. harveyi and V. parahaemolyticus used in this study
| Strain | Name as received | Source | Country (yr) of isolation |
|---|---|---|---|
| VIB 571 | V. harveyi | Sea bass | Spain (1990) |
| VIB 572 | V. harveyi | Sea bream | Spain (1990) |
| VIB 645 | V. harveyi | Sea bass | Tunisia (1993) |
| VIB 646 | V. harveyi | Shark tank water | Denmark (1993) |
| VIB 647 | V. harveyi | Sea bream | Greece (1992) |
| VIB 648 | V. harveyi | Shark liver | Denmark |
| VIB 649 | V. harveyi | Sea bream | Malta (1993) |
| VIB 651 | V. harveyi | Shark tank water | Denmark (1994) |
| VIB 652 | V. harveyi | Sea bass | Italy |
| VIB 653 | V. harveyi | Sea bass | Turkey |
| VIB 658 | V. harveyi | Sea bream | France (1990) |
| VIB 659 | V. harveyi | Sea bass | Tunisia |
| VIB 661 | V. harveyi | Sea bass | Tunisia (1992) |
| VIB 304 | V. parahaemolyticus | LMGa 2850 |
LMG, culture collection of the Laboratorium voor Microbiologie, Universiteit Gent, Ghent, Belgium.
TABLE 2.
Recombinant plasmids constructed in this study
| Plasmid | Descriptiona |
|---|---|
| pVHHH1 | pUC19 containing a 2.3-kbp HindIII fragment carrying the 5′ end of the vhhA gene (nucleotides 1 to 1017) |
| pVH2H | pUC19 derivative with 1.6 kbp HindIII fragment carrying the 3′ end of the vhhA gene (nucleotides 1065 to 1254) |
| p645H1-1 | pCR2.1-TOPO containing a 1,257-bp PCR product carrying the ORF of vhhA (nucleotides −3 to 1254) |
| p645H1-15 | pCR2.1-TOPO containing a 1,257-bp PCR product carrying the ORF of vhhB (nucleotides −3 to 1254) |
| p645H2-1 | pCR2.1-TOPO containing a 1,404-bp PCR product carrying the ORF of vhhA (nucleotides −61 to 1343) |
Nucleotide positions are relative to the first nucleotide of the translational start codon of the vhh gene.
Isolation of DNA.
Total DNA was prepared from Vibrio spp. by the procedure of Ausubel et al. (2). The rapid alkaline extraction method of Birnboim and Doly (5) was used for the preparation of plasmid DNA from E. coli.
Construction of a genomic DNA library.
Total DNA from V. harveyi VIB 645 was completely digested using HindIII restriction endonuclease (MBI Fermentas, Sunderland, United Kingdom). The digested genomic DNA was mixed with HindIII-digested pUC19, which had also been treated with alkaline phosphatase (Roche Molecular Biologicals, Lewes, United Kingdom). This DNA mixture was ligated with T4 DNA ligase (Roche Molecular Biologicals) at 15°C overnight and used to transform E. coli NM522 by the method of Sambrook et al. (31).
Preparation of DIG-labeled DNA probes by PCR.
Nucleic acid probes are described in Table 3, together with the primers that were used in their construction. The VPHP and VP2P probes are specific to the central portion and the 3′ end of the V. parahaemolyticus tl (thermolabile hemolysin) gene (35), respectively, and were prepared by PCR amplification from total DNA of V. parahaemolyticus VIB 304. The design of the primers was based on the nucleotide sequence of the tl gene published by Taniguchi et al. (35). All PCR primers were custom synthesized by MWG-Biotech AG (Ebersberg, Germany). The PCR was performed in a volume of 50 μl using 1 ng of template DNA, 5 μl of 10× buffer (supplied with the Taq polymerase), a 200 μM concentration of each deoxynucleoside triphosphate, a 0.5 μM concentration of each primer, 2 μM MgCl2, and 2.5 U of Taq DNA polymerase (MBI Fermentas). The PCR conditions were 35 cycles of 1 min at 94°C, 30 s at 55°C, and 1 min at 72°C. A final extension of 7 min at 72°C was included. For the preparation of the digoxigenin (DIG)-labeled probes, 5 μl of 1 mM DIG-labeled dUTP (DIG-dUTP; Roche Molecular Biologicals) was included in the PCR. PCR products were resolved on a 1% (wt/vol) agarose gel, which was stained with ethidium bromide (1 μg/ml) before viewing on a UV transilluminator (UV Products, Prescott, Arizona).
TABLE 3.
DIG-labeled DNA probes hybridizing to the tl gene of V. parahaemolyticus.
| Probe | Primer | Sequences for PCR | Product size (bp) | Target for hybridizationa |
|---|---|---|---|---|
| VPHP | Forward (VPF1) | 5′-TGATCAGCACGCAAGAAAAC-3′ | 922 | Nucleotides 104 to 1025 |
| Reverse (VPR1) | 5′-GTTAGCGTCTCGAACAAGGC-3′ | |||
| VP2P | Forward (VP2F1) | 5′-ATACTCACGCCTTGTTCG-3′ | 252 | Nucleotides 998 to 1249 |
| Reverse (VP2R1) | 5′-GGTACTCGGCTAAGTTGTTG-3′ |
Nucleotide positions are relative to the first nucleotide of the translational start codon of the tl gene of V. parahaemolyticus (GenBank accession no. M36437), which contains an ORF of 1,254 nucleotides (excluding the stop codon).
Southern blotting and hybridization.
DNA samples digested with restriction endonucleases were subjected to electrophoresis through a 1% (wt/vol) agarose gel and transferred onto a nylon membrane (Duralon-UV; Stratagene, Cambridge, United Kingdom) using a modified method originally described by Southern (34). Hybridization was performed overnight at 58°C in a solution of 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.1% (wt/vol) N-laurylsarcosine, 0.02% (wt/vol) sodium dodecyl sulfate (SDS), and 2% (wt/vol) blocking reagent (Roche Molecular Biologicals). After hybridization, the membrane was washed twice for 5 min each at room temperature with low-stringency wash solution (2× SSC, 0.1% [wt/vol] SDS), and twice for 15 min each at 58°C with high-stringency wash solution (0.1× SSC, 0.1% [wt/vol] SDS). Detection of the DIG-labeled DNA was performed by an enzyme-linked immunoassay with an anti-DIG antibody conjugated to alkaline phosphatase (anti-DIG-AP; Roche Molecular Biologicals). The blots were developed at 37°C for 30 to 90 min using a chromogenic solution containing nitroblue tetrazolium (Sigma, Poole, United Kingdom) and 5-bromo-4-chloro-3-indolyl phosphate (p-toluidine salt; Sigma).
Colony blotting and hybridization.
Transformed cells were plated onto LB-Ap agar. Bacterial colonies were transferred onto circular nylon membranes (Duralon-UV [8.2-cm diameter]; Stratagene) by the method of Sambrook et al. (31). Colony blots were hybridized and developed in the same way as Southern blots. Each positive bacterial colony from the master plate was subcultured and screened a second time. A single, well-isolated, positive colony was picked from the second screen and used for further analysis.
Cloning of PCR products of the vhh genes.
PCR products were cloned into pCR2.1-TOPO using the TOPO TA Cloning Kit (Invitrogen) and as directed by the manufacturer. The design of PCR primers for the amplification of V. harveyi vhh genes was based on the sequences of the two partially cloned vhh gene fragments (VHHH1 and VH2H) from this study, and these primers are listed in Table 4. The template for the PCRs was V. harveyi VIB 645 DNA. The samples were subjected to 20 cycles of 1 min at 94°C, 30 s at 55°C, and 1 min at 72°C.
TABLE 4.
Nucleotide sequences of PCR primers for amplification of the vhh gene of V. harveyi
| Primer | Sequence (5′→3′) | Annealing sitea | Product size (bp) |
|---|---|---|---|
| VHF1 | ATCATGAATAAAACTATTACGTTACT | −3′→23 | 1,257 |
| VHR1 | GAAAGGATGGTTTGACAAT | 1254→1236 | |
| VHF2 | GAGGACGTTTGGTGAGATAA | −61→−42 | 1,404 |
| VHR2 | ACGACGAATACAATCTCTGG | 1343→1314 | |
| VHF3 | CTTATTAACGCCCAAAGTGC | −223→−204 | 1,677 |
| VHR3 | ATTGAGGTGCTCCAACAGAT | 1454→1435 |
Nucleotide positions are relative to the first nucleotide of the translational start codon of the vhh gene of V. harveyi.
Nucleotide sequence analysis.
Cloned DNA was sequenced by Cambridge BioScience (Cambridge, United Kingdom). Nucleotide sequences were translated using the ExPASy Translate tool (http://www.expasy.ch/tools/dna.html). Homology searches were performed using BLAST (http://www.ncbi.nlm.nih.gov/BLAST/). Multiple nucleotide or amino acid sequences were aligned using ClustalW (http://www.ebi.ac.uk/clustalw/).
RNA extraction.
V. harveyi was cultured on tryptone soy broth at 28°C overnight. Total RNA from 109 cells/ml was extracted using the RNeasy Mini Kit (Qiagen, Crawley, United Kingdom) according to the manufacturer's instructions.
Northern blotting and hybridization.
RNA (2 μg) was separated by electrophoresis through a guanidine thiocyanate agarose gel (1.2% [wt/vol] agarose) with Tris-borate-EDTA running buffer and blotted onto a nylon membrane (Stratagene). Hybridization was performed at 58°C overnight in a solution composed of 0.25 M Na2HPO4, 1 mM EDTA, 20% (wt/vol) SDS, and 1.0% (wt/vol) blocking reagent (pH 7.2) (6). After hybridization, the blot was washed three times for 20 min each in prewarmed washing buffer (20 mM Na2HPO4; 1 mM EDTA; 1% SDS, pH 7.2). The DNA-RNA hybrids were detected by chemiluminescence (6).
Hemolytic activity assay.
Fresh rainbow trout blood was collected by venipuncture from healthy animals maintained in a freshwater aquarium. The erythrocytes were washed three times with sterile phosphate-buffered saline (Oxoid). Blood agar plates were prepared by mixing 17 ml of melted LB agar (∼55°C) and 3 ml of 10% (vol/vol in phosphate-buffered saline) washed rainbow trout erythrocytes in a 9-cm-diameter petri dish. E. coli clones were inoculated onto the blood agar plate, and hemolytic activity was determined by the appearance of a lytic zone on the surface of the blood agar following incubation overnight at 28°C.
Nucleotide sequence accession numbers.
The nucleotide sequences reported in this work have been assigned GenBank accession numbers as follows: AF293430 for vhhA and AF293431 for vhhB.
RESULTS
Identification of hemolysin genes in V. harveyi.
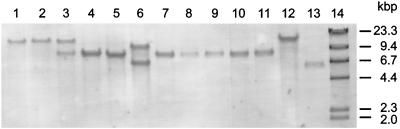
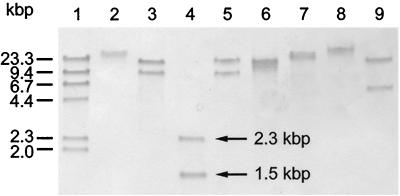
The VPHP probe (Table 3) was used to screen EcoRI digests of total DNA from the 13 V. harveyi strains listed in Table 1. At least one band was detected in all strains, indicating the presence of a hemolysin gene in V. harveyi that is very similar to the tl (thermolabile hemolysin) gene of V. parahaemolyticus. Two EcoRI fragments, with sizes of 8 and 15 kbp, were identified in VIB 645. Two EcoRI fragments, of 7 and 10 kbp, were also found in VIB 648. One band was observed in all other strains. Strains VIB 646, VIB 647, VIB 649, VIB 651, VIB 652, VIB 653, and VIB 658 produced a band at 8 kbp, whereas a 15-kbp band was detected in VIB 571, VIB 572, and VIB 659 and a 6-kbp band was detected in VIB 661 (Fig. 1). The VPHP probe was then used to examine total DNA from VIB 645 separately digested with eight further restriction enzymes (Fig. 2). In every case, two fragments were detected with the VPHP probe. This suggested that VIB 645 has two very similar, or possibly identical, copies of the hemolysin gene. The same is also likely to be true for VIB 648, although no further Southern hybridization analyses have been carried out on DNA from this strain. The two HindIII fragments detected in VIB 645 with the VPHP probe, with sizes of 1.5 and 2.3 kbp (lane 4 of Fig. 3), were selected for cloning in order to characterize the hemolysin genes.
FIG. 1.
Detection of hemolysin genes in strains of V. harveyi. Total DNA from each strain was digested with EcoRI and probed with VPHP. Lanes: 1, VIB 571; 2, VIB 572; 3, VIB 645; 4, VIB 646; 5, VIB 647; 6, VIB 648; 7, VIB 649; 8, VIB 651; 9, VIB 652; 10, VIB 653; 11, VIB 658; 12, VIB 659; 13, VIB 661; 14, DIG-labeled HindIII-digested λ DNA (molecular weight markers).
FIG. 2.
Southern hybridisation analysis of restriction enzyme-digested DNA from V. harveyi VIB 645 probed with VPHP. Lanes: 1, DIG-labeled HindIII-digested λ DNA (molecular weight markers); 2, BamHI; 3, EcoRI; 4, HindIII; 5, KpnI; 6, PstI; 7, SalI; 8, SmaI; 9, XbaI.
FIG. 3.
Nucleotide sequence and translation of the vhh gene. Differences between the nucleotide sequences of vhhA and vhhB are indicated in bold face type; the lower nucleotide appears in vhhA, and the upper one appears in vhhB. The position of the HindIII site in the vhh gene, starting at nucleotide 1020, is indicated by underlining.
Cloning of hemolysin genes from V. harveyi.
A genomic DNA library of V. harveyi VIB 645, consisting of HindIII DNA fragments inserted into pUC19, was used to transform E. coli NM522. Transformed E. coli cells were screened by colony hybridization using the VPHP probe. Thirteen positive clones were isolated from an initial screen of approximately 12,000 E. coli transformants. Plasmid DNA was prepared from each clone and analyzed by HindIII restriction enzyme digestion. All thirteen clones contained an identical 2.3-kbp fragment. Further efforts to clone the 1.5-kbp fragment were unsuccessful. The recombinant plasmid containing the 2.3-kbp fragment was designated pVHHH1 (Table 2), and the inserted DNA was sequenced (data not shown). A partial open reading frame (ORF) was identified in the 2.3-kbp HindIII fragment and was predicted to encode a polypeptide with 87% identity to the N-terminal 341 amino acids of the V. parahaemolyticus thermolabile (TL) hemolysin.
Since the 2.3-kbp HindIII fragment lacked the 3′ end of the ORF of the hemolysin gene, a second probe VP2P (Table 3), which hybridizes to the 3′ end of the V. parahaemolyticus tl gene, was constructed for cloning the missing portion of the gene from VIB 645. Southern hybridization analysis of HindIII-digested DNA prepared from VIB 645 and probed with VP2P produced two fragments, of 1.7 and 3.8 kbp. Screening of the genomic library of VIB 645 yielded six positive clones. All six clones contained the 1.7-kbp HindIII fragment; the 3.8-kbp HindIII fragment was not obtained. The recombinant plasmid containing the 1.7-kbp HindIII fragment was designated pVH2H (Table 2), and the inserted DNA was sequenced (data not shown). A partial open reading frame, encoding a polypeptide with 70% identity to the C-terminal 54 amino acids of the TL hemolysin, was identified.
In order to clone a complete copy of each of the two hemolysin genes that were thought to be present in VIB 645, an alternative strategy based on PCR was developed. Three sets of PCR primers (Table 4) were designed for amplification of the hemolysin genes in VIB 645, based on the nucleotide sequences of the cloned DNA in pVHHH1 and pVH2H (Table 4). PCR products obtained from VIB 645 DNA, using VHF1 and VHR1 as primers, were cloned into pCR2.1-TOPO. Eighteen clones were isolated that contained the expected 1.3-kbp insert. Plasmid DNA was prepared from four different clones, and the inserted DNA in each of them was partially sequenced. When the nucleotide sequences were aligned, it became apparent that there were two types, with nucleotide differences at seven positions (195, 324, 393, 414, 441, 447, and 471, relative to the first nucleotide of the translational start codon). Two plasmids, p645H1-1 and p645H15-1, each containing an insert with a different sequence, were selected for complete sequencing of the hemolysin genes. The genes contained within p645H1-1 and p645H15-1 were named vhhA and vhhB, respectively.
The PCR products obtained using the primers VHF2 and VHR2 were also cloned into pCR2.1-TOPO. Fifteen clones containing the expected 1.4-kbp insert were obtained. The DNA from six clones was partially sequenced, and all were found to contain the vhhB gene. One plasmid, p645H2-1, was selected for further study. Attempts to clone the PCR products obtained with the primers VHF3 and VHR3 into pCR2.1-TOPO were unsuccessful.
Characterization of cloned hemolysin genes.
The ORFs of vhhA and vhhB were both found to be 1,254 nucleotides long and are predicted to encode identical polypeptides of 418 amino acids with a deduced molecular mass of 47.3 kDa (Fig. 3). The nucleotide sequences of vhhA and vhhB are 98.8% identical and differ at only 15 nucleotide positions. The G+C content of both genes was 46%.
By comparing the nucleotide sequences of the cloned DNA contained in pVHHH1 and pVH2H with those of the vhhA and vhhB genes, it was found that both the HindIII fragments in the two plasmids carried a vhhA gene fragment (data not shown).
Analysis of the deduced amino acid sequence of VHH for homology to other sequences in the GenBank database revealed a high degree of identity (85.6%) to the TL hemolysin of V. parahaemolyticus (35) (Fig. 4). The VHH hemolysin also showed strong homology to the lecithinases of V. mimicus (65.3% identity) (11) and V. cholerae (64.3% identity). Three cysteine residues, at positions 49, 357, and 378 for VHH, were located at the same relative position in the four proteins.
FIG. 4.
Alignment of the deduced amino acid sequence of the V. harveyi VIB 645 VHH hemolysin with those of related proteins. VHH, hemolysin from V. harveyi VIB 645; VPTL, TL hemolysin from V. parahaemolyticus (35) (GenBank accession no. M36437); VML, lecithinase from V. mimicus (11) (GenBank accession no. AF035162); VCL, lecithinase from V. cholerae (GenBank accession no. U50074). Symbols: ∗, identical residues in all sequences; :, conserved substitutions; ., semiconserved substitutions; -, gaps introduced during the alignment process. Numbers on the right refer to the amino acid residue at the end of each line.
The vhh gene confers hemolytic activity on E. coli.
The plasmids p645H2-1 and p645H15-1, containing the ORFs of vhhA and vhhB respectively, each conferred weak hemolytic activity on E. coli (as determined on rainbow trout blood agar) (Fig. 5B and C). This was somewhat surprising given that all vhh promoter sequences were missing from both plasmids. The hemolytic activity conferred by p645H2-1, which contains the vhhB gene with 61 nucleotides of the promoter immediately upstream of the translational start codon, was substantially higher, confirming that the vhh gene is functional in E. coli (Fig. 5D). The zones of hemolysis were opalescent and reddish in color, resembling those of V. harveyi when grown on the same agar.
FIG. 5.
Expression of the vhh gene of V. harveyi in E. coli. Duplicate rainbow trout blood agar plates were inoculated as described in Materials and Methods. A. E. coli TOP10F′; B, E. coli TOP10F′ containing p645H1-1 (vhhA); C, E. coli TOP10F′ containing p645H1-15 (vhhB); D, E. coli TOP10F′ containing p645H2-1 (vhhA including 61 nucleotides of promoter DNA upstream of the translational start codon).
Expression of vhh genes in V. harveyi.
RNA transcripts from the vhh genes in strains VIB 571, VIB 645, VIB 646, and VIB 648 were assayed by Northern blot hybridization. Whereas VIB 648 produced a strong hybridization signal, no detectable RNA was found in extracts prepared from VIB 571, VIB 645, and VIB 646 (data not shown).
DISCUSSION
V. harveyi hemolysin has been regarded as a putative pathogenicity factor in salmonids (40). Therefore, it was considered important to identify the genetic basis for hemolysin production by V. harveyi. Initially four pairs of degenerate primers, designed to anneal to conserved sequences in 10 hemolysin genes from Vibrio and Aeromonas species, were used in an attempt to amplify DNA from V. harveyi by PCR, but no products were obtained (data not shown). Instead, probes specific for the V. mimicus vmhA hemolysin gene (12) and the V. parahaemolyticus tl hemolysin gene (35), were used to analyze DNA from V. harveyi by Southern blotting and hybridization. The vmhA gene was selected because it showed strong homology with six other hemolysin genes (data not shown), whereas the tl gene of V. parahaemolyticus was selected because this species is the most closely related to V. harveyi (25). Whereas the VPHP probe (produced by PCR amplification of the tl gene of V. parahaemolyticus) showed strong hybridization to the digests of total DNA from all of the V. harveyi isolates examined (Fig. 1), the V. mimicus vmhA probe (VMHP) did not hybridize to the DNA of any V. harveyi isolate (data not shown). This suggested that V. harveyi contains a hemolysin gene with strong similarity to the tl gene of V. parahaemolyticus. Therefore, the V. parahaemolyticus probe, VPHP, was employed for the initial cloning of the hemolysin gene from V. harveyi.
An interesting finding was the presence of two hemolysin gene copies in the most pathogenic strain, V. harveyi VIB 645, and only one copy in the majority of other V. harveyi strains. Strain VIB 648 also appeared to contain two gene copies, but these were arranged in a different manner compared to VIB 645, as judged from Southern blotting and hybridization analysis (Fig. 1). Both gene copies from VIB 645 (designated vhhA and vhhB) were individually cloned into E. coli. The nucleotide sequences of vhhA and vhhB were not identical but were very similar (98.8% identity), suggesting that the duplication of the vhh gene was a relatively recent event. The predicted amino acid sequences encoded by vhhA and vhhB were identical. The plasmid p645H2-1, which contained the ORF of vhhB together with 61 nucleotides of the promoter immediately upstream of the translational start codon, expressed high levels of hemolysin in E. coli, confirming that the vhhA gene was functional. We do not know at this stage if the two vhh genes contribute equally to hemolysin activity in V. harveyi. Further studies on the promoter sequences of the vhhA and vhhB genes may shed some light on this point.
Northern blot analysis revealed that VIB 648, which contains two vhh genes, expressed a high level of vhh-specific RNA transcript, whereas VIB 571, VIB 645, and VIB 646 produced no detectable vhh RNA under the experimental conditions used here. The result for VIB 645 was unexpected and suggests that the high level of hemolytic activity displayed by this strain is not a consequence of increased vhh gene expression. Conversely, for VIB 648, an increase in transcription, possibly for only one of the two vhh genes, is likely to be a contributing factor towards the high level of hemolytic activity shown by this strain.
The duplication of the vhh gene in V. harveyi is a phenomenon similar to that previously described for the hemolysin (hly) determinant of E. coli (13, 14) and the duplicated tdh genes of V. parahaemolyticus (23). However, unlike the tdh gene and the hly determinant, which have a much higher A+T content than the corresponding chromosomal DNA, the vhh gene had an A+T content (54.1%) similar to that of the chromosomal DNA (an average of 52 to 54%) (4).
The V. harveyi VHH protein shows extensive homology with the V. parahaemolyticus TL protein, also called lecithin-dependent hemolysin, which has been shown to confer thermolabile hemolytic activity (32). Previously, we have partially purified the VHH hemolysin of V. harveyi VIB 645 and found that the hemolytic activity was thermolabile (unpublished data). A BLAST search revealed that the VHH protein is also highly homologous to the lecithinases of V. mimicus and V. cholerae, suggesting that VHH is a lecithinase-like protein. The positions and numbers of cysteine residues in the four proteins were identical (except for an extra cysteine in VML towards the N-terminal end of the protein), suggesting that they share similar secondary structures. Interspecies relatedness has also been reported with the thermostable direct hemolysin originally identified in V. parahaemolyticus (22). Sequence homology with the gene encoding this hemolysin has been found in V. cholerae non-O1, V. mimicus, and V. hollisae (39).
Although gene duplication is acknowledged to be a mechanism that can lead to increased levels of the encoded product, this does not appear to be the case for overnight cultures of VIB 645. Nevertheless, it remains a distinct possibility that one of the vhh genes is expressed in a manner different than that of the other. There may be a high, but transient, level of expression of one gene that was not detected here by Northern blot analysis. Further studies on the molecular basis of hemolysin production, which could include sequencing and characterization of the promoter regions of the vhh genes, will be necessary to explain the high level of hemolytic activity shown by VIB 645.
ACKNOWLEDGMENTS
X.-H. Z. acknowledges the receipt of an Overseas Research Students Award (ORS, administered through Universities UK).
We thank Dawn Austin for providing the Vibrio strains, Robert Dorazi for helpful suggestions, and Nadine Randle for assistance with the Northern blotting and hybridization.
REFERENCES
- 1.Alvarez J D, Austin B, Alvarez A M, Reyes H. Vibrio harveyi: a pathogen of penaeid shrimps and fish in Venezuela. J Fish Dis. 1998;21:313–316. doi: 10.1046/j.1365-2761.1998.00101.x. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J, Smith J, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1987. [Google Scholar]
- 3.Baba K, Shirai H, Terai A, Takeda Y, Nishibuchi M. Similarity of the tdh gene-bearing plasmids of Vibrio cholerae non-O1 and Vibrio parahaemolyticus. Microb Pathog. 1991;10:61–70. doi: 10.1016/0882-4010(91)90066-j. [DOI] [PubMed] [Google Scholar]
- 4.Baumann P, Furniss A L, Lee J V. Genus 1. Vibrio Pacini 1854, 411AL. In: Krieg N R, Holt J G, editors. Bergey's manual of systematic bacteriology. I. Baltimore, Md: Williams and Wilkins; 1984. pp. 518–538. [Google Scholar]
- 5.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enger-Blum G, Meier M, Frank J, Müller G A. Reduction of background problems in nonradioactive Northern and Southern blot analyses enables higher sensitivity than 32P-based hybridizations. Anal Biochem. 1993;210:235–244. doi: 10.1006/abio.1993.1189. [DOI] [PubMed] [Google Scholar]
- 7.Hirono I, Masuda T, Aoki T. Cloning and detection of the hemolysin gene of Vibrio anguillarum. Microb Pathog. 1996;21:173–182. doi: 10.1006/mpat.1996.0052. [DOI] [PubMed] [Google Scholar]
- 8.Hispano C, Nebra Y, Blanch A R. Isolation of Vibrio harveyi from an ocular lesion in the short sunfish (Mola mola) Bull Eur Assoc Fish Pathol. 1997;17:104–107. [Google Scholar]
- 9.Ishimaru K, Muroga K. Taxonomical re-examination of two pathogenic Vibrio species isolated from milkfish and swimming crab. Fish Pathol. 1997;32:59–64. [Google Scholar]
- 10.Jiravanichpaisal P, Miyazaki T, Limsuwan C. Histopathology, biochemistry, and pathogenicity of Vibrio harveyi infecting black tiger prawn Penaeus monodon. J Aquat Anim Health. 1994;6:27–35. [Google Scholar]
- 11.Kang J H, Lee J H, Park J H, Huh S H, Kong I S. Cloning and identification of a phospholipase gene from Vibrio mimicus. Biochim Biophys Acta. 1998;1394:85–89. doi: 10.1016/s0005-2760(98)00100-3. [DOI] [PubMed] [Google Scholar]
- 12.Kim G T, Lee J Y, Huh S H, Yu J H, Kong I S. Nucleotide sequence of the vmhA gene encoding hemolysin from Vibrio mimicus. Biochim Biophys Acta. 1997;1360:102–104. doi: 10.1016/s0925-4439(97)00008-2. [DOI] [PubMed] [Google Scholar]
- 13.Knapp S, Kacker J, Then I, Müller D, Goebel W. Multiple copies of hemolysin genes and associated sequences in the chromosomes of uropathogenic Escherichia coli strains. J Bacteriol. 1984;159:1027–1033. doi: 10.1128/jb.159.3.1027-1033.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knapp S, Then I, Wels W, Michel G, Tschäpe H, Hacker J, Goebel W. Analysis of the flanking regions from different haemolysin determinants of Escherichia coli. Mol Gen Genet. 1985;200:385–392. doi: 10.1007/BF00425721. [DOI] [PubMed] [Google Scholar]
- 15.Kraxenberger-Beatty T, McGarey D J, Grier H J, Lim D V. Vibrio harveyi, an opportunistic pathogen of common snook, Centropomus undecimalis (Bloch), held in captivity. J Fish Dis. 1990;13:557–560. [Google Scholar]
- 16.Lee K K, Liu P C, Chen S N. Studies on the virulence factors of Vibrio for tiger prawn, Penaeus monodon and immunization trials. Rep Fish Dis Res. 1996;17:1–13. [Google Scholar]
- 17.Lee K K, Liu P C, Kou G H, Chen S N. Investigation on the major exotoxin of Vibrio harveyi 770527 isolated from diseased Penaeus monodon. Rep Fish Dis Res. 1997;18:33–42. [Google Scholar]
- 18.Liu P C, Lee K K, Yii K C, Kou G H, Chen S N. Isolation of Vibrio harveyi from diseased Kuruma prawns Penaeus japonicus. Curr Microbiol. 1996a;33:129–132. doi: 10.1007/s002849900087. [DOI] [PubMed] [Google Scholar]
- 19.Liu P C, Lee K K, Chen S N. Pathogenicity of different isolates of Vibrio harveyi in tiger prawn, Penaeus monodon. Lett Appl Microbiol. 1996b;22:413–416. [Google Scholar]
- 20.Liu P C, Lee K K, Tu C C, Chen S N. Purification and characterization of a cysteine protease produced by pathogenic luminous Vibrio harveyi. Curr Microbiol. 1997;35:32–39. doi: 10.1007/s002849900207. [DOI] [PubMed] [Google Scholar]
- 21.Montero A B, Austin B. Characterization of extracellular products from an isolate of Vibrio harveyi recovered from diseased post-larval Penaeus vannamei (Bonne) J Fish Dis. 1999;22:377–386. [Google Scholar]
- 22.Nishibuchi M, Kaper J B. Nucleotide sequence of the thermostable direct hemolysin gene of Vibrio parahaemolyticus. J Bacteriol. 1985;162:558–564. doi: 10.1128/jb.162.2.558-564.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishibuchi M, Kaper J B. Duplication and variation of the thermostable direct haemolysin (tdh) gene in Vibrio parahaemolyticus. Mol Microbiol. 1990;4:87–99. doi: 10.1111/j.1365-2958.1990.tb02017.x. [DOI] [PubMed] [Google Scholar]
- 24.Pass D A, Dybdahl R, Mannion M M. Investigations into the causes of mortality of the pearl oyster, Pinctada maxima (Jamson), in Western Australia. Aquaculture. 1987;65:149–169. [Google Scholar]
- 25.Pedersen K, Verdonck L, Austin B, Austin D A, Blanch A R, Grimont P A D, Jofre J, Koblavi S, Larsen J L, Tiainen T, Vigneulle M, Swings J. Taxonomic evidence that Vibrio carchariae Grimes et al. 1985 is junior synonym of Vibrio harveyi (Johnson and Shunk 1936) Baumann et al. 1981. Int J Syst Bacteriol. 1998;48:749–758. [Google Scholar]
- 26.Pizzutto M, Hirst R G. Classification of isolates of Vibrio harveyi virulent to Penaeus monodon larvae by protein profile analysis and M13 DNA fingerprinting. Dis Aquat Org. 1995;21:61–68. [Google Scholar]
- 27.Rader A E, Murphy J R. Nucleotide sequences and comparison of the hemolysin determinants of Vibrio cholerae E1 Tor RV79 (Hly−) and classical 569B (Hly−) Infect Immun. 1988;56:1414–1419. doi: 10.1128/iai.56.6.1414-1419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramesh A, Venugopalan V K. Response of enteric luminous bacteria to environmental conditions in the gut of the fish. J Appl Bacteriol. 1989;66:529–533. doi: 10.1111/j.1365-2672.1989.tb04574.x. [DOI] [PubMed] [Google Scholar]
- 29.Ruby E G, Morin J G. Luminous enteric bacteria of the marine fishes: a study of their distribution, densities, and dispersion. Appl Environ Microbiol. 1979;38:406–411. doi: 10.1128/aem.38.3.406-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saeed M O. Association of Vibrio harveyi with mortalities in cultured marine fish in Kuwait. Aquaculture. 1995;136:21–29. [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 32.Shinoda S, Matsouka H, Tsuchie T, Miyoshi S-I, Yamamoto S, Taniguchi H, Mizuguchi Y. Purification and characterization of a lecithin-dependent haemolysin from Escherichi coli transformed by a Vibrio parahaemolyticus gene. J Gen Microbiol. 1991;137:2705–2711. doi: 10.1099/00221287-137-12-2705. [DOI] [PubMed] [Google Scholar]
- 33.Sunaryanto A, Mariam A. Occurrence of a pathogenic bacteria causing luminescence in penaeid larvae in Indonesian hatcheries. Bull Brackishwat Aquacult Dev Centre. 1986;8:105–112. [Google Scholar]
- 34.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 35.Taniguchi H, Hirano H, Kubomura S, Higashi K, Mizuguchi Y. Comparison of the nucleotide sequences of the genes for the thermostable direct hemolysin and the thermolabile hemolysin from Vibrio parahaemolyticus. Microb Pathog. 1986;1:425–432. doi: 10.1016/0882-4010(86)90004-5. [DOI] [PubMed] [Google Scholar]
- 36.Vandenberghe J, Li Y, Verdonk L, Li J, Sorgeloos P, Xu H S, Swings J. Vibrios associated with Penaeus chinensis (Crustacea: Decapoda) larvae in Chinese shrimp hatcheries. Aquaculture. 1998;169:121–132. [Google Scholar]
- 37.Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto K, Wright A C, Kaper J B, Morris J G., Jr The cytolysin gene of Vibrio vulnificus: sequence and relationship to the Vibrio cholerae E1 Tor hemolysin gene. Infect Immun. 1990;58:2706–2709. doi: 10.1128/iai.58.8.2706-2709.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamasaki S, Shirai H, Takeda Y, Nishibuchi M. Analysis of the gene of Vibrio hollisae encoding the hemolysin similar to the thermostable direct hemolysin of Vibrio parahaemolyticus. FEMS Microbiol Lett. 1991;80:259–264. doi: 10.1016/0378-1097(91)90606-b. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X H, Austin B. Pathogenicity of Vibrio harveyi to salmonids. J Fish Dis. 2000;23:93–102. [Google Scholar]