Abstract
Autologous fat grafting is among the safest and most effective treatments for soft-tissue restoration and augmentation, and many efforts have been made to improve its efficiency, including adipose-derived stem cell (ASC) supplementation. Here, we investigated the role of Notch ligand Delta-like ligand 4 (Dll4) in angiogenesis within grafted fat and its effect on graft retention, as well as the effect of Dll4 inhibition on ASC supplementation. Using a murine fat graft model, we investigated the expression of Dll4 in fat grafts and assessed the graft volume, vascularity, and perfusion within the graft, and ASC differentiation patterns depending on the blockade of Dll4. The underlying mechanism of Dll4 inhibition on ASC supplemented fat grafts was investigated using transcriptome analysis. Dll4 was highly expressed in vascular endothelial cells (ECs) within grafted fat, where Dll4-blocking antibody treatment-induced angiogenesis, promoting fat graft retention. In addition, its effect on fat graft retention was synergistically improved when ASCs were concomitantly supplemented. The expression of junctional proteins was increased in ECs, and inflammatory processes were downregulated in grafted fat upon ASC supplementation and Dll4 inhibition. Dll4 inhibition induced vascularization within the grafted fat, thereby promoting graft retention and exhibiting synergistic effects with concomitant ASC supplementation. This study serves as a basis for developing new potential therapeutic approaches targeting Dll4 to improve graft retention after cell-assisted transfer.
Keywords: delta-like ligand 4, adipose tissue, transplantation, stem cells, angiogenesis
Graphical Abstract
Graphical Abstract.
Significance Statement.
Dll4 inhibition induces angiogenesis within the grafted fat, promoting graft retention. The angiogenic effect of adipose-derived stem cell (ASC) supplementation in fat grafting is improved by Dll4 inhibition. Dll4 inhibition improves ASC survival and facilitates proper differentiation of ASCs into adipocytes and endothelial cells.
Introduction
Autologous fat transfer is among the most effective and safest treatments for soft-tissue restoration and augmentation. This technique has numerous advantages, including biocompatibility, non-immunogenicity, and negligible donor-site morbidity; however, the challenge lies in selecting the tissue from which autologous fat with isolated stromal vascular fraction (SVF) should be isolated.1 Stromal vascular fraction is a heterogeneous cell mixture including adipose-derived stem cells (ASCs).2 Through several systematic reviews, it was confirmed that the retention rate of cell-assisted lipotransfer (CAL) is superior to that of conventional fat graft (60%-64% vs 44%-45%).3,4 Nevertheless, there is a significant portion of grafted fat resorption even in CAL; thus, further research is needed to improve graft retention.
Delta-like ligand 4 (Dll4) is a transmembrane protein that binds with Notch receptors 1 and 4.5 Notch signaling plays an essential role in cell-fate determination and differentiation of progenitor cells during the developmental process.6 Furthermore, the expression of Dll4 is primarily limited to vascular endothelial cells (ECs), suggesting that Dll4 is a key molecule for vasculogenesis and angiogenesis.7,8 Dll4-Notch signaling acts as a negative feedback regulator of vascular endothelial growth factor (VEGF) signaling. Thus, Dll4 inhibition activates VEGF signaling and profound angiogenic activity.5,9 Increased sprouting angiogenesis and interconnected vascular plexuses induced by Dll4 inhibition are excessive but non-functional in physiologic and tumor angiogenesis.10,11 On the other hand, in non-healing wound tissue, Dll4 inhibition provides early reperfusion by enhanced angiogenesis,12 which suggests that the aspects of angiogenesis induced by Dll4 inhibition may be different depending on tissue-specific differences in the microenvironment surrounding the vessels. To the best of our knowledge, there have been no studies on how Dll4 inhibition affects fat grafting.
Thus, we aimed to investigate the angiogenic effect of anti-Dll4 antibody on fat grafting and determine an appropriate dose of anti-Dll4 antibody to improve fat graft retention. In addition, we studied the effect of Dll4 inhibition on graft retention during CAL with ASC tracing and performed transcriptome analysis to gain insights into the underlying mechanisms.
Materials and Methods
Animal Model
Animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee (No. YUMC-AEC2020-001) of the Yeungnam University College of Medicine, Korea. All experiments were performed between December 2018 to January 2021, following the relevant guidelines and regulations. All mice used in this study were inbred C57BL/6J (B6) mice housed in specific pathogen-free animal facilities. In the graft experiments, wild-type (WT) donor mice were anesthetized using an intramuscular injection of a combination of ketamine (80 mg/kg) and xylazine (12 mg/kg). Fat tissues were harvested from the axilla and inguinal subcutaneous area of the donor mice and chopped into small pieces. Fragmented fat (200 μL) was injected into a recipient mouse at the supraperiosteal plane of the skull using a 16-gauge needle.
To trace ASCs, we used a previously described CAL animal model with a transgenic reporter mouse strain that expresses the DsRed fluorescent signal.13,14 Donor fat (WT fat) and ASCs (DsRed-ASCs) were harvested from the WT B6 and DsRed B6 mice, respectively. Wild-type B6 mice were used as the recipients. DsRed-ASCs were derived from a culture of SVF cells after harvesting, chopping, digestion with 0.2% collagenase type 1 (Worthington Biochemical Corporation, Lakewood, NJ, USA), and centrifugation of the inguinal fat of DsRed mice. Cultured DsRed-ASCs have the capacity to differentiate into adipocytes, osteocytes, and chondrocytes without an endothelial or hematopoietic lineage-positive subpopulation.13 Two hundred microliters of fragmented fat were mixed with 1.0 × 106 DsRed-ASCs and injected into a recipient mouse at the supraperiosteal plane of the skull.
To inhibit Dll4, 5 mg/kg of the anti-Dll4 antibody (α-Dll4 Ab, clone 26.82, US7803377 B2) was intraperitoneally injected into recipient mice (on the day of the fat graft, 1 week after the fat graft, and 2 weeks after the fat graft.)12 Depending on the group, α-Dll4 Ab was injected once on the day of the fat graft (group 1), twice on the day of fat grafting and 7 days after the fat graft (group 2), or 3 times on the day of fat grafting, and 1 week and 2 weeks after the fat graft (group 3). In the control group, an equal amount of control antibody (control IgG, 1-001-A, R&D Systems) was injected.
Next, WT recipient mice were used to assess Dll4 expression, graft volume, vascularity, lectin perfusion, and the proliferation and differentiation of ASCs (n = 6 for each group). Micro-computed tomography (microCT, NFR-Polaris-G90, NanoFocus Ray, Iksan, Korea) was performed to measure fat graft volume. Among the microCT images, the section with the largest graft was selected, and the graft volume was calculated using the formula 0.5 × A × B, where A is the longest diameter of the graft, and B is its perpendicular diameter. The grafts of recipient mice were harvested at postoperative week 8.
Histological Analyses
For immunofluorescence staining, wound tissues were fixed overnight in 4% paraformaldehyde, dehydrated in 20% sucrose solution overnight, and embedded in tissue-freezing medium (Leica, Wetzlar, Germany). Frozen blocks were cut into 20-μm-thick sections. The tissue sections were blocked in phosphate-buffered saline containing 0.2% Tween-20 and 5% goat or donkey serum (Jackson ImmunoResearch, West Grove, PA, MD, USA) for 1 h and then incubated overnight with the following primary antibodies: anti-Dll4 (goat; AF1389; R&D Systems, Minneapolis, USA), anti-CD31 (hamster; 2H8; Millipore, Billerica, USA), anti-perilipin (guinea pig; 20R-PP004; Fitzgerald Industries International, Acton, USA), anti-NCAM1 (goat; AF2408; R&D Systems, Minneapolis, USA), anti-Connexin43 (rabbit; 71-0700; Invitrogen, Waltham, USA), anti-FOXP3 (rabbit; ab215206; Abcam, Cambridge, UK), anti-CD3 (rat; MAB4841; R&D Systems, Minneapolis, USA), anti-CD206 (rat; MAB25351; R&D Systems, Minneapolis, USA), and anti-F4/80 (rabbit; SP115; Invitrogen, Waltham, USA). After several washes, the sections were incubated for an additional 2 h with the following secondary antibodies: Alexa Fluor 488- or Alexa Fluor 594-conjugated anti-goat IgG, Alexa Fluor 488- or Alexa Fluor 594-conjugated anti-hamster IgG, Alexa Fluor 647-conjugated anti-guinea pig IgG, Alexa Fluor 488- or Alexa Fluor 594-conjugated anti-rabbit IgG, or Alexa Fluor 488- or Alexa Fluor 594-conjugated anti-rat IgG (all secondary antibodies were obtained from Jackson ImmunoResearch, West Grove, USA). The nuclei were stained with 4ʹ, 6-diamidino-2-phenylindole (Invitrogen, Waltham, USA). A confocal microscope (LSM800, Zeiss, Oberkochen, Germany) was used to visualize the fluorescence images. Morphometric analyses were conducted using ImageJ software (National Institutes of Health, Bethesda, USA) in a blinded manner. Dll4 expression and DsRed-positive cells were calculated as percentages of the corresponding fluorescent-positive area per random 0.408-mm2 region. The vessel area was determined by CD31-positive area per random 0.408-mm2 region. Branching point, perilipin, DsRed co-positive cells, and CD31 and DsRed co-positive cells were counted in random 0.408-mm2 regions. The necrotic area was determined as a percentage of the perilipin-negative area in the perilipin-stained graft section images. The densities of the immunofluorescence signals were quantified using ImageJ software.
Assessment of Vascular Perfusion
For vascular perfusion, 100 µL of DyLight 488-conjugated tomato lectin (1 mg/mL, Vector Laboratories, Burlingame, USA) was intravenously administered 30 minutes before euthanasia. The vascular perfusion area was determined as the percentage of the lectin-positive area divided by the CD31-positive area. Mice were anesthetized and intracardially perfused with PBS to eliminate the circulating lectins. Two different images from each section were obtained to minimize local variation, and the mean lectin levels were measured. All signal intensities were measured using ImageJ software. The threshold was set at the same level.
Transcriptome Analysis
For the transcriptomic analysis of grafted fat, harvested mouse grafted fat, with or without 1.0 × 106 DsRed-ASC supplementation and injection of α-Dll4 Ab twice, were digested in 5 mL of enzyme buffer containing 0.2 mg/mL collagenase type-II (Worthington), 0.1 mg/mL DNase I (Roche), and 0.8 mg/mL dispase (Gibco) at 37 °C for 30 minutes. After complete digestion, the cell suspension was filtered through a 40-μm nylon cell strainer and RBC lysis was performed with suspension in ACK lysis buffer (Gibco) for 2 minutes at room temperature.
Total RNA concentration was calculated using the Quant-IT RiboGreen (Invitrogen). To determine the DV200 (% of RNA fragments >200 bp) values, samples were run on the TapeStation RNA screentape (Agilent). According to the manufacturer’s protocol, 100 ng of total RNA were subjected to sequencing library construction using the Agilent SureSelect RNA Direct kit. Briefly, the total RNA was first fragmented into small pieces using divalent cations at elevated temperatures. The cleaved RNA fragments were copied into first-strand cDNA using random primers. This was followed by second-strand cDNA synthesis. These cDNA fragments then go through an end repair process, the addition of a single “A” base, and then ligation of the adapters. The products were then purified and enriched by PCR to create a cDNA library.
To capture the mouse exonic region, we used the Agilent SureSelect XT Mouse All Exon Kit according to the standard Agilent SureSelect Target Enrichment protocol. Briefly, 250 ng of cDNA library was mixed with hybridization buffers, blocking mixes, RNase block, and 5 µL of SureSelect all exon capture library. Hybridization to the capture baits was conducted at 65 °C using a heated thermal cycler lid option at 105 °C for 24 h on a thermal cycler. The captured library was then washed and subjected to a second round of PCR amplification. The final purified product was quantified using KAPA Library Quantification kits for Illumina Sequencing platforms according to the qPCR Quantification Protocol Guide (KAPA BIOSYSTEMS, #KK4854). The purified products were quantified using the TapeStation D1000 ScreenTape (Agilent Technologies, #5067-5582). Indexed libraries were then submitted to Illumina NovaSeq (Illumina, Inc., San Diego, CA, USA), and paired-end (2 × 100 bp) sequencing was performed. mRNA-Seq reads were aligned using the Bowtie2 version 2.3. 4.1 Bowtie2 indices were either generated from the genome assembly sequence or the representative transcript sequences to align to the genome and transcriptome. The alignment file was used to assemble transcripts, estimate their abundances, and detect the differential expression of genes. Differentially expressed genes were determined based on counts from unique and multiple alignments using the DESeq2. The read count data were processed based on the quantile normalization method using StringTie version 2.1.3b.
For bioinformatics analysis, the gene expression data were annotated with official gene symbols and normalized (log2). Single-sample gene set enrichment analysis (ssGSEA)15 was performed with the REACTOME subsets of curated gene set (C2) collections of the Molecular Signature Database v6.1 (http://www.broadinstitute.org/gsea/msigdb). Heatmaps were made with normalized enrichment scores of each gene set.
Statistical Analysis
Experimental values are presented as the mean ± standard deviation. Differences were assessed using the Mann-Whitney U test between 2 groups or the Kruskal-Wallis test, followed by a rank-based version of Tukey’s honest significant difference test (ie, the Tukey-Kramer method). Statistical significance was set at P < .05. Statistical analyses were performed using the SPSS statistical software (version 22, IBM, Armonk, USA).
Results
Dll4 Expression in Grafted Fat Tissue
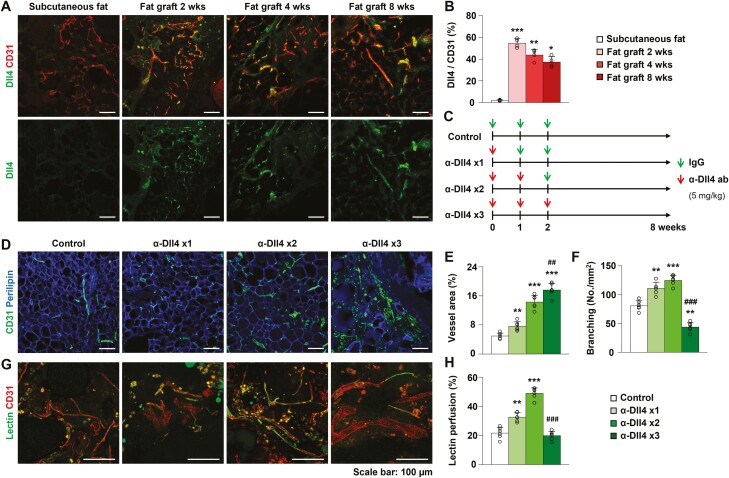
We examined Dll4 expression in donor fat and grafted fat tissues, 2, 4, and 8 weeks after implantation. There was minimal expression of Dll4 in the vessels of subcutaneous donor fat (Fig. 1A and 1B). In contrast, Dll4 was highly expressed in the vessels of 2, 4, and 8 weeks grafted fat (Fig. 1A and 1B). Dll4 expression in the vessel was strongest at 2 weeks after fat grafting, and was slightly lower at 4 and 8 weeks (P < .001, fat graft 2 weeks vs subcutaneous fat; P = .001, fat graft 4 weeks vs subcutaneous fat; P = .014, fat graft 8 weeks vs subcutaneous fat) (subcutaneous fat, 1.89 ± 0.46%; fat graft 2 weeks, 54.67 ± 4.07%; fat graft 4 weeks, 43.76 ± 5.16%, fat graft 8 weeks, 37.22 ± 5.13%, n = 4; Fig. 1B). These data suggest that, unlike untreated normal fat harboring stable blood vessels, active angiogenesis may occur in fat grafts, thereby enhancing expression of Dll4, which is known to negatively regulate active angiogenesis.16
Figure 1.
Expression and inhibitory effect of Dll4 in grafted fat tissue. (A, B) Representative images, and quantification of Dll4 in the subcutaneous fat and grafted fat at postoperative weeks 2, 4, and 8. (C) Schedule of fat grafting, administration of control IgG or anti-Dll4 antibody for experiments shown in Figs. 1 and 2. (D) Representative images of vascularity in the grafted fat after injection of control IgG, anti-Dll4 antibody once, twice, and 3 times. (E, F) Quantification of vessel area and branching in the grafted fat after injection of control IgG, anti-Dll4 antibody once, twice, and 3 times. (G, H) Representative images and quantification of lectin perfusion assay in the grafted fat after injection of control IgG, anti-Dll4 antibody once, twice, and 3 times. Values are presented as the mean ± standard deviation (n = 4-6). **P < .01, ***P < .001 vs control group. ## P < .01, ### P < .001 vs anti-Dll4 antibody twice injection group. Scale bars = 100 µm.
Anti-Dll4 Antibody Promotes Functional Angiogenesis in Fat Grafts
Based on the significant expression of Dll4 in vascular ECs within the fat graft, we evaluated the effect of Dll4 inhibition on vascularization within the fat graft. We administered α-Dll4 Ab to mice that underwent fat grafting and assessed its dose-dependent effect on angiogenesis within the fat graft (Fig. 1C). The mice were divided into 4 experimental groups according to the type of treatment: control IgG (5 mg/kg) (group 1), injection of α-Dll4 Ab (5 mg/kg) once (group 2), twice (group 3), and 3 times (group 4). α-Dll4 Ab or control IgG was injected intraperitoneally once a week from the day of fat grafting. As the amount of α-Dll4 Ab administration increased, the vessel area increased in the fat graft (P = .001, group 2 vs group 1; P < .001, group 3 vs group 1; P < .001, group 4 vs group 1) (group 1, 4.96 ± 0.77%; group 2, 7.66 ± 1.22%; group 3, 14.28 ± 1.88%, group 4, 17.63 ± 1.81%, n = 6) (Fig. 1D and 1E). Grafted fat tissues in Groups 2 and 3 had an increased number of vascular branches, but a substantial reduction was observed in group 4 compared to that in group 1 (P = .001, group 2 vs group 1; P < .001, group 3 vs group 1; P = .003, group 4 vs group 1; P < .001, group 4 vs group 3) (group 1, 80.89 ± 8.68 No./mm2; group 2, 110.98 ± 10.66 No./mm2; group 3, 124.93 ± 8.65 No./mm2, group 4, 43.90 ± 8.18 No./mm2; n = 6) (Fig. 1D and 1F). These data indicate that Dll4 inhibition promotes angiogenesis in grafted fat tissue; however, excessive doses of α-Dll4 Ab induce the formation of structurally abnormal blood vessels.
Given the changes in vascular density and structure caused by Dll4 inhibition, we further studied the effect of Dll4 inhibition on vascular function of grafted fat tissue using a lectin perfusion assay. Increased lectin perfusion was observed in grafted fat tissues of groups 2 and 3 compared with the control group (P = .001, group 2 vs group 1; P < .001, group 3 vs group 1) (group 1, 21.62 ± 3.89%; group 2, 32.55 ± 3.02%; group 3, 48.87% ± 3.73%; group 4, 19.80 ± 3.06%, n = 6) (Fig. 1G and 1H). However, 3 injections of α-Dll4 Ab did not improve perfusion but instead sharply decreased perfusion compared to 2 injections of α-Dll4 Ab (P = .632, group 4 vs group 1; P < .001, group 4 vs group 3) (Fig. 1G and 1H). These data suggest that the extent of Dll4 inhibition-induced vascular EC proliferation may affect the functions of ECs, particularly those associated with vascular perfusion. An appropriate dose of α-Dll4 Ab improves vascular perfusion by promoting functional angiogenesis during fat grafting.
Blockade of Dll4 Improves Fat Graft Retention
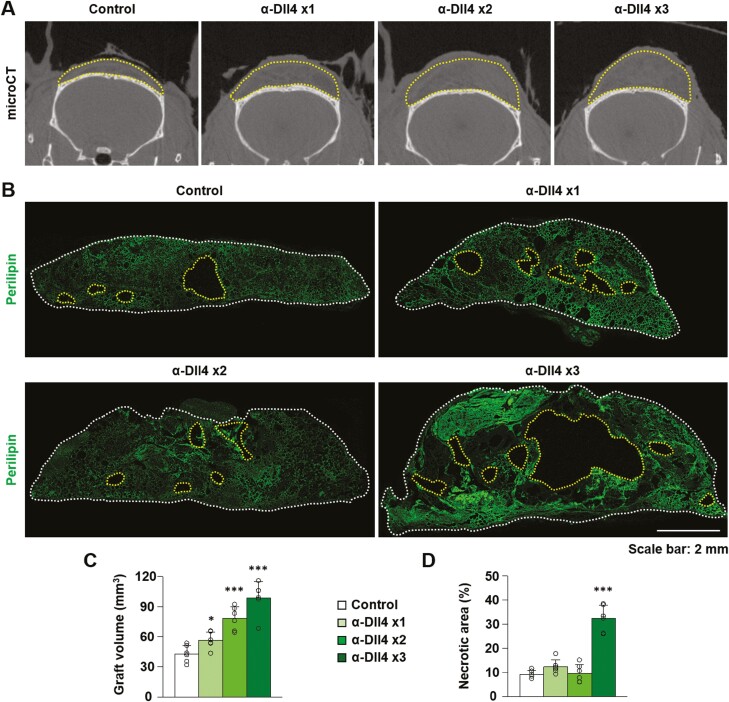
These findings led us to investigate whether increased vascularity and perfusion within fat grafts by Dll4 inhibition can facilitate fat graft retention. To answer this question, we assessed the remaining graft volume and necrotic area after fat grafting, depending on the blockade of Dll4. The graft volume was greater in the Dll4 inhibition groups than in the control group, and was largest in group 4 (P = .044, group 2 vs group 1; P < .001, group 3 vs group 1; P < .001, group 4 vs group 1; P = .044, group 4 vs group 3) (group 1, 42.84 ± 8.48 mm3; group 2, 56.37 ± fat graft retention 8.41 mm3; group 3, 78.15 ± 11.61 mm3, group 4, 98.62 ± 16.31 mm3, n = 6) (Fig. 2A-2C). However, the necrotic area in the grafted fat tissues increased by 32% in group 4 compared to that in other groups (P < .001, group 4 vs group 1) (group 1, 9.18 ± 1.57%; group 2, 12.31 ± 2.92%; group 3, 9.60 ± 3.72%, group 4, 32.38 ± 5.46%, n = 6) (Fig. 2B and 2D). These data demonstrate that a proper dose of Dll4 blockade can facilitate fat graft retention, while an excessive dose of α-Dll4 Ab may induce necrosis inside the graft. In our fat graft model, 2 injections of α-Dll4 Ab were the most appropriate to promote functional angiogenesis and improve fat graft retention. Therefore, 2 injections of α-Dll4 Ab were used for further experiments in this study.
Figure 2.
Dll4 inhibition enhances fat graft retention. (A) Representative images of micro-computed tomographic scans indicating graft volume (yellow-dotted lines) in the grafted fat after injection of control IgG, anti-Dll4 antibody once, twice, and 3 times. (B) Cross-sectional images with immunostaining of perilipin in the grafted fat after injection of control IgG, anti-Dll4 antibody once, twice, and 3 times. The graft margins (white dotted lines) and necrotic areas (yellow-dotted lines) are marked. (C, D) Quantification of graft volume and necrotic area in the grafted fat after injection of control IgG, anti-Dll4 antibody once, twice, and 3 times. Values are presented as the mean ± standard deviation (n = 6). *, P < .05, ***, P < .001 vs control group. Scale bars = 100 µm.
Effect of Dll4 Inhibition on Fat Graft Retention When Combined with ASC Supplementation
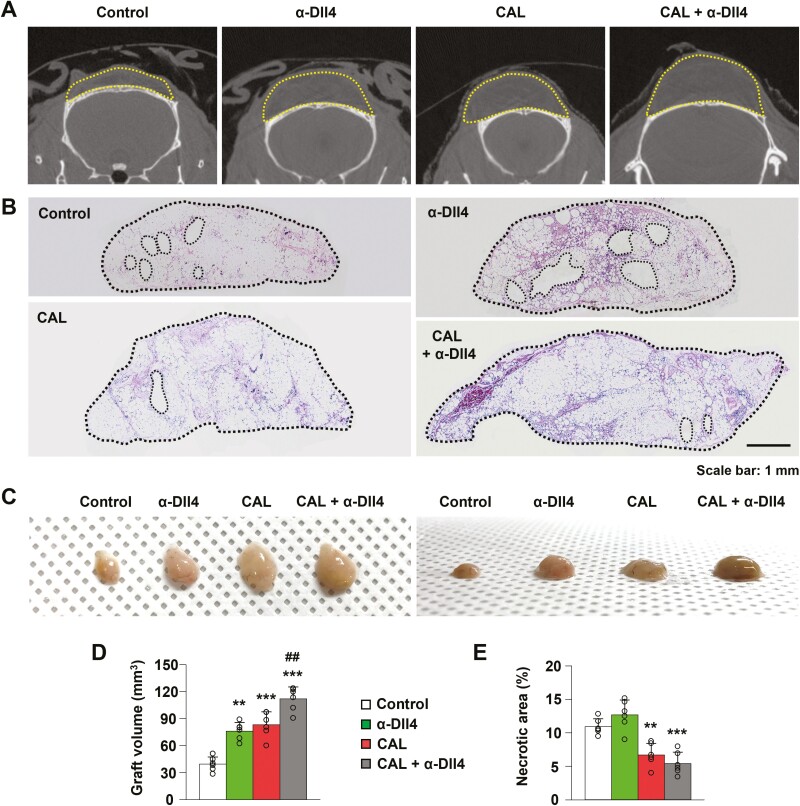
Adipose-derived stem cell supplementation during fat grafting is a well-established strategy to improve fat graft retention by inducing the differentiation of ASCs into vascular ECs and adipocytes.17 We tested whether Dll4 inhibition could affect fat graft retention when combined with ASC supplementation. The mice were divided into 4 groups: the control group, the Dll4 inhibition group (injection of α-Dll4 Ab twice), the CAL group (1.0 × 106 DsRed-ASC supplementation), and combination group (CAL and Dll4 inhibition). The graft volume was greater in the Dll4 inhibition, CAL, and combination groups than in the control group (P = .001, Dll4 inhibition group vs control group; P < .001, CAL group vs control group; P < .001, combination group vs control group) (control group, 39.46 ± 7.93 mm3; Dll4 inhibition group, 76.23 ± 9.59 mm3; CAL group, 83.36 ± 14.18 mm3, combination group, 112.01 ± 13.16 mm3, n = 6) (Fig. 3A-3D). The graft volume in the combination group was the largest (P = .003 combination group vs CAL group). These data indicate that Dll4 inhibition and ASC supplementation treatment improve fat graft retention.
Figure 3.
Effect of Dll4 inhibition and ASC supplementation on fat graft retention. (A) Representative images of micro-computed tomographic scans indicating graft volume (yellow-dotted lines) in the control, Dll4 inhibition, cell-assisted lipotransfer (CAL), and Dll4 inhibition plus CAL groups. (B, C) Cross-section images with hematoxylin and eosin staining and gross photo in the control, Dll4 inhibition, CAL, and Dll4 inhibition plus CAL groups. The graft margins (black-dotted lines) are marked. (D, E) Quantification of graft volume and necrotic area in the control, Dll4 inhibition, CAL, and Dll4 inhibition plus CAL groups. Values are presented as the mean ± standard deviation (n = 6). **P < .01, ***P < .001 vs control group. ##, P < .01 vs the CAL group. Scale bars = 100 µm.
Dll4 Inhibition Promotes Angiogenesis in CAL
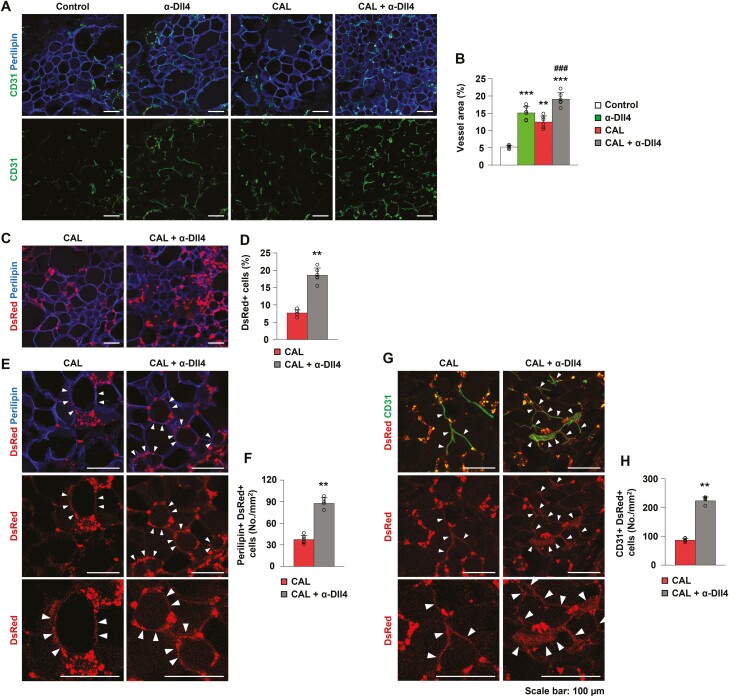
To elucidate the underlying mechanism of improved fat graft retention by combining Dll4 inhibition and ASC supplementation, we examined vascularity within the grafted tissues by immunofluorescence. Dll4 inhibition and ASC supplementation increased vessel area, and the density of the vessel area was highest in the combination group (P < .001, Dll4 inhibition group vs control group; P = .002, CAL group vs control group; P < .001, combination group vs control group; P < .001, combination group vs CAL group) (control group, 5.17 ± 0.45%; Dll4 inhibition group, 15.13 ± 1.90%; CAL group, 12.49% ± 1.76%, combination group, 19.09 ± 1.92%, n = 6) (Fig. 4A and 4B). Adipose-derived stem cell supplementation is known to induce adipogenesis and angiogenesis during fat grafting.13 These data demonstrate that the angiogenic effect of ASC supplementation in fat grafting can be improved by Dll4 inhibition.
Figure 4.
Dll4 inhibition promotes angiogenesis and augments ASC differentiation into endothelial cells and adipocytes in cell-assisted lipotransfer. (A, B) Representative images and quantification of vessel area in the control, Dll4 inhibition, cell-assisted lipotransfer (CAL), and CAL plus Dll4 inhibition (combination) groups. (C, D) Representative images and quantification of DsRed-positive cells originating from adipose-derived stem cells in the CAL and combination groups. (E, F) Representative images and quantification of DsRed and perilipin co-positive cells in the CAL and combination groups. (G, H) Representative images and quantification of DsRed and CD31 co-positive cells in the CAL and combination groups. Values are presented as the mean ± standard deviation (n = 6). **, P < .01, ***, P < .001 vs control group. ###, P < .001 vs the CAL group. Scale bars = 100 µm.
Dll4 Inhibition Facilitates the Differentiation of ASCs into Adipocytes and ECs in CAL
To determine the effect of Dll4 inhibition-induced angiogenesis on the cell number and differentiation of ASCs in CAL, we traced DsRed-ASCs using immunofluorescence. The cell number of DsRed-ASCs increased in the combination group compared with that in the CAL group (P = .004, CAL group, 7.67% ± 0.91%; combination group, 18.59 ± 2.07%, n = 6) (Fig. 4C and 4D). We considered DsRed and perilipin co-positive cells or DsRed and CD31 co-positive cells as evidence for the differentiation of ASCs into adipocytes and ECs, respectively. More DsRed and perilipin co-positive cells were present in the combination group than in the CAL group (P = .004, CAL group, 37.43 ± 5.69 No./mm2; combination group, 87.66 ± 8.03 No. mm2; n = 6) (Fig. 4E and 4F). In addition, more DsRed and CD31 co-positive cells were present in the combination group than in the CAL group (P = .004, CAL group, 86.57 ± 5.84 No./mm2; combination group, 223.09 ± 13.60 No. mm2; n = 6) (Fig. 4G and 4H). These data indicate that Dll4 inhibition improves ASC survival and facilitates proper differentiation of ASCs into adipocytes and ECs by enhancing angiogenesis in CAL, ultimately improving fat graft retention.
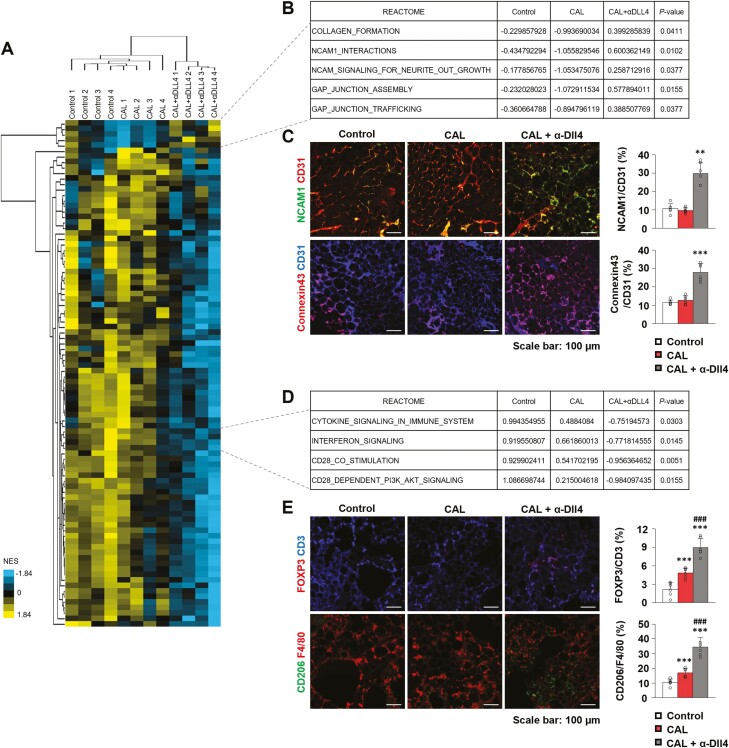
Transcriptome Analysis
To further investigate the molecular mechanism underlying the synergistic effect of CAL and Dll4 inhibition on fat grafts, we performed comparative transcriptomic analysis using grafted fat from the control group, CAL group (1.0 × 106 DsRed-ASC supplementation), and combination group (DsRed-ASC supplementation plus injection of α-Dll4 Ab twice). Heatmap of RNA-sequencing data indicates global changes in the transcriptome during CAL and Dll4 inhibition in grafted fat (Fig. 5A). Among the top upregulated gene sets in combination group compared with the control or CAL group, REACTOME pathways related to cell-to-cell or cell-matrix interactions, such as collagen formation, neural cell adhesion molecule 1 (NCAM1), or GAP junctions, were significantly enriched (Fig. 5B). Histopathologic analysis confirmed this, as NCAM1-positive or Connexin43 (GAP junction marker)-positive vessel areas were significantly increased by Dll4 inhibition and ASC supplementation (NCAM1, P < .001, control group, 10.76% ± 2.64%, CAL group, 9.67% ± 1.48%, combination group, 29.77% ± 5.93%, n = 6; Connexin43, P = .001; control group, 11.54% ± 1.31%; CAL group, 12.71% ± 2.32%; combination group, 27.95 ± 4.69%, n = 6) (Fig. 5C). Interestingly, REACTOME pathways related to inflammation, including cytokine, interferon, and T cell activation (CD28 co-stimulation and dependent signaling pathways), were downregulated in the combination group (Fig. 5D), implying that immune-related rejection of fat graft may be alleviated by Dll4 inhibition. Histopathologic analysis also supported this, as immune-suppressive regulatory T cells (FOXP3+CD3+) and anti-inflammatory M2-like macrophage (CD206+F4/80+) were significantly increased by ASC supplementation and Dll4 inhibition (FOXP3/CD3, P < .001, CAL group vs control group; P < .001, combination group vs CAL group, control group, 2.17% ± 1.13%; CAL group, 4.81% ± 0.81%; combination group, 8.96% ± 1.46%, n = 6; CD206/F4/80, P < .001, CAL group vs control group; P < .001, combination group vs CAL group, control group, 10.42% ± 2.26%; CAL group, 16.80% ± 2.98%; combination group, 34.52% ± 6.55%, n = 6) (Fig. 5E). Our transcriptomic analysis showed that the synergistic effect of Dll4 inhibition on CAL in fat grafts might be associated with enhanced vascular integrity via NCAM1 and GAP junction upregulation, thus relieving inflammation and increasing anti-inflammatory immune cells.
Figure 5.
The synergistic effect of Dll4 inhibition on CAL in fat grafts is associated with enhanced vascular integrity and relieved inflammation. (A) Heatmap of unsupervised clustering of normalized enrichment score (NES) of REACTOME pathways by single-sample gene set enrichment analysis (ssGSEA) among control, Dll4 inhibition, cell-assisted lipotransfer (CAL), and CAL plus Dll4 inhibition group samples (n = 4 each). (B) REACTOME pathways were significantly positively enriched among the CAL plus Dll4 inhibition group. Note that NCAM1 and GAP junction-related pathways were enriched after Dll4 inhibition. (C) Representative images and quantification of NCAM1 and Connexin43 in the control, CAL, and CAL plus Dll4 inhibition groups (n = 6). (D) Selected REACTOME pathways that were significantly negatively enriched among CAL plus Dll4 inhibition group. Note that inflammation related pathways including cytokine, interferon, and CD28-related pathways were suppressed after Dll4 inhibition. P-values were calculated comparing CAL samples and CAL plus Dll4 inhibition samples. (E) Representative images and quantification of FOXP3+CD3+ and CD206+F4/80+ cells in the control, CAL, and CAL plus Dll4 inhibition groups (n = 6). Values are presented as the mean ± standard deviation. **P < .01, ***P < .001 vs control group. ### P < .001 vs the CAL group. Scale bars = 100 µm.
Discussion
In this study, we demonstrated that the Notch ligand Dll4 is highly expressed in ECs of grafted adipose tissue and that blockade of Dll4 promotes fat graft retention while inducing angiogenesis in the grafted fat. Moreover, Dll4 inhibition significantly improved the effect of ASC supplemented fat transfer by increasing the proliferation and differentiation of ASCs in fat grafts and inducing angiogenesis. In addition, we confirmed that the expression of junctional proteins identified from transcriptome analysis was increased in the ECs of grafted fat treated with α-Dll4 Ab.
Many studies have been conducted to improve the survival of transplanted adipose tissue.18 The establishment of neovascularization and regulation of microenvironmental changes inside the fat graft have been considered as important factors related to the mechanism of fat graft survival.17 According to “graft survival theory,” fast and effective neovascularization in the fat graft is indispensable for fat graft survival because the graft can receive oxygen and nutrients only through diffusion from the surrounding capillaries until angiogenesis occurs.17,19 On the other hand, it is also possible to improve the survival of adipose tissue through differentiation and proliferation of ASCs supplemented with fat grafting, which is called ‘graft replacement theory.17,20 Since these 2 mechanisms are not independent of each other and can constantly interact, we thought that treatment that can have a positive effect on both of these mechanisms could be more effective in improving the efficiency of fat grafting. Our detailed experiment and analysis using a fat allograft animal model clearly showed that Dll4 inhibition promotes angiogenesis inside the fat graft and induces proliferation and differentiation of ASCs simultaneously, maximizing the survival of fat grafts. Furthermore, these results suggest that the blockage of Dll4 may be a new therapeutic approach to increase the survival of fat grafts.
It has been widely reported that Dll4 inhibition induces changes in blood vessels during vascular development or in pathologic conditions, such as tumors.12,21,22 In previous studies, it was reported that Dll4 inhibition in growing retinal vessels or tumor vessels results in the excessive formation of non-functioning vessels that do not perfuse into the tissue, exacerbating ischemia or causing tissue necrosis.12,23,24 Similarly, in this study, α-Dll4 Ab treatment increased the vascularity of the fat graft in a dose-dependent manner, but at the same time, the proportion of perfused vessels decreased accordingly. Nevertheless, when treated with an appropriate dose of α-Dll4 Ab we found fat graft survival could be enhanced while preventing excessive formation of non-perfused vessels and subsequent central necrosis. We believe that the microenvironment of the tissue in which normal vascular development occurs, or vascularized tumor tissue may differ from the adipose tissue immediately after grafting in terms of the amount of angiogenic growth factors and distribution of adhesion molecules; therefore, the effect of Dll4 inhibition on features of neovascularization may also be different. In fact, even in the same tissue within an individual mouse, similar expression level of Dll4 in ECs led to different features of vascular proliferation, remodeling, and response to external stress, depending on its location and microenvironmental stability inside the tissue.12,22,25 Further research is needed to elucidate what specifically determines the differences in EC response upon Dll4 inhibition.
The most intriguing finding in this study was that fat graft retention was maximized when Dll4 inhibition and ASC supplementation were combined. This indicates that Dll4 blockage-induced angiogenesis promoted the proliferation and differentiation of ASCs through an increase in oxygen and nutrient supply. On the other hand, changes in the microenvironment induced by ASC supplementation, such as the paracrine effect of various substances secreted from ASCs, might further stabilize the proliferating vessels derived from Dll4 inhibition, and cause a virtuous cycle. In fact, in this study, transcriptomic analysis revealed that the activities of junctional molecules were increased while the inflammatory process was weakened upon concomitant treatment with Dll4 Ab and ASC supplementation. Although the context-dependent relationship between the Dll4-NOTCH pathway and vascular integrity is controversial, histopathologic analysis corroborated our findings, with an increase in NCAM1- and Connexi43-positive vessels after Dll4 inhibition and ASC supplementation. In addition, it has also been reported that Dll4-Notch pathways are associated with promotion of pro-inflammatory activation.26 Furthermore, immune-suppressive regulatory T cells and anti-inflammatory M2-like macrophages were upregulated after Dll4 inhibition and ASC supplementation. These results imply that increased junctional stability and anti-inflammatory effects induced by Dll4 inhibition may provide a favorable microenvironment for grafted fat, supporting ASC survival and differentiation to adipocytes and vascular ECs, thereby promoting fat graft retention. Further studies on the molecular mechanisms are warranted.
In this study, we evaluated the role of Dll4 in CAL by systemically administrating the Dll4 Ab, which would not be feasible in a clinical setting. Systemic administration of Dll4 Ab was reported to have a minimal effect on other healthy organs in the animal model due to its preferential effect on the vessels undergoing active angiogenesis.10 However, local treatment would be more appropriate, as fat grafts are primarily performed in localized areas. This can maximize the effectiveness of treatment and reduce the harmful effects of systemic treatment. Further studies are needed to determine the specific methods of local treatment and confirm their effectiveness.
Conclusion
This study delineates the role of Dll4 inhibition in angiogenesis in grafted fat, promoting graft fat retention, which has a synergistic effect with concomitant supplementation of ASCs through enhanced angiogenesis, differentiation of ASCs, and stabilization of vascular ECs. This study provides a novel potential therapeutic approach to improve fat graft retention for the treatment of soft-tissue restoration and augmentation.
Acknowledgments
We would like to thank Professor Injune Kim from Korea Advanced Institute of Science and Technology for providing anti-Dll4 antibody and Professor Hak Chang from Seoul National University for providing DsRed mice.
Contributor Information
Choong-kun Lee, Division of Medical Oncology, Department of Internal Medicine, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea.
Bo-Yoon Park, Department of Plastic and Reconstructive Surgery, Yeungnam University College of Medicine, Daegu, Korea.
Taehee Jo, Department of Plastic and Reconstructive Surgery, Keimyung University School of Medicine, Daegu, Korea.
Cheol-Heum Park, Department of Plastic and Reconstructive Surgery, Yeungnam University College of Medicine, Daegu, Korea.
Ju-Hee Kim, Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea.
Kyu-Jin Chung, Department of Plastic and Reconstructive Surgery, Yeungnam University College of Medicine, Daegu, Korea.
Yong-Ha Kim, Department of Plastic and Reconstructive Surgery, Yeungnam University College of Medicine, Daegu, Korea.
Do Young Park, Department of Ophthalmology, Yeungnam University College of Medicine, Daegu, Korea.
Il-Kug Kim, Department of Plastic and Reconstructive Surgery, Yeungnam University College of Medicine, Daegu, Korea.
Funding
This work was supported by the National Research Foundation of Korea (NRF-2020R1C1C1004461 to Choong-kun Lee, NRF-2019M3E5D1A02068088 and 2022R1C1C1008365 to Do Young Park and NRF-2021R1A2C4002771 and 2019M3E5D1A02068102 to Il-Kug Kim).
Conflict of Interest
The authors declared no potential conflicts of interest.
Author Contributions
C.L., B.Y.P., T.J.: contribution to designing research studies and conducting experiments and acquiring data, data analysis, manuscript writing and final approval of the manuscript; C.H.P., J.H.K.: contribution to designing research studies and conducting experiments and acquiring data; K.J.C., Y.H.K.: contribution to designing research studies and manuscript writing and final approval of the manuscript; D.Y.P., I.K.K.: contribution to designing research studies and conducting experiments and data analysis, manuscript writing and final approval of the manuscript.
Data Availability
The data that support the findings of this study are available upon request from the corresponding authors.
References
- 1. Matsumoto D, Sato K, Gonda K, et al. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. J Tissue Eng. 2006;12(12):3375-3382. [DOI] [PubMed] [Google Scholar]
- 2. Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32(1):48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou Y, Wang J, Li H, et al. Efficacy and safety of cell-assisted lipotransfer: a systematic review and meta-analysis. Plast Reconstr Surg. 2016;137(1):44e-57e. [DOI] [PubMed] [Google Scholar]
- 4. Laloze J, Varin A, Gilhodes J, et al. Cell-assisted lipotransfer: friend or foe in fat grafting? Systematic review and meta-analysis. J Tissue Eng Regen Med. 2018;12(2):e1237-e1250. [DOI] [PubMed] [Google Scholar]
- 5. Lobov I, Renard R, Papadopoulos N, et al. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA. 2007;104(9):3219-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Artavanis-Tsakonas S, Rand MD, Lake RJ.. Notch signaling: cell fate control and signal integration in development. Science 1999;284(5415):770-776. [DOI] [PubMed] [Google Scholar]
- 7. Gale NW, Dominguez MG, Noguera I, et al. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci USA. 2004;101(45):15949-15954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shutter JR, Scully S, Fan W, et al. Dll4, a novel Notch ligand expressed in arterial endothelium. Genes Dev. 2000;14(11):1313-1318. [PMC free article] [PubMed] [Google Scholar]
- 9. Kim K, Kim I-K, Yang JM, et al. SoxF transcription factors are positive feedback regulators of VEGF signaling. Circ Res. 2016;119(7):839-852. [DOI] [PubMed] [Google Scholar]
- 10. Noguera-Troise I, Daly C, Papadopoulos NJ, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 2006;444(7122):1032-1037. [DOI] [PubMed] [Google Scholar]
- 11. Scehnet JS, Jiang W, Ram Kumar S, et al. Inhibition of Dll4-mediated signaling induces proliferation of immature vessels and results in poor tissue perfusion. Blood, Am J Hematol. 2007;109(11):4753-4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang JM, Ryu J, Kim I, Chang H, Kim IK.. Dll4 blockade promotes angiogenesis in nonhealing wounds of sox7-deficient mice. Adv Wound Care (New Rochelle) 2020;9(11):591-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hong KY, Yim S, Kim HJ, et al. The fate of the adipose-derived stromal cells during angiogenesis and adipogenesis after cell-assisted lipotransfer. Plast Reconstr Surg. 2018;141(2):365-375. [DOI] [PubMed] [Google Scholar]
- 14. Hong KY, Kim I-K, Park SO, Jin US, Chang H.. Systemic administration of adipose-derived stromal cells concurrent with fat grafting. Plast Reconstr Surg. 2019;143(5):973e-982e. [DOI] [PubMed] [Google Scholar]
- 15. Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545-15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hellström M, Phng LK, Hofmann JJ, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 2007;445(7129):776-780. [DOI] [PubMed] [Google Scholar]
- 17. Pu LL. Mechanisms of fat graft survival. Ann Plast Surg. 2016;77(Suppl 1):S84-S86. [DOI] [PubMed] [Google Scholar]
- 18. Khouri RK Jr., Khouri RE, Lujan-Hernandez JR, et al. Diffusion and perfusion: the keys to fat grafting. Plast Reconstr Surg Glob Open 2014;2(9):e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carpaneda CA, Ribeiro MT.. Percentage of graft viability versus injected volume in adipose autotransplants. Aesthetic Plast Surg. 1994;18(1):17-19. [DOI] [PubMed] [Google Scholar]
- 20. Suga H, Eto H, Aoi N, et al. Adipose tissue remodeling under ischemia: death of adipocytes and activation of stem/progenitor cells. Plast Reconstr Surg. 2010;126(6):1911-1923. [DOI] [PubMed] [Google Scholar]
- 21. Lee SH, Lee S, Yang H, et al. Notch pathway targets proangiogenic regulator Sox17 to restrict angiogenesis. Circ Res. 2014;115(2):215-226. [DOI] [PubMed] [Google Scholar]
- 22. Kim IK, Kim K, Lee E, et al. Sox7 promotes high-grade glioma by increasing VEGFR2-mediated vascular abnormality. J Exp Med. 2018;215(3):963-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoey T, Yen WC, Axelrod F, et al. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell 2009;5(2):168-177. [DOI] [PubMed] [Google Scholar]
- 24. Kangsamaksin T, Tattersall IW, Kitajewski J.. Notch functions in developmental and tumour angiogenesis by diverse mechanisms. Biochem Soc Trans. 2014;42(6):1563-1568. [DOI] [PubMed] [Google Scholar]
- 25. Yang JM, Park CS, Kim SH, et al. Dll4 suppresses transcytosis for arterial blood-retinal barrier homeostasis. Circ Res. 2020;126(6):767-783. [DOI] [PubMed] [Google Scholar]
- 26. Nakano T, Fukuda D, Koga J, Aikawa M.. Delta-like ligand 4-Notch signaling in macrophage activation. Arterioscler Thromb Vasc Biol. 2016;36(10):2038-2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding authors.