Abstract
Background
Mesenchymal stromal cells (MSCs) may reduce mortality in patients with COVID-19; however, early evidence is based on few studies with marked interstudy heterogeneity. The second iteration of our living systematic review and meta-analysis evaluates a framework needed for synthesizing evidence from high-quality studies to accelerate consideration for approval.
Methods
A systematic search of the literature was conducted on November 15, 2021, to identify all English-language, full-text, and controlled clinical studies examining MSCs to treat COVID-19 (PROSPERO: CRD42021225431).
Findings
Eleven studies were identified (403 patients with severe and/or critical COVID-19, including 207 given MSCs and 196 controls). All 11 studies reported mortality and were pooled through random-effects meta-analysis. MSCs decreased relative risk of death at study endpoint (RR: 0.50 [95% CI, 0.34-0.75]) and RR of death at 28 days after treatment (0.19 [95% CI], 0.05-0.78) compared to controls. MSCs also decreased length of hospital stay (mean difference (MD: −3.97 days [95% CI, −6.09 to −1.85], n = 5 studies) and increased oxygenation levels at study endpoint compared to controls (MD: 105.62 mmHg O2 [95% CI, 73.9-137.3,], n = 3 studies). Only 2 of 11 studies reported on all International Society for Cellular Therapy (ISCT) criteria for MSC characterization. Included randomized controlled trials were found to have some concerns (n = 2) to low (n = 4) risk of bias (RoB), while all non-randomized studies were found to have moderate (n = 5) RoB.
Interpretation
Our updated living systematic review concludes that MSCs can likely reduce mortality in patients with severe or critical COVID-19. A master protocol based on our Faster Approval framework appears necessary to facilitate the more accelerated accumulation of high-quality evidence that would reduce RoB, improve consistency in product characterization, and standardize outcome reporting.
Keywords: mesenchymal stromal cells, mesenchymal stem cells, extracellular vesicles, MSC-EVs exosomes, microvesicles, coronavirus disease 2019, COVID-19, severe acute respiratory syndrome coronavirus 2, SARS-CoV-2, acute respiratory distress syndrome, ARDS, sepsis, pneumonia
Graphical Abstract
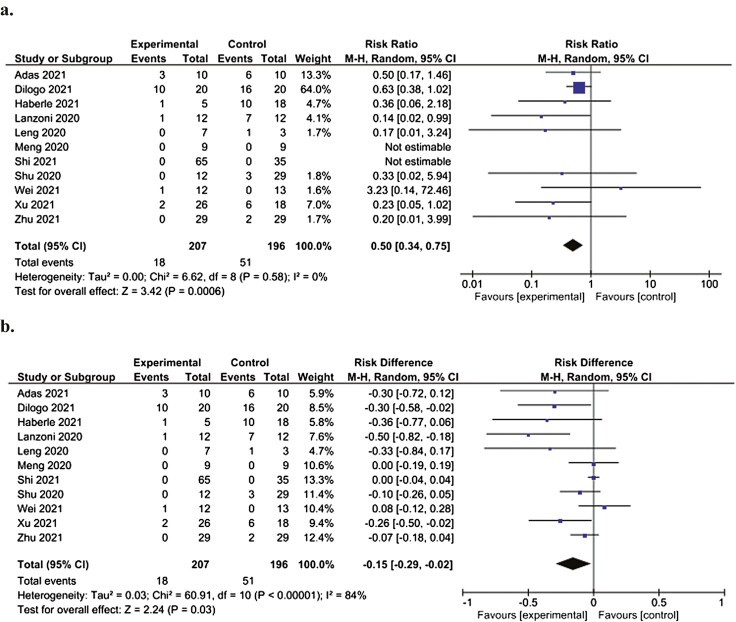
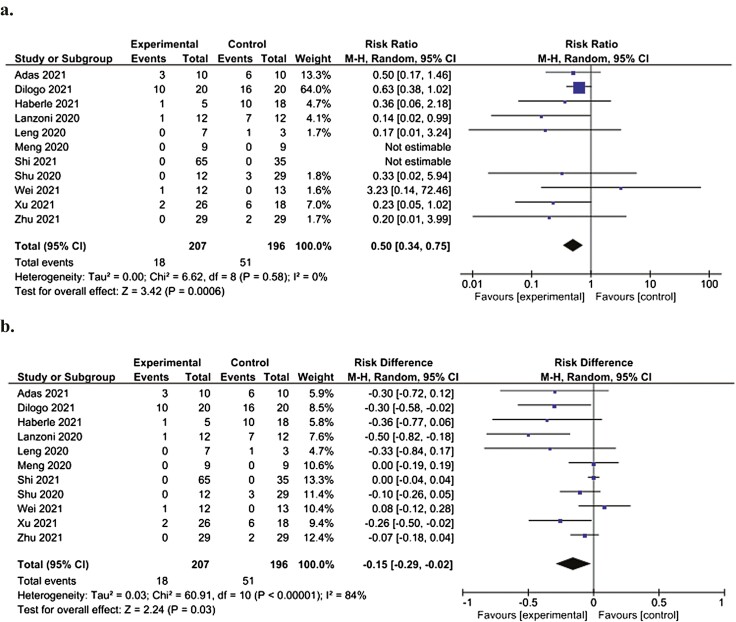
Forest plot demonstrating significantly decreased relative (a) and absolute (b) risk of death at the study endpoint in patients administered MSCs (experimental) compared to patients not administered MSCs (control). Control groups received standard of care for COVID-19 at the time of hospital admission, which varied depending on the institution.
Lessons Learned.
Mesenchymal stromal cells can likely reduce mortality in patients with severe or critical COVID-19.
To overcome differences between study designs and to enhance the consistency of the cell product used in these studies, a master protocol should be considered.
This could accelerate the ability to pool results from more similar studies to support regulatory approval of mesenchymal stromal cell to treat COVID-19.
Significance Statement.
Mesenchymal stromal cells (MSCs) may reduce mortality in patients with COVID-19 based on early evidence from a few studies. Our systematic review identified 11 controlled studies. Random effects meta-analysis revealed that MSCs decreased relative and absolute risk of death at study endpoint and relative risk of death at 28 days after treatment. Only 2 studies reported on standard criteria for MSC characterization. We conclude that MSCs can likely reduce mortality in patients with severe or critical COVID-19. A master protocol would enhance pooling of evidence from studies that report outcomes in a similar manner and use MSCs that meet standard criteria.
Introduction
Since its initial emergence in December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), has killed millions of people and infected many hundreds of millions more.1 Despite intensive measures implemented to contain its spread, SARS-CoV-2 continues to infect people across the globe at an unprecedented rate.2,3 Although safe and effective vaccines have been developed and administered to billions of people,4-6 many areas are struggling to vaccinate enough of their population.7,8 Furthermore, COVID-19 vaccines display reduced efficacy in certain patient populations, such as those with autoimmune conditions, blood cancers, or transplant recipients.9,10 Moreover, SARS-CoV-2 variants of concern and potential future variants pose a continued risk to disease control.11,12 Taken together, there remains a continued urgent need for safe and effective therapies to reduce the morbidity and mortality associated with COVID-19.
From the onset of the pandemic, mesenchymal stromal cells (MSCs) were quickly viewed as a promising therapy to treat COVID-19 due to their proven immunomodulatory and regenerative capabilities across a wide variety of related diseases and conditions.13,14 Many clinical trials of MSC-based therapy have been registered15 but remain underpowered to determine safety and efficacy on their own due to the small sample size and there is considerable heterogeneity in terms of study design, intervention characteristics, and planned primary and secondary outcomes.
To determine the preliminary safety and efficacy of MSCs in the context of COVID-19, we performed an initial systematic review and meta-analysis examining all clinical studies (controlled and uncontrolled) examining the use of MSCs for COVID-19.16 Our initial search identified 9 studies (4 controlled), and meta-analysis demonstrated that MSCs significantly decreased the risk of death at study endpoint compared to controls. Moreover, MSCs were found to be safe, with no severe adverse events reported in any of the patients. However, this initial analysis had several limitations including a small number of controlled studies, heterogeneity in study design and intervention characteristics, potential ROB, and limited reporting of MSC product characterization in accordance with criteria from the International Society of Cellular Therapy (ISCT).17 We proposed a framework for the selection of high-quality studies that would yield a more robust evidence base that could be used in support of requests for regulatory approval of MSC products and that could accelerate more widespread use and availability of MSCs to treat COVID-19. This update to our living systematic review leverages our proposed framework for the accelerated synthesis of trial evidence for rapid approval—or FASTER Approval—that will provide more clear guidance on the extent to which MSC-based therapy could be considered for approval to treat COVID-19. Moreover, we identify potential elements of a master protocol that would ensure the rapid generation of high-quality evidence to support the timely launch of future informative trials of MSC-based applications.
Methods
This systematic review is reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines18 (Supplementary Table S3). The study protocol has been published in Systematic Reviews19 and is registered at the International Prospective Registry of Systematic Reviews (PROSPERO; CRD42021225431).
Literature Search Strategy
Our literature search strategy was performed as described previously16 and was updated to November 15, 2021 (from 1947 to November 15, 2021) (Supplementary Fig. S20). In this second iteration, we only included controlled clinical studies examining the use of MSCs as a therapeutic intervention for COVID-19.
Eligibility Criteria
Our eligibility criteria were the same as described previously, except we only included controlled clinical studies. Uncontrolled clinical studies, case series, reviews, commentaries, editorials, letters, case reports, conference abstracts, unpublished gray literature, and other study types (in vitro studies, preclinical animal studies, etc.) were excluded.
Outcomes
Mortality at the study endpoint and 28 days were our primary outcomes for analysis. Our secondary outcomes for analysis included the number of patients requiring hospital admission, number of patients requiring intensive care unit (ICU) admission, number of patients requiring mechanical ventilation, length of time in hospital, length of time in ICU, length of time on mechanical ventilation, circulating levels of immune cells, pro-inflammatory cytokines and anti-inflammatory cytokines, and adverse events.
Study Selection and Data Extraction
Study selection, data extraction, and data analysis steps were performed as described in the first edition of our living systematic review.16 ROB assessment for randomized controlled trials and non-randomized studies was performed using the ROB 220 and ROBINS I tools,21 respectively.
Data Analysis
The results from individual studies were pooled for meta-analysis using Review Manager (Version 5.4) Systematic Review Software (https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-5-download). For dichotomous outcomes, both risk ratios (RRs) and risk differences (RDs) between the control and experimental groups were calculated for each outcome. Both RDs and RRs were presented to account for studies that reported zero events in both control and experimental groups. For continuous outcomes, the mean difference (MD) or standardized mean difference (SMD) between control and experimental groups was calculated using random-effects meta-analyses. The SMD was applied for analyses of inflammatory markers (IL-6, ferritin, C-RP) as it was anticipated these outcomes would vary substantially according to the time of measurement. Moreover, the mean of medians (MoM) and the interquartile range (IQR) were reported along with MD and SMD for continuous outcomes to account for dispersion and help describe the shape of the distribution (normal, right skewed, left skewed, etc.). Pooled analysis was performed using the DerSimonian and Laird random-effects model.22 All data is presented with 95% CIs. Meta-analysis was only performed when 2 or more controlled studies reported the same outcome. Outcomes that were reported in less than 2 controlled studies or where adequate data for inclusion in the meta-analysis was not provided were analyzed in a descriptive manner using summary tables. Statistical heterogeneity was assessed using the I2 statistic. The thresholds for interpretation of I2 were: 0-40% (low heterogeneity), 30-60% (moderate heterogeneity), 50-90% (substantial heterogeneity), and 75-100% (considerable heterogeneity) Potential subgroup analyses (MSCs isolated from different tissue sources, MSCs compared to their secretome, MSCs that have been treated and/or genetically modified before administration or isolation of paracrine factors, according to COVID-19 severity, presence of co-morbidities, patient age, patient sex, based on geographic location and by type of funding support for the study) were determined a priori in our study protocol19 and were described in the first edition of our living systematic review.16 Publication bias was assessed using funnel plots for outcomes reported in 10 or more studies by plotting the effect measure (RD, SMD, etc.) against the standard error.
Role of the Funding Source
The funding sources had no role in the study design, analysis, interpretation of data, writing of the manuscript, or decision to submit the manuscript for publication.
Results
Literature Search
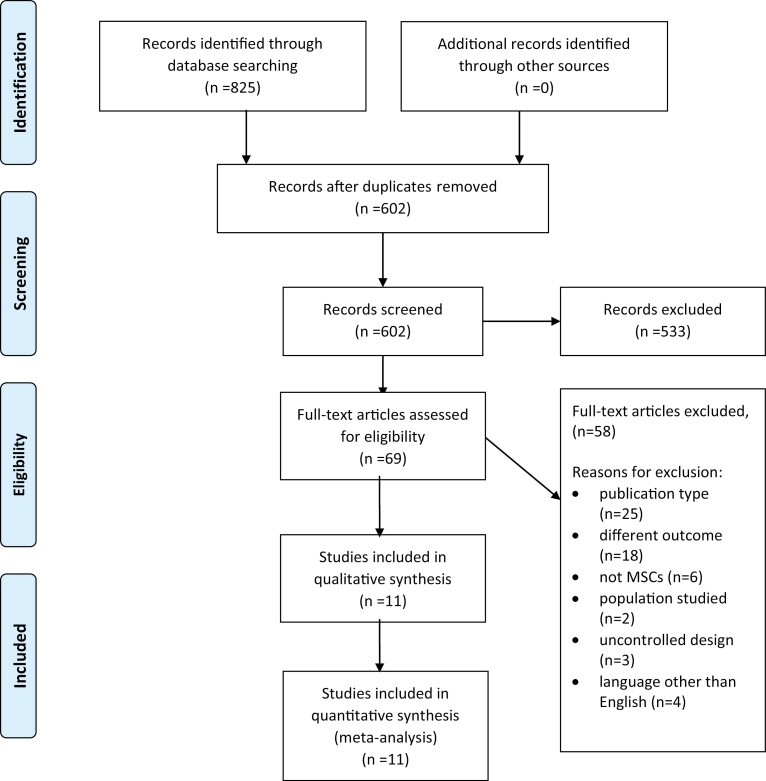
Our systematic search of the literature yielded 602 unique citations. After full text screening, 11 studies23-33 met all criteria for inclusion in our review. Reasons for study exclusion at the full-text stage were related to publication type (n = 25), outcomes reported (n = 18), non-MSC-based therapy (n = 6), did not involve patients with COVID-19 (n = 2), uncontrolled study design (n = 3) and language other than English (Chinese n = 2, Russian n = 2) (Fig. 1).
Figure 1.
Results of systematic search of the literature. MEDLINE and Embase and Cochrane Central Register of Controlled Trials, searched from 1947 up to November 15, 2021.
Study Characteristics
The characteristics of the 11 included studies are summarized in Table 1. Five of the studies were RCTs24,26,30,31,33 and 6 were non-randomized controlled trials.23,25,27-29,32 Study publication date ranged from March 9, 2020, to October 26, 2021. Seven of the studies were conducted in China,23-29 one in the US,30 one in Indonesia,31 one in Germany32 and one in Turkey.33
Table 1.
Characteristics of patients enrolled in clinical studies of MSCs as a therapeutic intervention for COVID-19.
| Patient characteristics | All groups | Control groups | MSC groups |
|---|---|---|---|
| Total patients, n | 403 | 196 | 207 |
| Sex, male, % | 56.9 | 55.4 | 58.4 |
| Mean age, years (SD) | 58.4 (7.1) | 60.1 (5.3) | 56.7 (8.5) |
| COVID-19 severity, n (%) | |||
| Mild | 39 (9.7) | 19 (9.7) | 20 (9.7) |
| Moderate | 20 (5.0) | 10 (5.1) | 10 (4.8) |
| Severe | 251 (62.3) | 124 (63.3) | 127 (61.4) |
| Critical | 93 (23.1) | 43 (21.9) | 50 (24.2) |
| Co-morbidities, n (%) | |||
| Hypertension | 109 (27.0) | 60 (30.6) | 49 (23.7) |
| Diabetes | 70 (17.4) | 34 (17.3) | 36 (17.4) |
| Obesity | 16 (4.0) | 5 (2.6) | 11 (5.3) |
| Chronic obstructive pulmonary disease | 4 (1.0) | 1 (0.5) | 3 (1.4) |
| Coronary artery disease | 9 (2.2) | 6 (3.1) | 3 (1.4) |
| Congestive heart failure | 10 (2.5) | 6 (3.1) | 4 (1.9) |
| Chronic kidney failure | 7 (1.7) | 5 (2.6) | 2 (1.0) |
| Other* | 45 (11.2) | 26 (13.3) | 19 (9.2) |
| Mean follow up, days (range) | 27.7 (14-60) | 26.9 (14-60) | 28.8 (14-60) |
* Includes current smoker, ex-smoker, pre-diabetes, asthma, tuberculosis, chronic bronchitis, hemorrhagic cerebral infarction, coronary heart disease, and chronic atrial fibrillation
Participant Characteristics
In total, there were 403 patients (mean age 58 ± 7; 229 male) enrolled across all study groups, with 207 patients (mean age 57 ± 9; 115 male) being administered MSCs and 196 patients (mean age 60 ± 5; 114 male) serving as controls. The overall distribution of COVID-19 disease severity was similar for patients in the intervention and control groups, with 62% and 23% of all patients having severe and critical disease severity, respectively (Table 1).
In terms of patient co-morbidities, there was a greater proportion of patients with hypertension in the control group compared to the intervention group (31% vs. 24%), a greater proportion of obese patients in the MSC group compared to the control group (5% vs. 3%), and a greater proportion of patients with other co-morbidities in the control group compared to the MSC group (13% vs. 9%). However, all other co-morbidities including diabetes, COPD, coronary artery disease, and chronic kidney failure were well balanced between the control and intervention groups (Table 1).
Intervention Characteristics
Intervention characteristics are summarized in Table 2. All 11 studies used MSCs, with no studies using MSC secreted factors (MSC-Extracellular Vesicles (MSC-EVs), MSC-Conditioned Medium (MSC-CM)). All MSCs were derived from allogeneic human tissues, including umbilical cord blood and/or tissue (n = 8),24-26,28-31,33 menstrual blood (n = 1)27 and bone marrow mononuclear cells (n = 1).32 One study did not report the specific tissue source from which its MSCs were derived.23 The passage number in ex vivo culture of the MSCs varied widely between studies (passage 3 to passage 6) (see Table 2), with 4 of the studies28-30,32 not reporting how many passages were performed before harvesting MSCs from ex vivo culture. In terms of the extent to which studies reported on specific ISCT criteria17 for MSC characterization, only 2 of the 11 studies reported sufficient information regarding all 4 minimal criteria established by the ISCT.17 Specific details regarding the number of studies reporting on each of the 4 individual ISCT criteria17 can be found in Table 2.
Table 2.
Intervention characteristics for clinical studies of patients administered MSCs as a therapeutic intervention for COVID-19.
| Intervention | Total studies, n |
|---|---|
| MSC tissue source | |
| Umbilical cord blood or tissue | 8 |
| Menstrual blood | 1 |
| Bone marrow mononuclear cells | 1 |
| Not described | 1 |
| MSCs fresh or frozen/cryopreserved | |
| Fresh only | 0 |
| Fresh or cryopreserved | 1 |
| Cryopreserved | 4 |
| Not stated | 6 |
| Product dose | |
| MSCs, cells/kg (n=7) | 1-3 × 106 |
| MSCs, total cells (n=4) | 30-100 × 106 |
| Route of administration | |
| Intravenous | 11 |
| No. MSC infusions, patients (%) | |
| 1 | 80 (38.6) |
| 2 | 15 (7.2) |
| 3 | 112 (54.1) |
| MSC passage number | |
| P3 or P4 | 2 |
| P5 | 3 |
| P3–P5 | 1 |
| P5-P6 | 1 |
| Not stated | 4 |
| ISCT criteria | |
| Fully met criteria (A–D below), n | 2 |
| (A) Plastic adherence | 3 |
| (B) Trilineage differentiation | 5 |
| (C) Positive/negative surface markers | 8 |
| (D) MSC viability | 6 |
Abbreviations: NA, not applicable; ISCT, International Society of Cellular Therapy.
MSC dosing and administration were reported differently between studies. The 2 formats in which MSC dosage were reported included cells/kg of body weight (n = 7; 1–3 × 106 cells/kg) and total cells/injection (n = 4; 30–100 × 106 cells). In 4 studies, MSCs were cryopreserved prior to administration and in 1 other study, the MSCs were either fresh or cryopreserved. The remaining studies (n = 7, 67%) did not report whether the MSCs they administered were fresh or cryopreserved. All 11 studies administered their MSCs intravenously. Most patients (n = 112, 54.1%) received 3 infusions of MSCs, although other studies reported administering 1 or 2 MSC infusions (see Table 2). The reported time from COVID-19 diagnosis to intervention was similar between control groups (median 9.8, range 1–47 days) and intervention groups (median 10.4, range 1–45) in the controlled studies.
Patients were administered other therapeutic agents in addition to MSCs in 10 of the 11 studies (91%). The specific therapeutic agents administered to patients varied considerably between studies and are summarized in Table 5. Two of the studies stated that they used other therapeutics in addition to MSCs but did not specify exactly which therapeutic agents were used.25,28 The mean follow-up period was 27.7 days (range 14–60), with mean follow-ups of 26.9 (range 14–60) and 28.8 (range 14–60) for the control and experimental groups respectively (Table 1)
Table 5.
Concomitant therapies reported in studies. Italics indicates the wording used in the reports.
| Study (ref) | Antiviral agents | Antibiotic agents | Glucocorticoids | Transfusion based interventions | Other interventions |
|---|---|---|---|---|---|
| (Leng)23 | None | None | None | None | None |
| (Shu)24 | Abidor/ Oseltamivir |
Moxifloxacin | Yes | None | None |
| (Meng)25 | Lopinavir/ Ritonavir |
None | Yes | None | None |
| (Shi)26 | Antiviral drugs | Antibiotics | Yes | None | None |
| (Xu)27 | Antiviral therapy | Antibacterial treatment | None | Extracorporeal blood purification system | Multiple* |
| (Wei)28 | Arbidol, Lopinavir– Ritonavir |
None | Yes | None | None |
| (Zhu)29 | α-Interferon, Ribavirin, Ganciclovir | Moxifloxacin, Piperacillin tazobactam, Levofloxacin | Yes | None | None |
| (Lanzoni)30 | BAT | BAT | BAT | BAT | BAT |
| (Dilogo)31 | Oseltamivir | Azithromycin | None | None | None |
| (Haberle)32 | None | None | Yes | None | Multiple** |
| (Adas)33 | Favipiravir, HCQ |
Piperacillin-tazobactam, | Yes | None | Enoxaparin |
Includes hormone, gut microflora modulator, Chinese medicine treatment, basic disease medication.
Includes immunosuppressive medication, ACE inhibitor, AT1 receptor blocker, beta-blocker, other antihypertensive drugs, Calcium antagonists, antiplatelet drugs.
Abbreviations: BAT, best available therapy; HCQ, hydroxychloroquine.
Outcome Reporting
Outcome reporting across studies was variable, with some outcomes like mortality and pro-inflammatory cytokines being reported in all 11 studies, while other outcomes including progression of clinical symptoms and viral load were reported in less than half of the studies. An overview of outcomes reported across studies is summarized in Table 3.
Table 3.
List of reported outcomes in clinical studies examining MSCs as a therapeutic intervention for COVID-19. Outcomes reported in each study are indicated by (•) and outcomes not reported are indicated by (-).
| Study | Mortality rate | Diagnosis to intervention (time) | Intervention to recovery (time) | No. pts hospitalized | No. pts on supplemental O2 | No. pts on ventilator | Time in hospital | Progression of symptoms | Improvement of symptoms | Time to clinical improvement | Oxygenation levels | Immune cell levels | Pro-inflammatory cytokines | Anti-inflammatory cytokine s | Viral load | Radiological outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Leng)23 | • | • | • | • | - | - | • | • | • | • | • | • | • | • | - | • |
| (Shu)24 | • | • | - | • | • | • | • | • | • | • | • | • | • | - | - | • |
| (Meng)25 | • | • | • | • | • | • | • | • | - | • | • | - | • | - | • | • |
| (Shi)26 | • | • | - | • | • | • | - | - | - | - | • | • | • | • | - | • |
| (Xu)27 | • | - | • | - | - | • | • | - | • | • | • | - | • | - | • | • |
| (Wei)28 | • | • | - | - | • | • | - | - | - | - | • | • | • | • | - | • |
| (Zhu)29 | • | • | • | - | - | - | • | - | • | • | - | - | • | • | - | • |
| (Lanzoni)30 | • | • | • | - | • | • | - | • | - | - | - | - | • | • | • | - |
| (Dilogo)31 | • | • | - | - | - | - | - | - | • | - | - | • | • | • | - | - |
| (Haberle)32 | • | - | - | - | - | • | - | - | - | - | • | • | • | - | - | - |
| (Adas)33 | • | - | - | • | - | • | • | - | • | - | • | • | • | • | • | - |
| Total | 11 | 8 | 5 | 5 | 5 | 8 | 6 | 4 | 6 | 5 | 8 | 7 | 11 | 7 | 4 | 7 |
Abbreviations: Pts, patients.
Primary Outcome Analysis: Mortality
All 11 studies reported mortality at study endpoint, which ranged from 14 to 60 days. The mortality rate at study endpoint for patients in the control groups was 51 of 196 patients (26%), compared to 18 of 207 patients (8.7%) in the groups administered MSCs. In meta-analysis (n = 11 studies), patients administered MSCs had a significant decrease in relative risk of mortality (RR: 0.50 [95% CI, 0.34-0.75, P = .0006, I2=0%]) (Fig. 2a) and absolute risk of death (RD: −0.15 [95% CI, −0.29 to −0.02, P = .03, I2 = 84%]) at study endpoint compared to controls (Fig. 2b). Meta-analysis of studies reporting risk of death at 28 days (n = 5 studies) also demonstrated a significant decrease in relative risk of death in patients administered MSCs compared to controls (RR: 0.19 [95% CI, 0.05-0.78, P = .02, I2 = 0%]) (Supplementary Fig. S1a) whereas no significant difference in absolute risk of death at 28 days was observed (RD: −0.09 [95% CI, −0.23 to 0.04, P = .19, I2 = 82%]) (Supplementary Fig. S1b).
Figure 2.
Forest plot demonstrating significantly decreased relative (a) and absolute (b) risk of death at study endpoint in patients administered MSCs (experimental) compared to patients not administered MSCs (control). Control groups received standard of care for COVID-19 at the time of hospital admission, which varied depending on the institution.
We addressed several potential factors in subgroup analysis to assess their impact on mortality. In all cases, the relative risk of death was reduced to a similar extent compared to controls (see Supplementary Figs. S2-S6). Specifically, we examined differences in the relative risk of death in subgroups of studies where the study endpoint was <28 or ≥28d, whether patients had ARDS or did not have ARDS, whether studies included patients with any severity of disease or just severe or critical disease, and whether cord blood tissue versus other cell sources were used to generate and expand MSCs, and whether a single infusion of MSCs compared to multiple infusions were administered.
Secondary Outcomes
Hospitalization
Four of the 11 studies reported on the number of patients still requiring hospital admission at study endpoint. No difference in relative risk (RR: 0.90 [95% CI, 0.65-1.26, P = .55, I2 = 46%]) (Supplementary Fig. S7) of requiring hospital admission at study endpoint for patients administered MSCs compared to controls was demonstrated in meta-analysis. However, length of stay in hospital (reported in 5 studies) was reduced in MSC groups compared to controls (MD: −3.97 days [95% CI, −6.09 to −1.85, P = .0002, I2 = 0%]; MSC: MoM = 25.7 days (IQR = 10.6); control: MoM = 28.4 days (IQR = 11.9)) (Supplementary Fig. S8).
Oxygenation Levels
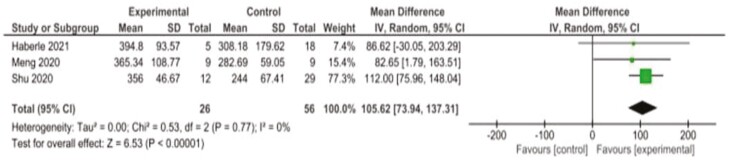
Eight studies reported changes in oxygenation levels from baseline to endpoint in their patients. Many measures were used to quantify the change in oxygenation, including PaO2/FiO2, FiO2 (%), SpO2 (%), maximum forced vital capacity (Vcmax) and diffusion lung capacity for carbon monoxide. Three of the studies reported PaO2/FiO2 and could be combined in meta-analysis which demonstrated that patients administered MSCs had a significantly higher oxygenation index at endpoint compared to controls (MD: 105.62 mmHg [95% CI, 73.9-137.3, P < .00001, I2 = 0%]; MSC: MoM = 372.0 mmHg (IQR = 19.4); control: MoM = 278.3 mmHg (IQR = 32.1)) (Fig. 3).
Figure 3.
Forest plot demonstrating increased oxygenation index at study endpoint in patients administered MSCs (experimental) and patients not administered MSCs (control). Control groups received standard of care for COVID-19 at the time of hospital admission, which varied depending on the institution.
Pro-inflammatory and Anti-inflammatory Cytokine Levels
All 11 studies reported changes in a range of pro-inflammatory cytokines from baseline to the end of the study period. Seven of the studies reported interleukin-6 (IL-6) levels, and meta-analysis revealed no significant decrease in IL-6 levels at study endpoint compared to controls (SMD: −0.22 [95% CI, −0.60-0.16, P = .25, I2 = 43%]; MSC: MoM = 73.8 (IQR = 51.9); control: MoM = 50.8 (IQR = 99.7)) (Supplementary Fig. S12). However, in a subset of studies, MSCs significantly reduced IL-6 levels compared to controls when measured 3–7 days following MSC administration (SMD: −0.62 [95% CI, −1.03 to −0.21, P = .003, I2 = 0%]; MSC: MoM = 29.5 (IQR = 34.5); control: MoM = 59.6 (IQR = 97.1)) (Supplementary Fig. S13). Five of the studies reported C-reactive protein (CRP) levels at study endpoint, and meta-analysis revealed that patients administered MSCs had significantly lower C-reactive protein (C-RP) levels at study endpoint compared to controls. (SMD: −0.41 [95% CI, −0.77 to −0.05, P = .02, I2 = 23%]; MSC: MoM = 36.8 (IQR = 61.8); control = 58.2 (IQR = 95.9)) (Supplementary Fig. S14).
Other Outcomes
Other outcomes measured across studies including admission to the intensive care unit, need for mechanical ventilation and/or ventilation parameters, immune cell levels, radiological parameters, virological and/or antibody responses, and clinical scale scores are summarized in the supplementary materials (pp. 1–4).
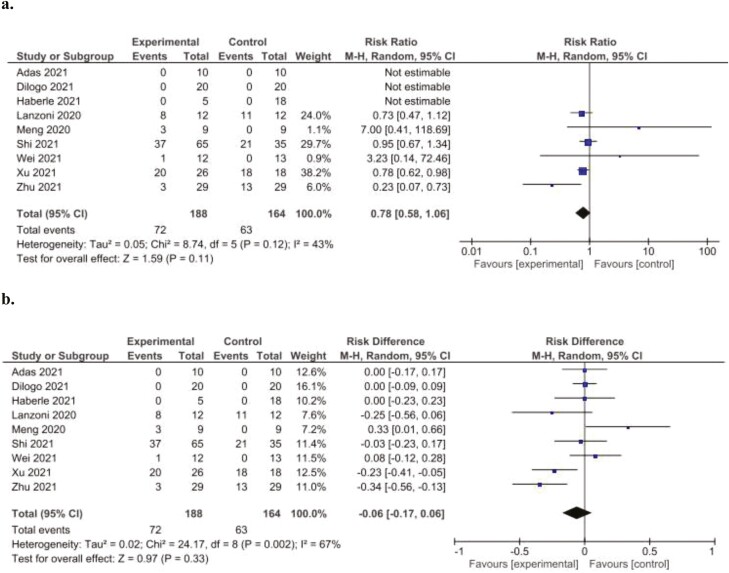
Adverse Events
Adverse event reporting was described for all studies and is summarized in Table 4. Adverse events occurred in 4 studies in association with MSC infusion. Meta-analysis demonstrated no significant difference in the relative risk of adverse events between treated and control groups (RR: 0.78 [95% CI, 0.58-1.06, P = .11, I2 = 43%]) (Fig. 4.). Specific adverse events occurring in patients administered MSCs included facial flushing, transient fever, hypoxemia, palpitations, and dizziness. However, these symptoms resolved in all patients spontaneously or with minimal supportive treatment following MSC administration. Only one of the studies reported the occurrence of treatment-related severe adverse events. However, this study did not specify which or how many severe adverse events occurred, only that “more serious adverse events were recorded for the placebo group than for the MSC group; however, the difference was not statistically significant.”29
Table 4.
Adverse events (AEs) and severe adverse events (SAEs) reported in clinical studies examining MSCs as a therapeutic intervention for COVID-19.
| Study | Safety lab values | Treatment-related AEs | Non-treatment-related AEs | Treatment-related SAEs | Non-treatment-related SAEs |
|---|---|---|---|---|---|
| (Leng)23 | • | • | • | • | • |
| (Shu)24 | • | • | • | • | • |
| (Meng)25 | - | - | • | • | - |
| (Shi)26 | - | • | - | • | • |
| (Xu)27 | - | - | - | • | • |
| (Wei)28 | - | • | • | • | - |
| Zhu29 | - | - | - | - | - |
| (Lanzoni)30 | • | - | - | • | - |
| (Dilogo)31 | • | • | • | • | • |
| (Haberle)32 | • | • | • | • | • |
| (Adas)33 | • | • | • | • | • |
| Total | 5 | 4 | 4 | 1 | 4 |
Figure 4.
Forest plot demonstrating no significant difference in relative (a) and absolute (b) risk of experiencing adverse events in patients administered MSCs (experimental) compared to controls. Control groups received standard of care for COVID-19 at the time of hospital admission, which varied depending on the institution.
Risk of Bias (RoB)
RoB was assessed for each outcome reported in RCTs using the Cochrane Risk of Bias 2 tool (ROB 2).20 The RoB analysis for our primary outcome of risk of death at the study endpoint can be seen in Supplementary Table S1. Four studies26,29,30,32 were found to have low RoB and 2 studies had an RoB of “some concerns,”24,29 as the method of randomization was unclear for both studies, and it was unclear whether there were deviations from intended interventions or selection of reported results in one of the studies. With regards to other outcomes that were subject to meta-analysis (eg, risk of death at 28 days, levels of pro-inflammatory cytokines, length of stay in the hospital, risk of requiring mechanical ventilation at study endpoint, etc.), each study had the same RoB classification for both individual RoB domains and overall RoB (n = 4 low RoB, n = 2 “some concerns” RoB; Supplementary Table S1). RoB was assessed for the non-randomized controlled studies using the ROBINS I tool.21 All studies23,25,27,28,31 were found to have a moderate RoB overall (Supplementary Table S2). All studies had potential bias due to confounding, measurement of outcomes (as studies did not mention blinding), and selection of reported results (as none of the studies reported a pre-registered protocol). Funnel plots for the primary analysis of mortality yielded asymmetrical distributions for both RR (Supplementary Fig. S19a) and RD Supplementary (Fig. S19b) effect measures, suggesting possible publication bias.
FASTER Approval Criteria Evaluation
In terms of the extent to which the overall evidence meets the criteria for high-quality evidence outlined in our proposed framework described in our first iteration of the living systematic review,16 referred to as the FASTER Approval criteria (Table 6), 2 of the domains were considered satisfactory (sample size, study populations), four of the domains were considered unclear (number of studies, study characteristics, outcome measurement, and RoB) and one of the domains was considered unsatisfactory (product characterization).
Table 6.
Assessment of proposed criteria in FASTER Approval framework for performing meta-analysis of high-quality studies of MSC-based therapy for COVID-19. Check marks indicate criteria satisfied, dark circle indicates uncertainty if criteria satisfied and “x” indicates criteria not met.
| Number of studies | • Sufficient number and similar enough to perform meta-analysis that achieves the required power for determining efficacy. See sample size. | ✓ 11 controlled studies identified. |
| Study characteristics | • Controlled with contemporary and similar control groups. Randomized is preferable. Concomitant therapies should be controlled. | ✓ RCTs: n = 5. ? Concomitant therapies not always controlled. |
| Sample size | • To reduce mortality from 20% to 10%, 199 subjects in intervention group(s) needed (24). | ✓ Sample size = 403 total, with 207 patients in treatment (MSC) arm. |
| Study populations | • Severe or critical COVID-19 in hospitalized patients (most common). | ✓ Most patients presented with severe (62.3%) or critical (23.1%) COVID-19. |
| Outcome measurement | • Mortality at day 28. • WHO response criteria. • Secondary: IL6 levels, hospitalization, ICU admission, pulmonary function at 1, 6, 12 months. • Safety and adverse event reporting. |
? Mortality at 28 days (n = 5). × WHO response criteria (n = 0). ? IL-6 levels (n = 7), hospitalization (n = 5), ICU admission (n = 4), pulmonary function at 1 (n=0), 6 (n = 0) and 12 (n = 0) months. ✓ Safety laboratory values (n = 5) and adverse event reporting (n = 9) |
| Product characterization | • MSCs produced and characterized according to ISCT criteria. | × Full criteria (n = 2) ✓ Positive/negative surface markers (n = 8), ? MSC viability (n = 6) ? Trilineage differentiation potential (n = 5), ? Plastic adherence (n = 3) |
| Risk of bias | • Studies with high risk of potential bias should not be included in meta-analysis. | ? High risk of bias: n = 4 (40%) |
Discussion
This second update to our living systematic review and meta-analysis suggests MSCs are safe and may be effective for the treatment of COVID-19. The number of studies in our analysis remains limited (n = 11), however, and most studies did not report sufficient characterization of MSC products in accordance with published ISCT minimal criteria.17 In particular, plastic adherence, cell viability, and tri-lineage differentiation potential were not reported in most studies. Moreover, outcome reporting varied between studies which limit the ability to perform meaningful meta-analyses regarding measures of clinical benefit. Some aspects of the study design can be further addressed to reduce the potential risk of bias and improve confidence in the evidence. Enhancing the likelihood of MSC-based therapy achieving regulatory approval and more widespread clinical use will require greater consistency in product characterization, outcome reporting, and overall study design, which could be facilitated with the design and implementation of a master protocol that could be shared by investigators working toward a common goal.
MSCs decreased both the relative and absolute risk of death at the study endpoint compared to controls in our meta-analysis and MSCs decreased the relative risk of death at 28 days. Although a limited number of other therapeutics including remdesivir34 and bamlanivimab35 have also demonstrated promise in terms of decreasing mortality in patients suffering from COVID-19, their mechanisms of action appear more limited in scope. Although the specific mechanisms and pathways interrogated by MSCs in patients with COVID-19 have yet to be confirmed, work in other related diseases and conditions indicates that MSCs ameliorate COVID-19 through a combination of immunomodulatory, antibacterial, tissue regenerative, and anti-apoptotic mechanisms.36,37 The pleiotropic effects of MSCs could explain the significant reduction in mortality observed for COVID-19 patients. More insight regarding specific mechanisms could allow further optimization of MSC therapy by leveraging key therapeutic mechanisms.38
Oxygenation at the study endpoint was improved in patients administered MSCs compared to controls and subgroup analysis indicated that MSCs were effective in reducing the risk of death at the study endpoint for patients experiencing ARDS. Mechanisms of how MSCs could improve oxygenation and ameliorate COVID-19-induced ARDS remain unknown but may involve attenuation of the cytokine storm and/or healing of damaged and dysfunctional lung tissue.36-38 Optimal management of COVID-19-induced ARDS is an area of active research39,40 and further studies that involve MSCs appear warranted.
MSCs did not decrease the levels of pro-inflammatory cytokines and immune biomarkers including IL-6, D-dimer, ferritin, and IFN-g when measured at various time points after treatment. Given the highly dynamic nature of these biomarkers at various points before, during, and after the height of the cytokine storm that can be influenced by IL-6 amplifier (IL-AMP) and by activated pro-inflammatory immune cells,41-44 we observed that patients administered MSCs had significantly reduced IL-6 levels compared to controls at early time points (<7 days), but not at later periods after MSC administration.
Interestingly, patients administered MSCs had significantly reduced CRP levels at the study endpoint compared to controls. CRP is an acute-phase protein that is produced by the liver and increases rapidly in response to inflammation, cell injury, and tissue necrosis.45-47 Binding of CRP to molecules on microorganisms activates the complement system, which plays an important role in innate host immunity. CRP levels are prognostic in patients with COVID-19 with higher CRP levels are associated with significantly worse disease severity, worse clinical outcomes, and higher mortality rates.48-53 Moreover, patients with elevated CRP levels have been associated with higher rates of extrapulmonary COVID-19 complications including venous thromboembolism (VTE) and acute kidney injury (AKI).54
Subgroup analysis from our review suggested that MSCs from umbilical cord tissue were inferior to MSCs from other tissue sources in reducing the risk of death at the study endpoint. Interestingly, previous reports have suggested that MSCs derived from umbilical cord tissue could be optimal for the treatment of COVID-19 given the ease of expansion and rapid doubling times along with low levels of surface ACE-R which should mitigate against infection by SARS-CoV-2.55,56 Moreover, many publications also suggest that cord tissue-derived MSCs are rich in anti-inflammatory cytokines.57 Given this contrast in perspectives, more work appears necessary to determine the optimal tissue source for MSCs to treat COVID-19.58,59
All patients received allogeneic MSCs. Allogeneic MSCs have advantages over autologous MSCs, including greater ease of acquiring the source material for product manufacturing and improved therapeutic efficacy compared to autologous MSCs, especially if isolated from individuals with advanced age or pre-existing medical conditions like diabetes or autoimmune diseases.60-63 Most importantly, third-party allogeneic MSCs may be stored and used when needed.64 This avoids the long process of manufacturing small personalized autologous MSC products—paramount for rapidly progressing diseases like COVID-19, where delayed treatment leads to increased morbidity and mortality.65,66 Although allogeneic MSCs appear to be effective in the context of COVID-19, the therapeutic implications of HLA-matching of MSCs between donors and recipients are currently less well understood and may be an important direction for future study to optimize MSC therapy.67 HLA matching of MSCs between donors and recipients may help avoid rejection of MSCs by the immune system and/or the occurrence of adverse events.67
Adherence to ISCT criteria17 remains low amongst studies included in our analysis, with only 2 studies reporting on all 4 criteria. Incomplete adherence and reporting of ISCT criteria are not restricted to the studies identified in our review, with 1 recent analysis demonstrating that 33% of MSC-based clinical trials included no ISCT characterization data.68 Poor adherence to ISCT criteria has been hypothesized to be one of the reasons why the success of MSC therapies in preclinical studies fails to translate to clinical settings.69,70 Of note, 6 of the 11 studies reported MSC viability, a criterion added to the most recent update to the ISCT guidelines.17 Administering viable MSCs increases the likelihood that cells will persist in vivo upon administration, which is likely critical to ensuring therapeutic success.71 Greater adherence and reporting of MSC product characterization according to all 4 ISCT criteria17 would augment confidence in the consistency of MSC products across studies.
The optimal timing of MSC administration to patients with COVID-19 remains uncertain.41,72 With their potential to attenuate the cytokine storm early in COVID-19 disease progression, some suggest administering MSCs at the onset of hypoxia to avoid intubation or mechanical ventilation.41,72 Concern regarding potential exacerbation of a hypercoagulable state, however, has been expressed73 given that MSCs from certain tissue sources are rich in tissue factor (TF/CD142).73 Future subgroup analysis examining the clinical outcomes and occurrence of adverse events in patients administered MSCs at different stages of COVID-19 disease would be informative and may help in the design of an optimized dosing schedule.
Our study has limitations worthy of mention. First, the number of studies and patients included in our analysis remains relatively small. This modest number of studies and patients limits our confidence in the observed effects. Slow accrual and delays in the completion and publication of studies remain an issue that impedes more rapid clinical translation of results. Indeed, a large number of registered controlled clinical trials were identified in a scoping review of the literature but remain unpublished.15 As more of these registered trials reach completion and are published, more refined estimates should be possible with regards to the safety and efficacy of MSC-based products as a therapeutic intervention for COVID-19. Given the limited study size of future RCTs, it appears meta-analysis will remain central to understanding the benefit of MSC therapy. Variable outcome reporting across the studies included in our review was also a major limitation and limited meta-analysis. The only outcome reported across all studies was mortality but standard time points for assessing mortality were not uniformly reported. Future studies should strive toward consensus on outcome reporting. Additional outcomes of interest could include the number of days free from mechanical ventilation, although we acknowledge that practices in ventilator support of critically ill patients with COVID continue to evolve. Moreover, only 2 studies reported on whether MSC products met all ISCT criteria,17 further limiting the confidence in our pooled results. Lastly, asymmetry was detected in the funnel plots for mortality which suggests possible publication bias.
In conclusion, this second iteration of our living systematic review and meta-analysis demonstrates continued promise with regard to the use of MSCs as a therapeutic intervention for COVID-19. Challenges persist, however, which limit the certainty of our conclusions, including a limited number of published studies, modest patient enrollment, and substantial interstudy heterogeneity with regard to study design, patient characteristics, outcome reporting, and adherence to ISCT criteria. Future iterations of our systematic review will analyze data only from studies that meet the criteria for high-quality studies outlined in our FASTER Approval framework. The development of a master protocol for MSC therapy should leverage aspects of our framework to ensure future studies report high-quality evidence. A strategy that ensures higher quality evidence for knowledge synthesis appears necessary to leverage the results from modest-sized trials and where evidence from meta-analysis could be used to support applications for regulatory approval and to the accelerate more widespread clinical application of MSC therapy for COVID-19.
Supplementary Material
Acknowledgments
Funding from Canadian Blood Services (Blood Efficiency Accelerator Program 2020) was provided by Health Canada and Provincial and Territorial Ministries of Health in Canada.
Contributor Information
Aidan M Kirkham, Department of Biochemistry, Microbiology and Immunology, University of Ottawa, Ottawa, ON, Canada; Clinical Epidemiology, Ottawa Hospital Research Institute, Ottawa, ON, Canada.
Adrian J M Bailey, Clinical Epidemiology, Ottawa Hospital Research Institute, Ottawa, ON, Canada.
Madeline Monaghan, Clinical Epidemiology, Ottawa Hospital Research Institute, Ottawa, ON, Canada.
Risa Shorr, Medical Information and Learning Services, The Ottawa Hospital, Ottawa, ON, Canada.
Manoj M Lalu, Department of Cellular and Molecular Medicine, University of Ottawa, Ottawa, ON, Canada; Department of Anesthesiology and Pain Medicine, University of Ottawa, Ottawa, ON, Canada; Regenerative Medicine, Ottawa Hospital Research Institute, Ottawa, ON, Canada; Department of Anesthesia, The Ottawa Hospital, Ottawa, ON, Canada.
Dean A Fergusson, Clinical Epidemiology, Ottawa Hospital Research Institute, Ottawa, ON, Canada; Department of Medicine, University of Ottawa, Ottawa, ON, Canada; Department of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, ON, Canada; Department of Medicine, The Ottawa Hospital, Ottawa, ON, Canada.
David S Allan, Department of Biochemistry, Microbiology and Immunology, University of Ottawa, Ottawa, ON, Canada; Clinical Epidemiology, Ottawa Hospital Research Institute, Ottawa, ON, Canada; Department of Medicine, University of Ottawa, Ottawa, ON, Canada; Regenerative Medicine, Ottawa Hospital Research Institute, Ottawa, ON, Canada; Department of Medicine, The Ottawa Hospital, Ottawa, ON, Canada.
Funding
Faculty of Medicine, University of Ottawa and Canadian Blood Services.
Conflict of Interest
D.S.A. is a paid medical consultant with Canadian Blood Services. The other authors declared no potential conflicts of interest.
Author Contributions
A.M.K. and D.S.A.: conceived the study design; A.M.K., A.J.M.B., and M.M.: performed study selection, data extraction, and data analysis. A.M.K. and D.S.A. were responsible for the initial drafting of the manuscript; D.S.A., A.J.M.B., M.M.L., and D.A.F.: provided important revisions to the protocol development as well as data extraction questions and reviewed data analysis and synthesis. All authors were involved in manuscript revisions before final approval.
Data Availability
Datasets available upon request from the corresponding author.
References
- 1. Ciotti M, Ciccozzi M, Terrinoni A, et al. The COVID-19 pandemic. Crit Rev Clin Lab Sci. 2020;57(6):365-388. [DOI] [PubMed] [Google Scholar]
- 2. Elrashdy F, Redwan EM, Uversky VN.. Why COVID-19 transmission is more efficient and aggressive than viral transmission in previous coronavirus epidemics?. Biomolecules. 2020;10(9):1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang Y, Wang Y, Chen Y, Qin Q.. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92(6):568-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soleimanpour S, Yaghoubi A.. COVID-19 vaccine: where are we now and where should we go? Expert Rev Vaccines. 2021;20(1):23-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Polack FP, Thomas SJ, Kitchin N, et al. . C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baden LR, El Sahly HM, Essink B, et al. . COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin Y, Hu Z, Zhao Q, et al. Understanding COVID-19 vaccine demand and hesitancy: a nationwide online survey in China. PLoS Negl Trop Dis. 2020;14(12):e0008961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lazarus JV, Ratzan SC, Palayew A, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021;27(2):225-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eberhardt CS, Balletto E, Cornberg M, Mikulska M.. Coronavirus disease 2019 vaccination in transplant recipients. Curr Opin Infect Dis. 2021;34(4):275-287. [DOI] [PubMed] [Google Scholar]
- 10. Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80(10):1330-1338. [DOI] [PubMed] [Google Scholar]
- 11. Gupta RK. Will SARS-CoV-2 variants of concern affect the promise of vaccines?. Nat Rev Immunol. 2021;21(6):340-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sanyaolu A, Okorie C, Marinkovic A, et al. The emerging SARS-CoV-2 variants of concern. Ther Adv Infect Dis. 2021;8:20499361211024372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rajarshi K, Chatterjee A, Ray S.. Combating COVID-19 with mesenchymal stem cell therapy. Biotechnol Rep (Amst). 2020;26:e00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kassem DH, Kamal MM.. Mesenchymal stem cells and their extracellular vesicles: a potential game changer for the COVID-19 crisis. Front Cell Dev Biol. 2020;8:587866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liao G, Zheng K, Lalu MM, Fergusson DA, Allan DS.. A scoping review of registered clinical trials of cellular therapy for COVID-19 and a framework for accelerated synthesis of trial evidence-FAST evidence. Transfus Med Rev. 2020;34(3):165-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kirkham AK, Monaghan M, Bailey AJM, Shorr R, Lalu MM, Fergusson DA, Allan DS.. Mesenchymal stromal cell-derived treatment for COVID-19: a living systematic review, meta-analysis and framework for accelerated regulatory approval. Cytotherapy. 2022;24(6):639-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Viswanathan S, Shi Y, Galipeau J, et al. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019;21(10):1019-1024. [DOI] [PubMed] [Google Scholar]
- 18. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirkham AM, Monaghan M, Bailey AJM, et al. Mesenchymal stromal cells as a therapeutic intervention for COVID-19: a living systematic review and meta-analysis protocol. Syst Rev. 2021;10(1):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 21. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. [DOI] [PubMed] [Google Scholar]
- 23. Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shu L, Niu C, Li R, et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meng F, Xu R, Wang S, et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct Target Ther. 2020;5(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shi L, Huang H, Lu X, et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Target Ther. 2021;6(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu X, Jiang W, Chen L, et al. Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: an exploratory clinical trial. Clin Transl Med. 2021;11(2):e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wei F, Kong D, Li T, et al. Efficacy and safety of umbilical cord mesenchymal stem cells for the treatment of patients with COVID-19. Clinics (Sao Paulo). 2021;76:e2604. 10.6061/clinics/2021/e2604.eCollection2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhu R, Yan T, Feng Y, et al. . Mesenchymal stem cell treatment improves outcome of COVID-19 patients via multiple immunomodulatory mechanisms. Cell Res. 2021; 26:1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lanzoni G, Linetsky E, Correa D, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021;10(5):660-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dilogo IH, Aditianingsih D, Sugiarto A, et al. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: a randomized controlled trial. Stem Cells Transl Med. 2021;10(9):1279-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Häberle H, Magunia H, Lang P, et al. Mesenchymal stem cell therapy for severe COVID-19 ARDS. J Intensive Care Med. 2021;36(6):681-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Adas G, Cukurova Z, Yasar KK, et al. The systematic effect of mesenchymal stem cell therapy in critical COVID-19 patients: a prospective double controlled trial. Cell Transplant. 2021;30:9636897211024942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Angamo MT, Mohammed MA, Peterson GM.. Efficacy and safety of remdesivir in hospitalised COVID-19 patients: a systematic review and meta-analysis. Infection. 2021; 31:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zuo L, Ao G, Wang Y, Gao M, Qi X.. Bamlanivimab improves hospitalization and mortality rates in patients with COVID-19: a systematic review and meta-analysis. J Infect. 2022;84(2):248-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang W, Lei W, Jiang L, et al. Therapeutic mechanisms of mesenchymal stem cells in acute respiratory distress syndrome reveal potentials for COVID-19 treatment. J Transl Med. 2021;19(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qin H, Zhao A.. Mesenchymal stem cell therapy for acute respiratory distress syndrome: from basic to clinics. Protein Cell. 2020;11(10):707-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gorman E, Millar J, McAuley D, O’Kane C.. Mesenchymal stromal cells for acute respiratory distress syndrome (ARDS), sepsis, and COVID-19 infection: optimizing the therapeutic potential. Expert Rev Respir Med. 2021;15(3):301-324. [DOI] [PubMed] [Google Scholar]
- 39. Fan E, Beitler JR, Brochard L, et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med. 2020;8(8):816-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Navas-Blanco JR, Dudaryk R.. Management of respiratory distress syndrome due to COVID-19 infection. BMC Anesthesiol. 2020;20(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moradinasab S, Pourbagheri-Sigaroodi A, Zafari P, Ghaffari SH, Bashash D.. Mesenchymal stromal/stem cells (MSCs) and MSC-derived extracellular vesicles in COVID-19-induced ARDS: mechanisms of action, research progress, challenges, and opportunities. Int Immunopharmacol. 2021;97:107694. 10.1016/j.intimp.2021.107694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hu B, Huang S, Yin L.. The cytokine storm and COVID-19. J Med Virol. 2021;93(1):250-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hojyo S, Uchida M, Tanaka K, et al. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020;40:37. 10.1186/s41232-020-00146-3.eCollection2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Attiq A, Yao LJ, Afzal S, Khan MA.. The triumvirate of NF-κB, inflammation and cytokine storm in COVID-19. Int Immunopharmacol. 2021;101(Pt B):108255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marnell L, Mold C, Du Clos TW.. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol. 2005;117(2):104-111. [DOI] [PubMed] [Google Scholar]
- 46. Gewurz H, Mold C, Siegel J, Fiedel B.. C-reactive protein and the acute phase response. Adv Intern Med. 1982;27:345-372. [PubMed] [Google Scholar]
- 47. Black S, Kushner I, Samols D.. C-reactive protein. J Biol Chem. 2004;279(47):48487-48490. [DOI] [PubMed] [Google Scholar]
- 48. Shang W, Dong J, Ren Y, et al. The value of clinical parameters in predicting the severity of COVID-19. J Med Virol. 2020;92(10):2188-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stringer D, Braude P, Myint PK, et al. . COPE Study Collaborators. The role of C-reactive protein as a prognostic marker in COVID-19. Int J Epidemiol. 2021;50(2):420-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sharifpour M, Rangaraju S, Liu M, et al. . Emory COVID-19 Quality & Clinical Research Collaborative. C-reactive protein as a prognostic indicator in hospitalized patients with COVID-19. PLoS One. 2020;15(11):e0242400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Knight V, Long T, Meng QH, Linden MA, Rhoads DD.. Variability in the laboratory measurement of cytokines. Arch Pathol Lab Med. 2020;144(10):1230-1233. [DOI] [PubMed] [Google Scholar]
- 52. Hardikar S, Song X, Kratz M, et al. Intraindividual variability over time in plasma biomarkers of inflammation and effects of long-term storage. Cancer Causes Contr. 2014;25(8):969-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Smilowitz NR, Kunichoff D, Garshick M, et al. C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J. 2021;42(23):2270-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rodriguez HC, Gupta M, Cavazos-Escobar E, El-Amin SF 3rd, Gupta A.. Umbilical cord: an allogenic tissue for potential treatment of COVID-19. Hum Cell. 2021;34(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hernandez JJ, Beaty DE, Fruhwirth LL, Lopes Chaves AP, Riordan NH.. Dodging COVID-19 infection: low expression and localization of ACE2 and TMPRSS2 in multiple donor-derived lines of human umbilical cord-derived mesenchymal stem cells. J Transl Med. 2021;19(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Corsello T, Amico G, Corrao S, et al. Wharton’s jelly mesenchymal stromal cells from human umbilical cord: a close-up on immunomodulatory molecules featured in situ and in vitro. Stem Cell Rev Rep. 2019;15(6):900-918. [DOI] [PubMed] [Google Scholar]
- 57. Abdelgawad M, Bakry NS, Farghali AA, Abdel-Latif A, Lotfy A.. Mesenchymal stem cell-based therapy and exosomes in COVID-19: current trends and prospects. Stem Cell Res Ther. 2021;12(1):469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Desterke C, Griscelli F, Imeri J, et al. Molecular investigation of adequate sources of mesenchymal stem cells for cell therapy of COVID-19-associated organ failure. Stem Cells Transl Med. 2021;10(4):568-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang J, Huang X, Wang H, et al. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res Ther. 2015;6:234. 10.1186/s13287-015-0240-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Choudhery MS, Khan M, Mahmood R, et al. Bone marrow derived mesenchymal stem cells from aged mice have reduced wound healing, angiogenesis, proliferation and anti-apoptosis capabilities. Cell Biol Int. 2012;36(8):747-753. [DOI] [PubMed] [Google Scholar]
- 61. Yang C, Yang Y, Ma L, et al. Study of the cytological features of bone marrow mesenchymal stem cells from patients with Neuromyelitis optica. Int J Mol Med. 2019;43(3):1395-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Liu Y, Li Z, Liu T, et al. Impaired cardioprotective function of transplantation of mesenchymal stem cells from patients with diabetes mellitus to rats with experimentally induced myocardial infarction. Cardiovasc Diabetol. 2013;12:40. 10.1186/1475-2840-12-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308(22):2369-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Alagesan S, Griffin MD.. Autologous and allogeneic mesenchymal stem cells in organ transplantation: what do we know about their safety and efficacy? Curr Opin Organ Transplant. 2014;19(1):65-72. [DOI] [PubMed] [Google Scholar]
- 65. Monedero P, Gea A, Castro P, et al. . COVID-19 Spanish ICU Network. Early corticosteroids are associated with lower mortality in critically ill patients with COVID-19: a cohort study. Crit Care. 2021;25(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gupta S, Wang W, Hayek SS, et al. . STOP-COVID Investigators. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181(1):41-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rowland AL, Miller D, Berglund A, et al. Cross-matching of allogeneic mesenchymal stromal cells eliminates recipient immune targeting. Stem Cells Transl Med. 2021;10(5):694-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wilson AJ, Rand E, Webster AJ, Genever PG.. Characterisation of mesenchymal stromal cells in clinical trial reports: analysis of published descriptors. Stem Cell Res Ther. 2021;12(1):360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Galipeau J, Sensébé L.. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lukomska B, Stanaszek L, Zuba-Surma E, et al. Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int. 2019;2019:9628536. 10.1155/2019/9628536. eCollection 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Giri J, Galipeau J.. Mesenchymal stromal cell therapeutic potency is dependent upon viability, route of delivery, and immune match. Blood Adv. 2020;4(9):1987-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shahani P, Datta I.. Mesenchymal stromal cell therapy for coronavirus disease 2019: which? when? and how much? Cytotherapy. 2021;23(10):861-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Moll G, Drzeniek N, Kamhieh-Milz J, et al. MSC therapies for COVID-19: importance of patient coagulopathy, thromboprophylaxis, cell product quality and mode of delivery for treatment safety and efficacy. Front Immunol. 2020;11:1091. 10.3389/fimmu.2020.01091. eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets available upon request from the corresponding author.