Abstract
Objective.
Recent reports in both cervical and endometrial cancer suggest that minimally invasive surgery (MIS) had an unanticipated negative impact on long-term clinical outcomes, including recurrence and death. Given increasing use of robotic surgery since the LAP2 trial, we sought to compare the intermediate and long-term outcomes between those who underwent robotic surgery or laparoscopy for Stage I endometrial cancer.
Methods.
We performed a retrospective review of patients from a single, large, academic, urban practice who underwent either laparoscopic or robot-assisted MIS (RA-MIS) for the treatment of endometrial carcinoma between 2006 and 2016, ensuring at least 5 years of potential follow-up. To adjust for differences in confounding variables between groups, propensity score-based inverse probability of treatment weighting (IPTW) was performed. Overall and recurrence-free survival were compared using Cox proportional hazards regression models adjusting for confounding weights.
Results.
1027 patients were included; 461 received laparoscopy and 566 received RA-MIS. RA-MIS use increased steadily during the study window, which resulted in longer mean surveillance in laparoscopy group (median 8.7 years versus 6.3 years, p < 0.001). RA-MIS was associated poorer recurrence-free (HR: 1.41, 95% CI: 1.12, 1.77) and overall survival (HR: 1.39, 95% CI: 1.06, 1.83). Disease-specific survival was also poorer in the RA-MIS group (HR: 3.51, 95% CI: 2.19, 5.63). Among those who recurred, median time to first recurrence was shorter in the RA-MIS group than the laparoscopy group (16.3 vs. 28.7 months, p = 0.07).
Conclusion.
RA-MIS was associated with poorer long-term patient outcomes. Our data in this lower-risk population indicate relevant clinical endpoints may be occurring during intermediate and long-term follow-up windows. These findings support a prospective evaluation of the long-term outcomes of RA-MIS.
Keywords: Endometrial cancer, Uterine cancer, Laparoscopy, Robotic, Minimally invasive
1. Introduction
Endometrial cancer is the most common cancer treated by gynecologic oncologists, with more 66,000 cases occurring annually in the United States [1]. Most patients present with prognostically favorable scenarios (low stage and grade), therefore the route-of-surgery is an important determinant of the total disease burden. The Gynecologic Oncology Group-LAP2 trial demonstrated both feasibility and short-term efficacy of minimally invasive surgery relative to open surgery, with clear improvement in peri- and post-operative morbidity [2]. Since the completion of the LAP2 study, the proportion of endometrial cancers treated via minimally invasive surgical approaches (MIS) has risen steadily, with current levels approaching 90% at high volume centers, spurred in part by the increasing use of robot-assisted minimally invasive surgery (RA-MIS) [3,4]. RA-MIS offers potential advantages to traditional laparoscopy including binocular view, additional degrees of rotational freedom, and decreased reliance on skilled assistance. There are however, disadvantages to RA-MIS including increased costs, especially when considering robot acquisition and maintenance costs [5,6], as well as loss of tactile sensation and increased operative times [7]. To date, most reports suggest that short term complication rates for patients with clinical Stage I endometrial cancer are similar between laparoscopic and RA-MIS approaches [8].
Recently, Ramirez et al. reported the results of a large prospective study demonstrating that MIS surgery was associated with poorer long-term oncologic outcomes in patients with early-stage cervical cancer despite reduced “up-front” morbidity when compared to open surgery [9]. This report was supported by the contemporaneous reporting of a large, retrospective, database report which came to similar conclusions [10]. Notably, in post-hoc analysis Melamed et al. observed that the detriment to survival was significant only in the RA-MIS while the confidence interval for the hazard ratio in the laparoscopy group crossed 1, suggesting that the laparoscopic and robotic groups may not result in similar outcomes. Taken together, these results suggest that the demonstration of short-term equivalency may underestimate the long-term impact of “route-of-surgery” decisions; and that even within the MIS format, there may be meaningful differences in outcomes.
The current study was undertaken to determine if the use of RA-MIS was associated with worse intermediate and long-term outcomes than traditional laparoscopy among patients with International Federation of Gynecologic Oncology (FIGO) Stage I endometrial cancer.
2. Methods
This study was approved by the Institutional Review Board at the University of Minnesota. We reviewed the records of all patients treated for uterine cancer by a single gynecologic oncology practice between 2006 and 2016. The initiation date was selected to coincide with group uptake of robotic surgery at our institution and to mitigate potential biases related to the learning curve. The termination date was selected to confirm that all living patients would have a minimum of 5 years of potential post-operative surveillance.
Patients were identified through a prospectively maintained department surgical database. Inclusion criteria included: a diagnosis of endometrial carcinoma (including endometrioid, clear cell, papillary serous, and/or carcinosarcoma), either primary surgery or secondary staging procedure by one of 12 gynecologic oncologists practicing during the study window, and Stage I disease (either by formal staging or by clinical impression in the absence of formal staging). Patients had to have undergone at least a complete hysterectomy to be included. Patients treated medically or with radiation alone were excluded from analysis. Additional exclusion criteria included stromal cancers (such as leiomyosarcoma or endometrial stromal sarcomas), synchronous tumors of the ovary and uterus (excluding borderline cancers of the ovary), metastatic involvement of the uterus from other cancers, and tumors of uncertain origin. All staging was standardized to FIGO 2009 criteria because surgical staging was changed during the study window.
We applied the following rules to standardize the groups: Patients who underwent more than one procedure, or who underwent conversion from an MIS approach to an open one, were categorized by the manner in which the uterus was removed. Patients who underwent any lymph node removal were considered to have undergone staging without regard to additional factors such as number of nodes, washings, omentectomy, or peritoneal biopsies. Patients with high-risk cell types (serous, clear cell, or carcinosarcoma) as their final diagnosis were categorized as grade 3; if an endometrioid tumor grade was listed across 2 categories (i.e., grade 2–3), the lower grade was reported.
Adjuvant therapies for patients with higher risk disease were at the discretion of the attending provider and were categorized as: none, vaginal brachytherapy (without pelvic irradiation), pelvic radiation (with or without vaginal brachytherapy), and chemotherapy (with or without radiation). Time to recurrence was measured from the date of surgery to the date of either pathologic or radiologic confirmation of recurrence, if the latter was used to inform treatment decisions. During the study window, the group practice was to evaluate patients in remission every 3–6 months for 2 years, followed by every 6 months to complete 5 years of total follow-up. The routine examination was clinical without the use of cytology or radiology in the absence of signs/symptoms of recurrence. Patients in follow-up at outside facilities were reviewed electronically or through shared notes; and were considered without evidence of disease if no new diagnoses of cancer or therapeutic treatments were initiated. Time to death was measured from surgery to death as determined in the medical or public records. Cause of death was categorized as due to disease or other cause. Deaths in the immediate post-operative period were considered death due to disease. All patients were censored at their last date of contact.
Sample size was dictated by the study window; as this was a retrospective analysis, power calculations were not undertaken. The primary outcome was recurrence-free survival (RFS). Secondary outcomes included: disease-specific survival (DSS) and overall survival (OS) as measured from the time of surgery. OS was calculated from the date of diagnosis to date of death or censored at the date of last follow-up if still alive or at 10 years. RFS was calculated from date of diagnosis to the date of first known recurrence or death, or censored at date of last follow-up if recurrence-free and alive or at 10 years.
Patient demographic and clinical data were summarized using descriptive statistics; means ± standard deviations (SD) and percentages are presented unless otherwise noted. OS and RFS were summarized using Kaplan-Meier methods and compared by surgery type using log-rank tests. We calculated confounding weights using an inverse probability of treatment weighting (IPTW) model to balance measured covariates. We estimated the propensity scores for undergoing robotic surgery using a logistic regression model and computed the inverse probability weight by using the inverse propensity score. The variables used to calculate the propensity scores were age at procedure (years), body mass index (BMI, kg/m2), disease stage (IA/IB), grade (1/2/3), histology (endometrioid/other), and receipt of adjuvant therapy (yes/no). Weights were calculated as the inverse of the propensity score. We assessed the weighted distributions of these variables to confirm balance; all were balanced with p-values >0.80 for comparisons by surgery type. Cox proportional hazards regression models accounting for weights were conducted to compare OS and RFS by surgery type.
We also compared disease-specific death between surgery groups using Fine and Gray’s sub-distribution hazard model to account for competing risks. We conducted a series of sensitivity analyses adding grade by surgery type interactions and further stratifying by grade and, to assess for the possible presence of a “learning curve bias” (that is that early RA-MIS patients were harmed by their surgeons’ relative in-experience), by excluding the first 20 robotic cases for each surgeon. Hazard ratios (HR) and 95% confidence intervals (CI) are reported unless otherwise noted. Data were analyzed using SAS 9.4 (Cary, NC) and p-values less than 0.05 were considered statistically significant.
3. Results
We identified 1142 patients who underwent treatment for surgical management of Stage I endometrial cancer during the study window; 1027 had sufficient data for the analysis, 461 (44.9%) underwent laparoscopy and 566 (55.1%) underwent RA-MIS. The proportion of cases employing RA-MIS increased steadily during the study window (Fig. 1). As a result, the median follow-up for patients undergoing laparoscopy was significantly longer than for patients who underwent RA-MIS, 8.7 years (Interquartile range (IQR) 5.3, 10.0) versus 6.3 years (IQR 4.6, 7.9) respectively (p < 0.001).
Fig. 1.
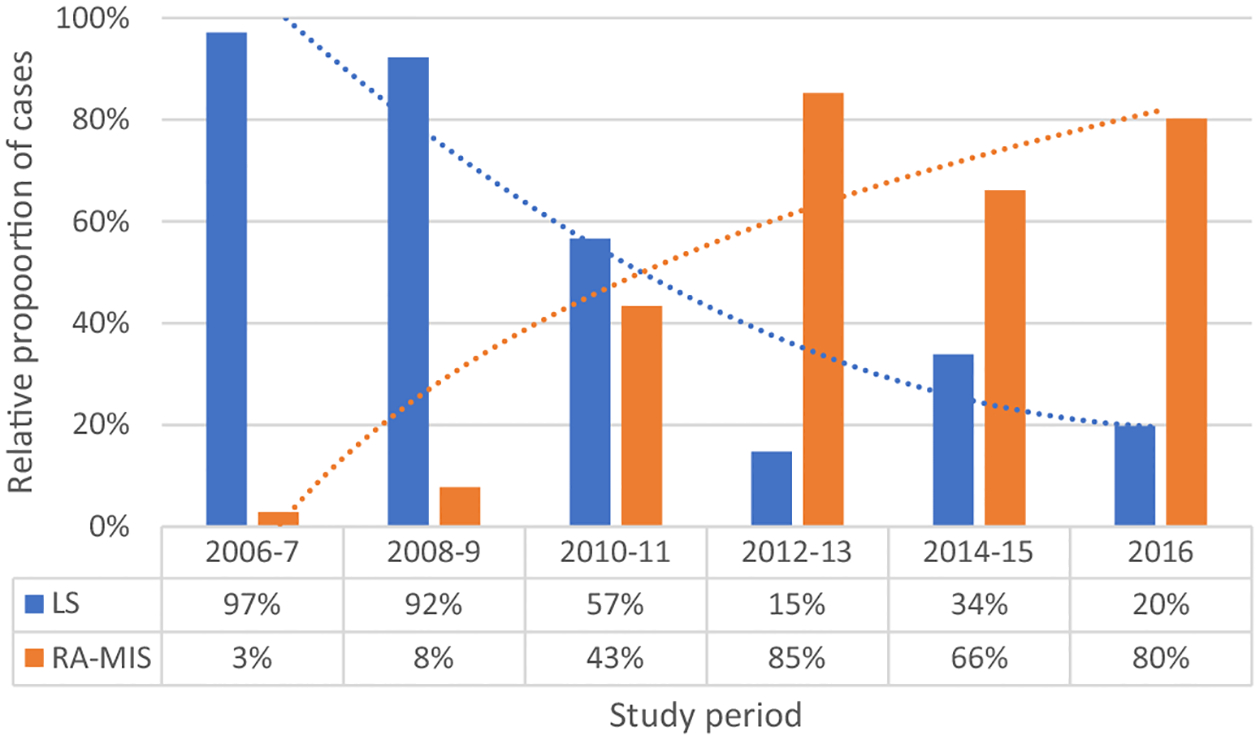
Trends in surgical strategy used over time.
LS, laparoscopic; RA-MIS, robot-assisted minimally invasive surgery.
Patients were on average 62 years old at the time of surgery, and had primarily grade 1 disease with endometrioid histology. (Table 1). The mean BMI rose throughout the study window, but patients in the RA-MIS were heavier overall (mean BMI 35.9 versus 33.3, p < 0.001). Surgical staging was performed in 67.7% of patients over the study period. The rate of surgical staging dropped following publication of the ASTEC trial, but fell by similar rates in both the laparoscopic (89.1% prior to 2010 versus 63.5% following 2010) and RA-MIS groups (84.6% prior to 2010 versus 62.5% following 2010) [11]. Sentinel lymph node sampling was rare during the study window (10 and 6 patients in the laparoscopy and RA-MIS arms respectively) and was not analyzed separately. Cross-over from one route of surgery was rare (approximately 1/250 cases), and consisted largely of conversion to open surgery, therefore no “intent to treat” analyses were performed.
Table 1.
Patient demographic and clinical characteristics by surgery type.
| Robotic N = 566 | Laparoscopic N = 461 | ||||
|---|---|---|---|---|---|
| Variable | N | Mean (SD) | N | Mean (SD) | p-value |
| Age at surgery, years | 566 | 62.3 (10.2) | 461 | 61.7 (10.6) | 0.35 |
| Body Mass Index, kg/m2 | 622 | 35.8 (9.2) | 461 | 33.4 (9.1) | <0.0001 |
| N | % | N | % | p-value | |
| Stage | 0.55 | ||||
| IA | 478 | 55.5 | 383 | 44.5 | |
| IB | 88 | 53.0 | 78 | 47.0 | |
| Grade | 0.04 | ||||
| 1 | 383 | 57.5 | 283 | 42.5 | |
| 2 | 105 | 47.7 | 115 | 52.3 | |
| 3 | 78 | 55.3 | 63 | 44.7 | |
| Histology | |||||
| Carcinosarcoma | 6 | 60% | 4 | 40% | |
| Clear cell | 7 | 58.3% | 5 | 41.7% | |
| Endometrioid | 521 | 50.7% | 418 | 40.7% | |
| Serous | 14 | 46.7% | 16 | 53.3% | |
| Undifferentiated | 0 | 0% | 3 | 100% | |
| Mixed | 17 | 54.8% | 14 | 45.2% | |
| Other/Missing | 1 | 50% | 2 | 50% | |
| Adjuvant Therapy | 0.70 | ||||
| No | 445 | 54.8 | 367 | 45.2 | |
| Yes | 121 | 56.3 | 94 | 43.7 | |
| Unknown | 1 | 4 | |||
| Type of Adjuvant Therapy | 0.53 | ||||
| None | 445 | 54.8 | 367 | 45.2 | |
| Vaginal brachytherapy (VBRT) alone | 67 | 60.4 | 44 | 39.6 | |
| Pelvic radiation +/− VBRT | 17 | 47.2 | 19 | 52.8 | |
| Chemotherapy +/− Radiation | 37 | 54.4 | 31 | 46.6 | |
Use of adjuvant treatment was not standardized during the study period. Overall use of adjuvant treatment was balanced, with 18.2% of laparoscopy patients versus 20.4% of RA-MIS patients receiving some form of adjuvant therapy following surgery (p = 0.70).
3.1. Outcomes
A total of 88 patients (8.6%) experienced recurrence during the study window. These included 30 patients (6.5%) and 58 patients (10.3%) in the laparoscopy and RA-MIS groups respectively. Patients treated via RA-MIS had poorer RFS compared to those treated via laparoscopy (p = 0.04), with some divergence by year 2 (proportion surviving disease free 0.92 [95% CI: 0.90, 0.94] vs. 0.96 [95 CI: 0.94, 0.97]) and sustained at year 10 (0.78 [95% CI: 0.73, 0.83] vs. 0.84 [95% CI: 0.80, 0.88]; Fig. 2). This relationship remained after accounting for confounding weights (adjusted HR: 1.41 [95% CI: 1.12, 1.77], p = 0.004; Table 2).
Fig. 2.
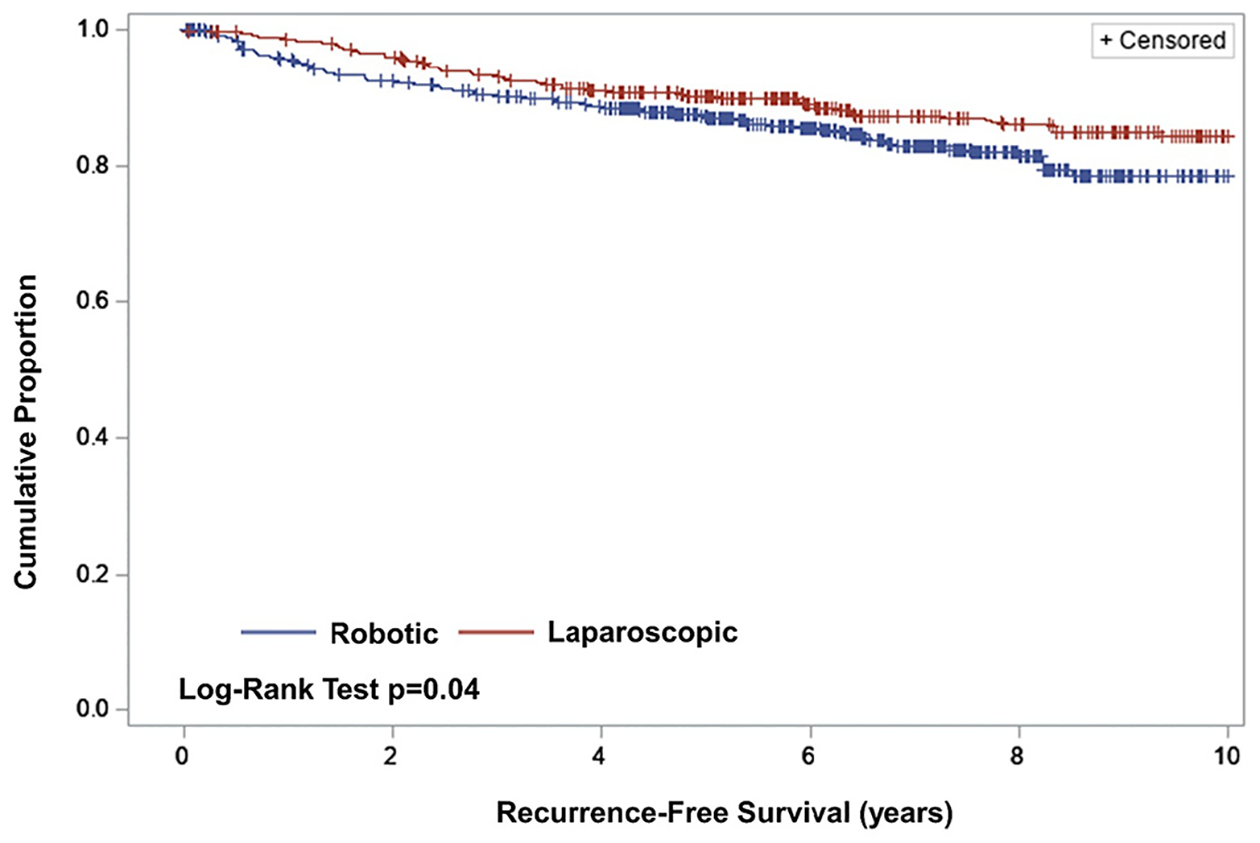
Recurrence-free survival by route-of-surgery.
Table 2.
Recurrence-free and overall survival for RA-MIS versus laparoscopy.
| Outcome | Hazard Ratio (95% CI) | p-value |
|---|---|---|
| Recurrence-free survival | 1.41 (1.12,1.77) | 0.004 |
| Overall Survival | 1.39 (1.06,1.83) | 0.02 |
Among patients who recurred, median time to first recurrence was shorter in the RA-MIS group than the laparoscopy group (16.3 vs. 28.7 months, p = 0.07). Though this may be an artifact of the longer duration of follow-up in the laparoscopy group both groups were followed greatly in excess of these estimates. Overall, 68% of recurrences in the laparoscopy arm and 55% in the RA-MIS group occurred greater than 24 months after surgery, while 28% and 24% respectively occurred after at least 5 years. These data suggest that previously reported 24–36 month outcomes data may be underestimating the long term impact of “route-of-surgery” decisions on clinically relevant outcomes in patients with early stage endometrial cancer. Furthermore, given that the median follow-up for the laparoscopy group is nearly 3-years longer, it is possible that the current study underestimates the relative hazard of RA-MIS, by virtue of fewer women/years at risk.
A total of 107 patients died during the study period, including 1 death in the immediate post-operative period, occurring in the laparoscopy group. The results for OS were similar to RFS, though less pronounced in the early period (Fig. 3). RA-MIS was associated with a significantly increased risk of death (HR: 1.39 [95% CI: 1.06, 1.83], p = 0.02; Table 2) when compared to patients treated with laparoscopy. Unsurprisingly for this lower-risk population, death from disease was uncommon, occurring in 12 patients (2.6%) in the laparoscopy group and 39 (6.9%) patients in the RA-MIS group. Competing risks analyses accounting for deaths from other causes indicated significantly worse DSS in the RA-MIS group (adjusted HR: 3.51 [95% CI: 2.19, 5.63]), suggesting that death from intercurrent ailments did potentially blunt the observed effect of RA-MIS on OS. Sensitivity analyses examining differences by grade (Table 3) and surgeon training by excluding early cases did not change conclusions (RFS HR = 1.59 [95% CI: 1.22–2.08], p = 0.0006; OS HR 1.64 [95%CI 1.20–2.25], p = 0.002).
Fig. 3.
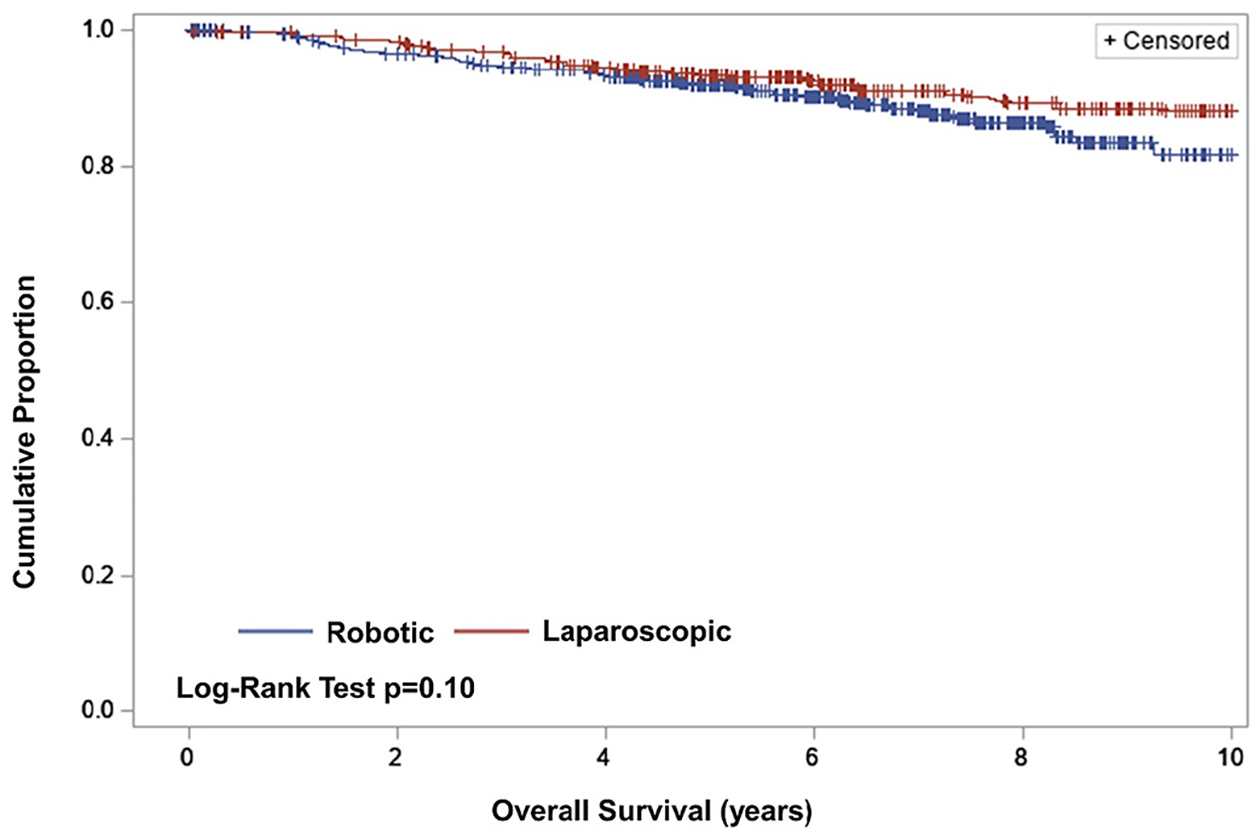
Overall survival by route-of-surgery.
Table 3.
Recurrence-free and overall survival for RA-MIS versus laparoscopy by grade.
| Recurrence-free survival Hazard Ratio (95% CI) |
Overall survival Hazard Ratio (95% CI) |
|
|---|---|---|
| Grade | ||
| 1 | 1.62 (1.14, 2.29) | 1.47 (0.94, 2.32) |
| 2 | 1.35 (0.89, 2.05) | 1.41 (0.88, 2.24) |
| 3 | 1.20 (0.76,1.90) | 1.31 (0.79,2.16) |
4. Discussion
We found that RA-MIS was associated with poorer clinical outcomes when compared to laparoscopy in the treatment of Stage I endometrial cancer. Though the global prognosis for patients with Stage I disease was predictably favorable, patients undergoing an RA-MIS route-of-surgery recurred more often, more quickly, and were more likely to die from their cancer. Our data further demonstrate that important clinical outcomes (recurrence and death) frequently occur outside of the of the 2–3 year window of follow-up which has been reported in the largest studies to date.
Between 1995 and 2010 minimally invasive surgery largely supplanted laparotomic staging for clinical Stage I endometrial cancer owing to obvious benefits with regard to surgical morbidity, post-operative recovery, patient satisfaction, and cost efficacy [12]. Two large, collaborative-group studies (LAP2 and the LACE study) were performed to establish safety and efficacy profiles for minimally invasive management of early stage endometrial cancer [2,13]. In 2009, Walker et al. reported the primary safety endpoints from LAP2, which demonstrated that despite longer operative times, laparoscopy was associated with decreased post-operative morbidity, shorter hospitalization, and similar completeness of staging. In 2012 however, the efficacy reports from LAP2 failed to demonstrate non-inferiority of laparoscopy with regard to RFS after a median of 59 months of follow-up (HR 1.14 with 90% lower bound 0.92, 95% upper bound 1.46) finding instead a modest 1.1% increase in risk of recurrence and without observed detriment to OS [13]. The LAP2 efficacy findings were largely supported by the LACE study, a second prospective, multi-center study assessing long-term outcomes after randomizing patients with clinical Stage I endometrioid endometrial cancer to surgical staging via either laparotomy or laparoscopy [14]. In this relatively low risk group, RFS at a median of 4.5 years was considered equivalent at 82% and 81% respectively, with no difference in overall survival. Despite the fact that both studies failed to demonstrate non-inferiority, both concluded that any relative increase in risk caused by converting from laparotomy to laparoscopy would be small, and potentially offset by reduced peri-operative morbidity.
The contemporaneous publication of these studies with the wide-spread adoption of RA-MIS led many surgeons to convert to the RA-MIS route based on presumed equivalency and multiple small series demonstrating short-term safety. Two recent studies in cervical cancer however call into question the long-term equivalency of “route-of-surgery” decisions. The LACC trial reported by Ramirez et al. demonstrated inferior RFS and OS in patients with surgically resectable cervix cancer who underwent minimally invasive surgery (84% via laparoscopy) versus laparotomy, despite similar intra-operative and peri-operative outcomes [9]. In reporting the retrospective data from the National Cancer Database, Melamed et al. found similarly poorer outcomes for patients undergoing minimally invasive surgery; but notably observed that while patients who underwent RA-MIS were significantly more likely to recur than patients treated via laparotomy (HR: 1.61, 95% CI: 1.18, 2.21), patients treated via laparoscopy were not (HR: 1.50, 95% CI: 0.97, 2.31). Interrupted time series analysis of these data demonstrated a significant decline in trends of relative survival between 2006 and 2010 which corresponds both to the adoption of minimally invasive radical hysterectomy and the adoption of RA-MIS in the present study [10].
Long term outcomes data comparing RA-MIS to laparoscopy in Stage I endometrial cancer are scarce. We were able to identify four moderate to large studies comparing the outcomes of patients treated with laparoscopy or RA-MIS. Notably, three of these found no significant difference in RFS or OS, but are limited by a number of factors, most notably a relatively short median follow-up and inclusion of higher stage disease [15–17]. The median reported follow-up in these studies ranged from 17.7 months to 38 months, which would have failed to detect significant portions (in some cases more than half) of the PFS and OS events seen in our study population. Similarly, combining the outcomes of higher and lower stage populations would potentially blunt the effect of the “route of surgery” decision in stage I patients – reflecting the significantly greater event rate in higher stage disease. Lastly, DSS was not addressed in these studies, perhaps reflecting the tendency of these patients to die of medical comorbidities, but was the most notable risk associated with RA-MIS in our study, after adjusting for competing risks.
The current study supports the conclusions of Song et al., who observed a significantly higher rate of relapse following RA-MIS than laparotomy at a median of 4.7 years of follow-up (10.4% versus 0% respectively, p = 0.005) for patients with Stage I endometroid endometrial cancers. Though overall survival was not different between the groups, it is notable that 5 of 8 recurrences in the RA-MIS group involved distant spread despite low-risk features, suggesting a poorer prognosis [18].
The current trial was not designed to identify the root cause of disparate outcomes, however multiple potential explanations implicated in the discrepancy between laparoscopy and “open” approaches, such as the use of a uterine manipulator, increased atmospheric pressure, and/or carbon dioxide exposure, were balanced in the present study, suggesting they play a less crucial role in the development of recurrence. The effect of prolonged relative operating times, and thus Trendelenburg positioning, as well as the potential effect of the absence of tactile sensation on tissue manipulation are poorly studied but may impact the two groups differently.
Our study benefits from both large sample size and long-term follow-up. Together these allow for analysis of less-common and delayed outcomes such as DSS, which are difficult to easily assess in prospective studies owing to operational costs and competing risks. The fact that the clinically meaningful outcomes (time to recurrence, RFS, OS and DSS) all demonstrated similar findings, with progressively increasing hazard ratios, suggests that shorter, smaller studies may miss the impact of “route-of-surgery” decisions. This is particularly relevant for diseases with favorable short-term outcomes and/or slow growth, as with most low-stage endometrial cancers. It is also encouraging that patients in our laparoscopy group experienced similar 3- and 5-year outcomes to those reported in the LAP2 trial, suggesting that the single-group nature of the study was not likely a biasing factor.
There are, however, multiple potential limitations that must be considered when evaluating this study. Though the patients were identified prospectively, the data was acquired retrospectively, subjecting it to typical input and omission biases. The IPTW approach assumes all confounding has been adjusted for in the model; while we have adjusted for all confounders that were measured, it is unlikely that we have fully accounted for differences between groups. There was no centralized review of the pathology, but as the reports were made prospectively, in advance of possible knowledge about recurrence, it would seem unlikely that this is a significant source of bias. Most patients had at least some follow-up at our institution, however there is some loss to follow-up which can lead to bias, however the numbers are relatively low and proportionally balanced between the groups, suggesting that poor follow-up was not likely a function of route-of-surgery. Lastly, while we tried to explore the possibility of a “learning curve” bias in the analysis, it is still possible that surgeons were more proficient (i.e. higher on the learning curve) with laparoscopy than RA-MIS during the study window by virtue of laparoscopy’s earlier incorporation into practice. Importantly our long-term laparoscopic outcomes are similar to those observed in LAP2, while the long-term RA-MIS outcomes are similar to those reported by Sun et al. suggesting these results may be typical.
Recent reports have suggested that using an RA-MIS route may increase the feasibility of minimally invasive surgery in obese and morbidly obese patients [19]. Because surgery route was not randomized, and all surgeons performed at least some laparoscopic and some RA-MIS surgeries during the study window, it is possible that unmeasured confounding variables, such as body mass index (BMI) or comorbidity indices biased the route-of-surgery decision; however, given the global transition from laparoscopy to RA-MIS during the study period, BMI was not likely a primary driver in the route-of-surgery decision. Similarly, we did not evaluate the impact of all known pathologic risk factors (lymph-vascular space involvement, positive washings, lower uterine segment involvement, etc), but as these would have been determined after the route-of surgery decision was executed, it is assumed that they could not bias the relationship between route-of surgery decision and clinical outcome.
Lastly, but importantly, the use of adjuvant treatment was non-standardized during this study. Given the recent suggestions that at least a subset of patient with Stage I endometrial cancer benefit from adjuvant therapy, it is possible that these choices could have biased our findings [20]. It should be noted however that the groups were relatively balanced for reported risk factors and that both the use of adjuvant therapy and the rates of adverse outcomes were higher in the RA-MIS group despite shorter follow-up.
5. Conclusions
Our data suggests that RA-MIS was negatively associated with multiple meaningful clinical outcomes, including OS and DSS, when compared with laparoscopy. Though the reasons for the discrepancies cannot be directly elucidated, these data add to a developing body of literature suggesting that route-of-surgery decisions may have unanticipated long-term consequences, despite similarity in short-term safety and efficacy. Taken together, these data support the development of a prospective, randomized trial examining the impact route-of-surgery decisions on clinical outcomes. Our data further demonstrate that meaningful outcomes (recurrence and death) continue to occur for years after surgery; as such, future studies in early-stage endometrial cancer may benefit from prolonging the duration of follow-up.
HIGHLIGHTS.
RA-MIS in early endometrial cancer was associated with poorer overall and disease specific survival than laparoscopy.
Differences in clinical outcomes persist after correction for known risk factors.
Delayed clinical endpoints (recurrence and death) were common, suggesting typical 24 and 60 month study endpoints are suboptimal.
Footnotes
Declaration of Competing Interest
None of the authors have conflicts, financial or otherwise, with regard to this manuscript.
References
- [1].Siegel RL, Miller KD, Fuchs HE, Jemal A, Cancer statistics, 2021, Cancer J. Clin 71 (2021) 7–33. [DOI] [PubMed] [Google Scholar]
- [2].Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, Spiegel G, Walker R, Barakat, Michael L. Pearl, Sudarshan K. Sharma, Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2, J. Clin. Oncol 27 (32) (2009) 5331–5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bergstrom J, Aloisi A, Armbruster S, Yen TT, Casarin J, Leitao MM, Tanner EJ, Matsumo R, Machado KK, Dowdy SC, Soliman PT, Wethington SL, Stone RL, Levinson KL, Fader AN, Minimally invasive hysterectomy surgery rates for endometrial cancer performed at National Comprehensive Cancer Network (NCCN) Centers, Gynecol. Oncol 148 (3) (2018) 480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Casarin J, Song C, Multinu F, Cappuccio S, Liu E, Butler KA, Glaser GE, Cliby WA, Langstraat CL, Ghezzi F, Fu AZ, Mariani A, Implementing robotic surgery for uterine cancer in the United States: better outcomes without increased costs, Gynecol. Oncol 156 (2) (2020. Feb) 451–458. [DOI] [PubMed] [Google Scholar]
- [5].Wright JD, Burke WM, Wilde ET, Lewin SN, Charles AS, Kim JH, Goldman N, Neugut AI, Th Herzog TJ, Hershman DL, Comparative effectiveness of robotic versus laparoscopic hysterectomy for endometrial cancer, J. Clin. Oncol 30 (8) (2012) 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wright JD, Ananth CV, Lewin SN, Burke WM, Lu YS, Neugut AI, Herzog TJ, Hershman DL, Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease, JAMA 309 (7) (2013) 689–698. [DOI] [PubMed] [Google Scholar]
- [7].Johnson L, Bunn WD, Nguyen L, Rice J, Raj M, Cunningham MJ, Clinical comparison of robotic, laparoscopic, and open hysterectomy procedures for endometrial cancer patients, J. Robot. Surg 11 (3) (2017) 291–297. [DOI] [PubMed] [Google Scholar]
- [8].Eoh KJ, Nam EJ, Kim SW, Shin M, Kim SJH, Kim JA, Kim YT, Nationwide comparison of surgical and oncologic outcomes in endometrial cancer patients undergoing robotic, laparoscopic, and open surgery: a population-based cohort study, Cancer Res. Treat 53 (2) (2021) 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, Buda A, Yan X, Shuzhong Y, Chetty N, Isla D, Tamura M, Zhu T, Robledo KP, Gebski V, Asher R, Behan V, Nicklin JL, Coleman RL, Obermair A, Minimally invasive versus abdominal radical hysterectomy for cervical cancer, N. Engl. J. Med 379 (20) (2018. Nov 15) 1895–1904. [DOI] [PubMed] [Google Scholar]
- [10].Alexander Melamed A, Margul DJ, Chen L, Keating NL, del Carmen MG, Yang J, Seagle BLL, Alexander A, Barber EL, Rice LW, Wright JD, Kocherginsky M, et al. , Survival after minimally invasive radical hysterectomy for early-stage cervical cancer, N. Engl. J. Med 379 (2018) 1905–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].ASTEC study group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK, Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomized study, Lancet 373 (9658) (2009) 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kornblith AB, Huang HQ, Walker JL, et al. , Quality of life of patients with endometrial cancer undergoing laparoscopic staging compared to laparotomy: A gynecologic oncology group study, J. Clin. Oncol 27 (32) (2009. Nov 10) 5337–5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, Barakat R, Pearl ML, Sharma SK, Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study, J. Clin. Oncol 30 (7) (2012. Mar 1) 695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Janda M, Gebski V, Davies LC, Forder P, Brand A, Hogg R, Jobling TW, Land R, Manolitsas T, Nascimento M, Neesham D, Nicklin JL, Oehler MK, Otton G, Perrin L, Salfinger S, Hammond I, Leung Y, Sykes P, Ngan H, Garrett A, Laney M, Ng TY, Tam K, Chan K, Wrede CD, Pather S, Simcock B, Farrell R, Robertson G, Walker G, Armfield NR, Graves N, McCartney AJ, Obermair A, Effect of Total laparoscopic hysterectomy vs total abdominal hysterectomy on disease-free survival among women with stage I endometrial cancer: A randomized clinical trial, JAMA 317 (12) (2017) 1224–1233. [DOI] [PubMed] [Google Scholar]
- [15].Chamber LM, Carr C, Freeman L, Jernigan A, Michener C, Does surgical platform impact recurrence and survival? A study of utilization of multiport, single port, and robotic assisted laparoscopy in endometrial cancer surgery, Am J Obstet Gyecol 221 (243) (2019) e1–11. [DOI] [PubMed] [Google Scholar]
- [16].Perrone E, Capasso I, Pacciuto T, Gioe A, Alletti SG, Restaino S, Scambia G, Fanfani F, Laparoscpy vs. robotic-assited laparoscopy in endometrial cancer staging: large retrospective single institution study, J. Gynecol. Oncol 32 (3) (2021) e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cardenas-Goicoechea J, Shepard A, Momeni M, Mandeli J, Chuang L, Gretz H, Fishman D, Rahaman J, Randall T, Survival analysis of robotic versus traditional laparoscopic staging for endometrial cancer, Am. J. Obstet. Gynecol 210 (160) (2014) e1–11. [DOI] [PubMed] [Google Scholar]
- [18].Song J, Le T, Hopkins L, Fung-Kee-Fung M, Lupe K, Gaudet M, C E, Samant R, A comparison of disease recurrence between robotic versus laparotomy approach in patients with intermediate-risk endometrial cancer, Int. J. Gynecol. Cancer 30 (2) (2020) 160–166. [DOI] [PubMed] [Google Scholar]
- [19].El-Achi V, Weishaupt J, Carter J, Saidi S, Robotic versus laparoscopic hysterectomy in morbidly obese women for endometrial cancer, J. Robot. Surg 15 (2021) 483–487. [DOI] [PubMed] [Google Scholar]
- [20].Son J, Chambers LM, Carr C, Michener CM, Yao M, Beavis A, Yen TT, et al. , Adjuvant treatment improves overall survival in women with high-intermediate risk early-stage endometrial cancer with lymphovascular space invasion, Int. J. Gynecol. Cancer 30 (11) (2020) 1738–1747. [DOI] [PubMed] [Google Scholar]


