Abstract
Background and objective
Endosonography with intrathoracic nodal sampling is proposed as the single test with the highest granuloma detection rate in suspected sarcoidosis stage I/II. However, most studies have been performed in limited geographical regions. Studies suggest that oesophageal endosonographic nodal sampling has higher diagnostic yield than endobronchial endosonographic nodal sampling, but a head‐to‐head comparison of both routes has never been performed.
Methods
Global (14 hospitals, nine countries, four continents) randomized clinical trial was conducted in consecutive patients with suspected sarcoidosis stage I/II presenting between May 2015 and August 2017. Using an endobronchial ultrasound (EBUS) scope, patients were randomized to EBUS or endoscopic ultrasound (EUS)‐B‐guided nodal sampling, and to 22‐ or 25‐G ProCore needle aspiration (2 × 2 factorial design). Granuloma detection rate was the primary study endpoint. Final diagnosis was based on cytology/pathology outcomes and clinical/radiological follow‐up at 6 months.
Results
A total of 358 patients were randomized: 185 patients to EBUS‐transbronchial needle aspiration (EBUS‐TBNA) and 173 to EUS‐B‐fine‐needle aspiration (FNA). Final diagnosis was sarcoidosis in 306 patients (86%). Granuloma detection rate was 70% (130/185; 95% CI, 63–76) for EBUS‐TBNA and 68% (118/173; 95% CI, 61–75) for EUS‐B‐FNA (p = 0.67). Sensitivity for diagnosing sarcoidosis was 78% (129/165; 95% CI, 71–84) for EBUS‐TBNA and 82% (115/141; 95% CI, 74–87) for EUS‐B‐FNA (p = 0.46). There was no significant difference between the two needle types in granuloma detection rate or sensitivity.
Conclusion
Granuloma detection rate of mediastinal/hilar nodes by endosonography in patients with suspected sarcoidosis stage I/II is high and similar for EBUS and EUS‐B. These findings imply that both diagnostic tests can be safely and universally used in suspected sarcoidosis patients.
Keywords: bronchoscopy and interventional techniques, diagnostic accuracy, EBUS, endoscopic ultrasound using the EBUS scope, endosonography, EUS‐B, sarcoidosis
Short abstract
This global RCT in patients with suspected sarcoidosis stage I/II with an indication for endosonographic nodal sampling showed a similarly high granuloma detection rate and sensitivity for diagnosing sarcoidosis with endobronchial ultrasound versus endoscopic ultrasound‐B. The findings imply that both diagnostic tests (endobronchial/oesophageal) can be used safely and universally in suspected sarcoidosis patients.
INTRODUCTION
Sarcoidosis is a systemic disease of unknown cause that is characterized by the formation of immune granulomas in various organs, mainly the lungs and the lymphatic system. 1 Given that the clinical manifestations of sarcoidosis are often non‐specific, demonstration of tissue granulomas is often required to establish the diagnosis. 2 Transoesophageal endoscopic ultrasound‐guided fine‐needle aspiration (EUS‐FNA) of mediastinal lymph nodes has a high diagnostic yield in patients with suspected sarcoidosis. 3 , 4 , 5 Since the development of endobronchial ultrasound‐transbronchial needle aspiration (EBUS‐TBNA) using a bronchoscope in 2004, this procedure has also proved to be successful for granuloma detection. Besides, EBUS‐TBNA has a higher diagnostic yield compared to conventional bronchoscopic techniques including (trans)bronchial lung biopsies (TBLB). 6 , 7 , 8 Frequently, both the hilar and mediastinal lymph nodes are affected in sarcoidosis, with the mean number of affected lymph nodes of 8, including the subcarinal lymph nodes in all cases. 9 Therefore, affected sarcoid lymph nodes can be sampled from both the oesophagus and the airways. EUS‐B‐guided nodal sampling is better tolerated by patients (no cough/shortness of breath) and often easier to perform in comparison to EBUS (absence of cartilage rings). 10 , 11 Several previous studies have compared endobronchial (EBUS‐TBNA) and transoesophageal (EUS‐FNA) approaches for indications such as undiagnosed mediastinal lymphadenopathy 10 or mediastinal staging of lung cancer, 12 but no such head‐to‐head comparisons have been performed in patients with suspected sarcoidosis.
Granuloma assessment on cytological specimens obtained by conventional FNAs can be challenging for pathologists. It is postulated that biopsy needles (ProCore) allow tissue acquisition including core biopsies—in addition to the cytological aspirates—thereby increasing the granuloma detection rate compared to conventional needles.
We performed a randomized clinical trial, comparing intrathoracic nodal sampling from the airways (EBUS) with an oesophageal (EUS‐B) approach, for the detection of non‐caseating granulomas in patients with suspected pulmonary sarcoidosis stage I/II using an EBUS scope. Additionally, patients were also randomized between the conventional 22‐G needle (EchoTip® Ultra Endobronchial HD Ultrasound Needle, Cook Medical, Limerick, Ireland) or the thinner 25‐G biopsy needle (EchoTip ProCore® Endobronchial HD Biopsy Needle, Cook Medical). 13 Based on previous studies, we hypothesized that EUS‐B sampling of intrathoracic lymph nodes has a higher granuloma detection rate compared to EBUS‐guided nodal sampling in patients with suspected sarcoidosis stage I/II.
METHODS
Study design
The International Sarcoidosis Assessment (ISA) trial is a prospective investigator‐initiated, unblinded, randomized clinical trial using a 2 × 2 factorial design. In this global study (14 hospitals, nine countries, four continents), patients were included in both university and general hospitals. Data were entered with web‐based case report forms. The study was registered under number NCT02540694 at ClinicalTrials.gov. This manuscript is reported according to the STARD 2015 (Standards for Reporting Diagnostic Accuracy studies) statement 14 and CONSORT (Consolidated Standards of Reporting Trials) statement.
Study participants
Consecutive patients, 18 years or older, with a clinical and radiological suspicion of sarcoidosis stage I (mediastinal or hilar lymphadenopathy) or stage II (lymphadenopathy and parenchymal abnormalities) and an indication for tissue verification of non‐caseating granulomas were eligible. Patients meeting any of the following criteria were excluded from participation in this study: obvious organ involvement of sarcoidosis with the possibility to confirm granulomas with a minimally invasive diagnostic procedure (e.g., skin lesion or superficial lymph node), pregnancy, a positive acid‐fast bacilli sputum test, contra‐indication for endosonography or a life expectancy of less than 6 months. Patients were recruited in outpatient clinics of respiratory medicine departments of participating hospitals. Diagnostic work‐up consisted of a conventional clinical evaluation (including medical history, physical examination and laboratory tests) with radiography and contrast‐enhanced computed tomography (CT) of the chest. The decision to obtain tissue for diagnostic purposes versus a clinical and radiological follow‐up was made in dialogue between the treating physician and the patient, weighing the risks and benefits of biopsy. The indication to obtain tissue was either to confirm sarcoidosis, to rule out other diseases, or before the start of treatment. Candidates for study participation were identified in 14 university and regional hospitals in the Netherlands, Belgium, Italy, Poland, Turkey, China, Japan, Canada and Australia. This investigator‐initiated trial was approved by the human research ethics committee or local institutional review board at each centre. This study was conducted in accordance with the amended Declaration of Helsinki. Written informed consent was obtained from every participant before randomization.
Study endpoints
The pre‐specified primary endpoint was the granuloma detection rate of EBUS‐guided sampling of intrathoracic lymph nodes compared to EUS‐B (using the EBUS scope)‐guided sampling, defined as the number of patients with granulomas detected relative to the total number of patients tested.
The pre‐specified secondary study endpoints were: (1) sensitivity of EBUS‐TBNA and EUS‐B‐FNA for diagnosing sarcoidosis, defined as the proportion of patients with granulomas relative to the total number of patients who received a final diagnosis of sarcoidosis; (2) granuloma detection rate of conventional 22‐G needle versus 25‐G ProCore needle in the overall population and in the EBUS and EUS‐B subgroups; (3) complication rate; (4) procedure duration (in minutes); and (5) sample quality of conventional 22‐G needle versus 25‐G ProCore needle in the overall population, defined as representative (i.e., [non‐]caseating granulomas, reactive lymph node or malignant disease) versus non‐representative samples (i.e., blood, squamous cells and bronchial epithelium).
Randomization
Both endoscopic procedures (EBUS and EUS‐B) were performed using a linear EBUS scope. Prior to endoscopy, patients were randomized using web‐based randomization software to an endobronchial (EBUS) or an oesophageal (EUS‐B) approach. In addition, patients in each arm were also randomized between the standard 22‐G COOK needle and the 25‐G bevel‐tipped COOK ProCore needle. We performed block randomization stratified by centre.
Endosonography
Sedation was performed following institutional practices; commonly, either deep sedation with propofol or mild sedation with low‐dose midazolam with or without opioids was used. All patients randomized to EBUS underwent a systematic endosonographic evaluation of accessible mediastinal/hilar nodes using the EBUS Assessment Tool (EBUSAT). 15 For patients randomized to EUS‐B, nodes were systematically assessed using the EUS Assessment Tool (EUSAT). 16 Endosonographic nodal characteristics were scored. Following endosonographic inspection, the most suspected, prominent and easily accessible nodes were selected for tissue sampling. In total, at least five nodal samples were taken per patient (at least four samples were sent in for cytology and one for microbiology). After insertion of the needle into the target lymph node, the stylet was pulled out slowly (slow pull technique). In case no or limited material was retrieved, suction using a 10‐ml syringe during the aspirations was advised for the next samples. Rapid on‐site cytological evaluation (ROSE) was available in some of the participating centres. In the absence of ROSE, it was advised to sample at least two different nodal stations, as this increases the diagnostic yield. 7 , 17 The aspirates were processed for both cytologic smears and cell block analysis. Cytology staining (Papanicolaou or Giemsa) and cell block preparation were performed following local practice. The local pathologist analysed nodal aspirates for clinical purposes. In addition, tissue samples were sent in routinely for Auramine/Ziehl–Neelsen staining, as well as culture and PCR testing (where available) for Mycobacterium tuberculosis.
Reference standard
Following endosonography—in the absence of granulomas—it was left to the local treating physician on how to proceed. A non‐diagnostic EBUS could be followed by EUS‐B or vice versa in the same session. Also, conventional bronchoscopy including TBLBs and biopsies of the bronchial mucosa could be performed in the same session.
Detection of granulomas does not equal diagnosing sarcoidosis, which also requires a compatible clinical and radiological manifestation and exclusion of other diseases capable of presenting similarly such as tuberculosis (TB) and lymphoma. 1 , 2 , 18 The diagnosis sarcoidosis was made by the (unblinded) treating physician based on the findings of the local pathologist and other available clinical information using the ERS/ATS/WASOG consensus statement. 19 The diagnosis of TB required a positive microbiology (PCR and/or culture) or a compatible clinic on baseline or follow‐up (worsening of typical clinical features, radiological features of progressive TB on CT) plus the resolution/improvement of clinical radiological findings during anti‐mycobacterial treatment. Clinical and radiological follow‐up was performed in all patients 6 months after inclusion to reassess the diagnosis. The diagnosis after 6 months as determined by the treating physician was considered the reference standard for the diagnosis of sarcoidosis.
Statistical analysis
Based on previous studies, we assumed that the sensitivity for granuloma detection of EBUS‐TBNA and EUS‐B‐FNA would be 75% and 90%, 8 , 20 respectively, and the sarcoidosis prevalence would be 90%. This would result in a granuloma detection rate of 67% (0.75 × 0.90) in the EBUS arm and of 81% (0.9 × 0.9) in the EUS‐B arm. This required a sample size of 167 patients per group with a power of 1 − B = 0.80, α = 0.05, two‐sided testing. Compensating for 5% drop‐outs, we aimed to include 350 patients. Chi‐square tests were used for the comparison of categorical data between EBUS and EUS‐B, including comparison of the detection rate and sensitivity. Patients in whom endosonography was initiated but in whom a complete procedure could not be performed were also included in the analysis. Analysis were performed with SPSS statistical package version 24.0 (IBM corporation). A p value of <0.05 was considered statistically significant.
RESULTS
Between May 2015 and August 2017, 381 consecutive patients with suspected sarcoidosis stage I/II were assessed for eligibility. Ultimately, 358 patients were included and randomized: 185 patients for EBUS (n = 96 standard 22 G needle; n = 89 25 G ProCore needle) and 173 patients for EUS‐B (n = 82 standard 22 G needle; n = 91 ProCore 25 G needle). In four patients, a different needle type or size was used. According to the protocol, these patients were analysed in the group into which they were randomized. At baseline, patients in both groups were well balanced for the major clinical characteristics (Table 1). Fatigue (in 49%) and cough (in 41%) were the most prevalent symptoms. Study flow of enrolled patients is presented in Figure 1.
TABLE 1.
Clinical characteristics
| Characteristics | EBUS (n = 185) | EUS‐B (n = 173) |
|---|---|---|
| Age, median (IQR), years | 49 (39–60) | 50 (41–61) |
| Sex, no. (%) | ||
| Male | 112 (61) | 96 (56) |
| Race, no. (%) | ||
| Caucasian | 138 (75) | 137 (78.5) |
| Asian | 30 (16) | 25 (15) |
| African | 12 (6.5) | 10 (6) |
| Other | 4 (2) | 1 (0.5) |
| Unknown | 1 (0.5) | 0 (0) |
| Symptoms, no. (%) | ||
| Fatigue | 86 (47) | 91 (53) |
| Cough | 78 (42) | 67 (39) |
| Dyspnoea | 56 (30) | 52 (30) |
| Weight loss | 25 (14) | 28 (16) |
| Night sweats | 30 (16) | 35 (20) |
| Laboratory | ||
| ACE, median (IQR), U/L a | 53 (34–76) | 54 (28–82) |
| Radiology, no. (%) | ||
| Sarcoidosis stage based on chest radiograph | ||
| Stage I | 97 (52) | 95 (55) |
| Stage II | 48 (26) | 32 (18) |
| Stage III | 2 (1) | 0 (0) |
| No enlarged LNs | 9 (5) | 8 (5) |
| No chest radiograph performed | 29 (16) | 38 (22) |
| Sarcoidosis stage based on chest CT | ||
| Stage I | 89 (48) | 87 (50) |
| Stage II | 92 (50) | 84 (49) |
| Stage IV | 1 (0.5) | 0 (0) |
| No enlarged LNs | 3 (1.5) | 1 (0.5) |
| No chest CT performed | 0 (0) | 1 (0.5) |
| Short axis of the largest LN on chest CT, median (IQR), mm | 19 (15–23) | 18 (15–22) |
| Enlarged LN in reach of EBUS resp. EUS‐B, no. (%) | ||
| Yes | 179 (97) | 165 (95) |
| No | 3 (1.5) | 5 (3) |
| Unknown | 3 (1.5) | 3 (2) |
Abbreviations: ACE, serum angiotensin‐converting enzyme; CT, computed tomography; EBUS, endobronchial ultrasound; EUS, endoscopic ultrasound; IQR, interquartile range; LN, lymph node.
ACE (U/L) was determined in 69 patients in the EBUS group and 57 patients in the EUS‐B group, respectively.
FIGURE 1.
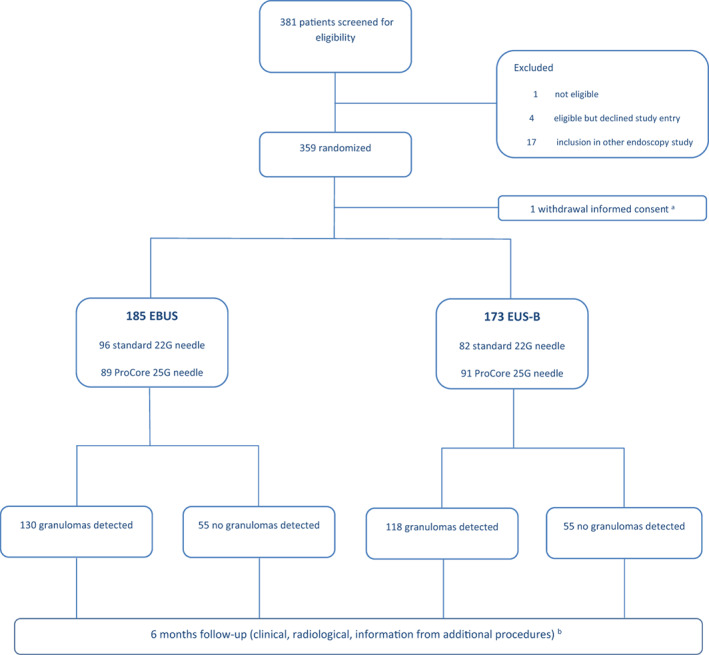
Flow diagram of enrolment and intervention in the International Sarcoidosis Assessment (ISA) trial. aOne patient refused endoscopy after randomization and was excluded from analysis. bSeventeen patients did not complete the study: three due to death and 14 lost to follow‐up
EBUS group
Among 185 patients undergoing an endobronchial procedure, the vast majority (179/185, 97%) of enlarged lymph nodes were located within the reach of EBUS, with a median short axis of the largest lymph node of 19 mm (interquartile range [IQR], 15–23). In 179 of 185 (97%) patients, the endoscopist was able to perform a complete systematic endosonographic evaluation of accessible mediastinal and hilar nodes using the EBUSAT 15 and perform five or more lymph node needle aspirations. The most prevalent ultrasound B‐mode characteristics identified for the lymph nodes in patients with a final diagnosis of sarcoidosis were hypervascularization in 43% (71/165), node clustering in 64% (105/165), oval shaped in 70% (115/165), homogeneity in 75% (123/165), distinct margins in 86% (142/165), absence of coagulation necrosis sign in 81% (134/165) and absence of central hilar structure in 70% (115/165) of patients. The mean number of sampled lymph node stations per patient was 2.2 (range, 1–4). Median procedure time was 20 min (IQR, 17–25). Prophylactic antibiotics were used in 31 (17%) of 185 patients. Further procedural endoscopy details are provided in Table 2. Of the 185 patients, 37 (20%) underwent an additional (endoscopy) procedure (e.g., EUS‐B [n = 25], bronchoscopy [n = 37], mediastinoscopy [n = 5]), resulting in granuloma detection in three additional patients not found by EBUS (one by EUS‐B, one by bronchoscopy and one by mediastinoscopy).
TABLE 2.
Endosonography findings
| Characteristics | EBUS (n = 185) | EUS‐B (n = 173) |
|---|---|---|
| Sedation, no. (%) a | ||
| None | 1 (0.5) | 2 (1) |
| Propofol | 74 (40) | 54 (31) |
| Midazolam | 117 (63) | 120 (69) |
| Opioid | 97 (52) | 90 (52) |
| General anaesthesia | 0 | 0 |
| Prophylactic antibiotics use, no. (%) | 31 (17) | 77 (45) |
| Endosonographic characteristics of sampled LNs with final diagnosis of sarcoidosis, no (%) b | ||
| Hypervascularized LN | 71 (43) | 61 (43) |
| Multiple clustered LN | 105 (64) | 95 (67) |
| Oval‐shaped LN | 115 (70) | 104 (74) |
| Homogeneous LN | 123 (75) | 117 (83) |
| Distinct margin LN | 142 (86) | 127 (90) |
| Absence of coagulation necrosis sign | 134 (81) | 125 (89) |
| Absence of hilar structure | 115 (70) | 111 (79) |
| Needle use randomization | ||
| Standard 22 G, no. (%) | 96 (52) | 82 (47) |
| ProCore 25 G, no. (%) | 89 (48) | 91 (53) |
| No. of sampled LN stations, mean (range) | 2.2 (1–4) | 1.8 (0–3) |
| On‐site cytological evaluation, no. (%) | 76 (41) | 67 (39) |
| Procedure time, median (IQR), min c | 20 (17–25) | 18 (14–24) |
| LN station (%) [median number of needle passes] | ||
| 2R | 2 (1) [1] | 2 (1) [3] |
| 2L | 1 (1) [3] | 0 (0) |
| 4R | 105 (57) [3] | 1 (1) [5] |
| 4L | 20 (11) [2] | 91 (53) [3] |
| 5 | 0 (0) | 0 (0) |
| 6 | 1 (1) [5] | 0 (0) |
| 7 | 155 (84) [3] | 168 (97) [3] |
| 8 | 0 (0) | 48 (28) [3] |
| 9 | 0 (0) | 1 (1) [2] |
| 10R | 13 (7) [3] | 0 (0) |
| 10L | 1 (1) [1] | 0 (0) |
| 11R | 46 (25) [2.5] | 0 (0) |
| 11L | 66 (36) [3] | 0 (0) |
Abbreviations: EBUS, endobronchial ultrasound; EUS, endoscopic ultrasound; IQR, interquartile range; LN, lymph node.
Patients could receive a combination of benzodiazepines with opioids, or propofol and opioids.
In the EBUS group, 165 patients had a final diagnosis of sarcoidosis, and in the EUS‐B group 141 patients had a final diagnosis of sarcoidosis.
Procedure time not recorded for 24 patients in the EBUS‐group, and for 12 patients in the EUS‐B group.
EUS‐B group
Among 173 patients undergoing an oesophageal procedure, the vast majority (165/173, 95%) of enlarged lymph nodes were located within the reach of EUS‐B, with a median short axis of the largest lymph node of 18 mm (IQR, 15–22). In 164 of 173 (94%) patients, the endoscopist was able to perform a complete systematic endosonographic evaluation of accessible mediastinal nodes using the EUSAT 16 and perform at least five lymph node needle aspirations. Common B‐mode findings for the lymph nodes of patients with a final diagnosis of sarcoidosis were hypervascularization in 43% (61/141), node clustering in 67% (95/141), oval shaped in 74% (104/141), homogeneity in 83% (117/141), distinct margins in 90% (127/141), absence of coagulation necrosis sign in 89% (125/141) and absence of central hilar structure in 79% (111/141) of patients. The mean number of sampled lymph node stations per patient was 1.8 (range, 0–3). Median procedure time was 18 min (IQR, 14–24). Prophylactic antibiotics were used in 77 (45%) of 173 patients. Further procedural endoscopy details are provided in Table 2. Of the 173 patients, 48 (28%) underwent an additional (endoscopy) procedure (e.g., EBUS [n = 35], bronchoscopy [n = 4], mediastinoscopy [n = 4]) resulting in granuloma detection in six additional patients not found by EUS‐B.
Final diagnoses
Final diagnoses determined at 6 months after randomization were sarcoidosis in 306 patients (86%), malignancy in 16 patients (4%), TB in two patients (0.5%) or another diagnosis in 34 patients (9.5%). The diagnosis of sarcoidosis was supported by the presence of granulomas in 280 of 306 patients, whereas in the remaining 26 patients it was based on clinical and radiological findings at baseline and during follow‐up or on the results of additional (endoscopy) procedures (other than the randomization arm procedure) (Table 3).
TABLE 3.
Granuloma detection rate, sensitivity and final diagnoses by group
| EBUS (n = 185) | EUS‐B (n = 173) | |
|---|---|---|
| Granuloma detection rate, no. (%, 95% CI) | 130 (70, 63–76) | 118 (68, 61–75) |
| Sensitivity for diagnosing sarcoidosis, % (95% CI) | 78 (71–84) | 82 (74–87) |
| Final diagnosis at 6 months after randomization, no. (%) | ||
| Sarcoidosis | 165 (89) | 141 (82) |
| Lymphoma | 4 (2) | 3 (2) |
| Lung cancer | 2 (1) | 5 (3) |
| Post‐inflammation/reactive nodal disease | 8 (4.5) | 13 (7) |
| Tuberculosis | 0 | 2 (1) |
| Other | 6 (3.5) | 5 (3) |
| Unknown | 0 | 4 (2) |
Abbreviations: EBUS, endobronchial ultrasound; EUS, endoscopic ultrasound; 95%CI, 95% confidence interval
Study outcomes
The granuloma detection rate was 70% (130/185; 95% CI, 63–76) for EBUS, and 68% (118/173; 95% CI, 61–75) for EUS‐B (p = 0.67; Table 3) and independent of the geographic localization. Sensitivity for diagnosing sarcoidosis was 78% (129/165; 95% CI, 71–84) for EBUS, and 82% (115/141; 95% CI, 74–87) for EUS‐B (p = 0.46; Table 3).
In the overall study population, the percentage of representative TBNA/FNA samples defined as (non‐)caseating granulomas, reactive lymph node or malignant disease was 90.4% (161/178; 95% CI, 85–94) in the standard 22‐G needle group and 91.1% (164/180; 95% CI, 86–94) in the 25‐G ProCore needle group (p = 0.829). Among patients in whom a standard 22‐G needle was used, granuloma detection rate was 70% (125/178; 95% CI, 63–76) in the overall study population, 70% (67/96; 95% CI, 60–78) in the EBUS procedural subgroup and 71% (58/82; 95% CI, 60–79) in the EUS‐B procedural subgroup (p = 0.89). Among patients in whom a 25‐G ProCore needle was used, granuloma detection rate was 68% (123/180; 95% CI, 61–75) in the overall study population, 71% (63/89; 95% CI, 61–79) in the EBUS procedural subgroup and 66% (60/91; 95% CI, 56–75) in the EUS‐B procedural subgroup (p = 0.48).
One serious adverse event was reported: a patient with a final diagnosis of sarcoidosis developed mediastinitis 10 weeks after an EBUS procedure with a standard 22‐G needle, requiring antibiotics; no prophylactic antibiotics had been administered in this patient prior to the endoscopy procedure. The patient recovered completely. No serious adverse events were reported in the EUS‐B group.
DISCUSSION
In this randomized clinical trial, we found that endobronchial (EBUS) and oesophageal (EUS‐B) sampling of intrathoracic lymph nodes have a similar granuloma detection rate and sensitivity in patients with presumed sarcoidosis stage I and II, irrespective of the needle type used. Adverse events were rare for both procedures, indicating that they can both be safely applied in this clinical setting. Procedure time of both approaches was in the same range.
Some of our findings are in line with the results of previous, non‐randomized, studies on the diagnostic accuracy of endosonography in granuloma detection in patients with suspected sarcoidosis. Two systematic reviews showed a diagnostic yield of EBUS‐TBNA ranging from 33% to 100% in individual studies, with a pooled diagnostic accuracy of 79% (95% CI, 71–86). 20 , 21 Multiple studies evaluated the diagnostic yield of transoesophageal EUS‐FNA using the conventional EUS scope, which ranged from 82% to 100% in patients with a final diagnosis of sarcoidosis. 3 , 4 , 5 A study on transoesophageal bronchoscopic ultrasound‐guided FNA using the EBUS scope (EUS‐B‐FNA) showed granulomas in 86% of patients with the final diagnosis of sarcoidosis. 22
To the best of our knowledge, EBUS‐TBNA and EUS‐B‐FNA have not been directly compared previously among patients with suspected sarcoidosis. The granuloma randomized clinical trial compared conventional bronchoscopy (with TBLB and endobronchial lung biopsies) with endosonography (EBUS or conventional EUS) and showed that the use of endosonographic nodal aspiration resulted in greater diagnostic yield for a tissue confirmation of sarcoidosis. In contrast to our findings, the authors reported in the endosonography group that EUS‐FNA (with a conventional EUS scope) seemed to have a higher diagnostic yield than EBUS‐TBNA (88% vs. 66%; p < 0.01). 8 However, patient selection bias can possibly explain this difference, as the decision to perform an oesophageal or endobronchial procedure was left to the local investigator. In sarcoidosis, the mean number of affected intrathoracic lymph node stations can be as high as 8 per patient and the subcarinal station (that can be reached both endobronchially and oesophageally) is affected in almost all patients with stage I/II. 9 This seems a likely explanation for the similar granuloma detection rate of both routes (EBUS and EUS‐B) that was found in the current trial.
EBUS‐TBNA and EUS‐B‐FNA are safe procedures. Mediastinitis/mediastinal abscess is described in 1% of patients after an EUS‐FNA procedure in sarcoidosis. 5 Mediastinitis as a complication of EBUS‐TBNA is described in 0.10% (95% CI, 0.02–0.17) of procedures for evaluation of mediastinal or hilar lymph node lesions, 23 although bacteraemia without clinically significant infection was found in 7% of patients. 24 In the present study, a single patient developed mediastinitis after an EBUS‐TBNA of a mediastinal lymph node (after 10 weeks).
Several limitations apply to the present study. The final diagnosis was sarcoidosis in the vast majority of cases as the majority of the participating centres were not located in a region with high prevalence of TB. Therefore, the results may not be reproducible to such settings. The diagnosis after 6 months as determined by the treating physician was considered the reference standard in this study. Although this strategy has been used in other studies on sarcoidosis diagnosis, we acknowledge that this contains a level of subjectivity. Based on previous literature, we assumed granuloma detection rates of 75% for EBUS‐TBNA and 90% for EUS‐B‐FNA in our sample size calculation. However, these were only 70% and 68%, respectively, in our study. Most likely, the retrospective nature of most previous studies resulted in selected patients and an overestimation of diagnostic yield, which cannot be extrapolated to a consecutive unselected sample of patients with suspected sarcoidosis. The outcomes of this study demonstrate that it is important to conduct multicentre, prospective, international studies as the results are likely to be a better reflection of the true diagnostic value than single‐centre retrospective studies.
To the best of our knowledge this is the largest randomized clinical trial on diagnostic approaches for sarcoidosis. Strong points of the study are its randomized design, multicentre and global setting. The current study was performed in a well‐defined study population, and endosonography procedures were performed with a structured and well‐documented approach. Patients were included in both general and university hospitals of four continents in the world.
There is an ongoing clinical debate whether it is essential to establish a pathological diagnosis in all patients with suspected sarcoidosis, as a clinical presentation of stage I/II disease can be characteristic based on clinical presentation and imaging only, and invasive diagnostic testing may be unnecessary. 25 , 26 However, sarcoidosis can mimic serious disorders which, if undiagnosed and untreated, may be associated with significant morbidity or mortality. In our study population, over 15% of patients had a final diagnosis other than sarcoidosis including lymphoma and lung cancer, underlining the need for appropriate and careful patient selection for invasive nodal sampling, both in a clinical setting and for study participation. In clinical practice, endoscopists may be inclined to initiate the endobronchial procedure with standard bronchoscopy, followed by EBUS. In our study, we only evaluated EBUS, and adding bronchoscopy with mucosal and transbronchial biopsies could increase granuloma detection rate. However, this added value is likely to be limited, as of the 37 patients in the EBUS group who underwent subsequent bronchoscopy, granulomas were found in only one patient. Our findings also imply that adding EBUS to EUS‐B (or the other way around) is not likely to improve granuloma detection rate to a considerable extent: 25 patients (14%) in the EBUS group underwent subsequent EUS‐B, revealing granulomas not detected by EBUS in only one patient. Of the 35 patients (20%) in the EUS‐B group who underwent subsequent EBUS, no additional granulomas not already detected by EUS‐B were found. These findings imply that performing additional endoscopic techniques may only be useful in selected patients, for example, in case lymph nodes cannot be sampled during the initial diagnostic procedure.
Despite a similar granuloma detection rate identified between EBUS and EUS‐B in the current trial, parameters affecting the choice of a diagnostic procedure for confirming sarcoidosis include endoscopists' competence, and availability of the diagnostic test and availability of moderate/deep sedation. An advantage of EBUS is that hilar (station #10) and inter‐lobar (station #11) lymph nodes, which are very commonly involved in sarcoidosis, can be sampled from the airways and not from the oesophagus. Besides, the endobronchial route allows to perform, in a single session, both EBUS‐TBNA and additional sampling procedures such as bronchoalveolar lavage and endobronchial and trans‐bronchial biopsies. This strategy has been shown to optimize the diagnostic yield in sarcoidosis, especially in stage II disease. 6 , 27 On the other hand, lymph nodes more difficult to sample (station #4L) or inaccessible (station #8L) from the airways can be sampled from the oesophagus through EUS‐B. Other advantages of an EUS‐B procedure are the fewer doses of anaesthetics and sedatives generally needed, and fewer oxygen desaturations. 11
In conclusion, granuloma detection rate of mediastinal/hilar nodes by endosonography in patients suspected of sarcoidosis stage I/II is high, geographic independent and does not significantly differ for endobronchial versus transoesophageal sampling routes. Parameters affecting the choice of a diagnostic procedure for confirming sarcoidosis include endoscopists' competence, patient's general status, availability of bronchoscopy/endosonography equipment and availability of sedation.
CONFLICT OF INTEREST
This is an investigator‐initiated study. The institution received a financial contribution for the database and a PhD student from Cook Medical. The institutions purchased the needles used in this study for a reduced fee. The complete design, execution, analysis and presentation of results were performed by the investigators without any input from Cook Medical or any other external party. The individual investigators made no other disclosures.
AUTHOR CONTRIBUTION
Kirsten Kalverda: Data curation (supporting); formal analysis (supporting); investigation (supporting); project administration (supporting); software (supporting); writing – review and editing (supporting). Artur Szlubowski: Investigation (equal); project administration (equal). Maciej Gnass: Investigation (equal); project administration (supporting). Kurt G. Tournoy: Conceptualization (supporting); investigation (equal); project administration (equal). Jiayuan Sun: Investigation (equal); project administration (supporting). Masahide Oki: Investigation (equal); project administration (supporting). Maarten K. Ninaber: Investigation (equal); project administration (supporting). Daniel Steinfort: Investigation (equal); project administration (equal). Barton Jennings: Investigation (equal); project administration (equal). Moishe Liberman: Investigation (equal); project administration (equal). Semra Bilaceroglu: Investigation (equal); project administration (equal). Peter I. Bonta: Conceptualization (supporting); investigation (equal); methodology (supporting); project administration (equal); writing – review and editing (supporting). Daniël A. Korevaar: Conceptualization (supporting); data curation (equal); formal analysis (equal); methodology (equal); writing – review and editing (equal). Rocco Trisolini: Investigation (equal); project administration (equal); supervision (equal); writing – review and editing (equal). Jouke T. Annema: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (lead); investigation (equal); methodology (equal); project administration (equal); resources (equal); software (supporting); supervision (lead); validation (equal); writing – original draft (supporting); writing – review and editing (equal). Laurence M. M. Crombag: Conceptualization (lead); data curation (lead); formal analysis (lead); funding acquisition (supporting); investigation (lead); methodology (lead); project administration (lead); resources (equal); software (lead); supervision (supporting); validation (lead); writing – original draft (lead); writing – review and editing (equal).
HUMAN ETHICS APPROVAL DECLARATION
This study was performed in accordance with the Declaration of Helsinki and approved by the Medical Ethical Committee of Amsterdam University Medical Centers. All adult participants provided written informed consent to participate in this study (Clinical trial registration: NCT02540694 at ClinicalTrials.gov). Participant registration took place from May 2015 to Aug 2017.
Crombag LMM, Mooij‐Kalverda K, Szlubowski A, Gnass M, Tournoy KG, Sun J, et al. EBUS versus EUS‐B for diagnosing sarcoidosis: The International Sarcoidosis Assessment (ISA) randomized clinical trial. Respirology. 2022;27:152–160. 10.1111/resp.14182
Associate Editor: Paul Thomas and Senior Editor: Phan Nguyen
DATA AVAILABILITY STATEMENT
The study protocol including the statistical analysis plan is available. The (deidentified) data that underlie the results reported in this article are available on request. The corresponding author will decide, depending on the type of planned analysis, whether these data will be shared.
REFERENCES
- 1. Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Muller‐Quernheim J. Sarcoidosis. Lancet (London, England). 2014;383(9923):1155–67. [DOI] [PubMed] [Google Scholar]
- 2. Crouser ED, Maier LA, Wilson KC, Bonham CA, Morgenthau AS, Patterson KC, et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(8):e26–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Annema JT, Veseliç M, Rabe KF. Endoscopic ultrasound‐guided fine‐needle aspiration for the diagnosis of sarcoidosis. Eur Respir J. 2005;25(3):405–9. [DOI] [PubMed] [Google Scholar]
- 4. Fritscher‐Ravens A, Sriram PV, Topalidis T, Hauber HP, Meyer A, Soehendra N, et al. Diagnosing sarcoidosis using endosonography‐guided fine‐needle aspiration. Chest. 2000;118(4):928–35. [DOI] [PubMed] [Google Scholar]
- 5. von Bartheld MB, Veseliç‐Charvat M, Rabe KF, Annema JT. Endoscopic ultrasound‐guided fine‐needle aspiration for the diagnosis of sarcoidosis. Endoscopy. 2010;42(3):213–7. [DOI] [PubMed] [Google Scholar]
- 6. Gupta D, Dadhwal DS, Agarwal R, Gupta N, Bal A, Aggarwal AN. Endobronchial ultrasound‐guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146(3):547–56. [DOI] [PubMed] [Google Scholar]
- 7. Tremblay A, Stather DR, MacEachern P, Khalil M, Field SK. A randomized controlled trial of standard vs endobronchial ultrasonography‐guided transbronchial needle aspiration in patients with suspected sarcoidosis. Chest. 2009;136(2):340–6. [DOI] [PubMed] [Google Scholar]
- 8. von Bartheld MB, Dekkers OM, Szlubowski A, Eberhardt R, Herth FJ, in 't Veen JC, et al. Endosonography vs conventional bronchoscopy for the diagnosis of sarcoidosis: the GRANULOMA randomized clinical trial. JAMA. 2013;309(23):2457–64. [DOI] [PubMed] [Google Scholar]
- 9. Trisolini R, Anevlavis S, Tinelli C, Orlandi P, Patelli M. CT pattern of lymphadenopathy in untreated patients undergoing bronchoscopy for suspected sarcoidosis. Respir Med. 2013;107(6):897–903. [DOI] [PubMed] [Google Scholar]
- 10. Madan K, Mittal S, Madan NK, Tiwari P, Jain D, Arava S, et al. EBUS‐TBNA versus EUS‐B‐FNA for the evaluation of undiagnosed mediastinal lymphadenopathy: the TEAM randomized controlled trial. Clin Respir J. 2020;14(11):1076–82. [DOI] [PubMed] [Google Scholar]
- 11. Oki M, Saka H, Ando M, Tsuboi R, Nakahata M, Oka S, et al. Transbronchial vs transesophageal needle aspiration using an ultrasound bronchoscope for the diagnosis of mediastinal lesions: a randomized study. Chest. 2015;147(5):1259–66. [DOI] [PubMed] [Google Scholar]
- 12. Korevaar DA, Crombag LM, Cohen JF, Spijker R, Bossuyt PM, Annema JT. Added value of combined endobronchial and oesophageal endosonography for mediastinal nodal staging in lung cancer: a systematic review and meta‐analysis. Lancet Respir Med. 2016;4(12):960–8. [DOI] [PubMed] [Google Scholar]
- 13. Dhooria S, Sehgal IS, Prasad KT, Muthu V, Gupta N, Bal A, et al. Diagnostic yield and safety of the ProCore versus the standard EBUS‐TBNA needle in subjects with suspected sarcoidosis. Expert Rev Med Devices. 2021;18(2):211–6. [DOI] [PubMed] [Google Scholar]
- 14. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Konge L, Clementsen PF, Ringsted C, Minddal V, Larsen KR, Annema JT. Simulator training for endobronchial ultrasound: a randomised controlled trial. Eur Respir J. 2015;46(4):1140–9. [DOI] [PubMed] [Google Scholar]
- 16. Konge L, Vilmann P, Clementsen P, Annema JT, Ringsted C. Reliable and valid assessment of competence in endoscopic ultrasonography and fine‐needle aspiration for mediastinal staging of non‐small cell lung cancer. Endoscopy. 2012;44(10):928–33. [DOI] [PubMed] [Google Scholar]
- 17. Trisolini R, Tinelli C, Cancellieri A, Paioli D, Alifano M, Boaron M, et al. Transbronchial needle aspiration in sarcoidosis: yield and predictors of a positive aspirate. J Thorac Cardiovasc Surg. 2008;135(4):837–42. [DOI] [PubMed] [Google Scholar]
- 18. Spagnolo P, Rossi G, Trisolini R, Sverzellati N, Baughman RP, Wells AU. Pulmonary sarcoidosis. Lancet Respir Med. 2018;6(5):389–402. [DOI] [PubMed] [Google Scholar]
- 19. Costabel U, Hunninghake GW. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14(4):735–7. [DOI] [PubMed] [Google Scholar]
- 20. Agarwal R, Srinivasan A, Aggarwal AN, Gupta D. Efficacy and safety of convex probe EBUS‐TBNA in sarcoidosis: a systematic review and meta‐analysis. Respir Med. 2012;106(6):883–92. [DOI] [PubMed] [Google Scholar]
- 21. Trisolini R, Lazzari Agli L, Tinelli C, De Silvestri A, Scotti V, Patelli M. Endobronchial ultrasound‐guided transbronchial needle aspiration for diagnosis of sarcoidosis in clinically unselected study populations. Respirology (Carlton, Vic). 2015;20(2):226–34. [DOI] [PubMed] [Google Scholar]
- 22. Oki M, Saka H, Kitagawa C, Kogure Y, Murata N, Adachi T, et al. Transesophageal bronchoscopic ultrasound‐guided fine needle aspiration for diagnosis of sarcoidosis. Respiration. 2013;85(2):137–43. [DOI] [PubMed] [Google Scholar]
- 23. Asano F, Aoe M, Ohsaki Y, Okada Y, Sasada S, Sato S, et al. Complications associated with endobronchial ultrasound‐guided transbronchial needle aspiration: a nationwide survey by the Japan Society for Respiratory Endoscopy. Respir Res. 2013;14:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Steinfort DP, Johnson DF, Irving LB. Incidence of bacteraemia following endobronchial ultrasound‐guided transbronchial needle aspiration. Eur Respir J. 2010;36(1):28–32. [DOI] [PubMed] [Google Scholar]
- 25. Mehta AC, Almeida FA. Choose wisely: endobronchial ultrasound‐guided transbronchial needle aspiration for sarcoidosis. Chest. 2014;146(3):530–2. [DOI] [PubMed] [Google Scholar]
- 26. Ribeiro Neto ML, Culver DA, Mehta AC. Sarcoidosis – no business of the bronchoscopist. J Thorac Cardiovasc Surg. 2012;144(5):1276–7; author reply 1277. [DOI] [PubMed] [Google Scholar]
- 27. Trisolini R, Lazzari Agli L, Cancellieri A, Poletti V, Candoli P, Paioli D, et al. Transbronchial needle aspiration improves the diagnostic yield of bronchoscopy in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21(2):147–51. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study protocol including the statistical analysis plan is available. The (deidentified) data that underlie the results reported in this article are available on request. The corresponding author will decide, depending on the type of planned analysis, whether these data will be shared.


